ABSTRACT
Introduction: Bovine neonatal pancytopenia (BNP) is a hemorrhagic disease that emerged in calves across Europe in 2007. Its occurrence is attributed to immunization of the calf’s mother with a vaccine produced using an allogeneic cell line. Vaccine-induced alloantibodies specific for major-histocompatibility class I antigens are transferred from the mother to the calf via colostrum, leading to profound depletion of peripheral blood and bone marrow cells that is often fatal.
Areas covered: Pubmed and Web of Science were used to search for literature relevant to BNP and the use of allogeneic vaccine cell lines. Following a review of the pathology and pathogenesis of this novel condition, we discuss potential risks associated with the use of allogeneic vaccine cell lines.
Expert commentary: Although BNP is associated with a specific vaccine, it highlights safety concerns common to all vaccines produced using allogeneic cell lines. Measures to prevent similar vaccine-induced alloimmune-mediated adverse events in the future are discussed.
1. The emergence of a novel disease
In 2007 a novel hemorrhagic disease emerged in calves across Europe, characterized by profound depletion of peripheral blood and bone marrow cells the disease was named bovine neonatal pancytopenia (BNP) [Citation1,Citation2]. By February 2011 the deaths of more than 4500 calves in Europe had been attributed to BNP [Citation3] and many more cases were undoubtedly unreported [Citation4]. From 2011 BNP cases also appeared in New Zealand [Citation5]. No infectious agent, myelosuppressive toxin or genetic defect could be identified as the cause of the disease [Citation1,Citation2,Citation6,Citation7] and, as the incidence on most affected farms was low (usually <10%), determining the pathogenesis proved to be challenging. It is now clear that BNP is caused by vaccine-induced maternal alloantibodies which are transferred from mother to calf via the colostrum (first milk rich in maternal antibodies). These alloantibodies, which are specific for major histocompatibility complex class I (MHC I) molecules, are generated in response to residual cell-line material found within a specific vaccine containing a novel adjuvant formulation [Citation8–Citation12]. BNP therefore highlights important issues regarding the safety of using allogeneic cell lines for vaccine production. In this review, the pathology and pathogenesis of BNP are described, followed by a discussion of the implications for vaccine safety in general and possible measures to avoid similar adverse effects in the future.
2. Clinical signs and pathology of BNP
Calves affected by BNP show no abnormalities at birth, their mothers are unaffected and there is no increase in abortion rate in affected herds [Citation1,Citation2,Citation13]. Onset of clinical disease is usually between 7 and 28 days of age with signs of external and internal hemorrhage and petechiation [Citation1,Citation2,Citation13,Citation14]. Rapid internal bleeding can result in affected calves being found dead with no external signs of hemorrhage, contributing to the under-reporting of cases [Citation4]. Intercurrent infections are common and often result in profound pyrexia [Citation2,Citation13,Citation15]. The majority (80–90%) of clinically affected calves die or are euthanized soon after the onset of symptoms [Citation2,Citation13], however in some cases recovery from the condition is possible [Citation2,Citation13].
Hematology from BNP cases reveals a profound thrombocytopenia and leukopenia [Citation2,Citation13] and histopathology of bone marrow shows extensive depletion of hematopoietic cells (). Experimental studies reproducing the disease demonstrated that as early as 4 h following ingestion of alloantibodies there is already a 75–95% reduction in peripheral blood lymphocytes, monocytes and neutrophils [Citation16,Citation17]. Detailed analysis of the time-course of hematological and bone marrow alterations indicates initial peripheral thrombocyte and leukocyte destruction followed by damage to hematopoietic precursors, with more profound damage to mononuclear lineages and relative sparing of granulocytes [Citation16]. While there is no damage to peripheral erythrocytes, cases develop a normocytic, normochromic, nonregenerative anemia, presumably reflecting a failure to respond to hemorrhage due to loss of early erythroid precursor cells. Cellular depletion of other lymphoid tissues appears to be variable and there is no evidence of primary damage to the parenchyma of other organs or to the vascular endothelium [Citation2,Citation13].
Figure 1. Histological findings from femoral bone marrow from: (a) a field case of BNP euthanized at approximately 3 weeks old, showing almost total loss of hematopoietic cells and their replacement by erythrocytes and proteinaceous fluid and (b) an unaffected healthy calf, showing normal high cellularity with little fatty stromal tissue apparent. Haematoxylin and Eosin. Bars = 100 µm. (reproduced with permission from BMJ Publishing Group Limited. Idiopathic bovine neonatal pancytopenia in a Scottish beef herd [Citation2]).
![Figure 1. Histological findings from femoral bone marrow from: (a) a field case of BNP euthanized at approximately 3 weeks old, showing almost total loss of hematopoietic cells and their replacement by erythrocytes and proteinaceous fluid and (b) an unaffected healthy calf, showing normal high cellularity with little fatty stromal tissue apparent. Haematoxylin and Eosin. Bars = 100 µm. (reproduced with permission from BMJ Publishing Group Limited. Idiopathic bovine neonatal pancytopenia in a Scottish beef herd [Citation2]).](/cms/asset/df225886-7aa9-4f78-a702-80db6fa4463b/ierv_a_1249859_f0001_oc.jpg)
3. The pathogenesis of Bovine Neonatal Pancytopenia
3.1. BNP is an alloimmune disease induced by antibodies absorbed from colostrum
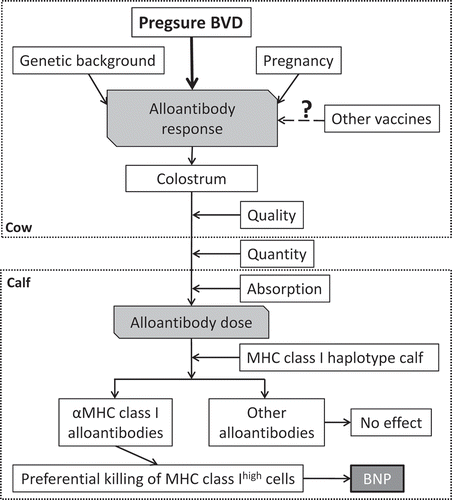
Early investigations found that colostrum obtained from cows that had previously given birth to a BNP-affected calf (BNP cows) causes BNP when fed to unrelated newborn calves [Citation15–Citation18]. Furthermore, serum and colostrum from BNP cows were shown to contain alloantibodies that bind to peripheral blood leukocytes, platelets and bone marrow cells [Citation9,Citation11,Citation12,Citation18–Citation21], and intravenous infusion of these antibodies was sufficient to induce the disease [Citation9]. The bovine epitheliochorial placenta presents a complete barrier to the transfer of immunoglobulins from the cow to the fetus, and thus the calf is completely dependent on ingestion of colostrum for maternal immunoglobulin transfer [Citation22], explaining why BNP only occurs postpartum. Moreover, differences in immunoglobulin concentrations in colostrum, the quantity ingested and the time from birth to suckling, which affects efficiency of immunoglobulin absorption, will all contribute to the final amount of maternal immunoglobulin absorbed by the calf [Citation23–Citation25]. Thus these factors will contribute to variability in the likelihood of a calf developing BNP and the extent of alloantibody-induced pathology () [Citation26].
3.2. Vaccination induces BNP-associated alloantibodies
Confirmation that BNP is mediated by colostrum derived alloantibodies prompted the question: what induced the production of alloantibodies by BNP cows? Epidemiological studies demonstrated a significant association between BNP and the administration of a specific vaccine (Pregsure© BVD, Pfizer Animal Health) to the mothers of affected calves [Citation26–Citation28] and showed that the incidence of BNP was higher in herds that received multiple doses of the vaccine [Citation10]. It was also notable that those European countries that remained free from BNP prohibited the use of this and all other BVD vaccines, due to alternative disease control policies. Pregsure© is an inactivated vaccine for the prevention of bovine viral diarrhea (BVD) virus and was launched in Europe in 2004 [Citation14] and in New Zealand in 2008 [Citation29]. To manufacture this vaccine, BVD virus is cultured in Madin–Darby bovine kidney (MDBK) cells [Citation30] and, following purification, combined with a novel proprietary adjuvant (Procison-ATM; a micro-fluidized oil-in-water adjuvant composed of Quil-A, Cholesterol, mineral oil [Citation31]). High levels of residual bovine proteins have been detected in Pregsure© [Citation8,Citation9,Citation32] and alloantibodies from BNP cows have been shown to recognize MDBK cells [Citation8,Citation9,Citation12,Citation19]. Furthermore, experimental immunization of calves with Pregsure© confirmed that vaccination induces alloantibodies that bind MDBK cells and leukocytes [Citation10,Citation19]. Together, these findings demonstrate that alloantigens present in Pregsure© induced maternal alloantibodies that, upon absorption from colostrum, caused BNP in calves.
Pregsure© was withdrawn from the European market in 2010, following reports of its association with BNP, but cases continue to be seen in previously vaccinated animals [Citation33].
3.3. Major histocompatibility complex class I molecules are the major target antigens in BNP
By using sera from BNP cows to immunoprecipitate antigens from the surface of MDBK cells [Citation8] and peripheral blood leukocytes [Citation9], MHC I molecules were identified as a target of BNP alloantibodies. Using transfected cell lines, sera from BNP cows were also shown to bind specifically to the MHC I alleles present on MDBK cells and to induce complement-mediated cytotoxicity [Citation11]. Furthermore, in vitro experiments showed that BNP alloantibodies preferentially target cells with high MHC I expression and that this correlates with the hematopoietic cell types affected in BNP [Citation11,Citation12,Citation16] ( and Section 2). Using a modified MDBK cell line with no MHC I expression (beta-2-microglobulin knockout) it was confirmed that MHC I is the dominant target of BNP alloantibodies and depending on the BNP dam accounts for 46–91% of the alloantibody specificity [Citation12]. Alloantibodies targeting alternative antigens (e.g. VLA-3) were also identified [Citation12], however only anti-MHC I alloantibodies were consistently found in BNP cows and showed correlation with BNP pathology. Thus, overall evidence indicates that BNP alloantibodies are induced by bovine cellular material, specifically MHC I molecules, found in Pregsure© and that these alloantibodies account for the damage to the hematopoietic system seen in BNP.
3.4. Following Pregsure© vaccination BNP cows develop high anti-MHC I alloantibody titers with heterogeneous specificities, and both the quantity and specificity of these alloantibodies determine the risk of BNP in the calf
Despite the widespread use of Pregsure© the number of BNP cases has been relatively low, with the incidence estimated to be below 0.3% [Citation19]. Even allowing for underreporting of cases, it became clear that factors other than Pregsure© vaccination alone must therefore determine whether BNP develops in the calf.
Following identification of MHC I as the antigenic target in BNP, it was hypothesized that epitope differences between the MHC I alleles expressed by MDBK cells and a given cow would determine the induction of alloantibodies and, conversely, that epitope similarities between MDBK cells and a given calf would determine its propensity to develop the disease [Citation8,Citation9]. Subsequently this was supported by the finding that Pregsure© vaccinated cows generate a unique profile of MHC I-specific alloantibodies, presumably reflecting the degree of alloantigen mismatch between their MHC I alleles and those of the vaccine cell line [Citation11,Citation12]. However, the situation in calves appears to be more complex with BNP-alloantibodies also showing varying degrees of cross reactivity with MHC I alleles not present on MDBK cells [Citation11,Citation12]. This is consistent with the nature of MHC I sequence polymorphism, where short stretches of amino acids are frequently shared by different alleles. Consequently, BNP cows and calves are both likely to have a wide range of different MHC I genotypes, which explains why no association has been found between specific MHC haplotypes and the occurrence of BNP [Citation34,Citation35] and why BNP is not a heritable trait for calves [Citation35].
Pregsure© vaccinated BNP cows have significantly higher serum alloantibody titers compared to both unvaccinated cows and Pregsure© vaccinated non-BNP cows, however alloantibodies are also found in Pregsure© vaccinated non-BNP cows [Citation11,Citation19,Citation35]. Among Pregsure© vaccinated cows there appears to be a continuum of both alloantibody titer and cross-reactivity, with those cows that produce clinically affected calves having both higher serum alloantibody titers and alloantibodies with broader specificity [Citation11]. Together, this suggests that the occurrence of BNP depends on the quantity and specificity of the alloantibody dose absorbed by the calf, implying that BNP is not a dichotomous disease but that calves fall within a continuum from unaffected to severe BNP cases depending on the effective cognate alloantibody dose. This is consistent with the identification of milder subclinical forms of BNP, where neonatal calves develop profoundly abnormal hematology, including depressed lymphocyte and monocyte counts but no clinical signs of the condition [Citation36,Citation37]. Results from other studies indicate that farms affected by BNP have increased levels of calf morbidity attributable to causes other than BNP [Citation4,Citation28] providing a credible basis for the suggestion that subclinical BNP could have an impact on calf health and productivity in situations where animals are exposed to pathogen challenge. In summary, the occurrence of BNP in the calf depends on the quantity of the alloantibodies absorbed from colostrum, which strongly depend on the alloantibody titer of the cow, and the specificity of these antibodies for the MHC I alleles of the calf ().
3.5. High titers of BNP-alloantibodies persist in cows for long periods of time
Following vaccination, serum antibody titers normally decline with time. However, cows vaccinated with Pregsure© appear to maintain high alloantibodies levels for years following last vaccination [Citation33], which explains why cases of BNP continue to be seen despite the withdrawal of Pregsure© from sale. Furthermore, pregnancy has been found to boost Pregsure© induced alloantibody titers, resulting in high titers immediately prior to parturition () [Citation33]. However the alloantibody specificity remains remarkably similar at subsequent pregnancies [Citation11], indicating that the fetus appears only to be boosting existing Pregsure©-induced alloantibodies, rather than stimulating novel alloantibodies. Whether other cattle vaccines, many of which are also produced using MDBK cells, play a role in maintaining or boosting existing BNP alloantibodies has not yet been thoroughly investigated, but vaccines other than Pregsure© have not been identified as risk factors for the occurrence of BNP [Citation26,Citation28].
Among cows vaccinated with Pregsure©, BNP cows also have higher BVD virus-specific antibody titers than non-BNP cows [Citation19], indicating that BNP cows responded with higher antibody levels in general following vaccination. It is possible that this reflects a genetic predisposition to respond robustly to vaccination, as both BNP and other antibody titers following vaccination have been shown to be heritable traits in cows [Citation35,Citation38,Citation39]. It is also notable that Pregsure© was an extremely efficacious vaccine, generating robust and sustained immune responses to BVD virus [Citation40] and significantly higher antibody titers than other BVD vaccines [Citation19], generally attributed to the novel adjuvant it contains. It is interesting to speculate that contaminant MHC I molecules within Pregsure© may themselves have immune stimulatory effects and so potentiate the immune response to viral antigens, particularly given the correlation between viral- and allo-antibody titers [Citation19].
4. Expert commentary – the safety of using allogeneic cell lines for vaccine production
The withdrawal of Pregsure© from the European market in June 2010 will probably result in a gradual reduction in the number of BNP cases, as cows vaccinated with the product are culled. Nevertheless, the production of many vaccines requires the use of allogeneic cell lines and, because complete purification to remove all residual cell-line contamination is difficult and costly, this raises the possibility of similar problems occurring with other vaccines. Very low alloantibody titers have been detected in cows and guinea pigs vaccinated with alternative BVD vaccines [Citation8,Citation19] and it could be speculated that such responses might play a role in BNP-like cases that cannot be linked to Pregsure© [Citation26,Citation41–Citation43].
Several licensed human vaccines are produced using allogeneic cell lines and the number of different allogeneic lines used has increased in recent years [Citation44]. Licensing procedures for human vaccines are more stringent than for veterinary vaccines and antigen purification standards are higher. However, it is difficult to assess what degree of cell-line contamination is safe and this may depend on the particular adjuvant or vaccine formulation, and the presence of preexisting alloimmune responses (e.g. following pregnancy) which might lower the threshold for responding to cell-line contaminants. Adjuvant and vaccine formulations that are able to induce immune responses to low antigen levels or induce very high antibody titers may pose an especially high risk in combination with allogeneic cell lines. Furthermore, vaccinating mothers to protect infants is increasing in humans [Citation45,Citation46] and this raises concerns for possible adverse alloimmune effects in the fetus or neonate.
Calves rely solely on colostrum for maternal antibodies and therefore receive a high alloantibody dose immediately after birth leading to the acute onset of BNP. In contrast, transfer of maternal antibodies in humans occurs during pregnancy, which would lead to continued exposure of the fetus to pathogenic alloantibodies during pregnancy. Accordingly, adverse effects associated with vaccine-induced alloimmune responses in humans are more likely to lead to early embryonic loss, miscarriage or chronic conditions. Naturally occurring MHC I specific alloantibodies have been associated with recurrent miscarriage and chronic chorioamnionitis in humans, although studies have given conflicting results [Citation47–Citation49]. Early embryonic loss and miscarriage are common in women and nonspecific symptoms add to the complication of monitoring for potential alloimmune related adverse vaccine effects. In addition, the frequency of adverse effects is likely to be low and may be complicated by the requirement for a specific genetic background, as is the case in BNP. Due to the transfer of antibodies during pregnancy in humans, preventing alloimmune-mediated disorders would be more challenging than the relatively straightforward colostrum substitution possible in cattle [Citation50]. Furthermore, the maintenance of high alloantibody titers in BNP dams for long periods suggests that any historical exposure to such a product could still result in pathology, and risks may therefore not be restricted to the period immediately following vaccination.
To our knowledge there are no reports of alloimmune related adverse effects due to vaccines produced on allogeneic cell lines in humans, however the possibility of such effects does not appear to have been thoroughly investigated. In the case of BNP, tolerance to self-antigens has not been broken and hence transplant patients and (pregnant) woman are likely to be most at risk of developing adverse reactions to such vaccines. Influenza vaccination has been shown to generate alloantibodies in a very small number of people and, although this prompted concerns regarding vaccination of transplant patients, there were no associations with adverse effects [Citation51–Citation53]. Comprehensive epidemiological studies are needed to test whether vaccines grown on allogeneic cell lines are associated with transplant rejection or poor pregnancy outcomes.
Testing for the induction of alloimmune responses following vaccination could also be incorporated as an obligatory assay during vaccine trials. However, since pathogenic alloimmune responses may only be induced in individuals with specific genetic backgrounds or from demographic groups not normally included in vaccine trials, and may also depend on exposure to additional risk factors, it is important to continue monitoring vaccines postlicensing. Testing for the presence of alloantibodies is relatively straightforward and a variety of assays have been employed in the case of BNP [Citation11,Citation19]. Thus, testing for alloantibody responses in humans during phase III vaccine trials, which generally involve many hundreds of individuals, should detect even a relatively low prevalence of responses. Indeed, while the prevalence of BNP pathology is low, the prevalence of alloantibody production in Pregsure© vaccinated cows is high; approximately 50% of Pregsure© vaccinated animals develop alloantibodies however these only cause pathology in a small proportion of calves depending on the absorbed alloantibody dose and their specific MHC I haplotype [Citation11,Citation12]. Determining safe threshold levels for alloantibody titers presents a greater challenge, and in fact interpretation of alloantibody titers still presents a challenge in transplant medicine [Citation54]. However in this context BNP could have potential as a model, given the possibility of performing controlled experiments in MHC defined/matched animals, and the detailed characterization of pathological changes observed during disease, especially subclinical disease. Although the occurrence of BNP is associated with a specific vaccine, it serves to emphasize safety concerns that are common to all vaccines produced using allogeneic cell lines and thus knowledge gained from this disease could aid prevention of other vaccine-induced alloimmune-mediated disorders. Furthermore it highlights the need for careful scrutiny of vaccines produced on allogeneic cell lines and underlines the importance of monitoring for adverse vaccine reactions, not just in vaccine recipients but also in their offspring, and the difficulties posed by such monitoring when signs may be nonspecific and cases are likely to be infrequent.
5. Five-year view
In veterinary medicine pregnant animals are routinely vaccinated to optimize passive transfer of protective antibodies via colostrum. In human medicine there is an increasing emphasis on the health benefits for both mother and fetus afforded by vaccination during pregnancy [Citation46,Citation55,Citation56] and in the next five years both the prevalence and range of vaccines given during pregnancy will likely increase. While this is obviously to be encouraged, the use of vaccines produced on allogeneic cell lines around pregnancy must be accompanied by careful considerations and it is likely that several steps will be taken in the coming years to ensure the safe use of such vaccines.
First, during development and prelicensure trials, novel vaccines should be tested for alloantigen contamination and for the induction of alloimmune responses. Using BNP as a model, it may be possible to define safe thresholds for both these parameters. Secondly, it is important to be vigilant for alloimmune related adverse events during trials and postlicensing. Following recent guidance from the National Institutes of Health [Citation56] it is likely that existing and future vaccines intended for use during pregnancy will include clinical trials on pregnant women. However, the long-term effects of maternal immunization among women and infants have not been fully characterized [Citation55], nor are risks posed by vaccine-induced alloimmune responses to transplant recipients. Additionally, pathogenic alloimmune responses may only be induced in specific populations not normally included in vaccine trials, and may depend on exposure to additional risk factors. Thus prelicensure trials need to be complemented with comprehensive epidemiological studies and continued monitoring of alloimmune-mediated adverse events postlicencing. Finally, research over the coming years is likely to further investigate alternative vaccine cell culture systems, such as the insect cell-baculovirus expression system used to produce novel influenza vaccines [Citation57], and manufacturing methods that reduce or remove potentially allogeneic contaminants and thus mitigate the risk of allogeneic adverse reactions.
Key issues
Bovine neonatal Pancytopenia (BNP) is a recently emerged vaccine-induced disease of calves that highlights an important vaccine safety issue.
BNP is attributed to immunization of the calf’s mother with a specific vaccine produced using an allogeneic cell line.
Cell-line contaminants present in this vaccine induce alloantibodies specific for Major-Histocompatibility class I antigens
The alloantibodies are transferred from the mother to the calf via the colostrum (first-milk), leading to profound depletion of the calf’s peripheral blood and bone marrow cells, which is often fatal.
Many vaccines are produced in potentially allogeneic cell lines, including several licensed human vaccines.
While there are currently no reports of vaccine-induced alloimmune adverse events in humans, the possibility of such effects does not appear to have been thoroughly investigated and are likely to affect specific populations that are not normally studied in licensing trials, such as transplant recipients and pregnant women.
The occurrence of BNP highlights the need for careful scrutiny of vaccines produced on allogeneic cell lines, the importance of monitoring for the induction of alloantibodies and the occurrence of adverse vaccine reactions in both recipients and their offspring, and the difficulties posed by monitoring for alloimmune-mediated adverse reactions.
Declaration of Interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Friedrich A, Rademacher G, Weber B, et al. Gehäuftes auftreten von häemorrhagischer diathese infole knockenmarkschädigung bei jungen kälbern. Tierarztl Umsch. 2009;64:423–431.
- Bell CR, Scott PR, Sargison ND, et al. Idiopathic bovine neonatal pancytopenia in a Scottish beef herd. Vet Rec. 2010;167:938–940. Epub 2011 Jan 26.
- Paul-Ehrlich-Institut. Investigating the cause of Bleeding Calf Syndrome. 2011. [cited 2016 Oct 14]. Available from: http://www.pei.de/EN/information/journalists-press/press-releases/2011/02-cause-bleeding-calf-syndrome.html
- Reichmann F, Sauter-Louis CFA, Rademacher G, et al. Bovine Neonatal Pancytopenia (BNP) in young calves in Germany on farms with high and low incidences of BNP. Lisbon: Associação Portuguesa de Buiatrics; 2012.
- Promed. Bovine Neonatal Pancytopenia (03): New Zealand. 2011. [cited 2016 Oct 14]. Available from: http://www.promedmail.org/
- Kappe EC, Halami MY, Schade B, et al. Bone marrow depletion with haemorrhagic diathesis in calves in Germany: characterization of the disease and preliminary investigations on its aetiology. Berl Munch Tierarztl Wochenschr. 2010;123:31–41. Epub 2010 Feb 9.
- Willoughby K, Gilray J, Maley M, et al. Lack of evidence for circovirus involvement in bovine neonatal pancytopenia. Vet Rec. 2010;166:436–437. Epub 2010 Apr 7.
- Deutskens F, Lamp B, Riedel CM, et al. Vaccine-induced antibodies linked to bovine neonatal pancytopenia (BNP) recognize cattle major histocompatibility complex class I (MHC I). Vet Res. 42;2011:97. Epub 2011 Sep 1.
- Foucras G, Corbière F, Tasca C, et al. Alloantibodies against MHC class I: a novel mechanism of neonatal pancytopenia linked to vaccination. J Immunol. 2011;187:6564–6570. Epub 2011 Nov 16.
- Kasonta R, Sauter-Louis C, Holsteg M, et al. Effect of the vaccination scheme on PregSure® BVD induced alloreactivity and the incidence of Bovine Neonatal Pancytopenia. Vaccine. 2012;30:6649–6655. Epub 2012 Oct 11.
- Bell CR, MacHugh ND, Connelley TK, et al. Haematopoietic depletion in vaccine-induced neonatal pancytopenia depends on both the titre and specificity of alloantibody and levels of MHC I expression. Vaccine. 2015;33:3488–3496. Epub 2015 Jun 10.
- Benedictus L, Luteijn RD, Otten H, et al. Pathogenicity of Bovine Neonatal Pancytopenia-associated vaccine-induced alloantibodies correlates with major histocompatibility complex class I expression. Sci Rep. 2015;5:12748. Epub 2015 Aug 4.
- Pardon B, Steukers L, Dierick J, et al. Haemorrhagic diathesis in neonatal calves: an emerging syndrome in Europe. Transbound Emerg Dis. 2010;57:135–146. Epub 2010 Mar 6.
- Doll K. Bovine Neonatal Pancytopenia. World Buiatrics Congr 2012. Lisbon: Associação Portuguesa de Buiatrics; 2012 Jun 3–8.
- Henniger P, Henniger T, Seehusen F, et al. Causes of death in calves with experimentally induced bovine neonatal pancytopenia (BNP). Berl Munch Tierarztl Wochenschr. 2014;127:61–69. Epub 2014 Feb 5.
- Bell CR, Rocchi MS, Dagleish MP, et al. Reproduction of bovine neonatal pancytopenia (BNP) by feeding pooled colostrum reveals variable alloantibody damage to different haematopoietic lineages. Vet Immunol Immunopathol. 2013;151:303–314. Epub 2013 Jan 1.
- Friedrich A, Büttner M, Rademacher G, et al. Ingestion of colostrum from specific cows induces Bovine Neonatal Pancytopenia (BNP) in some calves. BMC Vet Res. 2011;7:10. Epub 2011 Feb 22.
- Bridger PS, Bauerfeind R, Wenzel L, et al. Detection of colostrum-derived alloantibodies in calves with bovine neonatal pancytopenia. Vet Immunol Immunopathol. 2011;141:1–10. Epub 2011 Jan 29.
- Bastian M, Holsteg M, Hanke-Robinson H, et al. Bovine Neonatal Pancytopenia: is this alloimmune syndrome caused by vaccine-induced alloreactive antibodies? Vaccine. 2011;29:5267–5275. Epub 2011 May 25.
- Pardon B, Stuyven E, Stuyvaert S, et al. Sera from dams of calves with bovine neonatal pancytopenia contain alloimmune antibodies directed against calf leukocytes. Vet Immunol Immunopathol. 2011;141:293–300. Epub 2011 Mar 29.
- Assad A, Amann B, Friedrich A, et al. Immunophenotyping and characterization of BNP colostra revealed pathogenic alloantibodies of IgG1 subclass with specifity to platelets, granulocytes and monocytes of all maturation stages. Vet Immunol Immunopathol. 2012;147:25–34. Epub 2012 May 5.
- Chucri TM, Monteiro JM, Lima AR, et al. A review of immune transfer by the placenta. J Reprod Immunol. 2010;87:14–20. Epub 2010 Oct 20.
- Stott GH, Marx DB, Menefee BE, et al. Colostral immunoglobulin transfer in calves I. Period of absorption. J Dairy Sci. 1979;62:1632–1638. Epub 1979 Oct 1.
- Stott GH, Marx DB, Menefee BE, et al. Colostral immunoglobulin transfer in calves II. The rate of absorption. J Dairy Sci. 1979;62:1766–1773. Epub 1979 Nov 1.
- Stott GH, Marx DB, Menefee BE, et al. Colostral immunoglobulin transfer in calves. III. Amount of absorption. J Dairy Sci. 1979;62:1902–1907. Epub 1979 Dec 1.
- Jones BA, Sauter-Louis C, Henning J, et al. Calf-level factors associated with bovine neonatal pancytopenia–a multi-country case-control study. PLoS One. 8;2013:e80619. Epub 2013 Dec 7.
- Lambton SL, Colloff AD, Smith RP, et al. Factors associated with bovine neonatal pancytopenia (BNP) in calves: a case-control study. PLoS One. 2012;7:e34183. Epub 2012 May 19.
- Sauter-Louis C, Carlin A, Friedrich A, et al. Case control study to investigate risk factors for bovine neonatal pancytopenia (BNP) in young calves in southern Germany. Prev Vet Med. 2012;105:49–58. Epub 2012 Mar 6.
- Ministry for Primary Industries NZ. Recall of BVD vaccine in New Zealand. [cited 2016 Oct 14]. Available from: http://www.foodsafety.govt.nz/elibrary/industry/bvd-vaccine-nz.htm
- Madin SH, Darby NB Jr. Established kidney cell lines of normal adult bovine and ovine origin. Proc Soc Exp Biol Med. 1958;98:574–576. Epub 1958 Jul 1.
- Summary of product characteristics: Pregsure BVD. 2009. [cited 2016 Oct 24]. Available from: http://www.vmd.defra.gov.uk/ProductInformationDatabase/SPC_Documents/SPC_135334.doc
- Euler KN, Hauck SM, Ueffing M, et al. Bovine neonatal pancytopenia–comparative proteomic characterization of two BVD vaccines and the producer cell surface proteome (MDBK). BMC Vet Res. 2013;9:18. Epub 2013 Jan 25.
- Benedictus L, Rutten VPMG, Koets AP. Pregnancy boosts vaccine-induced Bovine Neonatal Pancytopenia-associated alloantibodies. Vaccine. 2016;34:1002–1005. Epub 2016 Jan 23.
- Ballingall KT, Nath M, Holliman A, et al. Lack of evidence for an association between MHC diversity and the development of bovine neonatal pancytopenia in Holstein dairy cattle. Vet Immunol Immunopathol. 2011;141:128–132. Epub 2011 Mar 1.
- Benedictus L, Otten HG, Van Schaik G, et al. Bovine Neonatal Pancytopenia is a heritable trait of the dam rather than the calf and correlates with the magnitude of vaccine induced maternal alloantibodies not the MHC haplotype. Vet Res. 2014;45:129. Epub 2014 Dec 18.
- Witt K, Weber CN, Meyer J, et al. Haematological analysis of calves with bovine neonatal pancytopenia. Vet Rec. 2011;169:228. Epub 2011 Jul 29.
- Bell CR, Kerr MG, Scott PR, et al. Evidence of a high incidence of subclinically affected calves in a herd of cattle with fatal cases of Bovine Neonatal Pancytopenia (BNP). BMC Vet Res. 2014;10:245. Epub 2014 Nov 2.
- Wagter LC, Mallard BA, Wilkie BN, et al. A quantitative approach to classifying Holstein cows based on antibody responsiveness and its relationship to peripartum mastitis occurrence. J Dairy Sci. 2000;83:488–498. Epub 2000 Apr 6.
- O’Neill RG, Woolliams JA, Glass EJ, et al. Quantitative evaluation of genetic and environmental parameters determining antibody response induced by vaccination against bovine respiratory syncytial virus. Vaccine. 2006;24:4007–4016. Epub 2006 Mar 4.
- Harmeyer S, Antonis AFG, Gadd T, et al. Fetal protection against BVDV fetal infection six months after vaccination with a novel inactivated BVDV vaccine. Tierarztl Umsch. 2004;59:663–+.
- Ammann VJ, Fecteau G, Hélie P, et al. Pancytopenia associated with bone marrow aplasia in a Holstein heifer. Can Vet J. 1996;37:493–495. Epub 1996 Aug 1.
- Shimada A, Onozato T, Hoshi E, et al. Pancytopenia with bleeding tendency associated with bone marrow aplasia in a Holstein calf. J Vet Med Sci/Jpn Soc Vet Sci. 2007;69:1317–1319. Epub 2008 Jan 8.
- Fukunaka M, Toyoda Y, Kobayashi Y, et al. Bone marrow aplasia with pancytopenia and hemorrhage in a Japanese Black calf. J Vet Med Sci/Jpn Soc Vet Sci. 2010;72:1655–1656. Epub 2010 Aug 13.
- Aubrit F, Perugi F, Léon A, et al. Cell substrates for the production of viral vaccines. Vaccine. 2015;33:5905–5912. Epub 2015 Jul 19.
- Keller-Stanislawski B, Englund JA, Kang G, et al. Safety of immunization during pregnancy: a review of the evidence of selected inactivated and live attenuated vaccines. Vaccine. 2014;32:7057–7064. Epub 2014 Oct 7.
- Rasmussen SA, Watson AK, Kennedy ED, et al. Vaccines and pregnancy: past, present, and future. Semin Fetal Neonatal Med. 2014;19:161–169. Epub 2013 Dec 21.
- Nielsen HS, Witvliet MD, Steffensen R, et al. The presence of HLA-antibodies in recurrent miscarriage patients is associated with a reduced chance of a live birth. J Reprod Immunol. 2010;87:67–73. Epub 2010 Jul 8.
- Lee J, Romero R, Xu Y, et al. Maternal HLA panel-reactive antibodies in early gestation positively correlate with chronic chorioamnionitis: evidence in support of the chronic nature of maternal anti-fetal rejection. Am J Reprod Immunol. 2011;66:510–526. Epub 2011 Sep 29.
- Lashley EELO, Meuleman T, Claas FHJ. Beneficial or harmful effect of antipaternal human leukocyte antibodies on pregnancy outcome? A systematic review and meta-analysis. Am J Reprod Immunol. 2013;70:87–103. Epub 2013 Mar 19.
- Bell CR, Scott PR, Kerr MG, et al. Possible preventive strategy for bovine neonatal pancytopenia. Vet Rec. 2010;167:758. Epub 2011 Jan 25.
- Danziger-Isakov L, Cherkassky L, Siegel H, et al. Effects of influenza immunization on humoral and cellular alloreactivity in humans. Transplantation. 2010;89:838–844. Epub 2010 Feb 25.
- Katerinis I, Hadaya K, Duquesnoy R, et al. De novo anti-HLA antibody after pandemic H1N1 and seasonal influenza immunization in kidney transplant recipients. Am J Transplant. 2011;11:1727–1733. Epub 2011 Jun 16.
- Vermeiren P, Aubert V, Sugamele R, et al. Influenza vaccination and humoral alloimmunity in solid organ transplant recipients. Transpl Int. 2014;27:903–908. Epub 2014 May 7.
- Tait BD, Süsal C, Gebel HM, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013;95:19–47. Epub 2012 Dec 15.
- Beigi RH, Fortner KB, Munoz FM, et al. Maternal immunization: opportunities for scientific advancement. Clin Infect Dis. 2014;59(Suppl 7):S408–S414. Epub 2014 Nov 27.
- Munoz FM, Sheffield JS, Beigi RH, et al. Research on vaccines during pregnancy: protocol design and assessment of safety. Vaccine. 2013;31:4274–4279. Epub 2013 Aug 3.
- Baxter R, Patriarca PA, Ensor K, et al. Evaluation of the safety, reactogenicity and immunogenicity of FluBlok® trivalent recombinant baculovirus-expressed hemagglutinin influenza vaccine administered intramuscularly to healthy adults 50-64 years of age. Vaccine. 2011;29:2272–2278. Epub 2011 Feb 1.