Abstract
Carbonic anhydrases (CAs, EC 4.2.1.1) have been studied for decades and have been classified as a superfamily of enzymes which includes, up to date, eight gene families or classes indicated with the Greek letters α, β, γ, δ, ζ, η, θ, ι. This versatile enzyme superfamily is involved in multiple physiological processes, catalysing a fundamental reaction for all living organisms, the reversible hydration of carbon dioxide to bicarbonate and a proton. Recently, the ι-CA (LCIP63) from the diatom Thalassiosira pseudonana and a bacterial ι-CA (BteCAι) identified in the genome of Burkholderia territorii were characterised. The recombinant BteCAι was observed to act as an excellent catalyst for the physiologic reaction. Very recently, the discovery of a novel ι-CAs (COG4337) in the eukaryotic microalga Bigelowiella natans and the cyanobacterium Anabaena sp. PCC7120 has brought to light an unexpected feature for this ancient superfamily: this ι-CAs was catalytically active without a metal ion cofactor, unlike the previous reported ι-CAs as well as all known CAs investigated so far. This review reports recent investigations on ι-CAs obtained in these last three years, highlighting their peculiar features, and hypothesising that possibly this new CA family shows catalytic activity without the need of metal ions.
1. Introduction
CAs represent a superfamily of enzymes considered among the most versatile on the planet. They are ubiquitously present in Archaea, Bacteria, and Eukaryote domains, with the function to accelerate a fundamental reaction for all living organisms, the reversible hydration of carbon dioxide (CO2) to bicarbonate (HCO3-) and a proton (H+), according to the following chemical reaction: CO2 + H2O ⇋ HCO3- + H+Citation1–7. The reversible spontaneous CO2 hydration reaction occurs with a rate of 0.15 s − 1 that cannot meet the fast demand of CO2 and HCO3- necessary for a complex sequences of metabolic pathways, which allow organisms to grow and reproduce, maintain their structures, and respond to environmental changesCitation6,Citation7. CA activity increases the velocity of the CO2 hydration reaction from 100,000 to one million times per second (kcat falling in the range of 104–106 s − 1) with respect to the uncatalyzed reaction, making this superfamily of enzymes among the fastest biocatalysts known in natureCitation7.
CAs are involved in multiple physiological processes in all organisms in which they are present, such as respiration, photosynthesis, CO2 and bicarbonate transport, pH and CO2 homeostasis, electrolyte secretion in various tissues and organs, bone resorption, and calcification, etc.Citation8. For example, carbon dioxide is a byproduct of sugar and fat breakdown in cells and needs to be removed from the mammalian cellsCitation9. At the level of peripheral tissues, the CO2 produced by cellular aerobic metabolism leaves the cells and enters the bloodstream by a pressure gradient effect. Approximately 90% of the CO2 flows into red blood cells and is converted to bicarbonate by CAsCitation9. The produced bicarbonate leaves the red blood cells via an anion exchanger (AE) protein and is transported from the bloodstream to the lungs. At the alveolar level, the concentration of CO2 is lower than in peripheral tissues, whereas there is a higher concentration of bicarbonate, which is pumped into the red blood cellCitation9. Here, through the action of the reverse reaction catalysed by CA, bicarbonate is transformed into water and CO2. The CO2 produced in this way is released into the bloodstream and, passing through the alveolus walls, is exhaled. Among others, gluconeogenesis, lipogenesis, and ureagenesis are several biosynthetic reactions that use pyruvate carboxylase (PC), acetyl-Co-A carboxylase (ACC), and carbamoyl-phosphate synthetase I and II, respectively, also use bicarbonate as substrate for carboxylation reactionsCitation8,Citation10–12. The bicarbonate is produced in a CA-dependent manner.Citation10–12
In plants, CO2 is stored as bicarbonate ions. In both terrestrial and aquatic plants, CA converts HCO3- ions to CO2, which is concentrated in the proximity of the enzyme RuBisCO (Ribulose Bisphosphate Carboxylase/Oxygenase) present in the stroma of the chloroplastsCitation13–15. As a result, the performance of RuBisCO carboxylation reaction is increased, whereas its oxygenation is suppressed. Eukaryotic unicellular photosynthetic organisms have evolved diverse Carbon Concentrating Mechanisms (CCMs) to increase CO2 concentration in the proximity of RuBisCO up to 1000-fold from the low CO2 levels present in the environment. In algae, the main component of the CCM is the pyrenoidCitation16,Citation17. In cyanobacteria, the equivalent of the pyrenoid is the carboxysome. Carboxysomes are composed of RuBisCO, CAs, active bicarbonate transporters, and structural envelope proteinsCitation18. The structure of the carboxysome envelope prevents the escape of CO2 from these organelles.
Another notable biological phenomenon in which CAs are involved is represented by coral calcificationCitation19–23. Calcium in seawater reacts with the HCO3- produced by coral CAs to form calcium carbonate and protons, which are extruded. CaCO3 is thereafter deposited and generates the hard outer surface of corals.Citation19
In bacteria, the CA catalysed reaction is the only known pathway to obtain and balance endogenous levels of CO2, H2CO3 (carbonic acid), HCO3-, and CO32-(carbonate) rapidlyCitation7,Citation24–26. In bacteria, CO2 enters and leaves the bacterial cell by passive diffusion, while bicarbonate is imported directly into the cell through bicarbonate transportersCitation27. Gram-negative bacteria have a periplasmic CA in their periplasmic space, for avoiding the loss of CO2 through diffusion. This enzyme converts faster the CO2 generated from the bacterial metabolism and that coming from the atmosphere into bicarbonate. HCO3- is thereafter pumped into the cytoplasm by bicarbonate transporters and, there, converted into CO2 by cytoplasmic forms of CAs belonging to the β- and/or γ-CA classesCitation15,Citation24,Citation27. Thus, the bicarbonate transporters and bacterial CA enzymes provide CO2 and HCO3- to sustain bacterial metabolismCitation15,Citation24,Citation27. The natural reaction of interconversion of CO2 and H2O into HCO3- and H+ cannot quickly supply CO2 and HCO3- to the bacterial metabolism, as already mentioned, since the reaction rate is too low at physiological pH.
From these examples, it is readily apparent the enzyme versatility of CAs, which are considered metabolic enzymes involved in many physiological processes indispensable for the lifecycle of most living organismsCitation7,Citation25.
The CA superfamily includes, up until now, eight gene families or classes indicated with the letters of the Greek alphabet (α, β, γ, δ, ζ, η, θ, ι)Citation1–5. The distribution of the CA classes is somewhat assorted in most investigated organisms, and except for mammals which encode only for α-CAs, most of them possess multiple representatives of two or even more genetic families. The genome of mammals encodes only for the α-CA class, of which 15 isoforms have been identifiedCitation8,Citation28–31. In plants, α and β-CAs have been recognisedCitation32. In Bacteria, Archaea, and cyanobacteria are present α, β, γ, and ι -CA classesCitation5–7,Citation32–34. Marine diatoms encode for α- δ-, ζ-, θ- and ι-CAsCitation35–37. In protozoa have been detected α- β and η-CAs. Probably, the η-CA-class, recently discovered, has a pivotal role in de novo purine/pyrimidine biosynthetic pathways in these organismsCitation38. In the fungal kingdom, the typical class is represented by β-CAs, and most fungi encode at least one β-CACitation39–41. In contrast, most filamentous ascomycetes contain multiple β-CA genes and, in some of them, it is possible to also find genes encoding for α-CAsCitation39–41.
The eight CA classes are phylogenetically unrelated and, thus, they can be classified as non-homologous isofunctional enzymes that catalyse the same reactionCitation1–7. This is an example of convergent evolution since CA classes show low sequence similarity in primary and possibly tertiary structures because they evolved in a different biological contexts, but catalysing the same reaction, with the active site residues showing a rather similar geometry. As mentioned above, CAs are metalloenzymes whose catalytic site contains a metal ion cofactor necessary for enzyme catalysisCitation5–7,Citation34. Usually, the Zn2+ ion cofactor is coordinated by three amino acid residues, which may be three His residues in the α-, γ-, δ- and, probably, θ-classes; one His, and two Cys residues in β- and ζ-CAs, and two His and one Gln residues in the η-classCitation42. Simultaneously, the fourth ligand is a water molecule/hydroxide ion acting as the nucleophile in the catalytic enzyme cycleCitation5,Citation6,Citation28,Citation34,Citation43,Citation44. Some CA-classes can also coordinate metal ions different from Zn2+, such as Co2+, Cd2+, Fe2+, and Mn2+. As described in the literature, α-, β-, δ-, η- and, perhaps θ-CAs use as ion cofactor the Zn2+; γ-CAs the Fe2+, although they can coordinate Zn2+ or Co2+, tooCitation31,Citation45–51. The ζ-CAs are active with either Cd2+ or Zn2+incorporated into the same apoprotein and are defined as cambialistic enzymesCitation52–54. From a structural point of view, the representative belonging to one CA-class shows a different folding and structure compared with those of other CA-classes. α-CAs are usually active as monomers or dimers; β-CAs are active only as dimers, tetramers, or octamers. The γ-CAs must be trimers for accomplishing their catalytic functionCitation46,Citation47,Citation50,Citation55. γ-CA monomers are characterised by a tandemly-repeated hexapeptide, which is crucial for the left-hand fold of the trimeric β-helix structuresCitation56. The X-ray structure of the θ-CAs resulted to be very similar to some β-CAsCitation57. The crystal structure of ζ-CA showed three slightly different active sites on the same polypeptide chainCitation54. No information is available so far on the structural organisation of δ- and η-CAs. Intriguingly, α-, η-, θ- and ι-CAs were reported to catalyse the esters/thioesters hydrolysis, while no esterase activity was detected for the other CA familiesCitation28,Citation58,Citation59.
2. The ultimately discovered class, the ι-CA
2.1. Lcip63 and BteCAι
In 2019 Gontero et al. discovered the ι-CAs (acronym LCIP63) by exploring the genome of the diatom Thalassiosira pseudonanaCitation59. LCIP63 was stated to prefer as ion cofactor Mn2+ to Zn2+, being localised in the chloroplast, and being only expressed at low concentrations of CO2, confirming their primary role in the diatom CCM.Citation59 These authors also reported LCIP63 homologs in the genome of other diatoms and algae, bacteria, and archaea. Most of the LCIP63 homologs identified in bacteria have been annotated in the data bank as SgcJ/EcaC oxidoreductase family with an unknown functionCitation59. In 2020, Capasso et al. demonstrated that the recombinant bacterial ι-CA (acronym BteCAι) identified in the genome of Burkholderia territorii resulted to be excellent catalyst for the hydration of CO2 to bicarbonate and protons with a kcat of 3.0 × 105 s − 1 and kcat/KM of 3.9 × 107 M − 1 s −1Citation60. Addition of Zn2+ or Ca2+ to the culture media for enzyme expression in E. coli allowed catalytically active enzyme. In contrast, by adding Mn2+, the enzyme activity was not present or the enzyme was found to contain zinc, probably from the traces of this ion present as impurity in the used reagentsCitation60. The protein resulted sensitive to inhibition with substituted benzene-sulphonamides and clinically licenced sulfonamide-, sulfamate- and sulfamide-type drugs, which are among the most investigated CA inhibitors (CAIs)Citation61. BteCAι inhibition profile showed several benzene-sulphonamides with an inhibition constant lower than 100 nMCitation61. In addition to sulphonamides and their bioisosteres, anion and small molecules (another group of CAIs) were investigated as BteCAι inhibitorsCitation62. The best inhibitors were sulphamic acid, stannate, phenylarsonic acid, phenylboronic acid, and sulfamide (KI values of 6.2–94 µM), whereas diethyldithiocarbamate, tellurate, selenate, bicarbonate, and cyanate were submillimolar inhibitors (KI values of 0.71–0.94 mM). The halides (except iodide), thiocyanate, nitrite, nitrate, carbonate, bisulphite, sulphate, hydrogensulfide, peroxydisulfate, selenocyanate, fluorosulfonate, and trithiocarbonate showed KI values in the range of 3.1–9.3 mMCitation62. These prompted us to propose that BteCAι is probably a Zn2+- and not Mn2+-containing enzyme,Citation60 as reported for diatom ι-CAs.Citation59
2.2. Primary structure features of ι-CAs
LCIP63 and the homologs identified as bacterial ι-CAs (like BteCAι) show a primary sequence that completely differs from any previously identified CA-class.Citation59,Citation60 For example, LCIP63, at its N-terminal part, displays the presence of an endoplasmic reticulum signal peptide (of 22 amino acid residues) and a chloroplast signal peptide (of 34 amino acid residues)Citation59. It is a multidomain protein with four, three, or two repeated domains, each of them homologous to the calcium/calmodulin-dependent protein kinase II Association Domain (CaMKII-AD)Citation59. The CaMKII-AD belongs to the NTF2-like protein superfamily, which is a group of proteins, sharing a common fold identified for the first time in the structure of the rat NTF2 (Nuclear Transport Factor 2)Citation63. Generally, the polypeptide chain of the bacterial ι-CAs present a pre-sequence of 19 or more amino acid residues at the N-terminal part and contains one or two repeated domains. The amino acid sequence is homologous to a group of proteins annotated as SgcJ/EcaC oxidoreductase family, with an unknown function. These proteins share a common structure with the NTF2-like superfamily, having a hydrophobic pocket that could constitute a putative substrate binding or catalytic active site.
reports the multialignment of ι-CA amino acid sequences from different species. It is evident that the ι-CAs do not show along the amino acid sequence the conserved residues essential for the catalytic mechanisms of all known CAs, such as the three histidine ligands (in α-, γ-, and δ-CAs), two histidine and one glutamine (of η-CA), or one histidine and two cysteines (from β-, ζ-, and θ-CAs). However, it is remarkable the presence in the C-terminal domain of all the amino acid sequences analysed and classified as ι-CAs (LCIP63-ιCAs and SgcJ/EcaC-ιCAs) of a consensus motif with the following residues: (H)HHSS, which seems to be a specific feature of ι-CAs ().
Figure 1. Multialignment of the ι-CA amino acid sequences from different species (bacteria, cyanobacteria, diatoms, and algae). In red, the putative residues of the catalytic triad (T106, Y124, S199); light blue colour, the H197 as the putative proton shuttle residue. In green are the other residues of the catalytic pocket (see Hirakawa et al. [Citation64]). The putative motif (H)HHSS is the typical consensus sequence characterising the ι-CAs. The asterisk (*) indicates identity at all aligned positions. The multialignment was performed with MUSCLE, version 3.1. See for the identification of the amino acid sequences used in the multialignment. The residue number system used refers to the AspCA enzyme.
![Figure 1. Multialignment of the ι-CA amino acid sequences from different species (bacteria, cyanobacteria, diatoms, and algae). In red, the putative residues of the catalytic triad (T106, Y124, S199); light blue colour, the H197 as the putative proton shuttle residue. In green are the other residues of the catalytic pocket (see Hirakawa et al. [Citation64]). The putative motif (H)HHSS is the typical consensus sequence characterising the ι-CAs. The asterisk (*) indicates identity at all aligned positions. The multialignment was performed with MUSCLE, version 3.1. See Table 1 for the identification of the amino acid sequences used in the multialignment. The residue number system used refers to the AspCA enzyme.](/cms/asset/aa94b6d7-6e91-457b-b23f-fbc33a7a9635/ienz_a_1972995_f0001_c.jpg)
3. ι-Cas (COG4337) with no metal ions within the catalytic site
Recently, a Japanese group identified novel CAs (acronym COG4337) encoded by the genome of the eukaryotic microalga Bigelowiella natans and the cyanobacterium Anabaena sp. PCC7120Citation64. COG4337 homologs from eukaryotic organisms resulted in multidomain proteins formed of up to five domains, while the prokaryotic homolog genes encode only a single domain of about 160 amino acid residuesCitation64. The Bigelowiella natans and Anabaena sp. PCC7120 CAs (indicated here as BnaCA and AspCA) showed the typical consensus HHSS characterising the ι-CAs () mentioned above. They showed CO2 hydration activity, which was investigated by determining the WAU (Wilburn-Anderson Units). The enzyme activity of BnaCA and AspCA resulted in 7 and 37 times respectively lower than that obtained for a mammalian CA. Still, it was in the same range of the θ-CA from Phaeodactylum tricornutum and ι-CA from T. pseudonanaCitation64. Moreover, and this was a great surprise, both enzymes BnaCA and AspCA resulted to be catalytically active without the metal ion cofactor, unlike other reported ι-CAs as well as any other known CAs investigated so farCitation64.
3.1. Phylogenetic analysis
From the amino acid alignment of the two metal-free ι-CAs with those of ι-CAs from different other species, it is readily apparent that the main residues involved in the catalytic pocket of the two enzymes identified by Hirakawa et al. are completely conserved in all the polypeptide chains considered in this paper (). A distinctive feature of the metal-free ι-CAs is the presence of an insertion absent in all the other presumably metal-containing ι-CAs (). The analysis of the hallmarks present in the amino acid sequences is far from being exhaustive, as it does not consider all the amino acid substitutions that differentiate the novel metal-free-CAs from those of other ι-CAs. Hence, we have constructed a most parsimonious tree to better investigate the relationships of the novel ι-CAs identified by Hirakawa et al. with other ι-CAs from other species, such as diatoms and bacteria (). In is presented the information needed for the identification of the amino acid sequences used in the phylogenetic analysis. The two metal-free CAs appear closely associated with each other, as shown in the dendrogram in . BnaCA and AspCA clustered in a branch distinct from all the other (presumably) metal-containing ι-CAs identified in diatoms and the bacterium species mentioned above. Thus, BnaCA and AspCA have several features typical of other ι-CAs, but in other aspects, they do not appear closely related to any other metal-ι-CAs. For this reason, they were annotated as a new subclass of the ι-CAsCitation64. Moreover, Del Prete et al. demonstrated that ι-CAs clustered in a group closely associated with the bacterial γ-CAsCitation60. Probably, from an ancestral γ-CA, during the evolution, a ι-CA originated developing a structural catalytic pocket, which evolved the CO2 hydration function, making possible the CO2 hydratase reaction without the metal ion cofactor as proposed by Hirakawa et al.Citation64.
Figure 2. Phylogenetic analysis of ι-CAs from various organisms. The dendrogram was constructed using the ι-CA amino acid sequences reported in .
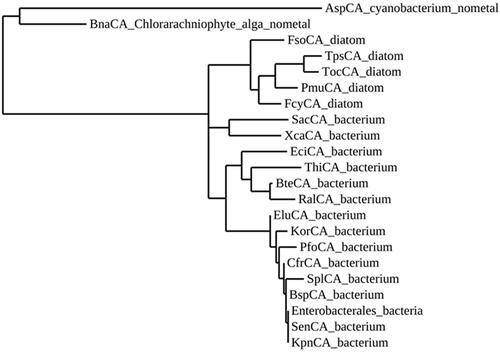
Table 1. Organisms, acronyms, and accession numbers of the amino acid sequences used in the phylogenetic analysis of ι-CAs.
3.2. Three-dimensional structure analysis
X-ray crystallographic structures were obtained for COG4337 proteins in the presence of bicarbonate and the anion inhibitor iodideCitation64. shows the three-dimensional structure of hNFT2 and CAMKII, two proteins belonging to the NTF2-like superfamily (, respectively); the three-dimensional structure of AspCA and BnaCA (the novel ι-CAs identified by Hirakawa et al.Citation64) obtained in the presence of bicarbonate and iodide anion (, respectively), the crystal structures of an SgcJ/EcaC oxidoreductase identified in the genome of Xanthomonas campestris () and BteCAι homology modelling () generated with a fully automated protein homology modelling server SWISS-MODEL (https://swissmodel.expasy.org) and using template structure the homologous enzyme from X. campestris. Here, we want to stress that with the aid of protonographyCitation65, a biochemical technique used to identify the activity and the oligomeric state of CAs on SDS-PAGE, it has been demonstrated that BteCAι can be present as a dimer as shown by the obtained homology modelCitation60.
Figure 3. Ribbon view of (A) hNFT2 (PDB 1GY5), (B) CAMKII in complex with acetate (PDB 2W2C), (C) AspCA (ι-CA from Anabaena cyanobacterium) in complex with HCO3- (PDB 7C5V), (D) BnaCA (ι-CA from microalga Bigelowiella natans) in complex with I- (PDB 7C5Y), (E) XcaCA (ι-CA from X. campestris) in complex with putative HCO3- (PDB 3H51), (F) homology model of BteCA (ι-CA from B. territorii) using XcaCA as template (51% identity).
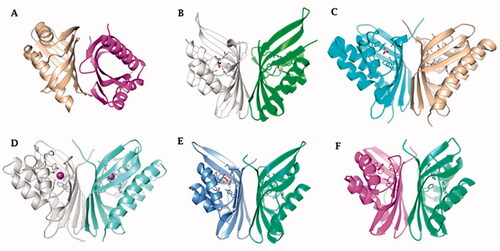
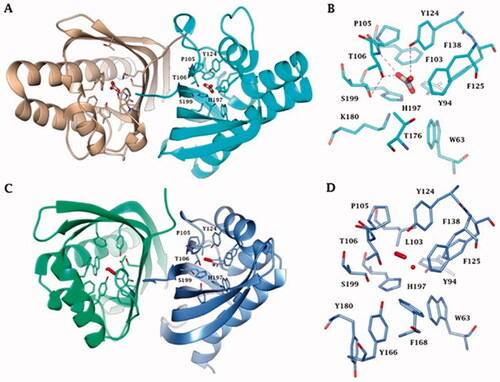
Interestingly, all the enzymes reported in are homodimers with a very high degree of structural homology and belong to the NTF2-like family (). The NTF2-like superfamily includes proteins widely found in both prokaryotic and eukaryotic organisms, which possesses highly versatile rolesCitation63. These proteins can perform a broad range of different functions because their three-dimensional folding form a channel that allows the introduction of differently molecular speciesCitation63. The NTF2-like family represents a classic example of divergent evolution in which proteins have similar general structures but diverge significantly in their functionsCitation63, which often can be determined only from the biochemical analysis of the proteins. Our groups in fact recently demonstrated, with the aid of a stopped-flow spectrophotometer, that the bacterial amino acid sequence annotated as SgcJ/EcaC oxidoreductase and characterised by the motif (H)HHSS is in fact a CA, which acts as a good catalyst for the CO2 hydration reactionCitation60. Intriguing, the homology model of BteCAι has a shape very similar to the crystal structure of the AspCA and BnaCA obtained with a bicarbonate molecule or iodide ion located inside the cone-shaped barrel, respectively. The most crucial evidence of AspCA and BnaCA structure is that the electron densities corresponding to metals were not detected in the structure cavity of both enzymes, confirming that the catalytic activity of these two enzymes is not dependent on the presence of the metal ion . Indeed, the residues of the catalytic pocket which are involved in the binding of bicarbonate and ion iodide are evidenced in . We want to stress that the model of BteCAι presented in 2020 did not allow us the insertion of the zinc ion in the interface of the two monomers to make possible the metal coordination with two histidines of a monomer and one histidine of the other monomerCitation60. However, it is also true that the BteCAι enzyme catalytic activity was observed by adding Zn2+ as described by Del Prete et al.Citation60. On the other hand, it may be possible that the zinc has not a catalytic but a structural function in the bacterial ι-CAs.
Figure 4. (A) Ribbon upper view and (B) binding site view of AspCA (ι-CA from Anabaena cyanobacterium) in complex with HCO3- (PDB 7C5V), (C) Ribbon upper view and (D) binding site view of XcaCA (ι-CA from X. campestris) in complex with putative HCO3- (PDB 3H51). Residue numbers according to AspCA (PDB 7C5V).
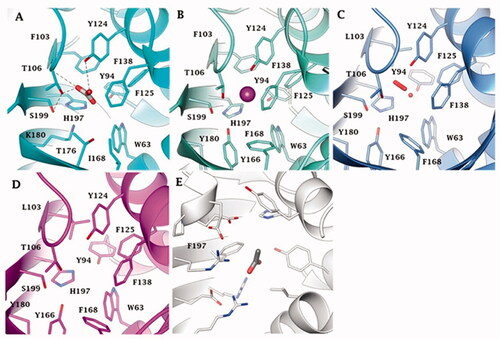
reports the metal-free ι-CAs, the ι-CAs from B. territorii, and the CaMKII (NTF2-like superfamily) binding pocket. The three-dimensional arrangement of AspCA and BnaCA evidenced a catalytic site with a shape of a cone whose cavity is formed by hydrophilic (Thr, Ser, His, Lys, and Tyr) and hydrophobic (Trp and Phe) residues (). Through the experiments of point mutation analysis, essential amino acids of the COG4337 catalytic pocket have been highlighted, and a putative catalytic mechanism for the CO2 hydration reaction has been proposed for these metal-free CAs (). In the known metallo-CAs, such as the human isoforms hCA I and hCA II, the initial step of the reaction involves the deprotonation of H2O in the active site to generate the nucleophile OH- ion. It attacks the CO2, producing the HCO3-; and the gatekeeper residues (Thr199 and Glu106) accept a hydrogen bond from the zinc-bound water, while the proton shuttle residue (His64) has the function to push away the protons from the active site. In the BnaCA and AspCA, metal-free CAs, the hydroxyl groups of the Thr106, Tyr124, and Ser199, present in the catalytic site, were proposed to be involved in the deprotonation of the active site water. The function of the proton shuttle is probably mediated by the H197 or the Tyr positioned on the molecular surface of the enzyme (). Hirakawa et al. assumed that the CO2 binding site could be the hydrophobic part of the catalytic pocket. Interestingly, the metal-free ι-CA probably does not exhibit the reversible dehydration reactionCitation64, of bicarbonate to CO2, which might be a peculiar feature of the metal free ι-CA, as all other known metallo-CAs catalyse both the CO2 hydration as well as bicarbonate dehydration reactionsCitation2,Citation7,Citation8.
Figure 5. Binding site view of (A) AspCA (ι-CA from Anabaena cyanobacterium) in complex with HCO3- (PDB 7C5V), (B) BnaCA (ι-CA from microalga Bigelowiella natans) in complex with I- (PDB 7C5Y), (C) XcaCA (ι-CA from X. campestris) in complex with putative HCO3- (PDB 3H51), (D) homology model of BteCA (ι-CA from B. territorii) using XcaCA as template (51% identity), (E) CAMKII in complex with acetate (PDB 2W2C). Residue numbers according to AspCA (PDB 7C5V).
4. Conclusions
The recent report of a metal-free CA belonging to the ι-classCitation64 and a very interesting proposal for the catalytic mechanism of these enzyme for the CO2 hydration reactions, prompted us to investigate in detail the phylogenetic relationship, primary, secondary and tertiary structures of the other two ι-CAs investigated in detail: the presumably manganese-containing enzyme from a diatom (T. pseudonana)Citation59, and the bacterial, presumably zinc-enzyme from Burkholderia territoriiCitation60. This analysis however also included many such sequences from other organisms, which have not yet been characterised in detail. It was thus observed that ι-CAs possess a rather relevant structural homology with the NTF2-like family of proteins, which have a great variety of functions and physiological rolesCitation63. Furthermore, the residues that have been proposed to be involved in the CO2 hydration reaction of the metal-free ι-CAs, were observed to be conserved in all sequences of such enzymes present in diatoms and bacteria. Coupled to the fact that by computational techniques we were unable to position zinc ions in the model of BteCAι, although we have determined the presence of one mole of Zn2+ per polypeptide chain of this protein (using atomic absorption spectrophotometry)Citation60 prompts us to hypothesise that probably in all ι-CAs the metal ions may not have a catalytic but a structural function (although in the X-ray crystal structure of AspCA no metal ions were present). It is also possible that the reported zinc or manganese ions necessary for the catalytic activity of some of the ι-CAs is an artefact due to the ubiquity of some metal ions present in traces in most reagents, solvent, glass, etc. However, we wish to stress here, the results reported by Jenssen et al.Citation59 and Del Prete et al.Citation60 are valid, even if those enzymes are metal free CAs. In fact, Hirakawa et al.Citation64 reported also adducts with anion inhibitors of AspCA (iodide and bicarbonate), and such an inhibition effect was also reported with various anions (inorganic and organic ones) for the presumably metal-containing ι-CAsCitation59–62. Thus, future work is needed to establish whether all ι-CAs are metal free, or whether some of them may use manganese or zinc ions within their active site, and the subclass reported by Hirakawa et al.Citation64 is a just a minority of such enzymes.
Acknowledgements
The authors are grateful to Valentina Brasiello and Giovanni Del Monaco for their technical assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s). CT Supuran is Editor-in-Chief of Journal of Enzyme Inhibition and Medicinal Chemistry and he was not involved in the assessment, peer review or decision making process of this paper. The authors have no relevant affiliations of financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Annunziato G, Angeli A, D'Alba F, et al. Discovery of new potential anti-infective compounds based on carbonic anhydrase inhibitors by rational target-focused repurposing approaches. Chem Med Chem 2016;11:1904–14.
- Ozensoy Guler O, Capasso C, Supuran CT. A magnificent enzyme superfamily: carbonic anhydrases, their purification and characterization. J Enzyme Inhib Med Chem 2016;31:689–94.
- Del Prete S, Vullo D, De Luca V, et al. Sulfonamide inhibition studies of the β-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. Bioorg Med Chem 2016;24:1115–20.
- Del Prete S, De Luca V, De Simone G, et al. Cloning, expression and purification of the complete domain of the eta-carbonic anhydrase from Plasmodium falciparum. J Enzyme Inhib Med Chem 2016;31(Sup 4):54–9.
- Capasso C, Supuran CT. An overview of the carbonic anhydrases from two pathogens of the oral cavity: Streptococcus mutans and Porphyromonas gingivalis. Curr Top Med Chem 2016;16:2359–68.
- Capasso C, Supuran CT. An overview of the alpha-, beta- and gamma-carbonic anhydrases from Bacteria: can bacterial carbonic anhydrases shed new light on evolution of bacteria? J Enzyme Inhib Med Chem 2015;30:325–32.
- Supuran CT, Capasso C. An overview of the bacterial carbonic anhydrases. Metabolites 2017;7:56.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81.
- Boron WF. Evaluating the role of carbonic anhydrases in the transport of HCO3-related species. Biochim Biophys Acta 2010;1804:410–21.
- Costa G, Carta F, Ambrosio FA, et al. A computer-assisted discovery of novel potential anti-obesity compounds as selective carbonic anhydrase VA inhibitors. Eur J Med Chem 2019;181:111565.
- Del Prete S, De Luca V, Iandolo E, et al. Protonography, a powerful tool for analyzing the activity and the oligomeric state of the γ-carbonic anhydrase identified in the genome of Porphyromonas gingivalis. Bioorg Med Chem 2015;23:3747–50.
- Supuran CT. Carbonic anhydrase inhibitors as emerging drugs for the treatment of obesity. Expert Opin Emerg Drugs 2012;17:11–5.
- Monti SM, De Simone G, Dathan NA, et al. Kinetic and anion inhibition studies of a β-carbonic anhydrase (FbiCA 1) from the C4 plant Flaveria bidentis. Bioorg Med Chem Lett 2013;23:1626–30.
- Vullo D, Flemetakis E, Scozzafava A, et al. Anion inhibition studies of two α-carbonic anhydrases from Lotus japonicus, LjCAA1 and LjCAA2. J Inorg Biochem 2014;136:67–72.
- Thoms S, Pahlow M, Wolf-Gladrow DA. Model of the carbon concentrating mechanism in chloroplasts of eukaryotic algae. J Theor Biol 2001;208:295–313.
- Matsuda Y, Hopkinson BM, Nakajima K, et al. Mechanisms of carbon dioxide acquisition and CO2 sensing in marine diatoms: a gateway to carbon metabolism. Philos Trans R Soc Lond B Biol Sci 2017;372:(1728):20160403.
- Tsuji Y, Nakajima K, Matsuda Y. Molecular aspects of the biophysical CO2-concentrating mechanism and its regulation in marine diatoms. J Exp Bot 2017;68:3763–72.
- Long BM, Forster B, Pulsford SB, et al. Rubisco proton production can drive the elevation of CO2 within condensates and carboxysomes. Proc Natl Acad Sci U S A 2021;118:(18):e2014406118.
- Del Prete S, Bua S, Alasmary FAS, et al. Comparison of the Sulfonamide Inhibition Profiles of the alpha-Carbonic Anhydrase Isoforms (SpiCA1, SpiCA2 and SpiCA3) Encoded by the Genome of the Scleractinian Coral Stylophora pistillata. Mar Drugs 2019;17:146.
- Del Prete S, Bua S, Zoccola D, et al. Comparison of the Anion Inhibition Profiles of the alpha-CA Isoforms (SpiCA1, SpiCA2 and SpiCA3) from the Scleractinian Coral Stylophora pistillata. Int J Mol Sci 2018;19:(7):2128.
- Del Prete S, Vullo D, Caminiti-Segonds N, et al. Protonography and anion inhibition profile of the α-carbonic anhydrase (CruCA4) identified in the Mediterranean red coral Corallium rubrum. Bioorg Chem 2018;76:281–7.
- Del Prete S, Vullo D, Zoccola D, et al. Kinetic properties and affinities for sulfonamide inhibitors of an α-carbonic anhydrase (CruCA4) involved in coral biomineralization in the Mediterranean red coral Corallium rubrum. Bioorg Med Chem 2017;25:3525–30.
- Del Prete S, Vullo D, Zoccola D, et al. Activation profile analysis of CruCA4, an alpha-carbonic anhydrase involved in skeleton formation of the mediterranean red coral, corallium rubrum. Molecules 2017;23:66.
- Supuran CT, Capasso C. New light on bacterial carbonic anhydrases phylogeny based on the analysis of signal peptide sequences. J Enzyme Inhib Med Chem 2016;31:1254–60.
- Campestre C, De Luca V, Carradori S, et al. Carbonic anhydrases: new perspectives on protein functional role and inhibition in helicobacter pylori. Front Microbiol 2021;12:629163.
- Del Prete S, Bua S, Supuran CT, Capasso C. Escherichia coliγ-carbonic anhydrase: characterisation and effects of simple aromatic/heterocyclic sulphonamide inhibitors. J Enzyme Inhib Med Chem 2020;35:1545–54.
- Wheatley NM, Eden KD, Ngo J, et al. A PII-like protein regulated by bicarbonate: structural and biochemical studies of the carboxysome-associated CPII protein. J Mol Biol 2016;428:4013–30.
- Supuran CT. Structure and function of carbonic anhydrases. Biochem J 2016;473:2023–32.
- McKenna R, Supuran CT. Carbonic anhydrase inhibitors drug design. Subcell Biochem 2014;75:291–323.
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77.
- Supuran CT. Carbonic anhydrases-an overview. Curr Pharma Design 2008;14:603–14.
- Supuran CT, Capasso C. Biomedical applications of prokaryotic carbonic anhydrases. Expert Opin Ther Pat 2018;28:745–54.
- Supuran CT, Capasso C. Carbonic anhydrase from porphyromonas gingivalis as a drug target. Pathogens 2017;6:30.
- Capasso C, Supuran CT. An overview of the selectivity and efficiency of the bacterial carbonic anhydrase inhibitors. Curr Med Chem 2015;22:2130–9.
- Rogato A, Del Prete S, Nocentini A, et al. Phaeodactylum tricornutum as a model organism for testing the membrane penetrability of sulphonamide carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2019;34:510–8.
- Angeli A, Pinteala M, Maier SS, et al. Inhibition of α-, β-, γ-, δ-, ζ- and η-class carbonic anhydrases from bacteria, fungi, algae, diatoms and protozoans with famotidine. J Enzyme Inhib Med Chem 2019;34:644–50.
- Berrino E, Bozdag M, Del Prete S, et al. Inhibition of α-, β-, γ-, and δ-carbonic anhydrases from bacteria and diatoms with N'-aryl-N-hydroxy-ureas. J Enzyme Inhib Med Chem 2018;33:1194–8.
- Angeli A, Del Prete S, Alasmary FAS, et al. The first activation studies of the η-carbonic anhydrase from the malaria parasite Plasmodium falciparum with amines and amino acids. Bioorg Chem 2018;80:94–8.
- Mogensen EG, Janbon G, Chaloupka J, et al. Cryptococcus neoformans senses CO2 through the carbonic anhydrase Can2 and the adenylyl cyclase Cac1. Eukaryot Cell 2006;5:103–11.
- Schlicker C, Hall RA, Vullo D, et al. Structure and inhibition of the CO2-sensing carbonic anhydrase Can2 from the pathogenic fungus Cryptococcus neoformans. J Mol Biol 2009;385:1207–20.
- Klengel T, Liang WJ, Chaloupka J, et al. Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr Biol 2005;15:2021–6.
- De Simone G, Di Fiore A, Capasso C, Supuran CT. The zinc coordination pattern in the η-carbonic anhydrase from Plasmodium falciparum is different from all other carbonic anhydrase genetic families. Bioorg Med Chem Lett 2015;25:1385–9.
- Buzas GM, Supuran CT. The history and rationale of using carbonic anhydrase inhibitors in the treatment of peptic ulcers. In memoriam Ioan Puscas (1932-2015). J Enzyme Inhib Med Chem 2016;31:527–33.
- Carta F, Supuran CT, Scozzafava A. Sulfonamides and their isosters as carbonic anhydrase inhibitors. Fut Med Chem 2014;6:1149–65.
- Pinard MA, Lotlikar SR, Boone CD, et al. Structure and inhibition studies of a type II beta-carbonic anhydrase psCA3 from Pseudomonas aeruginosa. Bioorg Med Chem 2015;23:4831–8.
- Ferraroni M, Del Prete S, Vullo D, et al. Crystal structure and kinetic studies of a tetrameric type II β-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. Acta Crystallogr D Biol Crystallogr 2015;71:2449–56.
- De Simone G, Monti SM, Alterio V, et al. Crystal structure of the most catalytically effective carbonic anhydrase enzyme known, SazCA from the thermophilic bacterium Sulfurihydrogenibium azorense. Bioorg Med Chem Lett 2015;25:2002–6.
- Żołnowska B, Sławiński J, Pogorzelska A, et al. Carbonic anhydrase inhibitors. Synthesis, and molecular structure of novel series N-substituted N'-(2-arylmethylthio-4-chloro-5-methylbenzenesulfonyl)guanidines and their inhibition of human cytosolic isozymes I and II and the transmembrane tumor-associated isozymes IX and XII. Euro J Med Chem 2014;71:135–47.
- De Luca L, Ferro S, Damiano FM, et al. Structure-based screening for the discovery of new carbonic anhydrase VII inhibitors. Euro J Med Chem 2014;71:105–11.
- Di Fiore A, Capasso C, De Luca V, et al. X-ray structure of the first `extremo-α-carbonic anhydrase', a dimeric enzyme from the thermophilic bacterium Sulfurihydrogenibium yellowstonense YO3AOP1. Acta Crystallogr D Biol Crystallogr 2013;69:1150–9.
- Supuran CT. Structure-based drug discovery of carbonic anhydrase inhibitors. J Enzyme Inhibition Med Chem 2012;27:759–72.
- Supuran CT. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin Drug Discov 2017;12:61–88.
- Bhatt A, Mahon BP, Cruzeiro VW, et al. Structure-activity relationships of benzenesulfonamide-based inhibitors towards carbonic anhydrase isoform specificity. Chembiochem 2017;18:213–22.
- Alterio V, Langella E, Viparelli F, et al. Structural and inhibition insights into carbonic anhydrase CDCA1 from the marine diatom Thalassiosira weissflogii. Biochimie 2012;94:1232–41.
- Lomelino CL, Mahon BP, McKenna R, et al. Kinetic and X-ray crystallographic investigations on carbonic anhydrase isoforms I, II, IX and XII of a thioureido analog of SLC-0111. Bioorg Med Chem 2016;24:976–81.
- Fu X, Yu LJ, Mao-Teng L, et al. Evolution of structure in gamma-class carbonic anhydrase and structurally related proteins. Mol Phylogenet Evol 2008;47:211–20.
- D’Ambrosio K, Di Fiore A, Buonanno M, et al. Eta and Teta-carbonic anhydrases. London: Elsevier; 2019.
- Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem 2016;31:345–60.
- Jensen EL, Clement R, Kosta A, et al. A new widespread subclass of carbonic anhydrase in marine phytoplankton. Isme J 2019;13:2094–106.
- Del Prete S, Nocentini A, Supuran CT, Capasso C. Bacterial ι-carbonic anhydrase: a new active class of carbonic anhydrase identified in the genome of the Gram-negative bacterium Burkholderia territorii. J Enzyme Inhib Med Chem 2020;35:1060–8.
- De Luca V, Petreni A, Nocentini A, et al. Effect of Sulfonamides and Their Structurally Related Derivatives on the Activity of iota-Carbonic Anhydrase from Burkholderia territorii. Int J Mol Sci 2021;22:571.
- Petreni A, De Luca V, Scaloni A, et al. Anion inhibition studies of the Zn(II)-bound ι-carbonic anhydrase from the Gram-negative bacterium Burkholderia territorii. J Enzyme Inhib Med Chem 2021;36:372–6.
- Eberhardt RY, Chang Y, Bateman A, et al. Filling out the structural map of the NTF2-like superfamily. BMC Bioinformatics 2013;14:327.
- Hirakawa Y, Senda M, Fukuda K, et al. Characterization of a novel type of carbonic anhydrase that acts without metal cofactors. BMC Biol 2021;19:105.
- De. Luca V, Del Prete S, Supuran CT, Capasso C. Protonography, a new technique for the analysis of carbonic anhydrase activity. J Enzyme Inhib Med Chem 2015;30:287–2.
