ABSTRACT
Introduction: The World Health Organization recommends hepatitis B virus (HBV) vaccines to be included in national immunization schedules everywhere, and has adopted the strategic goal of halting viral hepatitis as a major public health threat by 2030, under which vaccination plays a major role. Engerix™ B (GSK HepB, GSK, Belgium) was the first recombinant HBV vaccine to be licensed, and marked its 30th anniversary in 2016.
Areas covered: We conducted a systematic review of the literature summarizing 30 years of immunogenicity and safety data for GSK HepB in children and adolescents.
Expert commentary: Primary 3-dose vaccination of healthy infants and children, including infants born to HBsAg-positive mothers, using the standard 0, 1, 6 month schedule was associated with seroprotection rates ≥96.0%. In high-risk infants, vaccine efficacy at year 5 was 96.0% after 3-dose priming in infancy and immunoglobulin at birth. Lower seroprotection rates were observed in children with severe underlying disease including human immunodeficiency virus infection and cancer. GSK HepB had a clinically acceptable safety profile in all of the populations studied. HBV vaccines have demonstrated long-term impacts on rates of fulminant hepatitis, chronic liver disease and hepatocellular carcinoma. GSK HepB will continue to contribute to global HBV control for the foreseeable future.
1. Introduction
One-third of the global population is estimated to have been infected with the hepatitis B virus (HBV), making HBV the most common cause of liver infection worldwide [Citation1]. Complications of HBV infection, including fulminant hepatitis, cirrhosis, and liver cancer, contribute to more than 658,000 deaths annually [Citation2]. Worldwide, around 240 million individuals are estimated to have chronic HBV infection, with the highest incidence in developing countries in Asia and sub-Saharan Africa [Citation2].
The epidemiology and clinical outcomes of HBV infection vary regionally. In countries of moderate or high HBV endemicity (defined by the World Health Organization [WHO] as population HBV surface antigen [HBsAg] seroprevalence between 2–7% and ≥8%, respectively [Citation1]), transmission is primarily through neonatal vertical infection and horizontal infection during early childhood. In countries of low endemicity (<2% of the population is HBsAg positive), transmission is most commonly by the horizontal route, usually among older age groups due to sexual transmission or exposure to contaminated blood products or needles. The age at which infection occurs has a strong influence on the clinical outcome. Up to 90% of perinatally acquired HBV infections may become chronic, compared with approximately 30% of infections acquired before the age of 6 years of age, and fewer than 5% of infections acquired by healthy adults [Citation3]. Approximately 70% of deaths from HBV-related diseases such as liver cirrhosis and liver cancer occur in individuals who were infected as infants or during early childhood.
In 1991, WHO recommended HBV vaccines to be included in national immunization schedules everywhere, beginning with highly endemic countries [Citation4]. Since 1991, efforts by the WHO and the Global Alliance for Vaccines and Immunization have achieved substantial gains in HBV control. In 2015, 185 countries included HBV immunization in their national schedules, and global infant coverage of the third dose of HBV vaccine was estimated to be 83%, although global coverage of a birth dose remains low at 39% [Citation5,Citation6]. Countries in which HBV was previously highly endemic have seen large reductions in the incidence of acute HBV infection, in HBsAg seroprevalence and in the incidence of long-term complications after HBV vaccine introduction [Citation7–Citation9]. Some countries of low endemicity commence HBV immunization at the same time as other recommended pediatric vaccines at 2 months of age, combined with HBV screening of all mothers, to identify newborns at risk who require HBIG and vaccination at birth. Many countries also provide catch-up programs for unvaccinated adolescents.
GSK HepB (GSK, Belgium) was the first vaccine to be authorized that uses recombinant DNA technology [Citation10]. Since launch in 1986, approximately 1.1 billion doses have been distributed worldwide. In children up to and including 15 years of age, three doses of GSK HepB containing 10 μg HBsAg administered at 0, 1, and 6 months are recommended. Vaccination may also be given in an accelerated 0, 1, and 2 months schedule, with a fourth dose administered at 12 months, and in other schedules to allow coadministration of HBV vaccine with other recommended pediatric antigens. The WHO recommends that the first HBV vaccine dose be administered within 24 h of birth, followed by two or three additional doses at appropriate intervals [Citation1]. Hepatitis B-specific immunoglobulin (HBIG) administered at birth may provide additional benefits for newborns whose mothers are positive for HBsAg, and particularly if they are also positive for HBeAg [Citation1].
The first field trial with a yeast-derived HBV vaccine (the precursor of Recombivax HB®, Merck & Co., Inc.) in newborns from HBeAg-positive mothers was published in 1987 [Citation11], but this vaccine was given together with HBIG. The first proof that GSK HepB protects newborns from HBV infection was in a study conducted by Poovorawan et al., in infants of mothers who were HBsAg and e-antigen positive [Citation12]. GSK HepB was efficacious in this challenging post-exposure setting, without HBIG administered at birth.
Protection against HBV infection is mediated through antibodies and a post-vaccination anti-HBsAg (anti-HBs) antibody concentration ≥10 mIU/ml is associated with the development of immune memory and long-term protection [Citation13]. Antibody levels wane over time but respond robustly following challenge with an additional HBV vaccine full dose, even in individuals with anti-HBs levels that are low (<10 mIU/ml) or undetectable many years after vaccination [Citation14,Citation15]. The long incubation period between exposure to HBV and the development of infection means that immune memory responses are efficient in providing prolonged protection [Citation13]. These observations underlie current recommendations stating that booster vaccination of immunocompetent individuals after priming is not required [Citation1,Citation16].
GSK HepB has been studied in different populations, age groups, and settings. Systematic reviews of such data are important in the assessment of benefit and risk for licensed vaccines. We conducted a systematic review of the literature to summarize 30 years of efficacy, effectiveness, immunogenicity, and safety data using GSK HepB in children. Our aim was to collate information in a systematic way for all pediatric populations in which GSK HepB is used.
2. Methods
2.1. Search strategy
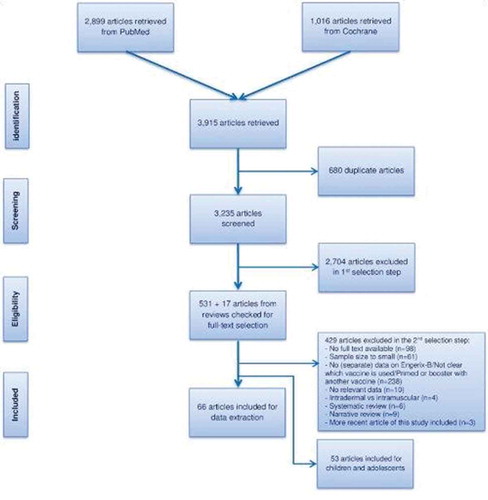
We searched the PubMed and Cochrane databases for relevant literature published between 1980 and 21 September 2016 using a search string combining terms on HBV and immunogenicity (Appendix 1). The search was limited to articles in English, articles with an abstract available, and the geographical scope was worldwide. The study was based on PRISMA guidelines. Gray literature sources were also accessed [Citation17–Citation21].
2.2. Inclusion and exclusion criteria
Inclusion criteria were articles containing data relevant to the study objectives if they were clinical trials, or of cohort, case–control, or cross-sectional design. Articles were included if the sample size of the GSK HepB group was ≥100 persons in studies of the general population, and ≥50 in special populations. Clinical trials were excluded if they were phase I/II trials, were studies comparing administration routes, or a booster dose of GSK HepB after priming with another vaccine. Case reports, editorials, letters, news, commentaries, and congress reports were excluded from the review. Articles that did not report separate data for GSK HepB, articles that reported genetic, biochemistry, molecular, or treatment studies, studies on vaccination coverage, laboratory methods or economic evaluations, were also excluded. Seroprevalence studies estimating vaccine effectiveness were not included if they did not have a control group.
2.3. Study selection and quality control
The procedures for study selection are reported in detail in van den Ende et al., in this issue. In brief, titles and abstracts identified through the search strategy and assessed as relevant to the study objectives underwent full-text review for critical appraisal. Because most studies were not of a classical design suited to appraisal using existing checklists, no checklists were used to assess the quality of the articles or to calculate a total quality score. Nevertheless, some articles were excluded based on their quality; for example if the methods section did not provide sufficient detail to understand the study. All systematic reviews were checked for relevant articles that may have been missed in our search.
2.4. Outcomes and definitions
The humoral antibody response to HBsAg is widely accepted as an immunological correlate of protection against HBV infection [Citation13]. Proof of protection against HBV also requires absence of HBsAg and anti-HBc after exposure. An increase in anti-HBs concentrations in the absence of a booster dose indicates HBV infection. In line with accepted guidelines, we defined seroprotection as being an anti-HBs antibody concentration ≥10 mIU/ml [Citation22], but also report additional cut-offs (for example, 100 mIU/ml and 1000 mIU/ml) when provided. We report anti-HBs antibody concentrations for all time points provided in each article, including after booster doses if administered. Long-term antibody persistence was defined as five or more years after the last vaccination.
3. Results
The search strategy was used to identify relevant articles across all age groups. In this paper, we present immunogenicity and safety data for GSK HepB that pertain to children and young adolescents. Results for adults are presented in another report.
We identified 64 unique articles () describing 60 studies of immunogenicity and/or safety data in children and adolescents. Eleven of these studies reported combined populations of adults and children for which we present the combined results. Thirty-four articles reported on the immunogenicity and/or safety of GSK HepB in the general population, and 45 included populations at high risk of HBV infection due to increased exposure or underlying disease. Key study characteristics for all included articles are provided in the Supplementary table. No relevant data were found in the gray literature.
3.1. Efficacy and effectiveness
Four articles describing three studies reported on GSK HepB efficacy, all which were conducted in high-risk infants born to HBsAg-positive mothers () [Citation23–Citation26]. These studies evaluated several alternative schedules and dosing regimens. Depending on the study, efficacy was calculated on assumed carrier rates at 1 year of between 65% and 90%. All infants received HBIG at birth, except for two study groups in the study reported by Poovorawan et al. [Citation26].
Table 1. Efficacy of GSK HepB in children and adolescents.
Estimated protective efficacy against chronic HBV carriage one year after the final vaccination using the 10-μg dose in standard schedules (0, 1, 6 or 0, 1, 2, and 12 months) was at least 94.8% without administration of HBIG at birth, and at least 97.4% when HBIG was administered at birth. The use of a 20-μg dose was also efficacious, with estimates that were similar for groups that received the standard pediatric dose (range 90% to 96.3% across schedules). Lee et al. [Citation25], reported sustained protective efficacy of 96% 5 years after the vaccination of newborns in various schedules using 10-μg or 20-μg doses ().
An additional study by Canho et al. used a four-dose (20 µg) vaccination schedule at 3, 4, 5, and 11 months after administration of HBIG at birth [Citation23]. After one year, the protective efficacy against chronic carriage was 90% (based on the observation that one-third of mothers who were additionally HBeAg-positive had HBV DNA levels below <5 pg/ml, and were therefore unlikely to transmit hepatitis B to their infants).
No studies reporting vaccine effectiveness of GSK HepB used as sole vaccine in children were identified.
3.2. Immunogenicity in the general population
3.2.1. Standard schedule and pediatric dose
Sixteen studies (17 articles) reported on seroprotection rates at varying times after primary vaccination according to a 0-, 1-, and 6-month schedule (). In nine studies, the seroprotection rate 1 month to 100 days after the complete vaccination course was at least 96.0%. In children 30–40 months of age vaccinated from birth, 83.8% continued to have anti-HBs antibodies ≥10 mIU/ml [Citation27]. Cheang et al. reported 96.4% seroprotection 1-year post-vaccination of newborn term infants [Citation28] (data presented in ).
Table 2. Immunogenicity of GSK HepB in children and adolescents in the general population.
Eight studies provided long-term antibody persistence data (seroprotection) from 5 up to approximately 20 years after primary vaccination using the 0-, 1-, and 6-months schedule (). The anti-HBs seroprotection rates in four studies with blood sampling at year 5 or 6.5 after immunization ranged between 83.3% and 88.2% [Citation32,Citation38,Citation41,Citation43]. Seroprotection at year 10 was 54.8% in Brazilian children and 86.4% in Canadian children [Citation31,Citation32]. Seroprotection rates decreased gradually over time, reaching 79.7% at year 15 in one study [Citation32] and between 33.7% and 75.5% 18–22-years post-primary immunization in two studies [Citation39,Citation42]. Three studies administered booster doses 5–20 years after primary vaccination [Citation32,Citation41,Citation42]. Booster vaccination induced marked increases in anti-HBs seroprotection rates, with the majority of subjects achieving concentrations ≥10 mIU/ml post-booster ().
3.2.2. Standard schedule and adult dose
Eight studies reported on immunogenicity in the general population using a standard 0-, 1-, and 6-month vaccination schedule and an adult (20 μg HBsAg) dose (). In seven of these studies in which blood samples were collected 1–2 months post-dose 3, seroprotection rates were 95.9% to 99.6%.
Three studies reported on long-term antibody persistence after vaccination with GSK HepB (20 μg) during adolescence (). One study reported 92% seroprotection five years after vaccination of 2–14-year olds [Citation38]. The seroprotection rate after immunization during adolescence (age not specified) was 94.3% at year 7, and 91.2% at year 9 [Citation44]. Ten years after primary vaccination at 12 years of age, Zanetti et al. reported a seroprotection rate of 89.2% [Citation48].
3.2.3. Alternative schedule and pediatric dose
Eleven studies reported on the immunogenicity of GSK HepB when the standard 10 μg dose was used in alternate schedules, usually schedules recommended for other routinely administered pediatric vaccines, such as the 3-, 5-, 11-months and 2-, 4-, 6-months schedules (). In one study that assessed the 3-, 5-, 11-month schedule, the seroprotection rate one month after dose 3 was 100% [Citation49]. Four studies of long-term antibody persistence after priming at 3, 5, and 11 months of age reported maintenance of seroprotective anti-HBs levels in 58.4% to 64.3% of subjects after 10 years, in 84% after 12–13 years, and in 75% 18 years after priming [Citation48–Citation50,Citation56]. In two studies in which a booster dose was administered at year 10, post-booster seroprotection rates were 98.9% [Citation49] and 97% [Citation48].
The 2-, 4-, 6-months schedule was assessed in two studies (). One month post-dose 3, the percentage of children with anti-HBs concentrations ≥10 mIU/mL was at least 99% [Citation51,Citation55]. Approximately 4-months post-dose 3 following a 0-, 2-, and 6-months schedule commencing at birth, the seroprotection rate was 93% [Citation53].
One study administered GSK HepB in an accelerated schedule at 0, 1, and 2 months [Citation48]. Immunization commencing at birth induced seroprotection in 91.3% of infants one month post-dose 3, increasing to 98.1% by age 7 months [Citation34].
Other 3-dose primary schedules (0, 6, 14 weeks commencing at birth [Citation52], 0, 12, and 24 months commencing during childhood or adolescence [Citation36]) all resulted in post-vaccination seroprotection rates of at least 95% one month after the third dose. Antibody persistence until 8 years after primary vaccination at 0, 3, 5 months or 0, 5, 24 weeks in Saudi children was 65% [Citation54].
3.2.4. Alternative schedule and adult dose
The adult dose of GSK HepB (20 μg HBsAg) was administered to adolescents in two studies using a 2-dose schedule of either 0, 6 months or 0, 12 months. Seroprotection rates one month after the final vaccine dose were 96.7% and 97.9%, respectively [Citation35,Citation58]. In one study in which children 1–12 years of age received three vaccine doses in the accelerated 0-, 1-, 2-months schedule, the seroprotection rate was 82.3% one month after completion [Citation57].
Gupta et al. evaluated anti-HBs levels in infants born to mothers that received two or three GSK HepB doses during the second/third trimesters of pregnancy [Citation59]. Seroprotection rates were not reported but the anti-HBs antibody geometric mean concentration (GMC) was 45.6 mIU/ml in newborns with mothers who had received two GSK HepB doses during pregnancy, and 94.1 mIU/mL in newborns whose mothers had received three doses [Citation59]. Antibody levels waned over the first 4 months after birth but appeared to persist at higher levels in infants whose mothers had received three, rather than two doses during pregnancy.
3.2.5. Schedule or dose unknown
Two studies reported 5–7 year antibody persistence after primary vaccination. Neither study provided information about the schedule used. In one study which specified three prior doses of GSK HepB 10 μg, the seroprotection rate 5–7 years after vaccination was 84.4% [Citation60]. Avazova et al. reported that the seroprotection rates (the definition of seroprotection was not provided) 6 years after vaccination commencing at birth was 74%, and was 100% 5–6 years after primary immunization at 1–4 years of age [Citation61].
3.3. Immunogenicity in children born to HBsAg-positive mothers
3.3.1. Standard schedule and pediatric dose
Poovorawan et al. evaluated the immunogenicity of GSK HepB administered at 0, 1, 6 months with or without HBIG at birth, in infants born to HBsAg-positive and HBeAg-positive mothers [Citation26,Citation68]. Seroprotection two months after the third dose was 98.2% in the group that did not receive HBIG at birth, and 96.6% in children who received HBIG at birth, decreasing to 87.5% and 80.0% respectively, by month 60. In participants who received a booster dose at month 60, the seroprotection rate 1 year later was 94.7% (group without HBIG) and 87.5% (group with HBIG), versus 85.0% and 68.4%, respectively, in those children who did not receive the month 60 booster dose [Citation68].
Fang et al. reported immunogenicity of GSK HepB administered at 0, 1, and 6 months to children <12 years of age [Citation69]. The seroprotection rate was 100% one month post-dose 3.
3.3.2. Standard schedule and adult dose
Fang et al. also reported immunogenicity of three doses of GSK HepB 20 μg administered to children <12 years of age [Citation69]. The seroprotection rate one month post-dose 3 was 100%.
Two further studies (three articles) administered 20 μg HBsAg to infants born to HBsAg-positive mothers. In one study the seroprotection rate was 90% one month post-dose 3 [Citation70]. In the study by Lee et al., the seroprotection rate was 96.3% at 12 months of age, persisting in 69% of children at year 5 [Citation24,Citation25].
3.3.3. Alternative schedule and pediatric dose
The same studies by Lee et al. and Poovorawan et al. also reported immunogenicity when GSK HepB was administered to infants at 0, 1, 2 months with a booster at 12 months of age () [Citation25,Citation68]. One to two months after the 12 month booster dose, the percentage of children with anti-HBs antibody concentrations ≥10 mIU/mL was at least 98% in both studies. By year 5, the seroprotection rate was 93.5% in one study and 84% in the other. A subset of children in the study by Poovorawan et al., received a booster dose at month 60 [Citation68]. Three years after the booster dose, the seroprotection rate was 100% versus 95.5% (group that did not receive HBIG at birth) and 87.5% (group with HBIG at birth) in children who did not receive the month 60 booster.
Table 3. Immunogenicity of GSK HepB in children born to HBsAg positive mothers.
In newborns of Belgian HBsAg-positive mothers, the seroprotection rate two months after dose 4 (four GSK HepB doses at 0, 1, 2 and 12 months of age) was 95% [Citation71].
3.3.4. Alternative schedule and adult dose
A 20-μg dose of GSK HepB was administered to newborn infants of HBsAg-positive mothers in a 3-, 4-, 5-, and 11-month schedule, or in a 0-, 1-, 2-, and 12-month schedule (). All infants also received HBIG at birth, and one group in the study reported by Canho et al. [Citation23] also received HBIG at 3 months of age . Two months after the fourth dose in a 0-, 1-, 2-, and 12-month schedule, the seroprotection rate was 92.6% and was maintained by 87% of participants at year 5 [Citation24,Citation25]. Two months after the booster dose at 11 months of age, Canho et al. [Citation23] concluded that the number of doses of HBIG did not influence the magnitude of post-vaccination anti-HBs GMC [Citation23].
3.4. Immunogenicity in preterm infants
3.4.1. Standard schedule and pediatric dose
Four studies assessed immunogenicity of GSK HepB when administered to preterm infants (). In two studies, the seroprotection rate in preterm infants after primary vaccination at 0, 1, and 6 months of age was 88.6% and 77.4%, versus 93.4% and 98.2% in term infants [Citation62,Citation63]. In a study by Chirico et al., that used the same schedule, the seroprotection rate one month post-dose 3 in a cohort of combined term and preterm infants was 98.2% [Citation64]. Five years after vaccination, the seroprotection rate in a sub-cohort of children who had seroconverted after vaccination was 74.4% [Citation65].
Table 4. Immunogenicity of GSK HepB in preterm infants.
The study by Cheang et al. also reported seroprotection rates at year 1 in 59 preterm infants. The seroprotection rate at year 1 was 98.3% in preterm infants versus 96.4% in infants born at term [Citation28].
3.4.2. Alternative schedule and pediatric dose
The study by Chirico et al. also evaluated immunogenicity using a 0-, 1-, and 3-months schedule (). Seroprotection in term and preterm infants was 97.8% one month post-dose 3, and 66.3% 5 years post-vaccination [Citation64,Citation65].
Two studies assessed the impact of birth weight on the immune response to GSK HepB (). Preterm infants (25–36-weeks gestation) received 3-dose primary vaccination using 10 μg GSK HepB, and a 20-μg booster dose at month 12 [Citation66]. One month after the booster dose the seroprotection rate was 97.5% in children whose initial birth weight had been ≤2000 g, and 100% in those with a birth weight >2000 g. Anti-HBs antibody GMCs were significantly lower in the group whose gestational birth weight was ≤2000 g [Citation66].
Lau et al. administered three doses of GSK HepB commencing at birth. However, vaccination was delayed until the weight reached at least 1000 g or 2000 g in respective groups [Citation67]. The first two vaccinations were given one month apart and the third dose after approximately a 4-month interval. One month after the third dose the seroprotection rate was 78.9% in children whose weight at vaccination commencement was at least 1000 g, and 90.5% in those with a starting weight of at least 2000 g, versus 95.8% in healthy infants born at term [Citation67].
3.5. Immunogenicity in children with human immunodeficiency virus (HIV) infection
3.5.1. Standard schedule and pediatric dose
Among HIV-infected children who had been receiving antiretroviral therapy for at least 3 months, and who had CD4 T cells ≥200 cells/mm3 (and/or ≥15%), seroprotection after three doses of GSK HepB administered at 0, 1, and 6 months was achieved by 71% of subjects () [Citation72]. In infants born to HIV-infected mothers, three vaccine doses (commencing at birth in 76.3% of children) induced seroprotection in 94.2% of children who were not infected with HIV, versus 41.1% in children who were HIV infected [Citation73].
Table 5. Immunogenicity of GSK HepB in children and adolescents with HIV.
3.5.2. Standard schedule and alternative dose
Adolescents and young adults with HIV infection received three doses of GSK HepB (20 μg or 40 μg) at 0, 1, and 6 months () [Citation74]. Participants had median CD4+ counts of 452–491 cells/mm3. The seroprotection rate one month post-dose 3 was 60% in recipients of the 20-μg dose, and 73.2% in recipients of the 40-μg dose (p = 0.04). Five years after vaccination, the seroprotection rates were 41.4% and 52.4%, respectively [Citation74].
Pollack et al. reported 62.6% seroprotection after three GSK HepB doses (20 μg) administered to adolescents and adults with HIV [Citation75]. The seroprotection rate was higher in women than in men (71.4% versus 56.8%). The CD4 T cell count at commencement of vaccination was significantly associated with development of seroprotective anti-HBs antibodies () [Citation75].
3.6. Immunogenicity in children with cancer
3.6.1. Standard schedule and pediatric dose
One study measured anti-HBs antibodies in children and adults with cancer immunized in a 0-, 1-, 6-month schedule using the age-recommended vaccine dose (). The seroprotection rate 2-months post-dose 3 was 49.0% across all age groups [Citation76].
Table 6. Immunogenicity of GSK HepB in children and adolescents with cancer.
3.6.2. Alternative schedule and alternative dose
In the same study, an alternative schedule of three vaccine doses (20 μg in children and 40 μg in adults) administered before and after chemotherapy (total of six doses) was assessed [Citation76]. At the end of the vaccination course, the seroprotection rate (all ages) was 75.9%. There was a significant difference in the percentage of patients who became HBsAg positive during the study (1.5% who received the 6-dose schedule versus 4.0% who received the standard schedule and dose as reported above), suggesting a clinical benefit of the additional doses and antigen content in this setting [Citation76].
Five studies assessed the immunogenicity of a range of alternative vaccination schedules in patients with cancer (). Four of the five studies were conducted in patients with acute lymphoblastic leukemia (ALL). All five studies administered a ‘double’ dose of the recommended vaccine (20 μg in children and 40 μg in adults), and administered at least one and up to three booster doses.
Seroprotection rates after four vaccine doses were lower in children receiving chemotherapy (31.5%) than in children not undergoing chemotherapy or who were in remission (87.5%) [Citation77]. The proportion of children and adolescents with ALL who seroconverted (definition not provided) after four ‘double’ doses of GSK HepB was 32% [Citation78]. A booster dose administered to non-responders did not result in seroconversion in any of the boosted subjects. Children and adults with newly diagnosed ALL achieved seroprotection after four vaccine doses in 10.53% of cases, and in 18.92% after five vaccine doses [Citation79,Citation80].
3.7. Immunogenicity in other at-risk groups
3.7.1. Alternative schedule
One article reported on GSK HepB immunogenicity in adolescents with diabetes mellitus (). In total, 12–13 years post-immunization at 3, 5, and 11 months of age, 58.2% of adolescents maintained anti-HBs antibodies ≥10 mIU/mL [Citation50].
Table 7. Immunogenicity of GSK HepB in other at-risk groups.
A follow-up study in 87 at-risk subjects who had participated in the pivotal studies reported by Poovorawan et al. in Thailand showed that 58.6% of individuals continued to have seroprotective anti-HBs antibodies 18–20 years post-vaccination [Citation85]. All subjects had been immunized at birth and had received a booster dose at 60 months of age.
3.7.2. Standard schedule and adult dose
Three articles describing one study reported on the immunogenicity of GSK HepB (20-μg dose) in institutionalized children and adults with an intellectual disability (). One month post-dose 3, 92.3% of residents had anti-HBs antibody concentrations ≥10 mIU/mL [Citation81,Citation82]. The seroprotection rate was lower in individuals with Down Syndrome (75.0%). The seroprotection rate at month 12 was 88.3%. Residents who were nonresponders and low responders (anti-HBs antibody concentration <100 mlU/mL) received a booster dose at month 12 [Citation81,Citation82]. Protective antibody levels were still high (92.4%) 11 years after the start of vaccination and six years after the booster dose [Citation83].
3.7.3. Schedule or dose not reported
Ertekin et al. used a standard vaccination schedule (neither the exact schedule nor dose were reported) to determine the immunogenicity in children with celiac disease. At an unspecified time after vaccination, the seroprotection rate was 61.5% (23.8% in male patients and 87.1% in female patients) [Citation84].
3.8. Safety of GSK HepB in children and adolescents
Eleven studies reported on the safety of GSK HepB in different populations of children and adolescents (). Two studies did not provide information on specific adverse events (AEs) and are not included in [Citation45,Citation86]. Three studies provided safety data in combined populations of children and adults and are not described further [Citation30,Citation81,Citation82].
Table 8. Solicited and unsolicited adverse events following immunization with GSK HepB in children and adolescents.
3.8.1. Solicited and unsolicited adverse events
The percentage of children/adolescents reporting any local reaction after vaccination ranged from 13.5% to 36.9% (). Post-injection site pain or soreness was reported by 0.2–47.8%, redness by 1.7–-22.0%, and swelling by 0.0–15.2% of participants. For studies reporting antibody persistence and efficacy, primary vaccination studies were checked for relevant safety information if they were identified by the search and met inclusion criteria.
The occurrence of any systemic reaction was reported in two studies (14.0% and 47.5%) [Citation29,Citation51]. The most commonly described systemic reactions reported by more than 10% in a study included irritability (22.9%), headache (15.7%), and asthenia (10.5%).
None of the studies describing safety in children and adolescents reported on the occurrence of unsolicited adverse events after vaccination.
Two studies reported on the occurrence of unspecified adverse events. Luna et al. reported 4–10 adverse events in newborns after dose 1, 14–42 adverse events after dose 2 and 22–41 after the third dose of GSK HepB [Citation45]. De Serres et al. reported that 24.2% or 47.5% of 8–10-year-old children had at least one adverse event up to 7 or 28 days after immunization, respectively [Citation86]. The attributable risk of adverse events after vaccination was estimated by evaluating the difference between the incidence of adverse events that occurred before and after vaccination over an equivalent time interval. The study concluded that while 47.5% children reported an adverse event within one month after vaccination, only 10.6% could be attributed to vaccination.
3.8.2. Serious adverse events (SAEs)
The number of SAEs reported in five studies ranged from 0 to 8 (). The number of vaccine related SAEs ranged from 0 to 5 in any study.
4. Conclusion
All of the efficacy studies reviewed here were conducted in infants at high risk for vertical transmission of HBV (HBsAg-positive mothers). Estimates of efficacy in these populations were at least 97.4% when HBIG was administered at birth with GSK HepB administered in a 0-, 1-, and 6-month schedule. The estimate of vaccine efficacy was lower, 90%, in one study in which the first GSK HepB vaccine dose was not provided until 3 months of age. Although HBIG was administered at birth, this schedule is not in-line with recommendations to provide HBV vaccination to infants of HBsAg-positive mothers as soon as possible after birth [Citation1], and may provide an explanation for the lower observed efficacy in this study.
In the studies reviewed here, primary 3-dose vaccination of healthy infants and children, including infants born to HBsAg-positive mothers, using the standard 0, 1 and 6 month schedule was associated with seroprotection rates of 96.0% or more, with evidence of long-term persistence of antibodies up to 22 years post-vaccination. In infants, seroprotection rates using the standard 0-, 1-, 6-month schedule were high regardless of whether the schedule commenced at birth or later in the first year of life. Booster vaccination of cohorts immunized in infancy up to 15 years earlier induced marked increases in anti-HBs GMCs, supporting the generally held view that primary vaccination induces long-term immune memory capable of robust immune responses on challenge [Citation1,Citation16].
Seroprotection rates achieved when GSK HepB was used in the 3, 5, 11, and 2, 4, 6 months primary schedules employed for other pediatric antigens were at least 99%. The seroprotection rate was lower following the third dose of an accelerated 0, 1, 2 months schedule, but increased to levels comparable with other schedules after the recommended booster dose at 12 months. These findings are in line with a recent meta-analysis conducted by the Cochrane group which did not identify differences in seroprotection rates of anti-HBs antibody GMCs when three doses were administered to infants in different primary schedules [Citation87].
Although not specifically addressed in our review, GSK HepB given concomitantly with other pediatric vaccines routinely administered in infancy, including Bacillus Calmette–Guérin, diphtheria, tetanus, pertussis, poliovirus, hepatitis A, measles, mumps, rubella, and Haemophilus influenzae type b vaccines [Citation88]. The recombinant HBsAg antigen bulk of GSK HepB is also combined with diphtheria, tetanus, pertussis, poliovirus, and Haemophilus influenzae type b antigens in GSK’s family of vaccines.
In some studies, the immune response to GSK HepB appeared to be lower in preterm and low birth weight infants than in term infants, whereas other studies indicated no difference in the response rate. The ability of preterm and low birth weight infants to respond to HBV vaccination remains an unresolved issue [Citation87], and the contribution of confounding factors including gestational age, birth weight and underlying comorbidities on immunogenicity remains an area of investigation [Citation89–Citation91].
The immune response to GSK HepB among children and adolescents with immunocompromising conditions including cancer, HIV, and Down Syndrome was lower than in the healthy population. Many of the studies conducted in these populations explored alternative schedules and doses in order to try and improve immunogenicity. Some studies suggested that the administration of additional doses or higher doses in these settings may improve immunogenicity [Citation74,Citation76]. The immune response was improved in individuals with HIV when the CD4 count at the start of vaccination was high [Citation75], and in individuals with cancer when vaccination was performed during periods of remission or when not undergoing chemotherapy [Citation77]. Further investigation is needed into how to optimize seroprotection in these groups.
GSK HepB was associated with an acceptable clinical safety profile in infants, children, and adolescents, and no safety concerns were raised in the studies we reviewed.
5. Expert commentary
Our systematic literature review summarized 30 years of clinical and post-marketing data reported for GSK HepB. A previous systematic review of the data published in 2001 found that the overall seroprotection rate among 3001 infants and 4945 children and adolescents immunized with GSK HepB in schedules approved in the product information was 95.8% and 98.6%, respectively [Citation92]. Our review builds on these results by describing an additional 15 years of data, as well as results from studies that assessed efficacy and a range of different schedules and vaccine dosages tested in various populations.
Interpretation of the studies we identified may be restricted by diverse study designs that differed in respects such as the timing of antibody testing, and use of different assays to measure anti-HBs, although the availability of a generally accepted correlate of protection overcomes some of these limitations. Few studies were identified that described the efficacy of GSK HepB in children and adolescents and no studies were identified that evaluated vaccine effectiveness of GSK HepB alone. Efficacy studies were not controlled, but used a historical cohort as the nonvaccinated group, or made an estimation of the expected infection rate in a nonvaccinated cohort. These assumptions potentially lower the quality of these studies, and may have led to under or overestimation of efficacy. Long-term, adequately controlled studies of vaccine efficacy are lacking. Overall, few studies were located that were conducted in special populations such as children with HIV or cancer.
The aim of HBV vaccination is to prevent persistent HBV infection, chronic hepatitis, and transmission. Breakthrough infections in immunized subjects have been documented in long-term studies, but clinical disease is rare, most commonly due to mother-to-infant transmission [Citation93,Citation94]. A population-based study showed that occult (HBsAg-negative) HBV infections occur at a lower rate in vaccinated than in unvaccinated individuals [Citation95]. Occult HBV infection has also been identified in vaccinated blood donors [Citation96]. 20-year follow-up of Thai infants immunized with GSK HepB showed that no subjects had developed chronic HBV, but there was evidence of possible breakthrough disease in 23% of participants living in a high exposure setting [Citation97]. No cases of carriage have been reported to date, and breakthrough cases of HBV in vaccinated individuals are considered not to be of clinical or public health importance [Citation14,Citation93].
More than 1 billion doses of recombinant hepatitis B vaccines have been administered since they were first licensed and these vaccines have an acceptable safety profile. Between 1995 and 1997, several cases of multiple sclerosis were diagnosed in students several weeks after HBV vaccination leading to concerns of a causal association. Extensive further investigations undertaken in large epidemiological studies failed to show any association between HBV vaccination and the onset or exacerbation of demyelinating diseases, including multiple sclerosis [Citation98–Citation100].
The introduction of HBV vaccines into infant vaccination schedules has resulted in marked reductions in the incidence of chronic HBV infection due to prevention of vertical and horizontal transmission early in life [Citation7]. Surveillance following 30 years of HBV vaccine use in Taiwan showed a 90% reduction in fulminant hepatitis, chronic liver disease and in mortality due to hepatocellular carcinoma among vaccinated cohorts [Citation101]. The WHO has adopted a strategic goal of halting viral hepatitis as a major public health threat by 2030 [Citation2]. Over the next 15 years WHO aims to reduce chronic HBV infections from 6–10 million to 0.9 million, and to reduce annual deaths resulting from chronic hepatitis (all causes) from 1.4 million to <0.5 million. Strategies to these goals include promoting high-impact interventions and hepatitis services to increase vaccination uptake, prevent vertical HBV transmission, improve blood safety and safe injections, improve access to early diagnosis and treatment of chronic HBV, and obtain sustainable funding for these services. Vaccination using currently available recombinant HBV vaccines will continue to play a critical role in achieving these goals.
6. Five-year view
Recombinant HBV vaccines are highly immunogenic in young infants. The currently available data support existing recommendations for the early administration of HBV vaccines to all children in all countries; a position confirmed in 2016 by a Cochrane analysis of HBV vaccine schedules [Citation87]. Infant HBV immunization will remain the cornerstone of global HBV control for the foreseeable future. Recombinant vaccines like GSK HepB will continue to be used in healthy and at-risk pediatric populations. Regular systematic reviews of the published data will be needed to bring together and integrate new data as it becomes available, with the aim of monitoring the benefits and risks of vaccination in specific population groups.
Key issues
GSK HepB was the first recombinant HBV vaccine to be licensed and has contributed to global HBV control for 30 years.
A systematic review of the literature identified 64 articles that reported on the efficacy, immunogenicity and/or safety of GSK HepB in children and adolescents.
Vaccine efficacy in at-risk infants of HBsAg-positive mothers was at least 97.4% when GSK HepB was administered in a 0, 1 and 6 month schedule with HBIG at birth.
At least 96% of healthy infants achieve anti-HBs antibody concentrations ≥10 mIU/ml after a 3-dose course administered at 0, 1 and 6 months or in schedules that allow co-administration with other recommended antigens. At-risk children born to HBsAg positive mothers show similar seroprotection rates compared with the general population.
Seroprotection rates were lower in children with HIV and cancer. Mixed results were observed in preterm and low birth weight infants.
GSK HepB has an acceptable clinical safety profile. No safety concerns were raised among healthy, at risk or immunosuppressed populations in the studies we reviewed.
Regular systematic reviews of published data are needed for the ongoing assessment of the benefit-risk ratio of vaccination in specific populations.
Declaration of interest
E.M Bunge and C.VD Ende and A.V Ahee are employees of Pallas, a commercial entity that has received grants from the GSK Group of Companies and which carried out part of the submitted work as a supplier to GSK Vaccines. C Marano and L. D Moerlooze are employees of the GSK group of companies and hold stock options/restricted shares from the sponsoring company. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed
Trademarks
Engerix is a trademark of the GSK groups of companies.
Supplementary_material.docx
Download MS Word (82.3 KB)Supplemental data
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- World Health Organization. Hepatitis B vaccines: WHO position paper–recommendations. Vaccine. 2010;28:589–590.
- World Health Organization. Global health sector strategy on Viral hepatitis 2016–2021. Towards ending viral hepatitis. WHO/HIV/2016.06.
- Hyams KC. Risks of chronicity following acute hepatitis B virus infection: a review. Clin Infect Dis. 1995;20:992–1000.
- Expanded programme on immunization. Global Advisory Group–part I. Wkly Epidemiol Rec. 1992;67:11–15. Epub 1992/01/17.
- World Health Organization. vaccine-preventable disease monitoring system, 2016 global summary.
- World Health Organization. Immunization coverage. Fact sheet Updated September 2016.
- Zanetti AR, Van Damme P, Shouval D. The global impact of vaccination against hepatitis B: a historical overview. Vaccine. 2008;26:6266–6273.
- Ng KP, Saw TL, Baki A, et al. Impact of the expanded program of immunization against hepatitis B infection in school children in Malaysia. Med Microbiol Immunol. 2005;194:163–168.
- Chien YC, Jan CF, Kuo HS, et al. Nationwide hepatitis B vaccination program in Taiwan: effectiveness in the 20 years after it was launched. Epidemiol Rev. 2006;28:126–135.
- Safary A, Beck J. Vaccination against hepatitis B: current challenges for Asian countries and future directions. J Gastroenterol Hepatol. 2000;15:396–401.
- Stevens CE, Taylor PE, Tong MJ, et al. Yeast-recombinant hepatitis B vaccine. Efficacy with hepatitis B immune globulin in prevention of perinatal hepatitis B virus transmission. JAMA. 1987;257:2612–2616.
- Poovorawan Y, Sanpavat S, Pongpunlert W, et al. Protective efficacy of a recombinant DNA hepatitis B vaccine in neonates of HBe antigen-positive mothers. JAMA. 1989;261:3278–3281.
- Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17:1055–1065.
- Van Damme P, Van Herck K. A review of the long-term protection after hepatitis A and B vaccination. Travel Med Infect Dis. 2007;5:79–84.
- Poovorawan Y, Chongsrisawat V, Theamboonlers A, et al. Persistence of antibodies and immune memory to hepatitis B vaccine 20 years after infant vaccination in Thailand. Vaccine. 2010;28:730–736.
- Are booster immunisations needed for lifelong hepatitis B immunity? European Consensus Group on Hepatitis B Immunity. Lancet. 2000;355:561–565.
- ECDC. European Centre for disease prevention and control [updated 2015 Mar 01]. Available from: http://www.ecdc.europa.eu/en/publications/surveillance_reports/Pages/index.aspx.
- World Health Organization. [cited 2016 Mar 21]. Available from: http://www.who.int/en/
- Centers for Disease Control and Prevention. [cited 2016Mar 21]. Available from: https://www.cdc.gov/
- Eurosurveillance 1996-2016. [cited 2016 Mar 21]. Available from: http://www.eurosurveillance.org/
- EUROHEP.NET. [cited 2016 Mar 21]. Available from: Hhttp://www.eurohep.net/
- Jack AD, Hall AJ, Maine N, et al. What level of hepatitis B antibody is protective? J Infect Dis. 1999;179:489–492.
- Canho R, Grosheide PM, Mazel JA, et al. Ten-year neonatal hepatitis B vaccination program, The Netherlands, 1982-1992: protective efficacy and long-term immunogenicity. Vaccine. 1997;15:1624–1630.
- Lee CY, Huang LM, Chang MH, et al. The protective efficacy of recombinant hepatitis B vaccine in newborn infants of hepatitis B e antigen-positive-hepatitis B surface antigen carrier mothers. Pediatr Infect Dis J. 1991;10:299–303. Epub 1991/04/01.
- Lee PI, Lee CY, Huang LM, et al. Long-term efficacy of recombinant hepatitis B vaccine and risk of natural infection in infants born to mothers with hepatitis B e antigen. J Pediatr. 1995;126:716–721. Epub 1995/05/01.
- Poovorawan Y, Sanpavat S, Pongpunglert W, et al. Long term efficacy of hepatitis B vaccine in infants born to hepatitis B e antigen-positive mothers. Pediatr Infect Dis J. 1992;11:816–821.
- Jaber L, Merlob P, Samra Z. Response to recombinant yeast‐derived hepatitis B vaccine in Arab infants. Int J Risk Saf Med. 1999;12:193–196.
- Cheang HK, Wong HT, Ho SC, et al. Immune response in infants after universal hepatitis B vaccination: a community-based study in Malaysia. Singapore Med J. 2013;54:224–226. Epub 2013/04/30.
- Tregnaghi MW, Voelker R, Santos-Lima E, et al. Immunogenicity and safety of a novel yeast Hansenula polymorpha-derived recombinant Hepatitis B candidate vaccine in healthy adolescents and adults aged 10-45 years. Vaccine. 2010;28:3595–3601. Epub 2010/03/02.
- Martins RM, Bensabath G, Arraes LC, et al. Multicenter study on the immunogenicity and safety of two recombinant vaccines against hepatitis B. Mem Inst Oswaldo Cruz. 2004;99:865–871. Epub 2005/03/12.
- Fagundes GD, Tabalipa Fde O, Silva J. Antibody levels in children after 10 years of vaccination against hepatitis B: a Brazilian community-based study. Rev Soc Bras Med Trop. 2012;45:260–262. Epub 2012/04/27.
- Gilca V, De Serres G, Boulianne N, et al. Antibody persistence and the effect of a booster dose given 5, 10 or 15 years after vaccinating preadolescents with a recombinant hepatitis B vaccine. Vaccine. 2013;31:448–451. Epub 2012/12/05.
- Duval B, Gilca V, Boulianne N, et al. Comparative long term immunogenicity of two recombinant hepatitis B vaccines and the effect of a booster dose given after five years in a low endemicity country. Pediatr Infect Dis J. 2005;24:213–218. Epub 2005/03/08.
- Goldfarb J, Baley J, Medendorp SV, et al. Comparative study of the immunogenicity and safety of two dosing schedules of Engerix-B hepatitis B vaccine in neonates. Pediatr Infect Dis J. 1994;13:18–22. Epub 1994/01/01.
- Heron L, Selnikova O, Moiseieva A, et al. Immunogenicity, reactogenicity and safety of two-dose versus three-dose (standard care) hepatitis B immunisation of healthy adolescents aged 11-15 years: a randomised controlled trial. Vaccine. 2007;25:2817–2822.
- Halsey NA, Moulton LH, O’Donovan JC, et al. Hepatitis B vaccine administered to children and adolescents at yearly intervals. Pediatrics. 1999;103:1243–1247. Epub 1999/06/03.
- Schiff GM, Sherwood JR, Zeldis JB, et al. Comparative study of the immunogenicity and safety of two doses of recombinant hepatitis B vaccine in healthy adolescents. J Adolesc Health. 1995;16:12–17. Epub 1995/01/01.
- Tsega E, Horton J, Nordenfelt E, et al. Antibody levels in Ethiopian children five years after vaccination with two different doses of hepatitis B vaccine: is there a need for booster vaccine? Can J Gastroenterol. 1998;12:57–60. Epub 1998/04/17.
- Dumaidi K, Al-Jawabreh A. Persistence of anti-HBs Among Palestinian medical students after 18-22 years of vaccination: a cross-sectional study. Hepat Mon. 2015;15:e29325. Epub 2016/02/03.
- Chiara F, Bartolucci GB, Cattai M, et al. Hepatitis B vaccination of adolescents: significance of non-protective antibodies. Vaccine. 2013;32:62–68. Epub 2013/11/06.
- Behre U, Bleckmann G, Crasta PD, et al. Long-term anti-HBs antibody persistence and immune memory in children and adolescents who received routine childhood hepatitis B vaccination. Hum Vaccin Immunother. 2012;8:813–818. Epub 2012/04/18.
- Hartal M, Yavnai N, Galor I, et al. Seroprevalence of anti-HBs antibodies at young adulthood, before and after a booster vaccine dose, among medical personnel vaccinated in infancy. Vaccine. 2015;33:4878–4885. Epub 2015/08/02.
- Ayerbe MC, Perez-Rivilla A. Assessment of long-term efficacy of hepatitis B vaccine. Eur J Epidemiol. 2001;17:150–156. Epub 2001/10/16.
- Gabbuti A, Romano L, Blanc P, et al. Long-term immunogenicity of hepatitis B vaccination in a cohort of Italian healthy adolescents. Vaccine. 2007;25:3129–3132. Epub 2007/02/13.
- Luna EJ, Moraes JC, Silveira L, et al. Efficacy and safety of the Brazilian vaccine against hepatitis B in newborns. Rev Saude Publica. 2009;43:1014–1020.
- Dobson S, Scheifele D, Bell A. Assessment of a universal, school-based hepatitis B vaccination program. JAMA. 1995;274:1209–1213. Epub 1995/10/18.
- Ferreira CR, Yoshida CF, Mercadante LA, et al. Immunization against hepatitis B in children from endemic zone: evaluation of the antibody response against the DNA recombinant vaccine (Engerix B-20 MCG). Rev Inst Med Trop Sao Paulo. 1993;35:89–92. Epub 1993/01/01.
- Zanetti AR, Mariano A, Romano L, et al. Long-term immunogenicity of hepatitis B vaccination and policy for booster: an Italian multicentre study. Lancet. 2005;366:1379–1384. Epub 2005/10/18.
- Avdicova M, Crasta PD, Hardt K, et al. Lasting immune memory against hepatitis B following challenge 10-11 years after primary vaccination with either three doses of hexavalent DTPa-HBV-IPV/Hib or monovalent hepatitis B vaccine at 3, 5 and 11-12 months of age. Vaccine. 2015;33:2727–2733. Epub 2014/06/26.
- Leonardi S, Vitaliti G, Garozzo MT, et al. Hepatitis B vaccination failure in children with diabetes mellitus? The debate continues. Hum Vaccin Immunother. 2012;8:448–452. Epub 2012/03/01.
- Vesikari T, Martin JC, Liss CL, et al. Safety and immunogenicity of a modified process hepatitis B vaccine in healthy infants. Pediatr Infect Dis J. 2011;30:e109–113. Epub 2011/05/10.
- Sapru A, Kulkarni PS, Bhave S, et al. Immunogenicity and reactogenicity of two recombinant hepatitis B vaccines in small infants: a randomized, double-blind comparative study. J Trop Pediatr. 2007;53:303–307. Epub 2007/05/05.
- Aristegui J, Muniz J, Perez Legorburu A, et al. Newborn universal immunisation against hepatitis B: immunogenicity and reactogenicity of simultaneous administration of diphtheria/tetanus/pertussis (DTP) and oral polio vaccines with hepatitis B vaccine at 0, 2 and 6 months of age. Vaccine. 1995;13:973–977. Epub 1995/08/01.
- Al-Faleh FZ, Al-Jeffri M, Ramia S, et al. Seroepidemiology of hepatitis B virus infection in Saudi children 8 years after a mass hepatitis B vaccination programme. J Infect. 1999;38:167–170. Epub 1999/07/29.
- Greenberg DP, Vadheim CM, Marcy SM, et al. Safety and immunogenicity of a recombinant hepatitis B vaccine administered to infants at 2, 4 and 6 months of age. The Kaiser-UCLA vaccine study group. Vaccine. 1996;14:811–816. Epub 1996/06/01.
- Da Villa G, Romano L, Sepe A, et al. Impact of hepatitis B vaccination in a highly endemic area of south Italy and long-term duration of anti-HBs antibody in two cohorts of vaccinated individuals. Vaccine. 2007;25:3133–3136. Epub 2007/02/07.
- Milne A, Brawner TA, Dumbill PC, et al. Comparison of the immunogenicity of reduced doses of two recombinant DNA hepatitis B vaccines in New Zealand children. J Med Virol. 1989;27:264–267. Epub 1989/03/01.
- Heron LG, Chant KG, Jalaludin BB. A novel hepatitis B vaccination regimen for adolescents: two doses 12 months apart. Vaccine. 2002;20:3472–3476. Epub 2002/09/26.
- Gupta I, Ratho RK. Immunogenicity and safety of two schedules of Hepatitis B vaccination during pregnancy. J Obstet Gynaecol Res. 2003;29:84–86. Epub 2003/05/21.
- Yazdanpanah B, Safari M, Yazdanpanah S. Persistence of HBV vaccine’s protection and response to hepatitis B booster immunization in 5- to 7-year-old children in the Kohgiloyeh and Boyerahmad province, Iran. Hepat Mon. 2010;10:17–21. Epub 2010/01/01.
- Avazova D, Kurbanov F, Tanaka Y, et al. Hepatitis B virus transmission pattern and vaccination efficiency in Uzbekistan. J Med Virol. 2008;80:217–224. Epub 2007/12/22.
- Blondheim O, Bader D, Abend M, et al. Immunogenicity of hepatitis B vaccine in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1998;79:F206–208. Epub 1999/04/09.
- Freitas da Motta MS, Mussi-Pinhata MM, Jorge SM, et al. Immunogenicity of hepatitis B vaccine in preterm and full term infants vaccinated within the first week of life. Vaccine. 2002;20:1557–1562. Epub 2002/02/23.
- Chirico G, Belloni C, Gasparoni A, et al. Hepatitis B immunization in infants of hepatitis B surface antigen-negative mothers. Pediatrics. 1993;92:717–719. Epub 1993/11/01.
- Belloni C, Pistorio A, Tinelli C, et al. Early immunisation with hepatitis B vaccine: a five-year study. Vaccine. 2000;18:1307–1311. Epub 2000/01/05.
- Golebiowska M, Kardas-Sobantka D, Chlebna-Sokol D, et al. Hepatitis B vaccination in preterm infants. Eur J Pediatr. 1999;158:293–297. Epub 1999/04/17.
- Lau YL, Tam AY, Ng KW, et al. Response of preterm infants to hepatitis B vaccine. J Pediatr. 1992;121:962–965. Epub 1992/12/01.
- Poovorawan Y, Sanpavat S, Chumdermpadetsuk S, et al. Long-term hepatitis B vaccine in infants born to hepatitis B e antigen positive mothers. Arch Dis Child Fetal Neonatal Ed. 1997;77:F47–51. Epub 1997/07/01.
- Fang JW, Lai CL, Chung HT, et al. Female children respond to recombinant hepatitis B vaccine with a higher titre than male. J Trop Pediatr. 1994;40:104–107. Epub 1994/04/01.
- Palmovic D, Crnjakovic-Palmovic J. Vaccination against hepatitis B: results of the analysis of 2000 population members in Croatia. Eur J Epidemiol. 1994;10:541–547. Epub 1994/10/01.
- Vranckx R, Alisjahbana A, Ngantung W, et al. Anti-HBs kinetics after HBV vaccination in neonates. Clin Diagn Virol. 1994;2:343–348. Epub 1994/10/01.
- Mutwa PR, Boer KR, Rusine JB, et al. Hepatitis B virus prevalence and vaccine response in HIV-infected children and adolescents on combination antiretroviral therapy in Kigali, Rwanda. Pediatr Infect Dis J. 2013;32:246–251. Epub 2012/09/15.
- Arrazola MP, De Juanes JR, Ramos JT, et al. Hepatitis B vaccination in infants of mothers infected with human immunodeficiency virus. J Med Virol. 1995;45:339–341. Epub 1995/03/01.
- Flynn PM, Cunningham CK, Rudy B, et al. Hepatitis B vaccination in HIV-infected youth: a randomized trial of three regimens. J Acquir Immune Defic Syndr. 2011;56:325–332.
- Pollack TM, Trang Le TT, Ngo L, et al. Response to hepatitis B vaccination among HIV-infected adults in Vietnam. J Virus Erad. 2016;2:102–106.
- Sodhi JS, Raja W, Zargar SA, et al. The efficacy of accelerated, multiple, double-dose hepatitis B vaccine against hepatitis B virus infection in cancer patients receiving chemotherapy. Indian J Gastroenterol. 2015;34:372–379. Epub 2015/11/05.
- Polychronopoulou-Androulakaki S, Panagiotou JP, Kostaridou S, et al. Immune response of immunocompromised children with malignancies to a recombinant hepatitis B vaccine. Pediatr Hematol Oncol. 1996;13:425–431. Epub 1996/09/01.
- Yetgin S, Tunc B, Koc A, et al. Two booster dose hepatitis B virus vaccination in patients with leukemia. Leuk Res. 2001;25:647–649. Epub 2001/06/09.
- Goyal S, Pai SK, Kelkar R, et al. Hepatitis B vaccination in acute lymphoblastic leukemia. Leuk Res. 1998;22:193–195. Epub 1998/05/21.
- Somjee S, Pai S, Kelkar R, et al. Hepatitis B vaccination in children with acute lymphoblastic leukemia: results of an intensified immunization schedule. Leuk Res. 1999;23:365–367. Epub 1999/05/06.
- Van Damme P, Vranckx R, Meheus A. Immunogenicity of a recombinant DNA hepatitis B vaccine in institutionalized patients with Down’s syndrome. Vaccine. 1990;8(Suppl):S53–55. discussion S60-52. Epub 1990/03/01.
- Van Damme P, Vranckx R, Safary A, et al. Protective efficacy of a recombinant deoxyribonucleic acid hepatitis B vaccine in institutionalized mentally handicapped clients. Am J Med. 1989;87:26s–29s. Epub 1989/09/04.
- Vellinga A, Van Damme P, Weyler JJ, et al. Hepatitis B vaccination in mentally retarded: effectiveness after 11 years. Vaccine. 1999;17:602–606. Epub 1999/03/13.
- Ertekin V, Tosun MS, Selimoglu MA. Is there need for a new hepatitis B vaccine schedule for children with celiac disease? Hepat Mon. 2011;11:634–637. Epub 2011/12/06.
- Chinchai T, Chirathaworn C, Praianantathavorn K, et al. Long-term humoral and cellular immune response to hepatitis B vaccine in high-risk children 18-20 years after neonatal immunization. Viral Immunol. 2009;22:125–130. Epub 2009/03/31.
- De Serres G, Duval B, Boulianne N, et al. Importance of attributable risk in monitoring adverse events after immunization: hepatitis B vaccination in children. Am J Public Health. 2001;91:313–315. Epub 2001/02/24.
- Optimizing the Hepatitis B vaccination schedules. Cochrane targeted update. 2016.
- Engerix B. Summary of product charactertistics. [cited 2017 Feb 19]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Engerix_B_30/WC500011088.pdf
- Losonsky GA, Wasserman SS, Stephens I, et al. Hepatitis B vaccination of premature infants: a reassessment of current recommendations for delayed immunization. Pediatrics. 1999;103:E14.
- Czajka H, Lauterbach R, Pawlik D. Vaccination of preterm infants by polyvalent vaccines: immunogenicity and safety- review of literature. Dev Period Med. 2014;18:360–366.
- Omenaca F, Garcia-Sicilia J, Boceta R, et al. Hepatitis B response of premature infants after primary and booster immunisation with a diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus/haemophilus influenzae type B vaccine. Infect Dis Obstet Gynecol. 2010;2010:802503.
- Coates T, Wilson R, Patrick G, et al. Hepatitis B vaccines: assessment of the seroprotective efficacy of two recombinant DNA vaccines. Clin Ther. 2001;23:392–403.
- Tosti ME, Alfonsi V, Lacorte E, et al. Acute hepatitis B after the implementation of universal vaccination in Italy: results from 22 years of surveillance (1993-2014). Clin Infect Dis. 2016;62:1412–1418.
- Su WJ, Liu CC, Liu DP, et al. Effect of age on the incidence of acute hepatitis B after 25 years of a universal newborn hepatitis B immunization program in Taiwan. J Infect Dis. 2012;205:757–762.
- Hsu HY, Chang MH, Ni YH, et al. Universal infant immunization and occult hepatitis B virus infection in children and adolescents: a population-based study. Hepatology. 2015;61:1183–1191.
- Stramer SL, Wend U, Candotti D, et al. Nucleic acid testing to detect HBV infection in blood donors. New Eng J Med. 2011;364:236–247.
- Poovorawan Y, Chongsrisawat V, Theamboonlers A, et al. Evidence of protection against clinical and chronic hepatitis B infection 20 years after infant vaccination in a high endemicity region. J Viral Hepat. 2011;18:369–375.
- Ascherio A, Zhang SM, Hernán MA, et al. Hepatitis B vaccination and the risk of multiple sclerosis. The New Eng J Med. 2001;344:327–332.
- DeStefano F, Verstraeten T, Jackson LA, et al. Vaccinations and risk of central nervous system demyelinating diseases in adults. Arch Neurol. 2003;60:504–509.
- Ramagopalan SV, Valdar W, Dyment DA, et al. Association of infectious mononucleosis with multiple sclerosis. A population-based study. Neuroepidemiol. 2009;32:257–262.
- Chiang CJ, Yang YW, You SL, et al. Thirty-year outcomes of the national hepatitis B immunization program in Taiwan. JAMA. 2013;310:974–976.
Appendix 1
Search strategy
PubMed Search
#1. Hepatitis B vaccine
Engerix-B [Supplementary Concept] OR Engerix[tiab] OR ((‘hepatitis B’[ti] OR Hep B[ti] OR HBs[ti]) AND (vaccin*[ti] OR immuni*[ti] OR immune[ti]))
#2. Outcomes
effectiv*[tiab] OR efficac*[tiab] OR immunogen*[tiab] OR safe*[tiab] OR adverse event*[tiab] OR Anti-HBs[tiab] OR adverse reaction*[tiab] OR reactogen*[tiab] OR seroprevalence[tiab]
Cochrane database search
#1. Hepatitis B vaccine
Engerix:ti OR ((hepatitis B:ti OR Hep B:ti OR HBs:ti) AND (vaccin*:ti OR immun*:ti))
#2. Outcomes
effectiv*:ti,ab OR efficac*:ti,ab OR immunogen*:ti,ab OR safe*:ti,ab OR adverse event*:ti,ab OR Anti-HBs:ti,ab OR adverse reaction*:ti,ab OR reactogen*:ti,ab OR seroprevalence:ti,ab