ABSTRACT
Introduction: GSK has developed a two-dose adjuvanted recombinant zoster vaccine (Shingrix, RZV) to protect people aged ≥50 years (50+) against herpes zoster (HZ) and its complications. RZV showed >90% efficacy against HZ, sustained over 4 years of follow-up, in all studied age groups.
Areas covered: This article reviews the scientific rationale underlying the design of RZV; the clinical evidence demonstrating immunogenicity, safety, and efficacy in persons 50+; and the public health implications and cost-effectiveness.
Expert commentary: A decline in varicella zoster virus (VZV) immunity is associated with increased risk of HZ in adults 50+ and immunocompromised individuals. RZV was designed to restore levels of anti-VZV cellular and humoral immunity to prevent VZV reactivation. RZV includes the recombinant gE glycoprotein antigen, and Adjuvant System AS01B which promotes cellular and antibody responses. In two Phase III studies in subjects aged 50+ and 70+ years, RZV efficacy against HZ compared to placebo was >90% and ≥89% against post-herpetic neuralgia (PHN). RZV is expected to dramatically impact HZ morbidity including its complications, and associated health-care costs. In the US population aged 50+ years, vaccination with RZV can be cost-effective compared to no vaccination and cost-saving compared to the currently available live-attenuated HZ vaccine (Zostavax, Merck).
Focus on the patient
What is the context?
Approximately one in three persons will develop herpes zoster (HZ, shingles), caused by the reactivation of latent varicella zoster virus, during their lifetime. This risk increases with age. HZ and its most common complication, post-herpetic neuralgia, negatively impact patient quality of life and cause a substantial economic burden to the health-care system. A live-attenuated HZ vaccine (Zostavax) has been available in some countries since 2006 but has several limitations. Here, we describe the scientific rationale underlying the design of Shingrix (RZV), a new vaccine to prevent HZ. Clinical evidence demonstrating immunogenicity, safety and efficacy, potential public health implications, and cost-effectiveness is summarized.
What is new?
RZV showed high efficacy (>90%) in preventing HZ in all age groups aged 50+ years, including the oldest adults, demonstrating that this vaccine can provide substantial benefit to a population usually less responsive to vaccination. Efficacy was sustained for the 4 years of study follow-up. The vaccine has an acceptable safety profile based on clinical trial data. The majority of common adverse reactions postvaccination such as injection-site reactions, myalgia, fatigue, and headache were mild-to-moderate in intensity and of short duration.
What is the impact?
Given its high and sustained efficacy, RZV demonstrates the potential for a significant individual and public health impact. RZV may prevent the negative impact of HZ on the quality of life of people aged 50+ years by preventing HZ and its related complications, as well as the associated health-care costs.
1. Unmet needs in herpes zoster prevention
The varicella zoster virus (VZV) is a member of the human herpesviridae family, which also includes herpes simplex 1 and 2. Primary infection with VZV causes systemic disease (varicella/chickenpox) and induces VZV-specific antibodies and T-cell responses [Citation1]. After a varicella episode, VZV virions establish latency in sensory ganglia, often for many decades. As part of the natural age-related decline in immunity, also called ‘immune senescence,’ VZV-specific T-cell immunity declines, and it is also decreased in subjects with impaired immunity, which may lead to clinical reactivation of latent VZV. When such reactivation occurs, herpes zoster (HZ) usually manifests as a painful blistering dermatomal rash, which may be complicated by refractory, long-term neuropathic pain (post-herpetic neuralgia [PHN]). PHN occurs in up to 10–20% of patients and in more than 30% of those who are 80+ years of age (YOA) [Citation2]. Other complications of HZ include secondary bacterial infection, ocular involvement with uveitis, neurological complications including encephalitis, vasculopathy leading to stroke or myocardial infarction, and disseminated infections [Citation3].
Cell-mediated immunity (CMI) is thought to be pivotal in preventing VZV reactivation [Citation1]. The risk of developing symptomatic HZ appears to be more associated with VZV-specific CMI rather than anti-VZV antibody levels, based on the observation that higher numbers of VZV-specific interferon-gamma (IFNγ)-producing CD4+ T-cells are associated with reduced HZ severity and reduced risk of PHN [Citation4–Citation6]. Increasing age is accompanied by immune senescence in which the capacity of the immune system, particularly the functioning of T-cells, decreases [Citation7]. While VZV-specific antibodies can remain stable in older age, cytokine-producing VZV-specific CD4+ cells decrease [Citation8]. Thus, in healthy individuals, the most important risk factor for viral reactivation is age. HZ incidence rates increase from approximately 2–5 per 1000 person-years at 40 YOA up to 10–16 per 1000 person-years at 85 YOA, with a lifetime risk of 30% [Citation9].
Individuals with underlying immunosuppressive or immunodeficient medical conditions, or who are undergoing medical treatments that decrease the functionality of CMI (e.g. malignancies, autoimmune diseases, human immunodeficiency virus [HIV] infection, and organ and stem-cell transplantation), are also at high risk of HZ and have HZ rates that are 1.5–5-times higher than that of the general population [Citation10–Citation13]. HZ is also more severe and of longer duration in immunocompromised individuals, and they are more likely to develop serious complications such as neurological or disseminated disease [Citation12,Citation14].
HZ and its associated complications have a sizeable impact on the quality of life of the patient and contribute substantially to the health economic burden in many countries [Citation15,Citation16]. It is estimated that 99% of all adults 40+ YOA have serological evidence of VZV infection and are therefore at risk of developing HZ [Citation17]. The incidence of HZ appears to have increased over the last decades even in countries where vaccination against varicella has long been available, for reasons that are not known. This increase is independent of the aging population, with the trend beginning prior to the widespread use of varicella vaccines in children [Citation9,Citation18]. Possible reasons include greater awareness of HZ among physicians and patients as a result of the availability of new treatments and vaccination [Citation19]. This increase is expected to continue, compounded by aging populations and longer life spans [Citation16]. However, these projections are based on populations who had wild-type VZV infection as children. It is not yet known if individuals vaccinated against VZV as children will develop HZ at the same rate as after wild-type infection, although one observational study suggested that the rate could be up to 80% lower in vaccinated than in unvaccinated children [Citation20].
Uncomplicated HZ, although painful, is a self-limiting disease, and treatment in the healthy individual is aimed at minimizing the symptoms and reducing the severity and duration of pain. In immunocompromised persons, treatment also aims to prevent the onset of serious, and sometimes disseminated, infectious complications. Antiviral drugs can reduce the duration and severity of dermatological symptoms caused by HZ if administered within 72 h of disease onset but were not conclusively shown to reduce the occurrence of PHN in meta-analyses of clinical trial data [Citation21,Citation22]. Active pain management is required to maintain quality of life and to reduce the risk of PHN. Narcotics and medication to treat neuropathic pain, such as tricyclic antidepressants or antiepileptics, may be required early during the course of the disease. However, pain control can be challenging and is frequently inadequate; in one study, only 14% of patients 65+ YOA were satisfied with their treatment for PHN [Citation23].
Prevention of HZ and reduction in disease severity can be achieved by vaccines capable of boosting VZV-specific CMI [Citation24]. The first vaccine to demonstrate the proof-of concept that zoster could be prevented by vaccination was the live-attenuated HZ vaccine (ZVL or zoster-vaccine live: Zostavax), licensed in 2006. ZVL has moderate efficacy in preventing HZ in immunocompetent adults that decreases with age (51.3% in adults 60+ YOA and 37.6% in those 70+ YOA) [Citation25]. Efficacy also wanes over time, decreasing to nonsignificant levels 6 years postvaccination [Citation26]. Because it is a live-attenuated vaccine, administration of ZVL may result in disseminated infection in individuals who are immunosuppressed or immunodeficient. VZV is therefore contraindicated in these persons [Citation27]. Thus, older adults and immunocompromised persons who are at most risk of developing HZ are not optimally protected by ZVL, and an alternative vaccine approach that provides higher and longer protection in these groups is warranted [Citation6].
The HZ adjuvanted recombinant zoster vaccine, RZV (Shingrix), composed of the VZV gE antigen and AS01B; an adjuvant that specifically boosts the cellular and humoral immune responses has been developed with the objective of providing high protection across all populations at risk of HZ. Large Phase III placebo-controlled efficacy studies demonstrated high efficacy (>90%) of RZV in preventing HZ in all studied age groups, including adults 70+ YOA and 80+ YOA [Citation28,Citation29]. An HZ vaccine that demonstrates high efficacy is expected to have a substantial impact on disease incidence and the associated health-care costs of managing the short- and long-term complications of HZ. This article reviews the scientific rationale underlying the design of RZV and the clinical evidence demonstrating immunogenicity, safety, and efficacy in persons 50+ YOA. The article also considers the potential public health impact and cost-effectiveness of RZV immunization.
2. Prevention of HZ: a need for improved vaccines
2.1. Live-attenuated VZV vaccine (ZVL)
The live-attenuated VZV vaccine is indicated for the prevention of HZ in people 50+ YOA in several countries. ZVL contains the same VZV Oka strain present in pediatric varicella vaccines, but at a higher dose (>19,400 plaque-forming units versus >1350 in the pediatric formulation) [Citation27,Citation30]. In placebo-controlled efficacy studies, ZVL reduced the risk of HZ by 69.8% in adults 50–59 YOA over a mean follow-up period of 1.3 years [Citation31], by 64% in adults 60–69 YOA and by 41% in those 70–79 YOA over a mean 3.13 years of follow-up [Citation25], and 18% in those 80+ YOA [Citation25,Citation27,Citation31] (). Efficacy against PHN was 66.5% among those 60+ YOA [Citation25]. Vaccine efficacy against HZ decreases after vaccination, down to 30.6% (95% confidence interval [CI] −6.0 to 54.6) after 6 years among adults 60+ YOA [Citation26,Citation32]. These effects have been confirmed in retrospective observational cohort studies using large health-care insurance databases [Citation33–Citation36].
Table 1. Estimates from placebo-controlled trials of vaccine efficacy in preventing herpes zoster or post-herpetic neuralgia in adults.
2.2. Scientific rationale for development of the adjuvanted recombinant zoster vaccine (RZV)
A non-live, adjuvanted recombinant vaccine approach was selected to potentially improve vaccine efficacy in older age groups and allow vaccination of immunocompromised persons compared to the standard of care. RZV was designed to restore both arms of the anti-VZV immune response: cell-mediated and humoral immunity, with the objective of preventing virus reactivation, and progression to HZ and subsequent complications. While CMI is likely the central mechanism in preventing VZV reactivation, it is plausible that antibodies are also required for the efficient elimination of infected cells, through a mechanism referred to as antibody-dependent cell-mediated cytotoxicity, which involves the recognition by natural killer (NK) cells of antibodies bound to infected cells [Citation24,Citation37]. This mechanism has been shown in vitro to kill VZV-infected fibroblasts [Citation38,Citation39]. In addition, NK cells can also be activated by VZV-specific CD4+ T-cells via the secretion of interleukin-2 (IL-2) [Citation40]. In general, NK cells specialize in defending the body against viral infections and are particularly important in herpes virus infections which can efficiently evade other host killing mechanisms [Citation41].
2.2.1. Glycoprotein e (gE)
VZV encodes nine envelope glycoproteins, of which three occur as heterodimers. The selected antigen, glycoprotein E (gE), is the most abundantly expressed VZV surface glycoprotein [Citation42]. gE is a major target for VZV-specific antibody and T-cell responses [Citation1,Citation42–Citation45] and was selected as vaccine antigen based on its role in viral replication and cell-to-cell transfer that promotes viral spread and the pathogenesis of skin lesions [Citation10,Citation46–Citation48]. Importantly, it is found on infected cells during reactivation and episodes of HZ. The antigen is produced by recombinant protein technology in a Chinese hamster ovarian cell line (CHO). In order to allow its secretion into cell-culture supernatant during manufacturing, the transmembrane anchor and carboxy-terminal domains were deleted from the original gE gene before transfecting the CHO cells [Citation43,Citation49].
2.2.2. Adjuvant selection
In a VZV-primed mouse model, vaccination with gE alone or gE adsorbed on Alum failed to reach the targeted immune profile (i.e. an enhanced CMI response) [Citation50]. Other Adjuvant Systems were therefore evaluated [Citation51]. Among these, AS01 was a promising candidate, given the clinical experience with another AS01-adjuvanted vaccine, the RTS,S malaria vaccine, that consistently showed that AS01 can enhance T-cell responses in addition to antibodies with an acceptable safety profile in the populations studied [Citation52,Citation53]. In the VZV-primed model, vaccination with gE combined with AS01 resulted in high gE-specific CMI responses including IFNγ-producing T-cells, which were significantly higher than responses when vaccinating with gE combined with other Adjuvant Systems (AS02, AS03, or AS04). The unique ability of RZV to enhance a strong T-cell response was further validated in humans, where adjuvanted RZV induced antigen-specific humoral and T-cell responses that were significantly higher compared to a live-attenuated VZV-vaccine (Varilrix) or unadjuvanted gE [Citation54].
AS01 is a liposome-based adjuvant that contains two immunostimulants: 3-O-desacyl–monophosphoryl lipid A (MPL) and Quillaja saponaria Molina, fraction 21 (QS-21). MPL is the detoxified derivative of lipopolysaccharide from Salmonella minnesota and stimulates innate immunity via Toll-like receptor 4 [Citation55]. QS-21 [Citation56] is a saponin molecule extracted and purified from the bark of the Quillaja saponaria Molina tree. Recent work has shown that QS-21 formulated in liposomes activates the caspase-1 pathway via a cholesterol-dependent mechanism and that its adjuvant effect is mediated by the activation of lymph node macrophages [Citation57,Citation58].
AS01 induces a rapid and transient activation of the innate immunity in the injected muscle and draining lymph node, leading to increased number of activated antigen-presenting cells [Citation59]. Activated antigen-presenting cells carrying gE in the draining lymph node are responsible for the generation of high levels of gE-specific CD4+ T-cells and antibodies [Citation59]. The adjuvant effect of AS01 results from synergistic interactions between MPL and QS-21 formulated in liposomes, leading to early IFNγ production by local resident cells in the lymph node. Blocking early IFNγ impairs T-cell responses enhanced by AS01 [Citation50,Citation60]. These data demonstrate that MPL and QS-21 are both required in AS01. Their synergistic effects are thought to be important in conferring protective immunity [Citation61].
2.2.3. Final composition
The RZV formulation is the combination of 50 µg of gE with a AS01 dosage of 50 µg of MPL and 50 µg of QS21 (AS01B). The selection of gE and AS01 dosage as well as the number of doses required for the primary vaccination course was established in Phase I–III studies conducted in adults 50+ YOA (see Section 5.3 and ) [Citation62,Citation63]. These studies further established immunogenicity in terms of CMI and humoral responses and confirmed that the reactogenicity profile of the RZV was clinically acceptable. The gE antigen is provided in a lyophilized form (white powder) in monodose vials. AS01B is provided in a liquid form in separate monodose vials (0.5 ml/dose) and is used for reconstitution prior to injection. The primary vaccination course selected for the pivotal Phase III clinical studies consisted of two doses given intramuscularly 2 months apart.
Table 2. Phase I/II studies of the adjuvanted recombinant zoster vaccine (RZV) in adults aged 50+ years.
3. Regulatory considerations
The clinical development of new vaccines is undertaken in accordance with guidance provided by regulatory agencies [Citation66,Citation67]. The safety of new adjuvanted vaccines is ultimately assessed on the final formulation administered to the subject [Citation68]. Phase I and II studies establish the optimal dosage and number of doses and route of administration in the population targeted to receive the vaccine: adults 50+ YOA and immunocompromised adult patients. These studies form the basis of the design of larger studies to demonstrate the efficacy and safety of the new vaccine in the target populations. As a result of the high incidence of HZ along with its typical clinical features, and the availability of diagnostic tests, conduct of pre-licensure studies of RZV vaccine efficacy was feasible. An extensive safety database in adults aged 50+ YOA was generated in line with regulatory guidelines for new vaccines. License applications for RZV were submitted to the US Food and Drug Administration, the European Medicines Agency, and Health Canada in 2016 and to the Therapeutic Goods Administration in Australia and the Pharmaceuticals and Medical Devices Agency in Japan in 2017 to seek marketing authorization for an indication of prevention of HZ in adults 50+ YOA. RZV received first approval for use in Canada and the USA in October 2017 [Citation69,Citation70].
Clinical research of RZV in immunocompromised persons 18+ YOA is progressing in parallel. Immunogenicity, efficacy and safety studies have been completed in patients with HIV infection and autologous hematopoietic stem cell transplant (HSCT) [Citation71,Citation72,Citation85,Citation86]. Studies in patients with renal transplant, solid tumors, and hematologic malignancies have been completed or are ongoing. A Phase III efficacy study in severely immunocompromised subjects (HSCT recipients) has been recently completed. These data will be submitted to and discussed with regulatory agencies worldwide as appropriate to complement the approved indication.
4. Preclinical toxicology
As part of the evaluation of the safety of RZV, more than 20 Good Laboratory Practices studies in animal models were conducted, including repeated-dose toxicity studies of RZV, AS01, or AS01 components, as well as local tolerance, male fertility, and reproductive toxicity studies. These studies did not reveal any specific concerns that would preclude the use of RZV in humans [Citation73]. The main findings of those studies were a dose-dependent mild cellular infiltration at the injection site and transient changes in some parameters such as temperature, C-reactive protein, and albumin/globulin ratio, all consistent with the mode of action of the adjuvanted vaccine. RZV or AS01 administered alone to rats showed no effects on fertility, pre- and postnatal development, or survival [Citation74].
5. RZV clinical development program in adults 50+ YOA
The RZV clinical development program was designed to support the marketing authorization of RZV as a prophylactic vaccine for the prevention of HZ and HZ-related complications in persons at risk of HZ, namely, individuals 50+ YOA. Two large Phase III studies demonstrated efficacy in older adults [Citation28,Citation29]. Additional studies are ongoing/completed to investigate the long-term duration of the immunogenicity and the efficacy of RZV, as well as safety and immunogenicity of vaccine coadministrations and of RZV when given at least 5 years after previous ZVL vaccination [Citation75–Citation77].
5.1. Methods to evaluate immunogenicity
Immunogenicity was assessed by measuring antigen-specific CMI in early studies and in the Phase III efficacy studies and humoral responses in all studies. Frequencies of gE-specific CD4+ T-cells expressing at least two activation markers from among IFNγ, IL-2, tumor necrosis factor-alpha, and Cluster of Differentiation 40 Ligand (CD40L) were measured using intracellular cytokine staining on thawed peripheral blood mononuclear cells as described previously [Citation62]. Anti-gE antibodies were measured by a validated in-house anti-gE enzyme-linked immunosorbent assay using recombinant gE.
5.2. Methods to evaluate safety
Safety was assessed at all stages of clinical development [Citation78]. The occurrence of local (pain, redness, and swelling) and systemic (fatigue, headache, myalgia, gastrointestinal symptoms, and fever) reactions was solicited and recorded by the participant on diary cards for 7 days after each dose. All other (unsolicited) adverse events were recorded for 30 days after each dose. Serious adverse events (SAEs) were recorded up to 1 year after the second dose. SAEs considered by the investigator to be related to vaccination, fatal SAEs, and the occurrence of potential immune-mediated diseases (pIMDs, collected because of the theoretical potential for the development of autoimmune diseases after vaccination with novel adjuvants [Citation79]) were recorded for the entire study period (at least 1 year).
5.3. Dose selection, dose interval, and administration route
The dosage of recombinant gE and of AS01B was selected based on the results of two studies by Chlibek et al. (referred to as Study A and B) [Citation62,Citation63]. Study A compared gE doses of 25, 50, and 100 μg as well as a one- or a two-dose course (2 months apart) in subjects 60+ YOA (). Study A established that higher CD42+ and anti-gE antibodies were achieved when two doses were administered and that, while there was a dose–response to the amount of recombinant gE adjuvanted to AS01B in terms of antibody levels, CD42+ levels did not increase significantly for doses above 50 μg [Citation63]. gE-specific CD8+ T-cells were not induced by the vaccine [Citation63]. The 50 μg gE dose was selected for further development as providing the lowest antigen dose with higher immune responses than the next lowest dose. Study B was an adjuvant dose confirmation study in subjects 50+ YOA comparing the immunogenicity of 50 µg of gE combined with either AS01B or AS01E (containing half the amount of MPL and QS-21) [Citation62]. In Study B, AS01B induced a significantly higher fold increase in gE-specific CD42+ and anti-gE antibodies as compared to AS01E () [Citation62].
The final formulation of 50 μg gE combined with AS01B (RZV) administered intramuscularly as a two-dose course given two months apart was selected for further development. RZV is supplied as a single-dose vial of lyophilized gE that is reconstituted with the accompanying vial of AS01B adjuvant to form a suspension. After reconstitution, a single dose of RZV is 0.5 ml. Both vaccine components are stored refrigerated between 2°C and 8°C. The shelf-life of RZV is 36 months. After reconstitution, RZV can be stored refrigerated for use within 6 h.
Across all studies, RZV CD42+ T-cell frequencies and antibody concentrations were similar in adults in 50–59, 60–69, and 70+ YOA, demonstrating little, if any, age-related decline in immune response to the vaccine [Citation62,Citation63].
The feasibility of subcutaneous administration was investigated by Vink et al. [Citation80], who showed that although the immune responses were similar when RZV was administered intramuscularly or subcutaneously, the subcutaneous injection was associated with higher incidences of local symptoms [Citation80].
Alternative dose intervals of 0–6 and 0–12 months were evaluated [Citation81]. The data indicate that compared to the 0–2-month interval, anti-gE antibody geometric mean concentrations (GMCs) were not inferior for the 0–6-month interval [Citation81]. Non-inferiority criteria were marginally missed when comparing the 0–2-month and 0–12-month schedules.
5.4. Immunogenicity
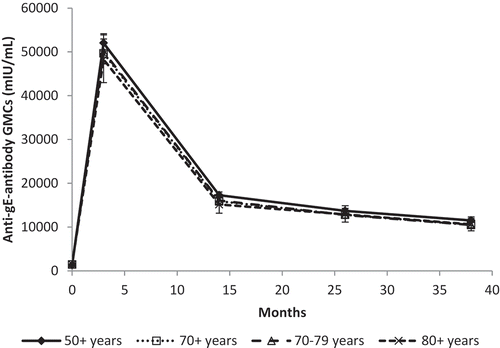
In addition to the assessment of immunogenicity in Phase I/II studies, immunogenicity was also assessed in a large population of adults (N = 1457) 50+ YOA who participated in Phase III trials [Citation28,Citation29]. Anti-gE antibody responses that were 40-fold higher than placebo were measured 1 month after the second dose of RZV. Antibody levels were lower after 1 year but persisted well above (8.7-fold) pre-vaccination levels up to 3 years post-dose 2 (). Subgroup analyses by age stratum (70+, 70–79, and 80+ YOA) were comparable with the overall 50+ YOA population, suggesting no or limited impact of age on antibody response and persistence ().
Figure 1. Anti-gE geometric mean antibody concentrations (GMCs) with 95% confidence intervals by age group (ATP cohort for immunogenicity, pooled results from ZOE-50 and ZOE-70).
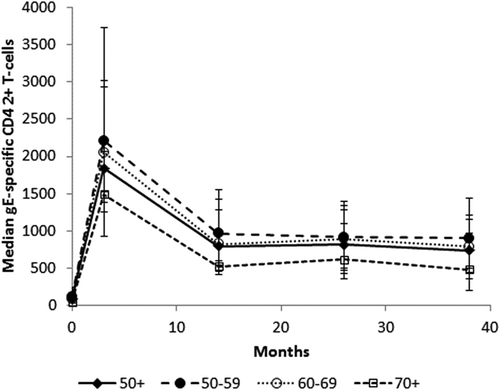
gE-specific CD42+ T-cells were substantially higher than at baseline or placebo 1 month after the second dose across all age strata (). In all age strata, observed gE-specific CMI responses decreased over time, but remained higher than pre-vaccination levels up to 3 years post-dose 2 (Month 38).
Figure 2. Frequency (median and interquartile range) of gE-specific CD42+ cells per 106 CD4 cells (ATP cohort for immunogenicity, ZOE-50).
Long-term follow-up of subjects vaccinated with RZV in Study B by Chlibek et al. has continued through Year 9 postvaccination [Citation77] (). gE-specific CD42+ cell frequencies and anti-gE antibody concentrations decreased until Year 4 and then plateaued until Year 9, remaining well above pre-vaccination levels: 3.4-fold for gE-specific CD42+ and 7.5-fold for anti-gE antibodies at Year 9 across all age strata [Citation77].
5.5. Clinical efficacy in adults 50+ YOA and 70+ YOA
Two Phase III placebo-controlled randomized studies of identical design, named ZOE-50 and ZOE-70, enrolled subjects 50+ YOA and 70+ YOA, respectively, in 18 countries (Australia, Brazil, Canada, Czech Republic, Estonia, Finland, France, Germany, Hong Kong, Italy, Japan, Mexico, Republic of Korea, Spain, Sweden, Taiwan, the United Kingdom, and the United States) [Citation28,Citation29]. All participants were to receive two intramuscular doses of RZV or placebo (lyophilized sucrose reconstituted in saline) 2 months apart.
The primary objective of each study was to evaluate vaccine efficacy in the prevention of HZ compared to placebo, as measured by the reduction in HZ risk. The studies were designed to allow a pooled analysis to provide more robust estimates of HZ vaccine efficacy in participants 70+ YOA and to evaluate pooled vaccine efficacy in the prevention of PHN compared to placebo in those 50+ and 70+ YOA. The pooled analysis also included an integrated safety analysis, allowing a more comprehensive assessment of the risk–benefit of the vaccine.
Participants were followed up for at least 30 months after dose 2 through monthly contacts and annual visits to the clinic. The mean follow-up period for efficacy was 3.2 years in ZOE-50 and 3.7 years in ZOE-70 [Citation28,Citation29].
5.5.1. End points
Suspected HZ cases were detected clinically and thereafter confirmed by PCR using validated sample collection methods [Citation82]. For cases where PCR results were either unavailable or indeterminate, a case was considered confirmed if unanimously agreed by five members of a case ascertainment committee who were blinded to group allocation. Vaccine efficacy was calculated on these confirmed HZ cases.
PHN was identified using the worst pain score from the Zoster Brief Pain Inventory [Citation83], using an 11-point Likert scale (0–10) to rate HZ pain and discomfort. PHN was defined as a worst pain score of ≥3 for pain that persisted or developed >90 days after HZ rash onset.
5.5.2. Results
5.5.2.1. Prevention of HZ
Among adults 50+ YOA in ZOE-50, 6 vaccinees versus 210 placebo recipients had confirmed HZ over the study period () [Citation28,Citation29]. The overall incidence of HZ was 0.3 per 1000 person-years in RZV recipients versus 9.1 per 1000 person-years in placebo recipients, resulting in efficacy of 97.2% (95% CI 93.7–99.0) in adults 50+ YOA over a period of approximately 4 years. Overall efficacy in adults 60+ YOA was 97.6% (95% CI 92.8–99.5) [Citation84]. Remarkably, vaccine efficacy was unchanged across age groups in ZOE-50: 96.6% (95% CI 89.6–99.3) in 50–59-year olds, 97.4% (95% CI 90.1–99.7) in 60–69-year olds, and 97.9% (95% CI 87.9–100.0) in 70+-year olds, suggesting no or limited impact of age on efficacy.
In the ZOE-50/ZOE-70 pooled analysis of 17,531 evaluable participants 70+ YOA, there were 25 cases of confirmed HZ in RZV recipients and 284 in placebo recipients over 3.7 years of follow-up, resulting in an overall vaccine efficacy of 91.3% (95% CI 86.8–94.5) (). As in ZOE-50, no impact of age was observed on vaccine efficacy, which was essentially the same across age groups: 91.3% (95% CI 86.0–94.9) in 70–79-year olds and 91.4% (95% CI 80.2–97.0) in those 80+ YOA. Efficacy was sustained such that during the fourth year of follow-up, vaccine efficacy was estimated as 87.9% (95% CI 73.3–95.4) in a sensitivity analysis of the data.
5.5.2.2. Prevention of PHN
In the pooled analysis of efficacy, there were 4 RZV recipients who developed PHN during the study, all of whom were 70+ YOA, versus 46 cases in the placebo group (10 were in 50–69-year olds) (). Vaccine efficacy of RZV versus placebo against the development of PHN after HZ was estimated to be 91.2% (95% CI 75.9–97.7) in 50+-year olds and 88.8% (95% CI 68.7–97.1) in 70+-year olds (). Vaccine efficacy against PHN was 100% in 50–59-year olds and 93.0% in 70–79-year olds. Estimates in 60–69- and 80+-year olds were also high (100% and 71.2%, respectively) but were not statistically significant due to the low number of cases observed ().
The long-term duration of efficacy beyond 4 years following ZOE-50 and ZOE-70 is not yet known. A study on long-term efficacy is ongoing in RZV recipients from both studies (NCT02723773).
5.6. Coadministration
Additional Phase III studies in adults 50+ YOA have evaluated RZV coadministered with quadrivalent influenza vaccine or with 23-valent pneumococcal polysaccharide vaccine [Citation76,Citation87]. Results indicate that coadministration with both vaccines was well tolerated and immunogenic, and no immunologic interference between the administered antigens has been observed [Citation76,Citation87]. A study to assess coadministration with reduced antigen content combined diphtheria–tetanus and acellular pertussis vaccine was recently completed (NCT02052596).
5.7. Safety
ZOE-50 and ZOE-70 together enrolled over 30,000 individuals of whom more than 14,000 received at least one dose of RZV. These studies provide efficacy and safety data over a median follow-up time of approximately 4 years. The data generated to date have not raised any safety concerns.
As may be expected with an adjuvanted vaccine, local and systemic solicited symptoms were higher in the RZV group versus the placebo group. Solicited injection-site pain was reported by 88.4% of RZV recipients 50–59 YOA, 82.8% of those 60–69 YOA, and 69.2% who were 70+ YOA versus up to 14.4% of placebo recipients (). The vast majority of local and systemic reactions were of short duration (median 1 day) and mild-to-moderate in intensity [Citation28]. Grade 3 local symptoms were reported by up to 10.3% of RZV recipients 50–59 YOA, 6.9% of those 60–69 YOA, and 4.0% of those 70+ YOA versus 0.5% or fewer of placebo recipients ().
Table 3. Vaccine reactogenicity in the reactogenicity subseta in adults (total vaccinated cohort; ZOE-50 and ZOE-70 [Citation88]).
Solicited systemic symptoms after vaccination were reported by more participants who received RZV than who received placebo. The most frequently reported solicited systemic symptoms occurring within 7 days after vaccination were myalgia, fatigue, and headache (). Of 50+ year olds in ZOE-50, 66.1% of RZV and 29.5% of placebo recipients reported a systemic reaction within 7 days after vaccination [Citation29]. In 70+-year olds in ZOE-70, systemic reactions were reported by 53.0% of HZ/zu recipients and 25.1% of placebo recipients [Citation28]. Most solicited systemic symptoms were mild-to-moderate in intensity. Grade 3 systemic symptoms were reported by up to 8.9% of RZV recipients 50–59 YOA, 5.3% of those 60–69 YOA, and 2.8% of those 70+ YOA versus 0.9%, 0.8%, and 0.4% of placebo recipients, respectively (). Reactogenicity did not appear to have a significant impact on compliance, as observed by approximately 90% compliance with the second dose among those study participants who experienced a grade 3 reaction following the first dose of RZV. The incidence of solicited local and general symptoms was lower in adults 70+ YOA compared with those 50–69 YOA ().
The safety profile of RZV is clinically acceptable, and the benefit–risk of vaccination of adults 50+ YOA with RZV is favorable. Overall, in a pooled analysis of ZOE-50 and ZOE-70, SAEs, pIMDs, and deaths occurred at similar rates between vaccine and placebo groups and as expected in the age groups studied [Citation89]. Events of interest were identified for further monitoring through pharmacovigilance activities in the post-licensure setting. These include pIMDs of particular interest in adults 50+ YOA and potentially serious inflammation-based pathology associated with these diseases.
6. RZV in immunocompromised persons
The initial clinical development focused on adults 50+ YOA, and clinical research is ongoing to determine the benefit of RZV in immunocompromised persons 18+ YOA. The clinical development program in immunocompromised individuals targets adults from 18 YOA and commenced after safety of RZV had been explored in Phase I/II studies in healthy adults. To date, clinical studies have shown that RZV is immunogenic and has an acceptable safety profile in immunocompromised persons with a range of underlying conditions (Studies C to G, ).
Table 4. Phase I/II/III studies of adjuvanted recombinant zoster vaccine (RZV) in immunocompromised adults 18+ years of age.
The safety, reactogenicity, and immunogenicity of RZV were also studied in 120 autologous hematopoietic cell transplant recipients 18+ YOA (Study D) [Citation71]. Participants were randomized to receive three doses of RZV, one dose of saline placebo followed by two doses of RZV, three doses of placebo, or three doses of gE/AS01E at 0, 1, and 3 months. Vaccination commenced 2–5 months after transplant. Two doses administered two months apart of RZV induced marked increases in anti-gE antibodies, with a 42-fold increase in GMC compared to placebo. High gE-specific CD42+ T-cell responses were observed after two doses of RZV in all patient groups, and a third RZV dose only modestly increased humoral and CMI responses (). Humoral and cellular immune responses persisted for up to 1 year after vaccination. The reactogenicity and safety profile of RZV in this population of hematopoietic cell transplant recipients was clinically acceptable. No negative impacts on the transplant were observed, and no safety concerns were identified during the study.
A total of 123 adults 18+ YOA with HIV were enrolled and randomized to receive three doses of RZV (74 patients) or saline placebo (49 patients) at Months 0, 2, and 6 (Study C) [Citation72]. Participants included patients receiving antiretroviral therapy (ART) with high (≥200 cells/mm3) or low (50–199 cells/mm3) CD4+ T-cell counts and patients who were ART-naive. Recruitment targets were not able to be met for the low CD4+ and ART-naive cohorts. The study collected immunogenicity and safety data until 18 months after the first dose of study vaccine. Two doses of RZV induced marked increases in anti-gE antibodies and in gE-specific CD42+ T-cells that were comparable to levels observed in healthy adults 50+ YOA (). The addition of a third dose did not induce substantial benefits over two doses in terms of the immune response. The reactogenicity and safety profile of RZV in HIV-infected adults was acceptable. No negative impacts on HIV disease progression were observed, and no safety concerns were identified.
Based on the results of these two studies, a two-dose schedule of RZV was selected for use in the Phase III studies in immunocompromised populations, with the second dose administered 1 to 2 months post-dose 1 to allow easier integration into the complex medical imperatives of these patients.
In adults with solid tumors vaccinated before or during chemotherapy (study F), available results show that two doses of RZV induce humoral and cellular immune responses, although vaccination during chemotherapy administration adversely impacted immune responses to the vaccine [Citation90] (). RZV had an acceptable safety profile in this population.
RZV administration was also immunogenic and well tolerated in adults with hematologic malignancies (results up to 6 months postvaccination available), although in those with non-Hodgkin B-cell lymphoma or chronic lymphocytic leukemia, the humoral response was substantially lower than in subjects with other underlying diseases [Citation91] ().
Two doses of RZV were evaluated in renal transplant recipients (Study G) [Citation92], which is the most common solid organ transplant and may be considered representative of other solid organ transplants due to similar nature of administered immunosuppressive therapies. Results from 1-year follow-up are not yet available, but data from the active phase show that RZV induced humoral and cellular responses in this population (). RZV had an acceptable safety profile, and no transplant rejections were reported during the active phase.
The available data to date suggest that RZV is immunogenic and has a clinically acceptable safety profile in immunocompromised patients with a range of underlying conditions. Vaccine efficacy in preventing HZ compared to placebo is being investigated in a cohort of HSCT recipients (NCT01610414). Results of this study will provide first indications about the efficacy of RZV in severely immunocompromised patients.
7. Health economic aspects
7.1. Hospitalization and mortality
The health-care burden attributable to HZ is substantial. Between 1% and 4% of people with HZ require hospitalization [Citation9,Citation93], and around 30% of these are persons with an immunocompromising condition. A literature review by Kawai et al. [Citation94] found that overall rates of HZ-related hospitalization ranged from 2.1 to 25.0 per 100,000 person-years and that hospitalization rates increased with age; hospitalization rates for HZ among 80+ YOA ranged from 15.7 to 144.2 per 100,000 person-years [Citation94]. In a study in Italy, 92.3% of all hospitalizations for HZ among immunocompetent adults 15+ YOA were in those 50+ YOA and 16.9% were due to PHN [Citation95].
Deaths due to HZ are infrequent but most often occur in older adults and in persons who are immunosuppressed [Citation96]. Analysis of data from North America, Europe, and the Asia-Pacific found HZ mortality rates between 0.02 and 0.47 per 100,000 person-years [Citation94]. Across Europe, HZ mortality rates among adults 50+ YOA vary widely from country-to-country, from 0.002 per 100,000 person-years in Poland to 0.070 per 100,000 person-years in Denmark [Citation97].
7.2. Productivity losses
Using data from the German statutory health insurance system, the average duration of sick leave was estimated as 12.5 days for HZ (range 8 days in those <20 YOA and 20 days in those 60–69 YOA) and around 2 months for PHN (range up to 205 days in those 60–69 YOA) [Citation98]. In a prospective Canadian study of 88 employed persons with HZ, 64% missed work and 76% reported decreased effectiveness at work (i.e. presenteeism) because of HZ or PHN. Mean hours of absenteeism and presenteeism per working individual were 27 and 34 h, respectively [Citation99]. Increased pain severity and longer duration of pain were associated with greater productivity loss.
7.3. Economic burden of HZ
Country-specific cost estimates indicate a substantial cost burden of HZ to the health-care system. In Italy, the total annual direct economic burden was estimated at €28.2 million, of which 76% (€21.5 million) was for the treatment of acute HZ [Citation95]. In Germany, the total annual burden of HZ and PHN to society was estimated to be €182 million [Citation98]. In the USA, HZ in 50+-year olds was estimated to result in an annual cost of $5 billion in 2013, of which $1.85 billion were medical care costs and $3.20 billion were indirect costs [Citation100]. This burden is expected to rise over the coming years as populations age [Citation16].
The key contributors to the cost burden of HZ and PHN are direct costs associated with inpatient and outpatient care (such as physician visits, diagnostic tests, and medication) [Citation15]. PHN, the most common complication of HZ, is associated with significant additional costs, although the overall cost of acute HZ is higher than that for PHN [Citation15,Citation101].
7.4. Public health impact of vaccination with RZV
The public health impact of RZV compared with a no vaccination strategy has been estimated for the USA, Canada, Germany, the UK, and Australia. All of these studies estimated the number of cases prevented over the remaining lifetime after vaccination. In the USA, it was estimated that vaccinating the entire cohort of adults 50+ YOA with RZV could prevent 11–15 million cases of HZ and 1.6–2.1 million case of PHN [Citation102]. In Canada, it was estimated that up to 0.8 million HZ cases and 190,000 PHN cases could be avoided, assuming 60% RZV vaccine coverage in adults 50+ YOA [Citation103]. Vaccinating 25% of German adults 50+ YOA could prevent 1.3–1.6 million cases of HZ and 226,000–296,000 cases of PHN [Citation104]. In the UK, vaccinating 72.8% of adults 60+ YOA (similar to the coverage for influenza vaccine in this age group) could prevent between 0.8 and 0.9 million HZ cases and 200,000–245,000 cases of PHN [Citation105]. Finally, an Australian study concluded that vaccinating all adults 60+ YOA with RZV could prevent 0.68–1.1 million cases of HZ [Citation106].
A more recent study compared the public health impact of introducing RZV or ZVL in the German population 50+ YOA [Citation107]. It was estimated that RZV could prevent 725,233 cases of HZ in adults 50–59 YOA, 533,162 cases in adults 60–69 YOA, and 486,794 in those 70+ YOA compared to 198,477, 196,000, and 104,640, respectively, using ZVL. The number needed to vaccinate (NNV) to prevent one HZ case was between 8 and 11 using RZV and 20 and 50 for ZVL. The NNV to prevent one PHN case was 39–53 for RZV and 94–198 for ZVL. The authors concluded that due to higher and sustained vaccine efficacy, RZV demonstrated the potential for a superior public health impact compared to ZVL.
GSK has developed the ZOster ecoNomic Analysis (ZONA) model, a deterministic Markov model to evaluate the cost-effectiveness of RZV (manuscript submitted). A hypothetical 1 million person cohort of US adults not previously vaccinated against HZ who were 60+ YOA was modeled over their remaining lifetimes from the year of vaccination with annual cycle lengths. Three different HZ vaccination strategies were compared: no vaccination, vaccination with RZV, and vaccination with ZVL. The ZONA model estimated that RZV vaccination would reduce disease burden resulting in a gain of 2291 quality-adjusted life years (QALYs) at a total societal cost of $27 million compared to no vaccination (i.e. an incremental cost-effectiveness ratio of $11,863 per QALY saved). The ZONA model estimated that RZV would reduce disease burden and result in a gain of 1261 discounted QALYs and societal cost savings of almost $96 million compared to ZVL. Consequently, when considering vaccinating US adults 60+ YOA who have not been previously vaccinated against HZ, vaccination with RZV is cost-effective relative to a no vaccination strategy and cost-saving relative to vaccination with ZVL. These findings were robust as demonstrated by sensitivity, scenario, and threshold analyses.
8. Production and manufacturing
Production and manufacturing procedures have implications for reliability of supply and product quality. Well-established recombinant protein technology simplifies manufacturing and promotes consistent production of large quantities of antigen [Citation108]. The recombinant gE protein is produced in CHO cells. Such cell lines are commonly used for production of complex recombinant proteins requiring specific three-dimensional folding and posttranslational modifications such as asparagine-associated glycosylation patterns to retain their antigenicity [Citation109]. The recombinant gE is then concentrated and purified, formulated into final bulk, and sterile filtered before being filled into monodose vials and lyophilized.
The process for preparing QS-21 involves extraction of the Quillaja saponaria tree bark followed by purification and lyophilization. MPL is prepared by bacterial culture and harvest of the S. minnesota R595. Lipopolysaccharide from S. minnesota R595 is extracted from these bacteria and subjected to sequential hydrolyses to produce crude MPL, which is purified by chromatography and extraction methods. The final liquid AS01B formulation containing a liposome vehicle and the two immunostimulants is sterile filtered before filling into vials. Quality control tests are performed throughout the production process to ensure consistent potency, purity, and safety [Citation110]. The final product can be stored for up to 36 months refrigerated to +2 to + 8°C. Upon administration, the lyophilized gE antigen is reconstituted with the liquid AS01B.
9. Expert commentary
It has been more than 10 years since the first HZ vaccine (ZVL) was first licensed. While showing moderate efficacy of 51.3% in 60+-year olds and 69% in 50–59-year olds in preventing HZ, there are limitations related to its lower efficacy in older age groups, especially in adults 70+ YOA, its rapidly waning efficacy, and its contraindication in immunosuppressed individuals, which are critical target groups for HZ vaccination. By using the recombinant gE antigen combined with an adjuvant specifically designed to improve humoral and CMI response, RZV has the potential to have a major impact on HZ-associated incidence and morbidity. Vaccination with RZV induced robust increases in specific humoral and CMI responses that appear to persist above baseline levels for many years. RZV vaccine efficacy against HZ of up to 97.2% and efficacy of up to 91.2% against PHN have been demonstrated in adults representative of the general 50+ YOA population (). In contrast to ZVL, increasing age does not seem to be detrimental to RZV efficacy ().
The clinical development program has to date included more than 15,000 adults who have received RZV in completed Phase III studies alone. As expected with an adjuvanted vaccine, solicited local and systemic reactions were more common in the RZV recipients, with the majority reporting local injection-site symptoms of pain, redness, and swelling. Systemic solicited symptoms of fatigue, myalgia, and headache were also common, but need to be interpreted in the context of the levels of those symptoms reported after placebo injection. The local and systemic reactogenicity following RZV may be explained by the transient stimulation of the innate immune responses elicited by AS01B which is required for the adjuvant effect of AS01B. This is therefore not unexpected. Indeed, extensive clinical experience using the AS01 adjuvant has shown that AS01-adjuvanted vaccines are more reactogenic than non-adjuvanted formulations or vaccines adjuvanted to aluminum [Citation111]. There were similar rates of SAEs, pIMDs, and deaths compared with placebo. High rates of uptake of the second vaccine dose in clinical trials suggest that local and systemic symptoms were tolerable and did not significantly affect vaccine compliance. Acceptance of the vaccine safety profile outside of clinical trials, as well as acceptance of the two-dose schedule, will continue to be evaluated in real-life settings. In view of the high incidence of HZ and the high risk of protracted PHN, as well as the high efficacy and clinically acceptable safety profile of the vaccine, the benefit–risk of vaccination of 50+-year olds with RZV is favorable. The safety of RZV when used in the real-world setting will continue to be monitored through pharmacovigilance activities.
10. Five-year view
The availability of RZV is a major step forward in HZ prevention. RZV could have a marked impact on HZ incidence and morbidity of vaccinated persons, having the potential to avoid the negative impact of HZ and related complications on their quality of life. In addition, associated reductions in health-care related costs can be expected. Clinical studies are ongoing to investigate how long efficacy persists. Vaccine safety will be continuously monitored through the ongoing systematic review of safety data arising from pharmacovigilance activities once the product is in use [Citation78]. The effectiveness of the vaccine in real-life use will also be closely monitored, allowing the evaluation of the vaccine in a broad range of situations.
To date, the available data are very promising. Ongoing and recently completed studies investigate the safety, immunogenicity, reactogenicity, and efficacy of RZV in patients 18+ YOA with various immunosuppressive conditions. Depending on the outcome of these studies, the benefit of RZV in children with renal transplant and with malignancies may also be explored.
The next 5 years may also see the availability of an inactivated whole virus HZ vaccine currently in development in immunosuppressed populations, investigated with administration of four vaccine doses [Citation112,Citation113].
In view of the globally aging population, it is timely that a new effective vaccine to prevent HZ has become available. Early estimates of impact suggest the potential to prevent substantially more HZ cases than ZVL, with favorable cost-effectiveness. The next 5 years should see the accumulation of information to quantitate these potential benefits.
Finally, the unique antigen–adjuvant RZV combination is the first vaccine to provide nearly complete protection against HZ to all categories 50+ YOA, which may be a sign that new adjuvant technologies are instrumental in helping overcome the immune hyporesponsiveness associated with older age. RZV could be the first of a series of new vaccines using this technology that are highly efficacious in (older) adults, potentially transforming the vaccination landscape for protection of older adults against infectious diseases.
Key issues
The RZV vaccine contains a recombinant, truncated version of the VZV envelope glycoprotein E (gE) and the AS01B adjuvant system. gE was selected as the vaccine antigen because it is implicated in viral replication and viral spread and is abundantly expressed during HZ episodes. AS01B was designed to enhance the immune responses to the antigen. Two vaccine doses are required for optimal immunogenicity and efficacy.
RZV induces high anti-gE antibody responses and high levels of gE-specific T cells expressing at least two activation markers. Humoral and CMI responses to RZV are similar across age groups including the oldest age groups aged 80+ YOA. RZV-induced cellular and humoral immune responses remained above pre-vaccination levels for at least 9 years postvaccination.
RZV was tested in two large Phase III efficacy trials in adults 50+ and 70+ YOA and demonstrated high efficacy of up to 97.2% in the prevention of HZ. Efficacy against PHN in older adults ranged from 88.8% to 91.2%, with up to 100% prevention in the younger age group 50–59 YOA. Currently available data show that efficacy persists to high levels 4 years after vaccination.
Vaccines containing AS01, including RZV, induce higher levels of local and systemic reactions than other adjuvants or unadjuvanted vaccines, likely due to the mode of action of the adjuvant. In clinical trials of RZV, uptake of the second vaccine dose was high despite substantial local and systemic reactions, suggesting that the reactogenicity is acceptable.
SAEs, pIMDs, and deaths occurred at similar rates to placebo, indicating a clinically acceptable safety profile.
The benefit–risk profile of RZV is favorable. RZV could have marked impacts on HZ incidence and burden of illness, helping to avoid the negative impact of HZ and related complications on quality of life, in particular that of the most vulnerable populations of adults 70+ YOA and potentially, immunocompromised individuals. In addition, the health-care and societal costs associated with HZ can be significantly reduced. Efficient systems to ensure compliance with the two-dose schedule are needed to optimize the impact of RZV vaccination.
Through its specific composition, RZV has overcome immune senescence, demonstrating high efficacy in the prevention of HZ for all ages in the studied 50+ YOA populations, and with evidence of only marginal waning efficacy during the study duration. This technology including the AS01B adjuvant could open the way forward to other innovative vaccines in older adults or immunocompromised subjects, where efficient vaccine prophylaxis has been limited by natural or drug-induced immune impairment.
Author’s contributions
All authors participated in the development and the review of the manuscript and approved the final submitted version. The corresponding author had final responsibility to submit for publication. Drafts were developed by a professional publication writer according to the recommendations, documentation, and outline provided by the lead author.
Declaration of interest
All authors were employees of the GSK group of companies at the time of the study. Nicolas Lecrenier, Romulo Colindres, Desmond Curran, Carine De Kesel, Jean-Philippe De Saegher, Marie Normand-Bayle, Lidia Oostvogels, Ventzislav Vassilev, Tomas Mrkvan, Carlota Vinals, and Alain Brecx own GSK shares/stock. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. A reviewer on this manuscript has disclosed a service contract with Merck, safety of live VZV vaccines, NIH funding to study enteric zoster and Ad hoc consulting on varicella and zoster vaccines.
Trademarks
Shingrix and Varilrix are trademarks of the GSK group of companies. Zostavax is a trademark of Merck Sharp & Dohme Ltd. QS-21 is licensed by GSK from Antigenics Inc., a wholly owned subsidiary of Agenus Inc., a Delaware, USA corporation.
Supplemental Material
Download Zip (1 MB)Acknowledgments
The authors thank the clinical study participants and everyone involved in the development of Shingrix within GSK and outside GSK. Writing assistance was provided by Joanne Wolter (Independent medical writer on behalf of GSK Vaccines) and editorial and coordination assistance were provided by Houda Khamis (XPE Pharma & Science on behalf of GSK Vaccines).
Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Arvin AM. Humoral and cellular immunity to varicella-zoster virus: an overview. J Infect Dis. 2008;197(Suppl 2):S58–60.
- Mallick-Searle T, Snodgrass B, Brant JM. Postherpetic neuralgia: epidemiology, pathophysiology, and pain management pharmacology. J Multidiscip Healthc. 2016;9:447–454.
- Johnson RW, Alvarez-Pasquin M-J, Bijl M, et al. Herpes zoster epidemiology, management, and disease and economic burden in Europe: a multidisciplinary perspective. Ther Adv Vaccines. 2015;3:109–120.
- Levin MJ, Oxman MN, Zhang JH, et al. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008;197:825–835.
- Weinberg A, Zhang JH, Oxman MN, et al. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis. 2009;200:1068–1077.
- Oxman MN. Zoster vaccine: current status and future prospects. Clin Infect Dis. 2010;51:197–213.
- Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol. 2007;211:144–156.
- Patterson-Bartlett J, Levin MJ, Lang N, et al. Phenotypic and functional characterization of ex vivo T cell responses to the live attenuated herpes zoster vaccine. Vaccine. 2007;25:7087–7093.
- Yawn BP, Gilden D. The global epidemiology of herpes zoster. Neurology. 2013;81:928–930.
- Cohen JI, Straus SE, Arvin AM. Varicella-zoster virus: Replication, pathogenesis, and management. In: Knipe DM, Howley PM, editors. Fields Virology. 5th ed. Philadelphia, PA: Lippincott-Williams & Wilkins; 2007.
- Yun H, Yang S, Chen L, et al. Risk of herpes zoster in autoimmune and inflammatory diseases: implications for vaccination. Arthritis Rheumatol. 2016;68:2328–2337.
- Habel LA, Ray GT, Silverberg MJ, et al. The epidemiology of herpes zoster in patients with newly diagnosed cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:82–90.
- Weitzman D, Shavit O, Stein M, et al. A population based study of the epidemiology of herpes zoster and its complications. J Infect. 2013;67:463–469.
- Tran TN, Ray GT, Horberg MA, et al. Complications of herpes zoster in cancer patients. Scand J Infect Dis. 2014;46:528–532.
- Gater A, Uhart M, McCool R, et al. The humanistic, economic and societal burden of herpes zoster in Europe: a critical review. BMC Public Health. 2015;15:193.
- Varghese L, Standaert B, Olivieri A, et al. The temporal impact of aging on the burden of herpes zoster. BMC Geriatr. 2017;17:30.
- Kilgore PE, Kruszon-Moran D, Seward JF, et al. Varicella in Americans from NHANES III: implications for control through routine immunization. J Med Virol. 2003;70(Suppl 1):S111–118.
- Yawn BP, Saddier P, Wollan PC, et al. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clinic Proc. 2007;82:1341–1349.
- Le P, Sabella C, Rothberg MB. Preventing herpes zoster through vaccination: new developments. Cleve Clin J Med. 2017;84:359–366.
- Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005–2009. J Infect Dis. 2013;208:1859–1868.
- Chen N, Li Q, Yang J, et al. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst Rev. 2014;2:CD006866.
- Werner RN, Nikkels AF, Marinović B, et al. European consensus-based (s2k) guideline on the management of herpes zoster – guided by the European Dermatology Forum (edf) in cooperation with the European Academy of Dermatology and Venereology (EADV), part 2: treatment. J Eur Acad Dermatol Venereol. 2017;31:20–29.
- Oster G, Harding G, Dukes E, et al. Pain, medication use, and health-related quality of life in older persons with postherpetic neuralgia: results from a population-based survey. J Pain. 2005;6:356–363.
- Levin MJ, Smith JG, Kaufhold RM, et al. Decline in varicella-zoster virus (VZV)-specific cell-mediated immunity with increasing age and boosting with a high-dose VZV vaccine. J Infect Dis. 2003;188:1336–1344.
- Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284.
- Schmader KE, Oxman MN, Levin MJ, et al. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis. 2012;55:1320–1328.
- ZOSTAVAX® (Zoster Vaccine Live). Product information. [ cited 2017 Apr 19]. Available from https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM132831.pdf.
- Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375:1019–1032.
- Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372:2087–2096.
- Varivax. Prescribing information. [ cited 2017 June 01]. Available from https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM142813.pdf.
- Schmader KE, Levin MJ, Gnann JW Jr., et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50–59 years. Clin Infect Dis. 2012;54:922–928.
- Morrison VA, Johnson GR, Schmader KE, et al. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis. 2015;60:900–909.
- Izurieta HS, Wernecke M, Kelman J, et al. Effectiveness and duration of protection provided by the live-attenuated herpes zoster vaccine in the Medicare population ages 65 years and older. Clin Infect Dis. 2017;64:785–793.
- Langan SM, Smeeth L, Margolis DJ, et al. Herpes zoster vaccine effectiveness against incident herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med. 2013;10:e1001420.
- Tseng HF, Harpaz R, Luo Y, et al. Declining effectiveness of herpes zoster vaccine in adults aged >/=60 years. J Infect Dis. 2016;213:1872–1875.
- Baxter R, Bartlett J, Fireman B, et al. Long-term effectiveness of the live zoster vaccine in preventing shingles: a cohort study. Am J Epidemiol. 2017;187:161–169.
- Duncan CJ, Hambleton S. Varicella zoster virus immunity: A primer. J Infect. 2015;71(Suppl 1):S47–53.
- Kamiya H, Starr SE, Arbeter AM, et al. Antibody-dependent cell-mediated cytotoxicity against varicella-zoster virus-infected targets. Infect Immun. 1982;38:554–557.
- Tilden AB, Cauda R, Grossi CE, et al. Demonstration of NK cell-mediated lysis of varicella-zoster virus (VZV)-infected cells: characterization of the effector cells. J Immunol. 1986;136:4243–4248.
- Ito M, Bandyopadhyay S, Matsumoto-Kobayashi M, et al. Interleukin 2 enhances natural killing of varicella-zoster virus-infected targets. Clin Exp Immunol. 1986;65:182–189.
- Orange JS. Natural killer cell deficiency. J Allergy Clin Immunol. 2013;132: 515–525.
- Fowler WJ, Garcia-Valcarcel M, Hill-Perkins MS, et al. Identification of immunodominant regions and linear B cell epitopes of the gE envelope protein of varicella-zoster virus. Virology. 1995;214:531–540.
- Haumont M, Jacquet A, Massaer M, et al. Purification, characterization and immunogenicity of recombinant varicella-zoster virus glycoprotein gE secreted by Chinese hamster ovary cells. Virus Res. 1996;40:199–204.
- Malavige GN, Jones L, Black AP, et al. Varicella zoster virus glycoprotein E-specific CD4+ T cells show evidence of recent activation and effector differentiation, consistent with frequent exposure to replicative cycle antigens in healthy immune donors. Clin Exp Immunol. 2008;152:522–531.
- Vafai A. Boosting immune response with a candidate varicella-zoster virus glycoprotein subunit vaccine. Vaccine. 1995;13:1336–1338.
- Berarducci B, Ikoma M, Stamatis S, et al. Essential functions of the unique N-terminal region of the varicella-zoster virus glycoprotein E ectodomain in viral replication and in the pathogenesis of skin infection. J Virol. 2006;80:9481–9496.
- Berarducci B, Rajamani J, Zerboni L, et al. Functions of the unique N-terminal region of glycoprotein E in the pathogenesis of varicella-zoster virus infection. Proc Natl Acad Sci USA. 2010;107:282–287.
- Mallory S, Sommer M, Arvin AM. Mutational analysis of the role of glycoprotein I in varicella-zoster virus replication and its effects on glycoprotein E conformation and trafficking. J Virol. 1997;71:8279–8288.
- Hasan UA, Harper DR, Wren BW, et al. Immunization with a DNA vaccine expressing a truncated form of varicella zoster virus glycoprotein E. Vaccine. 2002;20:1308–1315.
- Dendouga N, Fochesato M, Lockman L, et al. Cell-mediated immune responses to a varicella-zoster virus glycoprotein E vaccine using both a TLR agonist and QS21 in mice. Vaccine. 2012;30:3126–3135.
- Fochesato M, Dendouga N, Boxus M. Comparative preclinical evaluation of as01 versus other adjuvant systems in a candidate herpes zoster glycoprotein E subunit vaccine. Hum Vaccin Immunother. 2016;12:2092–2095.
- Kester KE, Cummings JF, Ofori-Anyinam O, et al. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis. 2009;200:337–346.
- Garçon N, Di Pasquale A. From discovery to licensure, the adjuvant system story. Hum Vaccin Immunother. 2017;2(13):19–33.
- Leroux-Roels I, Leroux-Roels G, Clement F, et al. A phase 1/2 clinical trial evaluating safety and immunogenicity of a varicella zoster glycoprotein e subunit vaccine candidate in young and older adults. J Infect Dis. 2012;206:1280–1290.
- Baldridge JR, McGowan P, Evans JT, et al. Taking a toll on human disease: toll-like receptor 4 agonists as vaccine adjuvants and monotherapeutic agents. Expert Opin Biol Ther. 2004;4:1129–1138.
- Garçon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev Vaccines. 2011;10:471–486.
- Detienne S, Welsby I, Collignon C, et al. Central role of CD169(+) lymph node resident macrophages in the adjuvanticity of the QS-21 component of AS01. Sci Rep. 2016;6:39475.
- Welsby I, Detienne S, N’Kuli F, et al. Lysosome-dependent activation of human dendritic cells by the vaccine adjuvant QS-21. Front Immunol. 2016;7:663.
- Didierlaurent AM, Collignon C, Bourguignon P, et al. Enhancement of adaptive immunity by the human vaccine adjuvant as01 depends on activated dendritic cells. J Immunol. 2014;193:1920–1930.
- Coccia M, Collignon C, Herve C, et al. Cellular and molecular synergy in AS01-adjuvanted vaccines results in an early IFNγ response promoting vaccine immunogenicity. NPJ Vaccines 2017;2:25.
- Didierlaurent AM, Laupèze B, Di Pasquale A, et al. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev Vaccines. 2017;16:55–63.
- Chlibek R, Bayas JM, Collins H, et al. Safety and immunogenicity of an AS01-adjuvanted varicella-zoster virus subunit candidate vaccine against herpes zoster in adults ≥50 years of age. J Infect Dis. 2013;208:1953–1961.
- Chlibek R, Smetana J, Pauksens K, et al. Safety and immunogenicity of three different formulations of an adjuvanted varicella-zoster virus subunit candidate vaccine in older adults: a phase II, randomized, controlled study. Vaccine. 2014;32:1745–1753.
- Chlibek R, Pauksens K, Rombo L, et al. Long-term immunogenicity and safety of an investigational herpes zoster subunit vaccine in older adults. Vaccine. 2016;34:863–868.
- Lal H, Zahaf T, Heineman TC. Safety and immunogenicity of an AS01-adjuvanted varicella zoster virus subunit candidate vaccine (RZV): a phase-I, open-label study in Japanese adults. Hum Vaccin Immunother. 2013;9:1425–1429.
- Food and Drug Administration. Guidance for industry for the evaluation of combination vaccines for preventable diseases: production, testing and clinical studies. U.S. Department of Health and Human Services, Food and Drug Administration: Center for Biologics Evaluation and Research; 1997.
- European Medicines Agency. Committee for medicinal products for human use. Guideline on clinical evaluation of new vaccines. London. [cited 2006 Oct 18]. (no. EMEA/CHMP/VWP/164653/2005).
- Tavares Da Silva F, Di Pasquale A, Yarzabal JP, et al. Safety assessment of adjuvanted vaccines: methodological considerations. Hum Vaccin Immunother. 2015;11:1814–1824.
- Press release. GSK announces first approval of Shingrix in Canada. Media release. 13 October 2017.[cited 2017 Oct 20]. Available from https://www.gsk.com/en-gb/media/press-releases/gsk-announces-first-approval-of-shingrix-in-canada/
- Press release. Shingrix approved in the US for prevention of shingles in adults aged 50 and over. 23 October 2017. [cited 2017 Oct 26]. Available from https://www.gsk.com/en-gb/media/press-releases/shingrix-approved-in-the-us-for-prevention-of-shingles-in-adults-aged-50-and-over/
- Stadtmauer EA, Sullivan KM, Marty FM, et al. A phase 1/2 study of an adjuvanted varicella-zoster virus subunit vaccine in autologous hematopoietic cell transplant recipients. Blood. 2014;124:2921–2929.
- Berkowitz EM, Moyle G, Stellbrink H-J, et al. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: a phase 1/2a randomized, placebo-controlled study. J Infect Dis. 2015;211:1279–1287.
- Giordano G, Segal L, Prinsen M, et al. Non-clinical safety assessment of single and repeated administration of gE/AS01 zoster vaccine in rabbits. J Appl Toxicol. 2017;37:132–141.
- Segal L, Thacker K, Fochesato M, et al. Intramuscularly administered herpes zoster subunit vaccine has no effects on fertility, pre- and post-natal development in Sprague-Dawley rats. Reprod Toxicol. 2017;69:297–307.
- Grupping K, Campora L, Douha M, et al. Immunogenicity and safety of the RZV adjuvanted herpes zoster subunit vaccine in adults previously vaccinated with a live-attenuated herpes zoster vaccine. J Infect Dis. 2017; 216:1343–1351.
- Marechal C, Lal H, Poder A, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine co-administered with the 23-valent pneumococcal polysaccharide vaccine in adults ≥50 years of age: a randomized trial. Vaccine. 2018;36:4278–4286.
- Pauksens K, Volpe S, Schwarz TF, et al. Persistence of immune response to an adjuvanted varicella‐zoster virus subunit candidate vaccine for up to year 9 in older adults. Open Forum Infect Dis. 2017;4(Suppl 1):S415.
- Di Pasquale A, Bonanni P, Garçon N, et al. Vaccine safety evaluation: practical aspects in assessing benefits and risks. Vaccine. 2016;34:6672–6680.
- Tavares Da Silva F, De Keyser F, Lambert P-H, et al. Optimal approaches to data collection and analysis of potential immune mediated disorders in clinical trials of new vaccines. Vaccine. 2013;31:1870–1876.
- Vink P, Shiramoto M, Ogawa M, et al. Safety and immunogenicity of a herpes zoster subunit vaccine in Japanese population aged >/=50 years when administered subcutaneously vs. intramuscularly. Hum Vaccin Immunother. 2017;13:574–578.
- Poder A, Geeraerts B, Lal H, et al. Immunogenicity and safety of 2 doses of an investigational herpes zoster subunit vaccine administered 2, 6 or 12 months apart in adults 50 years and older: results of a phase III, randomized, open-label, multicenter trial. Open Forum Infect Dis. 2016;3(Suppl 1):753.
- Mols JF, Ledent E, Heineman TC. Sampling of herpes zoster skin lesion types and the impact on viral DNA detection. J Virol Meth. 2013;188:145–147.
- Coplan PM, Schmader K, Nikas A, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain. 2004;5:344–356.
- McElhaney JE, Lal H, Cunningham A, et al. Efficacy, immunogenicity and safety of an investigational subunit adjuvanted herpes zoster vaccine in adults aged 60 years and older: results from the ZOE-50 and ZOE-70 efficacy studies. Open Forum Infect Dis. 2016;3(Suppl 1):127.
- de la Serna J, Campora L, Chandrasekar P, et al. Efficacy and safety of an adjuvanted herpes zoster subunit vaccine in autologous hematopoietic stem cell transplant recipients 18 years of age or older: first results of the phase 3 randomized, placebo-controlled ZOE-HSCT clinical trial. Abstract LBA2. BMT Tadem meetings; 2018 February 21-25; Utah, US. Available from: https://bmt.confex.com/tandem/2018/meetingapp.cgi/Paper/11724.
- Sullivan K, Abhyankar S, Campora L, et al. Immunogenicity and safety of an adjuvanted herpes zoster subunit vaccine in adult autologous hematopoietic stem cell transplant recipients: phase 3, randomized, placebo-controlled, ZOE-HSCT clinical trial. Abstract OS9-2. 44th Annual meeting of the European Society for Blood and Marrow Transplantation; March 18-21, 2018; Lisbon, Portugal.
- Schwarz T, Aggarwal N, Moeckesch B, et al. Randomized, phase III clinical trial to assess the immunogenicity and safety of an investigational subunit adjuvanted herpes zoster vaccine coadministered with a seasonal quadrivalent inactivated influenza vaccine in adults aged 50 years and older. Open Forum Infect Dis. 2016;3(Suppl 1):751.
- Shingrix Prescribing Information. [cited 2017 Nov 08]. Available from https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm581491.htm
- López-Fauqued M, Campora L, Delannois F, et al. Results of a safety pooled analysis of an adjuvanted herpes zoster subunit vaccine in more than 14,500 participants aged 50 years or older. Open Forum Infect Dis. 2017;4(Suppl 1):S416.
- Vink P. For the Zoster-028 study group. Immunogenicity and safety of a candidate subunit adjuvanted herpes zoster vaccine in adults with solid tumors vaccinated before or during immunosuppressive chemotherapy treatment: a phase II/III, randomized clinical trial. Open Forum Infect Dis. 2017;4(Suppl 1):S417-S418.
- Oostvogels L. For the Zoster-039 study group. Immunogenicity and safety of an adjuvanted herpes zoster subunit candidate vaccine in adults with hematologic malignancies: a phase III, randomized clinical trial. Open Forum Infect Dis. 2017;4(Suppl 1):S415.
- Vink P. For the Zoster-041 study group. Immunogenicity and safety of a candidate subunit adjuvanted herpes zoster vaccine (HZ/su) in adults post renal transplant: a phase III randomized clinical trial. Open Forum Infect Dis. 2017;4(Suppl 1):S417.
- Centers for Disease Control and Prevention. Shingles (Herpes Zoster). [cited 2017 Jun 05]. Available from https://www.cdc.gov/shingles/hcp/clinical-overview.html#complications
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4:e004833.
- Gialloreti LE, Merito M, Pezzotti P, et al. Epidemiology and economic burden of herpes zoster and post-herpetic neuralgia in Italy: a retrospective, population-based study. BMC Infect Dis. 2010;10:230.
- Mahamud A, Marin M, Nickell SP, et al. Herpes zoster-related deaths in the United States: validity of death certificates and mortality rates, 1979–2007. Clin Infect Dis. 2012;55:960–966.
- Bricout H, Haugh M, Olatunde O, et al. Herpes zoster-associated mortality in Europe: a systematic review. BMC Public Health. 2015;15:466.
- Ultsch B, Siedler A, Rieck T, et al. Herpes zoster in Germany: quantifying the burden of disease. BMC Infect Dis. 2011;11:173.
- Drolet M, Levin MJ, Schmader KE, et al. Employment related productivity loss associated with herpes zoster and postherpetic neuralgia: a 6-month prospective study. Vaccine. 2012;30:2047–2050.
- McLaughlin JM, McGinnis JJ, Tan L, et al. Estimated human and economic burden of four major adult vaccine-preventable diseases in the United States, 2013. J Prim Prev. 2015;36:259–273.
- Dworkin RH, White R, O’Connor AB, et al. Healthcare costs of acute and chronic pain associated with a diagnosis of herpes zoster. J Am Geriatr Soc. 2007;55:1168–1175.
- Varghese L, Nissen MD, Olivier A, et al. Public health perspective of phase III results of an investigational herpes zoster vaccine. Communicable Disease Control Conference 2015 June 1–2. Brisbane Australia. [cited 2017 May 06]. Available from https://www.phaa.net.au/eventsinfo/communicable-disease-control-conference-2.
- Van Oorschot D, Anastassopoulou A, Schlegel K, et al. The Public Health Impact Of A New Herpes Zoster Vaccine On The German Population. [cited 2017 May 05]. Available from: https://www.ispor.org/research_pdfs/54/pdffiles/PIH6.pdf
- Van Oorschot D, Tavares R, Varghese L, et al. Forecasting the potential public health impact of introducing a new herpes zoster vaccine to the Canadian population. Canadian Immunization Conference - 12th. 2016 December 7.
- Van Oorschot D, Hunjan E, Varghese L, et al. The Public Health Perspective Of An Investigational Herpes Zoster Vaccine In The United Kingdom (UK). [cited 2017 May 05]. Available from https://www.ispor.org/research_pdfs/54/pdffiles/PIH5.pdf
- Varghese L, Curran D, Yan S, et al. Estimating the potential public health impact of introducing the RZV vaccine in the US population aged ≥50 years. ID Week 2015. 2015 Oct 10.[cited 2017 May 02]. Available from: https://idsa.confex.com/idsa/2015/webprogram/Paper52887.html.
- Curran D, Van Oorschot D, Varghese L, et al. Assessment of the potential public health impact of herpes zoster vaccination in Germany. Hum Vaccin Immunother. 2017;14:1–9.
- Cunningham AL, Garcon N, Leo O, et al. Vaccine development: from concept to early clinical testing. Vaccine. 2016;34:6655–6664.
- Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 2004;22:1393–1398.
- Preiss S, Garcon N, Cunningham AL, et al. Vaccine provision: delivering sustained & widespread use. Vaccine. 2016;34:6665–6671.
- Leroux-Roels G, Marchant A, Levy J, et al. Impact of adjuvants on CD4(+) T cell and B cell responses to a protein antigen vaccine: results from a phase II, randomized, multicenter trial. Clin Immunol. 2016;169:16–27.
- Arnold N, Messaoudi I. Herpes zoster and the search for an effective vaccine. Clin Exp Immunol. 2017;187:82–92.
- Mullane KM, Winston DJ, Wertheim MS, et al. Safety and immunogenicity of heat-treated zoster vaccine (ZVHT) in immunocompromised adults. J Infect Dis. 2013;208:1375–1385.
