Abstract
The bacterial pathogen Neisseria gonorrhoeae encodes for an α-class carbonic anhydrase (CA, EC 4.2.1.1), NgCA, which was investigated for its inhibition with a series of inorganic and organic anions. Perchlorate and hexafluorophosphate did not significantly inhibit NgCA CO2 hydrase activity, whereas the halides, azide, bicarbonate, carbonate, stannate, perosmate, diphosphate, divanadate, perruthenate, and trifluoromethanesulfonate showed inhibition constants in the range of 1.3–9.6 mM. Anions/small molecules such as cyanate, thiocyanate, nitrite, nitrate, bisulphite, sulphate, hydrogensulfide, phenylboronic acid, phenylarsonic acid, selenate, tellurate, tetraborate, perrhenate, peroxydisulfate, selenocyanate, iminodisulfonate, and fluorosulfonate showed KIs in the range of 0.15–1.0 mM. The most effective inhibitors detected in this study were sulfamide, sulfamate, trithiocarbonate and N,N-diethyldithiocarbamate, which had KIs in the range of 5.1–88 µM. These last compounds incorporating the CS2- zinc-binding group may be used as leads for developing even more effective NgCA inhibitors in addition to the aromatic/heterocyclic sulphonamides, as this enzyme was recently validated as an antibacterial drug target for obtaining novel antigonococcal agents
1. Introduction
The metalloenzyme carbonic anhydrase (CA, EC 4.2.1.1) is common and widespread in prokaryotesCitation1,Citation2, organisms in which it catalyses the interconversion between CO2 and bicabornate, thus participating to crucial physiologic processes connected with pH regulation, metabolism, acclimation of the organism in various niches in which it lives, and in the case of pathogenic organisms, also acting as a virulence factorCitation3,Citation4. Bacteria encode for four CA genetic families, the α-, β-, γ- and ι-CAs, some of which have been characterised in detail for many of the common pathogens, such as Escherichia coli, Helycobacter pylori, Mycobaterium tuberculosis, Vibrio cholerae, Pseudomonas aeruginosa, Staphylococcus aureus, Porphyromonas gingivalis, Streptococcus spp., etc.Citation3–10. Considering bacterial CAs as potential drug targets for antiinfectives was already proposed some time agoCitation3, and although this idea initially met resistance from the scirntific community, a range of new data show indeed that inhibiting these enzymes may lead to significant bacteriostatic or bactericidal effects, the best examples being for the moment the sulphonamide CA inhibitors (CAIs) impairing the growth of H. pyloriCitation3,Citation4, E. coliCitation5, M. tuberculosisCitation6 and more recently, the vancomycin resistant enterococci (VRE)Citation11. For the last pathogens, which represent a major public health threat, the sulphonamide CAIs acetazolamide and some of its derivatives, as well as dorzolamide, outperformed linezolid, the drug of choice for treating VRE infections, as shown recently by Flaherty’s groupCitation11. All these promising new data offer the long-awaitedCitation12 proof-of-concept that inhibition of bacterial CAs may lead to antibiotics with a novel mechanisms of action, less prone to the development of drug resistance, as demonstrated for H. pylori and ethoxzolamide, case in which a certain level of mutations were observed in several bacterial genes, including the α-CA one, but the pathogen remained susceptible to the drug at low enough, clinically relevant concentrationsCitation4.
Neisseria gonorrhoeae is a pathogenic bacterium which became a global health concern as it provokes a sexually-transmitted disease difficult to treat due to increased levels of drug resistance to a wide range of antibiotics, including last generation cephalosporinsCitation13–15. Recently, we have demonstrated that the α-CA present in its genome, NgCA, is a druggable target, and its inhibition with sulphonamide inhibitors based on the acetazolamide scaffold induced a significant antigonococcal activity without toxicity to the host cellsCitation15. As there is a stringent need for novel antigonococcal agents, a deeper investigation of this enzyme and profiling of various calsses of inhibitors may be of great interest. Here we investigate anions and other small molecules as inhibitors of NgCA, considering that these compounds represent a classical type of CAIsCitation16.
2. Materials and methods
2.1. Chemistry
Anions and small molecules were commercially available reagents of the highest available purity from Sigma-Aldrich (Milan, Italy). Purity of tested compounds was higher than 99%.
2.2. Enzymology
NgCA was a recombinant enzyme obtained in-house as described earlierCitation15.
2.3. Ca activity and inhibition measurements
An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activityCitation17. Phenol red at a concentration of 0.2 mM was used as pH indicator, working at the absorbance maximum of 557 nm, with 10 mM Hepes (pH 7.4) as buffer, and in the presence of 10 mM NaClO4 for maintaining constant the ionic strength, following the initial rates of the CA-catalysed CO2 hydration reaction for a period of 10–100 s. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constants. For each inhibitor, at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The uncatalyzed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitors (10–20 mM) were prepared in distilled-deionized water and dilutions up to 0.01 µM were done thereafter with the assay buffer. Inhibitor and enzyme solutions were preincubated together for 15 min at room temperature prior to assay, in order to allow for the formation of the E-I complex. The inhibition constants were obtained by non-linear least-squares methods using PRISM 3 and the Cheng-Prusoff equation, whereas the kinetic parameters for the uninhibited enzymes from Lineweaver-Burk plots, as reported earlierCitation18–20, and represent the mean from at least three different determinations. NgCA concentration in the assay system was of 6.5 nM.
3. Results and discussion
The possibility to use CAIs for inhibiting the growth of N. gonorrhoeae was explored in vitro already in the 60 sCitation21, whereas only in the ‘90 s Carter’s groupCitation22 reported the presumed presence of CAs in these paathogens, by using a monospecific antibody prepared against the purified Neisseria sicca enzyme. In that studyCitation22 it has been shown that several all Neisseria spp., including various isolates of N. sicca, N. gonorrhoeae, N. meningitidis and N. lactamica, were sensitive to the sulphonamide CAI acetazolamide, but thereafter, the enzyme was purified and characterised only in 1997 by Lindskog’s groupCitation23, who showed that NgCA is an α-class CA possessing a high catalytic activityCitation23–25, with a kcat for the CO2 hydration reaction of 1.7 × 106 s − 1. In the same work it has been demonstrated that NgCA was inhibited by metal complexing anions such as cyanide, cyanate, thiocyanate, and azide (as determined by using the esterase actvity of the enzyme with 4-nitrophenyl acetate as substrateCitation23), whereas the X-ray crystal structure of the enzyme alone or in complex with acetazolamide was also reportedCitation25. These interesting studies, which could open the era of the CAIs as antiinfectives were however not continued, probably due to the retirement of Prof. Lindskog.
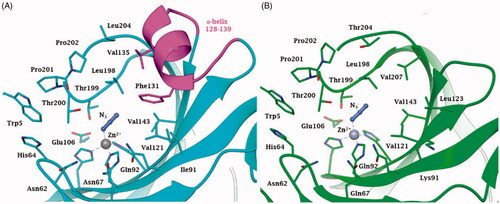
Indeed, the tridimensional structure of NgCA complexed with the anion inhibitor azide () shows an active site architecture quite similar to that of the dominant human (h) isoform, hCA II. The metal ion coordination and the binding mode of the anion inhibitor are identical in the two enzymes (), as well as the other residues involved in catalysis/inhibition, among which the dyad Glu106 – Thr199, which orientates the substrate CO2 for the nucleophilic attack and participates to the binding of inhibitors tooCitation12,Citation25. The main difference between the human and the bacterial enzyme is represented by the α-helix loop 128–139 () which is present in the human and absent in the bacterial enzyme (the same situation was observed also for the α-CA from H. pylori and exploited to obtain inhibitors with higher affinity for the bacterial than the human enzymeCitation4).
Figure 1. Active site view of A. hCA II (pdb 1RAY) and B. NgCA (pdb 1KOP) in adduct with the azide anion N3-. Amino acid residues of NgCA are renumbered according to the corresponding residues from hCA II. The zinc ion, represented as a grey sphere, is coordinated by three His residues, that are His94, His96 and His119, and the azide anion. Residues constituting the α-helix portion 128–139 are coloured magenta in hCA II, while being absent in NgCA.
Thus, considering the scarcity of NgCA inhibition data, for which only 4 anions were investigated using the esterase activityCitation23, as mentioned earlier, together with the extensive sulphonamide inhibition study reported recently by usCitation15, we investigated here the inhibition profile of this enzyme with a large series of inorganic metal-complexing, simple and complex anions, as well as small molecules known to act as CAIsCitation12,Citation16, such as sulfamide, sulphamic acid, phenylboronic acid, phenylarsonic acid and N,N-diethyl-dithiocarbamate (). The stopped-flow, CO2 hydrase assay was used to measure the inhibition constants shown in , and the same data for the inhibition of the red blood cell human isoforms hCA I and IICitation12,Citation16 were also included for comparison reasons. The following should be noted regarding the NgCA inhibition profile with anions and small molecules:
Table 1. Inhibition constants (KIs) of anion inhibitors against hCA I, II and NgCA by a stopped flow CO2 hydration assayCitation17.
anions known for their lower affinity to complexate metal ions, such as perchlorate and hexafluorophosphate, did not inhibit NgCA significantly up to 100 mM concentration of inhibitor in the assay system, which is also the case for their interaction with hCA I and II, as well as many other CAs belonging to all known classes. This is also the reason why we use perchlorate at 10 mM for maintaining constant the ionic strength in the stopped-flow assays, as mentioned in Materials and methods;
Inhibition constants in the range of 1.3–9.6 mM were measured for the following anions: all the halides, azide, bicarbonate, carbonate, stannate, perosmate, diphosphate, divanadate, perruthenate, and trifluoromethanesulfonate. The last anion is an interesting case, as it does not considerably inhibits hCA I and II (KIs > 100 mM) being thus an NgCA-selective (although weak) inhibitor. Among the halides, fluoride and iodide were weaker inhibitors than chloride and bromide (which was the most effective one among the halides). Bicarbonate, with a KI of 1.3 mM is a rather effective inhibitor (and this substrate of the enzyme is a much weaker hCA I and especially hCA II inhibitor, see ), which presumably may constitute a way to inhibit the activity of this highly effective enzyme by one of the reaction products, which might be physiologically significant. This hypothesis needs to be checked, but it is known for example that in the case of V. cholerae, bicarbonate, generated by the activity of the various CAs present in this pathogen, induces virulence gene expressionCitation1;
the following anions showed submillimolar (cyanide had a KI of 1 mM) affinity for NgCA: cyanate, thiocyanate, nitrite, nitrate, bisulphite, sulphate, hydrogensulfide, phenylboronic acid, phenylarsonic acid, selenate, tellurate, tetraborate, perrhenate, peroxydisulfate, selenocyanate, iminodisulfonate, and fluorosulfonate, for which KIs in the range of 0.15 – 1.0 mM were measured (). In the case of phenylboronic acid and phenylarsonic acid, there is also a selective inhibition of the bacterial enzyme versus the human isoforms, which were much less sensitive to inhibition with these two compounds. Based on these data we hypothesise that boronic acid derivativesCitation26 or benzoxaborolesCitation27, which were shown to act as CAIs for other enzymes, might lead to the development of more effective and possibly NgCA-selective inhibitors.
the most effective NgCA inhibitors among the tested anions/small molecules were sulfamide, sulfamate, trithiocarbonate and N,N-diethyldithiocarbamate, which had KIs in the range of 5.1 – 88 µM. Sulfamide and sulfamate incorporate the sulfamoyl (SO2NH2) moiety found in many of the most effective aromatic/heterocyclic sulphonamides CAIsCitation15,Citation16, whereas trithiocarbonate and N,N-diethyldithiocarbamate incorporate another interesting zinc-binding group for the design of CAIs, the CS2- moietyCitation28, which led to the discovery of dithiocarbamates, monothiocarbamates and tritiocarbonates as effective CAIsCitation29,Citation30. The very effective inhibitory activity of these small molecules against NgCA prompt us to propose them as interesting leads for developing more effective compounds that can inhibit the activity of this enzyme and presumably interfere with the life cycle of this pathogenic bacterium.
4. Conclusions
NgCA, a high activity α-CA present in the genome of the bacterial pathogen N. gonorrhoeae was investigated for its inhibition with a series of inorganic and organic anions. Perchlorate and hexafluorophosphate, did not inhibit NgCA significantly, whereas the halides, azide, bicarbonate, carbonate, stannate, perosmate, diphosphate, divanadate, perruthenate, and trifluoromethanesulfonate showed inhibition constants in the range of 1.3–9.6 mM. Anions/small molecules such as cyanate, thiocyanate, nitrite, nitrate, bisulphite, sulphate, hydrogensulfide, phenylboronic acid, phenylarsonic acid, selenate, tellurate, tetraborate, perrhenate, peroxydisulfate, selenocyanate, iminodisulfonate, and fluorosulfonate showed KIs in the range of 0.15–1.0 mM. The most effective inhibitors detected in this study were sulfamide, sulfamate, trithiocarbonate and N,N-diethyldithiocarbamate, which had KIs in the range of 5.1–88 µM. These last compounds incorporating the CS2- zinc-binding group may be used as leads for developing even more effective NgCA inhibitors, as this enzyme was recently validated as an antibacterial drug target for obtaining novel antigonococcal agents.
Acknowledgements
CT Supuran thank the Italian Ministry for University and Research (MIUR), project FISR2019_04819 BacCAD. The research program was also partially funded by a Purdue Institute for Drug Discovery Programmatic Grant and NIH/NIAID 1R01AI148523 (to D.P.F.).
Disclosure statement
CT Supuran is Editor-in-Chief of Journal of Enzyme Inhibition and Medicinal Chemistry and he was not involved in the assessment, peer review or decision making process of this paper. The authors have no relevant affiliations of financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- (a) Smith KS, Jakubzick C, Whittam TS, Ferry JG. Carbonic anhydrase is an ancient enzyme widespread in prokaryotes. Proc Natl Acad Sci USA 1999;96:15184–9. (b) Capasso C, Supuran CT. An overview of the alpha-, beta- and gamma-carbonic anhydrases from Bacteria: can bacterial carbonic anhydrases shed new light on evolution of bacteria? J Enzyme Inhib Med Chem 2015;30:325–32. (c) Abuaita BH, Withey JH. Bicarbonate Induces Vibrio cholerae virulence gene expression by enhancing ToxT activity. Infect Immun 2009;77:4111–20.
- (a) Merlin C, Masters M, McAteer S, Coulson A. Why is carbonic anhydrase essential to Escherichia coli? J Bacteriol 2003;185:6415–24. (b) Vullo D, Kumar RSS, Scozzafava A, et al. Sulphonamide inhibition studies of the β-carbonic anhydrase from the bacterial pathogen Clostridium perfringens. J Enzyme Inhib Med Chem 2018;33:31–6. (c) Supuran CT, Capasso C. Biomedical applications of prokaryotic carbonic anhydrases. Expert Opin Ther Pat 2018;28:745–54. (d) Del Prete S, Nocentini A, Supuran CT, Capasso C. Bacterial ι-carbonic anhydrase: a new active class of carbonic anhydrase identified in the genome of the Gram-negative bacterium Burkholderia territorii. J Enzyme Inhib Med Chem 2020;35:1060–8.
- (a) Supuran CT, Capasso C. Antibacterial carbonic anhydrase inhibitors: an update on the recent literature. Expert Opin Ther Pat 2020;30:963–82. (b) Supuran CT. Bacterial carbonic anhydrases as drug targets: toward novel antibiotics? Front Pharmacol 2011;2:34. (c) Campestre C, De Luca V, Carradori S, et al. Carbonic Anhydrases: New Perspectives on Protein Functional Role and Inhibition in Helicobacter pylori. Front Microbiol 2021;12:629163.
- (a) Rahman MM, Tikhomirova A, Modak JK, et al. Antibacterial activity of ethoxzolamide against Helicobacter pylori strains SS1 and 26695. Gut Pathog 2020;12:20. (b) Modak JK, Tikhomirova A, Gorrell RJ, et al. Anti-Helicobacter pylori activity of ethoxzolamide. J Enzyme Inhib Med Chem 2019;34:1660–7. (c) Modak JK, Liu YC, Supuran CT, Roujeinikova A. Structure-Activity Relationship for Sulfonamide Inhibition of Helicobacter pylori α-Carbonic Anhydrase. J Med Chem 2016;59:11098–109.
- (a) Del Prete S, Bua S, Supuran CT, Capasso C. Escherichia coliγ-carbonic anhydrase: characterisation and effects of simple aromatic/heterocyclic sulphonamide inhibitors . J Enzyme Inhib Med Chem 2020;35:1545–54. (b) Nocentini A, Del Prete S, Mastrolorenzo MD, et al. Activation studies of the β-carbonic anhydrases from Escherichia coli with amino acids and amines. J Enzyme Inhib Med Chem 2020;35:1379–86. (c) Del Prete S, De Luca V, Bua S, et al. The effect of substituted benzene-sulfonamides and clinically licensed drugs on the catalytic activity of CynT2, a carbonic anhydrase crucial for Escherichia coli Life Cycle. Int J Mol Sci 2020;21:4175.
- (a) Aspatwar A, Kairys V, Rala S, et al. Mycobacterium tuberculosis β-Carbonic Anhydrases: Novel Targets for Developing Antituberculosis Drugs. Int J Mol Sci 2019;20:5153. (b) Wani TV, Bua S, Khude PS, et al. Evaluation of sulphonamide derivatives acting as inhibitors of human carbonic anhydrase isoforms I, II and Mycobacterium tuberculosis β-class enzyme Rv3273. J Enzyme Inhib Med Chem 2018;33:962–71. (c) Aspatwar A, Hammarén M, Koskinen S, et al. β-CA-specific inhibitor dithiocarbamate Fc14-584B: a novel antimycobacterial agent with potential to treat drug-resistant tuberculosis. J Enzyme Inhib Med Chem 2017;32:832–40. (d) Carta F, Maresca A, Covarrubias AS, et al. Carbonic anhydrase inhibitors. Characterization and inhibition studies of the most active beta-carbonic anhydrase from Mycobacterium tuberculosis, Rv3588c. Bioorg Med Chem Lett 2009;19:6649–54. (e) Nishimori I, Minakuchi T, Vullo D, et al. Carbonic anhydrase inhibitors. Cloning, characterization, and inhibition studies of a new beta-carbonic anhydrase from Mycobacterium tuberculosis. J Med Chem 2009;52:3116–20.
- (a) Bonardi A, Nocentini A, Osman SM, et al. Inhibition of α-, β- and γ-carbonic anhydrases from the pathogenic bacterium Vibrio cholerae with aromatic sulphonamides and clinically licenced drugs - a joint docking/molecular dynamics study. J Enzyme Inhib Med Chem 2021;36:469–79. (b) Gitto R, De Luca L, Mancuso F, et al. Seeking new approach for therapeutic treatment of cholera disease via inhibition of bacterial carbonic anhydrases: experimental and theoretical studies for sixteen benzenesulfonamide derivatives. J Enzyme Inhib Med Chem 2019;34:1186–92. (c) Bua S, Berrino E, Del Prete S, et al. Synthesis of novel benzenesulfamide derivatives with inhibitory activity against human cytosolic carbonic anhydrase I and II and Vibrio cholerae α- and β-class enzymes. J Enzyme Inhib Med Chem 2018;33:1125–36. (d) Ferraroni M, Del Prete S, Vullo D, et al. Crystal structure and kinetic studies of a tetrameric type II β-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. Acta Crystallogr D Biol Crystallogr 2015;71:2449–56. (e) Del Prete S, Isik S, Vullo D, et al. DNA cloning, characterization, and inhibition studies of an α-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. J Med Chem 2012;55:10742–8.
- (a) Lotlikar SR, Kayastha BB, Vullo D, et al. Pseudomonas aeruginosa β-carbonic anhydrase, psCA1, is required for calcium deposition and contributes to virulence. Cell Calcium 2019;84:102080. (b) Murray AB, Aggarwal M, Pinard M, et al. Structural Mapping of Anion Inhibitors to β-Carbonic Anhydrase psCA3 from Pseudomonas aeruginosa. ChemMedChem 2018;13:2024–9. (c) Aggarwal M, Chua TK, Pinard MA, et al. Carbon Dioxide "Trapped" in a β-Carbonic Anhydrase. Biochemistry 2015;54:6631–8. (d) Pinard MA, Lotlikar SR, Boone CD, et al. Structure and inhibition studies of a type II beta-carbonic anhydrase psCA3 from Pseudomonas aeruginosa. Bioorg Med Chem 2015;23:4831–8.
- (a) Urbanski LJ, Bua S, Angeli A, et al. Sulphonamide inhibition profile of Staphylococcus aureus β-carbonic anhydrase. J Enzyme Inhib Med Chem 2020;35:1834–9. (b) Fan SH, Ebner P, Reichert S, et al. MpsAB is important for Staphylococcus aureus virulence and growth at atmospheric CO2 levels. Nat Commun 2019;10:3627.
- (a) Matsumoto Y, Miyake K, Ozawa K, et al. Bicarbonate and unsaturated fatty acids enhance capsular polysaccharide synthesis gene expression in oral streptococci, Streptococcus anginosus. J Biosci Bioeng 2019;128:511–7. (b) Capasso C, Supuran CT. An Overview of the Carbonic Anhydrases from Two Pathogens of the Oral Cavity: Streptococcus mutans and Porphyromonas gingivalis. Curr Top Med Chem 2016;16:2359–68. (c) Dedeoglu N, De Luca V, Isik S, et al. Cloning, characterization and anion inhibition study of a β-class carbonic anhydrase from the caries producing pathogen Streptococcus mutans. Bioorg Med Chem 2015;23:2995–3001. (d) Burghout P, Vullo D, Scozzafava A, et al. Inhibition of the β-carbonic anhydrase from Streptococcus pneumoniae by inorganic anions and small molecules: Toward innovative drug design of antiinfectives? Bioorg Med Chem 2011;19:243–8.
- (a) Kaur J, Cao X, Abutaleb NS, et al. Optimization of Acetazolamide-Based Scaffold as Potent Inhibitors of Vancomycin-Resistant Enterococcus. J Med Chem 2020;63:9540–62. (b) Abutaleb NS, Elkashif A, Flaherty DP, Seleem MN. In Vivo Antibacterial Activity of Acetazolamide. Antimicrob Agents Chemother 2021;65:e01715–20. (c) Abutaleb NS, Elhassanny AEM, Flaherty DP, Seleem MN. In vitro and in vivo activities of the carbonic anhydrase inhibitor, dorzolamide, against vancomycin-resistant enterococci. PeerJ 2021;9:e11059.
- (a) Supuran CT. Exploring the multiple binding modes of inhibitors to carbonic anhydrases for novel drug discovery. Expert Opin Drug Discov 2020;15:671–86. (b) Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81. (c) Mishra CB, Tiwari M, Supuran CT. Progress in the development of human carbonic anhydrase inhibitors and their pharmacological applications: Where are we today? Med Res Rev 2020;40:2485–565.
- (a) Golparian D, Sánchez-Busó L, Cole M, Unemo M. Neisseria gonorrhoeae Sequence Typing for Antimicrobial Resistance (NG-STAR) clonal complexes are consistent with genomic phylogeny and provide simple nomenclature, rapid visualization and antimicrobial resistance (AMR) lineage predictions. J Antimicrob Chemother 2021;76:940–4. (b) Turner JM, Connolly KL, Aberman KE, et al. Molecular Features of Cephalosporins Important for Activity against Antimicrobial-Resistant Neisseria gonorrhoeae. ACS Infect Dis 2021;7:293–308.
- (a) Yahara K, Ma KC, Mortimer TD, et al. Emergence and evolution of antimicrobial resistance genes and mutations in Neisseria gonorrhoeae. Genome Med 2021;13:51. (b) Jacobsson S, Cole MJ, Spiteri G, on behalf of The Euro-GASP Network, et al. Associations between antimicrobial susceptibility/resistance of Neisseria gonorrhoeae isolates in European Union/European Economic Area and patients' gender, sexual orientation and anatomical site of infection, 2009-2016. BMC Infect Dis 2021;21:273. (c) Aho EL, Ogle JM, Finck AM. The human microbiome as a focus of antibiotic discovery: Neisseria mucosa displays activity against Neisseria gonorrhoeae. Front Microbiol 2020;11:577762.
- Hewitt CS, Abutaleb NS, Elhassanny AEM, et al. Structure-activity relationship studies of acetazolamide-based carbonic anhydrase inhibitors with activity against Neisseria gonorrhoeae. ACS Infect Dis 2021;(in press).
- (a) De Simone G, Supuran CT. (In)organic anions as carbonic anhydrase inhibitors. J Inorg Biochem 2012;111:117–29. (b) Ozensoy Guler O, Capasso C, Supuran CT. A magnificent enzyme superfamily: carbonic anhydrases, their purification and characterization. J Enzyme Inhib Med Chem 2016;31:689–94. (c) Nocentini A, Angeli A, Carta F, et al. Reconsidering anion inhibitors in the general context of drug design studies of modulators of activity of the classical enzyme carbonic anhydrase. J Enzyme Inhib Med Chem 2021;36:561–80. (d) Supuran CT. Structure-based drug discovery of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012;27:759–72. (e) Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73.
- (a) Angeli A, Pinteala M, Maier SS, et al. Inhibition of α-, β-, γ-, δ-, ζ- and η-class carbonic anhydrases from bacteria, fungi, algae, diatoms and protozoans with famotidine. J Enzyme Inhib Med Chem 2019;34:644–50. (b) Urbański LJ, Di Fiore A, Azizi L, et al. Biochemical and structural characterisation of a protozoan beta-carbonic anhydrase from Trichomonas vaginalis. J Enzyme Inhib Med Chem 2020;35:1292–9.
- (a) Petreni A, De Luca V, Scaloni A, et al. Anion inhibition studies of the Zn(II)-bound ι-carbonic anhydrase from the Gram-negative bacterium Burkholderia territorii. J Enzyme Inhib Med Chem 2021;36:372–6. (b) De Luca V, Petreni A, Nocentini A, et al. Effect of Sulfonamides and Their Structurally Related Derivatives on the Activity of ι -Carbonic Anhydrase from Burkholderia territorii. Int J Mol Sci 2021;22:571.
- (a) Bonardi A, Nocentini A, Bua S, et al. Sulfonamide Inhibitors of Human Carbonic Anhydrases Designed through a Three-Tails Approach: Improving Ligand/Isoform Matching and Selectivity of Action. J Med Chem 2020;63:7422–44. (b) Bouzina A, Berredjem M, Nocentini A, et al. Ninhydrins inhibit carbonic anhydrases directly binding to the metal ion. Eur J Med Chem 2021;209:112875. (c) Gülçin İ, Scozzafava A, Supuran CT, et al. Rosmarinic acid inhibits some metabolic enzymes including glutathione S-transferase, lactoperoxidase, acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase isoenzymes. J Enzyme Inhib Med Chem 2016;31:1698–702. (d) Innocenti A, Gülçin I, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Antioxidant polyphenols effectively inhibit mammalian isoforms I-XV. Bioorg Med Chem Lett 2010;20:5050–3.
- Forkman A, Laurell AB. The effect of carbonic anhydrase inhibitor on the growth of Neisseriae. Acta Pathol Microbiol Scand 1965;65:450–6.
- Nafi BM, Miles RJ, Butler LO, et al. Expression of carbonic anhydrase in neisseriae and other heterotrophic bacteria. J Med Microbiol 1990;32:1–7.
- Chirică LC, Elleby B, Jonsson BH, Lindskog S. The complete sequence, expression in Escherichia coli, purification and some properties of carbonic anhydrase from Neisseria gonorrhoeae. Eur J Biochem 1997;244:755–60.
- Elleby B, Chirica LC, Tu C, et al. Characterization of carbonic anhydrase from Neisseria gonorrhoeae. Eur J Biochem 2001;268:1613–9.
- Huang S, Xue Y, Sauer-Eriksson E, et al. Crystal structure of carbonic anhydrase from Neisseria gonorrhoeae and its complex with the inhibitor acetazolamide. J Mol Biol 1998;283:301–10.
- (a) Supuran CT. Bortezomib inhibits bacterial and fungal β-carbonic anhydrases. Bioorg Med Chem 2016;24:4406–9. (b) Supuran CT. Bortezomib inhibits mammalian carbonic anhydrases. Bioorg Med Chem 2017;25:5064–7.
- (a) Alterio V, Cadoni R, Esposito D, et al. Benzoxaborole as a new chemotype for carbonic anhydrase inhibition. Chem Commun (Cam(b) 2016;52:11983–6. (b) Nocentini A, Cadoni R, Dumy P, et al. Carbonic anhydrases from Trypanosoma cruzi and Leishmania donovani chagasi are inhibited by benzoxaboroles. J Enzyme Inhib Med Chem 2018;33:286–9. (c) Nocentini A, Supuran CT, Winum JY. Benzoxaborole compounds for therapeutic uses: a patent review (2010- 2018). Expert Opin Ther Pat 2018;28:493–504.
- (a) Temperini C, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. X-ray crystal studies of the carbonic anhydrase II-trithiocarbonate adduct-an inhibitor mimicking the sulfonamide and urea binding to the enzyme. Bioorg Med Chem Lett 2010;20:474–8. (b) Innocenti A, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of transmembrane isoforms IX, XII, and XIV with less investigated anions including trithiocarbonate and dithiocarbamate. Bioorg Med Chem Lett 2010;20:1548–50.
- (a) Carta F, Aggarwal M, Maresca A, et al. Dithiocarbamates: a new class of carbonic anhydrase inhibitors. Crystallographic and kinetic investigations. Chem Commun (Cam(b) 2012;48:1868–70. (b) Carta F, Aggarwal M, Maresca A, et al. Dithiocarbamates strongly inhibit carbonic anhydrases and show antiglaucoma action in vivo. J Med Chem 2012;55:1721–30. (c) Syrjänen L, Tolvanen ME, Hilvo M, et al. Characterization, bioinformatic analysis and dithiocarbamate inhibition studies of two new α-carbonic anhydrases, CAH1 and CAH2, from the fruit fly Drosophila melanogaster. Bioorg Med Chem 2013;21:1516–21.
- (a) Vullo D, Durante M, Di Leva FS, et al. Monothiocarbamates strongly inhibit carbonic anhydrases in vitro and possess intraocular pressure lowering activity in an animal model of glaucoma. J Med Chem 2016;59:5857–67. (b) Carta F, Akdemir A, Scozzafava A, et al. Xanthates and trithiocarbonates strongly inhibit carbonic anhydrases and show antiglaucoma effects in vivo. J Med Chem 2013;56:4691–700.
