ABSTRACT
Introduction: Engerix B (GSK HepB, GSK, Belgium) was the first recombinant hepatitis B virus vaccine to be licensed, and marked its 30th anniversary in 2016. Vaccination of adult populations against HBV is usually implemented on a risk-based approach with varying degrees of success. Confirmation of ongoing vaccine effectiveness requires monitoring the performance of HBV immunization as reported in individual studies, using systematic methods.
Areas covered: We conducted a systematic review of the literature to summarize 30 years of immunogenicity and safety data for GSK HepB in adult populations.
Expert commentary: Primary 3-dose vaccination of healthy individuals is generally associated with seroprotection rates of 90% or more, although seroprotection decreases with older age. Accelerated 0, 1, 2-month or 0, 7 and 21-day schedules require the recommended booster dose to achieve similar rates of seroprotection. Lower rates of seroprotection were also observed in adults with underlying chronic disease and with a weakened immune system. GSK HepB had a clinically acceptable safety profile in all of the populations studied, including individuals with underlying co-morbidities and immunosuppression. GSK HepB will continue to contribute to global HBV control for the foreseeable future. Further investigation is needed into how to optimize seroprotection in less immune-competent groups.
1. Introduction
Hepatitis B is the most common liver infection worldwide and one-third of the global population is thought to have been infected [Citation1]. The World Health Organization (WHO) estimates that more than 658,000 individuals die annually from hepatitis B virus (HBV)-related complications, such as fulminant hepatitis, cirrhosis, and liver cancer [Citation2]. Chronic HBV infection is estimated to affect 240 million persons worldwide, most of whom reside in Asia and sub-Saharan Africa [Citation2]. New HBV infections in adults occur via horizontal infection through sexual transmission, or exposure to contaminated blood products or needles. Approximately 2.6 million individuals are coinfected with human immunodeficiency virus (HIV) and HBV, and these individuals are at higher risk of HBV-associated morbidity and mortality [Citation2].
The term ‘hepatitis B’ was first used in 1947. By 1965, the hepatitis B surface antigen (HBsAg) was identified by Blumberg (known initially as the Australia antigen) [Citation3]. In 1971, Krugmen et al. showed that boiled plasma from an HBsAg carrier provided protection against HBV infection when injected into humans [Citation4,Citation5]. Ten years later, the first serum subunit vaccine produced from the blood of HBV carriers was available. A landmark trial conducted in men who have sex with men estimated the efficacy of a subunit vaccine produced by MSD as 92.3%, providing convincing evidence of the means to prevent HBV infection through vaccination [Citation6]. Plasma-based vaccines were used widely more many years and were highly effective. However, the production process was time-consuming, was expensive, and was limited by available human plasma volumes and by the theoretical risk of possible human-to-human disease transmission, stimulating the search for alternative HBV vaccine technologies. To this end, several investigators including Rutter, Hall and colleagues in the United States, simultaneously identified the gene encoding HBsAg [Citation7], and using an existing yeast cell model, successfully produced HBsAg from genetically altered yeast cells [Citation8]. The recombinant HBsAg (a precursor of Recombivax™, MSD) was shown to protect chimpanzees from infection [Citation9], and vaccine development for use in humans rapidly followed. The first recombinant HBV vaccine brought to the European market was Engerix™ B (GSK HepB, GSK, Belgium), closely followed by Recombivax™ in the United States, both licensed in 1986.
Initial immunization strategies for HBV control in industrialized countries were targeted toward high-risk groups (men who have sex with men, intravenous drug users, health-care workers (HCW), relatives of individuals with chronic HBV infection, sex workers). The impact of these programs was limited because high coverage of at-risk populations proved hard to achieve and had little or no impact on transmission, particularly in highly endemic areas. In 1991, the WHO resolved on a global approach to HBV control and recommended HBV vaccines be included into national immunization schedules everywhere, beginning with highly endemic countries [Citation10]. Substantial gains in HBV infection control have been achieved over the last 25 years [Citation11]. Nevertheless, the number of people expected to die between 2015 and 2030 due to HBV-related conditions is about 20 million, unless current response rates to HBV control can be improved or a curative treatment is licensed [Citation2,Citation12].
HBV vaccines have been widely used alone or in combination vaccines in childhood vaccination programs, and the impact of these programs on HBV-associated liver disease can be measured and are published [Citation2–Citation4]. On the other hand, HBV adult vaccination is still implemented on a risk-based approach, with varying degrees of success and coverage in different countries. Because the impact of adult HBV immunization in terms of long-term safety, immunogenicity, and effectiveness cannot be measured in the context of national programs, it is important to continue monitoring the performance of HBV immunization as reported in individual studies using systematic and unbiased methods.
Studies of vaccinated cohorts demonstrated that virtually all subjects with an anti-HBsAg antibody (anti-HBs) level of at least 10 mIU/ml were protected against HBV disease [Citation13,Citation14]. A postvaccination anti-HBs antibody concentration ≥ 10 mIU/ml is associated with the development of immune memory and confers long-term protection [Citation14]. Individuals with low (<10 mIU/ml) or undetectable anti-HBs antibodies many years after vaccination respond robustly to challenge, indicating sustained immune memory responses [Citation15,Citation16]. Because of the long incubation period between exposure and development of HBV infection, immune memory responses are sufficient to provide prolonged protection [Citation14].
GSK HepB has made a substantial contribution to HBV containment globally. Since launch, approximately 1.1 billion doses of GSK HepB have been distributed worldwide. The indicated adult dose of GSK HepB consists of three doses of 20μg HBsAg administered at 0, 1, and 6 months, respectively. Accelerated schedules may be given at 0, 1, and 2 months or at 0, 7, and 21 days, but require a booster dose at 12 months.
We conducted a systematic review of the literature to summarize 30 years of efficacy, effectiveness, immunogenicity, and safety data using GSK HepB in adult populations aiming to collate information in a systematic way for all adult populations in which GSK HepB is used.
2. Methods
2.1. Search strategy
The PubMed and Cochrane databases were searched for relevant literature published between 1980 and 21 September 2016 using a search string combining terms on HBV and immunogenicity (Appendix). The search was limited to articles in English, articles with an abstract available, and the geographical scope was worldwide. The study was based on PRISMA guidelines.
Due to the very large number of available articles, we made decisions that limited the search to a manageable size; we searched for ‘Engerix B’ in the title and abstract but limited key terms (anti-HBs, hepatitis B, vaccine, and immunogenicity) to the title. While this may have led to some articles being missed, the identified articles nevertheless provide a comprehensive overview of GSK HepB immunogenicity and safety.
2.2. Inclusion and exclusion criteria
Articles were included if they contained data relevant to the study objectives and if they were clinical trials or of cohort, case–control, or cross-sectional design. Case reports, editorials, letters, news, commentaries, and congress reports were excluded from the review. Articles were also excluded if they did not report separate data for GSK HepB, if they were genetic, biochemistry, molecular or treatment studies, studies on vaccination coverage, laboratory methods, or economic evaluations. Other exclusion criteria were Phase I/II trials, articles comparing administration routes, articles on a booster dose of GSK HepB after priming with another vaccine, and articles in which the sample size of the GSK HepB group was <100 in studies of the general population and <50 in special populations. Seroprevalence studies estimating vaccine effectiveness were not included if they did not have a control group.
2.3. Selection process and quality control
Titles and abstracts identified through the search strategy were assessed for their relevance to the study objectives. Full text articles were critically appraised and relevant articles underwent data extraction and reference lists were examined for additional articles. The first 30% of titles and abstracts were screened in duplicate by two independent researchers. There was less than 5.0% discrepancy between the two researchers.
2.4. Gray literature
The following sources were also accessed: World Health Organization, United States Centers for Disease Control and Prevention, European Center for Disease Prevention and Control, Eurosurveillance, and The EuroHep.Net project [Citation17–Citation21].
2.5. Quality assessment
Most studies were not of a classical design (for example cohort or case–control studies) which could be critically appraised using existing checklists. Therefore, no checklists were used to assess the quality of the articles or to calculate a total quality score. However, some articles were excluded based on their quality, for example, if the article presented a narrative review with no methods section that described the way the authors collected the literature. All systematic reviews were checked for relevant articles that may have been missed in our search.
2.6. Outcomes and definitions
The humoral antibody response to HBsAg is widely accepted as an immunological correlate of protection against HBV infection. In line with accepted guidelines, we defined seroprotection as being an anti-HBs antibody concentration ≥ 10 mIU/ml [Citation13], but also report other cut-offs (100 and 1000 mIU/ml) when provided. We report anti-HBs for all time points provided in each article: usually 1 or 2 months after the final dose in the primary course, and after booster doses if administered. Long-term antibody persistence was defined as 5 or more years after the last vaccination.
3. Results
The search strategy was used to identify relevant articles across all age groups. In this paper we present immunogenicity and safety data for GSK HepB that pertains to adults. Results for children and adolescents are presented in another report.
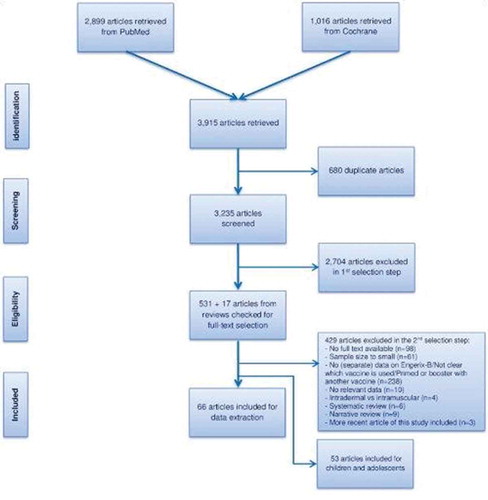
We identified 66 unique articles () describing 64 studies of immunogenicity and/or safety data in adults. Eleven of these studies reported combined populations of adults and children, for which we present the adult data if available separately, or the combined results. Twenty-three articles reported on the immunogenicity of GSK HepB in the general population, and 43 in special populations at risk for HBV infection either due to increased exposure or to immunosuppression. Only two articles described long-term follow-up of 5 years or more: one study in a healthy cohort and one in institutionalized adults with an intellectual disability [Citation22,Citation23]. Key study characteristics for all included articles are provided in the supplementary table. No relevant data were found in the gray literature. There were no studies reporting on the effectiveness of GSK HepB in adults.
3.1. Immunogenicity in the general population
3.1.1. Standard schedule and dose
Fourteen articles reported seroprotection (defined as anti-HBs antibody levels ≥ 10 mIU/ml) rates between 4 weeks and 8 months post-dose 3 following a 0-, 1-, and 6-month schedule (). In 10 articles, the seroprotection rate post-dose 3 was >90.0%. Very high seroprotection rates and geometric mean antibody concentrations (GMC) were observed in a study conducted in medical students in Italy [Citation24]. The high response may be because the population was young adults, and three-quarters of subjects were women, in whom high responses to HBV have been documented [Citation25]. One study in medical students only reported seroprotection rates before complete vaccination, these were 20.0%, 61.0%, and 82.0% at month 1, 2, and 3, respectively [Citation26]. In one of these studies with long-term persistence data, the rate of seroprotection 6.5 years after vaccination was 85.6% [Citation22].
Table 1. Immunogenicity of GSK HepB in adults in the general population.
Seroprotection rates <90.0% were reported in one study that enrolled 50- to 89-year-old adults (84.0% in ≥50 year olds and 67.7% in ≥65 year olds [Citation31]), and in three studies in adults aged ≥40 years (73.1% in a study conducted in three Asian countries [Citation32], 72.8% in Canada [Citation33], and 79.7% in three European countries [Citation37]). In all studies with data stratified by age or gender, seroprotection rates were lower in men than in women and progressively decreased as age increased [Citation31,Citation34,Citation35,Citation38].
The percentage of subjects with anti-HBs antibody concentrations ≥10 mU/ml after 1 year was reported in two articles; 59.0% in adults aged ≥40 years at the time of vaccination, and 98.6% in adults aged 16–40 years at the time of vaccination [Citation29,Citation33].
3.1.2. Standard schedule with alternative dose
Using a pediatric dose of 10 µg administered at 0, 1, and 6 months of age, 99.4% of 16- to 45-year-old subjects had anti-HBs concentrations ≥10 mIU/ml 1 month after the third dose [Citation39] ().
3.1.3. Alternative schedule and standard dose
Six articles reported on the immunogenicity of GSK HepB using the accelerated 0-, 1-, 2-month (0, 28, and 56 day) or 0-, 7-, 21-day schedules [Citation41–Citation46] (). Additional studies also investigated a 0-, 2- and 6-week schedule in medical students [Citation26], and a 0-, 14-, and 28-day schedule in adults [Citation46].
One month after the third vaccine dose, the percentage of subjects with anti-HBs concentrations ≥10 mIU/ml was approximately 65.0–85.0% using the 0-, 1-, 2-month schedule, approximately 77.0–90.8% using the 0-, 7-, 21-day schedule, 87.0% using the 0-, 2-, 6-week schedule, and around 79.0% after the 0-, 14-, 28-day schedule (). In four studies in which the recommended booster dose was administered after the third priming dose, the seroprotection rate 1 month post-booster was 93.4–100%. In one study with data stratified by age, seroprotection was higher in adults <40 years than in adults 40 years or older [Citation41].
3.2. Immunogenicity in HCW
3.2.1. Standard schedule and dose
Five articles reported on the immunogenicity of GSK HepB in HCW when used according to the standard dose and schedule [Citation47–Citation51] (). One month (or at an unspecified time) after dose 3, the percentage of subjects with anti-HBs concentrations ≥ 10 mIU/ml was ≥90.0% in four studies. The post-dose 3 seroprotection rate was 86.2% among 666 HCW in Pakistan, and 86.0% in HCW in the United Kingdom. There were 14.0% nonresponders in Pakistan; however, these results are difficult to interpret because anti-hepatitis B core antibody could not be assessed prior to enrolment, and occult HBV infection could not be ruled out [Citation51]. In the UK study, 8.5% were nonresponders and significantly lower post-dose 3 GMCs and lower seroprotection rates were observed in males compared to females [Citation50]. In other studies with data stratified by age or gender, anti-HBs responses were lower as age increased, and in men [Citation47–Citation49]. One year after vaccination of HCW in Germany, 93.0% retained anti-HBs concentrations ≥ 10 mIU/ml.
Table 2. Immunogenicity of GSK HepB in adult HCW.
3.2.2. Alternative schedule and standard dose
GSK HepB administered to medical students and HCW at 0, 1, and 12 months induced seroprotective antibodies in 94.0% 1 month post-dose 3 [Citation52] ().
3.3. Immunogenicity in patients with chronic renal disease (predialysis and patients receiving dialysis)
Patients with chronic renal failure (CRF) and those undergoing dialysis are known to respond suboptimally to HBV vaccines [Citation5]. Current recommendations indicate that these individuals should receive a higher dose and/or more doses of recombinant HBV vaccines than healthy individuals [Citation53,Citation54]. In this section, studies describing immunogenicity after three double doses (40 µg HBsAg) of GSK HepB, which is the indicated dosage in this population, are considered as the standard schedule and dose.
3.3.1. Standard schedule and dose
Using a 3-dose schedule and 40 µg HBsAg, the seroprotection rate in patients with CRF or in patients undergoing hemodialysis ranged from 60.9% to 85.5% (blood sampling time point ranging from 1 to 8–12 months post-dose 3, and unknown in one study) [Citation55–Citation57] (). One study stratified immunogenicity by the glomerular filtration rate (GFR) and showed that the percentage of subjects with anti-HBs concentrations ≥ 10 mIU/ml decreased as GFR decreased [Citation57]. The seroprotection rate 8–12 weeks post-dose 3 when the GFR was <15 ml/min was 67.0% in patients not receiving dialysis, versus 79.0% in those who had commenced dialysis [Citation57]. In one study, the seroprotection rate was 100% in patients <40 years of age, 75% in 40–60-year-olds, and 74% in those >60 years of age [Citation58].
Table 3. Immunogenicity of GSK HepB in adults with chronic renal disease (predialysis and [hemo]dialysis).
3.3.2. Standard dose and alternative schedule
Ten studies reported on the immunogenicity of 40 µg HBsAg (double-dose of GSK HepB) in different multidose schedules (). Seven of these studies assessed one or two consecutive 4-dose schedules at 0, 1, 2, and 6 months in patients with chronic renal disease and patients undergoing dialysis (peritoneal or hemodialysis) [Citation58,Citation60,Citation61,Citation63,Citation64,Citation67,Citation68]. In five studies, the seroprotection rate after four doses was 70.5–84.4% [Citation58,Citation61,Citation63,Citation64,Citation67]. In two studies, the seroprotection rates were 74.0% and 75.9% after four doses, and increased to 84.0% and 93.8% overall after a second course of four immunizations administered to nonresponders [Citation60,Citation68]. Three studies measured antibody persistence at month 12 or 13; the seroprotection rate ranged from 70.8% to 77.1% [Citation58,Citation63,Citation67], decreasing to 66.7% and 70.2% after 2 years (two studies [Citation58,Citation63]), and 52.0% after 3 years (one study [Citation63]).
Other four-dose schedules using 40 µg HBsAg included a UK study in which patients were immunized at 0, 1, 2, and 3 months, a multi-country study that assessed a 0-, 1-, 6-, 8-month schedule, and a study in Bangladesh that administered three doses at 0, 1, 2 months and a booster dose at month 6 if the antibody concentration was <100 mIU/ml. The percentage of subjects with anti-HBs concentrations ≥10 mIU/ml was 64.0%, 69.2%, and 80.0% (post-booster) in the respective studies [Citation62,Citation65,Citation66].
One study explored three doses of 20 µg HBsAg in patients with CRF undergoing dialysis [Citation59]. The percentage of subjects with anti-HBs concentrations ≥10 mIU/ml was 32.5% (6 weeks post-dose 3) ().
3.4. Immunogenicity in adults with type 2 diabetes mellitus
One controlled study was conducted in adults with and without type 2 diabetes mellitus [Citation69] (). Participants had normal renal function and received GSK HepB in a standard dose and schedule. One month post-dose 3, the seroprotection rate was 75.4% in diabetic participants and 82.0% in controls matched for age and body mass index (BMI) (not statistically significant). Subjects were less likely to achieve seroprotection if they had an increased BMI and if they were of older age [Citation69]. Among adults less than 40 years of age, the seroprotection rate was 88.5% in diabetic participants and 100% in controls, versus and 58.2% and 70.2%, respectively, in those aged 60 years or more.
Table 4. Immunogenicity of GSK HepB in adult transplant recipients and adults with type 2 diabetes mellitus.
3.5. Immunogenicity in transplant recipients
Two studies reported on the immunogenicity of GSK HepB in transplant recipients. Both studies used an alternative schedule and dose [Citation70,Citation71] (). In patients who underwent orthotropic liver transplantation, four doses (40 µg HBsAg) of GSK HepB at 0, 2, 4, and 24 weeks induced a seroprotection rate of 36.0% prior to transplantation, which decreased to 11.6% and 8.0%, after 1 and 2 years post-transplant, respectively [Citation70]. Multivariate analysis suggested that antibody persistence post-transplant may be influenced by genetic predisposition (HLA type) underlying liver disease and severity of disease. The impact of immunosuppressants was not analyzed [Citation70].
In the second study, liver transplant recipients received a 7-dose rapid schedule (0, 1, 2, 3, 4, 5, 6 months) or an accelerated schedule at 0, 7, 14, 28 days and 2, 3, 4 months (both schedules administered 40 µg HBsAg for the first four doses and 20 µg HBsAg for the last three doses). Response rates (defined as anti-HBs antibody concentrations > 60 IU/L) of the rapid and accelerated schedule were 24.6% and 8.8%, respectively (time point for testing not reported) [Citation71]. Multivariate analysis did not find evidence that demographic features, underlying indication of transplant, donor type, antiviral regimen, immunosuppressant, CD4+ count, or hepatitis B immunoglobulin dose influenced the response to vaccination [Citation71].
3.6. Immunogenicity in adults with liver disease
Two studies assessed GSK HepB in patients with liver disease patients using a 40 µg HBsAg dose administered at 0, 1, and 2 months (). Nonresponders either received a second vaccination course [Citation72], or a fourth dose of 80µg HBsAg [Citation73]. The first study reported a seroprotection rate of 44.0% after the first three doses and 62.0% after the second vaccination course [Citation72]. In the second study, the seroprotection rate was 62.0% (time point not reported). A total of 61.0% of the group who received a fourth dose seroconverted after the booster [Citation73].
Table 5. Immunogenicity of GSK HepB in adults with liver disease.
3.7. Immunogenicity in adults with inflammatory bowel disease (IBD)
3.7.1. Standard schedule and dose
Using the standard dose and schedule in patients with IBD, a seroprotection rate of 60.0% was reported 1–3 months post-dose 3 in a cohort of whom 70.0% were receiving immunosuppressive drugs [Citation74] (). Univariate analysis showed that the response rate was lower in patients receiving immunosuppressive therapy, but this was not confirmed in the multivariate analysis.
Table 6. Immunogenicity of GSK HepB in adults with IBD.
In a study that enrolled patients with IBD and healthy controls, the seroprotection rate in patients with IBD was 80.2% 2 months post-dose 3 compared with 94.1% in healthy controls [Citation75]. Ileal disease was the only factor associated with a lower vaccine response in the multivariate analysis.
3.7.2. Alternative schedule and dose
Two studies evaluated three doses of 40 µg HBsAg (double-dose of GSK HepB) administered at 0, 1, and 2 months [Citation74,Citation76] (). The seroprotection rate was 59.0% and 75.0% 1–3 months post-dose 3. In one of the studies, a second course was administered to patients with post-dose 3 concentrations < 100 mIU/ml. The cumulative percentage of patients with anti-HBs concentrations ≥ 10 mIU/ml was 79.0% after the second course [Citation76]. Older age and receipt of anti-tumor necrosis factor agents were associated with a lower response to immunization.
3.8. Immunogenicity in adults with HIV
3.8.1. Standard schedule and dose
In three studies that assessed GSK HepB administered according to the standard schedule and dose to patients with HIV, the seroprotection rate >2 weeks to 2 months post-dose 3 ranged from 34.0% to 62.6% [Citation77–Citation79] (). One study reported a seroprotection rate of 41.4% at week 72 (48 weeks after the third vaccination) [Citation77]. Pollack et al. identified the CD4 T-cell count at commencement of vaccination as the only predictor of response [Citation79]. The seroprotection rate decreased progressively as the CD4 T-cell count decreased, from 79.7% in patients with a CD4 count ≥300 cells/ml to 37.5% when the CD4 count was <100 cells/ml.
Table 7. Immunogenicity of GSK HepB patients with HIV infection.
3.8.2. Standard schedule and alternative dose
Two of these studies also assessed 40 μg GSK HepB administered at 0, 1, and 6 months. The seroprotection rate 1–2 months post-dose 3 was 46.9% and 73.2% in the respective studies [Citation77,Citation78] (). One of the studies reported that the seroprotection rate was 52.4% 48 weeks after vaccination [Citation77]. Compared to the standard dose, the double-dose of HBsAg improved the immune response in patients with low viral load (<10,000 copies) or CD4+ count ≥ 350 cells/mm3 [Citation78].
3.9. Immunogenicity in adults with cancer
3.9.1. Standard schedule and dose
One study conducted in India in cancer patients who received the standard schedule and dose reported a seroprotection rate of 49.0% 9 months after receiving chemotherapy [Citation80] ().
Table 8. Immunogenicity of GSK HepB in adults with cancer.
3.9.2. Alternative schedule and dose
The same study assessed an alternative schedule and dose in which six 40 µg HBsAg doses were administered 0, 1, and 3 weeks prior to chemotherapy and 0, 1, and 2 months after chemotherapy [Citation80] (). The seroprotection rate was 75.9% 9 months after chemotherapy [Citation80].
3.10. Immunogenicity in adults with an intellectual disability
3.10.1. Standard schedule and dose
Three articles describing one study reported on the immunogenicity of GSK HepB (standard dose and schedule) in institutionalized children and adults with an intellectual disability [Citation23,Citation81,Citation82] (). Two articles describe the results up to 2 years after vaccination [Citation81,Citation82], and the third article describes the results up to year 11 [Citation23]. Nonresponders and low responders (anti-HBs antibody concentration < 100 mlU/ml) received a booster dose at month 12, and all subjects received a booster dose at month 60.
Table 9. Immunogenicity of GSK HepB in adults with an intellectual disability.
One month post-dose 3, the seroprotection rate was 92.3% overall, but was lower in individuals with Down syndrome (75.0%). The seroprotection rate after 1 year was 88.3% and was 93.3% after 2 years [Citation81,Citation82]. Protective antibody levels were still high (92.4%) 11 years after the start of vaccination and 6 years after a booster dose [Citation23].
3.11. Immunogenicity in adults who are drug users
3.11.1. Standard schedule and dose
In current drug users in the United States of America, the anti-HBs seroprotection rate 6 months after the third dose of GSK HepB administered in a standard schedule and dose was 65.0% [Citation83] ().
Table 10. Immunogenicity of GSK HepB in adults who are drug users.
3.11.2. Alternative schedule and standard dose
In the same study, the seroprotection rate in USA drug users who received GSK HepB in the accelerated 0, 1, and 2 months schedule was 65.0% 6 months post-dose 3 [Citation83]. In a study in Spain using the same accelerated schedule, the seroprotection rate one month post-dose 3 was 58.0% [Citation44].
3.12. Immunogenicity in other high risk adults
3.12.1. Standard schedule and dose
Four articles reported on GSK HepB immunogenicity in populations at risk of HBV infection (). These included commercial sex workers, family members of chronic HBsAg carriers, alcoholic patients, and descendants of African slaves living in Remaining Quilombo communities in Central Brazil, where HBV endemicity is moderate-to-high [Citation49,Citation84–Citation86].
Table 11. Immunogenicity of GSK HepB in other at-risk groups.
The seroprotection rate 14–90 days post-dose 3 was 95.0–96.5% in sex workers and at-risk family members. The seroprotection rate amongst Quilombo community members was 83.1%, with a lower response significantly associated with male sex and age ≥40 years; the seroprotection rate was 90.0% in those aged <40 years, compared to 66.7% in adults >40 years of age. In alcoholic patients, 46.2% had anti-HBs concentrations ≥ 10 mIU/ml 6 months post-dose 3 [Citation85].
3.12.2. Alternative schedule and standard dose
The seroprotection rate in commercial sex workers 14–90 days after the third dose of GSK HepB administered in a 0, 1 and 4 months schedule was 89.0% and was 91.0% more than 90 days post-dose 3 [Citation84] (). In male prisoners, a three-dose schedule at 0, 15–90 days, and 5–12 months induced a seroprotection rate 1–2 months post-dose 3 of 76.0% [Citation88].
Pregnant women in India received two GSK HepB doses at 24 and 28–30 weeks gestation, or three doses beginning at 24 weeks of gestation and administered at 4–6 week intervals thereafter. The seroprotection rate, defined in this study as anti-HBs ≥ 15 mIU/ml, was 92.0% and 94.0% at the time of delivery, respectively, although anti-HBs antibody levels were higher in the mothers receiving three doses, and their infants [Citation87].
3.12.3. Alternative schedule and dose
In one study, alcoholic patients received four doses of 40 µg HBsAg (double-dose of GSK HepB) at 0, 1, 2, and 6 months. After 1 year, the seroprotection rate was 75.0% [Citation85]. In another study, subjects not sufficiently responding to three doses of GSK HepB (10 µg) were revaccinated with either three doses administered at 1-month intervals, or with a single dose. After revaccination with three doses, 75.1% were seroprotected compared to 62.2% in the one-dose group [Citation89].
3.13. Safety of GSK Hepb in adults
Twenty-three articles (22 studies) reported on the safety of GSK HepB in different adult populations (). Four articles (describing three studies) only reported on the occurrence of unspecified adverse events; these ranged from 0.0–12.5% after vaccination [Citation68,Citation78,Citation81,Citation82].
Table 12. Solicited and unsolicited adverse events following immunization with GSK HepB in adults.
3.13.1. Solicited adverse events
In the adult population vaccinated with GSK HepB, up to 40.9% of subjects reported having any local reaction after vaccination. The occurrence of injection site pain or soreness ranged from 1.5% to 39.8%, redness from 0.0% to 24.4%, and swelling from 0.0% to 12.0%.
Solicited systemic reactions were reported by 0.6–34.4% of adults vaccinated with GSK HepB. One of the most commonly reported systemic events was fatigue (10.5–25.2% of subjects). The occurrence of headache following vaccination was reported by 0.7–22.4% of subjects, fever by 0.0–14.8%, malaise by 1–13.1% and gastrointestinal events by <1.0–10.2% of subjects.
3.13.2. Unsolicited adverse events
One study reported on unsolicited events following GSK HepB. Forty-four unsolicited events were reported after 327 doses of GSK HepB in prehemodialysis and dialysis patients following a four-dose (40 µg) schedule [Citation63]. No other details were available.
3.13.3. Serious adverse events
The number of SAEs following vaccination with GSK HepB ranged from 0 to 58 in 13 studies. None of the SAEs were considered to be related to vaccination except for one SAE (reactive airways disease) reported in a healthy adult [Citation33].
4. Discussion
A previous systematic review of the data published in 2001 found that the overall seroprotection rate among a diverse population of 24,277 subjects (from 134 publications) immunized with GSK HepB was 95.8% [Citation91]. However, that review only provided information using schedules approved in the product information, and only included studies that used accelerated schedules when the recommended booster dose was administered. In our more recent review of the literature, we report the results from a similarly diverse group of subjects, but we have included all of the reported schedules and dosages limited to adults (immunogenicity and safety in children and adolescents are reviewed in a different report). Our approach updates a similar review published in 2003 [Citation92].
Primary three-dose vaccination of healthy individuals is generally associated with seroprotection rates of 90.0% or more, that gradually decreases with increasing age [Citation25,Citation47]. In the studies we identified, seroprotection after immunization of healthy young adult cohorts with three doses of GSK HepB at 0, 1, and 6 months was at least 90.0%, but was lower in cohorts that included adults >40 years of age and was lowest among adults aged 65 years or more. This was confirmed in an integrated analysis showing that seroprotection rates following primary vaccination with GSK HepB decrease with age; remaining at or above 90.0% until age 49 years, at or above 80.0% until 60 years of age, and decreasing thereafter [Citation93]. Studies with data reported according to gender confirmed previous observations that responses to HBV vaccines are lower in men [Citation25,Citation92]. Studies stratified by age and/or gender were mainly conducted in healthy populations. The two studies we identified that reported age stratified seroprotection in an at-risk population (patients undergoing hemodialysis or with diabetes) showed decreased responses in those aged 40 years or more, consistent with data in healthy adults [Citation58,Citation69].
Seroprotection rates following the third dose of an accelerated 0-, 1-, 2-month or 0-, 7-, and 21-day schedule were lower than after the standard 0-, 1-, and 6-month schedule. However, in the two studies in which subjects received the recommended booster dose, seroprotection rates increased to >90.0%, highlighting the importance of the booster dose when an accelerated schedule is used.
GSK HepB has been studied in a range of diverse populations at increased risk for infection, including healthy individuals at increased risk of exposure, such as HCW, sex workers, and family members of chronic carriers, and in groups in whom vaccine immunogenicity is known to be suboptimal due to underlying conditions affecting the integrity of the immune response. The most studied group is patients with CRF and patients undergoing dialysis in whom responses to recombinant HBV vaccines are known to be low [Citation5]. Current recommendations for this group reflect this observation and include the use of more doses, or higher dosage, or the use of an adjuvanted HBV vaccine (approved for use in Europe) [Citation53,Citation54]. Lower anti-HBs seroprotection rates were observed in patient populations with comorbidities including IBD, HIV, liver disease, transplant recipients, patients with cancer, as well as drug uses and individuals with Down syndrome. In a controlled study comparing the immune response to GSK HepB in patients with diabetes with a matched healthy control group, age appeared to be the main factor influencing the magnitude of the immune response [Citation69]. Many studies explored alternative schedules and doses among these patient groups in order to try and improve immunogenicity. In general, alternative schedules/doses achieved higher seroprotection rates than the standard dose and schedule in these populations. A meta-analysis of HBV vaccines in people with HIV confirmed that an increased response was achieved when the vaccine dose was 40μg [Citation94].
We identified no studies that measured the vaccine effectiveness of GSK HepB in adults that met our inclusion criteria. This is not unexpected given that adult HBV vaccination usually occurs on a risk-based individual approach outside of national immunization schedules. In countries of low HBV endemicity, long-term follow-up studies of large cohorts of immunized adults to evaluate effectiveness outcomes are difficult to perform. Population-based seroprevalence studies may be used to indirectly measure the impact of pediatric immunization programs, but are less useful for evaluating adult vaccination.
4.1. Strengths and limitations of the data
This review brings together a cross-section of articles from a range of countries and population groups, including groups at high risk of HBV infection due to increased risk of exposure or underlying immune compromise. The review summarizes three decades experience with GSK HepB.
Interpretation of the studies we identified is limited by diverse study designs that differed in respects such as the timing of antibody testing and use of different assays to measure anti-HBs, although the availability of a generally accepted correlate of protection overcomes some of these limitations.
Older age and male gender are known to be risk factors for a lower immune response to HBV vaccines, and these findings were also reported in the articles in our review that stratified their data by risk factor. As well as older age, the presence of comorbid conditions and male gender, there are numerous factors which are known to impact on the immunogenicity of HBV vaccines, such as underlying obesity and smoking [Citation25]. Many or most of the studies we reviewed did not report these demographic characteristics in their population, which may have influenced the immunogenicity of the vaccine in individual studies.
5. Expert commentary
The use of GSK HepB for adult immunization has contributed to global HBV control for the last 30 years. In this review, we demonstrate the ongoing immunogenicity of GSK HepB and its continued acceptable clinical safety profile. The review highlights how the immune response to HBV vaccination may be negatively impacted by gender, age, and a range of underlying comorbidities. Further investigation is needed into how to optimize seroprotection in these risk groups. Incomplete vaccine uptake by high-risk adults is a long-standing problem and requires continued vigilance by health-care professional to identify those at risk and promote vaccination in these groups [Citation95]. Adults are most likely to respond to HBV vaccines when vaccination is administered early during adulthood (before 40 years of age), particularly in men.
Since they were first licensed, the number of doses of HBV vaccines administered worldwide exceeds 1 billion and these vaccines have a well-documented acceptable safety profile [Citation96]. In the 1990s, several cases of multiple sclerosis were diagnosed in students in France, with apparent onset several weeks after HBV vaccination. Concerns of a causal association led to multiple large epidemiological studies to assess any potential risk. These studies did not show any association between HBV vaccination and the onset or relapse of demyelinating diseases, including multiple sclerosis [Citation96–Citation99].
There are 10 known HBV genotypes (types A–J) whose distribution varies globally. GSKHepB and other recombinant HBV vaccines are of the A2 genotype. Breakthrough infections with non-A2 genotypes among vaccinated individuals have been reported [Citation100]. However, HBV vaccination has proven highly effective in high endemicity countries, such as Asia and Africa, where non-A2 genotypes are prevalent [Citation101]. Available evidence indicates that the current HBV vaccines are effective in preventing infection and clinical disease by all known HBV genotypes [Citation101].
The WHO has adopted a strategic goal of halting viral hepatitis as a major public health threat by 2030 [Citation2]. The goal includes reducing chronic HBV infections from 6–10 million to 0.9 million, and a reduction in annual deaths due to chronic hepatitis B and C from 1.4 million to <0.5 million by 2030. The strategy includes describing and promoting high-impact interventions and hepatitis services to increase vaccination uptake, prevent vertical HBV transmission, improve blood safety and safe injections, and improve access to early diagnosis and treatment of chronic HBV. Achieving sustainable funding for these services and identifying areas where innovation could accelerate HBV control are also strategic goals. Vaccination using currently available recombinant HBV vaccines will continue to play a critical role in achieving these goals.
6. Five-year view
In view of the difficulties in accessing adults and achieving high rates of vaccine uptake, infant HBV immunization will remain the cornerstone of global HBV control. Recombinant vaccines like GSK HepB will continue to be used in the adult populations of healthy and at-risk individuals. The WHO does not recommend postvaccination testing for immunogenicity unless the results are relevant for clinical management of the individual – such as HCW, at-risk infants, patients undergoing dialysis and immunocompromised individuals [Citation1]. The European Consensus Group on Hepatitis B Immunity recommends annual anti-HBs testing in individuals who are immunocompromised, and revaccination if anti-HBs decreases to <10 mIU/ml [Citation102]. Current strategies for improving immunogenicity in poor responders include administering a higher vaccine dose and/or more vaccine doses in an effort to achieve seroprotection. New HBV vaccines developed for use in patients with chronic renal disease could be evaluated in other at-risk groups and in poor responders, in whom recombinant vaccines show reduced immunogenicity.
Systematic and unbiased reviews of published data allow assessment of the benefit versus risk of individual vaccines in specific populations. The currently available data support existing HBV vaccination schedules in healthy and at-risk populations. Nevertheless, there is a need to regularly and systematically review the available data in order to incorporate the findings of new studies, and to continue to monitor the benefit-risk ratio of vaccination in all, but particularly at-risk populations.
Key issues
GSK HepB was the first recombinant HBV vaccine to be licensed and has contributed to global HBV control for 30 years.
Regular systematic reviews of the literature are needed for the ongoing assessment of the benefit-risk ratio of vaccination in specific populations. A systematic review of the literature identified 66 articles that reported on the immunogenicity and/or safety of GSK HepB in adults
At least 90.0% of healthy young adults achieve anti-HBs antibody concentrations ≥10 mIU/ml after a 3-dose course administered at 0, 1 and 6 months. Accelerated immunization schedules require the administration of a booster dose to achieve equivalent seroprotection.
Seroprotection decreases as age increases, with male gender and the presence of co-morbidities including IBD, HIV, liver disease, transplant recipients, patients with cancer, drug users, and individuals with Down Syndrome.
GSK HepB has an acceptable clinical safety profile. No safety concerns were raised among healthy, at risk or immunosuppressed populations in the studies we reviewed.
Declaration of interest
E.M Bunge and C.VD Ende and A.V Ahee are employees of Pallas, a commercial entity that has received grants from the GSK Group of Companies and which carried out part of the submitted work as a supplier to GSK Vaccines. C Marano and L. D Moerlooze are employees of the GSK group of companies and hold stock options/restricted shares from the sponsoring company. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Trademarks
Engerix-B and Havrix are trademarks of the GSK groups of companies.
Supplementary_table.docx
Download MS Word (59.4 KB)Acknowledgments
The authors thank Syedah Maria Bokhari who originally conceived the idea for the manuscript, Joanne Wolter (medical writer on behalf of GSK), and Susana Montenegro Gouveia (from XPE Pharma & Science on behalf of GSK) for coordinating the publication development.
Supplemental data
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- World Health Organization. Hepatitis B vaccines: WHO position paper–recommendations. Vaccine. 2010;28:589–590.
- World Health Organization. Global health sector strategy on Viral hepatitis 2016–2021. Towards ending viral hepatitis, June 2016 (WHO/HIV/2016.06). Available from: http://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/
- Blumberg BS, Alter HJ, Visnich S. A “New” antigen in leukemia sera. JAMA. 1965;191:541–546.
- Krugman S, Giles JP, Hammond J. Viral hepatitis, type B (MS-2 strain). Studies on active immunization. Jama. 1971;217:41–45.
- Gerlich WH. Prophylactic vaccination against hepatitis B: achievements, challenges and perspectives. Med Microbiol Immunol. 2015;204:39–55.
- Szmuness W, Stevens CE, Harley EJ, et al. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N Engl J Med. 1980;303:833–841.
- Valenzuela P, Gray P, Quiroga M, et al. Nucleotide sequence of the gene coding for the major protein of hepatitis B virus surface antigen. Nature. 1979;280:815–819.
- Valenzuela P, Medina A, Rutter WJ, et al. Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature. 1982;298:347–350.
- McAleer WJ, Buynak EB, Maigetter RZ, et al. Human hepatitis B vaccine from recombinant yeast. Nature. 1984;307:178–180.
- Expanded programme on immunization. Global Advisory Group–Part I. Wkly Epidemiol Rec. 1992;67:11–15. Epub 1992/01/17.
- Zanetti AR, Van Damme P, Shouval D. The global impact of vaccination against hepatitis B: a historical overview. Vaccine. 2008;26:6266–6273.
- Hilleman MR. Critical overview and outlook: pathogenesis, prevention, and treatment of hepatitis and hepatocarcinoma caused by hepatitis B virus. Vaccine. 2003;21:4626–4649.
- Jack AD, Hall AJ, Maine N, et al. What level of hepatitis B antibody is protective? J Infect Dis. 1999;179:489–492.
- Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17:1055–1065.
- Van Damme P, Van Herck K. A review of the long-term protection after hepatitis A and B vaccination. Travel Med Infect Dis. 2007;5:79–84.
- Poovorawan Y, Chongsrisawat V, Theamboonlers A, et al. Persistence of antibodies and immune memory to hepatitis B vaccine 20 years after infant vaccination in Thailand. Vaccine. 2010;28:730–736.
- ECDC. European centre for disease prevention and control. [ updated 2015 Jun 01]. Available from: http://www.ecdc.europa.eu/en/publications/surveillance_reports/Pages/index.aspx
- World Health Organization. [ cited 2016 Mar 21]. Available from: http://www.who.int/en/
- Centers for Disease Control and Prevention. [ cited 2016 Mar 21]. Available from: https://www.cdc.gov/
- Eurosurveillance 1996-2016. [ cited 2016 Mar 21]. Available from: http://www.eurosurveillance.org/
- EUROHEP.NET. [ cited 2016 Mar 21]. Available from: http://www.eurohep.net/
- Ayerbe MC, Perez-Rivilla A. Assessment of long-term efficacy of hepatitis B vaccine. Eur J Epidemiol. 2001;17:150–156. Epub 2001/10/16.
- Vellinga A, Van Damme P, Weyler JJ, et al. Hepatitis B vaccination in mentally retarded: effectiveness after 11 years. Vaccine. 1999;17:602–606. Epub 1999/03/13.
- Chiaramonte M, Majori S, Ngatchu T, et al. Two different dosages of yeast derived recombinant hepatitis B vaccines: a comparison of immunogenicity. Vaccine. 1996;14:135–137. Epub 1996/02/01.
- Yang S, Tian G, Cui Y, et al. Factors influencing immunologic response to hepatitis B vaccine in adults. Sci Rep. 2016;6:27251.
- Harries AD, Clark M, Beeching NJ, et al. Early anti-HBs antibody response to accelerated and to conventional hepatitis B vaccination regimens in healthy persons. J Infect. 1991;23:251–254. Epub 1991/11/01.
- Treadwell TL, Keeffe EB, Lake J, et al. Immunogenicity of two recombinant hepatitis B vaccines in older individuals. Am J Med. 1993;95:584–588.
- Bock HL, Kruppenbacher J, Sanger R, et al. Immunogenicity of a recombinant hepatitis B vaccine in adults. Arch Intern Med. 1996;156:2226–2231. Epub 1996/10/28.
- Levie K, Gjorup I, Skinhoj P, et al. A 2-dose regimen of a recombinant hepatitis B vaccine with the immune stimulant AS04 compared with the standard 3-dose regimen of Engerix-B in healthy young adults. Scand J Infect Dis. 2002;34:610–614. Epub 2002/09/20.
- Moraes JC, Luna EJ, Grimaldi RA. Immunogenicity of the Brazilian hepatitis B vaccine in adults. Rev Saude Publica. 2010;44:353–359. Epub 2010/03/27.
- Gilbert CL, Klopfer SO, Martin JC, et al. Safety and immunogenicity of a modified process hepatitis B vaccine in healthy adults ≥50 years. Hum Vaccin. 2011;7:1336–1342. Epub 2011/12/22.
- Sablan BP, Kim DJ, Barzaga NG, et al. Demonstration of safety and enhanced seroprotection against hepatitis B with investigational HBsAg-1018 ISS vaccine compared to a licensed hepatitis B vaccine. Vaccine. 2012;30:2689–2696. Epub 2012/02/22.
- Heyward WL, Kyle M, Blumenau J, et al. Immunogenicity and safety of an investigational hepatitis B vaccine with a Toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared to a licensed hepatitis B vaccine in healthy adults 40-70 years of age. Vaccine. 2013;31:5300–5305. Epub 2013/06/04.
- Vermeiren AP, Hoebe CJ, Dukers-Muijrers NH. High non-responsiveness of males and the elderly to standard hepatitis B vaccination among a large cohort of healthy employees. J Clin Virol. 2013;58:262–264. Epub 2013/07/31.
- Rendi-Wagner P, Kundi M, Stemberger H, et al. Antibody-response to three recombinant hepatitis B vaccines: comparative evaluation of multicenter travel-clinic based experience. Vaccine. 2001;19:2055–2060. Epub 2001/03/03.
- Joines RW, Blatter M, Abraham B, et al. A prospective, randomized, comparative US trial of a combination hepatitis A and B vaccine (Twinrix) with corresponding monovalent vaccines (Havrix and Engerix-B) in adults. Vaccine. 2001;19:4710–4719.
- Van Der Wielen M, Van Damme P, Chlibek R, et al. Hepatitis A/B vaccination of adults over 40 years old: comparison of three vaccine regimens and effect of influencing factors. Vaccine. 2006;24:5509–5515. Epub 2006/05/27.
- Martins RM, Bensabath G, Arraes LC, et al. Multicenter study on the immunogenicity and safety of two recombinant vaccines against hepatitis B. Mem Inst Oswaldo Cruz. 2004;99:865–871. Epub 2005/03/12.
- Tregnaghi MW, Voelker R, Santos-Lima E, et al. Immunogenicity and safety of a novel yeast Hansenula polymorpha-derived recombinant hepatitis B candidate vaccine in healthy adolescents and adults aged 10-45 years. Vaccine. 2010;28:3595–3601. Epub 2010/03/02.
- Wang H, Cai B, Rao D, et al. Rapid immunization effects of a new type of 60 µg hepatitis B vaccine compared with traditional 20 µg hepatitis B vaccines in adults. Hum Vaccin Immunother. 2016;12:2921–2926.
- Young MD, Rosenthal MH, Dickson B, et al. A multi-center controlled study of rapid hepatitis B vaccination using a novel triple antigen recombinant vaccine. Vaccine. 2001;19:3437–3443. Epub 2001/05/12.
- Nothdurft HD, Dietrich M, Zuckerman JN, et al. A new accelerated vaccination schedule for rapid protection against hepatitis A and B. Vaccine. 2002;20:1157–1162.
- Connor BA, Blatter MM, Beran J, et al. Rapid and sustained immune response against hepatitis A and B achieved with combined vaccine using an accelerated administration schedule. J Travel Med. 2007;14:9–15. Epub 2007/01/24.
- Rodrigo JM, Serra MA, Aparisi L, et al. Immune response to hepatitis B vaccine in parenteral drug abusers. Vaccine. 1992;10:798–801. Epub 1992/01/01.
- Rustgi VK, Schleupner CJ, Krause DS. Comparative study of the immunogenicity and safety of Engerix-B administered at 0, 1, 2 and 12 months and Recombivax HB administered at 0, 1, and 6 months in healthy adults. Vaccine. 1995;13:1665–1668. Epub 1995/12/01.
- Bock HL, Loscher T, Scheiermann N, et al. Accelerated schedule for hepatitis B immunization. J Travel Med. 1995;2:213–217. Epub 1995/12/01.
- Averhoff F, Mahoney F, Coleman P, et al. Immunogenicity of hepatitis B vaccines. Implications for persons at occupational risk of hepatitis B virus infection. Am J Prev Med. 1998;15:1–8. Epub 1998/07/04.
- Knoll A, Hottentrager B, Kainz J, et al. Immunogenicity of a combined hepatitis A and B vaccine in healthy young adults. Vaccine. 2000;18:2029–2032. Epub 2000/03/09.
- Palmovic D, Crnjakovic-Palmovic J. Vaccination against hepatitis B: results of the analysis of 2000 population members in Croatia. Eur J Epidemiol. 1994;10:541–547. Epub 1994/10/01.
- Rogan PD, Duguid JK. Immunisation of staff of a regional blood transfusion centre with a recombinant hepatitis B vaccine. J Infect. 1991;22:5–9. Epub 1991/01/01.
- Zeeshan M, Jabeen K, Ali AN, et al. Evaluation of immune response to hepatitis B vaccine in health care workers at a tertiary care hospital in Pakistan: an observational prospective study. BMC Infect Dis. 2007;7:120. Epub 2007/10/27.
- Hess G, Hingst V, Cseke J, et al. Influence of vaccination schedules and host factors on antibody response following hepatitis B vaccination. Eur J Clin Microbiol Infect Dis. 1992;11:334–340. Epub 1992/04/01.
- Kundi M. New hepatitis B vaccine formulated with an improved adjuvant system. Expert Rev Vaccines. 2007;6:133–140.
- Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep. 2001;50:1–43.
- Fernandez E, Betriu MA, Gomez R, et al. Response to the hepatitis B virus vaccine in haemodialysis patients: influence of malnutrition and its importance as a risk factor for morbidity and mortality. Nephrol Dial Transplant. 1996;11:1559–1563. Epub 1996/08/01.
- Sorkhi H, Roushan MR, Al Hashemi GH, et al. Response to hepatitis B virus vaccination in haemodialysis patients with and without hepatitis C infection. East Mediterr Health J. 2008;14:798–803. Epub 2009/01/27.
- Hashemi B, Mahdavi-Mazdeh M, Abbasi M, et al. Efficacy of HBV vaccination in various stages of chronic kidney disease: is earlier better? Hepat Mon. 2011;11:816–820. Epub 2012/01/10.
- Peces R, De La Torre M, Alcazar R, et al. Prospective analysis of the factors influencing the antibody response to hepatitis B vaccine in hemodialysis patients. Am J Kidney Dis. 1997;29:239–245. Epub 1997/02/01.
- Fleming SJ, Moran DM, Cooksley WG, et al. Poor response to a recombinant hepatitis B vaccine in dialysis patients. J Infect. 1991;22:251–257. Epub 1991/05/01.
- Rault R, Freed B, Nespor S, et al. Efficacy of different hepatitis B vaccination strategies in patients receiving hemodialysis. Asaio J. 1995;41:M717–719. Epub 1995/07/01.
- Sezer S, Ozdemir FN, Guz G, et al. Factors influencing response to hepatitis B virus vaccination in hemodialysis patients. Transplant Proc. 2000;32:607–608. Epub 2000/05/17.
- Bel’eed K, Wright M, Eadington D, et al. Vaccination against hepatitis B infection in patients with end stage renal disease. Postgrad Med J. 2002;78:538–540. Epub 2002/10/03.
- Tong NK, Beran J, Kee SA, et al. Immunogenicity and safety of an adjuvanted hepatitis B vaccine in pre-hemodialysis and hemodialysis patients. Kidney Int. 2005;68:2298–2303. Epub 2005/10/14.
- Lin SY, Liu JH, Lin CC, et al. Comparison of hepatitis B surface antibody decay rates after vaccination between hemodialysis and peritoneal dialysis patients. Vaccine. 2011;29:3738–3741. Epub 2011/04/05.
- Nahar K, Jahan M, Nessa A, et al. Antibody responses after hepatitis B vaccination among maintenance haemodialysis patients. Bangladesh Med Res Counc Bull. 2011;37:88–91. Epub 2012/02/23.
- Gilbert CL, Stek JE, Villa G, et al. Safety and immunogenicity of a recombinant hepatitis B vaccine manufactured by a modified process in renal pre-dialysis and dialysis patients. Vaccine. 2014;32:6521–6526. Epub 2014/09/25.
- Janssen JM, Heyward WL, Martin JT, et al. Immunogenicity and safety of an investigational hepatitis B vaccine with a Toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared with a licensed hepatitis B vaccine in patients with chronic kidney disease and type 2 diabetes mellitus. Vaccine. 2015;33:833–837. Epub 2015/01/13.
- Garcia-Agudo R, Aoufi Rabih S, Araque Torres P, et al. Efficacy of a hepatitis B vaccination schedule with two cycles of four double doses of conventional vaccine and four doses of adjuvanted vaccine in chronic kidney disease patients evaluated for renal transplantation. Transplant Proc. 2012;44:2532–2534. Epub 2012/11/14.
- Van Der Meeren O, Peterson JT, Dionne M, et al. Prospective clinical trial of hepatitis B vaccination in adults with and without type-2 diabetes mellitus. Hum Vaccin Immunother. 2016;8:2197–2203.
- Arslan M, Wiesner RH, Sievers C, et al. Double-dose accelerated hepatitis B vaccine in patients with end-stage liver disease. Liver Transpl. 2001;7:314–320. Epub 2001/04/17.
- Feng L, Niu Y, Chen H, et al. Immunogenicity of different hepatitis B virus vaccination schedules in liver transplant recipients. Hepatol Res. 2013;43:495–501. Epub 2012/11/20.
- Dominguez M, Barcena R, Garcia M, et al. Vaccination against hepatitis B virus in cirrhotic patients on liver transplant waiting list. Liver Transpl. 2000;6:440–442. Epub 2000/07/29.
- De Maria N, Idilman R, Colantoni A, et al. Increased effective immunogenicity to high-dose and short-interval hepatitis B virus vaccination in individuals with chronic hepatitis without cirrhosis. J Viral Hepat. 2001;8:372–376. Epub 2001/09/14.
- Gisbert JP, Menchen L, Garcia-Sanchez V, et al. Comparison of the effectiveness of two protocols for vaccination (standard and double dosage) against hepatitis B virus in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35:1379–1385. Epub 2012/04/26.
- Belle A, Baumann C, Bigard MA, et al. Impact of immunosuppressive therapy on hepatitis B vaccination in inflammatory bowel diseases. Eur J Gastroenterol Hepatol. 2015;27:877–881. Epub 2015/06/30.
- Gisbert JP, Villagrasa JR, Rodriguez-Nogueiras A, et al. Efficacy of hepatitis B vaccination and revaccination and factors impacting on response in patients with inflammatory bowel disease. Am J Gastroenterol. 2012;107:1460–1466. Epub 2012/10/05.
- Flynn PM, Cunningham CK, Rudy B, et al. Hepatitis B vaccination in HIV-infected youth: a randomized trial of three regimens. J Acquir Immune Defic Syndr. 2011;56:325–332.
- Fonseca MO, Pang LW, De Paula Cavalheiro N, et al. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine. 2005;23:2902–2908. Epub 2005/03/23.
- Pollack TM, Trang LTT, Ngo L, et al. Response to hepatitis B vaccination among HIV-infected adults in Vietnam. J Virus Erad. 2016;2:102–106.
- Sodhi JS, Raja W, Zargar SA, et al. The efficacy of accelerated, multiple, double-dose hepatitis B vaccine against hepatitis B virus infection in cancer patients receiving chemotherapy. Indian J Gastroenterol. 2015;34:372–379. Epub 2015/11/05.
- Van Damme P, Vranckx R, Meheus A. Immunogenicity of a recombinant DNA hepatitis B vaccine in institutionalized patients with Down’s syndrome. Vaccine. 1990;8 Suppl:S53–55. discussion S60-52. Epub 1990/03/01.
- Van Damme P, Vranckx R, Safary A, et al. Protective efficacy of a recombinant deoxyribonucleic acid hepatitis B vaccine in institutionalized mentally handicapped clients. Am J Med. 1989;87:26s–29s. Epub 1989/09/04.
- Tran TQ, Grimes CZ, Lai D, et al. Effect of age and frequency of injections on immune response to hepatitis B vaccination in drug users. Vaccine. 2012;30:342–349. Epub 2011/11/15.
- Wouters K, Leuridan E, Herck K, et al. Compliance and immunogenicity of two hepatitis B vaccination schedules in sex workers in Belgium. Vaccine. 2007;25:1893–1900.
- Rosman AS, Basu P, Galvin K, et al. Efficacy of a high and accelerated dose of hepatitis B vaccine in alcoholic patients: a randomized clinical trial. Am J Med. 1997;103:217–222. Epub 1997/10/08.
- Motta-Castro AR, Gomes SA, Yoshida CF, et al. Compliance with and response to hepatitis B vaccination in remaining quilombo communities in Central Brazil. Cad Saude Publica. 2009;25:738–742. Epub 2009/04/07.
- Gupta I, Ratho RK. Immunogenicity and safety of two schedules of hepatitis B vaccination during pregnancy. J Obstet Gynaecol Res. 2003;29:84–86. Epub 2003/05/21.
- Bayas JM, Bruguera M, Martin V, et al. Hepatitis B vaccination in prisons: the Catalonian experience. Vaccine. 1993;11:1441–1444. Epub 1993/11/01.
- Hoebe CJ, Vermeiren AP, Dukers-Muijrers NH. Revaccination with Fendrix(R) or HBVaxPro(R) results in better response rates than does revaccination with three doses of Engerix-B(R) in previous non-responders. Vaccine. 2012;30:6734–6737. Epub 2012/09/18.
- Desombere I, Van Der Wielen M, Van Damme P, et al. Immune response of HLA DQ2 positive subjects, vaccinated with HBsAg/AS04, a hepatitis B vaccine with a novel adjuvant. Vaccine. 2002;20:2597–2602. Epub 2002/06/12.
- Coates T, Wilson R, Patrick G, et al. Hepatitis B vaccines: assessment of the seroprotective efficacy of two recombinant DNA vaccines. Clin Ther. 2001;23:392–403.
- Keating GM, Noble S. Recombinant hepatitis B vaccine (Engerix-B): a review of its immunogenicity and protective efficacy against hepatitis B. Drugs. 2003;63:1021–1051.
- Van Der Meeren O, Crasta P, Cheuvart B, et al. Characterization of an age-response relationship to GSK’s recombinant hepatitis B vaccine in healthy adults: an integrated analysis. Hum Vaccin Immunother. 2015;11:1726–1729.
- Ni JD, Xiong YZ, Wang XJ, et al. Does increased hepatitis B vaccination dose lead to a better immune response in HIV-infected patients than standard dose vaccination: a meta-analysis? Int J STD AIDS. 2013;24:117–122.
- Lu PJ, Byrd KK, Murphy TV, et al. Hepatitis B vaccination coverage among high-risk adults 18-49 years, U.S., 2009. Vaccine. 2011;29:7049–7057.
- Ascherio A, Zhang SM, Hernán MA, et al. Hepatitis B vaccination and the risk of multiple sclerosis. N Engl J Med. 2001;344:327–332.
- DeStefano F, Verstraeten T, Jackson LA, et al. Vaccinations and risk of central nervous system demyelinating diseases in adults. Arch Neurol. 2003;60:504–509.
- Ramagopalan SV, Valdar W, Dyment DA, et al. Association of infectious mononucleosis with multiple sclerosis. A population-based study. Neuroepidemiology. 2009;32:257–262.
- Confavreux C, Suissa S, Saddier P, et al. Vaccinations and the risk of relapse in multiple sclerosis. Vaccines in Multiple Sclerosis Study Group. N Engl J Med. 2001;344:319–326.
- Stramer SL, Wend U, Candotti D, et al. Nucleic acid testing to detect HBV infection in blood donors. N Engl J Med. 2011;364:236–247.
- Cassidy A, Mossman S, Olivieri A, et al. Hepatitis B vaccine effectiveness in the face of global HBV genotype diversity. Expert Rev Vaccines. 2011;10:1709–1715.
- Are booster immunisations needed for lifelong hepatitis B immunity? European Consensus Group on Hepatitis B immunity. Lancet. 2000;355:561–565.
Appendix
Search strategy
PubMed Search
#1. Hepatitis B vaccine
Engerix-B [Supplementary Concept] OR Engerix[tiab] OR ((‘hepatitis B’[ti] OR Hep B[ti] OR HBs[ti]) AND (vaccin*[ti] OR immuni*[ti] OR immune[ti]))
#2. Outcomes
effectiv*[tiab] OR efficac*[tiab] OR immunogen*[tiab] OR safe*[tiab] OR adverse event*[tiab] OR Anti-HBs[tiab] OR adverse reaction*[tiab] OR reactogen*[tiab] OR seroprevalence[tiab]
Cochrane database search
#1. Hepatitis B vaccine
Engerix:ti OR ((hepatitis B:ti OR Hep B:ti OR HBs:ti) AND (vaccin*:ti OR immun*:ti))
#2. Outcomes
effectiv*:ti,ab OR efficac*:ti,ab OR immunogen*:ti,ab OR safe*:ti,ab OR adverse event*:ti,ab OR Anti-HBs:ti,ab OR adverse reaction*:ti,ab OR reactogen*:ti,ab OR seroprevalence:ti,ab