Abstract
In the last decade, an increasing interest in compounds containing pyrazolo[4,3-e][1,2,4]triazine moiety is observed. Therefore, the aim of the research was to synthesise a novel sulphonyl pyrazolo[4,3-e][1,2,4]triazines (2a, 2b) and pyrazolo[4,3-e]tetrazolo[1,5-b][1,2,4]triazine sulphonamide derivatives (3a, 3b) to assess their anticancer activity. The MTT assay showed that 2a, 2b, 3a, 3b have stronger cytotoxic activity than cisplatin in both breast cancer cells (MCF-7 and MDA-MB-231) and exhibited weaker effect on normal breast cells (MCF-10A). The obtained results showed that the most active compound 3b increased apoptosis via caspase 9, caspase 8, and caspase 3/7. It is worth to note that compound 3b suppressed NF-κB expression and promoted p53, Bax, and ROS which play important role in activation of apoptosis. Moreover, our results confirmed that compound 3b triggers autophagy through increased formation of autophagosomes, expression of beclin-1 and mTOR inhibition. Thus, our study defines a possible mechanism underlying 3b-induced anti-cancer activity against breast cancer cell lines.
Introduction
Over the past 20 years, the number of new breast cancer cases has increased from 1 002 000 to 2 261 419Citation1,Citation2. The rapid increase in the incidence of breast cancer causes it one of the most diagnosed cancer. Moreover, breast cancer mortality has raised from 459 000 deaths in 2000 to 684 996 in 2020Citation1,Citation2. The growth in both mortality and morbidity of this disease may be related with heterogeneous nature of breast cancer tumours, associated with diverse gene expression. Variation of neoplasms results in patient’s responsiveness to chemotherapyCitation3.
According to the literature, MCF-7 and MDA-MB-231 are the most widely used breast cancer cell lines in in vitro studies of anticancer activity of novel compounds. MDA-MB-231 are grouped as triple negative B – basal b (ER(−), PR(−), and HER2(−)) and EGFR (+) cell lines, while MCF-7 is characterised as luminal a (ER(+), PR(+), and HER2(−))Citation3,Citation4. Moreover, invasive MDA-MB-231 cells use significantly more energy from aerobic glycolysis than non-invasive MCF-7. Altered metabolism of glucose by MDA-MB-231 is an adaptation to hypoxia. In both cell lines, glucose intake is decreased. Although MCF-7 cells have definitely lower glucose consumption under aerobic condition than MDA-MB-231Citation5.
Chemotherapy still plays crucial role in cancer treatment. Hence, the research, development and synthesis of new, potential anticancer agents with appropriate effectiveness, selectivity, and specificity of action are of prime importance. The inventive process of designing new compounds with biological activity against cancer cells requires understanding the molecular mechanism of neoplasm development. In the recent years, intriguing role in the preclinical research plays heterocyclic scaffold. Wide interest in this chemical structure is associated with progress of the new synthetic routes that allow to receive active pharmaceutical compounds with high biological activity and low toxicity. Statistics shows that heterocycles with at least one nitrogen atom in the five and six membered ring have tremendous part in drug discoveryCitation6,Citation7. Among them, triazine derivatives are worth to pay attention. Analysis of the published literature demonstrated potential antitumor activity of 1,2,4-triazine isomer. In recent years, 1,2,4-triazine derivatives and its benzo- and heterofused derivatives were designed and synthesised as potential anticancer drugsCitation8–16. In addition to their anticancer activity, 1,2,4-triazines have unique biological properties including: antibacterial activity, antiviral, anti-inflammatory, antihypertensive, antidiabetic, etc. One of the derivatives, remdesivir has been approved by FDA in May 2020 in emergency treatment patients having severe symptoms of COVID-19Citation17.
Important strategy in discovery of novel chemotherapeutics is combining pharmacophore group with anticancer compounds. This combination allows to increased biological activity by acting on multiple molecular targets, minimising drug resistance and side effects, and improving pharmacokinetic propertiesCitation18,Citation19. One of the most well-known pharmacophore group is sulphonamide group which is found in many medicine drugs. Their tremendous medical importance results from the pleiotropic effect documented in the literature which include anticancer activity Citation20–23. Therefore, it seems reasonable to combine sulphonamide group with triazine derivatives to increase biological activity of nitrogen containing compounds.
In continuation of our efforts in search of new effective anticancer agents, we synthesised new enantiomeric pyrazolo[4,3-e][1,2,4]triazine sulphonamides (2a,b) as pivotal intermediates for the preparation of novel pyrazolo[4,3-e]tetrazolo[1,5-b][1,2,4]triazine sulphonamide derivatives (3a,b) and evaluated their in vitro anticancer potential against MCF-7 and MDA-MB-231 cell lines. The promising activity obtained, prompted us to investigate their role in the apoptosis and autophagy.
Materials and methods
Synthesis
General
The melting points of the synthesised compounds were measured on Mel-Temp.1 H and 13C NMR spectra were measured in deuterated solvents on a Varian instrument (400 MHz for 1H and 100 MHz for 13C, Siedlce, Poland). The chemical shift values (δ) in both spectra are presented in ppm (part per million) and coupling constants (J) in Hz (Hertz). Tetramethylsilane (TMS) was used as internal standard. The measure of signal intensity in 1H NMR spectra is proportional to the number of protons which caused the signal. Molecular weights of compounds (2a, 2b, 3a, 3b) were evaluated by electrospray ionisation mass spectrometry (ESI/MS) on an Agilent Technologies 6538 UHD Accurate Mass Q-TOF LC/MS (Agilent Technologies, Inc., Santa Clara, CA). Elemental compositions are within ±0.4% of the calculated values. Compound 1 was synthesised according to the methods described in the literatureCitation24.
Synthesis of sulphonyl derivatives 2a,b
Compound 1 (200 mg, 0.515 mmol) was dissolved in anhydrous acetonitrile (10 mL) (Sigma Chemical Co., St. Louis, MO) and the corresponding amine (1.003 mmol) (Sigma Chemical Co., St. Louis, MO) was added. The obtained solution was stirred at room temperature until the substrate disappeared (control TLC) plates. When the reaction was completed, the solvent was evaporated and the remaining precipitate was purified by chromatography column using a mixture of methylene chloride:ethanol (50:1) as an eluent (Sigma Chemical Co., St. Louis, MO).
(S)-N-(1-Hydroxy-3-phenylpropan-2-yl)-4-(3-methyl-5-(methylsulfonyl)-1H-pyrazolo[4,3-e][1,2,4]triazin-1-yl)benzenesulfonamide (2a): yield 90%. Melting point: 130–134 °C. 1H NMR (DMSO): 2.38–2.43 (m, 1H), 2.75 (s, 3H), 2.79–2.83 (m, 1H), 3.20–3.28 (m, 1H), 3.30–3.38 (m, 2H), 3.56 (s, 3H), 4.85 (t, 1H, J = 6.4 Hz, OH exchanged with D2O), 6.95–7.08 (m, 5H), 7.78 (d, 2H, J = 8.8 Hz), 8.28 (d, 2H, J = 8.8 Hz). 13C NMR (DMSO): 11.12, 37.08, 40.82, 57.33, 63.27, 119.83, 126.03, 127.97, 128.08, 129.15, 138.15, 138.31, 139.69, 139.90, 145.98, 148.23, 160.95; HRMS (ESI, m/z) Calcd. for C21H22N6O4S2 [M++H] 503.11659. Found [M++H] 503.11719. Anal. Calcd. for C21H22N6O4S2: C, 50.19; H, 4.41; N, 16.72. Found: C, 50.10; H, 4.46; N, 16.50.
(R)-N-(1-Hydroxy-3-phenylpropan-2-yl)-4-(3-methyl-5-(methylsulfonyl)-1H-pyrazolo[4,3-e][1,2,4]triazin-1-yl)benzenesulfonamide (2b): yield 92%. Melting point: 124–128 °C. 1H NMR (DMSO): 2.39–2.44 (m, 1H), 2.76 (s, 3H), 2.79–2.84 (m, 1H), 3.20–3.28 (m, 1H), 3.30–3.38 (m, 2H), 3.63 (s, 3H), 4.85 (t, 1H, J = 6.4 Hz, OH exchanged with D2O), 6.96–7.10 (m, 5H), 7.79 (d, 2H, J = 8.8 Hz), 8.29 (d, 2H, J = 8.8 Hz).13 C NMR (DMSO): 11.12, 37.08, 40.81, 57.33, 63.26, 119.83, 126.03, 127.97, 128.07, 129.15, 138.15, 138.31, 139.69, 139.90, 145.97, 148.23, 160.94; HRMS (ESI, m/z) Calcd. for C21H22N6O4S2 [M++H] 503.11659. Found [M++H] 503.11730. Anal. Calcd. for C21H22N6O4S2: C, 50.19; H, 4.41; N, 16.72. Found: C, 50.00; H, 4.53; N, 16.52.
Synthesis of pyrazolo[4,3-e]tetrazolo[1,5-b][1,2,4]triazine sulphonamide 3a,b
The appropriate compound 2a or 2b (0.29 mmol) was dissolved in anhydrous ethanol (25 mL), sodium azide (22 mg, 0.35 mmol) (both of Sigma Chemical Co., St. Louis, MO) was added to the solution. The reaction mixture was refluxed. The progress of the reaction was controlled by TLC until all substrate reacted. Then solvent was evaporated on evaporator and the final compound (3a or 3b) was purified on column chromatography using methylene chloride:ethanol (100:1) (Sigma Chemical Co., St. Louis, MO).
(S)-N-(1-Hydroxy-3-phenylpropan-2-yl)-4-(7-methyl-5H-pyrazolo[4,3-e]tetrazolo[1,5-b][1,2,4]triazin-6-yl)benzenesulfonamide (3a): yield 82%. m.p. 172–176 °C.
1H NMR (MeOH-d4): 2.50–2.55 (m, 1H), 2.86 (s, 3H), 2.90–2.95 (m, 1H), 3.46–3.50 (m, 2H), 3.55–3.62 (m, 1H), 4.28 (t, 1H, J = 6.4 Hz, OH exchanged with D2O), 7.02–7.12 (m, 5H), 7.82 (d, 2H, J = 8.8 Hz), 8.24 (d, 2H, J = 8.8 Hz). 13C NMR (DMSO): 11.10, 37.09, 57.34, 63.33, 118.75, 126.08, 128.12, 128.17, 129.17, 138.34, 139.07, 139.50, 142.03, 146.98, 147.20, 147.77; HRMS (ESI, m/z) Calcd. for C20H20N9O3S [M++H] 466.14043. Found [M++H] 466.14079. Anal. Calcd. for C20H19N9O3S: C, 51.61; H, 4.11; N, 27.08. Found: C, 51.78; H, 4.39; N, 26.80.
(R)-N-(1-hydroxy-3-phenylpropan-2-yl)-4-(7-methyl-5H-pyrazolo[4,3-e]tetrazolo[1,5-b][1,2,4]triazin-6-yl)benzenesulfonamide (3b): yield 86%. m.p. 170–172 °C.
1H NMR (CDCl3): 2.73–2.76 (m, 1H), 2.83–2.85 (m, 1H), 3.52–3.55 (m, 2H), 3.57–3.61 (m, 1H), 3.68–3.70 (m, 1H), 4.80 (t, 1H, J = 6.4 Hz, OH exchanged with D2O), 7.15–7.19 (m, 5H), 7.88 (d, 2H, J = 8.8 Hz), 8.32 (d, 2H, J = 8.8 Hz). 13C NMR (DMSO): 11.09, 37.08, 57.34, 63.32, 118.75, 126.07, 128.11, 128.16, 129.17, 138.34, 139.06, 139.49, 142.03, 146.97, 147.19, 147.76; HRMS (ESI, m/z) Calcd. for C20H20N9O3S [M++H] 466.14043. Found [M++H] 466.14055. Anal. Calcd. for C20H19N9O3S: C, 51.61; H, 4.11; N, 27.08. Found: C, 51.70; H, 4.35; N, 26.84.
Cell culture of breast cancer cells (MCF-7, MDA-MB-231), normal breast cells (MCF-10a)
MCF-7, MDA-MB-231 human breast cancer cells were purchased from ATCC (Manassas, VA) and cultured DMEM (Corning, Kennebunk, ME) with 10% heat-inactivated foetal bovine serum (Eurx, Gdańsk, Poland) and 100 μg/mL antibiotics (penicillin/streptomycin) (Corning, Kennebunk, ME). Both cell lines were incubated under optimal condition, i.e. 37 °C in a humidified atmosphere (90–95%) containing 5% of CO2. To perform experiments, cells were plated in six-well plates until they reach 80% of confluency.
MCF-10A normal human breast cancer cells were provided by ATCC and cultured in MEGM supplemented with Mammary Epithelial Cell Growth Kit (H-insulin, l-glutamine, epinephrine, apo-transferrin, rH-TGFα, extract P, and hydrocortisone hemisuccinate) and 10% of FBS and 100 µg/mL antibiotics (penicillin and streptomycin). MCF-10A was incubated under the same conditions as cancer cells.
Viability assay
The MTT viability assay was done as described previouslyCitation25,Citation26. In brief, cells were incubated with novel synthesised compounds 2a, 2b, 3a, 3c and reference drug cisplatin. Following 24-h incubation with tested compounds, the medium was discarded from the cell cultures. Then, into each well with cells was added 1 mL of PBS and 50 µL of MTT solution (5 mg per mL). Cells were incubated at 37 °C and 5% CO2 in humidified atmosphere for a defined time appropriate for each cell line. After incubation, the solution above the cells was removed and obtained purple formazan crystals were dissolved in 1 mL DMSO. The absorbance was read at OD = 570 nm on Evolution 201 Spectrophotometer (Thermo Scientific, Waltham, MA).
Apoptosis
MCF-7 and MDA-MB-231 cell lines were incubated with or without novel compounds (2b, 3b) and cisplatin at concentration 0.25 µM and 0.5 µM for 24 h and stained with Annexin V-FITC/PI. The analysis was performed using flow cytometry Apoptosis Detection Kit II (BD Biosciences, San Diego, CA) as described previouslyCitation27.
Total ROS activity
The effect of 3b and cisplatin on ROS induced cellular damage of two breast cancer cells (MCF-7 and MDA-MB-231) was performer as described previouslyCitation27. In brief, cells were washed two times with cold PBS solution and incubated in a solution of ROS Green dye in Assay Buffer and incubated for 1 h in optimal cell growth parameters, protected from light. After incubation, cells were washed with assay buffer. Subsequently, fluorescence emission intensities at 490 nm excitation and 520 nm emission were recorded in 500 μL assay buffer using flow cytometer BD FACSCanto II flow cytometer and FACSDiva software (BD Biosciences Systems, San Jose, CA).
Activity of caspase-9, -10, and -3/7
MCF-7 and MDA-MB-231 cells were plated in six-well plates at density 1 × 106 and cultured in DMEM for 24 h. Following incubation, cells were treated with compound 3b and cisplatin at concentration 0.25 µM and 0.5 µM. Then to identify the involvement of caspase family in inducing apoptosis, cells were pre-treated with Z-VAD-FMK (100 µM) 2 h before adding compoundsCitation28. The activation of caspases 9, 10, and 3/7 was detected as described beforeCitation14.
Autophagy
The effect of novel compound 3b at 0.25 µM and 0.5 µM concentration on the induction of autophagy in MCF-7 and MDA-MB-231 pre-treated 1 h with or not autophagy inhibitor 3-MA for 1 h (3-methyladenine, Sigma-Aldrich, St. Louis, MO) at 1 mM concentrationCitation29 was assessed by ICT’s Autophagy Assay, Red using a flow cytometer. The test was performed as previouslyCitation25. Briefly, cells were washed with PBS and suspended in 490 µL of PBS. To each probe, 10 µL of diluted Autophagy Probe, Red solution was added. Prepared samples were incubated at 37 °C for 60 min. Following incubation, cells were suspended in diluted cellular assay buffer and centrifuged. Then, supernatant was removed, and cells were resuspended in diluted cellular assay buffer and centrifuged again. This step was repeated three times. Finally, cell pellet was resuspended in 0.5 mL of diluted cellular assay buffer. The analysis was performed with a flow cytometer (excitation/emission 590 nm/620 nm).
p53, NF-κB, mTOR, and Bax
To evaluate the molecular mechanism of induction of apoptosis of tested compounds, the level of p53, NF-κB, mTOR, Bax, and beclin-1 was measured using appropriate antibody according to the manufacturer’s instruction. In brief, tryspinised cells were centrifuged, then supernatant was discarded, and cells were suspended in 4% FA (formaldehyde). Prepared samples were incubated 15 min at RT. Following incubation, the 2.5 mL PBS was added to each sample and centrifuged. Then, supernatant was discarded, and cells were resuspended in 90% ice-cold methanol to perform permeabilisation and left for 1 h in ice. To remove methanol, cells were rinsed with excess PBS by centrifugation. Following centrifugation, the supernatant was discarded. Then, cells were resuspended in 100 µL of appropriate diluted (1:100 in PBS) antibody conjugate (p53 (7F5) Rabbit mAb – Alexa Fluor 488 Conjugate #5429 Cell Signalling Technology, Danvers, MA; NF-κB p65 (D14E12) XP Rabbit mAb – Alexa Fluor 647 Conjugate #8801 Cell Signalling Technology, Danvers, MA; mTOR (7C10) Rabbit mAB – Alexa Fluor 647 #5048 Cell Signalling Technology, Danvers, MA; FITC Anti-Bax antibody (T22-A) #ab139543, Abcam, Cambridge, UK) and incubated 1 h in the dark at RT. Afterwards, cells were washed by centrifugation in 2 mL of incubation buffer. Finally, supernatant was removed and 300 µL PBS was added to each sample and pipetted. The analysis was performed by flow cytometry (BD FACSCanto II, Becton Dickinson Biosciences Systems, San Jose, CA) using FACSDiva software ver. 6.1.3.
Beclin-1
The fixation and permeabilisation were performed as described above. Cells were resuspended in 100 µL of diluted primary antibody (1:100 in PBS, anti-beclin 1 antibody (2A4) #ab114071, Abcam, Cambridge, UK) and incubated 30 min in the dark at RT. The cells were washed three times by centrifugation in PBS. Then to each probe, 100 µL of diluted fluorochrome-labelled secondary antibody (1:100 in PBS; goat anti-mouse IgG H&L – Alexa Fluor 488 #ab150113, Abcam, Cambridge, UK) was added and incubated 30 min in dark at RT. Then, cells were rinsed with 2.5 mL PBS. In the last step, supernatant was discarded and cells were resuspended in 300 µL PBS and pipetted. The analysis was carried out by flow cytometry.
3D spheroids of both cancer cell lines: MCF-7 and MDA-MB-231
The 3D cell culture was carried out with 6-Well Bio-Assembler Kit in accordance with the manufacturer’s instructions (Greiner Bio-One GmbH, Frickenhausen, Germany). First, MCF-7 and MDA-MB-231 were cultured in 2D using procedure and supplied to 80% confluency and treated with nanoshuttle at concentration of 1 µL/10 000 cell and incubated overnight. Following incubation, the medium was removed, and the cells were detached by trypsin–EDTA solution and resuspended in DMEM. The cells were counted using Scepter 2.0 Handheld Automated Cell Counter (Merck, Darmstadt, Germany) and plated at the concentration 1 × 106 cells per well in six-well plate. Then, cells were gently tilted in the flask to evenly distribute the nanoparticle and incubated (37 °C, 5% CO2, 95–98% humidity) for 1 h with magnet on the top of the plate. The cells self-assemble to spheroids. The obtained 3D cell culture was incubated until analysisCitation30.
The spheroids were incubated with tested drugs (2b, 3b) and cisplatin at 0.25 µM and 0.5 µM concentrations. The visualisation of the morphological changes in 3D cell culture of MCF-7 and MDA-MB-231 was evaluated using microscope (Nikon Eclipse Ti, Tokyo, Japan).
Statistical analysis
The mean ± standard deviation (SD) for all data is presented. One-way and two-way analysis of variance (ANOVA) was conducted followed by Dunnett’s test. GraphPad 8.0.1 Prism software (GraphPad Software, San Diego, CA) was used for calculation. The analysis of spheroids area was carried out using ImageJ software from the NIH (Bethesda, MD).
Results
Chemistry
Sulphonamides 2a,b and 3a,b were prepared using previously published procedureCitation12–14 and the synthetic route is briefly presented in Scheme 1. The synthesis was started with the preparation of the pivotal intermediate benzenesulfonyl chloride (1), which was obtained by the chlorosulfonation reaction using our previously published procedureCitation12–14,Citation24. The preparation of the sulphonamide intermediates (2a,b) was performed via N-sulfonation reactions of compound 1 with appropriate enantiomeric phenylalaninol derivative in acetonitrile at room temperature (Scheme 1). Further, these compounds 2a,b were reacted with sodium azide in ethanol to afford the final tricyclic sulphonamides 3a,b. The structures of compounds 2a,b/3a,b were confirmed by spectroscopic techniques. In the 1H NMR spectrum, the products 2a,b showed similar chemical shift values for aromatic protons to those of compound 1 but slightly shifted upfield. Additionally, at 7.00–7.10 ppm, a multiplet was observed corresponding to the five protons of the phenyl ring present in the amine moiety. Analogous to our previous research resultsCitation12,Citation14, tautomeric equilibrium between azide derivatives and appropriate fused tetrazoles was observed in the 1H NMR spectra. We have observed additional low-intensity signals on 1H NMR spectra that correspond to the suitable azido derivatives forming in solution from tetrazole derivatives.
Biological studies
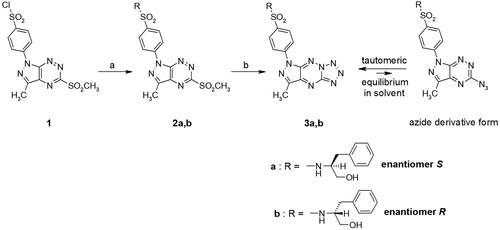
In a continuation of our previous studies on bioactive compounds of pyrazolo[4,3-e]tetrazolo[1,5-b][1,2,4]triazine derivativesCitation12,Citation14, the cytotoxic activity of enantiomeric sulphones (2a,2b) and their appropriate tricyclic derivatives (3a,3b) of (S)- and (R)-phenylalaninol was investigated against two breast cancer cell lines (MCF-7 and MDA-MB-231) and normal breast cell line (MCF-10A). In and , it is shown that survival of MCF-7 and MDA-MB-231 was significantly inhibited after 24 h incubation with 2a, 2b, 3a and 3b in concentration-dependent manner. The results revealed the inhibitory concentrations (IC50) of 2a, 2b, 3a, 3b were 6.0 ± 0.38 µM, 5.5 ± 0.39 µM, 0.35 ± 0.08 µM, and 0.25 ± 0.07 µM () in MCF-7 and 9.2 ± 0.78 µM, 7.8 ± 0.53 µM, 0.71 ± 0.14 µM, and 0.31 ± 0.14 µM in MDA-MB-231, respectively.
Figure 1. Viability of MCF-7 (A), MDA-MB-231 (B) breast cancer cells, and MCF-10A (C) normal breast cells incubated for 24 h with different concentrations of 2a, 2b and cisplatin. Mean values ± SD from three independent experiment (n = 3) done in duplicate are presented.
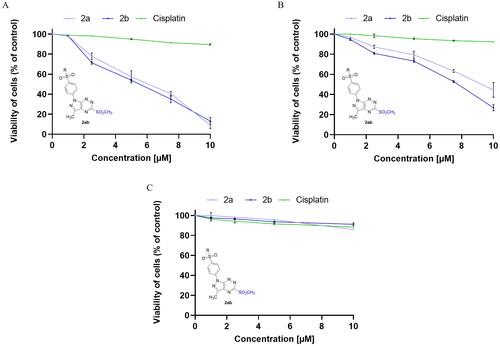
Figure 2. Viability of MCF-7 (A), MDA-MB-231 (B) breast cancer cells, and MCF-10A (C) normal breast cells incubated for 24 h with different concentrations of 3a, 3b and cisplatin. Mean values ± SD from three independent experiment (n = 3) done in duplicate are presented.
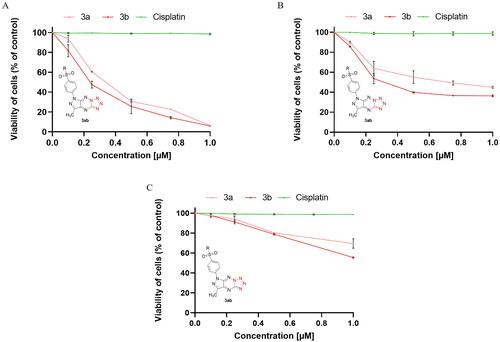
Table 1. The cytotoxic activity of new derivatives of pyrazolo[4,3-e][1,2,4]triazine.
According to the outcomes, the cytotoxic effect of (R)-enantiomers (2b,3b) was slightly higher than (S)-enantiomers (2a,3a) in both breast cancer cell lines (MCF-7 and MDA-MB-231). The pyrazolo[4,3-e]tetrazolo[1,5-b][1,2,4]triazine derivatives (3a, 3b) have stronger cytotoxic activity against both cancer cell lines compared to the sulphonyl from which they were derived (2a,b). The 3b inhibited MCF-7 and MDA-MB-231 cancer cell viability to the highest rate. Moreover, 3b exerted weaker cytotoxic effect on normal breast cells (IC50 = 2.3 ± 0.04 µM) than on cancer cells. According to the previous studies, the IC50 value for cisplatin (used as reference drug) was 82 µM in MDA-MB-231 cell line Citation31, 93 µM in MCF-7 Citation31 and above 100 µM in MCF-10A Citation25 after 24 h incubation.
For further studies as the most potent compound, 3b and its respective sulphonamide 2b were selected. Their effect on the molecular mechanism of apoptosis and autophagy was evaluated.
For quantitative determination of the apoptotic effects of 2b and 3b on MCF-7 and MDA-MB-231, flow cytometric assay was performed using double staining annexin V-FITC and propidium iodide (AV/PI). This study makes it possible to distinguish between four cell populations: viable cells, cells in early apoptosis, cells in late apoptosis, and necrotic cells. The results indicated that 3b induced apoptosis in both breast cancer cell lines was much greater than its respective sulphonyl 2b. It is intriguing that the number of apoptotic cells was related to the type of cell line. Compound 3b at 0.25 µM concentration caused an increase in apoptotic cells to 50.6 ± 3.5% in MCF-7 cell line and 11.8 ± 1.3% in MDA-MB-231. Whereas at concentration of 0.5 µM, 98.9 ± 1.6% of early and late apoptotic cells were noted in MCF-7 and 29.5 ± 0.9% in MDA-MB-231. The poorer proapoptotic activity was observed in both breast cancer cell lines exposed to compound 2b. This derivative of pyrazolo[4,3-e][1,2,4]triazine caused an elevation in the number of apoptotic cells to 9.8 ± 1.2% at 0.25 µM concentration in MCF-7 and 5.7 ± 0.6% in MDA-MB-231. At 0.5 µM concentration, 14.6 ± 1.7% of apoptotic cells were observed in MCF-7 and 5.8 ± 1.8% in MDA-MB-231. For the reference drug, 7.1 ± 0.9% apoptotic cells were noted after 24 h exposure at 0.5 µM in MCF-7 and 7.6 ± 0.6% in MDA-MB-231. As can be seen from , tetrazole derivative (3b) had marked stronger proapoptotic effect against both breast cancer cell lines than its sulphonyl derivative (2b). Thus, all further experiments were only performed with the 3b.
Figure 3. Flow cytometric analysis of induction of apoptosis in MCF-7 (A) and MDA-MB-231 (B) breast cancer cells incubated for 24 h with 2b, 3b and cisplatin (0.25 μM and 0.5 μM) stained with annexin V-FITC and propidium iodide. Mean percentage values from three independent experiments done in duplicate are presented. *p < 0.05 vs. control group and ***p < 0.001 vs. control group.
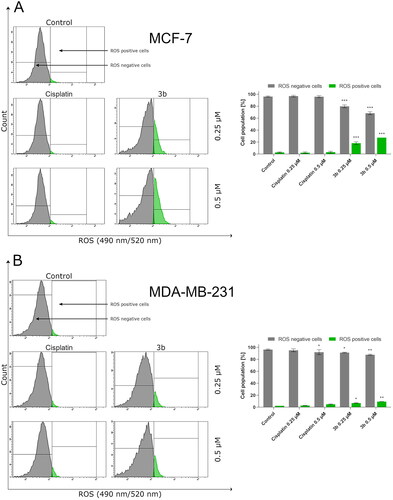
Recent scientific reports show that one of the clue factors involved in the induction of cancerous transformation are reactive oxygen species – ROSCitation32. These elements are essential for tumour cell proliferation survival, invasion, and metastasis. On the other hand, free radicals and compounds which lead to oxidative stress may induce PCD by triggering extrinsic or intrinsic pathway of apoptosis. Based on the dual role that ROS has, we evaluated the effect of 3b on the oxidative stress in MCF-7 and MDA-MB-231 cells. Following incubation with 3b, the flow cytometry revealed that 3b increased ROS generation in a dose-dependent manner. In MCF-7 at 0.25 µM concentration, 19.8 ± 2.2% cells have generated ROS and at 0.5 µM 27.5 ± 1.3%. On MDA-MB-231, there was observed 7.0 ± 0.6% cells with ROS at 0.25 µM concentration and 9.4 ± 0.7% at 0.5 µM. It is worth mentioning that the number of cells with ROS was almost threefold higher in MCF-7 cell line than in MDA-MB-231 in both concentrations of tested compound (3b). The obtained results are shown in .
Figure 4. Flow cytometric analysis of ROS induction in MCF-7 (A) and MDA-MB-231 (B) breast cancer cells incubated for 24 h with 3b and cisplatin (0.25 μM and 0.5 μM). Mean percentage values from three independent experiments done in duplicate are presented. *p < 0.05 vs. control group, **p < 0.01 vs. control group, ***p < 0.001 vs. control group.
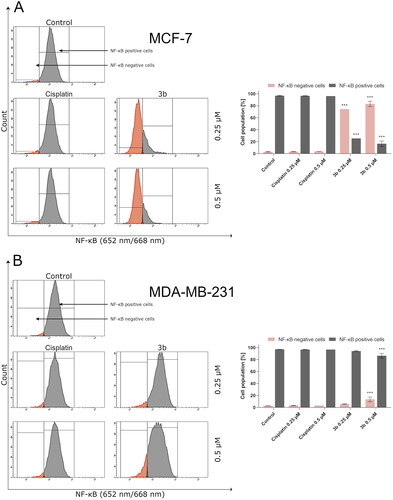
Moreover, the last data suggest that ROS can activate or deactivate nuclear factor κ light chain enhancer of activated B cells Citation33. In cancer cells, NF-κB is overactivated. This protein possesses antiapoptotic properties by stimulating expression of antiapoptotic proteins of Bcl-2 family and inhibiting the JNK signalling pathwayCitation34–36. In 2018, Huang and Xin demonstrated that intracellular ROS may inhibit signalling pathway associated with NF-κBCitation37. During the flow cytometry analysis of activity of NF-κB in breast cancer cell lines incubated with tested compound, it was shown that pyrazolo[4,3-e]tetrazolo[1,5-b][1,2,4]triazine derivative (3b) leads to decreased number of cells with active NF-κB (). In MCF-7 cells incubated 24 h with 3b at concentration 0.25 µM, there was observed 25.5 ± 4.5% of cells with active NF-κB and at 0.5 µM 19.8 ± 4.4% while in control was 97.2 ± 0.6%. After 24 h exposure of MDA-MB-231 to 3b at 0.25 µM and 0.5 µM, the percentage of cells with active NF-κB was 94.8 ± 0.6% and 89.6 ± 4.1%, respectively. Studies proved that 3b decreased the activity of NF-κB in both cell lines. The effect was more noticeable in MCF-7 cell line.
Figure 5. Anti-NF-κB antibody flow cytometric analysis of MCF-7 (A) and MDA-MB-231 (B) breast cancer cells compared to a negative control cell after 24 h of incubation with 3b and cisplatin (0.25 μM and 0.5 μM). Mean percentage values from three independent experiments done in duplicate are presented. ***p < 0.001 vs. control group.
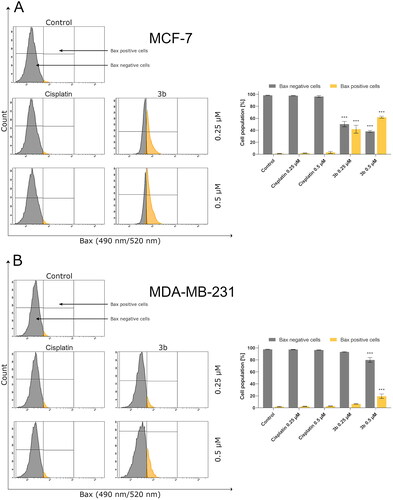
The intrinsic pathway of apoptosis could also be activated by ROS through an increase of protein Bax Citation38. This protein promotes apoptosis by creating pores in outer mitochondrial membrane and consequently increasing its permeabilityCitation39. Thus, in the next step of the research, the effect of novel compound 3b on Bax level in MCF-7 and MDA-MB-231 cells was evaluated. The number of cells with active Bax after exposition to cisplatin (24 h) was at the control level in both breast cancer cells. In contrast, incubation with 3b resulted in dose-dependent increase of Bax either in MCF-7 and MDA-MB-231 as shown in . The incubation with 3b (0.25 µM) increased number of cells with active Bax to 46.4 ± 6.6% in MCF-7 and 6.3 ± 0.4% in MDA-MB-231. After exposure of MCF-7 and MDA-MB-231 to higher concentrations, this value increased to 63.0 ± 1.6% and 16.9 ± 3.7%, respectively.
Figure 6. Anti-Bax antibody flow cytometric analysis of MCF-7 (A) and MDA-MB-231 (B) breast cancer cells compared to a negative control cell after 24 h of incubation with 3b and cisplatin (0.25 μM and 0.5 μM). Mean percentage values from three independent experiments done in duplicate are presented. ***p < 0.001 vs. control group.
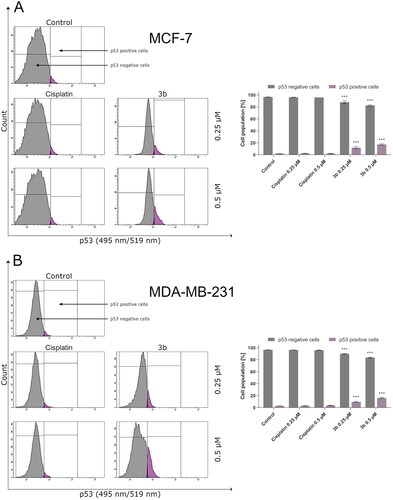
In addition, tumour suppressor p53 is a regulator of Bax gene expression in vitro and in vivoCitation40. According to the literature, the p53 protein activates the transcription genes involved in cell cycle regulation, apoptosis, and DNA repair in response to various stress factors. This mechanism ensures stability of genetic materials and prevents division of mutated cells and in consequence development of cancerCitation41. In human, invasive breast cancer TP53 is most common mutated geneCitation42. This mutation is associated with metastasis and poor overall patient’s survival. It is worth noting that in 80% of triple negative breast cancer cases, there is mutation in TP53. Therefore, activation of p53 may be an interesting molecular target of anticancer therapy. In this study, we have proved that novel compound 3b activates p53 in MCF-7 and MDA-MB-231 cell lines ().
Figure 7. Anti-p53 antibody flow cytometric analysis of MCF-7 (A) and MDA-MB-231 (B) breast cancer cells compared to a negative control cell after 24 h of incubation with 3b and cisplatin (0.25 μM and 0.5 μM). Mean percentage values from three independent experiments done in duplicate are presented. ***p < 0.001 vs. control group.
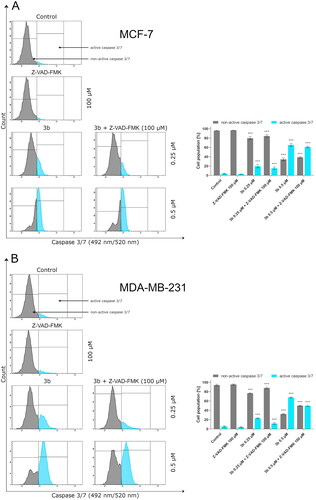
Protein p53 initiates intrinsic apoptosis pathway involving Bax and caspase 9, 3/7. Caspases play crucial role in apoptosis. They are not only the initiators of the process but also the main executors. The initiator caspases are activated by separate protein complexes. Caspase 10 is activated in death signalling protein (DISC) while caspase 9 in the apoptosome. Both caspases (10 and 9) lead to initiation of executor caspases 3 and 7. Therefore, in this study, we have evaluated the effect of the new derivative (3b) on the activation of these initiator and executioner caspases in breast cancer cell lines. The 24 h incubation of MCF-7 and MDA-MB-231 with 3b induced number of cells with active caspases 9, 10, and 3/7 in both breast cancer cell lines compared to control. There were 12.9 ± 0.8% of cells with active caspase 10 at 0.25 µM concentration and 70.8 ± 1.1% at 0.5 µM in MCF-7. In MDA-MB-231, there was 21.2 ± 1.4% (0.25 µM) and 50.8 ± 3.8% (0.5 µM) cells with active caspase 10 (). Moreover, 24 h exposure of cells to compound 3b was shown to result in an increase in caspase 9 activity at both concentration in oestrogen-independent cell line (MDA-MB-231) and oestrogen dependent (MCF-7) cell line. The highest percentage of active form of caspase 9 was observed at 0.5 µM in MCF-7 and was 72.1 ± 2.3%. While in MDA-MB-231 at the same concentration, 55.9 ± 1.2% of cells had active caspase 9 (). The induction of executioner caspases in the MCF-7 is related with activation of caspase 7 since this cell line does not express caspase 3Citation43. Despite the lack of expression of caspase 3 in MCF-7, it was observed that 24 h incubation of cells with 3b leads to increase in activity of executive caspase 7. In MDA-MB-231 cell line, we have also observed increase of cells with active form of caspase 3 and 7. At 0.25 µM concentration, there was 23.8 ± 0.8% cells with active executive caspases and at 0.5 µM there was 68.4 ± 1.2% ().
Figure 8. The activity of caspase 10 in MCF-7 (A) and MDA-MB-231 (B) breast cancer cells incubated with 3b (0.25 μM and 0.5 μM) in the absence and presence of Z-VAD-FMK (100 μM) for 24 h. Mean percentage values from three independent experiments done in duplicate are presented. ***p < 0.001 vs. control group.
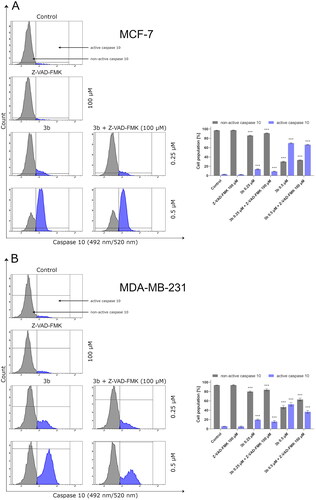
Figure 9. The activity of caspase 9 in MCF-7 (A) and MDA-MB-231 (B) breast cancer cells incubated with 3b (0.25 μM and 0.5 μM) in the absence and presence of Z-VAD-FMK (100 μM) for 24 h. Mean percentage values from three independent experiments done in duplicate are presented. ***p < 0.001 vs. control group.
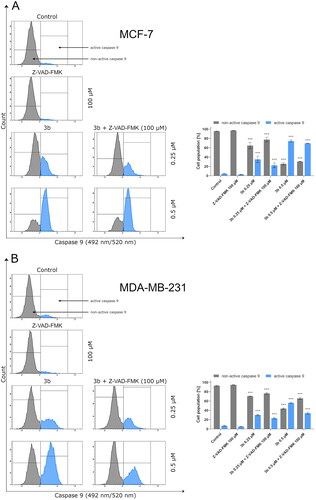
Figure 10. The activity of caspase 3/7 in MCF-7 (A) and MDA-MB-231 (B) breast cancer cells incubated with 3b (0.25 μM and 0.5 μM) in the absence and presence of Z-VAD-FMK (100 μM) for 24 h. Mean percentage values from three independent experiments done in duplicate are presented. ***p < 0.001 vs. control group.
To determine the role of the caspases in the activation of apoptosis by 3b, we pre-treated MCF-7 and MDA-MB-231 cells with irreversible pan-caspase inhibitor Z-VAD-FMK (50 µM) for 60 min. Following incubation, cells were treated with novel synthesised compound 3b for 24 h. In all instances, Z-VAD-FMK inhibitor decreased the activation of caspase 10, 9, and 3/7 in response to 3b in both cancer cell lines ().
The obtained results revealed that compound 3b initiation of apoptosis was slightly inhibited by pan-caspase inhibitor Z-VAD-FMK. Similar results were obtained by van der WaltCitation44. The researchers showed that Sutherlandia frutescens activate apoptosis in malignant melanoma cells which is related with activation of caspases. The use of a caspase inhibitor as in the case of the use of compound 3b resulted in a decrease in the activity of initiator and execution caspasesCitation44. The rate of inhibition of caspase activity depended on the type of caspase. These data suggest that compound 3b may induce apoptosis primarily through a caspase-dependent pathway and partially via caspase independent pathway in MCF-7 and MDA-MB-231 cells.
Many cancers result from failure of the apoptosis induction. The apoptotic signal may be blocked by increase of antiapoptotic molecules such as Bcl-2, IAP, FLIP, or decrease of proapoptotic molecules, i.e. Bax. In this case, autophagy may increase the efficacy of treatment by induction of apoptosis or be even alternative process leading to elimination of tumour cells Citation45. However, it can be also involved in tumour formation. The process of autophagy is responsible for maintaining intracellular homeostasis under stress conditions Citation46. Considering the ambiguous importance of autophagy in cancer treatment, we evaluated the effect of compound 3b on this process.
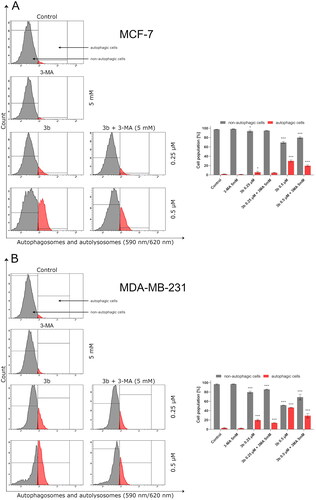
After 24 h incubation, it was observed that 3b in both concentrations (0.25 µM and 0.5 µM) induced autophagy in MCF-7 and MDA-MB-231 cells. The highest rate of autophagosomes was noted in MDA-MB-231 cells incubated with 3b at 0.5 µM (47.1 ± 0.6%). In the case of MCF-7 incubated with this compound at same concentration, 30 ± 2.1% autophagic cells were registered. Pre-treatment of MCF-7 and MDA-MB-231 with autophagy inhibitor (3-MA, 1 mM, 1 h) resulted in inhibition of the autophagy in cells treated with 3b. MDA-MB-231 treated with 3b (0.5 µM) and 3-MA generated 25.9 ± 4.7% of autophagic cells, while in MCF-7 incubated with 3b (0.5 µM) + 3MA, there was one and a half times less autophagic cells than in cells incubated with 3b (0.5 µM). The results are presented in .
Figure 11. Flow cytometric analysis of autophagy induction in MCF-7 (A) and MDA-MB-231 (B) breast cancer cells incubated with 3b (0.25 μM and 0.5 μM in the absence or presence of 3-MA (5 mM)). Mean percentage values from three independent experiments done in duplicate are presented. *p < 0.05 vs. control group, ***p < 0.001 vs. control group.
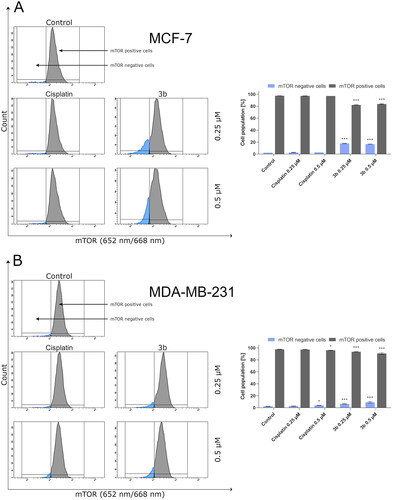
It has been reported that overactivation of PI3K/Akt/mTOR signalling pathway is commonly observed in cancer cells Citation47. Based on our previous results, on the induction of production of autophagosomes by 3b in both breast cancer cell lines, we have evaluated effect of this derivative on mTOR inhibition. It has been shown that compound 3b at both used concentrations (0.25 µM and 0.5 µM) leads to a decrease in the number of cells with active mTOR ().
Figure 12. Anti-mTOR antibody flow cytometric analysis of MCF-7 (A) and MDA-MB-231 (B) breast cancer cells compared to a negative control cell after 24 h of incubation with 3b and cisplatin (0.25 μM and 0.5 μM). Mean percentage values from three independent experiments done in duplicate are presented. *p < 0.05 vs. control group, ***p < 0.001 vs. control group.
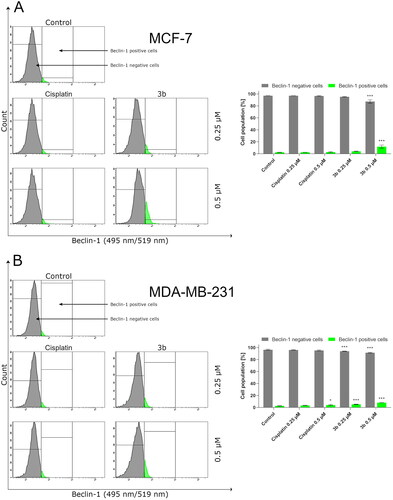
One of the autophagy marker’s is beclin-1. In our study, we have proved that compound 3b increases the number of cells with active beclin-1 in dose-dependent manner in both breast cancer cells. In MCF-7 after 24 h incubation with cisplatin and 3b at 0.5 µM concentration, we observed 4.1 ± 0.5% and 13.9 ± 2.6% cells with active beclin-1, respectively. While in MDA-MB-231, we noted 3.6 ± 0.7% cells with active beclin-1 after treatment with cisplatin (0.5 µM) and 8.1 ± 0.1% in 3b (0.5 µM). The obtained results are presented in .
Figure 13. Anti-beclin-1 antibody flow cytometric analysis of MCF-7 (A) and MDA-MB-231 (B) breast cancer cells compared to a negative control cell after 24 h of incubation with 3b and cisplatin (0.25 μM and 0.5 μM). Mean percentage values from three independent experiments done in duplicate are presented. *p < 0.05 vs. control group, ***p < 0.001 vs. control group.
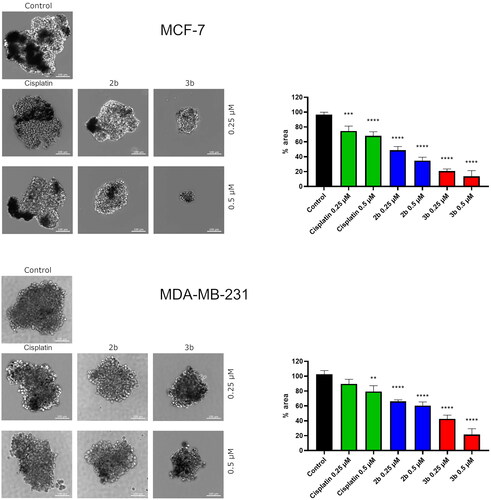
Recent progress in drug discovery has resulted in 3D in vitro culturing tools. Three-dimensional cell culture more closely reflected the conditions in the human body than monolayer 2D. Thus, we investigated the effect of novel compounds 2b and 3b on spheroid formation in MCF-7 and MDA-MB-231. The obtained results revealed that both derivatives, the sulphonyl (2b) and tetrazole (3b), lead to slower formation of spheroids compared to untreated cells. Moreover, the edges of MCF-7 spheroids exposed to 2b and 3b were frayed and blurred vs. control. Cisplatin used as reference drug at 0.25 µM and 0.5 µM concentration slightly affected growth of spheroids in MCF-7 and MDA-MB-231. The strongest inhibition of spheroid formation was observed in MCF-7 cells incubated with 3b at 0.5 µM concentration. The spheroids were approximately 86.37 ± 7.7% smaller compared to the control ().
Figure 14. Microscopy images of cell invasion of spheroids incubated for 24 h with 2b, 3b and cisplatin (0.25 μM and 0.5 μM). Representative photographs are shown; scale bar: 100 μm. Microscopy images of cell invasion of spheroids incubated for 24 h with 2b, 3b and cisplatin (0.25 μM and 0.5 μM). Representative photographs are shown; scale bar: 100 μm. **p < 0.01 vs. control group, ***p < 0.001 vs. control group, ****p < 0.0001 vs. control group.
Discussion
Nowadays, a significant part of scientific research is focused on prevention, early detection, and effective treatment of cancer. According to estimates of GLOBOCON, the number of breast cancer deaths in the world has increased by almost 84% compared to the year 2000Citation1,Citation48. In addition to this, despite the great progress in the treatment of cancer and dynamic development of pharmacology, modern medicine still does not have effective and minimally invasive anticancer therapy. Currently used therapy has low specificity towards cancer cells which causes undesirable side effects. Thus, one of the greatest challenges of the modern oncology is synthesis of effective and safe anticancer drugs for patients. It is worth noting that heterocyclic systems possess variable biological activity and are widely used in pharmaceuticals, antibiotics, and in agrochemicals. In addition to this, almost 75% of FDA-approved drug by contain a nitrogen heterocycle Citation49. Among N-heterocycles, 1,2,4-triazine derivatives present an interesting group of anticancer drugs. Currently, numerous studies are underway to utilise this moiety in designing the most optimal lead structure with anticancer activityCitation11,Citation13–15,Citation50–52. In 2020, one of the derivatives of 1,2,4-triazine was approved in cancer treatment. Blueprint Medicines Corporation registrated Ayvakit in gastrointestinal stromal tumours (GIST). The active substance of this drug is avapritinib which is a kinase inhibitor of PDGFRα Citation53. The high efficacy of the compound in comparison to other tyrosine kinase inhibitors has led to further clinical trial using this compound in the treatment of other advanced solid tumours such as lung, breast, melanoma, and sarcomaCitation54,Citation55.
Nevertheless, important strategy in the search for chemotherapeutics is the approach based on combining fragments of known drugs in a single molecule. It is intended to design compounds with increased biological activity than the original compounds. Combining natural pyrazolo[4,3-e][1,2,4]triazine system with pharmacophore groups provides an opportunity to design new chemical compounds with potential anticancer activity. Mojzych et al. presented synthesis of aza-sildenafil analogues and aniline substituted pyrazolo[4,3-e][1,2,4]triazine sulphonamides. These compounds exerted moderate antitumor activity in breast cancer cells and potential mechanism of action was associated with inhibition of tumour associated carbonic anhydrase isoforms (IX and XII) Citation56. In 2010, Gucký et al. presented series of pyrazolo[4,3-e][1,2,4]triazine derivatives which exerted cytotoxic activity against five cancer cell lines (T-lymphoblastic leukaemia, T-lymphoblastic leukaemia daunorubicin-resistant clone, myeloid leukaemia, myeloid leukaemia paclitaxel resistant clone, and lung adenocarcinoma)Citation57. As can be seen, the incorporation of a sulphonamide group to pyrazolotriazine moiety leads to an increase in the antitumor activity of the original compounds. In addition to this, the structure of pyrazolo[4,3-e][1,2,4]triazine can be modified by incorporation of tetrazole or triazole ring. A 2011 study showed that the insertion of a terminal tetrazole or triazole ring in replacement of SCH3 group resulted in a significant increase in cytotoxic activity against breast cancer cell line. For the triazole derivative, the IC50 value is 4 µM while for tetrazole it is 0.5 µM Citation58. These premises prompted our research team to use pyrazolo[4,3-e]tetrazolo[1,5-b][1,2,4]triazine system conjugated with sulphonamide group to synthesise new derivatives with potential biological activityCitation12–14,Citation51. Our previous studies on bioactive compounds of pyrazolo[4,3-e]tetrazolo[1,5-b][1,2,4]triazine derivatives Citation13,Citation14,Citation51 against colon cancer cell lines were very promising. In view of high anticancer activity of these compounds and exploiting literature on the synthesis and functionalisation of the hitherto little known pyrazolo[4,3-e][1,2,4]triazine system, we designed novel compounds 2a, 2b, 3a, and 3b. Chlorosulfonyl derivative (1) in reaction with amines led to sulphonyl derivatives (2a,b), which were used in a nucleophilic substitution reaction of methylsulfonyl group with sodium azide. The reaction directly led to the final tetrazolium derivatives (2a,b). Based on conducted experiments, the new synthetic derivatives 2a, 2b, 3a, and 3b have shown high cytotoxic activity against MCF-7 and MDA-MB-231 breast cancer cells in the micromolar concentration range (IC50 from 0.25 µM to 9.2 µM). Moreover, synthesised compounds indicated low cytotoxic effect on normal cell lines. The nucleophilic substitution of SO2CH3 group with sodium azide was shown to yield tetrazolium derivatives of pyrazolo[4,3-e][1,2,4]triazine (3a,b) with stronger cytotoxic properties than sulphonamide derivatives (2a,b). Compound 3a was 17 times stronger than 2a against MCF-7 and almost 13 times against MDA-MB-231. Compound 3b was 22 times stronger than 2b in MCF-7 and 25 times against MDA-MB-231. It is worth mentioning that all synthesised compounds have notable higher cytotoxic activity than reference drug (cisplatin). Czarnomysy et al. reported that IC50 of cisplatin of MCF-7 cells was 93 µM and 82 µM of MDA-MB-231 cells after 24 h incubationCitation31. Preliminary MTT assay results allowed us to select 2b as most active sulphonyl derivative and 3b as the most active tetrazole derivative.
Research on the effect of drug chirality on their pharmacological activity has been conducted for many years. The differences in the pharmacological activity of particular isomers may have serious consequences for the treatment. For this reason, stereochemistry has become an important part of the drug development. Chiral biologically active compounds can be classified into one of the following categories: both enantiomers exhibit favourable pharmacological activity, only one of the enantiomers is pharmacologically active, both enantiomers have desirable pharmacological activity but different mechanism and one of the enantiomers has therapeutic effect and the other acts as an antagonistCitation59,Citation60. In our studies, we have proved that both pairs of enantiomers 2ab and 3ab have similar cytotoxic activity against breast cancer cell lines (MCF-7 and MDA-MB-231) and normal cells (MCF-10A). However, the R-enantiomers of synthesised compounds exerted slightly higher anticancer activity than their respective S-isomers.
Currently, approaches utilised in cancer chemotherapy are likely to affect the apoptosisCitation61. Thus, to compare biological activity of sulphonamide derivative (2b) and its corresponding tetrazolium derivative (3b), their effect on the molecular mechanism of apoptosis in both breast cancer cells was evaluated. The study revealed that both 2b and 3b derivatives have proapoptotic effect against MCF-7 and MDA-MB-231. Recent research indicates that exposure of cancer cells to chemotherapeutics induces apoptosis mediated by ROSCitation27. Increased level of ROS by drugs may be due to mitochondrial generation of ROS and depletion of antioxidant systems. ROS is engaged in activation of p53, JNK, and proapoptotic Bcl-2 proteins (i.e. Bax) and oxidation of cardiolipin which induce release of cytochrome C to cytosol and initiate intrinsic pathway of apoptosis. Moreover, ROS may also trigger apoptosis through extrinsic pathway by transmembrane death receptors (i.e. TNFR, TRAIL)Citation62,Citation63. The links between ROS and apoptosis prompted us to evaluate the effect of 3b on the level of ROS after 24 h incubation in MCF-7 and MDA-MB-231 breast cancer cells. Our results confirmed the previous research reportsCitation27,Citation64,Citation65, that anticancer activity of chemotherapeutics may be related with induction of reactive oxygen species. Compound 3b at 0.5 µM increased ROS level in MCF-7 12 times and four times in MDA-MB-231 compared to respective control.
As it was mentioned, programmed cell death is related with morphological and biochemical modifications which embrace upregulation of Bax, p53, and caspasesCitation66. To identify that apoptosis induced by 3b flow cytometry methods were used (activity levels of caspase 9, 10, 3/7 and protein activation p53, Bax), the obtained results revealed that novel synthesised compound 3b induces the level of Bax and p53 proteins in both breast cancer cell lines in dose-dependent manner. Our findings are consistent with those of Zaki et al. who synthesised 1,2,4-triazinone derivatives and proved that these compounds induce apoptosis in MCF-7 breast cancer cell by increasing level of proteins involved in intrinsic pathway of programmed cell death, i.e. Bax and p53Citation67.
One of the main enzymes engaged in apoptosis are caspases. They participate by their cysteine moiety in the catalytic process and the digestion of the proteins directly after asparagineCitation68. Keeping this in view, we have proved that compound 3b induces apoptosis by activation of cascade caspase. This results present promising strategy for the treatment of cancer. However, to understand the role of caspases in the induction of apoptosis by 3b in MCF-7 and MDA-MB-231, pan-caspase inhibitor Z-VAD-FMK was used. We confirmed that Z-VAD-FMK inhibitor slightly reduced the number of cells with active caspase −3/7, −9, and −10 indicating that caspase may be a potential molecular mechanism of 3b. The decrease in caspase activity was more significant in MDA-MB-231 cells than in MCF-7 cells.
One of the significant links between cancer progression and cell death is NF-κB. It is nucleus of B cells that is the transcriptional factor. In the cancer cells, overexpression of NF-κB is observed which suppresses programmed cell death by exciting expression of IAP, cFLIP, and TNF – inhibitory genesCitation69. Recent scientific reports of in vitro studies suggest inhibition of NF-κB may sensitise cancer cells to chemotherapy which occur by launching apoptosis in these cells. Bortezomib in head and neck squamous cell carcinomaCitation70 has anticancer effect by inhibition of NF-κB, activation of apoptosis and inhibition of proliferation of cancer cells. The results presented in this paper indicate that compound 3b also inhibits the number of cells with active NF-κB.
According to the recommendations of the Nomenclature Committee on Cell Death (NCDD) besides apoptosis, other types of cell death have been distinguished including autophagyCitation71. Nowadays, the role of autophagy in development of cancer is not clearCitation72. To evaluate whether the mechanism of action of novel pyrazolo[4,3-e]tetrazolo[1,5-b][1,2,4]triazine on the breast cancer cells is related to autophagic response, we measured the number of autophagosomes and level of beclin-1 after 24 h incubation with compound 3b. We found that 3b stimulates autophagy process in MCF-7 and MDA-MB-231 by inducing autophagosome formation and induction of beclin-1 level in dose-dependent manner. In addition to this, we investigated the possibility that 3-MA could inhibit autophagy induced by 3b. Co-treatment with autophagy inhibitor (3-MA) resulted in decrease of formation of autophagosomes in both cancer cell lines. As the concentration of 3b increased, the proautophagic effect was more noticeable. The role of autophagy in cancer is still inconclusive. However, many scientific reports indicate that autophagy is a basis for anticancer activityCitation25,Citation27,Citation72,Citation73. One of the factors involved in either apoptosis or autophagy is mTOR. Overexpression of PI3K-Akt-mTOR pathway induced the oncogenesis in various cancers: breast, gastric, melanoma, and hepatocellular Citation74. Increased activity of this pathway is a common result of mutations observed in cancer cells. Hence, inhibition of this signalling pathway not only induces apoptosis in cancer cells but also stimulates autophagy. We discovered that incubation with 3b downregulates the number of cells with active mTOR in a dose dependent manner in both breast cancer cell lines at 24 h. These results indicate that the pyrazolo[4,3-e][1,5-b][1,2,4]triazine derivative (3b) might induce autophagy via PI3K/AKT/mTOR pathway.
Another protein required for the initiation of autophagy is beclin 1. Under physiological conditions, this protein remains in complex with the anti-apoptotic proteins Bcl-2 and Bcl-xl. Under the influence of stress factors, e.g. chemotherapeutics beclin 1 is released from the complex and induce formation of autophagosomesCitation75,Citation76. Our research showed that compound 3b slightly induced the level of beclin 1 in both breast cancer cell lines.
The intermediate form between monolayer and in vivo is multicellular tumour spheroids. Morphologically co-cultures in spheroids are composed of the cells with different phenotypes, proliferating cells, and necrotic cells located in the middle of spheroid. The responses of spheroids and tumours growing in vivo to administrated antitumor agents are similarCitation77–81. According to this, we proved that novel synthesised compounds 2b and 3b reduced the size of MCF-7 and MDA-MB-231 spheroids. The strongest inhibition of spheroid formation in both breast cancer cell lines was caused by compound 3b at 0.5 µM.
Conclusions
We have proved that new sulphones (2ab) and tetrazole derivatives (3ab) have potent anticancer activity against breast cancer. It was shown that new pairs of enantiomer derivatives 2a,b and 3a,b possess cytotoxic properties against MCF-7 and MDA-MB-231 in the micromolar concentration range (IC50 0.25 µM from to 6.0 µM). In addition, novel synthesised compounds have less effect on the survival of normal cells: MCF-10A. Preliminary MTT assay results allowed us to select R-enantiomers for further more detailed biological research. Studies on molecular mechanism of action of 2b and 3b revealed that both synthesised compounds lead to initiation of apoptosis in two breast cancer cell lines. This enabled compound 3b to be selected as the most active. Compound 3b induced apoptosis through activation of extrinsic and intrinsic pathway. This compound increased the activity of p53, Bax, and ROS and decreased NF-κB which play crucial role in activation of apoptosis. Moreover, the compound 3b decreased the level of mTOR which play main role in autophagy. It is known that process of autophagy is impaired in cancer cells. The results revealed that 3b induces formation of autophagosomes in MCF-7 and MDA-MB-231. Preincubation of cells with an autophagy inhibitor (3-MA) caused inhibition of 3b induced autophagosome formation. Beclin-1 also plays a crucial role in the process of autophagy. We proved our novel compound 3b increased the level of this protein in both breast cancer cells, but the effect was negligible. It suggests that activation of autophagy by 3b via beclin-1 is minor. The inhibitory effect of 3-MA was more prominent in cells incubated with the compound 3b at a concentration of 0.5 µM. The obtained results extend the existing knowledge on the molecular mechanism of anticancer activity of pyrazolo[4,3-e]tetrazolo[1,5-b][1,2,4-triazine] derivatives. Further studies to understand the molecular pathways involved in the anticancer activity and effect of the described group of compounds on the expression of genes involved in the apoptosis and autophagy are necessary and will be continued.
Author contributions
Conceptualisation, R.C., M.M., K.K.-M., A.B.; methodology, R.C., A.S., D.R. M.M.; investigation, A.S., D.R., R.C., M.M.; writing – original draft preparation, A.S., R.C., M.M.; writing – review and editing, R.C., A.B., K.B., M.M, K.K.-M.; visualisation, D.R., A.S., R.C., M.M.; supervision, R.C., K.B., M.M., A.B.; funding acquisition, K.B., A.B., R.C., M.M. All authors have read and agreed to the published version of the manuscript.
Supplemental Material
Download PDF (490.7 KB)Disclosure statement
The authors report no conflicts of interest.
Data availability statement
Department of Synthesis and Technology of Drugs, Medical University of Bialystok, Kilinskiego 1, 15-089 Bialystok, Poland.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020;71(6):1–23.
- Mathers CD, Boschi-Pinto C, Lopez AD, Murray CJL, World Health Organization. Cancer incidence, mortality and survival by site for 14 regions of the world; 2001. Geneva. https://apps.who.int/iris/handle/10665/67775.
- Dai X, Cheng H, Bai Z, Li J. Breast cancer cell line classification and its relevance with breast tumor subtyping. J Cancer. 2017;8(16):3131–3141.
- Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn H-J, Panel members. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24(9):2206–2223.
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4(11):891–899.
- Heravi M, Zadsirjan V. Prescribed drugs containing nitrogen heterocycles: an overview. RSC Adv. 2020;10(72):44247–44311.
- Kerru N, Gummidi L, Maddila S, Gangu KK, Jonnalagadda SB. A review on recent advances in nitrogen-containing molecules and their biological applications. Molecules. 2020;25(8):1909.
- Mojzych M, Bernat Z, Karczmarzyk Z, Matysiak J, Fruziński A. Synthesis, structural characterization, and biological activity of new pyrazolo[4,3-e][1,2,4]triazine acyclonucleosides. Molecules. 2020;25(1):221.
- Mojzych M, Ceruso M, Bielawska A, Bielawski K, Fornal E, Supuran CT. New pyrazolo[4,3-e][1,2,4]triazine sulfonamides as carbonic anhydrase inhibitors. Bioorg Med Chem. 2015;23(13):3674–3680.
- Mojzych M, Dolashki A, Voelter W. Synthesis of pyrazolo[4,3-e][1,2,4]triazine sulfonamides, novel sildenafil analogs with tyrosinase inhibitory activity. Bioorg Med Chem. 2014;22(23):6616–6624.
- Bernat Z, Szymanowska A, Kciuk M, Kotwica-Mojzych K, Mojzych M. Review of the synthesis and anticancer properties of pyrazolo[4,3-e][1,2,4]triazine derivatives. Molecules. 2020;25(17):3948.
- Gornowicz A, Szymanowska A, Mojzych M, Bielawski K, Bielawska A. The effect of novel 7-methyl-5-phenyl-pyrazolo[4,3-e]tetrazolo[4,5-b][1,2,4]triazine sulfonamide derivatives on apoptosis and autophagy in DLD-1 and HT-29 colon cancer cells. Int J Mol Sci. 2020;21(15):5221.
- Gornowicz A, Szymanowska A, Mojzych M, Czarnomysy R, Bielawski K, Bielawska A. The anticancer action of a novel 1,2,4-triazine sulfonamide derivative in colon cancer cells. Molecules. 2021;26(7):2045.
- Hermanowicz JM, Szymanowska A, Sieklucka B, Czarnomysy R, Pawlak K, Bielawska A, Bielawski K, Kalafut J, Przybyszewska A, Surazynski A, et al. Exploration of novel heterofused 1,2,4-triazine derivative in colorectal cancer. J Enzyme Inhib Med Chem. 2021;36(1):535–548.
- Cascioferro S, Parrino B, Spanò V, Carbone A, Montalbano A, Barraja P, Diana P, Cirrincione G. An overview on the recent developments of 1,2,4-triazine derivatives as anticancer compounds. Eur J Med Chem. 2017;142:328–375.
- Szymanowska A, Gornowicz A, Bielawska A, Bielawski K. [1,2,4]triazines – as potential drugs in cancer chemotherapy. Postepy Hig Med Dosw. 2021;75:64–84.
- Rai GS, Maru JJ. Synthetic strategies for pyrrolo[2,1-f][1,2,4]triazine: the parent moiety of antiviral drug remdesivir. Chem Heterocycl Compd. 2020;56(12):1517–1522.
- Fortin S, Bérubé G. Advances in the development of hybrid anticancer drugs. Expert Opin Drug Discov. 2013;8(8):1029–1047.
- Nepali K, Sharma S, Sharma M, Bedi PMS, Dhar KL. Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur J Med Chem. 2014;77:422–487.
- Supuran CT, Casini A, Scozzafava A. Protease inhibitors of the sulfonamide type: anticancer, antiinflammatory, and antiviral agents. Med Res Rev. 2003;23(5):535–558.
- Scozzafava A, Owa T, Mastrolorenzo A, Supuran CT. Anticancer and antiviral sulfonamides. Curr Med Chem. 2003;10(11):925–953.
- Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev. 2003;23(2):146–189.
- Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem. 2007;15(13):4336–4350.
- Mojzych M, Rykowski A. Synthesis of functionalized 1H-pyrazolo[4,3-e][1,2,4]triazines and their fused derivatives via IPSO-substitution of methylsulfonyl group with O-, N-, S- and C-nucleophiles. ChemInform. 2004;35(50):35.
- Czarnomysy R, Radomska D, Muszyńska A, Hermanowicz JM, Prokop I, Bielawska A, Bielawski K. Evaluation of the anticancer activities of novel transition metal complexes with berenil and nitroimidazole. Molecules. 2020;25(12):2860.
- Radomska D, Czarnomysy R, Szymanowska A, Radomski D, Domínguez-Álvarez E, Bielawska A, Bielawski K. Novel selenoesters as a potential tool in triple-negative breast cancer treatment. Cancers. 2022;14(17):4304.
- Czarnomysy R, Muszyńska A, Rok J, Rzepka Z, Bielawski K. Mechanism of anticancer action of novel imidazole platinum(II) complex conjugated with G2 PAMAM-OH dendrimer in breast cancer cells. Int J Mol Sci. 2021;22(11):5581.
- Shirato K, Imaizumi K, Abe A, Tomoda A. Phenoxazine derivatives induce caspase-independent cell death in human glioblastoma cell lines, A-172 and U-251 MG. Oncol Rep. 2007;17(1):201–208.
- Hseu Y-C, Lin R-W, Shen Y-C, Lin K-Y, Liao J-W, Thiyagarajan V, Yang H-L. Flavokawain B and doxorubicin work synergistically to impede the propagation of gastric cancer cells via ROS-mediated apoptosis and autophagy pathways. Cancers. 2020;12(9):2475.
- Haisler WL, Timm DM, Gage JA, Tseng H, Killian TC, Souza GR. Three-dimensional cell culturing by magnetic levitation. Nat Protoc. 2013;8(10):1940–1949.
- Czarnomysy R, Bielawski K, Muszynska A, Bielawska A, Gornowicz A. Biological evaluation of dimethylpyridine–platinum complexes with potent antiproliferative activity. J Enzyme Inhib Med Chem. 2016;31(Suppl. 3):150–165.
- Gupta SC, Hevia D, Patchva S, Park B, Koh W, Aggarwal BB. Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid Redox Signal. 2012;16(11):1295–1322.
- Kumar Rajendran N, George BP, Chandran R, Tynga IM, Houreld N, Abrahamse H. The influence of light on reactive oxygen species and NF-κB in disease progression. Antioxidants. 2019;8(12):640.
- Takai A, Marusawa H, Minaki Y, Watanabe T, Nakase H, Kinoshita K, Tsujimoto G, Chiba T. Targeting activation-induced cytidine deaminase prevents colon cancer development despite persistent colonic inflammation. Oncogene. 2012;31(13):1733–1742.
- Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: balancing life and death – a new approach to cancer therapy. J Clin Invest. 2005;115(10):2625–2632.
- De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, Cong R, Franzoso G. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature. 2001;414(6861):308–313.
- Huang M, Xin W. Matrine inhibiting pancreatic cells epithelial–mesenchymal transition and invasion through ROS/NF-κB/MMPs pathway. Life Sci. 2018;192:55–61.
- George BP, Abrahamse H. Increased oxidative stress induced by rubus bioactive compounds induce apoptotic cell death in human breast cancer cells. Oxid Med Cell Longev. 2019;2019:6797921.
- Westphal D, Kluck RM, Dewson G. Building blocks of the apoptotic pore: how Bax and Bak are activated and oligomerize during apoptosis. Cell Death Differ. 2014;21(2):196–205.
- Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, Hoffman B, Reed JC. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994;9(6):1799–1805.
- Wang Z, Sun Y. Targeting p53 for novel anticancer therapy. Transl Oncol. 2010;3(1):1–12.
- Duffy MJ, Synnott NC, Crown J. Mutant p53 in breast cancer: potential as a therapeutic target and biomarker. Breast Cancer Res Treat. 2018;170(2):213–219.
- Jänicke RU. MCF-7 breast carcinoma cells do not express caspase-3. Breast Cancer Res Treat. 2009;117(1):219–221.
- van der Walt NB, Zakeri Z, Cronjé MJ. The induction of apoptosis in A375 malignant melanoma cells by Sutherlandia frutescens. Evid Based Complement Alternat Med. 2016;2016:4921067.
- Brech A, Ahlquist T, Lothe RA, Stenmark H. Autophagy in tumour suppression and promotion. Mol Oncol. 2009;3(4):366–375.
- Kim R. Recent advances in understanding the cell death pathways activated by anticancer therapy. Cancer. 2005;103(8):1551–1560.
- Chen N, Karantza-Wadsworth V. Role and regulation of autophagy in cancer. Biochim Biophys Acta. 2009;1793(9):1516–1523.
- Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: GLOBOCAN 2000. Int J Cancer. 2001;94(2):153–156.
- Omar A. Anticancer activities of some fused heterocyclic moieties containing nitrogen and/or sulfur heteroatoms. Al-Azhar J Pharm Sci. 2020;62(2):39–54.
- Bernat Z, Mieszkowska A, Mazerska Z, Matysiak J, Karczmarzyk Z, Kotwica-Mojzych K, Mojzych M. Chiral pyrazolo[4,3-e][1,2,4]triazine sulfonamides—their biological activity, lipophilicity, protein affinity, and metabolic transformations. Appl Sci. 2021;11(6):2660.
- Hermanowicz JM, Pawlak K, Sieklucka B, Czarnomysy R, Kwiatkowska I, Kazberuk A, Surazynski A, Mojzych M, Pawlak D. MM-129 as a novel inhibitor targeting PI3K/AKT/mTOR and PD-L1 in colorectal cancer. Cancers. 2021;13(13):3203.
- Szymanowska A, Gornowicz A, Bielawska A, Bielawski K. [1,2,4]triazines – as potential drugs in cancer chemotherapy. Postępy Higieny i Medycyny Doświadczalnej. 2021;75(1):64–84.
- Dhillon S. Avapritinib: first approval. Drugs. 2020;80(4):433–439.
- von Mehren M, Heinrich MC, Shi H, Iannazzo S, Mankoski R, Dimitrijević S, Hoehn G, Chiroli S, George S. Clinical efficacy comparison of avapritinib with other tyrosine kinase inhibitors in gastrointestinal stromal tumors with PDGFRA D842V mutation: a retrospective analysis of clinical trial and real-world data. BMC Cancer. 2021;21(1):291.
- ClinicalTrials.gov. Avapritinib for the treatment of CKIT or PDGFRA mutation-positive locally advanced or metastatic malignant solid tumors; 2021 [Internet] [accessed 2021 Dec 12]. https://clinicaltrials.gov/ct2/show/NCT04771520?term=triazine&draw=2&rank=5
- Mojzych M, Bielawska A, Bielawski K, Ceruso M, Supuran CT. Pyrazolo[4,3-e][1,2,4]triazine sulfonamides as carbonic anhydrase inhibitors with antitumor activity. Bioorg Med Chem. 2014;22(9):2643–2647.
- Gucký T, Řezníčková E, Džubák P, Hajdúch M, Kryštof V. Synthesis and anticancer activity of some 1,5-diaryl-3-(3,4,5-trihydroxyphenyl)-1H-pyrazolo[4,3-e][1,2,4]triazines. Monatsh Chem. 2010;141(6):709–714.
- Mojzych M. Cytotoxic activity of some pyrazolo[4,3-e][1,2,4]triazines against human cancer cell lines. J Chem Soc Pak. 2011;33:123–128.
- Nguyen LA, He H, Pham-Huy C. Chiral drugs: an overview. Int J Biomed Sci. 2006;2(2):85–100.
- Wainer IW. Stereoisomers in clinical oncology: why it is important to know what the right and left hands are doing. Ann Oncol. 1993;4(Suppl. 2):7–13.
- Pfeffer CM, Singh ATK. Apoptosis: a target for anticancer therapy. Int J Mol Sci. 2018;19(2):448.
- Yang H, Villani RM, Wang H, Simpson MJ, Roberts MS, Tang M, Liang X. The role of cellular reactive oxygen species in cancer chemotherapy. J Exp Clin Cancer Res. 2018;37(1):266.
- Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016;1863(12):2977–2992.
- Huang S-M, Hsu P-C, Chen M-Y, Li W-S, More SV, Lu K-T, Wang Y-C. The novel indole compound SK228 induces apoptosis and FAK/paxillin disruption in tumor cell lines and inhibits growth of tumor graft in the nude mouse. Int J Cancer. 2012;131(3):722–732.
- Wang Y, Luo YH, Piao XJ. Novel 1,4‑naphthoquinone derivatives induce reactive oxygen species‑mediated apoptosis in liver cancer cells. Mol Med Rep. 2019;19(3):1654–1664.
- Mohan S, Abdelwahab SI, Kamalidehghan B, Syam S, May KS, Harmal NSM, Shafifiyaz N, Hadi AHA, Hashim NM, Rahmani M, et al. Involvement of NF-κB and Bcl2/Bax signaling pathways in the apoptosis of MCF7 cells induced by a xanthone compound pyranocycloartobiloxanthone A. Phytomedicine. 2012;19(11):1007–1015.
- Zaki I, Abdelhameid MK, El-Deen IM, Abdel Wahab AHA, Ashmawy AM, Mohamed KO. Design, synthesis and screening of 1,2,4-triazinone derivatives as potential antitumor agents with apoptosis inducing activity on MCF-7 breast cancer cell line. Eur J Med Chem. 2018;156:563–579.
- Boice A, Bouchier-Hayes L. Targeting apoptotic caspases in cancer. Biochim Biophys Acta Mol Cell Res. 2020;1867(6):118688.
- Leber B, Lin J, Andrews DW. Embedded together: the life and death consequences of interaction of the Bcl-2 family with membranes. Apoptosis. 2007;12(5):897–911.
- Van Waes C, Chang AA, Lebowitz PF, Druzgal CH, Chen Z, Elsayed YA, Sunwoo JB, Rudy SF, Morris JC, Mitchell JB, et al. Inhibition of nuclear factor-kappaB and target genes during combined therapy with proteasome inhibitor bortezomib and reirradiation in patients with recurrent head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2005;63(5):1400–1412.
- Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25(3):486–541.
- Buzun K, Gornowicz A, Lesyk R, Bielawski K, Bielawska A. Autophagy modulators in cancer therapy. Int J Mol Sci. 2021;22(11):5804.
- Deng S, Shanmugam MK, Kumar AP, Yap CT, Sethi G, Bishayee A. Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer. 2019;125(8):1228–1246.
- Ciołczyk-Wierzbicka D, Zarzycka M, Gil D, Laidler P. mTOR inhibitor Everolimus-induced apoptosis in melanoma cells. J Cell Commun Signal. 2019;13(3):357–368.
- Doherty J, Baehrecke EH. Life, death and autophagy. Nat Cell Biol. 2018;20(10):1110–1117.
- Liu W, Li S, Qu Z, Luo Y, Chen R, Wei S, Yang X, Wang Q. Betulinic acid induces autophagy-mediated apoptosis through suppression of the PI3K/AKT/mTOR signaling pathway and inhibits hepatocellular carcinoma. Am J Transl Res. 2019;11(11):6952–6964.
- Zhang X, Wang W, Yu W, Xie Y, Zhang X, Zhang Y, Ma X. Development of an in vitro multicellular tumor spheroid model using microencapsulation and its application in anticancer drug screening and testing. Biotechnol Prog. 2005;21(4):1289–1296.
- Madsen SJ, Sun C-H, Tromberg BJ, Cristini V, De Magalhães N, Hirschberg H. Multicell tumor spheroids in photodynamic therapy. Lasers Surg Med. 2006;38(5):555–564.
- Anada T, Masuda T, Honda Y, Fukuda J, Arai F, Fukuda T, Suzuki O. Three-dimensional cell culture device utilizing thin membrane deformation by decompression. Sens Actuators B. 2010;147(1):376–379.
- Santini MT, Rainaldi G, Indovina PL. Apoptosis, cell adhesion and the extracellular matrix in the three-dimensional growth of multicellular tumor spheroids. Crit Rev Oncol Hematol. 2000;36(2–3):75–87.
- Kim JB. Three-dimensional tissue culture models in cancer biology. Semin Cancer Biol. 2005;15(5):365–377.