ABSTRACT
Introduction: Fifteen million people each year receive post-exposure prophylaxis (PEP) to prevent rabies, yet the disease remains neglected and highly under-reported.
Areas covered: In this systematic literature review, we assessed the immunogenicity, efficacy, and safety of a purified chick embryo cell-culture rabies vaccine (PCECV) for PEP against rabies by intramuscular (IM) or intradermal (ID) administration. We performed meta-analyses to compare immunogenicity according to the route of vaccine administration, study population, and PEP regimen, such as number of doses, and concomitant rabies immunoglobulin.
Expert commentary: There were 54 estimates of immune responses to vaccination, which showed that in the overall population, after starting PEP with PCECV by the IM or ID route (≥2.5 IU per dose), almost all individuals had rabies virus neutralizing antibody (RVNA) titers above the World Health Organization (WHO) recommended serological threshold for an adequate immune response to vaccination (RVNA ≥0.5 IU/ml by day 14). In the overall population, PCECV had an acceptable safety profile. However, given that there are 59,000 human rabies deaths reported annually, the challenge is to improve access to PCECV for PEP against human rabies.
Focus on the Patient Section
What is the context?
Rabies is endemic worldwide and transmitted by the bites and licks from infected mammals. When the rabies virus enters the central nervous system, it causes rabies, which is almost always fatal. Early postexposure prophylaxis by thorough wound cleaning and vaccination prevents the development of the disease.
What is new?
In this recent review of published studies, most showed that vaccinated people had antibody responses above the WHO recommended threshold considered adequate to protect against rabies from 14 days after vaccination, regardless of whether vaccine was given into a muscle (IM) or the skin (ID). On days 7 and 90 after vaccination, immune responses appeared higher for IM than ID vaccination. The clinical significance of the higher immune response is not known. In most studies, no serious adverse events were reported. Local redness and swelling, headache, and swollen lymph nodes seemed more frequent in ID than IM vaccination.
What is the impact?
The challenge to reducing rabies deaths is to ensure that people at risk – such as those in remote rural communities – are able to access rabies vaccines after exposure to infection.
1. Introduction
The rabies virus is endemic in domestic dog populations in most low-income countries, although rabies can infect any mammal [Citation1]. In humans, the viral infection causes encephalitis which is almost always fatal, leading to an estimated 59,000 human deaths and 3.7 million disability-adjusted life years annually [Citation2–Citation4]. Although about 15 million people receive postexposure prophylaxis (PEP) every year after suspected exposure to the rabies virus, the disease remains neglected and highly underreported among poor rural communities with livestock economies [Citation2–Citation5].
Concentrated and purified cell culture and embryonated egg-based rabies vaccines have been administered to millions of people worldwide for PrEP and PEP against rabies. Cell-culture vaccines are propagated from human diploid cells (human diploid cell vaccines), purified Vero cells (purified Vero cell rabies vaccine), embryonated duck eggs (purified duck embryo vaccine [PDEV]), or chick embryo cells (purified chick embryo cell vaccine; PCECV). Cell-culture-based human rabies vaccines are widely reported to be immunogenic and well tolerated, and their administration is part of the PEP protocol recommended by the World Health Organization (WHO) [Citation6].
The rabies PEP protocol involves careful and adapted management of the wound such as thoroughly washing, applying antiseptics, avoiding sutures, and the use of antibiotics, as well as a complete course of human rabies vaccine for category II exposures (nibbling of uncovered skin, minor scratches, or abrasions without bleeding); a complete course of human rabies vaccine plus the concomitant administration of rabies immunoglobulin (RIG) is recommended for category III exposures (transdermal bites or scratches, contamination of mucous membrane with saliva from licks, licks on broken skin, and exposures to bats) [Citation7].
Traditionally, rabies vaccine had been administered by the intramuscular (IM) route; however, intradermal (ID) administration has routinely been used in a number of countries such as Thailand, Sri Lanka, the Philippines, and India. Protocols for ID administration were established to reduce the direct costs of vaccine since the volume of vaccine dose needed by the ID route (0.1 mL) is lower than that needed by the IM route (0.5 or 1.0 mL, depending on the vaccine used) [Citation8–Citation11]. However, although ID administration is recommended by the WHO, the technique requires training and is not approved in all countries [Citation7]. Indeed, ID administration requires highly trained medical staff and medical supervision meaning that it is not appropriate everywhere, that is, adoption of ID administration in local rabies strategies depends upon factors such as the burden of rabies, population distribution, clinical infrastructure, and funding [Citation12].
The WHO recommends that rabies vaccines should have a minimum antigen content of ≥2.5 IU per dose to provide adequate rabies virus neutralizing antibody (RVNA) titers (≥0.5 IU/mL of serum) at 14 days after the first dose of vaccine [Citation6]. This adequate level of antibodies is based on observations that rabies has not been reported among vaccinated individuals with RVNA titers above a threshold of 0.5 IU/mL of serum [Citation1,Citation13]. Dose potency and immune response to vaccination have been assessed in meta-analyses of rabies vaccines with potency ranging from <2.5 IU to ≥5 IU per dose. Results demonstrated that a minimum potency of 2.5 IU per dose was needed to elicit an adequate immune response to vaccination by both the IM and ID routes [Citation7,Citation14].
The purified chick embryo cell-culture rabies vaccine (PCECV; Rabipur, GSK) is used for PEP administered by the IM or ID route [Citation15]. Studies show that most individuals achieve adequate RVNA concentrations of ≥0.5 IU/mL by Day 14 (D14) after the first dose of PCECV [Citation15]. Vaccination regimens for PCECV for PEP are shown in Supplement 1. PCECV by both routes of administration has been extensively studied as part of PEP protocols in rabies-endemic regions in healthy volunteers, people at occupational risk such as those who work with animals, and people exposed to rabies, as well as special populations such as malnourished children and pregnant women. To evaluate PCECV for rabies PEP, we conducted a systematic review of studies reporting the proportion of participants with RVNA ≥0.5 IU/mL, survival rates of rabies-exposed cases who did/did not receive PCECV (efficacy), and PCECV safety. We performed meta-analyses to compare immunogenicity endpoints by route of administration and by the coadministration of RIG.
2. Methods
2.1. Literature search
The databases PubMed and Embase were systematically searched for primary articles and reviews reporting on the immunogenicity, efficacy, or safety of PCECV as part of a PEP protocol. The literature search was conducted according to the PRISMA guideline [Citation16]. The search was restricted to English publications published from 1 January 1980 to 7 January 2016, using a combination of search strings consisting of terms used for the diseases, rabies vaccination, and study outcomes, and terms used for the exclusion of animal studies. The complete list of search strings is presented in Supplement 2. A gray literature search was also conducted (Supplement 2).
2.2. Selection procedure
Relevant references were selected by a three-step selection procedure, involving the screening of the title and abstract, and the full article, and further screening during the data extraction phase (Supplement 3). The first step excluded all articles describing PrEP, those not mentioning the number of cases and how many were administered PEP, non-pertinent publication types (e.g. letters to the editor, comments), case reports, animal studies, genetic studies, biochemistry or molecular studies, or modeling studies not providing original data. The second step determined whether the article answered one of the review questions and if so, the article was selected for critical appraisal. Reasons for exclusion at this stage were those from step 1, and the following: not PCECV, multiple vaccines administered in the study and results not stratified, no quantitative data provided. The third step was performed to exclude articles presenting similar results from identical studies by keeping only the one presenting the more complete results and to check previously identified systematic reviews/meta-analyses for relevant references possibly missed in the search.
Screening was performed by two independent researchers. The first 10% of references in the first step were screened in duplicate, and results were compared and discussed before the remaining ones were assessed. All selected references from the two researchers were included for full text selection. The first 10% of full text articles were also critically appraised in duplicate by two researchers and results were compared and discussed early in the process, with discrepancies or disagreements being resolved by a third researcher. The rest of the references were appraised by only one of the researchers. The process of selection and inclusion/exclusion of articles was registered in the reference library.
The critical appraisal of included articles was performed using methodology checklists from the Scottish Intercollegiate Guidelines Network (SIGN) [Citation17]. All studies were graded on a four-category scale (unacceptable, low quality, acceptable, and high quality). Unacceptable studies were rejected.
2.3. Data extraction
Data extraction was performed by one researcher and reviewed by all the authors. Standardized data extraction tables were used to summarize results by study outcome (immunogenicity, efficacy, safety), route of administration (IM, ID, not recorded [NR]), population (nonexposed, exposed, at risk), and study design (intervention study, observational study). The following immunogenicity-related outcomes were included: the RVNA response in geometric mean titers (GMTs) and the percentage of vaccinated individuals with adequate RVNA titers (≥0.5 international units [IU]/mL) at D14 and other intervals following vaccination. Vaccine efficacy, assessed by survival following rabies exposure and vaccination and safety outcomes (adverse events [AEs]), were also reviewed.
Outcomes were presented by population type: in healthy volunteers (no prior exposure to rabies), bite cases (individuals exposed to rabies prior to vaccination), and people at risk of rabies (individuals at higher risk of rabies exposure due to the nature of their occupation or from other reasons, like animal handlers, veterinary doctors, and health-care workers in contact with rabies-exposed patients). When available, data on special populations (e.g. immunocompromised or HIV-infected individuals, pregnant women, and children) were also summarized.
2.4. Data analysis
The possibility of performing meta-analysis was evaluated for each outcome measure. If a meta-analysis was not possible, results were summarized in summary tables or were described narratively.
For meta-analysis, the following variables were considered relevant: route of administration, population, regimen (including number of injections), dose potency (<2.5 IU/mL [low], ≥2.5-<5 IU/mL [normal], and ≥5 IU/mL [high], administration of rabies immunoglobulin (RIG; human [HRIG], equine [ERIG], or none), age group, testing days, the type of assay used to measure RVNA, and SIGN score.
The percentages of vaccinated individuals with RVNA titers ≥0.5IU/mL measured on D7, D14, and D90 after vaccination were meta-analyzed for studies that had comparable populations, interventions, and outcome measures. Overall estimates were calculated with 95% confidence intervals (CI) by using a random effects model based on the fixed-effect inverse-variance method. The summarized outcomes were shown in forest plots and I2 was presented as a measure of heterogeneity, graded as low (I2 = 0–40%), moderate (30–60%), substantial (50–90%), or high (75–100%) [Citation18]. A p-value of ≤.10 for the chi-squared test was considered as a cut-off point for statistically significant heterogeneity.
Sensitivity analyses were performed in the case of significant heterogeneity, based on differences in vaccine dose, dose potency, the administration of RIG and RVNA assay, and the quality of studies based on the SIGN criteria. When subgroups of specific populations were too small for analysis, healthy volunteers and people at risk were combined in a subgroup analysis for unexposed population.
Stata version 13.1 was used to perform the meta-analyses.
3. Results
3.1. Search results
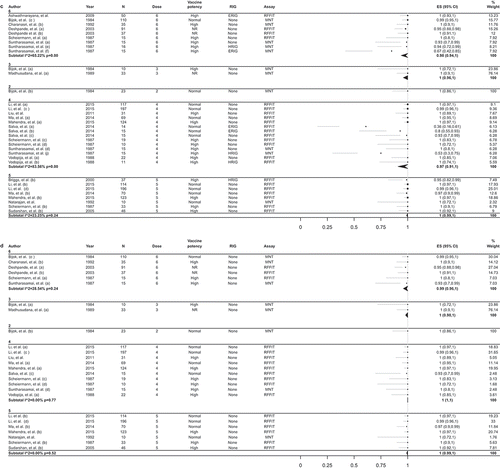
The search yielded 2036 articles from PubMed and 1565 from Embase, resulting in 3601 unique hits, of which 124 articles were included in the full text assessment. Reasons for exclusion at this stage are given in Supplement 4. Of the 48 studies considered eligible (), 33 were intervention studies, 14 were observational, and 1 had both interventional and observational components [Citation19].
Figure 1. Flow chart of search and selection procedure.
n, number of items in each category; PCECV, purified chick embryo cell vaccine; PrEP, pre-exposure prophylaxis; RVNA, rabies virus neutralizing antibodies; GMT, geometric mean titer.
Most studies included estimates of outcomes for more than one subgroup, leading to up to 55 estimates for immunogenicity, 10 for efficacy, and 48 for safety ().
The majority of intervention studies were conducted in Asia (China, India, Philippines, Thailand) [Citation7,Citation9,Citation10,Citation20–Citation40] and eight studies in Europe (Austria, Croatia, Czech Republic, Germany, Lithuania, Serbia) [Citation8,Citation41–Citation47]. All the observational studies were conducted in Asian countries [Citation19,Citation48–Citation61].
3.2. Immunogenicity
3.2.1. Rabies virus neutralizing antibody geometric mean titers
Meta-analysis for RVNA levels could not be performed because of missing data; for example, there was not enough information on RVNA levels per study participant in the individual studies to calculate the required standard deviations or standard errors.
All interventional studies using IM administration of PCECV according to various vaccination schedules reported RVNA GMTs ≥0.5 IU/mL on D14, D90, and D365, for all groups except one (RVNA GMT was 0.36 IU/mL at D14 in 14 adults), for which PCECV was administered concomitantly with ERIG, according to Zagreb regimen [Citation35]. GMT values at D14 varied greatly among age groups and with vaccination schedules and assay used to assess RVNA levels (Supplement 5, Table S1). Observational studies on bite cases in individuals of all ages also reported RVNA GMTs ≥0.5 IU/mL on D14, D90, and D365 (Table S2).
In interventional studies using ID administration of PCECV, RVNA GMTs ≥0.5 IU/mL were reported on D14 and D90, regardless of the regimen used, with six studies reporting values ≥0.5 IU/mL up to D365. RVNA GMT values at D90 ranging from 4.9 to 17.7 IU/mL were collected from observational studies (Table S3).
Five interventional studies (three conducted among healthy volunteers [Citation21,Citation36,Citation40], one among bite cases, and one among individuals at risk [Citation9,Citation39]) directly compared immunogenicity of PCECV between groups with either ID or IM administration (). A meta-analysis could not be performed because of difference in time of the conducted measurement and difference in the population studied.
Table 1. RVNA GMTs from studies comparing IM and ID administration of PCECV.
A summary of immunogenicity of IM and ID PEP PCEVC as measured by RVNA GMTs according to vaccination regimen is provided in Figure S1.
3.2.2. Proportion of participants with rabies virus neutralizing antibody titers ≥0.5 IU/mL following intramuscular administration of PCECV
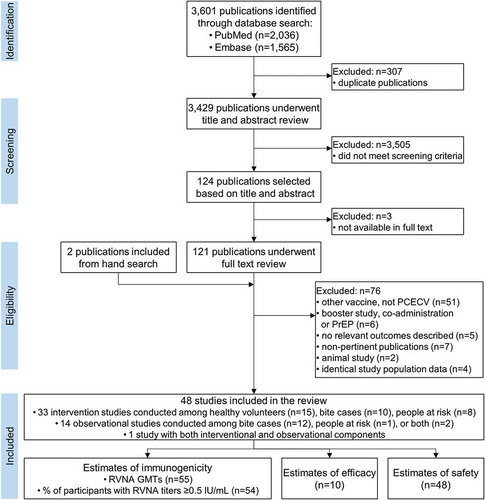
Among the 17 interventional studies included in the meta-analysis, the overall estimate of the proportion of individuals with RVNA titers ≥0.5 IU/mL at D14, irrespective of population, was 99% (95% CI: 97–100%; I2 = 71.6%; p < 0.001) ()). Omitting studies providing RIG with the vaccine improved the overall value (100% [95% CI: 100–100%]) and decreased heterogeneity (I2 = 0%; p = 0.60) ()). The percentage of individuals with adequate RVNA titers at D14 among healthy volunteers, bite cases, and people at risk were 99% (95% CI: 98–100%; I2 = 71.6%; p < 0.001), 99% (95% CI: 95–100%; I2 = 35.4%; p = 0.19), and 96% (95% CI: 89–100%; I2 = 73.9; p < 0.001), respectively ()); heterogeneity in the healthy volunteers population decreased to 0% (p = 0.69) when studies providing RIG were omitted ()). The overall estimates on D7 and D90 were 44% and 100%, respectively ().
Table 2. Overview of the estimates for the percentage of participants with RVNA titers ≥0.5 IU/mL following vaccination with PCEV by IM and ID administration in interventional studies.
Figure 2. Estimates for the proportion of individuals with RVNA titers ≥0.5 IU/ml on day 14 following vaccination with PCECV by intramuscular administration, stratified by population and including (a) or omitting (b) studies providing RIG at first administration, and stratified by number of doses and including (c) or omitting (d) studies providing RIG at first administration.
RVNA, rabies virus neutralizing antibodies; ES, estimate for the proportion of individuals with RVNA titers ≥0.5 IU/ml; PCECV, purified chick embryo cell vaccine; RIG, rabies immunoglobulin; N, number of participants with available results; MNT, standard mouse neutralization test; RFFIT, rapid fluorescent focus inhibition test; ERIG, equine RIG; HRIG, human RIG; NR, not reported.
In stratified analysis by reported vaccine dose potency performed on all populations, the percentage of vaccines with RVNA titers ≥0.5 IU/mL at D14 was ≥98%, for doses of high and normal potency, and at D7, titers ≥0.5 IU/mL were reached in 64% and 15% of the people vaccinated with high potency and normal potency PCECV doses, respectively ().
Studies were also stratified by the number of doses provided to each vaccinee, irrespective of population. The overall estimates for the proportion of individuals with RVNA titers ≥0.5 IU/mL on D14 for four-, five- (including Zagreb or [2-[1-]] or Essen [[1-]-[1-]-1] regimens), or six-dose vaccination schemes were >97% ()). When studies providing RIG with the vaccine were omitted from the analysis, overall estimates and measures of heterogeneity within populations improved ()). A limited number of studies provided information on a two- or three-dose scheme ().
No significant differences were found between the four-, five-, and six-dose schemes, yet a six-dose schedule had slightly lower estimates than the other regimens. No stratified analysis could be performed for the percentage of participants with RVNA titers ≥0.5 IU/mL on D7 and D90, because limited data were available ().
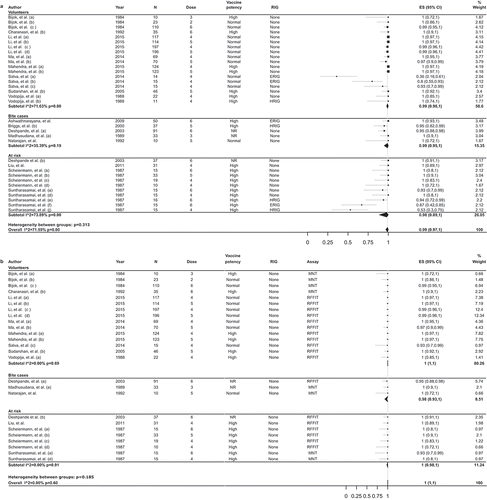
The overall estimates for the proportion of vaccines with adequate RVNA levels on D14 were 100% (95% CI: 100–100%; I2 = 0%; p = 0.60) for no RIG administered, 77% (95% CI: 37–100%; I2 = 92.6%; p < 0.001) for ERIG, and 90% (95% CI: 69–100%; I2 = 77.7%; p < 0.001) for HRIG () [Citation20,Citation21,Citation23,Citation26,Citation27,Citation31,Citation34–Citation36,Citation39,Citation42,Citation44,Citation45,Citation47,Citation62]. For healthy volunteers and people at risk combined, the estimated rate of adequate RVNA levels for PCECV with no additional RIG was 100% at D14 and 50% at D7 ().
Figure 3. Estimates for the proportion of individuals with RVNA titers ≥0.5 IU/ml on day 14 following vaccination with PCECV by intramuscular administration, stratified by RIG administration.
RIG, rabies immunoglobulin; RVNA, rabies virus neutralizing antibodies; ES, estimate for the proportion of individuals with RVNA titers ≥0.5 IU/ml; PCECV, purified chick embryo cell vaccine; CI, confidence interval; N, number of participants with available results; MNT, standard mouse neutralization test; NR, not reported; RFFIT, rapid fluorescent focus inhibition test.
Among the six observational studies using IM regimens of PCECV [Citation19,Citation48,Citation54,Citation57,Citation59,Citation61], five reported estimates of the assessed end point at D14, while one study did not report this information [Citation59].
3.2.3. Proportion of participants with rabies virus neutralizing antibody titers ≥0.5 IU/mL following intradermal administration of PCECV
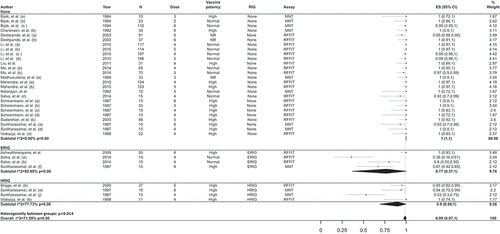
Among the 14 interventional studies included in the meta-analysis, the overall proportion of participants with adequate RVNA levels on D14 was 100% (95% CI: 99–100%; I2 = 0.0%; p = 0.91). Overall estimates for healthy volunteers, bite cases, and people at risk separately were 100% (95% CI: 99–100%; I2 = 0.0%; p = 0.63), 100% (95% CI: 99–100%; I2 = 0.0%; p = 1.00), and 100% (95% CI: 99–100; I2 = 0.0%; p = 0.75), respectively ()).
Figure 4. Meta-analysis of the proportion of individuals with RVNA titers ≥0.5 IU/ml on day 14 following vaccination with PCECV by intradermal administration, stratified by population (a) and number of doses (b).
RVNA, rabies virus neutralizing antibodies; ES, estimate for the proportion of individuals with RVNA titers ≥0.5 IU/ml; PCECV, purified chick embryo cell vaccine; CI, confidence interval; N, number of participants with available results; MNT, standard mouse neutralization test; RFFIT, rapid fluorescent focus inhibition test; ERIG, equine RIG; HRIG, human RIG; NR, not reported.
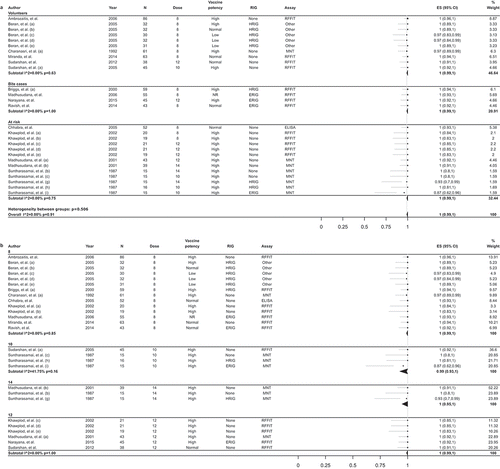
Stratified analysis by dose potency was performed only for D14 on the combined populations and showed estimates of 100% for both normal and high potency PCECV dose ().
Stratified analyses by the number of doses administered yielded overall percentages of individuals with adequate immune response to vaccination of 100% (95% CI: 99–100%, I2 = 0.0%; p = 0.85), 99% (95%CI: 93–100%, I2 = 41.8%; p = 0.16), and 100% (95% CI: 99–100%, I2 = 0.0%; p = 1.00) for the 8- (administered in 4–5 visits), 10- (2-2-2-2-2 or a 4-4-1-1), and 12-dose (administered in ≥3 visits) schemes, respectively ()). Only two studies provided estimates for a 14-dose (4-4-4-1-1 or 8-4-1) vaccination scheme ().
When RIG administration was used for stratified analyses, the overall estimates on D14 were 100% (95% CI: 100–100%; I2 = 0.0%; p = 0.99) for no RIG, 100% (95% CI: 96–100%; I2 = 51.7%; p = 0.10) for ERIG, and 100% (95% CI: 98–100%; I2 = 0%; p = 0.60) for HRIG (). One study in which either ERIG or HRIG was administered within the same study population reported that 99% (95% CI: 95–100%) of subjects had RVNA titers ≥0.5 IU/mL at D14 [Citation10].
Figure 5. Estimates for the proportion of individuals with RVNA titers ≥0.5 IU/ml on day 14 following vaccination with PCECV by intradermal administration, stratified by RIG administration.
RVNA, rabies virus neutralizing antibodies; ES, estimate for the proportion of individuals with RVNA titers ≥0.5 IU/ml; PCECV, purified chick embryo cell vaccine; RIG, rabies immunoglobulin; CI, confidence interval; N, number of participants with available results; RFFIT, rapid fluorescent focus inhibition test; MNT, standard mouse neutralization test; ELISA, enzyme-linked immunosorbent assay; NR, not reported.
Five observational studies reported on the adequacy of immune response for PCECV [Citation50,Citation52,Citation53,Citation58,Citation60]. Among them, three reported adequate RVNA levels in GMTs from D14 [Citation50,Citation52,Citation58], and one study reported adequate RVNA levels throughout the whole study period (3 years) [Citation53]. In one study, RVNA titers ≥0.5 IU/mL were reported after 2 months up to 12 months [Citation60].
A meta-analysis to compare the ID and IM routes of administration could not be performed because of the difference in duration of the measurements and difference in the populations studied.
3.2.4. Assessment methods for rabies virus neutralizing antibody titers
The RVNA assessment was done by using rapid fluorescent focus inhibition test (RFFIT) in 29 (24 interventional and 5 observational) studies and mouse neutralization test (MNT) in 11 (6 interventional and 5 observational) studies. One intervention study used the enzyme-linked immunosorbent assay (ELISA) [Citation22], and one used a method employing an international reference serum containing 2 IU/mL of RVNA to calculate the titer of RVNA in each sample [Citation41]. One observational study used an in vitro serum neutralization test as RVNA assay [Citation55], while another one did not report the assay [Citation51]. For studies that used the RFFIT and MNT, there was no difference between the tests for the reported RVNA titers at Day 14 (data not shown).
3.3. Efficacy
Two interventional [Citation9,Citation20] and four observational [Citation19,Citation48,Citation57,Citation61] trials reported 100% survival throughout the study period following IM administration of PCECV. Only one interventional study reported bite cases who received PCECV administered ID, with a 100% survival rate during the study [Citation9]; four observational studies reported 100% survival after 1 year [Citation51], 3 years [Citation53], and throughout the study period [Citation19,Citation60].
In an observational study in India, 32 patients with exposure to laboratory-confirmed rabid dogs received wound care and PCECV by multisite ID administration, and HRIG or ERIG was also administered to 22 patients. The source of the exposure was 12 dogs, 9 of which were strays and 3 were unimmunized pets. The dogs inflicted multiple bite injuries, including transdermal bites and severe lacerated wounds on the face or forearms. All of the patients were followed up for 3 years and were alive and doing well [Citation53].
In the Phetchabun province, Thailand, between 1996 and 2001 there were an estimated 587,528 dogs, of which 8.9% were strays, 91.1% were house dogs, and 71.0% were vaccinated for rabies [Citation51]. During the same period, there were a reported 8157 bite cases, of which 51% were category III exposures; although only 3.0% received RIG, all of the patients who received vaccine (ID route) survived; however, 2 deaths were reported among poor subsistence farmers who did not seek PEP after dog bites [Citation51].
3.4. Safety
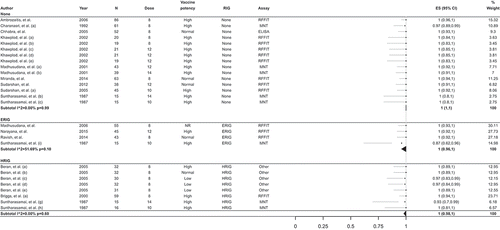
When PCECV was administered according to IM regimens, the most frequent local AEs reported in the 15 interventional studies reviewed were itching, erythema, and pain. The most reported systemic AEs were (in decreasing order of occurrence): myalgia, headache, fever, or having a feverish feeling. One study reported an incidence of 15–16% for severe local and systemic AEs. Four studies reported no serious AEs (SAEs) (). Across the seven observational studies reporting on this outcome, pain was reported as the most common local AE in five studies, while headache and fever were less frequently reported. Two studies reported minor AEs that were self-limiting, one study reported no AEs, and no studies reported any SAEs ().
Table 3. Adverse reactions after PCECV by IM administration, reported in interventional studies.
Table 4. Adverse reactions after PCECV by IM administration reported in observational studies.
In studies reporting ID administration of PCECV, across the 16 interventional studies identified, the most frequently reported local AEs were erythema, itching, induration, lymphadenopathy, and pain, while the most common systemic AEs reported were headache and fever or having a feverish feeling (). Erythema, induration, and lymphadenopathy were more frequently reported for ID compared with studies assessing the safety of IM-administered PCECV ( and ). In a study conducted among healthy hospital staff, veterinary students, and patients with possible risk of infection, pain was more frequently reported in the IM group than in the ID group (p < 0.001); however, ID caused more local irritation, erythema, and induration (92%) compared with IM vaccination (66%) (p < 0.02) [Citation39].
Table 5. Adverse reactions after PCECV by ID administration reported in interventional studies.
3.5. Special populations
Twelve studies reported outcomes relevant to our objectives in HIV-infected adults, pregnant women, and malnourished children.
One observational study was conducted among 27 HIV-infected adults who received PCECV by ID administration according to an eight-site regimen (8-8-8-8-8) on D0, D3, D7, D14, and D30) and reported RVNA titers ≥0.5 IU/mL on D14, D90, and D180, although 3 individuals did not maintain these levels at D365 [Citation58]. Adequate RVNA titers were achieved at D14 and D30 in all patients, regardless of the CD4+ cell count. However, at D90, 100% of individuals with a CD4+ cell count of ≤200 cells/µl and 94% of those with >200 cells/µL had RVNA titers ≥0.5 IU/mL, and these percentages decreased at subsequent time points [Citation58]. PCECV was well tolerated in HIV-infected individuals and no SAEs related to the vaccine were reported. Furthermore, there were no aggravations of the HIV infection, and mean CD4+ cell counts increased significantly after vaccination [Citation58].
One observational study in Chinese pregnant women receiving PCECV following exposure reported safety data. No immediate reaction occurred in any of the women and none of the AEs reported by 46.4% of participants required medical intervention. The AEs were local pain (22.5%), fatigue (18.3%), fever (2.8%), local erythema (1.4%), and headache (1.4%). No miscarriages, stillbirths, or fetal malformations were reported, and birth weights were within ranges considered normal for neonates in China [Citation49].
We identified 10 studies reporting data in children aged 1–17 years [Citation10,Citation19,Citation25,Citation29,Citation31,Citation40,Citation53,Citation54,Citation56,Citation61]. At Day 14, all children had Day 14 RVNA titers ≥0.5 IU/mL, but not all studies reported seroconversion rates. One study among malnourished children aged from 8 months to 16 years reported a GMT of 15.3 IU/mL on D14 and 16.5 IU/mL on D30 [Citation54] following IM administration of PEP. A second study conducted among a subgroup of healthy children aged 1–5 years reported a GMT of 12.0 IU/mL on D14 and 13.0 IU/mL on D42 for the IM Zagreb regimen (2-1-1). For the Essen regimen (1-1-1-1-1) a GMT of 14.0 IU/mL was reported on D14 and 24.0 IU/mL on D42 [Citation25]. For both regimens at Day 14, the seroconversion rate was 100% (95% CI: 97–100). In children aged 5–15 years receiving IM PCECV on D0, D3, D7, D14, D30, and D90, GMTs of 14.5 and 121.9 IU/mL were achieved after three and six doses, respectively [Citation31]. In 10 children aged 7–13 years receiving ID immunization who were followed for 3 years, GMTs were 9.5 IU/mL at D28 and 0.9 IU/mL at the end of the third year [Citation53]. One study on children aged 1–13 years having received two to six doses of PCECV (no recorded administration route) found a GMT of 1.98 IU/mL during 2 years of follow-up [Citation56]. Several studies which were only partly conducted in children did not report separate data for this age group, but all studies conducted in special populations reported that RVNA titers ≥0.5 IU/mL were achieved by all participants by D14.
Mild to moderate solicited local and systemic reactions, with pain being the most reported solicited local AE (38% and 40% of children following the Zagreb and Essen regimens, respectively) and fatigue as the most common systemic AE (15% and 13% of children after the Zagreb and Essen regimens respectively), were reported in a study on healthy children [Citation25]. Unsolicited AEs were noted in 3% (Zagreb regimen) and 8% (Essen regimen) of children, with upper respiratory tract infection being the most frequent, regardless of regimen. No SAEs were reported [Citation25]. A study conducted among bite cases reported fever mostly in children aged <5 years; no further information on safety of the vaccine could be retrieved from the manuscript [Citation48]. Among 235 children ≤13 years of age, bitten by dogs and receiving PCECV, only 7% experienced mild to moderate AEs (pain, induration, fever, or rash) [Citation56]. In a study among exposed malnourished children, no life-threatening AEs were observed in any of the children; all AEs were minor and resolved within a few days [Citation54].
4. Discussion
This systematic review and meta-analysis was conducted to assess the current evidence for the use of PCECV administered by the IM or ID route for PEP against rabies. Across the studies identified for this review, there were 54 reports of immune responses to vaccination including healthy volunteers, or individuals at risk such as those occupationally exposed to rabies cases, and bite cases. Our analysis showed that in almost all individuals, 14 days after starting the vaccine regime, PCECV elicited RVNA titers above the WHO recommended serological threshold for an adequate immune response to vaccination (≥0.5 IU/mL serum) [Citation7]. The studies reviewed showed that if administered promptly within a PEP protocol, PCECV with potency ≥2.5 IU per dose by the IM route or the ID route should be entirely effective in preventing rabies.
4.1. Immunogenicity
In the analysis of the proportion of individuals with RVNA titers ≥0.5 IU/mL, few studies provided a direct comparison of IM with ID, so we performed stratified analyses by IM or ID route of administration. In healthy volunteers and healthy volunteers and at-risk populations combined, higher percentages of individuals with adequate immune response were also observed for IM versus ID. It was not possible to analyze bite cases and people at risk separately due to the lack of data. The proportion of the overall populations with adequate RVNA titers by D7 was estimated at 0.44 (CI: 0.24–0.65) for the IM route and 0.39 (CI: 0.13–0.69) for the ID route; however, only small differences (not significant) were observed for the ID and IM routes, as most of the population had RVNA titers ≥0.5 IU/mL by D14. At Day 90, the overall proportion of vaccines with adequate RVNA titers was 1.00 (CI: 1.00–1.00) for IM and 0.97 (CI: 0.92–1.00) for ID. In addition, the population size for the IM route of administration was larger than for the ID route which may have influenced the power of the analyses.
The WHO recommends that rabies vaccines should have a minimum antigen content of ≥2.5 IU per dose to elicit adequate immune response and protection against rabies. Because ID regimens use about a fifth of the antigen needed to achieve adequate RVNA levels by the IM route, after the introduction of ID regimens, various clinical trials assessed the antigenicity and immunogenicity of ID vaccines and showed that increases in vaccine potency above ‘normal’ levels for IM use were not needed to elicit an adequate immune response. For example, a study of healthy volunteers, who received 0.1 mL ID doses of PCECV corresponding to a potency of 0.32 IU per dose, showed that every participant achieved RVNA titers ≥0.5 IU/mL by D14 [Citation41]. Previous meta-analyses have shown that vaccines by IM administration with high potency (≥5 IU per dose) were not associated with higher RVNA titers at D14 and D90 compared with normal potency vaccine, and by ID administration, there was no linear relationship between high and normal vaccine potency and immunogenicity [Citation7,Citation14]. In our review, for the IM route at D7, high potency vaccines elicited slightly higher proportions of vaccinated individuals with RVNA titers ≥0.5 IU/mL than normal potency vaccines (≥2.5–<5 IU/mL), although it was not possible to compare the two routes of administration due to a lack of data for ID vaccination. At D14, stratification by vaccine potency did not provide evidence of a difference in estimates between the two routes of administration. Moreover, the potency of PCECV should be sufficient to elicit adequate RVNA levels if stored and handled appropriately (stored at 2–8°C, used no later than 8 h after reconstitution, and when the dose is drawn following aseptic technique).
Data have been reported indicating that HIV-infected individuals with low CD4+ counts do not produce the same levels of RVNA titers after vaccination compared with healthy individuals, although the data were not peer reviewed so were not included in our review [Citation63]. The data included showed that adequate RVNA titers were achieved at D14 and D30 in all HIV-infected individuals, regardless of the CD4+ cell count [Citation58].
While vaccine immunogenicity might be different due to the medical history of the vaccinated individual, such history is provided in most studies. However, based on the limited studies available, post-vaccination RVNA titers do not appear different between healthy volunteers and bite cases.
4.2. Efficacy
There were not enough data reported for survival efficacy to allow a meta-analysis, although among the 10 studies reporting survival data of rabies virus exposed individuals, all patients who received PCECV for PEP survived. All reported cases with confirmed rabies virus exposure received PEP and survived, although a limitation of the survival rate studies was that rabies virus in the biting animal was not confirmed for all cases. In addition, whereas 100% survival was observed for PCECV PEP, it should be noted that while death is almost inevitable among individuals once they develop the symptoms of rabies, the risk of developing the disease in an unvaccinated individual after exposure to the virus depends on various factors such as the route of exposure, category of exposure, and genetic factors in the virus and host [Citation64,Citation65]. Based on a study in India in 1946–51, among unvaccinated people who were bitten by infected animals, 43.2% died [Citation66], and in a study of 2174 bite cases in India in 1923, the death rate among unvaccinated people was 6.2% [Citation67]. In a study in Thailand in 1987, among 20 people with bites from confirmed rabid dogs, 9 (45%) died, all of whom had been severely bitten on the face, neck, or arms, compared with on the legs or feet among those who survived [Citation68]. More recently, in a model-based analysis of annual dog bite counts in Bhutan, it was estimated that without human rabies vaccine PEP, 223 dog bites would result in 19.24 deaths/year [Citation69].
4.3. Vaccine regimens
In previously unvaccinated individuals, PCECVs are usually given by the IM route using the five-dose Essen regimen or the four-dose Zagreb regimen, or by the ID route based on the updated Thai Red Cross (TRC) regimen. The five-dose PEP regimen in unvaccinated people was first replaced by the four-dose regimen in the United States (USA), where the Advisory Committee on Immunization Practices estimated that assuming total compliance with four doses, protection against rabies could be achieved at a reduced cost of about $16.6 million to the health-care system [Citation70]. The WHO now advocates four- or five-dose regimens for PEP after category 2 and 3 exposures [Citation7]. The WHO convened a Strategic Advisory Group of Experts working group to reexamine current recommendation in June 2016 and new guidelines were published in 2018 [Citation5]. The key recommendations emphasize the use of ID applications as being more cost- and dose-effective than IM administrations for both PEP and PrEP provide updates in RIG dosage and administration guidelines and propose the generation of accelerated regimens [Citation65].
In our review, various vaccine regimens were reported, including the Zagreb regimen (2-1-1), the Essen regimen (1-1-1-1-1), or with a booster dose on D90, TRC regimen of 0.1 mL of cell culture vaccines administered ID at two body sites on days 0, 3, 7, and 28 (2-2-2-0-2 or 2-2-2-0-1-1 with a booster dose on D90), and the Kempegowda Institute of Medical Sciences (KIMS)-ID regimen with 0.1 mL of PCECV administered ID at two body sites (deltoids) on days 0, 3, 7, 14, and 28 (2-2-2-2-2). After D14, more doses were related to higher proportions of individuals with adequate RVNA levels, although this could have been influenced by the provision of RIG; in a second analysis comprising only studies that did not provide RIG, there was no difference in estimates between four- and five-dose regimens, whereas six doses led to a slightly lower proportion of individuals with RVNA titers ≥0.5 IU/mL than the other regimens at Day 14. For the group that did not receive RIG, there was no difference in the proportion of vaccines with adequate RVNA levels between four- and five-dose regimens.
4.4. Concomitant use of RIG
After category 3 exposure in rabies unvaccinated or in immunocompromised people, RIG should be administered concomitantly with the PCECV regimen [Citation6]. RIG (a dose calculated based on body weight) should be mainly administered into or around the wound as soon as possible on the basis that passive immunity provided by RIG will prevent the rabies virus from getting into the central nervous system; any remaining RIG can also be administered IM at a separate site from the vaccine, although systemic use is unlikely to be as effective as direct administration at the wound [Citation71]. In a study of healthy volunteers, however, the reported proportion of participants with adequate RVNA levels suggested that the proximity of the ERIG and PCECV administration sites may affect the immune response to vaccination – that is, ERIG appeared to suppress vaccine-induced RVNA titers when administered at the same site as the vaccine, whereas this was not observed when ERIG was administered at a different site to the vaccine [Citation35]. In another study, whereas RVNA titers were enhanced by HRIG or ERIG at D7 among healthy volunteers who received PCECV by the IM or ID routes, on D14 and D28, RVNA titers were lower in the groups receiving HRIG or ERIG compared with vaccine alone [Citation39].
In our analysis, in the overall population who did not receive RIG, the proportion of participants with RVNA titers ≥0.5 IU/mL at D7 was higher for IM than for ID, with a similar trend seen in the analysis of healthy volunteers and people at risk combined. No analysis of individuals who received RIG could be performed due to a lack of data. Studies providing HRIG or ERIG with vaccine by the ID route tended to report higher estimates of this end point at D14, than by the IM route. Furthermore, the proportion of vaccines with RVNA titers ≥0.5 IU/mL was lower when RIG and PCECV were coadministered than PCECV alone in the ID subgroup, and appeared higher in those who received vaccine by the IM route combined with HRIG compared with ERIG. Notably, the proportion of individuals with adequate RVNA levels at D14 was lowest in healthy volunteers who received RIG at the same site as ID administration of PCECV [Citation35], and in healthy volunteers who received HRIG or ERIG as part of a four- or eight-dose regimen where PCECV was administered ID or IM [Citation39]. These studies suggest that passive immunization with RIG can interfere with vaccine-induced antibody production and highlights the need for caution regarding dosage and administration site when providing RIG concomitant to PCECV vaccine.
4.5. Safety
The review identified 48 reports of reactogenicity and safety of PCECV, including four studies that assessed children or pregnant women [Citation25,Citation48,Citation49,Citation54]. The most frequently reported systemic AEs were myalgia, headache, fever, or having a feverish feeling, and overall, our descriptive analysis indicated that adverse reactions including headache, erythema, induration, and lymphadenopathy may be more often reported among individuals receiving PCECV by ID than IM administration. Unsolicited AEs were noted in 3% (Zagreb regimen) and 8% (Essen regimen) of children, with upper respiratory tract infection being the most frequent, regardless of regimen. PCECV-administered ID by a modified eight-site regimen was well tolerated in HIV-infected individuals and no SAEs related to the vaccine were reported [Citation58], although it should be noted that ID administration is not approved for immunocompromised individuals and those taking anti-malaria drugs. In rabies-exposed pregnant women, no miscarriages, stillbirths, or fetal malformations were reported, and birth weights were within the normal ranges for Chinese newborns. Overall, the studies included in the review showed that PCECV had an acceptable safety profile, and is suitable for use in special populations including pregnant women.
4.6. Limitations
The main limitation of the review was the low number of studies with data on bite cases such that the overall estimates for the proportion of individuals with RVNA titers ≥0.5 IU/mL were based largely on healthy volunteers and people at risk. In addition, because of the limited number of studies that directly compared the routes of administration, we performed stratified analyses by IM or ID route of administration, so the interpretation of IM versus ID should consider possible biases associated with the heterogeneity of studies (e.g. population, regimen, dose potency, RIG, age group, testing days, RNVA assay type). Notably, the meta-analyses included more studies of the IM route than the ID route, a greater heterogeneity was observed in the IM than the ID subgroup, and due to missing data for continuous immunogenicity, no meta-analysis could be performed on RVNA GMTs. A further limitation of our review was that a large number of the studies included were of low quality, with negative scores on a selection of criteria of the SIGN checklist. No studies were excluded based on quality because stratifying the analyses by study quality did not affect the results.
A potential source of variance and misclassification is the type of test used to measure RVNA titers, as although both methods are recommended by WHO, the RFFIT test is documented to be more sensitive with less variability and higher titers than the MNT test [Citation72]. Moreover, there is little standardization or proficiency testing for the RFFIT between laboratories which may also introduce additional variance, which may not have affected the analysis of the proportion of patients in studies reported to seroconvert (≥0.5 IU/mL), but it may account for some of the difficulties trying to analyze GMTs.
5. Expert commentary
PCECV to prevent rabies is administered to about 15 million people each year as part of a PEP protocol, yet despite this, rabies remains neglected and highly underreported among poor rural communities with livestock economies [Citation2–Citation5]. In countries such as Thailand and Sri Lanka, rather than traditional IM administration of PCECV, the ID route is used as this reduces the volume of antigen needed per dose compared with doses for IM use, and thus reduces the direct costs of vaccine [Citation5,Citation8–Citation11].
Our literature search yielded too few studies with survival data among exposed individuals to analyze PCECV efficacy; however, among the 10 studies that explored the survival rates, there were no treatment failures reported in rabies-exposed individuals. In one study including 8157 bite cases, there were two deaths among subsistence farmers who did not seek PEP after dog bites. This highlights the need for better access to PEP among people in remote areas [Citation51]. The review also showed that almost all individuals achieved adequate RVNA levels by D14 after starting a PEP protocol with PCECV given by the IM or ID route; the proportion of vaccinated individuals with RVNA titers ≥0.5 IU/mL on D14 for PCECV given IM as 4-, 5-, or 6-dose regimens was >97%, and for ID administration as 8-, 10-, 12-, and 14-dose regimens, the proportion was 100%. Most individuals who received RIG had adequate RVNA levels on D14, but in two studies comparing RIG with no RIG in healthy volunteers, reduced RVNA levels were observed in individuals who received RIG at the same site as ID vaccine [Citation35], and in those who received HRIG or ERIG as part of a four- or eight-dose regimen with ID or IM vaccine [Citation39]. In the overall population, PCECV had an acceptable safety profile, and PCECV by IM and ID administration were well tolerated in special populations, including pregnant women and malnourished children.
The results of our analysis suggest that if administered promptly within a PEP protocol, PCECV at ≥2.5 IU per dose by the IM or the ID route should be effective in preventing rabies. RIG should be used for category 3 exposures to provide passive protection against rabies and to protect against the virus entering the central nervous system. However, care should be taken to avoid excessive doses of RIG and to administer the vaccine at a site distal to the wound when RIG is administered to prevent suppression of active antibody production. Our review showed that after rabies virus exposure, PCECV within a PEP protocol was 100% effective at preventing death, and almost all individuals across study populations had adequate RVNA levels 14 days after receiving PCECV. Given that there are 59,000 human rabies deaths reported annually [Citation2–Citation4], the challenge to reducing the burden of this neglected disease is to improve access to highly effective PEP treatments among remote rural communities, including countries with urban areas that are dog rabies-free.
Whereas better access to vaccine and awareness of PEP protocols is important for reducing human rabies deaths in poor rural communities, reducing the canine reservoir of rabies is key to eliminating human rabies in populations. Human exposures to rabies virus are most often caused by dogs, and whereas PEP protocols are highly effective at preventing infection in humans, the use of human rabies vaccines for either PrEP or PEP has no effect on the canine rabies reservoir. Mass vaccination of dogs against rabies in countries where dog rabies is endemic is a cost-effective way to reduce the personal and financial burden of rabies in humans, as demonstrated in Tanzania and Bhutan [Citation73]. The One Health collaborative, involving public health and veterinary agencies, has developed a framework for the implementation of mass dog vaccination, with the objective of achieving 70% coverage in low-income countries [Citation74]. The long-term aim of the One Health program in participating countries is to eliminate human rabies associated with dogs by 2030 [Citation74].
6. Five-year view
It is clear that the current vaccine schedules against rabies are effective, yet universal access to PEP remains as one of the challenges in reaching the goal of zero human rabies deaths by 2030. To achieve this, over the next 5 years funding mechanisms need to improve to create sustainable models for procurement and distribution to help countries access the vaccines. Improving access to vaccines may be enhanced by the global adoption of ID administration, thereby reducing the volume of vaccine required per patient. This approach has been successfully implemented in Asia for the last 20 years.
Further abbreviating PEP schedules over the next 5 years could also reduce the cost of the clinically necessary overtreatment of rabies, as emphasized by recent key recommendations on rabies immunization by the WHO [Citation5,Citation75]. For example, the US Advisory Committee on Immunization Practices recommendation of the four-dose Essen regimen was based on evidence showing that the regimen provided adequate protection after suspected exposure. In addition, increasing the use of PrEP beyond Europe and North America could also have a significant effect in regions with high rabies prevalence in local dog or bat populations [Citation76]. For example, limited data from the Philippines have shown that risk group-focused PrEP vaccination programs can reduce human rabies incidents [Citation77,Citation78].
Improving animal rabies control programs to reduce the risk of rabies to humans is a global health goal; however, improving access to vaccines for PEP among people at risk of rabies exposure should reduce human rabies deaths in poor rural communities. In many low-income countries, government funding of rabies vaccines is limited. Gavi, the Vaccine Alliance, included improving access to rabies vaccine in its vaccine investment strategy in 2008 and 2013, and has worked to improve knowledge of operational feasibility, the public health impact, and the cost of rabies vaccination programs in low-income countries. As such, GAVI is scrutinizing the available data, and a decision about the inclusion of rabies in the vaccine investment strategy is expected during 2018 [Citation79].
Key issues
Vaccine efficacy data are sparse, but available studies reported 100% survival among individuals who received PCECV for post-exposure prophylaxis after rabies exposure.
Immunogenicity data from 54 studies found seroconversion rates of >97% at 14 days after starting IM PCECV as 4-, 5-, or 6-dose regimens, and 100% with ID administration as 8-, 10-, 12- and 14-dose regimens.
Prompt administration of PEP with PCECV at ≥2.5 IU/mL per dose by either IM or ID route was effective in preventing rabies.
PCECV was well-tolerated, including in HIV-infected persons, pregnant women, and malnourished children; most frequently reported systemic AEs were myalgia, headache, and feverishness.
Trademark statement
Rabipur is a registered trademark of the GSK group of companies.
Declaration of interest
S Preiss, C Maranp, P Mukherjee and P Buchy are employees of GSK group of companies and hold shares and options from the sponsoring company. M Vonk Noordergraaf and R van Horn are employees of Pallas, a commercial entity that has received grants from the GSK group of companies and which carried out the submitted work as a supplier to GSK vaccines; Pallas also received grants from Sanofi Pasteur and Daiichy Sankyo, outside the submitted work. L H Chen is an advisor to Shoreland Inc.; Member of the Work Group on Flaviviruses for the Advisory Committee on Immunization Practices. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors had full access to the data, were involved with developing this manuscript, gave final approval before submission and are accountable for all aspects of the work.
Supplemental Material
Download MS Word (317.8 KB)Acknowledgments
The authors thank Juan Vargas and Laurence de Moerlooze for their contribution to the early phases of the preparation of this assessment. Annick Moon (independent medical writer) and Petronela M. Petrar (XPE Pharma & Science for GSK) provided writing assistance and Susana Montenegro Gouveia (XPE Pharma & Science for GSK) provided publication coordination and editorial support.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- World Health Organization. WHO expert consultation on rabies. Second report. World Health Organ Tech Rep Ser.2013; 1–139. back cover.
- Hampson K, Coudeville L, Lembo T, et al. Estimating the global burden of endemic canine rabies. PLoS Negl Trop Dis. 2015;9:e0003709.
- Hampson K, Dobson A, Kaare M, et al. Rabies exposures, post-exposure prophylaxis and deaths in a region of endemic canine rabies. PLoS Negl Trop Dis. 2008;2:e339.
- Hampson K, Dushoff J, Cleaveland S, et al. Transmission dynamics and prospects for the elimination of canine rabies. PLoS Biol. 2009;7:0462.
- World Health Organization. Rabies vaccines: WHO position paper. Weekly epidemiological record 2018 [cited 2018 Apr]. Available from: http://apps.who.int/iris/bitstream/handle/10665/272371/WER9316.pdf?ua=1
- World Health Organization. Rabies vaccine: WHO position paper. Wkly Epidemiol Rec. 2010;32:309–320.
- Ravish HS, Vijayashankar V, Madhusudana SN, et al. Safety and immunogenicity of purified chick embryo cell rabies vaccine (VaxiRab N) administered intradermally as post exposure prophylaxis. Hum Vaccin Immunother. 2014;10:2433–2437.
- Ambrozaitis A, Laiskonis A, Balciuniene L, et al. Rabies post-exposure prophylaxis vaccination with purified chick embryo cell vaccine (PCECV) and purified Vero cell rabies vaccine (PVRV) in a four-site intradermal schedule (4-0-2-0-1-1): an immunogenic, cost-effective and practical regimen. Vaccine. 2006;24:4116–4121.
- Briggs DJ, Banzhoff A, Nicolay U, et al. Antibody response of patients after postexposure rabies vaccination with small intradermal doses of purified chick embryo cell vaccine or purified Vero cell rabies vaccine. Bull World Health Organ. 2000;78:693–698.
- Quiambao BP, Dimaano EM, Ambas C, et al. Reducing the cost of post-exposure rabies prophylaxis: efficacy of 0.1 ml PCEC rabies vaccine administered intradermally using the Thai Red Cross post-exposure regimen in patients severely exposed to laboratory-confirmed rabid animals. Vaccine. 2005;23:1709–1714.
- Warrell MJ, Riddell A, Yu LM, et al. A simplified 4-site economical intradermal post-exposure rabies vaccine regimen: a randomised controlled comparison with standard methods. PLoS Negl Trop Dis. 2008;2:e224.
- Madhusudana SN, Mani RS. Intradermal vaccination for rabies prophylaxis: conceptualization, evolution, present status and future. Expert Rev Vaccines. 2014;13:641–655.
- World Health Organization. WHO position paper on rabies vaccine. Grading of scientific evidence. 2010 [updated 2010 Aug 6. Cited 2017 Jun 7]. Available from: http://wwwwhoint/immunization/rabies_grad_efficacypdf?ua=1
- Sudarshan MK, Mahendra BJ, Madhusudana SN, et al. Assessing the relationship between antigenicity and immunogenicity of human rabies vaccines. Results of a meta-analysis. Hum Vacci. 2005;1:187–190.
- Giesen A, Gniel D, Malerczyk C. 30 years of rabies vaccination with Rabipur: a summary of clinical data and global experience. Expert Rev Vaccines. 2015;14:351–367.
- Stewart LA, Clarke M, Rovers M, et al. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. Jama. 2015;313:1657–1665.
- Scottish Intercollegiate Guidelines Network. Critical appraisal: notes and checklists. 2015. Available from: http://www.sign.ac.uk/methodology/checklists.html
- Deeks J, Higgins J, Altman D. Chapter 9: analysing data and undertaking meta-analysis. In: Higgins JPT GSe, editor. Cochrane handbook for systematic reviews of interventions. Version 5.0.1. London: The Cochrane Collaboration; Updated Sep 2008.
- Sehgal S, Bhattacharya D, Bhardwaj M. Ten year longitudinal study of efficacy and safety of purified chick embryo cell vaccine for pre- and post-exposure prophylaxis of rabies in Indian population. J Commun Dis. 1995;27:36–43.
- Ashwathnarayana DH, Madhusudana SN, Sampath G, et al. A comparative study on the safety and immunogenicity of purified duck embryo vaccine [corrected] (PDEV, Vaxirab) with purified chick embryo cell vaccine (PCEC, Rabipur) and purified Vero cell rabies vaccine (PVRV, Verorab). Vaccine. 2009;28:148–151.
- Charanasri U, Meesomboon V, Kingnate D, et al. Intradermal simulated rabies postexposure prophylaxis using purified chick embryo rabies vaccine. J Med Assoc Thai. 1992;75:639–643.
- Chhabra M, Ichhpujani RL, Bhardwaj M, et al. Safety and immunogenicity of the intradermal Thai red cross (2-2-2-0-1-1) post exposure vaccination regimen in the Indian population using purified chick embryo cell rabies vaccine. Indian J Med Microbiol. 2005;23:24–28.
- Deshpande AK, Londhe VA, Akarte S, et al. Comparative evaluation of immunogenicity, reactogenecity and safety of purified chick embryo cell rabies vaccine and neural tissue rabies vaccine. J Assoc Physicians India. 2003;51:655–658.
- Khawplod P, Tantawichien T, Wilde H, et al. Use of rabies vaccines after reconstitution and storage. Clin Infect Dis. 2002;34:404–406.
- Li R, Li Y, Wen S, et al. Immunogenicity and safety of purified chick-embryo cell rabies vaccine under Zagreb 2-1-1 or 5-dose Essen regimen in Chinese children 6 to 17 years old and adults over 50 years: a randomized open-label study. Hum Vaccin Immunother. 2015;11:435–442.
- Liu H, Huang G, Tang Q, et al. The immunogenicity and safety of vaccination with purified Vero cell rabies vaccine (PVRV) in China under a 2-1-1 regimen. Hum Vacci. 2011;7:220–224.
- Ma J, Wang H, Li J, et al. A randomized open-labeled study to demonstrate the non-inferiority of purified chick-embryo cell rabies vaccine administered in the Zagreb regimen (2-1-1) compared with the Essen regimen in Chinese adults. Hum Vaccin Immunother. 2014;10:2805–2812.
- Madhusudana SN, Anand NP, Shamsundar R. Evaluation of two intradermal vaccination regimens using purified chick embryo cell vaccine for post-exposure prophylaxis of rabies. Natl Med J India. 2001;14:145–147.
- Madhusudana SN, Sanjay TV, Mahendra BJ, et al. Comparison of safety and immunogenicity of purified chick embryo cell rabies vaccine (PCECV) and purified Vero cell rabies vaccine (PVRV) using the Thai Red Cross intradermal regimen at a dose of 0.1 ML. Hum Vacci. 2006;2:200–204.
- Madhusudana SN, Sanjay TV, Mahendra BJ, et al. Simulated post-exposure rabies vaccination with purified chick embryo cell vaccine using a modified Thai Red Cross regimen. Int J Infect Dis. 2004;8:175–179.
- Madhusudana SN, Tripathi KK. Post exposure studies with human diploid cell rabies vaccine and purified chick embryo cell vaccine: comparative serological responses in man. Zentralbl Bakteriol. 1989;271:345–350.
- Miranda EA, Lacuesta TLV, Suquila JT, et al. Safety and immunogenicity of purified Vero cell rabies vaccine versus purified chick embryo cell rabies vaccine using pre-exposure and post exposure regimen among healthy volunteers in San Lazaro Hospital. Philippine J Intern Med. 2014;52:1–7.
- Narayana A, Manoharan A, Narayan MS, et al. Comparison of safety and immunogenicity of 2 WHO prequalified rabies vaccines administered by one week, 4 site intra dermal regimen (4-4-4-0-0) in animal bite cases. Hum Vaccin Immunother. 2015;11:1748–1753.
- Natarajan M, Mukundan P, John TJ. Immune response to purified chick embryo cell culture rabies vaccine manufactured in India. Indian J Med Res. 1992;95:51–53.
- Salva EP, Dimaano EM, Villarama JBR, et al. An evaluation of the safety and potency of equine rabies immunoglobulin through measurement of suppression on vaccine-induced antibody production among healthy volunteers. Philippine J Intern Med. 2014;52:1–7.
- Sudarshan MK, Madhusudana SN, Mahendra BJ, et al. Evaluation of a new five-injection, two-site, intradermal schedule for purified chick embryo cell rabies vaccine: a randomized, open-label, active-controlled trial in healthy adult volunteers in India. Curr Ther Res Clin Exp. 2005;66:323–334.
- Sudarshan MK, Narayana DH, Madhusudana SN, et al. Evaluation of a one week intradermal regimen for rabies post-exposure prophylaxis: results of a randomized, open label, active-controlled trial in healthy adult volunteers in India. Hum Vaccin Immunother. 2012;8:1077–1081.
- Suntharasamai P, Chaiprasithikul P, Wasi C, et al. A simplified and economical intradermal regimen of purified chick embryo cell rabies vaccine for postexposure prophylaxis. Vaccine. 1994;12:508–512.
- Suntharasamai P, Warrell MJ, Viravan C, et al. Purified chick embryo cell rabies vaccine: economical multisite intradermal regimen for post-exposure prophylaxis. Epidemiol Infect. 1987;99:755–765.
- Venkataswamy MM, Madhusudana SN, Sanyal SS, et al. Cellular immune response following pre-exposure and postexposure rabies vaccination by intradermal and intramuscular routes. Clin Exp Vaccine Res. 2015;4:68–74.
- Beran J, Honegr K, Banzhoff A, et al. Potency requirements of rabies vaccines administered intradermally using the Thai Red Cross regimen: investigation of the immunogenicity of serially diluted purified chick embryo cell rabies vaccine. Vaccine. 2005;23:3902–3907.
- Bijok U, Barth R, Gruschkau H, et al. Clinical trials in healthy volunteers with the new purified chick embryo cell rabies vaccine for man. J Commun Dis. 1984;16:61–69.
- Lalosevic D, Lalosevic V, Lazarevic-Ivanc L, et al. BHK-21 cell culture rabies vaccine: immunogenicity of a candidate vaccine for humans. Dev Biol (Basel). 2008;131:421–429.
- Mahendra BJ, Narayana DA, Agarkhedkar S, et al. Comparative study on the immunogenicity and safety of a purified chick embryo cell rabies vaccine (PCECV) administered according to two different simulated post exposure intramuscular regimens (Zagreb versus Essen). Hum Vaccin Immunother. 2015;11:428–434.
- Scheiermann N, Baer J, Hilfenhaus J, et al. Reactogenicity and immunogenicity of the newly developed purified chick embryo cell (PCEC)-rabies vaccine in man. Zentralbl Bakteriol Mikrobiol Hyg A. 1987;265:439–450.
- Vodopija I, Baklaic Z, Vodopija R. Rabipur: a reliable vaccine for rabies protection. Vaccine. 1999;17:1739–1741.
- Vodopija I, Sureau P, Smerdel S, et al. Interaction of rabies vaccine with human rabies immunoglobulin and reliability of a 2-1-1 schedule application for postexposure treatment. Vaccine. 1988;6:283–286.
- Hu Q, Liu MQ, Zhu ZG, et al. Comparison of safety and immunogenicity of purified chick embryo cell vaccine using Zagreb and Essen regimens in patients with category II exposure in China. Hum Vaccin Immunother. 2014;10:1645–1649.
- Huang G, Liu H, Cao Q, et al. Safety of post-exposure rabies prophylaxis during pregnancy: a follow-up study from Guangzhou, China. Hum Vaccin Immunother. 2013;9:177–183.
- Kamoltham T, Khawplod P, Wilde H. Rabies intradermal post-exposure vaccination of humans using reconstituted and stored vaccine. Vaccine. 2002;20:3272–3276.
- Kamoltham T, Singhsa J, Promsaranee U, et al. Elimination of human rabies in a canine endemic province in Thailand: five-year programme. Bull World Health Organ. 2003;81:375–381.
- Madhusudana SN, Anand NP. Response to purified chick embryo cell rabies vaccine administered intradermally for post-exposure prophylaxis. Natl Med J India. 1997;10:115–116.
- Madhusudana SN, Anand NP, Shamsundar R. Economical multi-site intradermal regimen with purified chick embryo cell vaccine (Rabipur) prevents rabies in people bitten by confirmed rabid animals. Int J Infect Dis. 2002;6:210–214.
- Sampath G, Parikh S, Sangram P, et al. Rabies post-exposure prophylaxis in malnourished children exposed to suspect rabid animals. Vaccine. 2005;23:1102–1105.
- Sehgal S, Bhardwaj M, Bhatia R. Clinical evaluation of purified chick embryo cell antirabies vaccine for post-exposure treatment. J Commun Dis. 1988;20:293–300.
- Sehgal S, Bhardwaj M, Bhattacharya D. Immunogenicity and feasibility of purified chick embryo cell vaccine. Indian Pediatr. 1994;31:133–137.
- Selvakumar R, John TJ. Immune response to purified chick embryo cell culture rabies vaccine (Rabipur) in dog-bite victims. Indian J Med Res. 1989;89:217–220.
- Sirikwin S, Likanonsakul S, Waradejwinyoo S, et al. Antibody response to an eight-site intradermal rabies vaccination in patients infected with human immunodeficiency virus. Vaccine. 2009;27:4350–4354.
- Tanphaichitra D, Siristonpun Y. Study of the efficacy of a purified chick embryo cell vaccine in patients bitten by rabid animals. Intern Med. 1987;02:163–1658.
- Tanterdtham S, Chaiprasithikul P, Yuthavong K, et al. Follow-up of protective antibody level after post-exposure vaccination with purified tissue culture rabies vaccine (PCEC) small doses intradermally. J Med Assoc Thai. 1991;74:498–501.
- Wasi C, Chaiprasithikul P, Auewarakul P, et al. The abbreviated 2-1-1 schedule of purified chick embryo cell rabies vaccination for rabies postexposure treatment. Southeast Asian J Trop Med Public Health. 1993;24:461–466.
- Briggs DJ, Dreesen DW, Nicolay U, et al. Purified chick embryo cell culture rabies vaccine: interchangeability with human diploid cell culture rabies vaccine and comparison of one versus two-dose post-exposure booster regimen for previously immunized persons. Vaccine. 2000;19:1055–1060.
- Deshpande A, Briggs D, Branzhoff A Investigation of immune responses to purified chick embryo cell tissue culture rabies vaccine (PCECV) in HIV infected individuals using a simulated post-exposure regimen. Proceedings of XIII International AIDS conference Durban, 2000 Jul 9–14; South Africa.
- Salomao C, Nacima A, Cuamba L, et al. Epidemiology, clinical features and risk factors for human rabies and animal bites during an outbreak of rabies in Maputo and Matola cities, Mozambique, 2014: implications for public health interventions for rabies control. PLoS Negl Trop Dis. 2017;11:e0005787.
- Manning SE, Rupprecht CE, Fishbein D, et al. Human rabies prevention–United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports 2008; 57:1–28.
- Veerararghavan N. Phenolized vaccine treatment of people exposed to rabies in Southern India. Bull World Health Organ. 1954;10:789–796.
- Cornwall J. Statistics of anti-rabic inoculations in India. Br Med J. 1923;2(3268):298.
- Sitthi-Amorn C, Jiratanavattana V, Keoyoo J, et al. The diagnostic properties of laboratory tests for rabies. Int J Epidemiol. 1987;16:602–605.
- Tenzin, Dhand NK, Gyeltshen T, et al. Dog bites in humans and estimating human rabies mortality in rabies endemic areas of Bhutan. PLoS Negl Trop Dis. 2011;5:e1391.
- Rupprecht CE, Briggs D, Brown CM, et al. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the advisory committee on immunization practices. MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports 2010; 59:1–9.
- Saesow N, Chaiwatanarat T, Mitmoonpitak C, et al. Diffusion and fate of intramuscularly injected human rabies immune globulin. Acta Trop. 2000;76:289–292.
- Louie RE, Dobkin MB, Meyer P, et al. Measurement of rabies antibody: comparison of the mouse neutralization test (MNT) with the rapid fluorescent focus inhibition test (RFFIT). J Biol Stand. 1975;3:365–373.
- Lavan RP, King AI, Sutton DJ, et al. Rationale and support for a One Health program for canine vaccination as the most cost-effective means of controlling zoonotic rabies in endemic settings. Vaccine. 2017;35:1668–1674.
- World Health Organization and World Organisation for Animal Health. Global elimination of dog-mediated human rabies. Report of the rabies global conference. [ updated 1995 Dec 10–11; cited 2018 Apr]. Available From: http://apps.who.int/iris/bitstream/handle/10665/204621/WHO_HTM_NTD_NZD_2016.02_eng.pdf;jsessionid=519FC33AD28731BAAB4215B8006DC8D3?sequence=1
- World Health Organization. Rabies: guide for post-exposure prophylaxis. [ Cited 2017 Jun 7]. Available from: http://wwwwhoint/rabies/human/postexp/en/
- Centers for Disease Control and Prevention. Rabies, Medical care. [ Cited 2015 Dec 17]. Available from: http://www.cdc.gov/rabies/medical_care/index.html
- Davlin S, Lapiz SM, Miranda ME, et al. Factors associated with dog rabies vaccination in Bhol, Philippines: results of a cross-sectional cluster survey conducted following the island-wide rabies elimination campaign. Zoonoses Public Health. 2013;60:494–503.
- Davlin SL, Lapiz SM, Miranda ME, et al. Knowledge, attitudes, and practices regarding rabies in Filipinos following implementation of the Bohol Rabies Prevention and Elimination Programme. Epidemiol Infect. 2014;142:1476–1485.
- World Health Organization. Gavi’s learning agenda drives change for rabies. [ Cited 2018 Apr]. Available from: http://wwwwhoint/rabies/news/Gavis_learning_agenda_drives_change_for_rabies/en/