ABSTRACT
Introduction: Lack of cross protection between foot and mouth disease (FMD) virus (FMDV) serotypes as well as incomplete protection between some subtypes of FMDV affect the application of vaccine in the field. Further, the emergence of new variant FMD viruses periodically makes the existing vaccine inefficient. Consequently, periodical vaccine strain selection either by in vivo methods or in vitro methods become an essential requirement to enable utilization of appropriate and efficient vaccines.
Areas covered: Here we describe the cross reactivity of the existing vaccines with the global pool of circulating viruses and the putative selected vaccine strains for targeting protection against the two major circulating serotype O and A FMD viruses for East Africa, the Middle East, South Asia and South East Asia.
Expert commentary: Although in vivo cross protection studies are more appropriate methods for vaccine matching and selection than in vitro neutralization test or ELISA, in the face of an outbreak both in vivo and in vitro methods of vaccine matching are not easy, and time consuming. The FMDV capsid contains all the immunogenic epitopes, and therefore vaccine strain prediction models using both capsid sequence and serology data will likely replace existing tools in the future.
1. Introduction
1.1. Foot and mouth disease
Foot and mouth disease (FMD) is a highly contagious disease that predominantly affects animals of the order artiodactyla, with the primary hosts being cattle, sheep, pigs, and goats [Citation1]. Globally FMD is one of the most economically important livestock diseases and is currently endemic in all parts of the world except North America, Oceania, Europe and most parts of South America [Citation2](). Although FMD does not have a high mortality rate in adult animals, morbidity rates can be very high (up to 100%) often with dramatical reduction in the productivity of infected herds mainly through reduced milk yield, draught power, and weight gain [Citation3]. FMD also seriously affects the economic capability of the agricultural sector of enzootic countries by impeding exports of livestock and livestock products. Global losses are seen through both direct (reduced production and altered herd structure) and indirect (control costs and reduced market access) costs that are estimated to be US$6.5–21 billion annually [Citation3].
Figure 1. Distribution of seven endemic pools of FMD (reproduced from http://www.wrlfmd.org/).
FMD is not only a concern to those countries where the disease is enzootic. The 2001 outbreak of FMD in the United Kingdom is estimated to have cost the country around £2.7 billion and led to the slaughter of around 4 million animals [Citation4]. Such wholesale slaughter programs, like those that have been used in the EU since 1991 are becoming unpalatable to a population increasingly concerned about animal welfare. This has placed an increased emphasis on the need to develop new vaccines and control strategies.
1.2. Foot and mouth disease virus
The causative agent of FMD is foot and mouth disease virus (FMDV), a member of the genus Aphthovirus in the family Picornaviridae. It is a nonenveloped single stranded positive sense RNA virus containing a genome, ~ 8.3 kb in length enclosed in a viral capsid [Citation5]. The genome contains a single open reading frame (ORF) that codes for four structural (VP1–4) and 8–10 non-structural proteins. The viral capsid comprises 60 copies of each of the 4 structural proteins. One copy of each of the structural protein form a protomer, 5 protomers form a pentamer, and 12 pentamers form the complete capsid. VP1, 2 and 3 are on the surface of the virus and are comprised essentially of 8 antiparallel β sheets linked to each other by loop structures to form a β barrel, whereas VP4 is internal and has little secondary structure [Citation5].
Due to the lack of proof reading ability of the FMDV RNA polymerase FMDV populations exhibit high levels of genetic diversity [Citation6,Citation7]. There are seven immunologically distinct serotypes: O, A, C, SAT (Southern African Territories) 1–3 and Asia-1; serotype O is the most prevalent, followed by serotype A [Citation2,Citation8,Citation9]. Serotype C is thought to be extinct being last reported in 2004 [Citation2,Citation10]. Although the most prevalent world-wide, serotype O is not the most antigenically diverse of the serotypes; serotype A and SAT2 have been reported to be antigenically highly diverse [Citation11]. These serotypes share an approximate 86% amino acid homology to each other [Citation12]; however, some of the capsid proteins exhibit more variation, with VP1 varying by around 30–50% between serotypes [Citation13]. Within each of these seven serotypes, genetically there are several topotypes (genetically and geographically distinct evolutionary lineages) which are further divided into various genotypes (lineages or strains; these terms are used interchangeably), with up to 61 described so far [Citation13]. Within a serotype, viruses with approximately 15% nucleotide differences in VP1 sequence may be grouped into a different genotype except up to 20% nucleotide difference in SAT serotype where a greater degree of genetic diversity is observed [Citation13]. Generally different FMDV topotypes circulate within a defined geographical area and usually do not jump borders. Based on this seven virus pools have been identified () and only specific FMDV serotypes are present within a pool although occasional spread to neighboring areas is not uncommon. Globalization of commerce and trade, migration of people and lack of cross-border animal movement control play a big role in the epidemiology of FMDV.
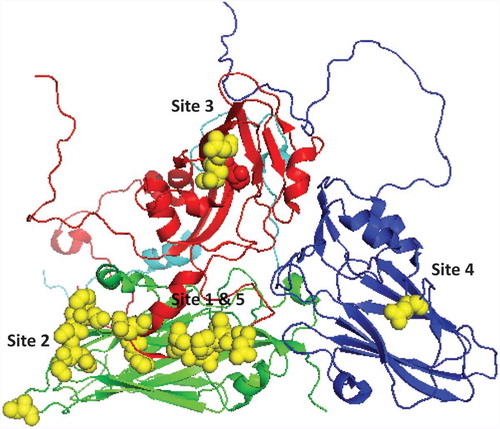
1.3. Neutralizing antigenic sites of FMDV
Crystallographic studies have identified the structure of the FMDV capsid [Citation14] and immunological epitopes have been mostly found on the surface oriented interconnecting loops between structural elements. Till date five neutralizing antigenic sites (1–5) have been reported for FMDV through monoclonal antibody (mAb) neutralization resistant (mar) mutant studies () [Citation15–Citation19]. Out of these, site 1 is linear and trypsin sensitive whereas the rest are conformation dependent. The βG-βH loop and carboxy terminus of VP1 contribute to antigenic site 1 with critical residues at positions 144, 148, 154, and 208. Amino acid residues at position 70–73, 75, 77, and 131 of VP2 contribute to antigenic site 2. In addition amino acid residues at position 79 and 134 have been reported to influence binding of site 2 mAbs [Citation20]. Antigenic site 3 is formed in part by residue 43 and 44 of the βΒ-βC loop of VP1 whereas amino acid residues at position 56 and 58 of VP3 have been reported to be critical for antigenic site 4. Antigenic site 5, characterized by an amino acid at position 149 of VP1, is probably formed by interaction of the VP1 loop region with other surface-oriented amino acids. Recently attempts have been made to predict epitopes using capsid sequence data and 3-D structure of FMD, and these epitopes can be tested through mutational analysis by reverse genetics [Citation9,Citation21,Citation22]. Using a similar approach a new epitope (VP2 191) has been reported to be a part of antigenic site 2 in serotype O and A [Citation21,Citation23].
2. FMDV vaccines
FMD vaccines consist of chemically inactivated purified whole virus preparations. The high level of antigenic variation in FMDV means that currently there is no universal vaccine that can afford protection against all serotypes of the virus. Indeed current vaccines can fail to cross protect some strains of the same serotype [Citation24,Citation25]. Vaccines currently consist of varying formulations of inactivated viruses of different serotypes. Unfortunately, unlike the broadening of protection that has been witnessed following multiple challenges [Citation26], vaccination with multiple serotypes or strains has not much effect on broadening the protection conferred by each vaccine individually [Citation27–Citation29]. However, it has been demonstrated that by increasing the potency of the vaccines the range of protection elicited by vaccination can be increased [Citation30,Citation31].
Current vaccines have been used successfully to aid in the eradication of the virus from many areas of the globe [Citation32]. However, these vaccines require the growth of large quantities of virus (requiring high containment facilities) and must be adapted constantly to keep the vaccine strains current to those in circulation. These vaccines also only provide a short-lived protection (4–6 months) [Citation25] and require cold chain delivery making the vaccination logistically problematic and costly in endemic areas. Additionally studies have demonstrated that the virus can persist in the epithelium of the pharynx in over 50% of cattle exposed to the virus, even in immunized animals [Citation33].
In areas experiencing reintroduction of FMD, the decision of whether or not to vaccinate can have profound implications for trade and disease free status. The 2001 outbreak in the United Kingdom and the Netherlands brought about a change in EU policy and it is now possible for a country to be declared free from disease 6 months after an outbreak that has utilized a vaccinate to live policy (EU directive 2003/85/EC). This taken with the development of non-structural protein ELISAs that are able to serologically differentiate between infected and vaccinated cattle [Citation34] means that it may become the favored policy to utilize current vaccines as emergency vaccines. This could be economically very favorable for countries hit by the reintroduction of infection through preventing the wholesale slaughter of animals
Ideally future vaccines should aim to fulfill a wide range of criteria, being able to provide a universal protection against all strains of FMD with long lasting immunity. Additionally these vaccines should also be cheap to produce and delivered in a cost-effective manner. However any vaccine that could improve upon those currently available would be advantageous [Citation35].
3. Vaccine matching and selection
In endemic countries vaccination is the cheapest method of disease control. However antigenic diversity between serotypes and genotypes means that vaccination with another serotype or other genotypes of the same serotype may fail to control the disease [Citation20,Citation24,Citation36]. In addition, new variant viruses are emerging periodically and antigenic mismatch is one of the main reasons of vaccine failure [Citation37]. Consequently, the vaccine strain requirements differ according to the serotypes and genotypes of virus prevailing in or threatening different regions, and vaccines have to be selected with care [Citation24,Citation25]. The FMD free countries usually store various strains of inactivated FMDV antigen over liquid nitrogen in an antigenic reserve/vaccine bank, ready for immediate formulation into vaccine if required during an emergency. The vaccine strains stored in reserves, or banks, are generally chosen on the basis of the perceived coverage against a number of different field isolates circulating globally. However, the wide antigenic variation within and outside the different serotypes highlights the importance of continually monitoring FMD worldwide [Citation24,Citation25]. The antigenicity of FMDV can change because of the frequency of mutations that occur in its RNA genome, which sometimes allows evasion of the immunity provided by the vaccines resulting in emergence of immunologically distinct variants [Citation37]. It is therefore necessary to monitor the field isolates for their relatedness to the available vaccines in order to assess if any vaccine will provide protection against any new isolate. In any FMDV incursion, where vaccination is to be implemented as a control measure, it is important to match the vaccine, antigenically, as closely as possible to the causative field isolate.
Use of matching vaccine is crucial for successful implementation of vaccination based FMD control policy. There are two different methods to determine the most appropriate vaccine to combat the disease: in vivo and in vitro. In in vivo matching test the animals are vaccinated with the vaccine to be tested for a defined period of time and challenged with the field virus. The in vitro matching tests measure the cross-reactivity of a bovine postvaccinal sera with the field strain in question, and the results are usually expressed as antigenic relationship values (r1-values). An ‘r1-value' is defined as: the reactivity of serum against the heterologous (field) strain divided by the reactivity of serum against homologous (vaccine) strain [Citation38].
3.1. In vivo vaccine matching
In vivo cross-protection test is the direct comparative matching test where the vaccinated animals are challenged with the outbreak virus. Whilst highly effective at assessing protection the vaccination and challenge of animals with live virus is time consuming, costly and requires extensive infra-structure, including provision of appropriate biosecurity procedures and practices which are always not available. So far few studies have reported attempts to measure cross-protection directly. Brehm et al. (2008) conducted several heterologous protection studies using FMDV serotype A viruses, and in all cases the in vitro r1-value suggested that the vaccine virus was not a good match [Citation30]. In this study cattle were vaccinated with one serotype A vaccine and challenged by another heterologous strain. In six out of the eight cases PD50 above 6 were obtained, which means the response is above what is needed for the emergency vaccine bank. This study demonstrated that high potency emergency vaccines could provide cross-protection between different subtypes of serotype A strains even though the r1-value suggested that the vaccine was not a good match [Citation30]. Similarly in-vivo studies in South America involving serotype O vaccines reported appropriate protection following challenge whereas the in-vitro matching values (r1-value) were below cut-off [Citation39]. In contrast Nagendrakumar and colleagues (2011) conducted a cross protection study vaccinating animals with O1/Manisa vaccine and challenging with O1/Campos virus [Citation31]. Though the r1-value suggested the vaccine to be a good match, there was a poor correlation between serology and in vivo cross-protection results. This study also reported that cross-protection required very high potency vaccines. Similarly in a heterologous challenge experiment animals vaccinated with a highly potent (> 6 PD50) FMD O1/Manisa vaccine were protected upon challenge with an O/SEA/MYA-98 virus (O/SKR/2010), although the in vitro r1-value was at the lower end of an acceptable threshold (~ 0.3) [Citation40] and partially protected when challenged with the O/ME-SA/Ind-2001 lineage field virus where the r1-value was very low [Citation41]. Similarly a further study in pigs reported an incomplete protection when pigs vaccinated with a highly potent (> 6 PD50) FMD O1/Manisa vaccine were challenged with the O/SEA/MYA-98 virus (O/VIT/2010) [Citation42]. These studies indicate a lack of certainty on the correlation of the results between the in vitro and in vivo tests, and therefore the design of such experiments warrants further investigation.
3.2. In vitro vaccine matching
The humoral immune response is thought to be an important component in vaccinal protection to FMDV as there is a strong correlation between antibody titer and protection against virus generalization in cattle challenged at 21 days after vaccination [Citation43]. This holds true whether antibody titers are calculated based on in vitro virus neutralization tests (VNT) or binding assays (ELISA) [Citation43–Citation45]. Furthermore, adoptive transfer of immune anti-FMDV serum or mAbs prior to challenge with FMDV can confer protection to mice and guinea pigs [Citation46]. As observed with numerous other viral infections, the precise mechanism of in vivo protection remains largely unknown, although it has been speculated that opsonization of free virus may be involved, since protection occurs at dilutions of antibodies below that needed for in vitro neutralization [Citation44]. If so, then antibodies that merely bind as well as those that are neutralizing in vitro may have a role in protection. Therefore, serological tests using postvaccinal serum can provide very useful information in terms of cross-reactivity of the vaccine.
Several indirect vaccine matching (in vitro) tests can be performed serologically. These tests measure the reactivity of a bovine post-vaccinal serum (BVS) raised against the vaccine strain to be tested, with the field virus in question. In FMD, three serological methods namely (i) two-dimensional virus neutralization test (2D-VNT), (ii) liquid phase blocking enzyme linked immunosorbent assay (LPBE) and (iii) complement fixation tests (CFT) are the standard methods by which candidate vaccine strains are evaluated for selecting cross-protective vaccine strain [Citation38]. Using in vitro vaccine matching, a one-way indirect antigenic relationship value (“r1-value”) is determined for each reference BVS.
In order to conduct vaccine matching tests, the field isolates need to be serotyped and adapted to cell culture growth conditions to enable virus titers to be reached for serological assessment. Many factors can influence the results including variation in the cell lines used between different laboratories, and poor viral growth in cell culture leading to requirements to adapt the isolates to different cell lines. Most laboratories usually use bovine thyroid cells (BTY) or pig kidney cells (IBRS) or baby hamster kidney cells (BHK), and the results could vary depending on the cell line used for isolating/growing the virus, and also in the vaccine matching tests. In most cases the test virus may require 2–3 passages in cell culture minimally to achieve the titer required for the test. In a pool of different heterogenous genomes (quasispecies) within a strain the propagation leads to selection of viruses that are fit to bind and replicate within the cell [Citation6,Citation7]. The number of propagations carried out is therefore kept to a minimum, but the exact effect that this process has on the viral populations is largely undefined [Citation6,Citation7]. Further these viruses need to be titrated on multiple occasions to determine optimal dilution for serological testing. Importantly, the development of these tools cannot compete with the natural transmission rate of FMDV in an outbreak scenario and under emergency outbreak conditions, the results from any previous outbreaks for which vaccine matching tests were completed may help inform on vaccine strain selection provided that the challenge virus remains unchanged.
3.2.1. VNT
This is a widely used method for FMDV vaccine matching and detects protective immune response in vitro. Vaccine match is evaluated by comparison of the serum titer of a field strain to that of the vaccine strain against which the vaccinate serum was prepared. A VNT is carried out to determine the serum titer at a virus dose of 100TCID50 [Citation47]. A 100TCID50 is chosen because this is located at the linear part of the curve between the neutralization titer and the virus dose. This test is the gold standard for vaccine matching in FMDV and is preferred over LPBE and/or CFT. The VNT uses five virus doses and linear regression in what is known as a two dimensional checkerboard titration. This method has the advantage of being able to predict the neutralization titer at a fixed virus dose (100TCID50) and allows for greater flexibility in the virus titration that may occur due to cell variation. However, this method is very labor intensive and time consuming. The results of this test are usually interpreted as r1 value and a value equal to or greater than 0.3 suggests that the vaccine is most likely to protect against the field virus in question, depending on the number of repeats [Citation48,Citation49]. An r1-value less than 0.3 indicates a dissimilar match indicating urgency to develop a new vaccine.
3.2.2. LPBE
Liquid Phase Blocking ELISA detects any residual antigen remaining after overnight reaction between dilutions of serum and a pretitrated virus dose (Kitching et al., 1988) and is used in different FMD reference laboratories for vaccine matching. This test has the advantage over virus neutralization tests in that the test is rapid (the result can be read in one day versus waiting three days in VNT) and uses smaller volumes of postvaccination sera that are mostly available in limited amount. In the LPBE an r1-value of 0.4 is considered indicative of a good vaccine match; an r1-value between 0.2 and 0.4 showing significant differences from the vaccine strain but there may a good enough level of cross protection depending on the potency of the vaccine [Citation50].
No significant difference is usually observed between the VNT and LPBE results [Citation51]; however, there are occasional mismatches between the two sets of results which is probably because in the neutralizing assay the virus needs to be able to escape serum neutralization and replicate in cells and cause cytopathic effect, while the LPBE only measures binding of antibodies to immobilized antigen. In cases where the VNT and ELISA methods do not give congruous outputs, it is difficult to conclude which method gives the best indicator of protection in the host although neutralization of live virus must be considered a more appropriate indicator of serum neutralization. Ultimately, these serological tests quantify the ability of BVS to neutralize or bind field strains of FMD. However, they do not measure any potential antibody-mediated opsonization, which is thought important for a protective immune response (37). Along with the role of cellular immunity, antibody mediated opsonization could be one of the reasons of the discrepancies between VNT/LPBE titers and protection in the field.
3.2.3. CFT
Similar to LPBE, CFT also measures the relationship between a vaccine strain and the field isolate using a guinea-pig antiserum raised against the vaccine strain [Citation38]. In CFT, an r1-value of 0.25 is used as a cut-off point and r1-values greater than 0.25 indicate the field isolate to be antigenically close to the vaccine strain, and use of the vaccine is likely to confer protection against challenge with the field strain [Citation38]. The use of this test is now largely restricted to the South American countries, however much less in recent years. The CFT is usually recommended as a screening test.
The VNT and LPBE are currently the gold standard tests for FMDV vaccine matching, however the antisera required are very expensive to produce, inherently variable, and the tests are not always reproducible. Therefore it is very difficult to standardize the tests or have full confidence in the results without performing numerous experimental repeats. In addition, another disadvantage of both the assays (VNT and LPBE) is that they may not accurately reflect what will happen in vivo. Cellular immunity such as antibody-mediated opsonization and phagocytosis of the pathogen also play a role in vivo. A way to quantify and combine these results with the VNT and LPBE is also not easy. Further these serological tests can be laborious, tedious and time consuming, which is not ideal in a situation where a rapid response and deployment of an appropriate vaccine is needed. Therefore there is an urgency to improve existing methods or develop new methods for rapid vaccine strain selection.
3.3. Alternative approaches
3.3.1. mAb based ELISA
Though useful information is obtained from the serological matching tests (VNT, ELISA, CFT), there are various factors that may affect the results obtained from these in vitro tests. A major problem is the standardization of the polyclonal serum raised against the vaccine viruses including both the sera collected from vaccinated target species (usually cattle) and also the antisera raised in rabbits and guinea-pigs for use as capture or detector antibodies in ELISA/CFT. There is often animal to animal variation as well as batch to batch variation of the antigen. An approach to overcome these limitations to reproducibility involves the use of consistent and readily replenishable reagents such as monoclonal antibodies (mAb) for vaccine matching test [Citation20]. Monoclonal antibodies have been used in antigenic profiling studies for FMDV [Citation20,Citation52,Citation53]. In a previous study we explored the possibility of replacing the polyclonal antibody (PAb)-based neutralization assay with the mAb based method; however, it was clear that the mAb based system for FMD vaccine matching was not yet comparable with the gold standard neutralization assay system suggesting further improvement [Citation20]. Potential improvements include (i) inclusion of a wider panel of mAbs with at least one mAb for each antigenic site in order to obtain a wider spectrum of the mAb reactivity, (ii) inclusion of more than one mAb for each antigenic site, (iii) use of universal ligands that can ensure trapping of equivalent amount of virus particles on to the ELISA plate. Considering the ultimate goal of this system is to measure the reactivity in terms of protection inclusion of mAbs recognizing protective epitopes seems a sensible approach. Though a strong correlation has been reported between virus neutralizing antibody and protection in bovids, one of the main target species [Citation54] non-neutralizing epitope/(s) have also been suggested to be contributing to FMD vaccine protection [Citation46]. However very few studies have tried to identify the epitopes recognized by non-neutralizing mAbs and their contribution to FMD vaccine protection and further work in this area is warranted to explore these issues. Another possibility to improve the currently used mAb based ELISA system would be equalization of the amount of virus captured to the plate. The mAb-based system uses serotype-specific polyclonal rabbit antisera as the capture antibody. Based on the antigenic relatedness of the field isolate/(s) with the vaccine virus the amount of virus trapped may vary greatly. Though every effort was made in this study to equalize the amount of virus trapped by the capture antibody, it could be variable [Citation20]. Therefore, there is a need to use a universal ligand which may ensure trapping of equal amount of virus. Work is ongoing in our laboratory to improve the antigen profiling ELISA system using a universal ligand and a wider panel of mAbs.
Considering new viruses are emerging periodically [Citation6,Citation37], this approach does not seem feasible and practical as there is requirement of availability of well-defined mAbs that are capable of recognizing differences between the vaccine virus and the field isolate, preferably sets of available mAbs for each and every vaccine strain, and for each antigenic site. In addition the immunodominance of each antigenic site in relation to protection also need to be resolved and this may vary between serotypes. Currently, limited information is available only for serotype O in this respect [Citation55]. Producing panels of mAbs specific for each and every vaccine strains is unrealistic, and as such newer approaches using modern techniques needs to be explored.
3.3.2. New approaches
3.3.2.1. Antibody avidity test and isotype ELISA
During the maturation of the humoral immune response, there is an antibody selection process that results in synthesis of antibodies with increased antigen-antibody association strength. This antigen-antibody association strength can be expressed as antibody avidity. The avidity of pathogen-specific antibodies have been implicated as playing an important role in antibody-mediated protection where the induction of high-avidity antibodies is desired [Citation56,Citation57], and has been demonstrated to correlate well with virus neutralizing antibodies for other pathogens including Bovine Viral Diarrhoea virus (BVDV), Vesicular Stomatitis virus (VSV), Measles, Tick-borne encephalitis virus (TBEV) [Citation56–Citation59]. The avidity of antibodies has been associated with the opsonizing capacity of the antibodies, although this has been poorly studied in relation to FMDV capsid epitopes. Antibody avidity is usually determined after exposure of antigen-antibody complexes to chemicals that destroy the low-avidity bonds between the antigen and antibody. It is hypothesized that the avidity and isotype of the antibodies induced by FMDV vaccination could be related to the protective potential of the vaccine [Citation60]. Usually antibody avidity increases with the vaccine dose and unprotected animals have been shown to have low-avidity antibodies [Citation60]. Similarly unprotected animals exhibited a proportionately lower IgG1/IgG2 ratio than protected animals [Citation59]. The test has the advantage of being rapid and less time consuming than the gold standard neutralization test as it does not involve use of live viruses or cells. Therefore it has the potential to be used as alternative to existing serological tests for FMD vaccine strain selection where usually both high and low avidity antibodies are measured. This test requires sucrose-density gradient (SDG) purified 146S particles to serve as antigen in the ELISA [Citation60] and making SDG purified antigen for a large number of outbreak strains for routine vaccine matching is logistically impractical. Therefore, this technique may be adopted as a complementary test to the currently used serological assays for vaccine matching [Citation61]. However, from a research perspective the avidity of different monoclonal and polyclonal antibodies may provide additional information to elucidate the induction of vaccine-induced cross-protection in the target species [Citation61].
3.3.2.2. Cartography
Another method by which vaccine selection can be undertaken is by analysis of serological relationships between viruses using antigenic cartography techniques as applied to human influenza vaccine selection [Citation62,Citation63]. Antigenic cartography is a computational technique which facilitates quantification and simple visualization of antigenic relationships of vaccine strains and field viruses on a map of two or more dimensions based on their antigenic distance generated from normalized serological data [Citation62,Citation64]. This technique allows measurement of antigenic relationships between antibody and antigen by calculating coordinates in multiple dimensions relating to different physio-chemical properties of the antigen/antibody bond. The advantage of this technique is that multiple relationships are revealed so that a limited amount of serological testing on a new virus can reveal its antigenic similarity with many vaccine strains and field viruses. Currently, antigenic cartography is used for human seasonal and pandemic influenza vaccine strain selection and has contributed to our understanding of the global circulation of Influenza A (H3N2) virus. The technique can also detect emerging variants based on differences in antigenic distances. When antigenic cartography is supplemented with careful sequence comparison at least in defined antigenic sites, the residue positions responsible for variation could be identified [Citation65,Citation66]). The technique was applied to human influenza [Citation62], equine influenza [Citation67], swine influenza virus [Citation68] using serum titers obtained from heamagglutination inhibition (HI) test, and has also been applied to other viruses like rabies [Citation69] using virus neutralization test data to characterize and understand differences in antigenic relationships and evolution. Recently, manipulation of antigenic sites within lyssaviruses has been attempted to assess the role of different antigenic epitopes [Citation70] and similar approaches may be utilized for other viruses including FMDV. The only report of antigen cartography in FMDV using serum (VNT) titers was carried out assessing serotype A [Citation66], however this could be extended to other FMDV serotypes. It is anticipated that the antigenic map for FMDV can be utilized with the same success as influenza and may indicate potential options for cross reactivity of defined antigens.
3.3.2.3. Sequence based approaches
Sequence-based vaccine strain selection is the latest development and probably a potential area for further research as all the residues responsible for variability and escape from vaccine protection are defined by sequence data. The “r1-value” is affected by antigenic variation which in turn is the consequence of genetic evolution leading to amino acid substitution [Citation71]. Such amino acid substitutions can be deduced from gene sequence data and used for antigenic site prediction and related analysis. Sequence data can be generated rapidly and economically and is used for genetic characterization for detecting emerging variants. The sequence data can also be used for vaccine strain selection with or without inputting serology data, although establishing predictive models based on both serological and capsid sequence data might help in rapid selection of suitable vaccine strains.
3.3.2.3.1. FMD vaccine strain selection from capsid sequence
In silico predictive models of viral cross-reactivity, which derive antigenic distances from sequence data and phylogenetic history for FMDV has been developed for serotypes SAT1 and SAT2 [Citation72]. In this model, although serological data was used at early stages of model development, subsequent generation of serological data is not required, and by feeding the program with new isolate amino acid sequence data, it predicts whether the existing vaccine can provide protection or not as the model refers to critical residue changes in the antigenic sites and/or epitopes. The technique also involves prediction of epitopes by combining serological test results and changes in amino acid sequence [Citation72]. Alternatively, it is performed by filtering residues important for antigenicity using bioinformatics methods. The results of the predicted epitopes in SAT1 and SAT2 viruses by this approach were consistent with antigenic sites previously determined by mar-mutant studies [Citation72–Citation74], and provides a quick and alternative method for antigenic site identification and vaccine strain selection.
This work has now been extended to serotype O [Citation74] and A [Citation21,Citation75]. Work carried out to predict vaccine match for serotype O, and cross-validated predictions using these models, have shown it to be as good as the serological assay itself, with its predictions falling within the consensus 95% confidence limits derived from the whole dataset. Such predictions have included multiple repeats of each test with confidence interval being similar to those generated through testing using classical laboratory-based serology tests (71.8% vs. 71.4%). However, further work is required to enhance the resolution beyond this (e.g. by including serological data for additional, antigenically distant sera as all the antisera used in this study are clustered together antigenically), before the approach can be used routinely in diagnostic laboratories. This work has also led to the identification of residues in antigenic sites 2 and 1 (in order of significance) on the surface of the virion that are of antigenic interest. It has also enabled the quantification of the effect of the mutation of these residues with corresponding alteration in serological neutralization. This was supported by our recent study of the antibody repertoire of bovine post-vaccinal sera [Citation55]. One novel epitope identified by the prediction model is the residue at position VP3 220 which is supported by other observations i.e. critical residue (VP3 218) in serotype Asia 1 by mar-mutant studies [Citation17], by epitope prediction softwares using FMDV crystal structure data of 4 different serotypes (O, A, C, and SAT 1) [Citation9], and also in serotype A [Citation21]. The significance of predicted amino acid residues can be tested in vitro by reverse genetics. Once tested and validated, the new models would then allow rapid selection of suitable vaccine strains from capsid sequence data without the need for new serology work with the existing vaccines. Moreover, the necessity to develop a new vaccine strain could also be identified as well as the suitability of a field isolate for adaptation and development of a new vaccine strain.
4. Global vaccine strain selection
The vaccine strains used in a particular geographical region mainly depend on the serotypes and genotypes circulating in the region. Accordingly there is no universal vaccine for FMD control as different vaccine strains are used in different geographical region. Currently no coherent information is available regarding the use of vaccine strains in different country/continent, and available information may vary from one country to another. The World Reference Laboratory for FMD (WRL-FMD) usually recommends vaccine strains for inclusion in the antigen reserves, and for different regional pools. However this is generally treated as a guideline whilst in reality the situation could be different in the field as many outbreaks are not reported or investigated. Generally, FMD-free countries have access to the antigen and vaccine reserves that could provide protection against the viruses that are thought to be a threat to their country. However with globalization of trade and increasing migration of people there is always the possibility of introduction of new strains of FMDV to a region. Therefore vaccine strains with broad immunological coverage are preferred, and it is important to assess the capability of the vaccine viruses to elicit effective cross-protection against several circulating strains, including extracontinental isolates [Citation76,Citation77]. Currently there are not many studies reporting systematic vaccine matching work using viruses from across the globe. In addition, where available, reports do not cover all the serotypes/strains circulating in the region.
4.1. Serotype O
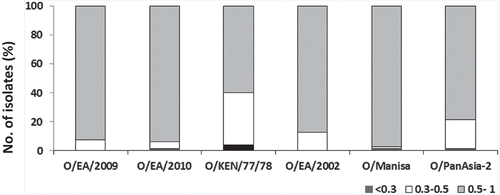
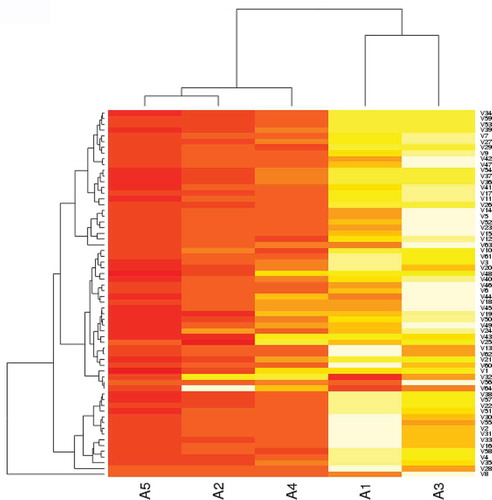
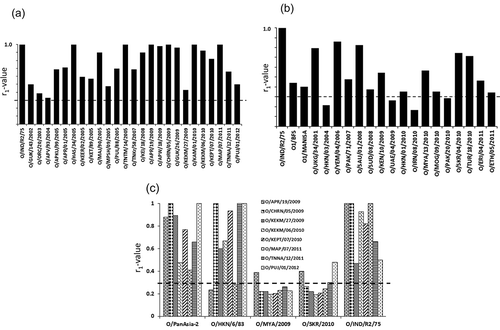
This serotype causes more than 60% outbreaks worldwide [Citation2] and exists as 11 genetically distinct topotypes which are further divided into different genotypes. Currently there are three main serotype O vaccines in use across the globe. (a) O/Campos (Euro-SA topotype), selected and harmonized for use in South America; (b) O/Manisa or O/PanAsia-2 (ME-SA topotype), mainly used in the Middle East, Asia including South East Asia and also in some countries in Africa; (c) O/IND/R2/75 (ME-SA topotype), mainly used in India. South America, China and India only allow production and use of approved vaccine strains originating from their country or region. In addition some countries use locally produced vaccines in their control programs. Generally serotype O vaccines are broadly cross reactive often exhibiting in-vitro protection against a number of genotypes [Citation13,Citation76–Citation79]. O/Manisa has been effectively used as a broadly reactive vaccine for more than five decades; however it has not been able to protect against the viruses circulating in the Middle East in the last few years resulting in development of the O/PanAsia-2 vaccine. Similarly the use of O/Manisa vaccine in the Far East (South Korea and Japan) has not provided optimum cross-protection [Citation79–Citation81] emphasizing the requirement of a broadly reactive serotype O vaccine for the region. In a previous study using 74 serotype O viruses collected across the globe; Africa (n = 13), Asia (n = 26), Europe-South America (n = 20), and Southeast Asia (n = 15) we evaluated four established serotype O vaccines (O/BFS, O/Kaufbeuren, O/Manisa, O/PanAsia-2) and one putative serotype O vaccine, O/UKG/2001 [Citation74]. The results revealed O/PanAsia-2 to be the best vaccine followed by O/Manisa (). In South America the O/Campos vaccine has been used for routine vaccination for more than five decades and still matches with the viruses circulating in the region. In a recent study this vaccine was tested against single virus isolates representing three topotypes (SEA, ME-SA, and Cathay), and exhibited acceptable in vitro cross-protection [Citation76]. However the Cathay topotype virus O/Taiwan showed an average r1-value of 0.3 (two values: 0.35 and 0.25) which indicates that this vaccine may not protect against infection with Cathay viruses unless highly potent antigens are included in the vaccine, and a strong response to vaccination is elicited [Citation30,Citation31,Citation40].
Figure 3. Heatmap and clustering analysis of VNT titers of 74 serotype O viruses with five antisera. Viruses are of global origin and clustered according to their neutralization profiles along the vertical axis. Similarly the antisera are arranged according to their abilities to neutralize the panel of viruses along the horizontal axis. A1: O/BFS, A2: O/Manisa, A3: O/Kaufbeuren, A4: O/UKG, A5: O/PanAsia-2. Darker color indicates higher VNT titer.
Recently another strain of the ME-SA topotype, O-Ind-2001d originating from the Indian subcontinent has spread to new areas and caused new outbreaks. The O-Ind-2001 viruses have been circulating in India since 2001 and outcompeted PanAsia from the field in 2009 [Citation82]. Gradually this lineage spawned four different (a–d) sublineages out of which O-Ind-2001d first emerged in the Middle East (Iran) as early as 2009 and North Africa (Libya) in 2013 [Citation83]. These viruses have also spread to several countries in the Middle East (UAE, Saudi Arabia, Baharin, Jordan) and in North Africa (Algeria, Morocco, Tunisia). These O-Ind-2001d viruses have also spread to the Southeast Asia region, initially being detected in the People’s Democratic Republic of Lao (Lao PDR) in 2015 with spread since to neighboring countries including China, Myanmar, South Korea, Thailand, Russia, and Vietnam [Citation79] thereby complicating the epidemiological situation and also emphasizing the importance of a matching vaccine for use in FMD control programs. Vaccine matching studies involving O-Ind-2001d viruses isolated from India between 2009 and 2012 revealed O/PanAsia-2 and O/IND/R2/75 vaccine to be a good match [Citation79].
4.1.1. East Africa
In East Africa four East African topotypes (EA 1-4) of serotype O viruses are known to be circulating [Citation78]. In addition ME-SA topotype (PanAsia) viruses have been detected in Libya and Egypt probably because of trade links with the Middle East countries. Another strain of the ME-SA topotype, Ind2001d, originating from the Indian subcontinent has also been reported as circulating in Libya since 2013. These Ind2001d viruses have also spread to North African countries including Tunisia, Algeria, and Morocco. Most of the FMD outbreaks in the region are caused by serotype O followed by serotype A and SAT 2 [Citation2,Citation8]. There is no clear information available detailing the use and availability of vaccines in this region. There are vaccine production plants in Ethiopia and Kenya that mainly produce vaccine strains isolated from their own country () though Ethiopia has recently imported O/Manisa and A22, and may not cater to the need of other East-African countries. In addition candidate vaccine strains, two each from serotype O, A and SAT2 were evaluated recently in vaccine matching tests using a limited number of field isolates from Ethiopia [Citation8], although no information is currently available as to whether they have been taken forward for vaccine production. Some countries also import vaccine from Botswana (BVI). Regular vaccine matching studies are not carried out and no report is available on the efficacy of these vaccines to protect from the circulating viruses. We have carried out vaccine matching studies using 80 serotype O viruses collected over a 20-year period between 1993 and 2012 by 2D-VNT using postvaccination bovine sera raised against six vaccine strains [Citation78]. Out of these, O/KEN/77/78 is the only established vaccine originating from the region. The vaccine strain of ME-SA topotype, O/Manisa and O/PanAsia-2 were also included to test their suitability to be potentially used as vaccines in the region. In addition, antisera generated against three putative strains representing the predominant topotypes (two from EA-2 and one from EA-3) were also included in this study. All the six antisera exhibited cross-reactivity with the circulating field isolates though only about 60% of the viruses exhibited r1-values in the range of 0.5–1 (good match) against the established vaccine (O/KEN/77/78) from the region, whereas the other vaccines matched well with > 75% viruses in the same range, O/Manisa being the best followed by O/Pan Asia-2 (). A relatively high number of isolates (~ 36%) exhibited low positive r1-values in the range of 0.3–0.5 (medium match) against O/KEN/77/78. In particular, this vaccine (O/KEN/77/78) also showed comparatively lower percent in vitro match against the predominant topotypes (EA-2 and EA-3) circulating in the region (). Therefore, continuous vaccine matching studies of the outbreak strains was suggested as being essential in order to continue using O/KEN/77/78 vaccine in FMD control program in the region. Serotype O Ethiopian candidate vaccine strain belonging to EA-3 and EA-4 topotype have been reported to be a good match (in-vitro) with the serotype O Ethiopian isolates [Citation8]. The Indian type O vaccine (O/IND/R2/75) has also been reported to cross react with viruses from East Africa, mainly Eritrea, Ethiopia, Kenya, and Sudan [Citation77]. Therefore, it appears that the antigenic nature of the type O viruses circulating in the region has not changed greatly, and in addition to the type O vaccine originating from the region the vaccines belonging to the ME-SA topotype can also be used in East Africa to control the disease.
Table 1. FMD vaccines used in the East African countries.
4.1.2. The Middle East
The Middle East currently acts as a hub for viruses coming from South Asia and also from North and East Africa [Citation84] as exemplified by the detection of O-Ind-2001 and A-Genotype VII viruses from the Indian subcontinent, and SAT 2 viruses from Africa probably introduced via trade links. The serotypes circulating in this region are mainly O, A and Asia 1 with occasional incursion of SAT 2 viruses such as seen in Bahrain and Palestine in 2012, and Oman in 2015 (http://www.wrlfmd.org/fmd_genotyping). There are two broadly cross-reacting vaccines of ME-SA topotype, O/Manisa and O/PanAsia-2 originating from Turkey. The broadly cross reactive O/Manisa vaccine was found to be not a good match for the currently circulating viruses resulting in the development of O/PanAsia-2 vaccine. The serotype O outbreaks in the region are mainly controlled by these two vaccines though some countries use locally produced vaccines.
4.1.3. South Asia/Indian subcontinent
The serotypes circulating in this region are O, A, and Asia 1. Serotype O has been reported to be the cause of more than 90% outbreaks in the last three years (72). The predominant topotype circulating in the region is ME-SA. Two lineages (O-Ind-2001 and O-PanAsia-2) of ME-SA topotype are currently circulating in the region out of which O-Ind-2001 was first recorded in 2001. In 2008, this lineage re-emerged and out-competed PanAsia from the field by 2009 [Citation85] and continued to be the predominant genotype in the region. This genotype has diversified into four sublineages (a–d) in due course of time out of which O-Ind-2001d has recently spread to the Gulf States, North Africa and Southeast Asia. Like South America, Indian authorities harmonize and select FMD vaccine strains to be used in the subcontinent. India only allows production and use of approved vaccine strains originating from the region. The currently used vaccine strains are O/IND/R2/75, A/IND/40/2000, and Asia 1/IND/63/1972 for serotype O, A, and Asia 1, respectively. The Indian serotype O vaccine O/IND/R2/75 has been reported to be broadly cross-reactive (67, 72). Vaccine matching studies using 35 serotype O Indian viruses collected over a 3-year period (2010–2012) revealed the Indian serotype O vaccine, O/IND/R2/75 to be a good match and their use was recommended in the control program [Citation85]. In a recent study we evaluated the suitability of the Indian vaccine strain, O/IND/R2/75 by vaccine matching studies (2D-VNT) using 25 serotype O viruses collected from India over a period of 11 years (2002–2012) [Citation77]. The results revealed the Indian vaccine, O/IND/R2/75 to be broadly cross-reactive, matched with the currently circulating viruses from the Indian subcontinent (). In addition, we also studied the protective (in vitro) ability of this vaccine (O/IND/R2/75) with 17 serotype O viruses collected over a 11-year period (2001–2011) from three continents, Asia (n = 13), Europe (n = 2), and East Africa (n = 4). The vaccine was a good match with 79% viruses employed in the study including viruses representing topotypes, East Africa-2, -3, and MYA-98 strains () [Citation77].
Figure 5. Antigenic relationship (r1) values of serotype O Indian isolates (A) and isolates from other countries (B) against O/IND R2/75 vaccine antisera and (C) serotype O-Ind-2001d viruses against five post-vaccinal bovine antisera. The horizontal dotted line indicates the cut-off value of 0.3, above which the vaccine is considered to be a good match. Reproduced from (92) under Creative Commons Attribution License (CC BY).
The O-Ind-2001d viruses are responsible for the majority of the recent serotype O outbreaks in India [Citation82] and have also been detected in the Middle East, North Africa and Southeast Asia emphasizing the importance of a matching vaccine for use in FMD control programs. A previous vaccine matching study involving eight O-Ind-2001d viruses isolated from India between 2009 and 2012 revealed O/PanAsia-2 and O/IND/R2/75 vaccine to be a good match () [Citation79]. In a recent study cattle vaccinated with a highly potent (homologous 17 PD50/dose) O1/Manisa vaccine were partially protected upon challenge with a O/ME-SA/Ind-2001 lineage virus from North Africa with an estimated heterologous potency of about 3.5 PD50/dose [Citation41]. Therefore further work is warranted to ascertain the suitability of the existing serotype O vaccines to protect from the currently circulating O-Ind-2001d viruses, and to establish if there is a need to develop a new vaccine.
4.1.4. Southeast Asia (SEA)
The serotypes circulating in this region are O, A, and Asia 1. Asia 1 has not been reported since 2006 though circulation of this topotype in Cambodia is suspected. Three topotypes (SEA/Mya-98, Cathay, and ME-SA/PanAsia and ME-SA/Ind-2001d) from serotype O and one topotype (Asia/Sea-97) from serotype A are currently circulating in the region. The SEA topotype was the predominant topotype in Southeast Asia, however, in 1999–2002 the viruses of the PanAsia lineage caused extensive outbreaks especially in South Korea and Japan, and are circulating in the region [Citation86]. Similarly the O/Mya-98 viruses were originally restricted to mainland Southeast Asia; however, in 2010–2011 this genotype caused devastating outbreaks in FMD-free countries in the Far East, South Korea, and Japan [Citation87,Citation88]. Another strain of ME-SA topotype, O-Ind-2001d originating from the Indian subcontinent has been circulating in mainland Southeast Asia since 2015, and recently this genotype has also been detected in China and South Korea in 2017 thereby further complicating the epidemiological situation of the region. There are various vaccine strains available for use in the region [Citation79]. However reports of systematic vaccine matching studies are rare for this region. In a recent study, we evaluated four existing (O/HKN/6/83, O/IND/R2/75, O/SKR/2010, and O/PanAsia-2) and one putative (O/MYA/2009) vaccine strains using a total of 85 serotype O FMD viruses collected over a 20 year period between 1993 to 2012 from SEA, East Asia, and the Far East by virus neutralization tests [Citation79]. Broad cross-reactivity was observed (); O/PanAsia-2 exhibited a good match with over 95% viruses followed by O/HKN/6/83 (92%) and the putative strain O/MYA/2009 (89%). The O/SKR/2010 vaccine was found to be the least cross-reactive exhibiting no match with 41% of the isolates employed in this study [Citation79].
4.2. Serotype A
This serotype is antigenically very diverse with more than 32 genotypes [Citation13] and is changing more rapidly than serotype O as exemplified by the emergence of various antigenically distinct strains in the Middle East and in South America in the last two to three decades. A22/Iraq vaccine had been the vaccine of choice for use in the Middle East for nearly three decades until emergence of A-Iran-96 and A-Iran-99 genotypes that resulted in development of A/Iran/96 and A/Iran/99 vaccines, respectively. Later on in 2003 another antigenically distinct genotype A-Iran-05 emerged that resulted in development of A/TUR/2006 vaccine [Citation89]. Recently circulating ARD-07, BAR-08, SIS-10, and SIS-12 viruses have been reported to exhibit lower cross-reactivity with A/TUR/2006 antisera emphasizing the requirement for development of another vaccine strain for the region [Citation37,Citation90]. Similarly in South America emergence of antigenic variants in 2000–2001 led to development of a new vaccine strain, A/ARG/2001 in Argentina [Citation36].
The serotype A viruses in the Middle East are evolving very rapidly as exemplified by emergence of several antigeni-cally distinct strains in the last five decades; A22, A/Iran-87, A/Iran-96, A/Iran-99, A/Iran-05 all requiring development of new vaccine strain to control the outbreaks [Citation37,Citation90]. Several sub-lineages of the A-Iran-05 have been detected during the last 10–15 years, with some spread to North African countries, some apparent sublineages die out alongside reports of new sublineages are appearing. This further complicates the epidemiological situation requiring constant surveillance and monitoring of the outbreaks in the region [Citation37,Citation90]. In a previous study, we evaluated two widely used serotype A vaccine strains (A22 and A/TUR/2006) using 57 serotype A viruses collected over a 16 year period between 1996 and 2011 from the region by 2D VNT [Citation90]. The A22 vaccine was found not to be a good match with majority of the viruses employed in the study. A/TUR/2006 vaccine was a good match with most viruses except viruses belonging to BAR-08 and ARD-07 sub-lineage (). In a further study in 2016, we evaluated these two vaccine strains and a putative strain of FAR-09 sublineage using 15 viruses (collected in 2012–2013) belonging to SIS-10 and SIS-12 sublineage [Citation37]. None of these vaccines were found to be a good match with the viruses of SIS-10 and SIS-12 sublineage indicating the inadequacy of the existing vaccine strains to provide protection against the circulating field isolates. Molecular analysis of the capsid sequences of these viruses identified mutations in the neutralizing antigenic sites that could have been the reason for the antigenic shift of these viruses [Citation37,Citation90,Citation91]. Consequently we evaluated another putative strain, A/IRN/07/2013 belonging to the SIS-10 sub-lineage that exhibited 100% in vitro protection against circulating field isolates. This emphasizes the requirement of the development of new serotype A vaccines every 5–10 years in the region; therefore constant surveillance and monitoring of the outbreaks together with regular vaccine matching studies could identify the inadequacy of the existing vaccine strains to provide protection against the circulating field viruses in a timely manner to facilitate new vaccine strain development.
Figure 7. (A) Bayesian phylogenetic tree of the A-Iran-05 viruses and (B) respective r1-values against A22/Iraq (white bars) and A/TUR/2006 (black bars) antisera. The sublineages were defined by WRLFMD on the basis of VP1 sequences and are labeled on the respective branches in this figure. The horizontal dotted line indicates the cut-off value of 0.3, above which the vaccine is considered to be a good match. Reproduced from (90) under Creative Commons Attribution License (CC BY).
Currently three lineages (I, IV, and VII) of the AFRICA topotype and one lineage (A-Iran-05) of the ASIA topotype are circulating in the East African region. All locally produced vaccines are of historic viruses () and vaccine matching work is rarely undertaken to determine the suitability of these vaccines to be used in FMD control programs. In a recent study, we measured the ability of the three existing vaccine strains (A-ERI-1998, A-ETH-06–2000, and A-KEN-05–1980) and four putative candidate vaccine strains (A-EA-2007, A-EA-1984, A-EA-2005, and A-EA-1981) of serotype A FMDV to cross-protect (in vitro) against 56 circulating viruses (collected over a period of 14 years between 1998 and 2012) by 2D VNT [Citation92]. The existing vaccine strains were least cross-reactive, only 5–46% of the viruses employed in this study exhibited an r1-value ≥ 0.3 (). A-EA-2007 was the best vaccine followed by A-EA-1981 and A-EA-1984. However, none of the vaccines were found to be a good match with the A-Iran-05 viruses circulating in the region indicating that (a) multiple strains from the serotype may need to be included, (b) different strains may need to be included in vaccine formulation for different countries. Therefore, careful consideration and informed decision based on epidemiological information including topotypes and genotypes currently circulating in countries, and regular vaccine matching studies are critical for the success of FMD control programs in the region.
Figure 8. Antigenic relationship (r1) values of 56 East Africa type A isolates. The serological match (r1-values) in the range of < 0.3 and > 0.3 for the seven vaccine strains are shown.
In South America, the A24 Cruzeiro vaccine is still used in their vaccination program whereas some countries like Argentina are also including the new A/ARG/2001 vaccine strain in their vaccine formulation. Similarly India uses A/IND/40/2000 as the vaccine strain in their control program [Citation93]; however this vaccine is not matching with the currently circulating field isolates in the neighboring countries, especially G-VII viruses that are rapidly spreading to other parts of the continent. This A/ASIA/G-VII has been circulating in India since 2003; however, it emerged in 2015, being first detected in the Middle East and rapidly spread to West Eurasia. This virus has been now detected in several countries including Armenia, Iran, Saudi Arabia, and Turkey. However, this virus has not moved to other continents/areas as was observed in serotype O-Ind-2001d viruses. Currently, there are no matching vaccines for these viruses including the Indian vaccine strain (A/IND/40/2000), although the Indian authorities are in the process of evaluating some candidate strains for use in their control programs. Clearly close monitoring and surveillance of this sublineage is crucial for global FMD control.
4.3. Serotype SAT 1–3
The SAT viruses were normally confined to sub-Saharan Africa with African buffalo as the natural host [Citation94,Citation95], however a number of SAT1 and SAT2 outbreaks have been recorded in the Middle East. Out of the three SAT serotype viruses present in Africa serotype SAT3 is still restricted to Southern Africa, being mainly detected in countries like Botswana, Malawi, Namibia, Mozambique, South Africa, Zambia, and Zimbabwe with the exception of Uganda in East Africa. SAT1-2 viruses have been circulating all over Africa with occasional spread to the Middle Eastern countries probably through trade links [Citation96]. Currently there is no information available on the use of vaccines. In addition to the vaccines described in , other available SAT-1 vaccines include SAT-1/South Africa, SAT-1/Nigeria and SAT-1/Kenya; SAT-2: SAT-2/Saudi Arabia, SAT-2/Eritrea; SAT-3: SAT-3/Zimbabwe. Reports of systematic vaccine matching study involving SAT viruses are scant. Studies involving a limited number of SAT-1 and SAT-2 field isolates revealed the viruses to be very dissimilar to the vaccine strains [Citation72,Citation74,Citation97].
4.4. Serotype Asia-1
Asia 1 viruses are mainly confined to the Asian continent. They have been considered to be antigenically less diverse and so far only one topotype of the virus has been detected in the field [Citation13]. Currently, available vaccines are Asia 1 Shamir, Asia1-YNBS/58 and Asia 1-IND 8/79; Asia 1 Shamir being used all over Asia whereas use of Asia 1-IND and Asia1-YNBS/58 are mainly restricted to the Indian subcontinent and China, respectively. To the best of our knowledge there is no peer reviewed publication reporting matching Asia 1 vaccines for the Asia 1 viruses currently circulating in Asia. However, local Reference Laboratories carry out limited vaccine matching work using available isolates.
5. Expert commentary
As the antigenic diversity of FMDV is a major concern for FMD control, regular vaccine matching and strain selection studies appropriate for each region is essential for disease control. Although in vivo cross protection studies are more appropriate methods for vaccine matching and selection, current methods of FMD vaccine matching and selection are being carried out by in vitro neutralization test or ELISA. In the face of an outbreak both in vivo and in vitro methods of vaccine matching are not easy, and time consuming. Therefore, easy and rapid selection of vaccine strains in an outbreak situation is required. The FMDV capsid contains all the immunogenic epitopes, and therefore sequence based method of vaccine selection may be a promising tool for future studies although alignment with structural capsid models may improve confidence in sequence-based prediction. Vaccine strain prediction models using both capsid sequence and serology data will likely replace existing tools in the future. Regional vaccine matching and selection studies using relevant circulating field viruses need to be carried out regularly to ensure the vaccine strain selected is a good match with the circulating field viruses.
6. Five-year view
Systematic vaccine matching and putative strain selection for serotype Asia1 viruses for Asia and Middle East, and SAT1-3 viruses for East Africa and West Africa may be completed. Provision of inclusion of more putative antisera from circulating viruses in the reference laboratory banks and in vaccine industries may be helpful to select appropriate vaccine strain. New methods of vaccine strain selection using capsid sequence data may allow rapid selection of appropriate strain/s. Rapid substitution of the capsid sequence of the most appropriate putative strain with the existing full length cDNA clone or with an existing viral vector vaccine that expresses FMDV capsid using reverse genetics technology may help to reduce length of time required for the adaptation of new strain/s in the cell culture.
Key issues
High antigenic variation in foot and mouth disease virus (FMDV) causes a major problem in vaccine strain selection to control the disease.
Current in vitro methods (i.e. VNT and ELISA) for vaccine matching and selection are time consuming, and can have poor repeatability and reproducibility.
Inclusion of new antisera from circulating strains from specific region are necessary to include in in vitro testing.
Comparison of sequence data generated from FMDV capsids may help in the rapid selection of a vaccine strain.
Development of in sillico models for vaccine strain selection using genetic and antigenic data may be helpful for appropriate and rapid vaccine strain selection.
We have recently focused on serotype O and A FMD vaccine matching and selection for most of the regions across the globe; regionally organized vaccine matching and selection are required for serotype Asia 1 and SAT1-3 viruses.
The O/PanAsia-2 vaccine appears to be broadly cross-reactive with numerous viruses circulating worldwide.
Serotype A strains are genetically and antigenically very diverse and appear to alter antigenicity rapidly, therefore, regular vaccine matching and selection (regional) studies are necessary.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Grubman MJ, Baxt B. Foot-and-mouth disease. Clin Microbiol Rev. 2004;17:465–493.
- Rweyemamu M, Roeder P, Mackay D, et al. Epidemiological patterns of foot-and-mouth disease worldwide. Transbound Emerg Dis. 2008;55:57–72.
- Knight-Jones TJ, Rushton J. The economic impacts of foot and mouth disease - what are they, how big are they and where do they occur? Prev Vet Med. 2013;112:161–173.
- Davies G. The foot and mouth disease (FMD) epidemic in the United Kingdom 2001. Comp Immunol Microbiol Infect Dis. 2002;25:331–343.
- Belsham GJ. Distinctive features of foot-and-mouth-disease virus, a member of the picornavirus family - aspects of virus protein-synthesis, protein processing and structure. Prog Biophys Mol Biol. 1993;60:241–260.
- Domingo E, Escarmis C, Baranowski E, et al. Evolution of foot-and-mouth disease virus. Virus Res. 2003;91:47–63.
- Morelli MJ, Wright CF, Knowles NJ, et al. Evolution of foot-and-mouth disease virus intra-sample sequence diversity during serial transmission in bovine hosts. Vet Res. 2013;44:12.
- Ayelet G, Soressa M, Sisay T, et al. FMD virus isolates: the candidate strains for polyvalent vaccine development in Ethiopia. Acta Tropica. 2013;126:244–248.
- Borley DW, Mahapatra M, Paton DJ, et al. Evaluation and use of in-silico structure-based epitope prediction with foot-and-mouth disease virus. PLoS One. 2013;8:e61122.
- Sangula AK, Siegismund HR, Belsham GJ, et al. Low diversity of foot-and-mouth disease serotype C virus in Kenya: evidence for probable vaccine strain re-introductions in the field. Epidemiol Infect. 2011;139:189–196.
- Doel TR. FMD vaccines. Virus Res. 2003;91:81–99.
- Yang M, Holland H, Clavijo A. Production of monoclonal antibodies against whole virus particles of foot-and-mouth disease virus serotype O and A and their potential use in quantification of intact virus for vaccine manufacture. Vaccine. 2008;26:3377–3382.
- Knowles NJ, Samuel AR. Molecular epidemiology of foot-and-mouth disease virus. Virus Res. 2003;91:65–80.
- Lea S, Hernandez J, Blakemore W, et al. The structure and antigenicity of a type-C foot-and-mouth-disease virus. Structure. 1994;2:123–139.
- Aktas S, Samuel AR. Identification of antigenic epitopes on the foot and mouth disease virus isolate O1/Manisa/Turkey/69 using monoclonal antibodies. Rev Sci Tech. 2000;19:744–753.
- Crowther JR, Farias S, Carpenter WC, et al. Identification of a fifth neutralizable site on type O foot-and-mouth disease virus following characterization of single and quintuple monoclonal antibody escape mutants. J Gen Virol. 1993;74(Pt 8):1547–1553.
- Grazioli S, Fallacara F, Brocchi E. Mapping of antigenic sites of foot-and-mouth disease virus serotype Asia 1 and relationships with sites described in other serotypes. J Gen Virol. 2013;94:559–569.
- Kitson JD, McCahon D, Belsham GJ. Sequence analysis of monoclonal antibody resistant mutants of type O foot and mouth disease virus: evidence for the involvement of the three surface exposed capsid proteins in four antigenic sites. Virology. 1990;179:26–34.
- Mahapatra M, Seki C, Upadhyaya S, et al. Characterisation and epitope mapping of neutralising monoclonal antibodies to A24 Cruzeiro strain of FMDV. Vet Microbiol. 2011;149:242–247.
- Mahapatra M, Aggarwal N, Cox S, et al. Evaluation of a monoclonal antibody-based approach for the selection of foot-and-mouth disease (FMD) vaccine strains. Vet Microbiol. 2008;126:40–50.
- Bari FD, Parida S, Asfor AS, et al. Prediction and characterization of novel epitopes of serotype A foot-and-mouth disease viruses circulating in East Africa using site-directed mutagenesis. J Gen Virol. 2015;96:1033–1041.
- Opperman PA, Rotherham LS, Esterhuysen J, et al. Determining the epitope dominance on the capsid of a serotype SAT2 foot-and-mouth disease virus by mutational analyses. J Virol. 2014;88:8307–8318.
- Asfor AS, Upadhyaya S, Knowles NJ, et al. Novel antibody binding determinants on the capsid surface of serotype O foot-and-mouth disease virus. J Gen Virol. 2014;95:1104–1116.
- Paton DJ, Valarcher JF, Bergmann I, et al. Selection of foot and mouth disease vaccine strains–a review. Rev Sci Tech. 2005;24:981–993.
- Parida S. Vaccination against foot-and-mouth disease virus: strategies and effectiveness. Expert Rev Vaccines. 2009;8:347–365.
- Cottral GE, Gailiunus P. Experimental multiple infection of animals with foot-and-mouth disease viruses. Proc 75th Annu Meeting US Anim Health Assoc. 1972;75:441–468.
- Black L, Nicholls MJ, Rweyemamu MM, et al. Foot-and-mouth disease vaccination: a multifactorial study of the influence of antigen dose and potentially competitive immunogens on the response of cattle of different ages. Res Vet Sci. 1986;40:303–307.
- Lyons NA, Ludi AB, Wilsden G, et al. Evaluation of a polyvalent foot-and-mouth disease virus vaccine containing A Saudi-95 against field challenge on large-scale dairy farms in Saudi Arabia with the emerging A/ASIA/G-VII viral lineage. Vaccine. 2017;35:6850–6857.
- Waters R, Ludi AB, Fowler VL, et al. Efficacy of a high-potency multivalent foot-and-mouth disease virus vaccine in cattle against heterologous challenge with a field virus from the emerging A/ASIA/G-VII lineage. Vaccine. 2018;36:1901–1907.
- Brehm KE, Kumar N, Thulke HH, et al. High potency vaccines induce protection against heterologous challenge with foot-and-mouth disease virus. Vaccine. 2008;26:1681–1687.
- Nagendrakumar SB, Srinivasan VA, Madhanmohan M, et al. Evaluation of cross-protection between O1 Manisa and O1 Campos in cattle vaccinated with foot-and-mouth disease virus vaccine incorporating different payloads of inactivated O1 Manisa antigen. Vaccine. 2011;29:1906–1912.
- Sobrino F, Saiz M, Jimenez-Clavero MA, et al. Foot-and-mouth disease virus: a long known virus, but a current threat. Vet Res. 2001;32:1–30.
- Kitching P, Hammond J, Jeggo M, et al. Global FMD control - Is it an option? 4th International Veterinary Vaccines and Diagnostics Conference. Oslo, NORWAY: Elsevier Sci Ltd; 2006. p. 5660–5664.
- Paton DJ, De Clercq K, Greiner M, et al. Application of non-structural protein antibody tests in substantiating freedom from foot-and-mouth disease virus infection after emergency vaccination of cattle. Vaccine. 2006;24:6503–6512.
- de Los Santos T, Diaz-San Segundo F, Rodriguez LL. The need for improved vaccines against foot-and-mouth disease. Curr Opin Virol. 2018;29:16–25.
- Mattion N, Konig G, Seki C, et al. Reintroduction of foot-and-mouth disease in Argentina: characterisation of the isolates and development of tools for the control and eradication of the disease. Vaccine. 2004;22:4149–4162.
- Mahapatra M, Statham B, Li Y, et al. Emergence of antigenic variants within serotype A FMDV in the Middle East with antigenically critical amino acid substitutions. Vaccine. 2016;34:3199–3206.
- OIE. Foot and mouth disease. In: Manual of diagnostic tests and vaccines for terrestrial animals. 6th ed. Paris, France; 2012. Available from: http://www.oie.int/en/standard-setting/terrestrial-manual/access-online/
- Maradei E, Malirat V, Beascoechea CP, et al. Characterization of a type O foot-and-mouth disease virus re-emerging in the year 2011 in free areas of the Southern Cone of South America and cross-protection studies with the vaccine strain in use in the region. Vet Microbiol. 2013;162:479–490.
- Horsington J, Zhang Z, Bittner H, et al. Early protection in sheep against intratypic heterologous challenge with serotype O foot-and-mouth disease virus using high-potency, emergency vaccine. Vaccine. 2015;33:422–429.
- Fishbourne E, Ludi AB, Wilsden G, et al. Efficacy of a high potency O-1 Manisa foot-and-mouth disease vaccine in cattle against heterologous challenge with a field virus from the O/ME-SA/Ind-2001 lineage collected in North Africa. Vaccine. 2017;35:2761–2765.
- Wilna V, Hong NT, Geoffrey FT, et al. Efficacy of a high potency O1 Manisa monovalent vaccine against heterologous challenge with a FMDV O Mya98 lineage virus in pigs 4 and 7 days post vaccination. Vaccine. 2015;33:2778–2785.
- Pay TW, Hingley PJ. Foot and mouth disease vaccine potency tests in cattle: the interrelationship of antigen dose, serum neutralizing antibody response and protection from challenge. Vaccine. 1992;10:699–706.
- McCullough KC, De Simone F, Brocchi E, et al. Protective immune response against foot-and-mouth disease. J Virol. 1992;66:1835–1840.
- Barnett PV, Statham RJ, Vosloo W, et al. Foot-and-mouth disease vaccine potency testing: determination and statistical validation of a model using a serological approach. Vaccine. 2003;21:3240–3248.
- Dunn CS, Samuel AR, Pullen LA, et al. The biological relevance of virus neutralisation sites for virulence and vaccine protection in the guinea pig model of foot-and-mouth disease. Virology. 1998;247:51–61.
- Rweyemamu MM, Booth JC, Head M, et al. Microneutralization tests for serological typing and subtyping of foot-and-mouth disease virus strains. J Hyg (Lond). 1978;81:107–123.
- Rweyemamu MM. Antigenic differentiation of foot and mouth disease virus strains by serum neutralisation. Thesis submitted to Royal College of Veterinary Surgeons, London. 1983. p. 160.
- Rweyemamu MM. Antigenic variation in foot-and-mouth disease: studies based on the virus neutralization reaction. J Biol Stand. 1984;12:323–337.
- Ferris NP, Donaldson AI. The world reference laboratory for foot and mouth disease: a review of thirty-three years of activity (1958–1991). Rev Sci Tech. 1992;11:657–684.
- Kitching RP, Rendle R, Ferris NP. Rapid correlation between field isolates and vaccine strains of foot-and-mouth disease virus. Vaccine. 1988;6:403–408.
- Seki C, Robiolo B, Periolo O, et al. Rapid methodology for antigenic profiling of FMDV field strains and for the control of identity, purity and viral integrity in commercial virus vaccines using monoclonal antibodies. Vet Microbiol. 2009;133:239–251.
- Yang M, Xu W, Goolia M, et al. Characterization of monoclonal antibodies against foot-and-mouth disease virus serotype O and application in identification of antigenic variation in relation to vaccine strain selection. Virol J. 2014;11:136.
- Pay TW, Hingley PJ. The use of serum neutralizing antibody assay for the determination of the potency of foot and mouth disease (FMD) vaccines in cattle. Dev Biol Stand. 1986;64:153–161.
- Mahapatra M, Hamblin P, Paton DJ. Foot-and-mouth disease virus epitope dominance in the antibody response of vaccinated animals. J Gen Virol. 2012;93:488–493.
- Bachmann MF, Kalinke U, Althage A, et al. The role of antibody concentration and avidity in antiviral protection. Science (New York, NY). 1997;276:2024–2027.
- Polack FP, Hoffman SJ, Crujeiras G, et al. A role for nonprotective complement-fixing antibodies with low avidity for measles virus in atypical measles. Nat Med. 2003;9:1209–1213.
- Franco Mahecha OL, Ogas Castells ML, Combessies G, et al. Single dilution avidity-blocking ELISA as an alternative to the Bovine Viral Diarrhea virus neutralization test. J Virol Methods. 2011;175:228–235.
- Saika S, Matsunaga T, Ogawa T, et al. [Gelatin particle agglutination (PA) antibody: comparison to neutralizing antibody (NT) and hemagglutination inhibition (HI) antibody and relationship to IgG avidity]. Kansenshogaku Zasshi J Jpn Assoc Infect Dis. 2008;82:310–316.
- Lavoria MA, Di-Giacomo S, Bucafusco D, et al. Avidity and subtyping of specific antibodies applied to the indirect assessment of heterologous protection against foot-and-mouth disease virus in cattle. Vaccine. 2012;30:6845–6850.
- Brito BP, Perez AM, Capozzo AV. Accuracy of traditional and novel serology tests for predicting cross-protection in foot-and-mouth disease vaccinated cattle. Vaccine. 2014;32:433–436.
- Smith DJ, Lapedes AS, de Jong JC, et al. Mapping the antigenic and genetic evolution of influenza virus. Science (New York, NY). 2004;305:371–376.
- Cai Z, Zhang T, Wan XF. A computational framework for influenza antigenic cartography. PLoS Comput Biol. 2010;6:e1000949.
- Cai Z, Zhang T, Wan XF. Antigenic distance measurements for seasonal influenza vaccine selection. Vaccine. 2012;30:448–453.
- Harvey WT, Benton DJ, Gregory V, et al. Identification of low- and high-impact Hemagglutinin amino acid substitutions that drive antigenic drift of influenza A(H1N1) viruses. PLoS Pathog. 2016;12:e1005526.
- Ludi AB, Horton DL, Li Y, et al. Antigenic variation of foot-and-mouth disease virus serotype A. J Gen Virol. 2014;95:384–392.
- Lewis NS, Daly JM, Russell CA, et al. Antigenic and genetic evolution of equine influenza A (H3N8) virus from 1968 to 2007. J Virol. 2011;85:12742–12749.
- de Jong JC, Smith DJ, Lapedes AS, et al. Antigenic and genetic evolution of swine influenza A (H3N2) viruses in Europe. J Virol. 2007;81:4315–4322.
- Horton DL, McElhinney LM, Marston DA, et al. Quantifying antigenic relationships among the lyssaviruses. J Virol. 2010;84:11841–11848.
- Evans JS, Wu G, Selden D, et al. Utilisation of Chimeric Lyssaviruses to assess vaccine protection against highly divergent Lyssaviruses. Viruses. 2018;10(3). pii: E130. doi: 10.3390/v10030130.
- Li C, Hatta M, Burke DF, et al. Selection of antigenically advanced variants of seasonal influenza viruses. Nat Microbiology. 2016;1:16058.
- Reeve R, Blignaut B, Esterhuysen JJ, et al. Sequence-based prediction for vaccine strain selection and identification of antigenic variability in foot-and-mouth disease virus. PLoS Comput Biol. 2010;6:e1001027.
- Maree FF, Blignaut B, Esterhuysen JJ, et al. Predicting antigenic sites on the foot-and-mouth disease virus capsid of the South African Territories types using virus neutralization data. J Gen Virol. 2011;92:2297–2309.
- Reeve R, Borley DW, Maree FF, et al. Tracking the antigenic evolution of foot-and-mouth disease virus. PLoS One. 2016; 11.
- Rahman T, Mahapatra M, Laing E, et al. Evolutionary non-linear modelling for selecting vaccines against antigenically variable viruses. Bioinformatics. 2015;31:834–840.
- Galdo Novo S, Malirat V, Maradei ED, et al. Antigenic and immunogenic spectrum of foot-and-mouth disease vaccine strain O1 Campos against representative viruses of topotypes that circulated in Asia over the past decade. Vaccine. 2017;35:2303–2307.
- Mahapatra M, Yuvaraj S, Madhanmohan M, et al. Antigenic and genetic comparison of foot-and-mouth disease virus serotype O Indian vaccine strain, O/IND/R2/75 against currently circulating viruses. Vaccine. 2015;33:693–700.
- Lloyd-Jones K, Mahapatra M, Upadhyaya S, et al. Genetic and antigenic characterization of serotype O FMD viruses from East Africa for the selection of suitable vaccine strain. Vaccine. 2017;35:6842–6849.
- Mahapatra M, Upadhyaya S, Aviso S, et al. Selection of vaccine strains for serotype O foot-and-mouth disease viruses (2007–2012) circulating in Southeast Asia, East Asia and Far East. Vaccine. 2017;35:7147–7153.
- Park J-N, Lee S-Y, Chu J-Q, et al. Protection to homologous and heterologous challenge in pigs immunized with vaccine against foot-and-mouth disease type O caused an epidemic in East Asia during 2010/2011. Vaccine. 2014;32:1882–1889.
- Ryoo S, Kim T, Nah JJ, et al. Complete genome sequence of a foot-and-mouth disease virus of serotype O isolated from Gimje, Republic of Korea, in 2016. Genome Announc. 2017;5.
- Subramaniam S, Mohapatra JK, Sharma GK, et al. Evolutionary dynamics of foot-and-mouth disease virus O/ME-SA/Ind2001 lineage. Vet Microbiol. 2015;178:181–189.
- Knowles NJ, Bachanek-Bankowska K, Wadsworth J, et al. Outbreaks of foot-and-mouth disease in Libya and Saudi Arabia during 2013 due to an exotic O/ME-SA/Ind-2001 lineage virus. Transbound Emerg Dis. 2016;63:E431–E5.
- Di Nardo A, Knowles NJ, Paton DJ. Combining livestock trade patterns with phylogenetics to help understand the spread of foot and mouth disease in sub-Saharan Africa, the Middle East and Southeast Asia. Revue Scientifique Et Technique-Office International Des Epizooties. 2011;30:63–85.
- Subramaniam S, Mohapatra JK, Das B, et al. Genetic and antigenic analysis of foot-and-mouth disease virus serotype O responsible for outbreaks in India during 2013. Infect Genet Evol. 2015;30:59–64.
- Knowles NJ, Samuel AR, Davies PR, et al. Pandemic strain of foot-and-mouth disease virus serotype O. Emerg Infect Dis. 2005;11:1887–1893.
- Knowles NJ, He J, Shang Y, et al. Southeast Asian foot-and-mouth disease viruses in Eastern Asia. Emerg Infect Dis. 2012;18:499–501.
- Paton DJ, King DP, Knowles NJ, et al. Recent spread of foot-and-mouth disease in the Far East. Vet Rec. 2010;166:569–570.
- Knowles NJ, Nazem Shirazi MH, Wadsworth J, et al. Recent spread of a new strain (A-Iran-05) of foot-and-mouth disease virus type A in the Middle East. Transbound Emerg Dis. 2009;56:157–169.
- Upadhyaya S, Ayelet G, Paul G, et al. Genetic basis of antigenic variation in foot-and-mouth disease serotype A viruses from the Middle East. Vaccine. 2014;32:631–638.
- Jamal SM, Ferrari G, Ahmed S, et al. Evolutionary analysis of serotype A foot-and-mouth disease viruses circulating in Pakistan and Afghanistan during 2002–2009. J Gen Virol. 2011;92:2849–2864.
- Bari FD, Parida S, Tekleghiorghis T, et al. Genetic and antigenic characterisation of serotype A FMD viruses from East Africa to select new vaccine strains. Vaccine. 2014;32:5794–5800.
- Biswal JK, Sanyal A, Rodriguez LL, et al. Foot-and-mouth disease: global status and Indian perspective. Indian J Anim Sci. 2012;82:109–131.
- Thomson GR, Vosloo W, Bastos AD. Foot and mouth disease in wildlife. Virus Res. 2003;91:145–161.
- Vosloo W, Boshoff K, Dwarka R, et al. The possible role that buffalo played in the recent outbreaks of foot-and-mouth disease in South Africa. Ann N Y Acad Sci. 2002;969:187–190.
- Brito BP, Rodriguez LL, Hammond JM, et al. Review of the global distribution of foot-and-mouth disease virus from 2007 to 2014. Transbound Emerg Dis. 2017;64:316–332.
- Fargeaud D. Selection of vaccine strains of foot and mouth disease virus for use in southern Africa. Rev Sci Tech. 1995;14:521–538.