Abstract
A series of sulfenimide derivatives (1a-i) were investigated as inhibitors of human (hCA-I, hCA-II) and bovine (bCA) carbonic anhydrase enzymes. The compounds were synthesised by the reaction of substituted thiophenols with phthalimide by means of an effective, simple and eco-friendly method and the structures were confirmed by IR, 1H NMR, 13C NMR, MS and elemental analysis. All derivatives except for the methyl derivative (1b) exhibited effective inhibitory action at low micromolar concentrations on human isoforms, but only four derivatives (1e, 1f, 1h, 1i) inhibited the bovine enzyme. The bromo derivative (1f) was found to be strongest inhibitor of all three enzymes with KI values of 0.023, 0.044 and 20.57 µM for hCA-I, hCA-II and bCA, respectively. Results of our study will make valuable contributions to carbonic anhydrase inhibition studies for further investigations since inhibitors of this enzyme are important molecules for medicinal chemistry.
Introduction
Carbonic anhydrase (CA) enzymes catalyse the reversible hydration of carbon dioxide to bicarbonate and protonCitation1. Eight evolutionarily unrelated classes of CA families (α, β, γ, δ, ζ, η, θ, and ι) have been identified so farCitation2,Citation3. CA isoforms are involved in many physiological functions and homeostasis in animals, including respiration, bone resorption and calcification, electrolyte transport in various epithelia and biosynthesis of essential biomolecules as well as metabolic waste detoxification and tumorigenicityCitation4–7. The inhibitors of CA isoenzymes are being used to develop a new class of medicines for epilepsy and glaucoma, two clinically significant conditions. Therefore, new CA inhibitors should be developed as potential therapeutic medicines. A wide variety of chemical ligands have been used to inhibit the catalytic activity of CAs such as anions, phenols, bischalcones, benzenesulfonamides, phthalocyanines and uracil derivativesCitation5–12.
Sulfenimides (thiophthalimides) are divalent sulphur-containing compounds linked to trivalent nitrogen. Although both sulphur and nitrogen have lone pairs of electrons, the electronegativity of the two atoms differs, leading to bond polarisation and the formation of the reactive S-N bond, which may be broken down into electrophiles and nucleophiles. Some sulfenimides have a role as sulfenyl-transfer reagents and may be used to obtain sulfonamidesCitation13. N-Alkylthio- and N-arylthioimides are used as sulphur-transfer reagents. They react with thiols, hydrosulphides, alkoxides, amines, arenesulfinates, active methylene compounds, enamines, and organometallic compounds to give the corresponding sulfenylated productsCitation14. In addition, sulfenimides have a wide range of industrial, agrochemical and medical applications. They have been used as accelerating vulcanisation in the rubber industry and in the agrochemical industry as insecticides and fungicidesCitation15,Citation16.
Many biological conditions have been treated with a class of organic chemical compounds made up of cyclic imides, such as phthalimide derivatives. Moreover, a number of possible derivatives of these compounds have been synthesised in order to inhibit acetylcholinesterase and butyrylcholinesterase and limit the antiproliferative activity on both tumour and normal cellsCitation17. Previous studies have investigated the preparation and biological activity of cyclic imides containing primary sulphonamide moieties as CA inhibitorsCitation18,Citation19.
As previously mentioned, inhibitors of CA enzymes are of particular interest due to their potential to be used in medicinal chemistry, and our aim in this study is to extend earlier researches to explore novel candidates. For this reason, we analysed the effects of nine sulfenimide derivatives, which has never been tested as CA inhibitors, on human CA I, II and bCA.
Materials and methods
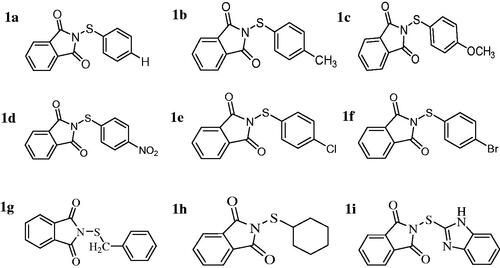
Human CA enzymes were purified from erythrocytes using affinity chromatography as previously describedCitation20. bCA and other chemicals were obtained from Sigma-Aldrich. Synthesis and characterisation of the sulfenimides were performed as previously describedCitation21,Citation22 ().
Hydratase activity assay
CA activity was measured by the method described by Wilbur and AndersonCitation23. CO2–hydratase activity as an enzyme unit (EU) was calculated by using the equation (t0-tc/tc) where t0 and tc are the times for pH change of the nonenzymatic and the enzymatic reactions, respectively. The inhibitory effects of the compounds 1a-i were examined using different inhibitor concentrations. The compounds were prepared in 1 mg/ml DMSO and diluted 1000 times with distilled water. The CA activities without inhibitors were taken as 100% activity. For each inhibitor, an activity percent – [Inhibitor] graph was drawn. The IC50 values were obtained using the curve-fitting method and KI values were calculated from Cheng Prussof equationCitation24 ().
Table 1. CA enzyme inhibition data with sulfenimides (KI values).
Results and discussion
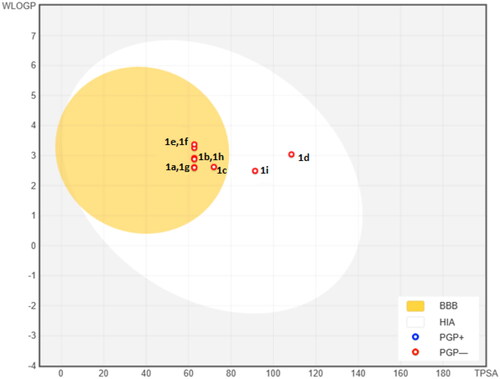
Here we synthesised the sulfenimides using the simple methods described in the literatureCitation25–29. The pharmacokinetic properties of our compounds are shown in . As can be seen from the table, many derivatives tend to have high gastrointestinal absorption, blood brain permeability, skin permeation and inhibition of cytocrom P450 proteinsCitation30–32. A boiled-egg model has been also proposed for the compounds using Swissdock prediction software (). According to the model, compounds 1d and 1i has the highest probability of being absorbed by the gastrointestinal tract and other derivatives might have the highest probability to permeate to the brain.
Table 2. Pharmacokinetic properties of the compounds obtained from SwissADME database.
Phthalimide derivatives have been reported as transmembrane inhibitors at low nanomolar/subnanomolar concentrations of tumour-associated isoforms hCA IX and XII and less effectively inhibited cytosolic isoforms hCA I and IICitation33,Citation34. Additionally, pyridine-N-oxide-2-thiophenol, a thiophenol derivative, was investigated as inhibitor of CA I-XIV by Carta et al.Citation35. They reported that the two mitochondrial isoforms (hCA VA – hCA VB) and isoform hCA III, were not inhibited significantly by the simple coumarin used as control but were inhibited in the low micromolar range by the thiophenol derivative. The membrane-associated isoform hCA IV was inhibited by the pyridine-N-oxide-2-thiophenol, with an inhibition constant of 6.13 µM. The thiophenol derivative of pyridine-N-oxide was a micromolar inhibitor of the tumour-associated hCA IX and hCA XII, with inhibition constants in the range of 1.72–5.40 µM. Furthermore, despite the fact that one is cytosolic (hCA XIII) and the other is transmembrane (hCA XIV), the inhibitory profiles of these two CAs with thiophenol derivative of pyridine-N-oxide were very comparableCitation36. In another study by Demirdağ et al., the inhibitory effects of some sulphonamides on sheep kidney CA enzyme were investigated. The IC50 values for the benzenesulfonamide compounds used in this study were determined to be between 1.348 and 69.31 µMCitation37.
Inhibitory effects of the obtained sulfenimides on human and bCA activities were analysed in vitro; IC50 values were calculated from the graphs and KI values were calculated using Cheng Prusoff equation. The activity% – [inhibitory] regression analysis graphs were provided for the most effective inhibitor of three enzymes (1f) in . The KI values of all compounds are shown in . Compounds 1f, 1i, 1e, 1c, 1g, 1h, 1a and 1d showed quite strong inhibition on hCA-I with KI values ranging from 0.0254 to 0.1194, respectively. 1f was found to be the most effective while 1d exhibited the weakest action. As for hCA-II, the KI values for 1f, 1a, 1g, 1i, 1c, 1h, 1e and 1d were in the range of 0.0044–0.152 µM, respectively. As seen, similar behaviour was observed for hCA-I and II isoforms such that the most effective inhibitor was 1f and the weakest was 1d for both two human enzymes. As clearly seen, the nitro derivative showed the weakest activity on both human isoforms. A quite much weaker inhibiton was observed for the bCA. Only four out of nine compounds showed inhibitory action on bCA with KI values ranging from 20.57 to 265.96 µM. The compound 1f was the most effective and 1i was the weakest inhibitor. Overall results show that compound 1f (bromo derivative) was the most effective inhibitor for all three enzymes suggesting the efficacy of the halogen atoms.
Figure 2. Activity %-[C14H8BrNO2S (µM) (1f)] regression analysis graph for hCA-I in the presence of five different concentrations.
![Figure 2. Activity %-[C14H8BrNO2S (µM) (1f)] regression analysis graph for hCA-I in the presence of five different concentrations.](/cms/asset/0f5bf5c4-b0dd-4771-b4aa-00502e6e4c0a/ienz_a_2194573_f0002_c.jpg)
Figure 3. Activity %-[C14H8BrNO2S (µM) (1f)] regression analysis graph for hCA-II in the presence of five different concentrations.
![Figure 3. Activity %-[C14H8BrNO2S (µM) (1f)] regression analysis graph for hCA-II in the presence of five different concentrations.](/cms/asset/5285d056-6b9d-43db-bc66-d679d06e3f36/ienz_a_2194573_f0003_c.jpg)
Figure 4. Activity %-[C14H8BrNO2S (µM) (1f)] regression analysis graph for bCA in the presence of five different concentrations.
![Figure 4. Activity %-[C14H8BrNO2S (µM) (1f)] regression analysis graph for bCA in the presence of five different concentrations.](/cms/asset/92cd85ca-553a-4c4c-a6bb-bb20f73395b1/ienz_a_2194573_f0004_c.jpg)
Figure 5. The BOILED-Egg predictive model for sulfenimide derivatives. The white region is the physicochemical space of molecules with highest probability of being absorbed by the gastrointestinal tract, and the yellow region is the physicochemical space of molecules with highest probability to permeate to the brain. Yolk and white areas are not mutually exclusive.
Conclusion
The sulfenimides (1a-i) used in our study showed effective inhibition on hCA-I and -II and a rather weaker inhibition on bCA. Nevertheless, the compounds can be novel candidates of medicinal CA inhibitors due to inhibiting human isoforms I and II at nanomolar levels. The KI values of the compounds indicate that the strongest inhibitor was the bromo derivative 1f against all three enzymes. This study confirms that sulfenimides incorporating a phthalimide-based scaffold may lead to novel CA inhibitors which could be used for medicinal purposes.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Supuran CT. Carbonic anhydrase inhibitors. Bioorg Med Chem Lett. 2010;20(12):3467–3474.
- Del Prete S, Vullo D, Fisher GM, Andrews KT, Poulsen S-A, Capasso C, Supuran CT. Discovery of a new family of carbonic anhydrases in the malaria pathogen plasmodium falciparum-the η-carbonic anhydrases. Bioorg Med Chem Lett. 2014;24(18):4389–4396.
- Hirakawa Y, Senda M, Fukuda K, Yu HY, Ishida M, Taira M, Kazushi K, Senda T. Characterization of a novel type of carbonic anhydrase that acts without metal cofactors. BMC Biol. 2021;19(1):1–15.
- Supuran CT. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov. 2008;7(2):168–181.
- Arslan T, Celik G, Celik H, Şentürk M, Yaylı N, Ekinci D. Synthesis and biological evaluation of novel bischalcone derivatives as carbonic anhydrase inhibitors. Arch Pharm. 2016;349(9):741–748.
- Ekinci D, Çavdar H, Talaz O, Şentürk M, Supuran CT. NO-releasing esters show carbonic anhydrase inhibitory action against human isoforms i and ii. Biorg Med Chem. 2010;18(10):3559–3563.
- Soydan E, Olcay AC, Bilir G, Taş Ö, Şentürk M, Ekinci D, Supuran CT. Investigation of pesticides on honey bee carbonic anhydrase inhibition. J Enzyme Inhib Med Chem. 2020;35(1):1923–1927.
- Akbaba Y, Balaydın HT, Menzek A, Göksu S, Şahin E, Ekinci D. Synthesis and biological evaluation of novel bromophenol derivatives as carbonic anhydrase inhibitors. Arch Pharm. 2013;346(6):447–454.
- Fidan I, Salmas RE, Arslan M, Senturk M, Durdagi S, Ekinci D, Senturk E, Cosgun S, Supuran CT. Carbonic anhydrase inhibitors: design, synthesis, kinetic, docking and molecular dynamics analysis of novel glycine and phenylalanine sulphonamide derivatives. Bioorg Med Chem. 2015;23(23):7353–7358.
- Yaseen R, Ekinci D, Senturk M, Hameed AD, Ovais S, Rathore P, Samim M, Javed K, Supuran CT. Pyridazinone substituted benzenesulfonamides as potent carbonic anhydrase inhibitors. Bioorg Med Chem Lett. 2016;26(4):1337–1341.
- Işık S, Vullo D, Durdagi S, Ekinci D, Şentürk M, Çetin A, Şentürk E, Supuran CT. Interaction of carbonic anhydrase isozymes i, ii, and ix with some pyridine and phenol hydrazinecarbothioamide derivatives. Bioorg Med Chem Lett. 2015;25(23):5636–5641.
- Alper Türkoğlu E, Şentürk M, Supuran CT, Ekinci D. Carbonic anhydrase inhibitory properties of some uracil derivatives. J Enzyme Inhib Med Chem. 2017;32(1):74–77.
- Yakan H, Kütük H. Comparison of conventional and microwave-assisted synthesis of some new sulfenamides under free catalyst and ligand. Monatsh Chem. 2018;149(11):2047–2057.
- Suwa S, Sakamoto T, Kikugawa Y. Alcl3-mediated aromatic phenylthiation with n-phenylthiophthalimide. Chem Pharm Bull. 1999;47(7):980–982.
- Craine L, Raban M. The chemistry of sulfenamides. Chem Rev. 1989;89(4):689–712.
- Koval’ I. Reactions of thiols. Russ J Org Chem. 2007;43:319–346.
- Júnior RV, Moura GL, Lima NB. Insights into the spontaneity of hydrogen bond formation between formic acid and phthalimide derivatives. J Mol Model. 2016;22(11):1–9.
- Alaa A-M, Angeli A, El-Azab AS, El-Enin MAA, Supuran CT. Synthesis and biological evaluation of cyclic imides incorporating benzenesulfonamide moieties as carbonic anhydrase i, ii, iv and ix inhibitors. Biorg Med Chem. 2017;25(5):1666–1671.
- Alaa A-M, El-Azab AS, El-Enin MAA, Almehizia AA, Supuran CT, Nocentini A. Synthesis of novel isoindoline-1, 3-dione-based oximes and benzenesulfonamide hydrazones as selective inhibitors of the tumor-associated carbonic anhydrase ix. Bioorg Chem. 2018;80(1):706–713.
- Dizdaroglu Y, Albay C, Arslan T, Ece A, Turkoglu EA, Efe A, Senturk M, Supuran CT, Ekinci D. Design, synthesis and molecular modelling studies of some pyrazole derivatives as carbonic anhydrase inhibitors. J Enzym Inhib Med Chem. 2020;35(1):289–297.
- Klose J, Reese CB, Song Q. Preparation of 2-(2-cyanoethyl) sulfanyl-1h-isoindole-1, 3-(2h)-dione and related sulfur-transfer agents. Tetrahedron. 1997;53(42):14411–14416.
- Kutuk H, Turkoz N. Microwave-assisted synthesis of disulfides. Phosphorus Sulfur Silicon Relat Elem. 2011;186(7):1515–1522.
- Wilbur KM, Anderson NG. Electrometric and colorimetric determination of carbonic anhydrase. J Biol Chem. 1948;176(1):147–154.
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108.
- Harpp DN, Friedlander BT, Smith RA. The synthesis of thiocyanates from sulfenyl chlorides and trimethylsilyl cyanide. Synthesis. 1979;1979(3):181–182.
- Barrett AGM, Dhanak DG, Taylor SJ. (phenylthio)nitromethane – (benzene, [(nitromethyl)thio]). Org Synth . 1990;68(1):8–13.
- Kittleson A. Parasiticidal compounds containing the nsccll3 group. US Patent Office; US2553770A:2553770, 1951.
- Behforouz M, Kerwood JE. Alkyl and aryl sulfenimides. J Organ Chem. 1969;34(1):51–55.
- Lee C, Lim YN, Jang HY. Copper‐catalyzed synthesis of n‐formyl/acylsulfenamides and‐thiosulfonamides. Eur J Org Chem. 2015;2015(27):5934–5938.
- Aina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017; 7(7):42717.
- Daina A, Michielin O, Zoete V. iLOGP: a simple, robust, and efficient description of n-octanol/water partition coefficient for drug design using the GB/SA approach. J Chem Inf Model. 2014;54(12):3284–3301.
- Daina A, Zoete V. A BOILED-Egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem. 2016;11(11):1117–1121.
- Alaa A-M, El-Azab AS, Ceruso M, Supuran CT. Carbonic anhydrase inhibitory activity of sulfonamides and carboxylic acids incorporating cyclic imide scaffolds. Bioorg Med Chem Lett. 2014;24(22):5185–5189.
- El-Azab AS, Alaa A-M, Ayyad RR, Ceruso M, Supuran CT. Inhibition of carbonic anhydrase isoforms i, ii, iv, vii and xii with carboxylates and sulfonamides incorporating phthalimide/phthalic anhydride scaffolds. Biorg Med Chem. 2016;24(1):20–25.
- Carta F, Vullo D, Maresca A, Scozzafava A, Supuran CT. New chemotypes acting as isozyme-selective carbonic anhydrase inhibitors with low affinity for the offtarget cytosolic isoformmII. Bioorg Med Chem Lett. 2012;22(6):2182–2185.
- Ekinci D, Çavdar H, Durdagi S, Talaz O, Şentürk M, Supuran CT. Structure–activity relationships for the interaction of 5, 10-dihydroindeno [1, 2-b] indole derivatives with human and bovine carbonic anhydrase isoforms I, II, III, IV and VI. Eur J Med Chem. 2012;49:68–73.
- Demirdağ R, Çomaklı V, Şentürk M, Ekinci D, İrfan Küfrevioğlu Ö, Supuran CT. Characterization of carbonic anhydrase from sheep kidney and effects of sulfonamides on enzyme activity. Bioorg Med Chem. 2013;21(6):1522–1525.