ABSTRACT
Introduction: The 2015 Global Meningococcal Initiative (GMI) meeting discussed the global importance of meningococcal disease (MD) and its continually changing epidemiology.
Areas covered: Although recent vaccination programs have been successful in reducing incidence in many countries (e.g. Neisseria meningitidis serogroup [Men]C in Brazil, MenA in the African meningitis belt), new clones have emerged, causing outbreaks (e.g. MenW in South America, MenC in Nigeria and Niger). The importance of herd protection was highlighted, emphasizing the need for high vaccination uptake among those with the highest carriage rates, as was the need for boosters to maintain individual and herd protection following decline of immune response after primary immunization.
Expert commentary: The GMI Global Recommendations for Meningococcal Disease were updated to include a recommendation to enable access to whole-genome sequencing as for surveillance, guidance on strain typing to guide use of subcapsular vaccines, and recognition of the importance of advocacy and awareness campaigns.
1. Introduction
Meningococcal disease (MD) has a rapid onset with potentially life-changing consequences. MD is fatal in as many as 50–80% of untreated cases [Citation1], and case fatality rates even in treated individuals are ~10–15% [Citation2,Citation3]. In addition, MD causes great morbidity, with 12–20% of survivors suffering significant clinical sequelae (e.g. paralysis, deafness, mental impairment, amputations, and seizures) [Citation2,Citation4–Citation8]. According to the World Health Organization (WHO), there are no accurate estimates of the global burden of MD, a situation that is due to inadequate surveillance in many parts of the world. However, MD is often considered as endemic globally, although epidemics occur frequently in the meningitis belt in sub-Saharan Africa, as will be discussed further in this paper. Prevention strategies, in particular vaccination, have been shown to be extremely effective in controlling MD [Citation9].
The most common presentations of invasive MD are meningitis and sepsis [Citation10]. Localized and chronic infections resulting in pneumonia, endophthalmitis, arthritis, pericarditis, or myocarditis may also occur [Citation3,Citation10]. Although MD affects individuals of all ages, the highest rates of disease are found in infants <1 year old [Citation1,Citation11]. Peaks in incidence are also seen in adolescents as well as the elderly in some countries [Citation12–Citation16].
The causative agent in MD is the bacterium Neisseria meningitidis. In a phenomenon known as carriage, N. meningitidis usually colonizes the mucosa of the human upper respiratory tract without resulting in MD. Carriage is frequent and involves ~10% of the general population [Citation17]; although rates are variable by age and setting, it is highest in adolescents and young adults (e.g. ≤27%), but far lower in older adults (e.g. ≤8%) and infants (<5%) [Citation18,Citation19]. Transmission of the bacterium from an infected individual to another person occurs via direct contact with droplet respiratory secretions [Citation20].
Genetic analysis of carriage strains has revealed a diverse range of organisms, with only a few of these found to be linked to MD [Citation20,Citation21]. Twelve serogroups of N. meningitidis have been identified, with six of these – N. meningitidis serogroups (Men) A, B, C, W, X, and Y – being responsible for virtually all invasive disease [Citation1,Citation11]. The epidemiology of MD is dynamic, with continuing changes in incidences of N. meningitidis serogroups and the emergence of new strain variants [Citation1,Citation22].
Although acquisition of meningococci usually results in asymptomatic carriage, local inflammation occurs in some cases along with invasion of mucosal surfaces, which provide access to the bloodstream [Citation23] and can result in invasive MD (e.g. fulminant sepsis and/or meningeal inflammation) [Citation23]. Additionally, a number of environmental factors such as exposure to cigarette smoke [Citation24] that can cause inflammation of the nasopharyngeal mucosal surfaces have also been associated with increased risk of invasive MD.
Vaccination remains the key method for prevention of MD, and various vaccines and vaccine strategies have been developed. The key desired effects of vaccination are to protect those vaccinated from invasive MD when they are exposed, as well as to reduce acquisition and carriage, particularly of hyperinvasive isolates, and onward transmission. The coverage of the various types of anti-MD vaccines are summarized in . Polysaccharide vaccines (PSVs) have been available for >40 years and variously cover one or more of serogroups [Citation1]. Protein-conjugate capsular vaccines are available [Citation25], and when possible, these vaccines should be used in preference to the polysaccharide form, as they are more immunogenic, provide longer-lasting immunity and a stronger response to booster vaccination (i.e. more immune cell activity and antibody production), and do not induce hyporesponsiveness (i.e. show a poor or absent immune response) upon repeated use [Citation11]. Combination conjugate vaccines are also available [Citation26]. Vaccines using outer membrane vesicles (OMVs) have been used in outbreak control against specific strains since the 1980s [Citation27]. In addition, two vaccines designed to offer broad protection against MenB are available and were developed using subcapsular meningococcal antigens [Citation28,Citation29].
Table 1. Meningococcal disease vaccine coverage and manufacturers.
The Global Meningococcal Initiative (GMI) was established in 2009 to promote the prevention of MD worldwide through education, research, international cooperation, and vaccination [Citation11]. The GMI is an international group of clinicians and scientists with expertise in MD immunology, microbiology, epidemiology, public health, and vaccination. Since its inception, several global and regional meetings have been held and these have resulted in the publication of recommendations, including the GMI Global Recommendations for Meningococcal Disease (), as well as regional situation reports [Citation11,Citation30–Citation33].
Table 2. The GMI Global Recommendations for MD [Citation33].
In November 2015, the GMI convened an Expert Meeting in London, UK, titled ‘Prevention of Meningococcal Disease – Importance of Herd Protection.’ The objectives of this meeting were to discuss the importance of herd protection and the potential impact this may have on MD; provide an update on surveillance, epidemiology, prevention, and control strategies from around the globe; highlight the lessons learned and experience gained from immunization strategies used in other countries; examine the health economics aspects of meningococcal vaccination strategclinic-based, and a national referenceies; and emphasize the critical need for disease awareness and advocacy with regard to MD prevention and control.
2. Methods
Participants included more than 20 clinicians and scientists with expertise in various aspects of MD. The experts represented institutions in Africa, the Asia-Pacific region, Europe, Latin America, and North America. The meeting content comprised expert presentations, workshop sessions, and roundtable discussions.
3. Results
3.1. Surveillance, epidemiology, and control: a global picture
A series of presentations were given on MD surveillance, epidemiology, and control in different regions and countries from around the globe.
3.1.1. Latin America
Much of the data for Latin America are from the Sistema de Redes de Vigilancia de los Agentes Responsables de Neumonias y Meningitis Bacterianas (SIREVA II) network. This network includes regional reference laboratories employing deoxyribonucleic acid (DNA)-based diagnostic technologies, such as polymerase chain reaction (PCR). Diagnostic methodologies for MD are described in detail elsewhere [Citation34].
In Chile, N. meningitidis is subject to laboratory surveillance and requires immediate notification. Control measures implemented include vaccination (polyvalent conjugate vaccine used providing protection from MenA, C, W, and Y [note: other MD vaccines are licensed in Chile, but currently only the MenACWY conjugate is recommended and funded by the government]) and the chemoprophylaxis of close contacts to prevent secondary cases. According to data from the Instituto de Salud Pública de Chile (ISPCH), since its first detection in 2010, the clone serogroup:serotype:subtype W:2a:P1.5,2:sequence type(ST)-11 has undergone aggressive expansion, displacing serogroup B as the main cause of MD, with an incidence rate of >0.5/100,000 in 2014; in 2015, again according to ISPCH data, MenB also appeared to be increasing, with MenW possibly decreasing. A campaign using quadrivalent conjugate vaccine aimed at children aged between 9 months and 5 years was launched in late 2012; in 2014, an MD vaccination program using the MenACWY conjugate vaccine was included in the national immunization program (NIP) for all children aged ≥1 year.
Since 2012, a National System of Health Surveillance has been in place in Argentina, where surveillance is both laboratory- and clinic-based, and a national reference laboratory receives an estimated 50% of isolates. Overall disease incidence is currently low (<0.7/100,000; data from the Ministerio de Salud de la Nación, Argentina); as in Chile, infants and young children are most affected. Epidemiology has been dynamic in the last 5 years, with increased MenW circulation (W:2a:P1.5,2:ST-11 and W:2a:P1.2:ST-11 accounted for 78% of all isolates). In Argentina, the MenW strains are distinct from the MenW strain that was first identified in Europe following the Hajj pilgrimage in 2000 (the so-called Hajj outbreak strain) [Citation35], as is also the case with the strains in Chile. Argentina recently announced the plan to introduce the quadrivalent conjugate vaccine for infants at 3, 5, and 15 months old, with an adolescent dose at 11 years.
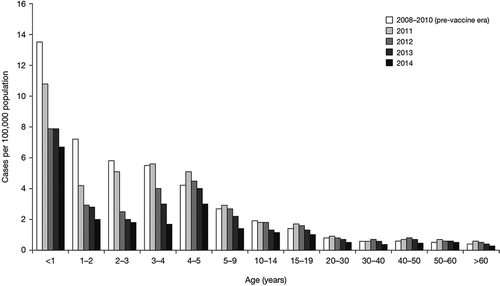
In Brazil, MenC remains the most common cause of disease; disease due to MenW has not increased as much as in Argentina or Chile, although the same MenW strain is involved [Citation35]. Infant immunization with MenC conjugate vaccine follows schedule of immunization at 3 and 5 months, with a booster dose at 12 months, and a single dose for toddlers ages 12–23 months, but without a ‘catch-up’ campaign in older age groups [Citation36]. The introduction of the MenC vaccine in Brazil in 2010 provided an immediate reduction in incidence rates of MD, especially in those children targeted for vaccination (). Carriage rates in adolescents, in a study performed 2 years after the initiation of the infant immunization program, showed a prevalence of 10%, where serogroups were identified, serogroup C was the most common (1.32%), followed by serogroups B (0.99%), E (0.74%), Y (0.49%), and W (0.25%) [Citation37]. Although plain PSVs offered protection against disease, they did not prevent acquisition of carriage of MenC in the 2010 outbreaks [Citation38], which is why only conjugate vaccines are now used to control outbreaks.
Figure 1. Incidence rates before and after routine MenC vaccination in Brazil, 2008–2014. Adapted with permission from Safadi MA, Berezin EN, Arlant LH. Meningococcal Disease: Epidemiology and Early Effects of Immunization Programs. J Pediatric. Infect Dis Soc. 3(2), 91–93 (2014). Supplemented with unpublished data for 2013–2014.
In Mexico, MD is reportable through the Mexican National Epidemiologic Surveillance System; however, the true burden of MD is unknown. Not all isolates are submitted to the national reference laboratory, Instituto de Diagnóstico y Referencia Epidemiológicos. As a consequence, a limited number of isolates receive further characterization. In general, the number of reported MD cases has increased since 2002, although incidence rates are still extremely low, ranging from 0.01 to 0.04 per 100,000 in the 2010–2014 period (data from the Secretariat of Health, Mexico). Following the Metropolitan area outbreak in 2010, MenC has emerged as the prevalent strain, with some cases of MenY and MenB. Since 2010, a national response strategy has been developed that includes the availability of vaccines, but they are only used in case of outbreaks and, more recently, offered for travelers to high-risk countries. It is the opinion of the GMI, therefore, that N. meningitidis colonization in children and young adults might be a better indicator to detect at-risk target populations, in addition to demonstrating the presence and potential trigger of outbreaks. Such colonization data also suggest that inclusion of MD vaccination in NIPs could be a more effective protection strategy than reservation of vaccination for at-risk groups only.
3.1.2. Asia-Pacific
Approximately 4 billion people live in Asia [Citation39], and there are two WHO regional offices covering Asia, namely the South-East Asia Region and the Western Pacific Region [Citation40]. In South-East Asia, no country has been in either the WHO high (defined as >10 cases/100,000 per year in this region [Citation41]) or moderate (2–10 cases/100,000 per year in this region [Citation41]) endemic rate categories for MD in the past 20 years. The true burden of MD is, however, unknown in the Asia-Pacific region for several reasons, including under-reporting, weak surveillance systems, lack of guidelines and standard case definitions, as well as lack of awareness. In some countries in the region (such as South Korea), MD has recently become a major public health issue due to more frequent outbreaks. In a move to rectify this situation, a set of recommendations for surveillance, prevention, and control in the Asia-Pacific region was developed at a regional meeting of the GMI in South Korea in 2014 [Citation42].
Korea and Thailand are considered to have low endemic rates (defined for these countries as <2 cases/100,000 per year [Citation41]). On the other hand, in the Western Pacific Region, New Zealand and Mongolia are considered high-endemic areas, Australia is categorized as moderate, and the majority of countries have low endemic rates (again defined as <2 cases/100,000 per year), including China, Japan, the Philippines, Singapore, and Taiwan [Citation41]. Five of the major MD serogroups (A, B, C, Y, and W) are variedly present in Asia. MenA dominates in China, India, Bangladesh, Mongolia, and the Philippines during epidemic years, while MenB is seen in Australia and New Zealand. MenB, as a cause of sporadic cases, is seen in China, Indonesia, Japan, Malaysia, the Philippines, Singapore, Taiwan, and Thailand. MenC is likewise seen in China and Singapore; MenY has been documented in Japan and Taiwan and MenW in Singapore and Taiwan (2014 meeting of the GMI, manuscript submitted [Citation41]).
As with the quality of epidemiological data, vaccination programs are also variable within the region [Citation43]. Only a few countries have meningococcal vaccination in their NIPs. China has routine mass countrywide immunization using PSV MenA in infants aged 6–18 months, given as two doses at 3-monthly intervals, and PSV MenA and C in young children, given as two doses at 3 and 6 years [Citation44]. By comparison, Australia uses a combined Haemophilus influenzae type b and MenC conjugate (Menitorix®, ) in their NIP at 1 year [Citation45]. Several meningococcal vaccines are available in the region, and the two predominating types are quadrivalent meningococcal (ACWY) conjugate vaccines (MCV4) and quadrivalent meningococcal PSVs (MPSV4), but the conjugate is preferred. Generally, across the region, its use is only for certain conditions (e.g. asplenia, and for other persons at risk), as well as for selected populations, such as those performing the Hajj pilgrimage and travelers to endemic countries [Citation43].
MD is not a reportable disease in Bangladesh; therefore, there are no national data on prevalence and incidence. However, cases are captured from the ongoing surveillance at a network of multiple hospitals in urban and rural Bangladesh (Saha SK, et al. unpublished data, cited with permission). Antibiotic use prior to specimen collection presents a barrier to obtaining viable meningococcal isolates, and most cases are now detected by PCR, with only a few isolates recovered from blood samples. MenA was predominant (90%; 152/167) from 1994 to 2005. However, in subsequent years (2006–2015), MenB emerged gradually and established itself as the dominant serogroup (62%; 100/162) in Bangladesh. More than 50% of MD cases occur in the first year of life, and incidence in infants aged <1 year ranges from 18 to 24/100,000 (Saha SK, et al. unpublished data).
In Japan, cases are reported via the National Epidemiological Surveillance on Infectious Disease. The incidence of MD is low; 7–21 cases of meningococcal meningitis were reported annually between 1999 and 2012, but numbers have increased since meningococcemia was added as a notifiable disease condition in April 2013. Incidence was 0.028/100,000 in 2014, and the predominant serogroup was MenY, followed by MenC and MenB. MenW is rarely reported. There have only been a few carriage studies to date; in these, an overall carriage rate of ~0.4–0.8% was observed [Citation46,Citation47].
Quadrivalent meningococcal vaccines have been approved in Japan since 2014 and should be available on request to anyone who qualifies for the conditions shown on the package insert; however, who should be vaccinated remains a key question, and there is a need to define high-risk populations. Indeed, there are currently no recommendations for meningococcal vaccination in Japan, except for travelers to West Africa [Citation48].
3.1.3. Europe
Incidence of MD is currently low in many parts of Europe, with differing distributions of serogroups and strains, and different vaccination policies in place. The official European case definition was last updated in 2012 [Citation49], and there is continuing movement across Europe to adopt this common definition. The current clinical definition includes any person with at least one of the following: meningeal signs, hemorrhagic rash, septic shock, or septic arthritis. The laboratory criteria must include at least one of the following: isolation of N. meningitidis from a normally sterile site, including purpuric skin lesions; detection of N. meningitidis nucleic acid from a normally sterile site, including purpuric skin lesions; detection of N. meningitidis antigen in cerebrospinal fluid; and detection of gram-negative stained diplococci in cerebrospinal fluid [Citation49].
In the United Kingdom, the introduction of MenC conjugate vaccination in 1999 through routine immunization and a large catch-up campaign has resulted in significant and sustained disease reduction and the induction of herd protection [Citation50]. Routine vaccination strategies have also been implemented in France, Germany, the Netherlands, and Spain, and these have dramatically reduced MenC MD incidence, particularly in the countries that have achieved high vaccine uptake rates among adolescents and young adults [Citation49]. The key to maintaining this success will likely be to prevent acquisition of carriage by maintaining high antibody levels in adolescents. Hence, many countries, including the United Kingdom and Canada, have introduced booster vaccinations in this population.
In Russia, reporting of MD is mandatory; however, there is a lack of typing facilities at the local level, although the availability of PCR-based methods is increasing. Therefore, the reported incidence is likely an underestimate, at 0.3–0.8/100,000 across the different regions, with most cases reported in young children [Citation51]. The known serogroups are MenA, B, C, and W; MenW:clonal complex (cc)11 was first detected in Moscow in 2007, with the number of cases increasing in 2014–2015 ([Mironov K, unpublished data] [Citation52]). Although several vaccines are licensed, including multivalent conjugate types, the national vaccine strategy covers only at-risk groups, areas where the disease is endemic, and military recruits. Current barriers to vaccination are reported to include the underestimation of disease burden and limited pharmacoeconomic data. Improved surveillance systems, better physician and public awareness, and cost-effectiveness studies are also needed.
3.1.4. Africa
In South Africa, MD is endemic, with peaks in the winter and spring months. Any suspected MD is notifiable, and guidelines for treatment, prevention, and control are in place. National laboratory-based surveillance has been available since 1999 and enhanced surveillance has been in place at 25 hospital sites since 2003. Routine vaccinations are available for at-risk groups. Incidence rates in South Africa vary by province, but are currently low overall (0.36/100,000 in 2014). The majority of disease is caused by MenW, followed by MenB; 67–77% of disease is caused by MenA, C, W, or Y. The last peak in incidence, in 2006, was attributed to MenW [Citation53], and MenW ST-11 (related to the so-called Hajj strain) remains the most prevalent, with infection rates highest in infants and young children [Citation54]. Importantly, human immunodeficiency virus infection has been associated with an increased incidence of invasive MD, a higher risk of bacteremia, and a higher case fatality rate than in uninfected populations [Citation55,Citation56].
Surveillance between 1998 and 2008 in Mozambique revealed 63 cases of MD, which were serogroup A, W, and Y. Of these, MenW was again the most prevalent (38/43; 88% cases), followed by MenA (3/43; 7.0% cases) [Citation57]. As in South Africa, MenW ST-11 strains appeared to be the most prevalent (as of 2005) [Citation57].
In a number of countries worldwide, including several on the African continent, the Invasive Bacterial Vaccine-Preventable Diseases hospital sentinel surveillance program [Citation58] is enabling PCR to be used for surveillance for a number of diseases, including MD.
The sub-Saharan meningitis belt has a unique MD situation and special strategies in place, as discussed in Section 3.2.2.
3.1.5. North America
In North America (excluding Mexico), surveillance systems are in place and considered robust; active surveillance has been in place in the Unites States of America since 1995, and surveillance has been carried out in Canada since 1924 [Citation59]. The incidence of invasive MD has remained low over the past several decades and is continuing to decline [Citation59]. This decline is thought to be multifactorial, including the introduction of mass vaccination campaigns and changes in behavioral risk factors [Citation59]. MenB and MenC are the predominant serogroups reported in the region; however, localized outbreaks caused by various clones belonging to different serogroups are observed [Citation59]. Outbreaks in recent years have led to the implementation of outbreak control and routine immunization vaccination programs in the Unites States of America and Canada. In the Unites States of America, routine anti-MenA,C,W,Y conjugate vaccination is recommended for otherwise healthy children at age 11 years, with a booster at age 16 (and catch-up program during adolescence) or from infancy onwards for those with certain high-risk conditions; MenB vaccination for those ages 16 through 23 is subject to individual clinical decisions [Citation26]. Schedules vary across the regions and provinces of Canada, but in general anti-MenC vaccination is recommended during the first year and anti-MenA,C,W,Y conjugate vaccination during adolescence [Citation60].
3.2. Today’s remaining concerns, and key prevention and control strategies
3.2.1. Prevention of carriage and the introduction of herd protection
Conjugate vaccines are considered superior to plain PSVs in most aspects and also prevent acquisition of carriage and promote (indirect) herd protection () [Citation61,Citation62]. Consequently, their use is supported by the GMI [Citation11]. In the United Kingdom, for example, adolescent boosters have been introduced to maintain herd protection that forms an integral part of the MenC control strategy [Citation63]. Additionally, in Canada, an adolescent booster dose of MenC or MenACWY conjugate vaccine has been recommended in all provinces and territories [Citation60].
Figure 2. Impact of MenC conjugate vaccines in reducing carriage, leading to herd protection in the UK. (a) Reduction in MenC carriage [Citation61] (immunized individuals aged 15–19 years). (b) Direct and herd protection [Citation62] against MenC (attack rates in infants and overall attack rate reduction in age group 2 months to 18 years).
![Figure 2. Impact of MenC conjugate vaccines in reducing carriage, leading to herd protection in the UK. (a) Reduction in MenC carriage [Citation61] (immunized individuals aged 15–19 years). (b) Direct and herd protection [Citation62] against MenC (attack rates in infants and overall attack rate reduction in age group 2 months to 18 years).](/cms/asset/3c765aa9-c010-4341-9f5c-4cbcd3543367/ierv_a_1258308_f0002_b.gif)
In general, carriage is most frequent in young adults, with a prevalence of ~24% and approaching 100% in closed or semi-closed populations, such as military recruits and university students [Citation18]. Since most transmission occurs in the carriage state, reducing carriage is pivotal to effective vaccination strategies. In such situations, conjugate vaccines provide herd protection by providing long-lasting protection and reducing nasopharyngeal carriage [Citation61,Citation64,Citation65], for example, through the presence of high levels of mucosal antibodies, thus reducing total transmission in the population.
Carriage studies can support and guide the introduction of meningococcal conjugate vaccines by showing which groups have the highest prevalence and are driving circulation of meningococci. They can also be used to determine the impact of the introduction of conjugate vaccines on carriage in vaccination programs. There are, however, few studies of meningococcal carriage in some ages/populations, but those that are available provide useful data. The lack of studies overall can be due to difficulties in sampling a representative population. Sample sizes of several thousand or more are necessary to evaluate changes when the prevalence of pathogens targeted by a vaccine is low; multicenter studies are preferable, because of the variability between sites. Sample collection and transport and analytical methods can all impact carriage data collection; it is also essential to have rigorous quality control. Due to atypical strains, identification of N. meningitidis carriage remains problematic, and methods need to be standardized. In general, detection and characterization methods such as PCR [Citation66], multi-locus sequence typing (MLST) [Citation67], and whole-genome sequencing (WGS) [Citation68] appear to be the most specific.
3.2.2. MenA and the sub-Saharan meningitis belt
The sub-Saharan meningitis belt comprises 26 countries across the sub-Saharan region and is characterized by very low rainfall and humidity in the dry season. Cases peak, and epidemics occur more frequently, in the dry season. During the 1996–1997 MD epidemic, there were >250,000 cases of MenA, prompting African governments and the WHO to demand a new conjugate vaccine for Africa [Citation69]. MenW, MenX, and MenC have also caused epidemics in the sub-Saharan meningitis belt. Between 2004 and 2010, 19 sequence types belonging to 6 clonal complexes were identified, with MenA of the ST-5 cc identified as the predominant disease-causing strain, responsible for ~80% of epidemics [Citation67,Citation70]. The most recent large-scale MenA epidemic was in 2009 [Citation71]: during this year alone, nearly 90,000 cases of meningitis were reported – 50,000 of which were in Nigeria [Citation71].
In response to the threat of MenA epidemics, MenAfriVac® () was developed and licensed in India and awarded pre-qualification by the WHO in 2010 [Citation69]. The introduction strategy for MenAfriVac® was to induce rapid direct and indirect (i.e. herd) protection by vaccinating individuals aged 1–29 years in mass campaigns [Citation72] spanning over 1–4 years, and to protect new birth cohorts through a routine expanded program of immunization (EPI) or follow-up campaigns. This staggered approach to vaccination was, in part, due to large populations being spread over a wide area. Risk assessments were put in place [Citation73] to define priority areas and to estimate target populations before vaccine introduction, and by November 2015, 237 million people had been vaccinated [Citation74]. Enhanced surveillance and outbreak response capacity are an essential part of epidemic preparedness and response, enabling a quick response to new outbreaks and provision of adequate treatment and containment. Case-based surveillance is being undertaken in some countries, such as Burkina Faso and Niger, and uses epidemiological and laboratory data. Countries are supported by the WHO and international collaborating laboratories. The incidence of MenA has now dramatically decreased [Citation65,Citation69], and the vaccine has also had a dramatic impact in reducing carriage [Citation65]. Between 1 January and 12 May 2013, there were 9249 suspected meningitis cases with a case fatality ratio of 9.3% (857 deaths) across 18 countries – the lowest number of cases recorded during the epidemic season in the last 10 years [Citation75], with the majority of cases occurring during 2009 () [Citation76]. Under enhanced surveillance in 2014, 7585 meningitis cases and 610 deaths were reported across the African meningitis belt, with the WHO region-specific epidemic threshold of >100 cases/100,000 [Citation41] crossed in districts of Burkina Faso, Cameroon, the Central African Republic, Chad, Ethiopia, Gambia, Ghana, Guinea, Mali, Niger, Nigeria, Senegal, South Sudan, and Sudan [Citation77]. The WHO recommendations for EPI and ‘catch-up’ schedules and dosages are being rolled out across the sub-Saharan meningitis belt [Citation72,Citation78]. Following the introduction of MenAfriVac®, the WHO now recommends that the vaccine is incorporated into the routine EPI schedule within 5 years. Modeling suggests that, if routine EPI is not followed with subsequent immunization, epidemics could occur within 15 years following mass campaigns [Citation59,Citation72].
Figure 3. Effect of MenAfriVac® vaccination on number of meningitis cases (data for African countries under enhanced MD surveillance) [Citation76].
Reproduced with permission from WHO surveillance bulletins. http://wwwmeningvaxorg/epidemic-updatesphp (2016) http://www.meningvax.org/epidemic-updates.php., last accessed 4 October 2016.
![Figure 3. Effect of MenAfriVac® vaccination on number of meningitis cases (data for African countries under enhanced MD surveillance) [Citation76].Reproduced with permission from WHO surveillance bulletins. http://wwwmeningvaxorg/epidemic-updatesphp (2016) http://www.meningvax.org/epidemic-updates.php., last accessed 4 October 2016.](/cms/asset/51d66c20-aa10-4f64-88d0-3f9dcb9c1395/ierv_a_1258308_f0003_b.gif)
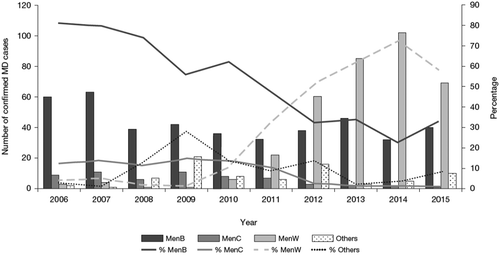
Subsequent to MenAfriVac® introduction, MenW became the predominant strain across the sub-Saharan meningitis belt. However, MenC appears to have reemerged recently, having not been responsible for outbreaks in the sub-Saharan meningitis belt since 1979 [Citation79]; in Nigeria, it increased from 452 suspected cases in 2013 to 796 suspected cases in 2014. Then in 2015, the outbreak expanded rapidly, with 2845 suspected cases in Nigeria, and 8502 cases in the bordering country of Niger (totaling 11,347 suspected cases) [Citation80]. This is a new MenC strain (ST-10217) [Citation80] that seems to have spread from a single source in Nigeria; it is genetically unrelated to the epidemic clones found in Africa in previous decades or to the rare serogroup C isolates that have circulated elsewhere in the world since the 1980s [Citation81]. It probably originated from a carrier isolate that has acquired serogroup C capsule and other virulence genes by recombination (Caugant DA, unpublished data, cited with permission). The clone is still evolving and has now spread to neighboring countries. Affected populations are unlikely to have immunity against MenC, and the prospect of a major epidemic is of great concern.
3.3. The rising concern of MenW: epidemiology and control
3.3.1. MenW epidemiology
Serogroup W was discovered in 1968 and, until 2000, was responsible for a relatively small number of cases worldwide. A pivotal recent event was the emergence and epidemic situation of MenW in Hajj pilgrims in Saudi Arabia, the United Kingdom, and France in 2000, subsequently in sub-Saharan Africa, and elsewhere globally [Citation82,Citation83]. The Hajj cluster of N. meningitidis represents an expansion of one MenW clone within the ST-11/ET-37 complex [Citation35,Citation84]. However, some other recent MenW outbreaks (such as seen in Brazil, Portugal [Citation85,Citation86], Sweden [Citation87], and Taiwan [2014 meeting of the GMI, manuscript submitted] [Citation41]) have been due to other ST-11 variants. Although the hypervirulent clone W:2a:P1.5,2:ST-11 emerged 10 years ago in Brazil [Citation88], incidence rates have become far higher in Argentina and Chile. In Chile, for example, the MenW incidence rate had risen to >0.5/100,000 by 2014, compared with <0.1/100,000 in 2010 (data from the ISPCH, Laboratorio de Agentes de Meningitis Bacteriana, Santiago, Chile); by 2012, 58% of MD was MenW [Citation89]. The MenW ST-11/ET37 cc now appears to be endemic in the Southern Cone region [Citation90]. In the United Kingdom, MenW has also been increasing; the new isolates belong to the ST-11/ET-37 complex but, again, appear to be different from the Hajj strain, although close to the South American isolates [Citation35].
Thus, there appears to be an ongoing multifocal emergence of new MenW isolates by means of an old event of capsule switching, and MenW/cc11 isolates – other than those from the Hajj outbreak – have contributed to a significant proportion of MenW/cc11 cases globally. Surveillance therefore needs to combine exhaustive reporting and typing, and, if real insight is to be gained from what is taking place with MenW, WGS typing is required. To date, isolates from waves of MenW/cc11 infection have been found to have differences in the genes encoding factor H-binding protein (fHbp), fetA, nitric oxide reductase, and nitrite reductase [Citation35,Citation91].
3.3.2. MenW control
Vaccination strategies for controlling MenW and other N. meningitidis strains using multivalent conjugate vaccines are being rolled out in a number of countries. An immunization campaign began in 2012 in Chile with the quadrivalent conjugate vaccine (MenACWY; Menveo®; ), initially targeting children aged from 9 months to <5 years. The incidence of MD in this age group before and after introduction of quadrivalent N. meningitidis (MenACWY) vaccination is shown in . Since 2012, approximately 1 million children have been vaccinated, and vaccine uptake of 95% has been attained in these age groups (data from ISPCH Laboratorio de Agentes de Meningitis Bacteriana). In addition, since 2012, a temporary vaccination strategy was established in which children aged from 9 months to 5 years were vaccinated, and infants from 9 months old were given a second dose to increase protection. The vaccines Menactra® () and Menveo® have been used as part of this temporary vaccination strategy. On 1 January 2014, vaccination became part of the national immunization schedule and was mandatory for all children aged ≥1 year, with a one-dose schedule of Nimenrix® () implemented. Overall, however, the number of cases of MenW is still increasing in Chile in children between 9 months and 5 years of age (data from ISPCH Laboratorio de Agentes de Meningitis Bacteriana) (). In Argentina, between 2012 and 2014, there were 848 cases of MD, with an incidence rate of 0.7/100,000 population; 43% of these were in infants aged <2 years [Citation92]. Around 50% of cases were MenW, and 41% were MenB [Citation92]. Argentina recently announced the decision to implement quadrivalent conjugate vaccination in infants (at 3, 5, and 15 months old). Adolescents aged ≥11 years are to receive one dose [Citation92]. In the United Kingdom, MenW has increased rapidly since 2011/2012 [Citation93]. A vaccination program was introduced in August 2015, for teenagers and university freshers, using MenA,C,W,Y conjugate vaccines, with the intention of inducing direct and herd protection [Citation63]. Although it is anticipated that conjugate vaccines will have a similar effect on the carriage of W as they have had on serogroups A, C, and Y, this has yet to be demonstrated.
Figure 4. Incidence of MD in Chile in children between 9 months and <5 years of age before and after introduction of quadrivalent Neisseria meningitidis (MenACWY) vaccination in 2012 (unpublished data from Instituto de Salud Pública de Chile, Laboratorio de Agentes de Meningitis Bacteriana, Santiago, Chile).
MD: meningococcal disease; Men: Neisseria meningitidis serogroup.
3.4. Decreasing the threat of MenB: implementation of MenB vaccines
Capsular vaccines cannot be used for MenB due to similarities of the polysaccharide with human polysialic acid on neural cell adhesion molecules; therefore, vaccines targeting MenB have been developed using subcapsular proteins. Earlier vaccines based solely on OMVs could only offer protection against homologous strains and needed multiple doses to induce broader protection [Citation27]. Bexsero®, a multicomponent vaccine, was developed by reverse vaccinology and comprises an OMV used in a New Zealand outbreak and three recombinant proteins – the neisserial heparin-binding antigen (NHBA), the Neisseria adhesin A (NadA), and the fHbp [Citation29]. Clinical studies were conducted in infants, toddlers, and adolescents [Citation94]; >5000 infants/toddlers and 19,000 adolescents/adults have now been vaccinated. Bexsero® was approved by the European Medicines Agency in 2013 for individuals ≥2 months of age, and by the US FDA in 2015 for ages 10–25 years [Citation95,Citation96]. The vaccine is reactogenic, with transient fever seen in infants peaking ~6 h after vaccination and resolving within 2–3 days, particularly after the primary dose; the reaction can be increased by concomitant administration with other routine infant vaccinations, but is manageable with paracetamol, with only 2–3% of patients requiring additional medical attention [Citation94]. A second subcapsular vaccine, Trumenba®, contains two fHbp variants [Citation29,Citation97], and was approved by the FDA in 2014 for use in adolescents and young adults aged 10–25 years [Citation98].
Measurement of strain coverage with subcapsular vaccines can be complex, as they comprise multiple antigens that vary between strains and may be expressed at variable levels. The Meningococcal Antigen Typing System (MATS), which is based on an enzyme-linked immunosorbent assay, and either phenotypic or genotypic PorA typing [Citation99], has been used for strain characterization to evaluate potential strain coverage with Bexsero®. Using this method, worldwide strain coverage has been estimated at ~66–91% [Citation93]. Some coverage of non-MenB strains by the vaccine has also been reported, with 70% coverage of MenW and MenY strains, but only ~20% for MenC. Coverage of African epidemic MenX isolates has also been suggested [Citation100].
In 2013, MenB outbreaks occurred at two universities in the Unites States of America (Princeton, NJ, and the University of California, Santa Barbara); Bexsero® vaccination was conducted under the FDA’s expanded access investigational new-drug protocol, and approximately 5500 and 17,000 individuals were vaccinated at these universities, respectively. For the Princeton outbreak, MATS analysis showed that the strains were sufficiently reactive with the Bexsero® fHbp and NHBA to invoke an effective immune response, but were mismatched for the NadA and PorA antigens [Citation101]. Vaccination uptake rates of 97% (first dose) and 92% (second dose) were achieved. Carriage was not formally assessed, but the occurrence of a ninth case in an unvaccinated Princeton student who had close contact with vaccinated undergraduates implies that the strain continued to circulate after vaccination [Citation102,Citation103]. Since September 2015, Bexsero has been introduced into the United Kingdom’s routine infant immunization strategy [Citation63] and enhanced surveillance – that includes genotyping and MATS – is ongoing as part of this program [Citation104].
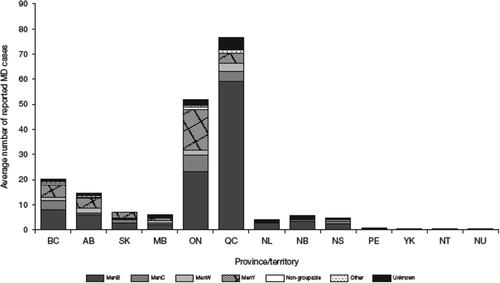
Vaccination programs (e.g. for MenC [consisting of one to three doses of MenC conjugate vaccine in infancy, with a booster dose of MenC conjugate or MenACWY conjugate vaccine at 12–15 years old]) have been in place for a number of years in Canada; MenB, however, has recently become the most prevalent serogroup in Quebec and Ontario (data from Public Health Agency of Canada, 2014 shown in ) [Citation105,Citation106], although the reasons for differences in prevalence from other provinces are unclear. Most of the isolates recently characterized in Quebec have been ST-269 clones that express two variants of the antigens fHbp and NHBA potentially covered by Bexsero® [Citation107]. Bexsero® was licensed in 2013 in Canada and vaccination is proposed for high-risk groups [Citation105,Citation108,Citation109], with current recommendations based on various criteria, including low disease burden, program cost, lack of effectiveness and reactogenicity data [Citation108,Citation109]. In one area in Quebec, MenB MD incidence reached >3.5/100,000 in the period 2006–2012, and a decision was taken to use Bexsero® based on several strands of evidence, including phenotyping and genotyping of strains. More than 50,000 people between the ages of 2 months and 20 years were vaccinated on a two-dose schedule [Citation102]. The vaccine uptake rate for one dose was 82%, but this went down to 70% for at least two doses, mainly due to low uptake in older adolescents and young adults [Citation102]. Based on a modeling analysis, it was estimated that the vaccination campaign reduced disease incidence by 77% [Citation110]. In May 2014, more than a year after the start of the immunization campaign, no new MenB cases had been observed among vaccinees, with two cases observed among non-vaccinated adults [Citation110]. The strain therefore continues to circulate and, as in the US university outbreaks, there is no evidence of indirect protection. Despite the lack of data supporting herd protection with these MenB vaccines, there are data suggesting that OMV vaccines can reduce carriage [Citation111].
Figure 5. Average annual number of invasive MD cases reported in Canadian provinces, 2007–2011. © All rights reserved. The recommended use of the multicomponent meningococcal B (4CMenB) vaccine in Canada: common guidance statement. Public Health Agency of Canada, 2014. Reproduced with permission from the Minister of Health, 2016. http://publications.gc.ca/collections/collection_2014/aspc-phac/HP40-103-2014-eng.pdf (2014), last accessed 4 October 2016.
AB: Alberta; BC: British Columbia; MB: Manitoba; MD: meningococcal disease; NB: New Brunswick; NL: Newfoundland and Labrador; NS: Nova Scotia; NT: Northwest Territories; NU: Nunavut; ON: Ontario; PE: Prince Edward Island; QC: Quebec; SK: Saskatchewan; YK: Yukon.
3.5. Advocacy and awareness in MD
Around the world and in many different countries, many organizations play important roles in raising awareness of MD and advocating for clinicians, as well as for patients and their families. The Meningitis Research Foundation (MRF), for example, is a UK-based charity that funds research, promotes education and awareness of MD, and provides support for those affected by meningitis. For example, in the case of Bexsero® in the United Kingdom, following the interim statement from the UK Joint Committee on Vaccination and Immunisation that vaccination was unlikely to be cost-effective at any vaccine price, the MRF highlighted the consequences and costs of survival with life-limiting sequelae. In addition, the MRF conducted campaigns to engage with clinicians and experts in the field, as well as public campaigns based on real-life personal stories in a range of settings. This coordinated collective effort by parent and patient advocacy, health experts, and key opinion leaders contributed to the decision to approve Bexsero® for use in the United Kingdom’s vaccination program [Citation112].
On a global scale, another such organization, the Confederation of Meningitis Organisations (CoMO) represents 45 organizations from 28 countries [Citation113]. CoMO has a scientific advisory panel comprising experts from around the globe and uses nongovernment organizations and ‘people advocate’ conferences, as well as meetings with politicians in Europe and campaigning on social media. It provides a global platform for its member organizations to campaign on meningitis awareness and vaccination and organizes World Meningitis Day annually in April [Citation114]. CoMO has commissioned research into the long-term cost of meningitis [Citation115], and is currently advocating for a ‘life-course’ immunization initiative (for infants, adolescents, and the elderly) [Citation75].
3.6. The role of modeling in MD control and prevention
To improve understanding of the epidemiology of an infection, make predictions about future incidence under particular conditions or interventions, and identify data gaps, transmission dynamic mathematical models can be developed and applied. Modeling studies of meningococcal infection reinforce the importance of herd protection following mass ‘catch-up’ campaigns and illustrate that conjugate vaccines’ effect on carriage was crucial to the success of the MenC and MenAfriVac® vaccine programs [Citation18,Citation72,Citation116]. Such models are used to best effect in combination with data from surveillance, clinical trials, and carriage studies [Citation61,Citation64,Citation65]. The clinical evidence for the impact of subcapsular vaccines (such as Bexsero®) on MenB carriage is currently unclear as few data have been published [Citation117]; however, models can help us to identify knowledge gaps and enable testing of how hypothetical effects on carriage might impact different potential vaccination strategies.
A workshop on modeling vaccination strategies held during the meeting explored how modeling can be used in combination with surveillance and vaccine information to guide decision-making. Transmission models should be used for MD, as the occurrence of infection depends on carriage in other members of the population; risks of infection are dynamic and nonlinear [Citation72,Citation116,Citation118]. In the United Kingdom, models have been used to synthesize different types of evidence (e.g. in disease burden, natural history, and vaccine effects); to make predictions about vaccine impact and also to aid optimum implementation of 4CMenB vaccination; modeling has been used to evaluate factors including herd protection; to compare schedules, strategies, and policy options; and to estimate the cost-effectiveness of vaccination [Citation107,Citation116,Citation118,Citation119]. It was agreed by the GMI that, to predict future incidence and develop a vaccination strategy during an outbreak, incidence and carriage data, and the response threshold selected, are the most important factors. It was also agreed that, when deciding whom to vaccinate and which vaccine to use, the availability of sufficient vaccine and the vaccine’s ability to interrupt carriage were the most important factors. Regarding health economic analyses, it was noted that these are only reliable when working from robust data. The GMI agreed that economic modeling could be conducted, but it requires reliable data on the burden of the disease and should not be the sole driver of decision-making.
4. Discussion
The presentations on surveillance, epidemiology, and control from around the globe given at this GMI meeting highlight disparities in the quality and availability of surveillance networks and technologies, such as PCR and WGS. The current GMI Global Recommendations for Meningococcal Disease [Citation11] underline the need to increase the availability and quality of laboratory surveillance in order to understand the true burden of MD (). The role of enhanced surveillance in demonstrating the success of the MenAfriVac® campaigns [Citation71] and in detecting emerging MenW outbreaks in Latin America and the United Kingdom [Citation120] illustrates the importance of high-quality data that should include typing by WGS [Citation120]. There is a need for continued vigilance in the face of the emergence of new clones requiring maintenance of high-level surveillance where it is already attained and improvement in countries where systems are weak. Also, although vaccination programs are effective in combating MD, meningococcal vaccines are still not available as part of routine childhood vaccination programs in many countries.
As the GMI, we have developed a number of recommendations. First, conjugate vaccines are recommended by the GMI, as in most aspects they are superior to plain PSVs (). In addition, the GMI recognizes herd protection as being a significant component of control in MD, with evidence presented from several studies and countries showing that adolescent doses provide individual and herd protection. It is important to note, however, that a number of factors, including disease prevalence, carriage rates, and vaccine type, may influence the level of vaccination coverage required to attain herd immunity [Citation121] (e.g. in the African meningitis belt, a vaccination rate of 70% was considered to have afforded herd immunity [Citation72]).
The success of MenAfriVac® in Africa and MenC vaccination in several European countries, Australia, and Canada demonstrates the importance of building herd protection through adolescent and ‘catch-up’ campaigns. It was noted that, in different countries, adolescent vaccinations were undertaken at different ages (e.g. at 11 years in Argentina, but in older teenagers in the United Kingdom). A number of factors seem to govern such decisions, including the practicalities of timing to attain maximum vaccine uptake rates. We suggest that, as socioeconomic factors – such as age, differences in levels of close physical contact, starting smoking, and so on – may differ between and even within countries, local differences need to be taken into account when devising vaccination strategies to ensure that herd protection is optimized. Although carriage studies are complex to undertake, evaluation of the age distribution of carriage, estimation of the case–carriage ratios, and identification of the clones being carried are essential to fully understand the relationships between carriage and outbreak strains. We also believe that MLST [Citation67] and WGS [Citation120] may be the most effective DNA analysis methods for carriage studies.
The extensive data presented on MenW from around the globe again highlight the importance of routine and ‘catch-up’ vaccination programs for both direct and indirect protection. The data show that the recent expansion of MenW has been through the emergence of sub-clones that are spreading globally [Citation120]. Again, such observations highlight the need for active surveillance systems that can provide accurate data through WGS, for example. Intriguingly, one strain can appear to behave differently in different countries; for example, the hypervirulent MenW strain that emerged in Brazil has become far more prevalent in recent years in Argentina, Chile, and the United Kingdom. Yet again, such observations underline the need for strong local surveillance networks and locally tailored control measures. In addition, combination of WGS and new molecular techniques such as proteomic gene expression analysis may provide additional detail on the biological characteristics of individual strains and thus further aids our understanding of emergence events [Citation122].
The report presented on the outbreak of MenW at the 2015 World Scout Jamboree in Japan [Citation87] illustrates the ever-present risk and need for preventive measures such as vaccination for participants in events where large numbers young people are gathered. It also underlines the importance of maintaining immunity in adolescents and young adults.
Subcapsular MenB vaccines are now available and have been used effectively in outbreak control [Citation101,Citation102,Citation110], and are also now included in routine infant vaccination recommendations in the United Kingdom. To evaluate the level of coverage provided by these vaccines, such as in an outbreak situation, accurate data on presence and expression of the various vaccine-related antigens in the active strain are required. For this, again, good-quality surveillance with access to MATS and DNA analysis technologies, especially WGS, are required. There is still uncertainty about the ability of these vaccines to eliminate MenB acquisition/carriage, and further data are needed in this area.
The importance of raising awareness and of advocacy in promoting the prevention and control of MD was highlighted by the work of the MRF and the CoMO. Indeed, these organizations continue to play an important role in activities to support the introduction and expansion of vaccination and surveillance programs.
As noted above, modeling can be used to help us understand the impact of carriage/acquisition reduction on indirect protection and to enable longer term predictions. The GMI agreed that models are used to best effect in combination with data from various sources, including disease surveillance, clinical trials, and carriage studies.
5. Summary
During the 2015 GMI meeting, presentations showed how vaccination programs have been successful in reducing MD incidence in many countries; however, it was also described how new MenW clones present a threat, as does the emergence of a new MenC strain in the African meningitis belt. As a result of the findings presented, the GMI recognized the importance of ongoing vigilance and called for continued support and expansion of vaccination and surveillance programs. The importance of building herd protection and stopping acquisition for the prevention of transmission of MD was also discussed. The GMI agreed that vaccination of those age groups with the highest carriage rates (particularly adolescents) is important for this. With this in mind, the GMI also called for vaccination programs for protection during large gatherings of young people in close contact. Presentations from the MRF and the CoMO showed how such organizations are key to ensuring continuation and growth in all of these areas.
Updates to the GMI Global Recommendations for Meningococcal Disease were determined during the meeting based on the findings presented (). The GMI agreed that there is a need for a recommendation to enable access to WGS as part of surveillance programs and also for DNA sequence data to be publicly available. It was also agreed that guidance on the antigen expression criteria that indicate use of subcapsular vaccines should be included in the recommendations. Finally, the GMI agreed that support for MD advocacy and awareness campaigns should be included in the GMI Global Recommendations for Meningococcal Disease.
Table 3. The GMI Updated Global Recommendations regarding strategies for the prevention of MD and the importance of herd protection.
6. Expert commentary
MD remains an important health concern in many regions across the globe, particularly Africa, where morbidity and mortality rates are still high, as well as in Asia, where the true burden of MD is uncertain. However, health policy leaders, scientists, and clinicians in these regions (and individual countries) can learn from the experiences, insights, and strategies of others from across the globe where MD has been prevented and controlled with great success. Indeed, much can be learnt from the Latin American experience and control of MenW, as well as from the control of MenA in the African meningitis belt, of MenC in Australia, Brazil, Canada, and Europe, and of MenB in the United Kingdom and Canada.
Since the introduction of meningococcal vaccines, the world has seen a substantial reduction in the burden of MD. Outbreaks continue to occur in many areas of the world. The reasons for this are multifactorial and include relatively low vaccination uptake rates; poor surveillance and control systems; lack of standardized case definitions and diagnostic assessments; lack of herd protection; failure to vaccinate those currently at risk; strain changes; rise of serogroup(s) not covered by currently used vaccines; and the general unpredictability of MD epidemiology.
Moving forward, a key strategy to further reduce/interrupt MD transmission, beyond the levels noted today, would be to induce herd protection in populations where it is currently lacking. Data suggest that herd protection can be achieved with conjugate vaccines by immunizing those who are most likely to be carriers and thus targeting the driving force of transmission (i.e. adolescents and young adults), as opposed to immunizing those in whom only direct protection is gained (i.e. infants and young children). Some countries are currently implementing (or at least recommending) such strategies (e.g. the Unites States of America and United Kingdom). Of course, achieving and sustaining herd protection will be a challenge in itself and is likely to require high vaccination uptake rates in populations where vaccine uptake is currently low. While the experience in many countries, both developed and developing, proves that decreasing the overall incidence of MD and control of outbreaks is possible, coordinated, sustained, and long-term strategies will be required in each country in order to reach the goals of lowering mortality and morbidity due to MD.
7. Five-year view
In the next 5 years, the epidemiology of MD will most likely continue to be dynamic and change across the globe, as enhanced surveillance systems and prevention and control strategies are being implemented. Furthermore, it is likely that localized outbreaks that are potentially controllable through vaccination programs will continue to occur in places where systems are weak or lacking. In addition, the emergence of new strains is likely to be an issue, such as the ST-10217 in Nigeria and Niger and the variants of ST-11/ET-37 cc MenW, and the spread of other hypervirulent strains. Constant vigilance and high-quality MD surveillance will be needed.
New multi-omic technologies and bioinformatics tools continue to develop, with genetic techniques such as real-time PCR and WGS, as well as gene expression methods such as transcriptomic and proteomic analyses, which enable even more in-depth strain characterization, becoming more widely available. Such techniques could become more accessible to surveillance laboratories at least on a regional level, if not nationally, within the next 5 years, and should improve not only surveillance but also understanding of emergence of particular strains. It would be hoped that availability of higher quality epidemiological data from such sources could be used as a driver for implementation of effective routine and emergency vaccination programs. In parallel, the increasing availability of carriage data, together with the availability of new DNA technologies and modeling data, should enable effective targeting of age groups and populations in whom carriage is greatest, and where immunization would be best employed to develop effective herd protection. The GMI agrees that vaccination of the age groups with the highest carriage rates (particularly adolescents) is important for this and should be implemented, or at least recommended, in more countries in the next 5 years.
The GMI recognizes the importance of ongoing vigilance and has called for continued support and expansion of vaccination and surveillance programs. Organizations such as the MRF and the CoMO will be key to ensuring the continuation and growth of surveillance and vaccination programs during the coming years.
When employed in organized vaccination programs, new vaccines, such as the quadrivalent MenACWY conjugate vaccines and the MenA conjugate MenAfriVac®, have already made dramatic contributions to the control of MD through providing direct protection and, potentially, herd protection. It is anticipated that if such programs are continued and expanded, impact on MD will also continue. The new MenB vaccine Bexsero® has also now shown effectiveness in real-world outbreak situations, and, within the next 5 years, its effects within a national infant immunization program and on carriage should be better understood.
Finally, availability of high-quality surveillance data in the future is also necessary so that a state of preparedness is maintained and suitable vaccines are available or can be rapidly made available should newly emergent strains become a threat (e.g. MenC in the sub-Saharan meningitis belt).
All of these points are covered by the GMI Global Recommendations for Meningococcal Disease. If they are implemented, through a combination of improved disease surveillance, the availability of conjugate vaccines, and advocacy to build disease awareness, it might be possible to have a substantial impact on the incidence of MD globally within the next 5 years.
Key issues
The GMI is an international group of clinicians and scientists with expertise in MD immunology, microbiology, epidemiology, public health, and vaccination; it was established to promote the prevention of MD worldwide through education, research, international cooperation, and vaccination.
The GMI has previously produced a set of Global Recommendations for Meningococcal Disease ().
More than 20 clinicians, scientists, and public health experts representing institutions in Africa, the Asia-Pacific region, Europe, and Latin America convened in November 2015 to discuss topics including herd protection, surveillance, epidemiology, prevention, and control strategies.
Although vaccination programs have been successful in reducing MD incidence in many countries, it was agreed that new MenW sub-clones present a threat, as does the emergence of a new MenC strain in the sub-Saharan meningitis belt.
The GMI recognizes the importance of ongoing vigilance in the face of this dynamic disease and called for continued support and expansion of vaccination and surveillance programs.
Building herd protection and stopping carriage are important, as they prevent transmission of MD; therefore, vaccination of those age groups with the highest carriage rates (particularly adolescents) is necessary, as are vaccination programs for protection during large gatherings of young people in close contact.
A number of additions to the GMI Global Recommendations for Meningococcal Disease were agreed:
There is a need for a recommendation to enable access to WGS as part of surveillance programs, and also for DNA sequence data to be publicly available.
Guidance on the antigen expression criteria that indicate use of subcapsular vaccines should be included in the recommendations.
The GMI recognizes the importance of MD advocacy and awareness campaigns and agrees that support for such activities should be included in the GMI Global Recommendations for Meningococcal Disease.
Declaration of interest
The GMI is funded by an educational grant from Sanofi Pasteur; however, the group is not led in any way by the company. GMI members determine meeting agenda items and lead the discussions and outputs. Medical writing support was provided by Shelley Lindley, PhD, of PAREXEL, which was funded by Sanofi Pasteur. R Borrow and J Findlow perform contract research on behalf of Public Health England for Baxter Biosciences, GlaxoSmithKline, Novartis, Pfizer, Sanofi Pasteur, and Sanofi Pasteur MSD. DA Caugant has performed in the past contract research on behalf of the Norwegian Institute of Public Health for Novartis, Pfizer, and Sanofi Pasteur. H Christensen has received an honorarium from Sanofi Pasteur for running a GMI modeling workshop paid to her employer. Furthermore, H Christensen is supported by the National Institute for Health Research [PDF-2012-05-245] and is a member of the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Evaluation of Interventions at the University of Bristol, UK. S Sidorenko has received grants from Pfizer, outside the submitted work. P De Wals has received research grants and reimbursements of travel expenses from vaccine manufacturers including GlaxoSmithKline, Novartis, Sanofi Pasteur, and Pfizer, as well as from governmental agencies including the Quebec Ministry of Health and Social Services, Health Canada, and the Public Health Agency of Canada. J Carlos has received research grant support from GlaxoSmithKline, Novartis, and Sanofi Pasteur; however, no conflict of interest relevant to this manuscript is reported. M-K Taha performs contract research on behalf of Institut Pasteur for GlaxoSmithKline, Novartis, Pfizer, and Sanofi Pasteur. C Trotter received a consulting payment from GlaxoSmithKline in 2013 for critical review of a health economic model of meningococcal vaccines and an honorarium from Sanofi Pasteur in 2015 for running a GMI modeling workshop. JA Vázquez Moreno performed contract research on behalf of Institute of Health Carlos III for Baxter Biosciences, GlaxoSmithKline, Novartis, Pfizer, and Sanofi Pasteur. A von Gottberg received research funding from Pfizer. MAP Sáfadi has received grants to support research projects from GlaxoSmithKline, Novartis, Pfizer, and Sanofi Pasteur. This work is produced by the authors under the terms of the research training fellowships issued by the NIHR. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, NIHR, the Department of Health, or Public Health England. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgments
We confirm that all authors contributed in the development of this manuscript and that they all approved the final article.
Additional information
Funding
References
- Flexner S, Jobling JW. An analysis of four hundred cases of epidemic meningitis treated with the anti-meningitis serum. J Exp Med. 1908;10(5):690–733.
- Centers for Disease Control and Prevention. Meningococcal disease. In: Hamborsky J, Kroger A, Wolfe C, editors. Epidemiology and prevention of vaccine-preventable diseases. 13th ed. Washington, DC: Public Health Foundation; 2016.
- Manchanda V, Gupta S, Bhalla P. Meningococcal disease: history, epidemiology, pathogenesis, clinical manifestations, diagnosis, antimicrobial susceptibility and prevention. Indian J Med Microbiol. 2006;24(1):7–19.
- Abio A, Neal KR, Beck CR. An epidemiological review of changes in meningococcal biology during the last 100 years. Pathog Glob Health. 2013;107(7):373–380.
- Al-Tawfiq JA, Clark TA, Memish ZA. Meningococcal disease: the organism, clinical presentation, and worldwide epidemiology. J Travel Med. 2010;17(Suppl):3–8.
- Cartwright K, Noah N, Peltola H. Meningococcal disease in Europe: epidemiology, mortality, and prevention with conjugate vaccines. Report of a European advisory board meeting Vienna, Austria. Vaccine. 2000 [Oct 6-8];19(31):4347–4356.
- Pollard AJ. Global epidemiology of meningococcal disease and vaccine efficacy. Pediatr Infect Dis J. 2004;23(12 Suppl):S274–S279.
- Sáfadi MA, Barros AP. Meningococcal conjugate vaccines: efficacy and new combinations. J Pediatr (Rio J). 2006;82(3 Suppl):S35–S44.
- Stefanelli P, Rezza G. Impact of vaccination on meningococcal epidemiology. Hum Vaccin Immunother. 2016;12(4):1051–1055.
- Pace D, Pollard AJ. Meningococcal disease: clinical presentation and sequelae. Vaccine. 2012;30(Suppl 2):B3–B9.
- Harrison LH, Pelton SI, Wilder-Smith A, et al. The global meningococcal initiative: recommendations for reducing the global burden of meningococcal disease. Vaccine. 2011;29(18):3363–3371.
- National Advisory Committee on Immunization (NACI). An update on the invasive meningococcal disease and meningococcal vaccine conjugate recommendations. An Advisory Committee Statement (ACS). Can Commun Dis Rep. 2009;35(ACS–3):1–40.
- Bilukha OO, Rosenstein N. Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2005;54(RR–7):1–21.
- Cohn AC, MacNeil JR, Harrison LH, et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998-2007: implications for prevention of meningococcal disease. Clin Infect Dis. 2010;50(2):184–191.
- Cordeiro SM, Neves AB, Ribeiro CT, et al. Hospital-based surveillance of meningococcal meningitis in Salvador, Brazil. Trans R Soc Trop Med Hyg. 2007;101(11):1147–1153.
- Kimmel SR. Prevention of meningococcal disease. Am Fam Physician. 2005;72(10):2049–2056.
- Yazdankhah SP, Caugant DA. Neisseria meningitidis: an overview of the carriage state. J Med Microbiol. 2004;53(Pt 9):821–832.
- Christensen H, May M, Bowen L, et al. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(12):853–861.
- Trotter CL, Greenwood BM. Meningococcal carriage in the African meningitis belt. Lancet Infect Dis. 2007;7(12):797–803.
- Caugant DA, Maiden MC. Meningococcal carriage and disease–population biology and evolution. Vaccine. 2009;27(Suppl 2):B64–B70.
- Read RC. Neisseria meningitidis; clones, carriage, and disease. Clin Microbiol Infect. 2014;20(5):391–395.
- Halperin SA, Bettinger JA, Greenwood B, et al. The changing and dynamic epidemiology of meningococcal disease. Vaccine. 2012;30(Suppl 2):B26–B36.
- Pizza M, Rappuoli R. Neisseria meningitidis: pathogenesis and immunity. Curr Opin Microbiol. 2015;23:68–72.
- Murray RL, Britton J, Leonardi-Bee J. Second hand smoke exposure and the risk of invasive meningococcal disease in children: systematic review and meta-analysis. BMC Public Health. 2012;12:1062.
- Chang Q, Tzeng YL, Stephens DS. Meningococcal disease: changes in epidemiology and prevention. Clin Epidemiol. 2012;4:237–245.
- Centers for Disease Control and prevention website [Internet]. Advisory Committee on Immunization Practices. Vaccine acronyms: vaccines included in the immunization schedules for children, adolescents, and adults. [ cited 2015 May]. Available from: http://www.cdc.gov/vaccines/acip/committee/guidance/vac-abbrev.pdf
- Holst J, Oster P, Arnold R, et al. Vaccines against meningococcal serogroup B disease containing outer membrane vesicles (OMV): lessons from past programs and implications for the future. Hum Vaccin Immunother. 2013;9(6):1241–1253.
- Folaranmi T, Rubin L, Martin SW, et al. Use of serogroup B meningococcal vaccines in persons aged ≥10 years at Increased risk for serogroup B meningococcal disease: recommendations of the advisory committee on immunization practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(22):608–612.
- MacNeil JR, Rubin L, Folaranmi T, et al. Use of serogroup B meningococcal vaccines in adolescents and young adults: recommendations of the advisory committee on immunization practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(41):1171–1176.
- John TJ, Gupta S, Chitkara AJ, et al. An overview of meningococcal disease in India: knowledge gaps and potential solutions. Vaccine. 2013;31(25):2731–2737.
- Sáfadi MA, de los Monteros LE, López EL, et al. The current situation of meningococcal disease in Latin America and recommendations for a new case definition from the Global Meningococcal Initiative. Expert Rev Vaccines. 2013;12(8):903–915.
- Sáfadi MA, Bettinger JA, Maturana GM, et al. Evolving meningococcal immunization strategies. Expert Rev Vaccines. 2015;14(4):505–517.
- Sáfadi MA, O’Ryan M, Valenzuela Bravo MT, et al. The current situation of meningococcal disease in Latin America and updated Global Meningococcal Initiative (GMI) recommendations. Vaccine. 2015;33(48):6529–6536.
- Vázquez JA, Taha MK, Findlow J, et al. Global Meningococcal Initiative: guidelines for diagnosis and confirmation of invasive meningococcal disease. Epidemiol Infect. 2016;144(14):3052–3057.
- Lucidarme J, Hill DM, Bratcher HB, et al. Genomic resolution of an aggressive, widespread, diverse and expanding meningococcal serogroup B, C and W lineage. J Infect. 2015;71(5):544–552.
- Sáfadi MA, Berezin EN, Arlant LH. Meningococcal disease: epidemiology and early effects of immunization programs. J Pediatric Infect Dis Soc. 2014;3(2):91–93.
- de Moraes JC, Kemp B, de Lemos APS, et al. Prevalence, risk factors and molecular characteristics of meningococcal carriage among Brazilian adolescents. Pediatr Infect Dis J. 2015;34(11):1197–1202.
- Sáfadi MA, Carvalhanas TR, Paula de Lemos A, et al. Carriage rate and effects of vaccination after outbreaks of serogroup C meningococcal disease, Brazil, 2010. Emerg Infect Dis. 2014;20(5):806–811.
- World Population Statistics website [Internet]. World Population Statistics. Population of Asia. 2016. [cited 2016 Jan]. Available from: http://www.worldpopulationstatistics.com/population-of-asia/
- World Population Statistics website [Internet]. World Health Organization. WHO regional offices. [ cited 2016]. Available from: http://www.who.int/about/regions/en/
- Jafri RZ, Ali A, Messonnier NE, et al. Global epidemiology of invasive meningococcal disease. Popul Health Metr. 2013;11(1):17.
- Borrow R, Lee JS, Vázquez JA, et al. Meningococcal disease in the Asia-Pacific region: Findings and recommendations from the Global Meningococcal Initiative. Vaccine. 2016;34(48):5855–5862.
- World Health Organization website [Internet]. World Health Organization. WHO vaccine-preventable diseases: monitoring system. 2016 global summary. [ cited 2016 Jul]. Available from: http://apps.who.int/immunization_monitoring/globalsummary.
- World Health Organization Western Pacific Region website [Internet]. World Health Organization Western Pacific Region. China, 2014. [cited 2015]. Available from: http://www.wpro.who.int/immunization/documents/epi_country_poster_2014_chn.pdf.
- Australian Government Department of Health website [Internet]. Australian Government Department of Health. National immunisation program schedule. [ cited 2016 Sep]. Available from: http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/nips.
- Tanaka H, Kuroki T, Watanabe Y, et al. Isolation of Neisseria meningitidis from healthy persons in Japan. Kansenshogaku. Zasshi. 2005;79(8):527–533.
- Takahashi H, Haga M, Sunagawa T, et al. Meningococcal carriage rates in healthy individuals in Japan determined using loop-mediated isothermal amplification and oral throat wash specimens. J Infect Chemother. 2016;22(7):501–504.
- Hamada A, Fukushima S. Present situation and challenges of vaccinations for overseas travelers from Japan. J Infect Chemother. 2015;21(6):405–409.
- European Centre for Disease Prevention and Control. Surveillance of invasive bacterial diseases in Europe, 2012. Stockholm: ECDC; 2015.
- Borrow R, Abad R, Trotter C, et al. Effectiveness of meningococcal serogroup C vaccine programmes. Vaccine. 2013;31(41):4477–4486.
- Koroleva I. Meningococcal infection and purulent bacterial meningitis in Russia: analytical review. Moscow: Central Research Institute of Epidemiology, Russian Inspectorate for the Protection of Consumer Rights and Human Welfare; 2014.
- Matosova SV, Mironov KO, Platonov AE, et al. Molecular biological monitoring of Neisseria meningitidis in Moscow in the period 2011 to 2015. Epidemiologia I Infekcionnye Bolezni. 2016;2:4–9.
- Meiring S, Cohen C, de Gouveia L et al. A decade of invasive meningococcal disease surveillance in South Africa: 2003–2012. The 16th International Congress on Infectious Diseases (ICID); Apr 2-5, 2014, Cape Town, South Africa.
- von Gottberg A, du Plessis M, Cohen C, et al. Emergence of endemic serogroup W135 meningococcal disease associated with a high mortality rate in South Africa. Clin Infect Dis. 2008;46(3):377–386.
- Cohen C, Singh E, Wu HM, et al. Increased incidence of meningococcal disease in HIV-infected individuals associated with higher case-fatality ratios in South Africa. AIDS. 2010;24(9):1351–1360.
- Simmons RD, Kirwan P, Beebeejaun K, et al. Risk of invasive meningococcal disease in children and adults with HIV in England: a population-based cohort study. BMC Med. 2015;13:297.
- Ibarz-Pavón AB, Morais L, Sigaúque B et al. Epidemiology, molecular characterization and antibiotic resistance of Neisseria meningitidis from patients ≤15 years in Manhiça, rural Mozambique. PLoS One. 2011;6:(6), e19717.
- Murray J, Agocs M, Serhan F, et al. Global invasive bacterial vaccine-preventable diseases surveillance–2008-2014. MMWR Morb Mortal Wkly Rep. 2014;63(49):1159–1162.
- Baccarini C, Ternouth A, Wieffer H, et al. The changing epidemiology of meningococcal disease in North America 1945-2010. Hum Vaccin Immunother. 2013;9(1):162–171.
- Government of Canada website [Internet]. Public Health Agency of Canada. Canadian immunization guide. [cited 2016 Feb]. Available from: http://www.phac-aspc.gc.ca/publicat/cig-gci/p04-meni-eng.php
- Maiden MC, Ibarz-Pavon AB, Urwin R, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008;197(5):737–743.
- Ramsay ME, Andrews NJ, Trotter CL, et al. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. Bmj. 2003;326(7385):365–366.
- GOV UK website [Internet]. Public Health England. Meningococcal meningitis and septicaemia notifiable. [ cited 2015 Sep]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/462629/2904512_Green_Book_Chapter_22_v6_0W.PDF
- Kristiansen PA, Diomande F, Ba AK, et al. Impact of the serogroup A meningococcal conjugate vaccine, MenAfriVac, on carriage and herd immunity. Clin Infect Dis. 2013;56(3):354–363.
- Daugla DM, Gami JP, Gamougam K, et al. Effect of a serogroup A meningococcal conjugate vaccine (PsA-TT) on serogroup A meningococcal meningitis and carriage in Chad: a community study [corrected]. Lancet. 2014;383(9911):40–47.
- Jeppesen CA, Snape MD, Robinson H, et al. Meningococcal carriage in adolescents in the United Kingdom to inform timing of an adolescent vaccination strategy. J Infect. 2015;71(1):43–52.
- Caugant DA, Kristiansen PA, Wang X, et al. Molecular characterization of invasive meningococcal isolates from countries in the African meningitis belt before introduction of a serogroup A conjugate vaccine. PLoS One. 2012;7(9):e46019.
- Kretz CB, Retchless AC, Sidikou F, et al. Whole-genome characterization of epidemic neisseria meningitidis serogroup C and resurgence of serogroup W, Niger, 2015. Emerg Infect Dis. 2016;22(10):1762–1768.
- Meningitis Vaccine Project website [Internet]. PATH. A public health breakthrough. [ cited 2015]. Available from: http://www.meningvax.org/
- World Health Organization website [Internet]. World Health Organization. WHO meningococcal meningitis. Fact sheet 141. [ cited 2015 Nov]. Available from: http://www.who.int/mediacentre/factsheets/fs141/en/
- Lingani C, Bergeron-Caron C, Stuart JM, et al. Meningococcal meningitis surveillance in the African meningitis belt, 2004-2013. Clin Infect Dis. 2015;61(Suppl 5):S410–S415.
- Karachaliou A, Conlan AJ, Preziosi MP, et al. Modeling long-term vaccination strategies with MenAfriVac in the African meningitis belt. Clin Infect Dis. 2015;61(Suppl 5):S594–S600.
- Global Advisory Committee on Vaccine Safety. 3-4 December 2009. Wkly Epidemiol Rec. 2010;85(5):29–33.
- World Health Organization website [Internet]. World Health Organization. Affordable and effective vaccine brings Africa close to elimination of meningitis A. [ cited 2015 Nov]. Available from: http://www.who.int/features/2015/meningitis-africa-elimination/en/
- World Health Organization website [Internet]. World Health Organization. Meningococcal disease: 2013 epidemic season in the African Meningitis Belt. [cited 2016 November]. Available from: http://www.who.int/csr/don/2013_06_06_menin/en/.
- Meningitis Vaccine Project website [Internet]. PATH. WHO surveillance bulletins. [ cited 2016]. Available from: http://www.meningvax.org/epidemic-updates.php.
- World Health Organization. Meningococcal disease control in countries of the African meningitis belt, 2014. Wkly Epidemiol Rec. 2015;90(13):123–131.
- World Health Organization website [Internet]. World Health Organization Strategic Advisory Group of Experts (SAGE) on Immunization. SAGE; 2014 Oct. [cited 2014 Oct]. Geneva: World Health Organization. Available from http://www.who.int/immunization/sage/meetings/2014/october/Yellow-bookSAGE2014_final.pdf
- World Health Organization website [Internet]. World Health Organization. Serogroup C in the meningitis belt: facing the challenge. Report of meeting held in Geneva, October 2015. [cited 2015 Nov]. Available from: http://www.who.int/csr/disease/meningococcal/Facing_NmC_Epidemics_Meningitis_Belt.pdf
- World Health Organization. Preparedness for outbreaks of meningococcal meningitis due to Neisseria meningitidis serogroup C in Africa: recommendations from a WHO expert consultation. Wkly Epidemiol Rec. 2015;90(47):633–636.
- Funk A, Uadiale K, Kamau C, et al. Sequential outbreaks due to a new strain of Neisseria meningitidis serogroup C in northern Nigeria, 2013-14. PLoS Curr. 2014;6. doi:10.1371/currents.outbreaks.b50c2aaf1032b3ccade0fca0b63ee518.
- Taha MK, Achtman M, Alonso JM, et al. Serogroup W135 meningococcal disease in Hajj pilgrims. Lancet. 2000;356(9248):2159.
- Taha MK, Parent Du Chatelet I, Schlumberger M, et al. Neisseria meningitidis serogroups W135 and A were equally prevalent among meningitis cases occurring at the end of the 2001 epidemics in Burkina Faso and Niger. J Clin Microbiol. 2002;40(3):1083–1084.
- Mayer LW, Reeves MW, Al-Hamdan N, et al. Outbreak of W135 meningococcal disease in 2000: not emergence of a new W135 strain but clonal expansion within the electophoretic type-37 complex. J Infect Dis. 2002;185(11):1596–1605.
- Ferreira E, Dias R, Giorgini D, et al. Neisseria meningitidis serogroup W135 in Portugal: presence of the ST-11/ET-37 clonal complex. Pathol Biol (Paris). 2008;56(2):94–96.
- Lemos AP, Harrison LH, Lenser M, et al. Phenotypic and molecular characterization of invasive serogroup W135 Neisseria meningitidis strains from 1990 to 2005 in Brazil. J Infect. 2010;60(3):209–217.
- National Institute of Infectious Diseases website [Internet]. Kanai M, Hachisu Y, Fukusumi M et al. Meningococcal disease cases in Scotland and Sweden, following attendance at the World Scout Jamboree, Yamaguchi, Japan, July 28-August 8, 2015. [ cited 2015 Aug]. Available from: http://www.nih.go.jp/niid/en/id/997-disease-based/sa/bac-%20%20%20%20megingitis/idsc/iasr-in/5879-pr4272e.html
- Weidlich L, Baethgen LF, Mayer LW, et al. High prevalence of Neisseria meningitidis hypervirulent lineages and emergence of W135:P1.5,2:ST-11clone in Southern Brazil. J Infect. 2008;57(4):324–331.
- Valenzuela MT, Moreno G, Vaquero A, et al. [Emergence of W135 meningococcal serogroup in Chile during 2012]. Rev Med Chil. 2013;141(8):959–967.
- Abad R, Lopez EL, Debbag R, et al. Serogroup W meningococcal disease: global spread and current affect on the Southern Cone in Latin America. Epidemiol Infect. 2014;142(12):2461–2470.
- Mustapha MM, Marsh JW, Krauland MG, et al. Genomic epidemiology of hypervirulent serogroup W, ST-11 Neisseria meningitidis. EBioMedicine. 2015;2(10):1447–1455.
- LinkedIn SlideShare website [Internet]. Sáfadi MA. Emergence of a new virulent meningicoccal W sequence type 11 in South America: experience, control measures and impact. [ cited 2015 Nov]. Available from: http://www.slideshare.net/meningitis/dr-marco-safadi-mrfs-meningitis-septicaemia-in-children-adults-2015
- Ladhani SN, Beebeejaun K, Lucidarme J, et al. Increase in endemic Neisseria meningitidis capsular group W sequence type 11 complex associated with severe invasive disease in England and wales. Clin Infect Dis. 2015;60(4):578–585.
- Vesikari T, Esposito S, Prymula R, et al. Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: results of two randomised trials. Lancet. 2013;381(9869):825–835.
- FDA website [Internet]. US Food and Drug Administration. FDA News Release: FDA approves a second vaccine to prevent serogroup B meningococcal disease. [ cited 2015 Jan]. Available from: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm431370.htm
- European Medicine Agency website [Internet]. European Medicines Agency. Summary of the European public assessment report (EPAR) for Bexsero. [ cited 2015 Mar]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002333/human_med_001614.jsp&mid=WC0b01ac058001d124
- Shirley M, Dhillon S. Bivalent rLP2086 vaccine (Trumenba®): a review in active immunization against invasive meningococcal group B disease in individuals aged 10-25 years. BioDrugs. 2015;29(5):353–361.
- FDA website [Internet]. US Food and Drug Administration. FDA News Release: First vaccine approved by FDA to prevent serogroup B meningococcal disease. [ cited 2014 Oct]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm420998.htm.
- Donnelly J, Medini D, Boccadifuoco G, et al. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc Natl Acad Sci U S A. 2010;107(45):19490–19495.
- Hong E, Giuliani MM, Deghmane AE, et al. Could the multicomponent meningococcal serogroup B vaccine (4CMenB) control Neisseria meningitidis capsular group X outbreaks in Africa? Vaccine. 2013;31(7):1113–1116.
- Patel M. Use of a novel serogroup B meningococcal vaccine in response to two university outbreaks in the US. XIXth International Pathogenic Neisseria Conference; 2014 Oct 12-17; Asheville, NC, USA.
- McNamara LA, Shumate AM, Johnsen P, et al. First use of a serogroup B meningococcal vaccine in the US in response to a university outbreak. Pediatrics. 2015;135(5):798–804.
- Medini D, Stella M, Wassil J. MATS: global coverage estimates for 4CMenB, a novel multicomponent meningococcal B vaccine. Vaccine. 2015;33(23):2629–2636.
- GOV UK website [Internet]. Public Health England. National enhanced surveillance of vaccination programmes targeting invasive meningococcal disease in England. [cited 2015 Sep]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/457723/MeningoEnhancedSurveillancePlan_01092015_v1.1.pdf
- INSPQ website [Internet]. Institut National de Santé Publique Québec. Rapport intérimaire de surveillance de la sécurité de la première dose du vaccin contre le méningocoque de sérogroupe B au Saguenay-Lac-Saint-Jean. [cited 2014 Sep]. Available from: https://www.inspq.qc.ca/publications/1885
- INSPQ website [Internet]. Institut National de Santé Publique Québec. Avis sur la pertinence d’une intervention visant à contrôler une incidence élevée d’infections invasives à méningocoque de sérogroupe B dans l’Est du Québec. [cited 2014 Apr]. Available from: https://www.inspq.qc.ca/publications/1801
- Law DK, Lefebvre B, Gilca R, et al. Characterization of invasive Neisseria meningitidis strains from Quebec, Canada, during a period of increased serogroup B disease, 2009-2013: phenotyping and genotyping with special emphasis on the non-carbohydrate protein vaccine targets. BMC Microbiol. 2015;15:143.
- Pan_Canadian Public Health Network website [Internet]. Meningococcal B Pilot Project Task Group. The recommended use of the multicomponent meningococcal B (4CMenB) vaccine in Canada. [ cited 2014 Mar]. Available from: http://publications.gc.ca/collections/collection_2014/aspc-phac/HP40-103-2014-eng.pdf
- Government of Canada website [Internet]. Public Health Agency of Canada. Advice for the use of the multicomponent meningococcal serogroup B (4CMenB) vaccine. [ cited 2014 Apr]. Available from: http://publications.gc.ca/site/eng/463960/publication.html
- de Wals P. Results of a mass immunization campaign with a 4-components serogroup B meningococcal vaccine in Quebec, Canada. Rio de Janeiro, Brazil: World Society for Pediatric Infectious Diseases; 2015 Nov 18-21.
- Delbos V, Lemee L, Benichou J, et al. Impact of MenBvac, an outer membrane vesicle (OMV) vaccine, on the meningococcal carriage. Vaccine. 2013;31(40):4416–4420.
- Meningitis Research Foundation website [Internet]. Meningitis Research Foundation. MenB. [ cited 2015]. Available from: http://www.meningitis.org/menb.
- Confederation of Meningitis Organisations website [Internet]. Confederation of Meningitis Organisations. About us. [ cited 2016]. Available from: http://www.comomeningitis.org/about-us/
- Confereration of Meningitis Organisations website [Internet]. Confederation of Meningitis Organisations. World Meningitis Day 2015. [cited 2015 May]. Available from: http://www.comomeningitis.org/news-and-events/world-meningitis-day/wmd-2015/
- Confederation of Meningitis Organisations website [Internet]. Booy R, Robinson P, Gold R, et al. The meningitis B debate. [ cited 2015 Sep]. Available from: http://www.comomeningitis.org/blog/2015/09/the-meningococcal-b-vaccine-debate/
- Trotter CL, Edmunds WJ. Reassessing the cost-effectiveness of meningococcal serogroup C conjugate (MCC) vaccines using a transmission dynamic model. Med Decis Making. 2006;26(1):38–47.
- Read RC, Baxter D, Chadwick DR, et al. Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. Lancet. 2014;384(9960):2123–2131.
- Trotter CL, Gay NJ, Edmunds WJ. Dynamic models of meningococcal carriage, disease, and the impact of serogroup C conjugate vaccination. Am J Epidemiol. 2005;162(1):89–100.
- Christensen H, Hickman M, Edmunds WJ, et al. Introducing vaccination against serogroup B meningococcal disease: an economic and mathematical modelling study of potential impact. Vaccine. 2013;31(23):2638–2646.
- Mustapha MM, Marsh JW, Harrison LH. Global epidemiology of capsular group W meningococcal disease (1970-2015): multifocal emergence and persistence of hypervirulent sequence type (ST)-11 clonal complex. Vaccine. 2016;34(13):1515–1523.
- Trotter CL, Maiden MC. Meningococcal vaccines and herd immunity: lessons learned from serogroup C conjugate vaccination programs. Expert Rev Vaccines. 2009;8(7):851–861.
- Taha MK, Claus H, Lappann M, et al. Evolutionary events associated with an outbreak of meningococcal disease in men who have sex with men. PLoS One. 2016;11(5):e0154047.
