ABSTRACT
Introduction: Tick-borne encephalitis (TBE), which is endemic across large regions of Europe and Asia, is most effectively prevented through vaccination. Three-dose primary TBE vaccination schedules are either rapid (0,7,21-days) or conventional (0,28–84-days, 9–12-months). The second dose can also be administered at 14 days for faster priming and sero-protection).
Areas covered: We used a three-step selection process to identify 21 publications comparing the immunogenicity and/or safety of different schedules.
Expert commentary: Priming with two or three TBE vaccine doses was highly immunogenic. After conventional priming (0–28 days), 95% adults and ≥95% children had neutralization test (NT) titers ≥10 at 14 days post-dose-2 compared with 92% adults and 99% children at 21 days post-dose-3 (rapid schedule). Most subjects retained NT titers ≥10 at day 300. A single booster dose induced a strong immune response in all subjects irrespective of primary vaccination schedule or elapsed time since priming. GMT peaked at 42 days post-dose-1 (i.e., 21 days post-dose 3 [rapid-schedule], or 14–28 days post-dose-2 [conventional-schedule]), and declined thereafter. Adverse events were generally rare and declined with increasing doses. In the absence of data to recommend one particular schedule, the regimen choice will remain at the physician’s discretion, based on patient constraints and availability.
1. Introduction
Tick-borne encephalitis (TBE), caused by infection with one of the three described TBE virus (TBEV) subtypes (European, Siberian or Far Eastern), is predominantly transmitted through the bite of an infected Ixodes sp tick, for example Ixodes ricinus [Citation1]. The majority of infected individuals will remain asymptomatic and some will suffer a short period of flu-like symptoms before full recovery [Citation2]. However, up to 10% will progress to central nervous system involvement including meningitis, encephalitis, or myelitis [Citation1–Citation3]. The clinical outcome is dependent on several host factors, including patient’s age (severity increases with advancing years), immune status, genetic predisposition, for example chemokine receptor 5 [CCR5] expression, and CNS response, as well as the infecting TBEV subtype (case fatality is higher after infection with the Far Eastern subtype) [Citation1,Citation4,Citation5]
TBE is endemic across Europe and Asia ( [Citation6]), and the associated infections occur most commonly in the summer when ticks are most active [Citation1]. Although TBE reporting varies geographically, and fluctuates annually [Citation7], it is apparent that the incidence of the disease has increased over the past thirty years. Reasons for this increase remain unclear but climatic changes, and increased surveillance and notification are contributing factors [Citation1,Citation7,Citation8].
Figure 1. Global distribution of tick-borne encephalitis. Dotted line defines the border for the tick-borne encephalitis endemic area. Ixodes distribution in China is uncertain. Data for this figure were derived from of reference [Citation6] and reprinted from The Lancet, Vol 371/Issue 9627, Lars Lindquist and Olli Vapalahti, Tick-borne encephalitis /p1861–1871, with permission from Elsevier.
![Figure 1. Global distribution of tick-borne encephalitis. Dotted line defines the border for the tick-borne encephalitis endemic area. Ixodes distribution in China is uncertain. Data for this figure were derived from Figure 2 of reference [Citation6] and reprinted from The Lancet, Vol 371/Issue 9627, Lars Lindquist and Olli Vapalahti, Tick-borne encephalitis /p1861–1871, with permission from Elsevier.](/cms/asset/eb04802f-f554-435c-94de-03b6508ae28f/ierv_a_1358620_f0001_oc.jpg)
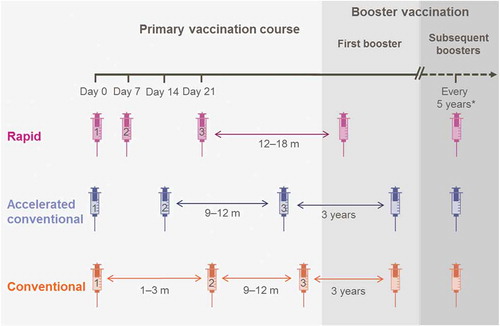
The incidence of TBE in Europe, for which reporting is mandatory in most regions, is generally stable, but tends to be higher in Eastern countries (5.3–22.0 per 100,000 population; see supplementary materials for incidence review data) [Citation7,Citation9–Citation21], in older individuals aged between 40 and 70 years [Citation7,Citation17] and slightly higher in men than women [Citation10,Citation17]. TBE has traditionally represented an underestimated health risk in most countries, and given the lack of specific treatment, the World Health Organization (WHO) has stated that vaccines are the only effective method to prevent infection [Citation22]. European TBE vaccines, based on the Neudörfl or K23 strains, although undergoing modifications in formulation, have been available for many years. Encepur vaccine (GSK Belgium), referred as GSK TBEvac throughout, which is based on the TBEV K23 strain, was originally formulated with a polygeline stabilizer. However, post-marketing surveillance suggested that the vaccine could, albeit rarely, reinforce preexisting allergic reactions or induce allergic reactions in predisposed individuals [Citation23], therefore, since 2001, the vaccine has been polygeline free [Citation24]. The reformulated vaccine is indicated for active immunization against TBEV in adolescents and adults ≥12 years (GSK TBEvac Adults; 1.5 µg in 0.5 mL dose) or in children aged 1–11 years (GSK TBEvac Children; 0.75 µg in 0.25 mL dose). The primary vaccination course in both adults and children consists of three doses, which can be administered according to three different immunization schedules (). With the conventional schedule, the dose intervals between doses 1 and 2 are 1–3 months, and then 9–12 months between doses 2 and 3 (for example 0, 28, and 300 days). Alternatively, the dose interval between doses 1 and 2 can be reduced to 14 days, then 9–12 months between doses 2 and 3 (0, 14, and 300 days). The first booster dose after the conventional schedules is administered after 3 years. Finally the rapid immunization schedule, which is used for individuals who require a 3-dose immunization schedule at short notice, comprises vaccine dosing at 0, 7 and, 21 days, followed by the first booster dose after 12–18 months. Subsequent boosters for conventional and rapid schedules are administered at 5 year intervals.
In order to systematically report and consolidate the existing evidence on the efficacy, immunogenicity, effectiveness, and safety of the vaccine administered according to the different schedules, we undertook this systematic literature review, which to our knowledge is the first of its kind.
2. Methods
We were interested in publications reporting the efficacy, immunogenicity, effectiveness, and safety of three different administration schedules of GSK TBEvac in adults and children and therefore collected, appraised, and summarized the best available evidence using international standards, such as the The Cochrane Collaboration guidelines for performing systematic reviews and preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [Citation25,Citation26]. We were only interested in publications on GSK TBEvac, as this is the only vaccine for which a rapid schedule (0, 7, 21 days) is described and the focus of this review. However, when we encountered publications that are comparative studies of GSK TBEvac versus other vaccines, namely the Pfizer vaccine, we decided to not discard these data and included it for completeness and transparency. For more detailed information, please see the supplementary materials.
2.1. Information sources and search strategy
We used the search terms shown in to three electronic databases: PubMed, Embase, and the Cochrane Library. Additional publications were identified using other non-indexed citations (gray literature) as well as searching the following websites: World Health Organization (WHO; http://www.who.int); European Centre for Disease Protection and Control (ECDC; http://www.ecdc.europa.eu), and Eurosurveillance (http://www.eurosurveillance.org). Publications were limited to studies undertaken in Europe, by publication date (January 2000 [one year before the polygeline-free formulation was launched] until present) and English language
Table 1. Search terms.
2.2. Selection
We used a three-step selection procedure to identify relevant publications. First, we screened titles and abstracts for relevant terms and clinical study designs, and obtained publications of interest for full-text assessment. At this stage, we excluded publications describing vaccines other than GSK TBEvac; animal, genetic, biochemistry or molecular studies; laboratory methods; economic evaluations; Phase I/II trials; vaccine coverage or boosting in subjects who received another priming vaccine.
The second selection step was full-text screening, when we determined whether the paper assessed our objective and then undertook a critical appraisal. We excluded narrative reviews and publications with incomprehensible methodology at this stage. In our final selection stage, which was undertaken during data extraction, we removed duplicate publications and checked the reference lists of meta-analysis or good-quality systematic reviews to identify any missed relevant articles.
2.3. Study quality assessment
We used the Scottish Intercollegiate Guidelines Network (SIGN) checklists [Citation27], to critically appraise methodological quality wherever possible to avoid selecting badly reported or poor quality articles (Appendix S1). We qualitatively scored responses to questions on predefined aspects of a study to provide an overall indication of study quality. This ranged from ‘high’ [++; most criteria met (little or no risk of bias/the results are unlikely to change with further research)], through ‘acceptable’ [+; most criteria met (some flaws in study/associated risk of bias/conclusions may change with further study)] to ‘low’ (0; most criteria not met/significant flaws in study design).
The final decision on whether to include or exclude a study was made by an experienced epidemiologist alone, or in consultation with a second expert.
2.4. Data extraction/collection/quality control
We created evidence tables to capture relevant participants, interventions, comparisons, outcomes and study design (PICOS), study characteristics from the outcome trials according to the vaccine primary administration schedule in adults and children. Two independent researchers screened the first third of titles and abstracts in duplicate and compared their results before a single researcher completed the first assessment step. The full-text review, which was performed by a single researcher, and subsequent data extraction by multiple researchers, were fully validated by a senior reviewer.
3. Results
3.1. Identification of relevant publications
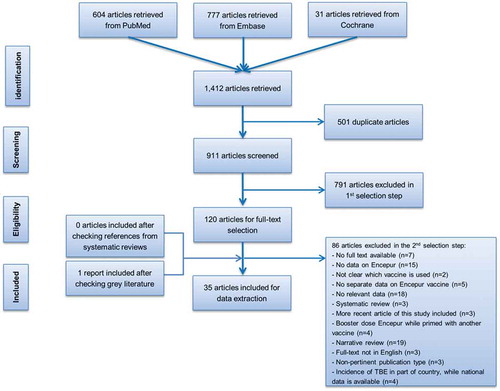
We initially selected 1,412 publications from our database searches and after duplicates had been removed we screened the remaining 911 articles (). We identified 120 articles for full-text assessment and using our predefined exclusion criteria 86 articles were further excluded. An additional report from the gray literature was included at this stage. The 35 reviewed articles included 21 reporting the immunogenicity and/or safety of GSK TBEvac [Citation23,Citation24,Citation28–Citation46] and 14 describing the incidence of TBE in Europe [Citation7,Citation9–Citation21]. No articles describing the efficacy or effectiveness in either adults or children were identified, but indirect evidence of protection is provided by immunogenicity results [usually neutralization test (NT) titers ≥10] [Citation22].
3.2. Immunogenicity following primary vaccination in adults
It should be noted that the identified publications used diverse analytical techniques and different definitions or cut-off values to measure seroprotection which have been specified in the text. In the selected studies, children were aged from 1 to 11 years and adults ≥12 years. These age ranges reflect the indications in adults and children, respectively, for GSK TBEvac.
3.2.1. Three-dose rapid administration schedule
Two publications described immunogenicity after the full three-dose primary vaccination course administered according to the rapid schedule (). Zent et al. 2003 described the results of three clinical trials involving 15 centers in three countries in a single publication [Citation28]. In the per-protocol study population, geometric mean titers (GMT) measured using reciprocal values 21 days after completion of the 0-, 7-, 21-day rapid schedule had reached 68 [95% confidence intervals (CI) 58–79] and 51 [95% CI 43–61] in 90 adolescents (exact age not specified) and 110 adults, respectively. In another study of 66 adults mean age 32.8 years, GMT (measured using NT) reached 44 at 21 days after completion of the rapid schedule [Citation31]. By 21 days after completion of the rapid schedule, 92% subjects reached NT TBE ≥10 and by 300 days, 74% subjects retained NT TBE ≥10 [Citation31].
Table 2. Immunological responses in adults following primary vaccination with GSK TBEvac delivered according to different administration schedules.
3.2.2. Three-dose conventional schedules
A single study compared the immunogenicity of three different conventional schedules, which differed in the timing of the second vaccine dose: conventional at 0, 28, and 300 days (N = 66; mean age 32.5 years); accelerated at 0, 14, and 300 days (N = 133; mean age 31.9 years); modified at 0, 21, and 300 days (N = 133; mean age 32.8 years) () [Citation31]. At day 321, i.e., 21 days after the last vaccine dose, GMTs (NT) increased by approximately 40-fold compared with pre-dose 3, and there were no differences in GMTs between the three groups (values not shown) [Citation31].
3.2.3. Two-dose data
Three trials measured immunogenicity after two doses (separated by different dosing intervals of the conventional 3-dose schedules () [Citation31–Citation33]. In one trial, two doses of polygeline-containing vaccine separated by 20–35 days were administered to 977 subjects as part of a large multicenter comparison with the FSME-IMMUN TBE vaccine (Pfizer TBE vaccine; Pfizer, based on the Neudörfl virus strain) [Citation32]. At 6-months post-dose 2, GMT measured using NT was 35.5 after GSK TBEvac and 22.9 after Pfizer TBE vaccine [Citation32]. Jilkova et al. compared two doses of both vaccines in a retrospective observational study in individuals aged 60 years and above [Citation33]. At 4–8 weeks after the second dose of GSK TBEvac, administered in routine clinical practice at 28–84 days after the first dose, results using ELISA developed with the Neudörfl strain (used in Pfizer TBE vaccine) showed that 18% of all subjects had antibody levels below the threshold of protection, and that mean antibody levels and/or seropositivity rates for Pfizer TBE vaccine were significantly higher than for GSK TBEvac. However, when the ELISA developed with the K23 strain (used in GSK TBEvac) was used, there were no significant differences between the vaccines. The authors concluded that it is important to routinely confirm protective TBE antibody levels after the second vaccine dose in elderly vaccinees [Citation33].
In the trial comparing three different conventional schedules 0 and 14, 21 or 28 and 300 days, on Day 42, 79, 82, and 95% of subjects had NT titers ≥10 at 28, 21 and 14 days after their second vaccine dose, respectively. At Day 300, just before the third vaccine dose, 59, 60, and 71% of subjects retained NT titers ≥10 following the 0–14, 0–21, and 0–28 schedules, respectively [Citation31].
3.3. Immunogenicity following booster vaccination in adults
3.3.1. After priming with the rapid administration schedule
GSK TBEvac was administered as a booster dose to 222 adults who had been primed with either the old or the polygeline-free formulation, 12–18 months previously () [Citation29,Citation30]. Before priming, 186 subjects had been seronegative (NT<2) and 19 had been seropositive (NT≥2). At the time the booster was administered, 99% originally seronegative and all originally seropositive subjects had NT≥2. All subjects showed a strong immune response to the booster [Citation29,Citation34,Citation35].
Table 3. Immunological responses in adults following booster vaccination with GSK TBEvac (primary vaccination delivered according to different administration schedules).
A follow-up study of 323 subjects (≥15 years), who had received one of four different primary schedules [Citation31] and were first boosted 5 years previously, carried out serological testing. Forty-nine subjects had received primary immunization according to the rapid schedule and their first booster dose of GSK TBEvac either at 12–18 months post-primary, as recommended (N = 40), or at approximately 3-years post-primary (N = 9). Approximately 94% of subjects who had received their booster at 12–18 months recorded NT ≥10 from day 0 of the follow-up study until year 5. All subjects who received booster vaccination at approximately 3-years post-primary recorded NT ≥10 from day 21 until 5-years post-booster [Citation36].
A single booster dose of the adult formulation of GSK TBEvac was given to 178 subjects who had received primary TBE vaccination using the rapid schedule 2–11 years previously; these subjects had not received a booster at 12–18 months [Citation37]. A total of 162 subjects were included in the immunogenicity analysis, of whom 96% had NT titers ≥10 pre-booster; all were seropositive at three weeks post-booster. However, when the subjects were stratified according to the time since primary immunization, the fold increases in GMT were higher when the time elapsing was shorter (17-fold after 2–3 years; 12-fold after 4–5 years and 9.9-fold after ≥6 years). Notably, GMT increases were similar in adults below or ≥50 years of age [Citation37].
A second booster dose with the polygeline-free formulation was given to 148 subjects (mean age 36 years) who had received the old vaccine at 0, 7, 21 days and as a first booster at month 15 [Citation38]. All subjects were seropositive before the second booster, but GMT responses were more pronounced in younger subjects [increasing from 149 (95% CI 126–173 U/mL) to 419 (95% CI 370–464 U/mL) in subjects 18–49 years and from 71 (95% CI 52–97 U/mL) to 286 (95% CI 233–352 U/mL) in subjects older than 50 years] [Citation38].
3.3.2. After priming with the conventional schedule
Subjects (≥15 years) who had received primary immunization with GSK TBEvac according to the conventional (0, 28, and 300 days, N = 55), accelerated conventional (0, 14, and 300 days, N = 109) or modified conventional (0, 21, and 300 days, N = 110) schedules received a first booster dose of the GSK TBE vaccine at approximately 3-years post-primary schedule [Citation36]. Before the booster, ≥97% subjects in each group had NT titers ≥10. From day 21 post-booster until 5 years post-booster, ≥95% subjects in each group had NT titers ≥10. The GMTs (measured using NT) were similar in all three groups at all time-points (overlapping CIs) [Citation36].
3.4. Immunogenicity following primary vaccination in children
3.4.1. Three-dose rapid administration schedule
Two studies described the three-dose rapid administration schedule in children () [Citation39,Citation40]. In the first, 21 days post-dose 3, 99% of the 82 children (mean age 6.1 years) had NT titers ≥10 [Citation39]. In the second study involving 404 children (mean age 5.3 years), at 21 days after the third primary vaccine dose, all 390 subjects in the immunogenicity population, who had been seronegative before priming had seroconverted, and all nine who were seropositive before priming had an ≥fourfold increase in TBE antibodies [Citation40]. In 312 children who received new GSK TBEvac, the GMT was 59 (53–66) [Citation40].
Table 4. Immunological responses in children following primary and booster vaccination with GSK TBEvac delivered according to different administration schedules.
By five years after primary dosing, all returning subjects retained NT titers ≥10 [Citation41].
3.4.2. Three-dose conventional schedules
A study from Hungary compared two types of three-dose conventional schedules with the rapid administration schedule () [Citation39]. Three weeks post-dose 3 delivered according to conventional (0–28–300 days) or modified conventional (0–42–300 days) dosing, all subjects (mean age 6.8 years) had NT titers ≥10 [Citation39]. Similar results were seen in a multicenter study from Germany where 3 weeks after the last priming dose administered by conventional (0–28–300 days) or accelerated conventional (0–14–300 days), all children (mean age 4.8 years) had NT titers ≥10 [Citation42].
Five years post-primary vaccination, 94–98% of all children retained NT titers ≥10, suggesting that an appropriate time interval for first boosting after a conventional/accelerated conventional schedule is 5 years [Citation43].
3.4.3. Two-dose data
Following two vaccine doses administered according to the conventional primary schedule (0–28 days), higher, more sustained antibodies were recorded than in children who had received two GSK TBEvac doses according to the accelerated conventional schedule (0–14 days) [Citation42,Citation44]. In a second study, 91% subjects (aged 1–11 years) had NT titers ≥10 five months after two primary vaccination doses of GSK TBEvac administered at 0 and 28 days [Citation45,Citation46].
In a study which compared titers after two doses of GSK TBEvac administered by conventional or modified conventional schedules, similar titer kinetics were observed from day 42 (14 and 21 days post-dose 2, respectively) [Citation39]. The peak at day 42 was followed by a decline to day 180 and a more gradual decline to day 300. However, the magnitude of the responses was different amongst the groups: by day 42, subjects who received the conventional schedule (0 and 28 days) had significantly higher GMTs (NT) than after the modified conventional schedules (0 and 21 days). By day 300, titers were similar in the conventional (15 U/mL [95% CI: 12–19]; p = 0.015) and modified groups (14 U/mL [95% CI: 12–16]; p < 0.001) [Citation39].
3.5. Immunogenicity following booster vaccination in children
3.5.1. After priming with the rapid schedule
A booster dose of GSK TBEvac children was administered 12–18 months post-rapid primary vaccination in 335 subjects, of whom 99% maintained NT titers ≥10 before boosting. GMTs increased rapidly post-booster to day 21, steadily declined to year 3 but then plateaued until year 5. Nevertheless, all 190 subjects who were analyzed at year 5 had NT titers ≥10 [Citation41].
3.5.2. After priming with the conventional schedule
We identified no studies meeting our review criteria which described booster dosing after primary vaccination administered according to a conventional schedule.
3.6. Safety in adults
Pooled safety data from the clinical trial program in 2,239 adults aged 12–76 years, who received at least one dose of GSK TBEvac according to the rapid primary immunization schedule showed that AE decreased with increasing number of vaccine doses () [Citation28].
Table 5. Incidence of adverse events in adults for four days after each primary and booster vaccine dose of GSK TBEvac.
Two doses of GSK TBEvac separated by 20–35 days were administered to 977 subjects as part of a large multicenter comparison with the Pfizer TBE vaccine [Citation32]. Local adverse events were experienced by 44.7 and 38.6% of the subjects after doses 1, and 2, respectively, in the GSK TBEvac group. The percentages in the Pfizer TBE vaccine were 35.6 and 31.7%, respectively). Systemic AE were recorded in 31.0 and 11.3% of GSK TBEvac subjects, and 13.6 and 9.2% Pfizer TBE vaccine subjects, respectively. The most frequent events after GSK TBEvac were headache, fatigue, malaise, muscle pain, and joint pain. Mild fever occurred in 5.6% of the subjects after the first dose of GSK TBEvac compared with 0.4% after the second dose. The percentages in the Pfizer TBE vaccine subjects were 0.8% and 0.5%, respectively [Citation32].
There were no differences in local and systemic reactions between 398 subjects (≥12 years) who received TBE vaccine according to four different primary vaccination schedules [Citation31]. Within three days of a first booster dose of GSK TBEvac, 64% of the subjects recorded at least one solicited AE [Citation31,Citation36]. The rates were higher in nine subjects who had received primary immunization with GSK TBEvac according to the rapid schedule group (78%), compared with the conventional (71%), modified conventional (64%), and accelerated conventional (58%) schedules. Notably, 40 subjects in the ‘rapid group’ had received their booster dose 18–24 months previously and were not monitored for safety. The most frequent local adverse events were pain (55%), erythema (8%), and swelling (6%); myalgia (17%) and headache (14%) were the most frequent systemic AE [Citation31,Citation36]. Other studies have reported similar findings after booster dosing. For example, a first booster dose of GSK TBEvac in 222 adults was well tolerated, with moderate/severe redness and swelling occurring in <1–3% subjects [Citation29,Citation30]. In 178 subjects who had received primary TBE vaccination by the rapid administration schedule between 2 and 11 years previously, most adverse events were mild and occurred at an expected frequency. The most frequent moderate-to-severe events were headache and malaise (both 5%) [Citation37].
GSK TBEvac was administered as a second booster to 148 subjects (mean age 36 years) who had received the old vaccine at 0, 7, 21 days and a first booster at month 15 [Citation38]. The majority of local and systemic reactions were mild and included redness (24%), swelling (11%), itching (5%), tiredness (21%), muscle ache (21%), and headache (10%).
3.7. Safety in children
Pooled safety results from two clinical trials involving 3,559 children who received three doses of GSK TBEvac for children according to the rapid schedule [Citation40], showed that the frequency of solicited events, which ranged between 1% and 32% (local) and 1–14% (systemic), decreased with increasing doses (). The most frequent local adverse event was injection site pain. Headache was the most frequent systemic adverse event in older children (7–12%); sleepiness was most frequent in those aged 1–2 years. Fever >38°C occurred more frequently after the first dose (15% in children aged 1–2 years and 5% in older children) than after subsequent doses (Dose 2: 14% and 4%, respectively; Dose 3: 10% and 3%, respectively) [Citation40].
Table 6. Incidence of adverse events in children for four days after each primary vaccine dose of GSK TBEvac.
In a study where three primary doses were administered to children according to conventional or modified conventional schedules, the most common adverse events after doses 1 and 2 were injection site pain, headache (older children), and sleepiness (children <3 years). There were no vaccine-related serious adverse events (SAEs) [Citation44]. Two doses of the GSK TBE vaccine, administered to 152 children according to the conventional (0, 28 day) schedule, were generally well tolerated. Injection site pain occurred most frequently and no SAEs occurred [Citation45].
A post-marketing surveillance study analyzed suspected adverse drug reactions involving immediate allergic reactions that were reported to the Pharmacovigilance department of Chiron Vaccines (GSK precedessor) from the German market. The analysis compared the incidence in the 2 years following the launch of the new polygeline-free pediatric vaccine with that for the old formulation. A reporting rate of approximately two cases per 100,000 doses sold was calculated for the old formulation compared with 0.08–0.24 cases per 100,000 doses sold with the new vaccine [Citation23].
4. Discussion
TBE is endemic across Europe and Asia, and represents an underestimated health risk [Citation1,Citation4]. In 2010, the highest incidence rates in Europe were recorded in the Eastern states [Citation7,Citation9–Citation21], and in older individuals [Citation7,Citation17]. European TBE vaccines, such as polygeline-free GSK TBEvac provide the only effective way to prevent TBE disease [Citation24]. In Austria the high vaccination coverage (>80%) has driven the sharp reduction in the incidence of TBE. Indeed, in the first 10 years of data collection (from 1972), the pre-vaccination era, the average incidence or TBE was 5.7 cases per 100,000. However, the incidence has fallen dramatically since the introduction of vaccination in the early 1980s, and was 0.9 cases per 100,000 in the 10 years preceding 2011 [Citation7].
This systematic literature review identified 21 publications reporting the immunogenicity and/or safety of GSK TBEvac [Citation23,Citation24,Citation28–Citation46]. We found no articles describing the efficacy or effectiveness of the vaccine, but indirect evidence of protection as provided by immunogenicity results (usually NT titers ≥10) [Citation22] were available for adults and children. In addition, limited field effectiveness studies from Austria and other European locations have been published [Citation7,Citation47].
Primary vaccination with three doses of GSK TBEvac was highly immunogenic in both adults and children, but the immunological responses in children were generally higher. For all schedules, the GMTs peaked at day 42 (21 days post-dose 3 in the rapid group, or 14–28 days post-dose 2 in the conventional-based groups), and then declined to days 180 and 300 (before the third vaccine dose in the conventional series) [Citation28–Citation46]. The percentage of adults with NT titers ≥10 fell from 92% at 21 days to 74% at 279 days post-dose 3 of the rapid schedule [Citation31]. In comparison 99% children retained NT titers ≥10 at these time points [Citation39]. For adults receiving two doses of the conventional schedules, percentages of subjects with NT titers ≥10 tended to be higher after conventional (0–28 day dosing) than modified conventional (0–21 day dosing) and accelerated conventional (0–14 day dosing) both on day 42 (95, 82, 79%, respectively) and day 300 before the third vaccine dose (71, 60, 59%, respectively) [Citation31]. The size of the immunological response post-dose 3 was not reported; it was only generally stated that the response was ‘strong’ [Citation31]. Such data are however available for children – and by day 321 (3 weeks after the third dose of vaccine administered by the conventional schedules), all subjects had NT titers ≥10 [Citation39–Citation44]. Data generated in children are more the limited and it would be interesting to measure the impact of increasing age on responses. A single study showed that GMTs tended to be lower in subjects aged above 50 years compared with those 18–49 of age, but the authors concluded that the findings were not clinically relevant [Citation38]. It is generally accepted that immune response to vaccination decreases with age and the concept of immunosenescence has been extensively studied [Citation48,Citation49].
Boosters are administered either at 12–18 months post rapid priming schedule or at 3 years post conventional priming schedules and then every 5 years thereafter. Long-term persistence data following primary vaccination were not found for adults after any schedule, but were available for up to 5 years after primary vaccination using conventional schedules in children. At this time point, 94–98% children still had NT titers ≥10, demonstrating excellent persistence and leading the authors to conclude that boosting could be extended from 3 to 5 years in this population [Citation42].
Booster data in children are limited to a single study, where a booster administered 12–18 months after priming with the rapid schedule produced multi-fold increases in GMT. All subjects acquired NT titers ≥10 within 21 days of boosting and these were retained until 5 years later [Citation41]. In adults, a single booster dose GSK TBEvac induced a strong immune response in all subjects irrespective of the primary vaccination schedule type or elapsed time between primary and booster dosing [Citation36,Citation37]. Immunological data showed that NT titers ≥10 persist in almost all subjects for over 5 years after boosting, suggesting that the three-year boosting recommendation could be extended.
The reported events after vaccination with GSK TBEvac were generally low and predictable and tended to decrease with increasing doses [Citation28,Citation40]. Local injection site reactions were more frequent than systemic reactions. Headache, muscle ache, fatigue, and malaise were the most frequent systemic events in adults, compared with sleepiness, headache, and changes in eating habits in children; low-grade fever occurred in up to 15% of subjects [Citation28,Citation32,Citation39,Citation40]. Serious adverse events were infrequent and rarely vaccine related.
4.1. Strengths and limitations
The main strength of this systematic review is that it is, to the best of our knowledge, the first such comprehensive review into the immunogenicity and safety of GSK TBEvac. We followed the rigorous methods proposed by PRISMA and Cochrane to identify the relevant studies. However, we were limited by the small number of publications identified, which was compounded when we sought to compare different administration schedules in adults and children. Additionally, we did not identify any controlled efficacy or effectiveness studies that met our prespecified criteria. The publications were typically at least 10 years old and often had unclear methodologies; they required close scrutiny to identify which follow-up studies corresponded to which primary vaccination trials. Such difficulties should be largely avoided with recent studies which have included study identifiers. The described studies used diverse analytical techniques and different definitions or cut-off values to measure seroprotection. We presented NT results as far as possible, as these are the surrogate markers of protection and generally included in the studies. However, in the studies sponsored by other manufacturers, additional ELISA-based methods were used [Citation32,Citation33,Citation44,Citation46].
There are many areas which would merit further research, in particular, the effects of age upon the response to both primary and booster vaccination. It would also be useful to define the exact period for booster dosing, it already appears that a 5 year interval provides excellent protection, but longer persistence studies could determine whether this interval could be extended even further. Real-world effectiveness or impact data would also be useful from a Public Health perspective.
5. Conclusions
A range of primary vaccination and booster schedules are available to help prevent TBE for children, adolescents, and adults. As evidence to support one administration schedule over another is lacking, the choice will remain at the doctor’s discretion, taking into account their personal preferences, traveler constraints, and vaccine availability.
6. Expert commentary
The incidence of TBE, which is endemic across large regions of Europe and Asia, is increasing for a number of reasons including climactic changes and increased surveillance. Additionally, the exposure of individuals to the transmitting Ixodes tick in forested areas, will increase as the current trend for outdoor leisure pursuits continues to grow. Vaccines, based on the Neudörfl or K23 strains, have been available for many years, with the one based on TBEV K23 being indicated for administration via rapid or conventional schedules. In general, residents of endemic regions receive their vaccination by the conventional schedule (intervals of 1–3 months between doses 1 and 2, and then 9–12 months between doses 2 and 3). However, in an emergency situation or for travelers, the rapid immunization schedule, comprising vaccination at 0, 7, and 21 days might be indicated. We conducted a literature analysis, to identify whether there were any major differences in the immunogenicity or safety profiles of the vaccine administered by the different schedules; i.e., that would recommend one regimen over another, when there is an open choice. Priming with two or three TBE vaccine doses was highly immunogenic: Titers peaked 3 weeks after the third rapid dose or 14–28 days after the second ‘conventional’ dose, at which time over 90% of all subjects had NT titers ≥10. The titers gradually fell thereafter, but, irrespective of schedule, most subjects in all groups retained NT titers ≥10 at ten months after the first dose. A single booster dose induced a strong immune response in all subjects irrespective of primary vaccination schedule type or elapsed time since priming. Adverse events were generally rare, predictable in nature and declined with increasing doses. We therefore concluded that there was no particular immunological or safety reason to recommend one particular schedule. Therefore, the choice of vaccination schedule should be made on a patient-by-patient basis by the physician, depending upon individual factors and needs.
7. Five-year view
The incidence of TBE, which has increased over the past 30 years, is likely to continue to grow – as a result of ongoing climactic changes, greater surveillance, and greater exposure to the transmitting ticks. Whilst residents of the endemic areas are probably aware of the need for vaccination, or covered by national vaccination schedules, over the coming years, countries with recommendations will increase to reflect the growing reach of the disease.
Further, due to the recent disease epidemiology changes, it is important to increase the awareness of the risks for travelers to endemic areas, possibility by internet recommendation and advice from travel organizers. Choice of vaccination administration schedule amongst travelers will probably remain dependent upon how soon they plan their trip. The available vaccines against the Western European subtype have already been available for decades and are well established, but countries which are exposed to the Siberian or Far-Eastern Ixodes tick subtypes, for example Russia, may continue to develop vaccines which provide protection against multiple subtypes. Overall, the increased disease incidence should be countered by greater protection through vaccination.
Key issues
TBE is endemic across large regions of Europe and is an underestimated health risk
Vaccination is the only effective method of preventing TBE
3-dose primary vaccination can be administered following rapid (0–7–21-day) or conventional (0, 14 or 28–84 days, 9–12 month) schedules
Immunological responses and safety profiles are comparable among schedules
Schedule selection depends on physician choice, patient constraints and availability
Trademark
Encepur is a trademark of the GSK group of companies. FSME-IMMUN is a trademark of Pfizer.
Declaration of interest
C Marano and L De Moerlooze are employees of GSK group of companies and hold shares and options from the sponsoring company. C Schludermann and I Galgani are employees of GSK group of companies. EM Bunge and L Hendricks have received grants from the GlaxoSmithKline Group of Companies and which carried out part of the submitted work as a supplier to GlaxoSmithKline Vaccines. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Encepur_Lit_Review_SupplementaryMethods_09June2017.docx
Download MS Word (38.8 KB)Acknowledgments
The authors would like to thank Julia Donnelly (freelance for XPE Pharma & Sciences on behalf of GSK Vaccines) for medical writing assistance and Susana Montenegro Gouveia for publication coordination (XPE Pharma & Sciences on behalf of GSK Vaccines).
Supplemental data
The supplemental data for this article can be accessed here
Additional information
Funding
Notes on contributors
Ilaria Galgani
C Marano, EM Bunge, L Hendricks, C Schludermann and L De Moerlooze were involved in the conception or the design of the study. EM Bunge, L Hendricks and C Schludermann participated in the data collection. EM Bunge and L Hendricks also performed the study/project and all authors participated in the analysis and interpretation of the data. All authors had full access to the data, were involved with developing this manuscript, gave final approval before submission and are accountable for all aspects of the work.
Eveline M. Bunge
C Marano, EM Bunge, L Hendricks, C Schludermann and L De Moerlooze were involved in the conception or the design of the study. EM Bunge, L Hendricks and C Schludermann participated in the data collection. EM Bunge and L Hendricks also performed the study/project and all authors participated in the analysis and interpretation of the data. All authors had full access to the data, were involved with developing this manuscript, gave final approval before submission and are accountable for all aspects of the work.
Lisa Hendriks
C Marano, EM Bunge, L Hendricks, C Schludermann and L De Moerlooze were involved in the conception or the design of the study. EM Bunge, L Hendricks and C Schludermann participated in the data collection. EM Bunge and L Hendricks also performed the study/project and all authors participated in the analysis and interpretation of the data. All authors had full access to the data, were involved with developing this manuscript, gave final approval before submission and are accountable for all aspects of the work.
Christopher Schludermann
C Marano, EM Bunge, L Hendricks, C Schludermann and L De Moerlooze were involved in the conception or the design of the study. EM Bunge, L Hendricks and C Schludermann participated in the data collection. EM Bunge and L Hendricks also performed the study/project and all authors participated in the analysis and interpretation of the data. All authors had full access to the data, were involved with developing this manuscript, gave final approval before submission and are accountable for all aspects of the work.
Cinzia Marano
C Marano, EM Bunge, L Hendricks, C Schludermann and L De Moerlooze were involved in the conception or the design of the study. EM Bunge, L Hendricks and C Schludermann participated in the data collection. EM Bunge and L Hendricks also performed the study/project and all authors participated in the analysis and interpretation of the data. All authors had full access to the data, were involved with developing this manuscript, gave final approval before submission and are accountable for all aspects of the work.
Laurence De Moerlooze
C Marano, EM Bunge, L Hendricks, C Schludermann and L De Moerlooze were involved in the conception or the design of the study. EM Bunge, L Hendricks and C Schludermann participated in the data collection. EM Bunge and L Hendricks also performed the study/project and all authors participated in the analysis and interpretation of the data. All authors had full access to the data, were involved with developing this manuscript, gave final approval before submission and are accountable for all aspects of the work.
References
- Fischer M, Rabe IB, Rollin PE. Tickborne encephalitis. In: Brunette GW, editor. Chief, centers for disease control and prevention. Oxford, UK: Oxford University Press;2016. Yellow Book. [cited 2016 Oct 25]. Available from: http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/tickborne-encephalitis
- Han MH, Zunt JR. Neurologic aspects of infections in international travelers. Neurologist. 2005;11:30–44.
- ECDC. Epidemiological situation of tick-borne encephalitis in the European Union and European Free Trade Association countries. [cited 2017 Feb 24]. Available from: http://www.ecdc.europa.eu/en/publications/Publications/TBE-in-EU-EFTA.pdf
- Amicizia D, Domnich A, Panatto D, et al. Epidemiology of tick-borne encephalitis (TBE) in Europe and its prevention by available vaccines. Human Vacc Immunother. 2013;9:1163–1171.
- Grygorczuk S, Osada J, Parczewski M, et al. The expression of the chemokine receptor CCR5 in tick-borne encephalitis. J Neuroinflammation. 2016;13:45.
- Lindquist L, Vapalahti O. Tick borne encephalitis. Lancet. 2008;371:1861–1871.
- Heinz FX, Stiasny K, Holzmann H, et al. Vaccination and Tick-borne encephalitis, Central Europe. Emerg Infect Dis. 2013;19:69–76.
- Süss J. Tick-borne encephalitis 2010: epidemiology, risk areas, and virus strains in Europe and Asia-an overview. Ticks Tick Borne Dis. 2011;2(1):2–15.
- Donoso Mantke O, Escadafal C, Niedrig M, et al. Tick-borne encephalitis in Europe, 2007 to 2009. Euro Surveill. 2011;16:39.
- Daniel M, Kříž B, Danielová V, et al. Sudden increase in tick-borne encephalitis cases in the Czech Republic, 2006. Int J Med Microbiol. 2008;298(SUPPL. 1):81–87.
- Grgic-Vitek M, Klavs I. High burden of tick-borne encephalitis in Slovenia–challenge for vaccination policy. Vaccine. 2011;29:5178–5183.
- Karelis G, Bormane A, Logina I, et al. Tick-borne encephalitis in Latvia 1973–2009: epidemiology, clinical features and sequelae. Eur J Neurol. 2012;19:62–68.
- Kriz B, Maly M, Benes C, et al. Epidemiology of tick-borne encephalitis in the Czech Republic 1970–2008. Vector Borne Zoonotic Dis. 2012;12:994–999.
- Zenz W, Pansi H, Zoehrer B, et al. Tick-borne encephalitis in children in Styria and Slovenia between 1980 and 2003. Pediatr Inf Dis J. 2005;24:892–896.
- European Centre for disease Prevention and Control (ECDC). Epidemiological situation of tick-borne encephalitis in the European Union and European Free Trade Association countries. Stockholm: ECDC; 2012. Available from: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/TBE-in-EU-EFTA.pdf
- Schuler M, Zimmermann H, Altpeter E, et al. Epidemiology of tick-borne encephalitis in Switzerland, 2005 to 2011. Eurosurveillance. 2014;19:13.
- Altpeter E, Zimmermann H, Oberreich J, et al. Tick related diseases in Switzerland, 2008 to 2011. Swiss Med Wkly. 2013;143:w13725.
- Dorko E, Rimarova K, Kizek P, et al. Increasing incidence of tick-borne encephalitis and its importance in the Slovak Republic. Central Eur J Public Health. 2014;22:277–281.
- Rezza G, Farchi F, Pezzotti P, et al. Tick-borne encephalitis in north-east Italy: a 14-year retrospective study, January 2000 to December 2013. Euro Surveill. 2015;20:40.
- Zielinski A, Czarkowski MP, Sadkowska-Todys M. Infectious diseases in Poland in 2011. Przegl Epidemiol. 2013;67(171–9):301–305.
- Zoldi V, Juhasz A, Nagy C, et al. Tick-borne encephalitis and Lyme disease in Hungary: the epidemiological situation between 1998 and 2008. Vector Borne Zoonotic Dis. 2013;13:256–265.
- WHO Position Paper. 2011. Vaccines against tick-borne encephalitis (TBE). [cited 2017 Jan 13]. Available from: http://www.who.int/immunization/TBE_duration_protection.pdf
- Zent O, Hennig R. Post-marketing surveillance of immediate allergic reactions: polygeline-based versus polygeline-free pediatric TBE vaccine. Vaccine. 2004;23:579–584.
- Zent O, Hennig R, Banzhoff A, et al. Protection against tick-borne encephalitis with a new vaccine formulation free of protein-derived stabilizers. J Travel Med. 2005;12:85–93.
- Higgins JPT, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from: http://handbook.cochrane.org
- Moher D, Liberati A, Tetzlaff J, et al. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Scottish Intercollegiate Guidelines Network. Critical appraisal: notes and checklists. [cited 2016 Oct 13]. Available from: http://www.sign.ac.uk/
- Zent O, Beran J, Jilg W, et al. Clinical evaluation of a polygeline-free tick-borne encephalitis vaccine for adolescents and adults. Vaccine. 2003;21:738–741.
- Zent O, Jilg W, Plentz A, et al. Kinetics of the immune response after primary and booster immunization against tick-borne encephalitis (TBE) in adults using the rapid immunization schedule. Vaccine. 2003;21:4655–4660.
- Zent O, Schwarz TF, Plentz A, et al. TBE booster immunization in adults–first experience with a new tick-borne encephalitis (TBE) vaccine, free of protein-derived stabilizer. Int J Med Microbiol. 2004;293(Suppl 37):134–138.
- Schöndorf I, Beran J, Cizkova D, et al. Tick-borne encephalitis (TBE) vaccination: applying the most suitable vaccination schedule. Vaccine. 2007;25:1470–1475.
- Loew-Baselli A, Konior R, Pavlova BG, et al. Safety and immunogenicity of the modified adult tick-borne encephalitis vaccine FSME-IMMUN: results of two large phase 3 clinical studies. Vaccine. 2006;24:5256–5263.
- Jilkova E, Vejvalkova P, Stiborova I, et al. Serological response to tick-borne encephalitis (TBE) vaccination in the elderly–results from an observational study. Exp Opin Biologic Ther. 2009;9:797–803.
- Zent O, Plentz A, Schwarz TF, et al. TBE booster immunization according to the rapid immunization schedule: are 3-year booster intervals really necessary? Vaccine. 2004;23:312–315.
- Plentz A, Jilg W, Schwarz TF, et al. Long-term persistence of tick-borne encephalitis antibodies in adults 5 years after booster vaccination with Encepur Adults. Vaccine. 2009;27:853–856.
- Beran J, Xie F, Zent O. Five year follow-up after a first booster vaccination against tick-borne encephalitis following different primary vaccination schedules demonstrates long-term antibody persistence and safety. Vaccine. 2014;32:4275–4280.
- Schöndorf I, Schonfeld C, Nicolay U, et al. Response to tick-borne encephalitis (TBE) booster vaccination after prolonged time intervals to primary immunization with the rapid schedule. Int J Med Microbiol. 2006;296(Suppl 40):208–212.
- Beran J, Douda P, Gniel D, et al. Long-term immunity after vaccination against tick-borne encephalitis with Encepur using the rapid vaccination schedule. Int J Med Microbiol. 2004;293(Suppl 37):130–133.
- Schoendorf I, Ternak G, Oroszlan G, et al. Tick-born encephalitis (TBE) vaccination in children: advantage of the rapid immunization schedule (i.e., days 0, 7, 21). Hum Vaccine. 2007;3:42–47.
- Zent O, Banzhoff A, Hilbert AK, et al. Safety, immunogenicity and tolerability of a new pediatric tick-borne encephalitis (TBE) vaccine, free of protein-derived stabilizer. Vaccine. 2003;21:3584–3592.
- Wittermann C, Petri E, Zent O. Long-term persistence of tick-borne encephalitis antibodies in children 5 years after first booster vaccination with Encepur Children. Vaccine. 2009;27:1585–1588.
- Wittermann C, Nicolay U, Hilbert AK, et al. Paediatric tick-borne encephalitis (TBE) vaccines: schedules to optimise protection. Int J Med Microbiol. 2008;298(SUPPL. 1):301–304.
- Wittermann C, Izu A, Petri E, et al. Five year follow-up after primary vaccination against tick-borne encephalitis in children. Vaccine. 2015;33:1824–1829.
- Wittermann C, Schöndorf I, Gniel D. Antibody response following administration of two paediatric tick-borne encephalitis vaccines using two different vaccination schedules. Vaccine. 2009;27:1661–1666.
- Pollabauer EM, Pavlova BG, Löw-Baselli A, et al. Comparison of immunogenicity and safety between two paediatric TBE vaccines. Vaccine. 2010;28:4680–4685.
- Prymula R, Pollabauer EM, Pavlova BG, et al. Antibody persistence after two vaccinations with either FSME-IMMUN(R) Junior or ENCEPUR(R) Children followed by third vaccination with FSME-IMMUN(R) Junior. Human Vaccin Immunother. 2012;8:736–742.
- Kollaritsch H, Paulke-Korinek M, Holzmann H, et al. Vaccines and vaccination against tick-borne encephalitis. Expert Rev Vaccines. 2012;11:1103–1119.
- Pinti M, Appay V, Campisi J, et al. Aging of the immune system. Focus on inflammation and vaccination. Eur J Immunol. 2016;46:2286–2301.
- Gnanasekaran G, Biedenbender R, Davidson HE, et al. Vaccinations for the older adult. Clin Geriatr Med. 2016;32:609–625.