ABSTRACT
Introduction: Menveo, quadrivalent meningococcal ACWY-CRM conjugate vaccine, was first licensed in 2010 in the United States and has a long track record of immunogenicity and safety in all age groups, including infants from 2 months of age.
Areas covered: This review presents clinical and post-marketing experience with MenACWY-CRM from 32 studies conducted in 20 countries that included individuals aged from 2 months to 75 years.
Expert commentary: This decade has seen an increased number of countries reporting serogroup W ST-11 clonal complex outbreaks of invasive meningococcal disease. As infant vaccination programs targeting the meningococcus are reevaluated, the role of quadrivalent meningococcal vaccines including MenACWY-CRM will be expanded. MenACWY-CRM was immunogenic in all populations and age groups studied, regardless of country of origin. MenACWY-CRM can be coadministered with many routinely used infant, toddler and adolescent vaccines, and traveler vaccines in adults, allowing for flexible use within national immunization programs and recommendations. Antibody persistence has been demonstrated up to 5 years post vaccination in all age groups. Booster doses induced robust increases in antibody titers for all four serogroups, indicative of effective priming and induction of immunological memory. The acceptable safety profile of MenACWY-CRM has been confirmed in large post-marketing safety studies.
1. The continuing burden of invasive meningococcal disease
Great progress has been made in the control and prevention of invasive bacterial diseases following routine immunization with conjugate vaccines targeting Streptococcus pneumoniae and Haemophilus influenzae type b (Hib). Today, Neisseria meningitidis is a leading cause of bacterial meningitis and septicemia in healthy children and adolescents. Although uncommon, invasive meningococcal disease (IMD) is a serious illness and each case is potentially life-threatening.
N. meningitidis is an encapsulated Gram-negative diplococcus and a commensal of the human upper respiratory tract. Rarely, invasive strains of N. meningitidis may enter the bloodstream causing septicemia and meningitis. Even when antibiotics are administered, mortality due to IMD reaches 10–20%, being highest in older adults and when accompanied by septicemia [Citation1]. Up to 20% of survivors suffer long-term neurological disability including deafness, cognitive deficit and seizures, as well as sequelae due to amputation and scarring [Citation2].
In industrialized countries, cases of IMD are usually sporadic, although localized outbreaks and epidemics occur when a virulent strain enters a previously unexposed population without underlying immunity [Citation3]. The incidence of IMD is usually <1/100,000 population in many industrialized countries during non-outbreak periods [Citation4,Citation5] but has exceeded 1000/100,000 population in the African meningitis belt during major epidemics [Citation6]. Incidence rates are highest in infants <1 year of age and young children, with a secondary peak observed during adolescence [Citation7]. Groups at highest risk for IMD include those with immunological deficits such as anatomical or functional asplenia and complement deficiency, travelers to endemic regions, Hajj pilgrims, and settings of increased exposure such as military barracks and student residences.
Despite being an uncommon disease, the health-care burden due to IMD is substantial in terms of treatment, mortality, or subsequent lifelong disability. Prevention through vaccination is the best defense against this aggressive disease that leaves little time for intervention after the first manifestation of signs/symptoms.
1.1. The impact of meningococcal vaccines on the epidemiology of IMD
The majority of endemic and epidemic IMD worldwide is caused by six meningococcal serogroups (A, B, C, W, Y, and X) [Citation8]. Meningococcal epidemiology is notably fluid, with substantial cyclical fluctuations in disease incidence and occurrence of outbreaks and epidemics. The epidemiology of IMD has also been altered profoundly by vaccination [Citation7,Citation9]; most significantly until now by monovalent serogroup C (MenC) vaccines available since 1999. Dramatic decreases in MenC IMD have been observed in countries where MenC conjugate vaccines have been implemented in universal mass vaccination (UMV) programs in infancy or early childhood, such as the United Kingdom (UK), Canada, Australia, and several countries in Europe [Citation10–Citation13]. Similar success has been observed in the African meningitis belt using monovalent serogroup A (MenA) conjugate vaccine [Citation9].
Globally, the importance of serogroups W and Y (MenW, MenY) has increased since the advent of conjugate vaccine immunization programs that focused on controlling MenC and MenA disease. In the last decade, the UK, Argentina, Chile, Australia, and some European countries have all experienced rapid increases in IMD due to an ST-11 MenW clonal complex [Citation14–Citation18]. IMD caused by MenW is also increasing in some areas of the meningitis belt where MenA disease has been controlled [Citation9]. Quadrivalent conjugate vaccines containing the meningococcal serogroups A, C, W, and Y capsular polysaccharides have been added to national immunization schedules in numerous countries to improve control of IMD, beginning with the United States (US) in 2005, the UK, and multiple other countries including Argentina, Australia, Saudi Arabia [Citation9,Citation16], and the Netherlands [Citation19].
1.2. Quadrivalent conjugate vaccine: MenACWY-CRM (Menveo)
There are three quadrivalent conjugate meningococcal vaccines available in the global market: all contain A, C, W, and Y polysaccharides but use different protein carriers: Nimenrix (Pfizer) conjugated to tetanus toxoid (MenACWY-TT), Menactra (Sanofi Pasteur) conjugated to diphtheria toxoid (MenACWY-DT), and Menveo (GSK) conjugated to nontoxic mutant of diphtheria toxin (MenACWY-CRM).
MenACWY-CRM was first licensed in 2010 and is currently licensed in more than 64 countries worldwide, with more than 37 million doses of MenACWY-CRM distributed since launch. At the time of writing, a single dose of MenACWY-CRM is approved for use from 2 years of age in European Union (EU) countries, and from 2 months to 55 years of age in the US and many other countries, including Argentina, Australia, and Saudi Arabia. MenACWY-CRM is indicated as a 4-dose series in infants at risk of IMD from 2 to 6 months of age and as a 2-dose series between 7 and 23 months of age in countries like the US, Saudi Arabia, and Argentina.
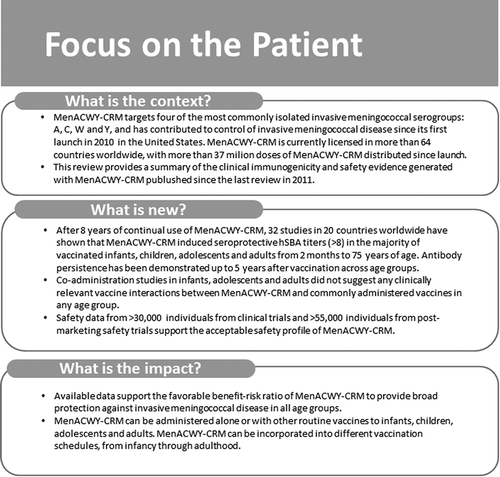
A wealth of experience with meningococcal conjugate vaccines has been accumulated during clinical development and after licensure. Meningococcal conjugate vaccines have demonstrated an acceptable safety profile and favorable benefit–risk balance. Conjugated quadrivalent vaccines are making a substantial contribution to reducing IMD globally, and MenACWY-CRM, along with other quadrivalent vaccines, is likely to play an increasing role as the epidemiology of IMD evolves. Since the last reviews of clinical data published in 2011 [Citation20,Citation21], MenACWY-CRM has received an indication for use in infants and other age groups in multiple countries. Substantial data evaluating the immunogenicity and safety of MenACWY-CRM in infants, antibody persistence, booster, and coadministration of MenACWY-CRM with other routine vaccines are now published. The aim of this review is to provide updated MenACWY-CRM clinical and post-licensure experience since 2011 and evaluate its benefit–risk ratio, providing a comprehensive summary of the available data in different age groups and in different countries. A summary contextualizing the results and potential clinical relevance and impact is displayed in the focus on the patient section ().
2. Clinical trials investigating MenACWY-CRM
The main sources for this updated clinical overview were published GSK-sponsored studies that were not included in the previous expert reviews [Citation20,Citation21]. Eight additional papers describing the immunogenicity and/or safety of MenACWY-CRM were also identified through a literature search performed in PubMed covering 1 January 2011 to 20 February 2018.
In total, 28 clinical trials and 4 safety surveillance studies have been published since the 2011 review. These studies describe the safety and/or immunogenicity and antibody persistence of MenACWY-CRM administered to individuals aged from 2 months through 75 years of age (–). Studies were conducted in 20 countries across Europe, the Americas, Asia, and the Asia-Pacific region.
Table 1. Studies supporting the immunogenicity and safety of MenACWY-CRM in healthy infants and toddlers.
Table 2. Studies supporting the immunogenicity and safety of MenACWY-CRM in children 2–10 years of age.
Table 3. Studies supporting the immunogenicity and safety of MenACWY-CRM in adolescents and adults.
2.1. Assessment of immunogenicity
Functional antibodies against each vaccine serogroup were measured by a serum bactericidal assay using human complement (hSBA) in all but two studies which used a baby rabbit complement source [Citation22,Citation23], and one study where the method was not specified [Citation24]. Historically, an hSBA assay cutoff dilution of 1:4 is considered a surrogate end point indicating clinical protection against MenC [Citation25]. SBA has proved to be the best method to evaluate the surrogate of protection for all serogroups [Citation26] and is usually extrapolated to all vaccine serogroups. However, when available, seroprotection rates are reported using a more conservative cutoff (1:8), which was used to determine seroprotection against all four serogroups in the context of MenACWY-CRM licensure.
2.2. Assessment of reactogenicity and safety
In most studies, reactogenicity was measured using diary cards from the day of vaccination (day 1) for 7 days (day 8). Solicited local reactions recorded in each study included pain at the injection site (tenderness in subjects <6 years of age), erythema, and induration. Solicited systemic reactions varied according to the age of the population evaluated in individual clinical trials and included sleepiness, persistent crying, irritability, change in eating habits, vomiting and diarrhea in infants, and chills, nausea, malaise or fatigue, myalgia, arthralgia, and headache at older ages. Fever (body temperature ≥38.0°C) was solicited in all age groups. Unsolicited adverse events (AEs) were usually collected for approximately 1 month after each vaccination, while serious adverse events (SAEs) were captured for the entire duration of each study. The study investigators had to determine the severity and seriousness of each AE (according to predefined criteria), and any potential causal association between vaccination and the AE.
2.3. MenACWY-CRM in infants and toddlers
2.3.1. Primary vaccination, antibody persistence, and booster
Three primary doses of MenACWY-CRM coadministered with routine vaccines (including combined diphtheria–tetanus–acellular pertussis [DTaP], inactivated poliovirus vaccine [IPV], hepatitis B [HBV], H. influenzae type b [Hib], 7-valent or 13-valent pneumococcal conjugate [PCV], and rotavirus vaccines) induced seroprotective antibody titers for each vaccine meningococcal serogroup in the majority of infants [Citation27–Citation30]. The percentages of infants with hSBA titers ≥1:8 after 3-dose priming ranged from 76% to 89% for MenA, 94% to 97% for MenC, 98% to 99% for MenW, and 94% to 98% for MenY in the different studies [Citation27–Citation30]. A fourth dose at 12 months of age induced robust increases in hSBA titers for each serogroup. The percentage of infants with hSBA titers ≥1:8 after the fourth dose was at least 89% for MenA and ranged from 95% to 100% for the other serogroups [Citation27–Citation30].
Two studies showed that the immune response using a 3-dose schedule (two doses of MenACWY-CRM in infancy with the third dose administered at 12–13 months of age) was non-inferior to the 4-dose schedule in terms of seroprotection rates and geometric mean antibody titers (GMTs) for serogroups C, W, and Y, and also for MenA in one study, while in the other study, there was no formal assessment of non-inferiority for serogroup A; however, the lower limit of the 95% confidence interval (CI) following a 3-dose series for serogroup A was 82% [Citation27,Citation29]. Across both studies, the percentages of toddlers with hSBA titers ≥1:8 after the third dose were 88–94% for MenA, 95–97% for MenC, 99% for MenW, and 99–100% for MenY.
A study of antibody persistence up to 5 years after 4-dose infant primary vaccination showed that around one-half of children continued to have seroprotective hSBA titers (≥1:8) for serogroups W and Y, and 26% for serogroup C, whereas hSBA titers waned rapidly for MenA (6% of children had hSBA ≥1:8 by age 5 years) () [Citation31]. Persistence of bactericidal antibodies after MenACWY-CRM vaccination in different age groups has been recently reviewed by Baxter et al. [Citation32]. Booster vaccination at 5 years of age induced large increases in hSBA titers of 42–113-fold for all serogroups, with titers that were higher than after the fourth dose at 12 months, indicating persistence of immune memory until at least 5 years of age.
Figure 2. hSBA immunogenicity in infants vaccinated with a 4-dose primary series and a booster dose at 60 months of age of MenACWY-CRM coadministered with routine vaccines (Month 60 booster cohort*). Data from Refs. [Citation30,Citation31].
*Four priming doses of MenACWY-CRM coadministered with DTaP–HBV–IPV, Hib, and PCV7. The Month 60 persistence cohort included participants vaccinated at 2, 4, 6 and 12, or 13 months of age.Post-3: One month after the third priming dose; Pre-4: at the time of the fourth dose; Post-4: 1 month after the fourth dose; M40/M60: at 40/60 months of age; Post-B: 1 month after the booster dose at month 60; GMT: geometric mean antibody titer; hSBA: serum bactericidal assay using human complement source.Error bars indicate 95% confidence intervals.
![Figure 2. hSBA immunogenicity in infants vaccinated with a 4-dose primary series and a booster dose at 60 months of age of MenACWY-CRM coadministered with routine vaccines (Month 60 booster cohort*). Data from Refs. [Citation30,Citation31].*Four priming doses of MenACWY-CRM coadministered with DTaP–HBV–IPV, Hib, and PCV7. The Month 60 persistence cohort included participants vaccinated at 2, 4, 6 and 12, or 13 months of age.Post-3: One month after the third priming dose; Pre-4: at the time of the fourth dose; Post-4: 1 month after the fourth dose; M40/M60: at 40/60 months of age; Post-B: 1 month after the booster dose at month 60; GMT: geometric mean antibody titer; hSBA: serum bactericidal assay using human complement source.Error bars indicate 95% confidence intervals.](/cms/asset/4a770884-5102-4858-a6ba-77ae0a0a71e0/ierv_a_1521280_f0002_b.gif)
Two doses of MenACWY-CRM were immunogenic when administered to unvaccinated toddlers [Citation27,Citation33]. In one study, the first dose was administered at 7–9 months [Citation33], and the second dose at 12 months of age, and in the second study the doses were administered at 12 and 15 months of age [Citation27]. The percentages of toddlers with hSBA titers ≥1:8 after the second dose were 88–97%% for MenA, and 96–100% for MenC, W, and Y.
Booster vaccination at 5 years of age after 2-dose priming of toddlers induced high levels of seroprotection (96–100% of children with hSBA titers ≥1:8) and GMTs that were similar to responses in 5-year old children primed with 4 doses in infancy [Citation31].
2.3.2. Reactogenicity and safety
In clinical trials MenACWY-CRM was associated with local and systemic reactions that were short-lived and largely of mild-to-moderate intensity. Overall, the most frequently reported injection site reaction after primary vaccination in infants and toddlers was tenderness (up to 68% of subjects) [Citation27,Citation29,Citation30,Citation33,Citation34]. The most frequently reported systemic reaction in most studies was irritability (30–77% of subjects across the studies). Body temperature ≥38.0°C was uncommon, reported for 5–15% of infants, and fever ≥38.5°C was rare. Multiple vaccine doses did not increase reactogenicity in infants.
Reactogenicity following a booster dose of MenACWY-CRM administered at 5 years of age was similar in children who had previously received MenACWY-CRM in infancy or between 2 and 10 years of age. Reactogenicity after the booster was also similar to that after a single dose in age-matched naïve controls [Citation31,Citation35].
Safety was evaluated in 7744 infants who received routine vaccines (DTaP, IPV, Hib, PCV7 at 2, 4, and 6 months and measles–mumps–rubella [MMR] at 12 month of age), with or without MenACWY-CRM, in a Phase 3 study conducted in the US, South America, and Asia [Citation34]. The percentage of children with severe systemic reactions was 16% among children who received MenACWY-CRM plus routine vaccines, versus 13% in those that received routine vaccines alone (group difference 3.0%, 95% CI −0.8–6.4%). Although the non-inferiority criterion was not met, a post-hoc analysis that controlled for center and group-by-center effects did show non-inferiority between the two groups in terms of severe systemic symptoms. The occurrence of unsolicited AEs, SAEs, and AEs leading to study withdrawal was similar in both groups, and no safety concerns were identified [Citation34].
2.3.3. Coadministration
MenACWY-CRM has been studied in coadministration with PCV7 or PCV13, DTaP-combination vaccines, IPV, HBV, Hib, and MMR/varicella vaccine combined with, or coadministered with, varicella vaccine (). Coadministration data for MenACWY-CRM have been recently reviewed by Gasparini et al. [Citation36].
There were no clinically relevant vaccine interactions on the immune response to MenACWY-CRM or to the coadministered vaccines [Citation36]. In one study assessing coadministration of MenACWY-CRM with DTaP, non-inferiority criteria were met for all pertussis antigens, when assessed in terms of geometric mean antibody concentrations (GMCs), and met for pertactin and FHA, but not pertussis toxoid and fimbriae, when assessed in terms of the percentages of participants who achieved a fourfold increase in hSBA titers [Citation28]. In another study, non-inferiority criteria were met for all pertussis antigens in the US arm, and for all antigens except pertactin in the Latin American arm [Citation27,Citation28,Citation30], when assessed in terms of GMCs. However, in terms of the percentage of participants who achieved a fourfold increase in titers, non-inferiority was demonstrated for all pertussis antigens in Latin American participants, and for all antigens except for pertactin in US participants of the same study [Citation27,Citation28,Citation30]. These results show no consistent trend across studies, and in the absence of an accepted serological correlate of protection, the clinical relevance of these findings is not known.
Non-inferiority criteria were met for immune responses to PCV7 after three doses, except for serotype 6B in one study [Citation30], for 6B and 23F in another [Citation28], and for 19A (after three doses) and serotypes 3 and 5 (after two doses) in a study of MenACWY-CRM administered concomitantly with PCV13 [Citation29]. Post-hoc analyses in two of these studies showed that all serotypes met non-inferiority criteria after accounting for strong center effects [Citation28,Citation29]. There are unlikely to be clinical implications of these findings because the percentages of subjects with pneumococcal serotype-specific antibody levels ≥0.35 μg/ml were at least 86% in all studies, and no serotype was consistently affected. Furthermore, all non-inferiority criteria were met after the dose administered in the second year of life.
The reactogenicity profile of MenACWY-CRM administered with routine vaccines to infants was similar to that of the routine vaccines administered alone [Citation27,Citation29,Citation30,Citation33].
2.3.4. B-cell responses and impact of maternal antibodies on vaccination of infants
Infants participating in a UK study received three doses of MenACWY-CRM at 2, 4, and 12 months of age [Citation37,Citation38]. A high percentage of infants had hSBA titers ≥1:4 after the second dose (69% for MenA and 95–99% for serogroups C, W, and Y) [Citation37]. Serogroup-specific memory B cells were only found in 25% of infants after the second dose but increased to 50–70% across serogroups after the third dose at 12 months of age. The expanded memory B-cell response after the third dose could be due to strong priming and/or to improved immunogenicity associated with older age.
In the same study, there was no consistent impact of maternal antibodies on the immune response of infants to MenACWY-CRM vaccination. Despite a moderate association between MenC hSBA at 2 months and a reduced post-dose 2 MenC hSBA level, 90% of infants achieved protective hSBA titers for MenC after the second MenACWY-CRM dose at 4 months of age [Citation38].
2.4. Children 2–10 years
2.4.1. Immunogenicity and antibody persistence
A single dose of MenACWY-CRM is recommended for primary vaccination of all age groups older than 2 years. Five studies were identified since the last review with immunogenicity data in children who received a single dose of MenACWY-CRM between 2 and 10 years of age (). The percentage of 2–10 year olds with hSBA titers ≥1:8 after vaccination ranged from 75% to 89% for MenA, 76% to 95% for MenC, 93% to 96% for MenW, and 65% to 83% for MenY [Citation39–Citation42].
Five years after vaccination at 2–5 or 6–10 years of age, 14% of 2–5 year olds and 22% of 6–10 year olds had hSBA titers ≥1:8 for MenA. The percentages for the other serogroups were 32% and 56% respectively for MenC, 74% and 80% for MenW, and 48% and 53% for MenY () [Citation35]. Overall, persistence of antibodies was higher in older children than in younger children, but antibody titers remained higher at year 5 than at baseline for all serogroups in both age groups.
Figure 3. hSBA immunogenicity in children vaccinated with one dose of MenACWY-CRM at 2–5 or 6–10 years of age. Data from Ref. [Citation35].
Postprimary: One month post vaccination; M60: 60 months after vaccination; Post-B: one month after the booster dose at year 5; GMT: geometric mean antibody titer; hSBA: serum bactericidal assay using human complement source.Vertical lines indicate 95% confidence intervals.
![Figure 3. hSBA immunogenicity in children vaccinated with one dose of MenACWY-CRM at 2–5 or 6–10 years of age. Data from Ref. [Citation35].Postprimary: One month post vaccination; M60: 60 months after vaccination; Post-B: one month after the booster dose at year 5; GMT: geometric mean antibody titer; hSBA: serum bactericidal assay using human complement source.Vertical lines indicate 95% confidence intervals.](/cms/asset/d6770396-5108-4d79-b479-e5225eeed83d/ierv_a_1521280_f0003_b.gif)
In another study, a booster dose of MenACWY-CRM, administered 5 years after primary vaccination at 2–10 years of age, induced marked increases in hSBA titers for all serogroups () [Citation35]. Geometric mean ratios of post/pre-booster vaccination hSBA titers ranged from 55 to 207. At least 98% of subjects had hSBA titers ≥1:8 for all serogroups after the booster dose, and GMTs after the booster dose were higher than those seen after primary vaccination.
A second dose of MenACWY-CRM may be given to children 2 through 5 years of age who are at continued high risk of IMD [Citation43]. Two studies, one by Halperin et al. [Citation44] and Johnston et al. [Citation41] investigated the immune responses to two doses of MenACWY-CRM in 2–5 year old children, as well as responses to a single dose. Immune responses to a single dose from the study by Halperin et al. have been previously reviewed by Broker et al. [Citation21]. In this study, a subset of 356 children aged 2–5 years received 2 doses of MenACWY-CRM 2 months apart. The percentages of children with hSBA titers ≥8 (sero-responders) across all serogroups were consistently higher after 2 doses (ranging from 89% to 98% across serogroups) than after a single dose (ranging from 60% to 89% across serogroups), although all analyses were descriptive [Citation44]. The second study by Johnston et al., formally evaluated the superiority of 2 MenACWY-CRM doses (n = 176) compared with a single dose (N = 183) in 2–5 year old children in the US [Citation41]. One month after the last meningococcal vaccination, and as per predefined criteria, superiority was demonstrated for serogroups C and Y, but not for serogroups A and W [Citation41]. At 1 year after the last meningococcal vaccination in the same study, persisting antibody levels were higher among children given two doses for serogroups A and C, compared with those administered a single dose.
2.4.2. Reactogenicity and safety
Injection site pain or tenderness was the most frequently reported local solicited reaction among 2–10 year olds who had received a single dose of MenACWY-CRM (reported for 29.9–43% of children) [Citation39–Citation41]. The most frequently reported systemic reactions were irritability (reported for 7–26% of children), sleepiness (4–33%) and vomiting (10%) in 2–5 year olds, and myalgia (13–28%) in 6–10 year olds. Fever ≥38°C was uncommon, reported by 3–8% of subjects across studies, and there were no reports of temperature ≥40°C in any of the subjects aged from 2 to 10 years who received MenACWY-CRM [Citation39–Citation42].
The reactogenicity profile of a booster dose administered 5 years after the first dose was similar to that observed after the first dose in age-matched vaccine-naïve controls [Citation35]. The most frequently reported local and systemic solicited reactions after the booster dose following priming with 1 dose were pain (53% in 7–10 year olds and 48% in 11–15 year olds), malaise (14% and 17%, respectively), and headache (9% and 19%, respectively). Most symptoms were mild-to-moderate in severity and there was only one report of fever after the booster dose in a group of 11–15 year old subjects primed with a single dose.
2.5. Adolescents and adults
2.5.1 Immunogenicity
Previous clinical trials have shown that a single dose of MenACWY-CRM induces hSBA titers ≥1:8 in almost all adolescents from 11 years of age for all vaccine serogroups, and in almost all adults, including older adults aged 56–65 years, for serogroups C, W, and Y, and in at least 69% of vaccinated individuals for serogroup A [Citation45].
Overall, 21 new publications were identified, describing 19 studies that have become available since the last review (). These studies included adolescents and adults from 11 to 75 years of age and were conducted in the US, UK, Korea, Taiwan, India, Russia, Germany, the Czech Republic, Italy, Australia, and France.
In registration studies conducted in Korea (11–55 year olds) [Citation46], Taiwan (11–18 year olds) [Citation39], India (11–75 year olds) [Citation42], and Russia (≥11 year olds) [Citation40], the percentages of subjects with hSBA titers ≥1:8 ranged from 77% to 92% for MenA, 86% to 99% for MenC, 95% to 99% for MenW and 86% to 95% for MenY. The robust responses to vaccination across a range of regions with diverse ethnicity and N. meningitidis epidemiology attest to the immunogenicity of MenACWY-CRM in all populations, irrespective of the geographic area.
2.5.2. Antibody persistence and booster vaccination
Antibody persistence has been measured up to 5 years after vaccination at 11–18 years of age [Citation47–Citation49]. In one study conducted in US subjects primed with either MenACWY-CRM or MenACWY-DT, the percentages of subjects with hSBA titers ≥1:8 at 3 years after vaccination were 28% for MenA, 64% for MenC, 82% for MenW, and 65% for MenY [Citation47]. However, at year 3 after primary vaccination, more MenACWY-CRM recipients had hSBA titers ≥1:8 for serogroups W and Y than MenACWY-DT recipients [Citation47]. By year 5 after vaccination, antibody levels in MenACWY-CRM recipients had plateaued, with little change from year 3 for any serogroup (32% for MenA, 59% for MenC, 82% for MenW, and 64% for MenY) [Citation48] (). A booster dose of MenACWY-CRM given at year 3 in a subset of participants from this study led to increases in hSBA titers for all serogroups (99–100% with hSBA ≥1:8 for all four serogroups post-booster), irrespective of whether MenACWY-CRM or MenACWY-DT was used for primary vaccination [Citation47]. Antibody levels appeared to be sustained after the MenACWY-CRM booster dose, with 79% to 100% of participants maintaining hSBA titers ≥1:8 by year 2 post-booster, which was higher than levels observed 21 months after priming (range 36–84%) [Citation48,Citation50].
Figure 4. hSBA immunogenicity and antibody persistence in adolescents vaccinated with one dose of MenACWY-CRM at 11–18 years of age (128 subjects). Data from Ref. [Citation48].
Postprimary: One month post vaccination; GMT: geometric mean antibody titer; hSBA: serum bactericidal assay using human complement source.Error bars indicate 95% confidence intervals.
![Figure 4. hSBA immunogenicity and antibody persistence in adolescents vaccinated with one dose of MenACWY-CRM at 11–18 years of age (128 subjects). Data from Ref. [Citation48].Postprimary: One month post vaccination; GMT: geometric mean antibody titer; hSBA: serum bactericidal assay using human complement source.Error bars indicate 95% confidence intervals.](/cms/asset/8c0b2df9-0a83-4d9a-86af-cd720f47406f/ierv_a_1521280_f0004_b.gif)
In another study, 5 years after priming with MenACWY-CRM between 11 and 18 years of age, 72–76% of subjects maintained hSBA titers ≥1:8 for serogroups C, W, Y, and 30% for serogroup A [Citation49]. A booster dose at year 5 induced hSBA titers ≥1:8 in 98–100% of participants across serogroups by 7 days after vaccination, with geometric titers that increased by 58–205-fold. One month after the booster dose at year 5, the hSBA GMTs increased 56–155-fold, across serogroups.
Studies in adolescents primed with diverse meningococcal vaccines, including MenACWY-CRM, MenACWY-DT, MenC-TT, MenC-CRM, or quadrivalent meningococcal polysaccharide vaccines, show that a MenACWY-CRM booster can induce hSBA titers ≥1:8 in almost all subjects. The booster response indicates successful induction of an anamnestic immune response irrespective of the vaccine used for priming [Citation24,Citation47,Citation49].
2.5.3. Coadministration
At the time of the previous review [Citation20], the immunogenicity and safety of MenACWY-CRM administered concomitantly with human papillomavirus vaccine and a reduced antigen content diphtheria–tetanus ± acellular pertussis containing vaccine (Tdap) had been assessed. Since then, the number of coadministration studies has increased such that coadministration data are now available for a range of vaccines commonly administered to adults including typhoid fever, yellow fever, Japanese encephalitis, rabies, hepatitis A and B, adult tetanus–diphtheria (Td) vaccine, PCV13, and 4-component meningococcal serogroup B protein vaccine (4CMenB) () [Citation36].
In coadministration studies reported by Alberer et al. [Citation51–Citation53], non-inferiority of the immune response was demonstrated for traveler vaccines (yellow fever and typhoid fever, rabies and Japanese encephalitis, and hepatitis A and/or B vaccines) administered concomitantly with MenACWY-CRM, compared with the traveler vaccines administered without MenACWY-CRM. Responses to MenACWY-CRM in all coadministration groups were similar to MenACWY-CRM administered alone [Citation51–Citation53]. As quadrivalent meningococcal vaccines are recommended for travelers visiting meningococcal-endemic regions, the coadministration of MenACWY-CRM with other traveler vaccines facilitates the delivery of multiple vaccines that may be required before departure.
Two studies published since the review by Gasparini et al. [Citation36] assessed coadministration of MenACWY-CRM with 4CMenB (Bexsero) [Citation22,Citation54]. An open trial was conducted in staff 18–64 years of age at Public Health England at risk of occupational exposure to meningococci. MenACWY-CRM and 4CMenB were coadministered at dose 1, followed by two further doses of 4CMenB at 3 and 6 months. This study assessed immune responses with an SBA using a rabbit complement source (rSBA). Two months after MenACWY-CRM and 4CMenB, more than 94% of subjects had seroprotective rSBA titers ≥1:8 for all four ACWY vaccine serogroups. The second study assessed MenACWY-CRM administered sequentially after three doses of 4CMenB [Citation54]. At least 96% of subjects had hSBA titers ≥1:8 for serogroups A, C, and W, and 83% for MenY. Because of the high percentages of subjects with seroprotective SBA titers against non-B serogroups observed in both studies, the authors hypothesized that the SBA response may have been augmented by the 4CMenB vaccine components [Citation22].
2.5.4. Reactogenicity and safety
The incidence of local and systemic solicited reactions among adolescents and adults who received a single dose of MenACWY-CRM was consistent with the reactogenicity profile described in the Product Information [Citation45]. Injection site pain/tenderness was the most frequently reported local solicited reaction in all studies, while myalgia, headache, and nausea were the most frequently reported systemic symptoms. MenACWY-CRM was also well tolerated as a booster dose 3 and 5 years after vaccination during adolescence, with a similar reactogenicity profile compared to the primary dose [Citation47,Citation49].
Across the studies reported here, only one participant in a study in India reported an SAE considered by the investigator at least possibly related to vaccination [Citation42]. This was a case of a subject who experienced a mild fever, chills, headache, and body aches 1 day after vaccination. The woman was admitted to hospital to rule out malaria. The laboratory tests were negative for malarial parasites and the discharge diagnosis was fever with chills. The subject recovered completely after 3 days [Citation42].
2.5.5. Carriage
The impact of MenACWY-CRM on nasopharyngeal carriage of meningococci was investigated in university students [Citation55,Citation56]. From 2 months until 12 months after vaccination, carriage of MenY decreased by 39.0% (95% CI 17.3–55.0) and carriage of combined serogroups CWY reduced by 36.2% (95% CI 15.6–51.7) compared to control [Citation55].
3. Post-marketing experience using ACWY-CRM
A safety surveillance of AEs after vaccination of the French military personnel identified 47 AEs after vaccination with MenACWY-CRM between 2011 and 2012; none of which was serious. A total of 119,640 MenACWY-CRM doses were administered during the surveillance period [Citation57]. No safety concerns were raised.
Two GSK-sponsored observational studies using a large US health-care claims database (Kaiser Permanente Southern California) also investigated post-marketing safety of MenACWY-CRM, administered to children and adolescents and young adults in a health-care setting in the US [Citation58,Citation59]. In the first study, the medical records of 387 children 2–10 years of age who received MenACWY-CRM between 30 September 2011 and 30 September 2014 were searched for the occurrence of SAEs or 26 prespecified AEs of interest up to 1 year post vaccination [Citation58]. The most frequent SAEs reported were trauma-related. One prespecified AE of interest was reported (asthma, 237 days post vaccination). No safety concerns were identified in this age group.
The second safety study conducted by the same institution used a self-controlled case series design to retrospectively assess safety of MenACWY-CRM in 11–21 year olds who had received a single dose of MenACWY-CRM between 30 September 2011 and 30 June 2013 [Citation59]. SAEs and 26 prespecified AEs of interest were identified within prespecified risk windows compared with the period from the end of the risk window until 1 year post vaccination. Of 48,899 individuals in the study, 72% had received MenACWY-CRM coadministered with another vaccine. No temporal associations between vaccination and AEs were observed with the exception of a higher risk of Bell’s palsy in subjects receiving concomitant vaccines (relative incidence 5.0, 95% CI 1.4–17.8), but not when MenACWY-CRM was administered alone (relative incidence 1.1, 95% CI 0.2–5.5). The observed association warrants further investigation as chance, concomitant vaccination, or an underlying medical history predisposing to Bell’s palsy have not been ruled out as confounding or contributory factors.
Finally, a US study investigated all adverse events reported to the Vaccine Adverse Event Reporting System (VAERS) from 1 January 2010 until 31 December 2015 [Citation60]. During this period approximately 8.2 million doses of MenACWY-CRM were distributed in the US. Of the 2614 AE reports received over the study period, 74% were in 11–18 year olds and 53% included concomitant administration of MenACWY-CRM with other vaccine(s). Overall, across the different age groups, the most frequently reported AEs were injection site reactions. There were no unexpected findings and no disproportionate reporting of any AEs, including facial palsy. The reported AEs were consistent with data from pre-licensure studies as reflected in the Product Information.
4. Strengths and limitations of the studies
The articles reported in this review summarize experience with MenACWY-CRM from a range of countries and population groups published since 2011. The majority of clinical studies were GSK-sponsored (legacy Novartis Vaccines) and therefore homogeneous in design and in the methods used to measure safety and immunogenicity end points, allowing evaluation of trends across the studies. Where differences between study designs exist, the availability of a generally accepted correlate of protection overcomes many potential limitations.
All of the reported studies were conducted in healthy individuals. At this time, special populations such as immunocompromised individuals or pregnant women have not been studied. While initial data from two studies suggest that MenACWY-CRM can be safely administered with (together or sequentially) 4CMenB without impacting the immune response to either vaccine [Citation22,Citation54], both studies were conducted in adults and further investigation of the immunogenicity and safety of this combination of vaccines is warranted in infants, children, and adolescents. Currently no data are available on the coadministration of MenACWY-CRM with the other multicomponent MenB protein vaccine rLP2086 (Trumenba, Pfizer) although a study of MenACWY-DT and rLP2086 showed that both vaccines could be coadministered without negative impact on the immune response, or safety concerns [Citation61].
5. Expert commentary
Reviews of published information allow assessment of the benefit versus risk of vaccination across diverse age groups and populations from different countries. Currently available immunogenicity and safety data support the use of MenACWY-CRM vaccine in infants, toddlers, children, adolescents, and adults, including older adults. Priming, persistence, and booster data show that MenACWY-CRM is immunogenic in all the populations and age-groups evaluated, regardless of country of origin, and induces both long-term persistence and immunological memory, and a robust, anamnestic and early booster response. The somewhat lower immunogenicity and shorter persistence of antibodies to the serogroup A polysaccharide compared with the other vaccine serogroups has been observed for all quadrivalent meningococcal conjugate vaccines containing serogroup A, especially when tested using hSBA [Citation32,Citation50,Citation62,Citation63]. The similarity of the observations suggests that the rapid decrease in hSBA titers for serogroup A is likely to be an antigen-specific feature rather than a vaccine-specific feature. Similar results have not been reported using SBA with rabbit complement.
During universal vaccination campaigns with MenA conjugate vaccine in the African population, a 94% reduction in the crude incidence rate of MenA disease and a 98% decrease in the prevalence of MenA carriage were observed, although antibodies against MenA declined sharply when assessed using hSBA but remained high when assessed using rSBA [Citation64–Citation66]. Therefore, the clinical relevance of the lower persistence of MenA hSBA titers is unknown.
Coadministration studies support the use of MenACWY-CRM in conjunction with infant and adolescent/adult traveler schedules. Data from over 32,000 infants, children aged 2–10 years, adolescents, and adults reported from clinical trials support the safety profile of MenACWY-CRM. MenACWY-CRM was well tolerated across age groups, both in single-dose and multidose schedules. No new safety concerns were identified in the studies reported in this review. Large post-marketing safety studies that include more than 55,000 individuals vaccinated with MenACWY-CRM [Citation57–Citation59], have confirmed the overall good safety profile identified during clinical development, and support the favorable benefit–risk ratio in all age groups.
It has proven difficult to assess effectiveness of quadrivalent meningococcal vaccines because of the low frequency of N. meningitidis disease, and because to date these vaccines have been used only infrequently in UMV programs. Effectiveness of approximately 90% in preventing MenC disease has been demonstrated for all MenC vaccines regardless of the conjugation protein [Citation67]. Vaccine effectiveness against serogroup A has also been demonstrated for monovalent MenA vaccine in Africa [Citation64], and a US study estimated that the effectiveness of another quadrivalent meningococcal vaccine (MenACWY-DT) within 3–4 years after vaccination was approximately 80–85% against MenC and MenY disease in adolescents [Citation68]. The first data indicating effectiveness of the MenW component have come from the UK, where ACWY vaccination (MenACWY-CRM or MenACWY-TT) was introduced for 13–14 year olds in 2015/2016 in response to an increase in MenW IMD [Citation16]. Coverage of the adolescent dose in 2016 was 70% amongst school attenders and 35% in school leavers [Citation69]. In the first year, there was a 68% decline in MenW case numbers among the target age group compared to projected levels, and MenW case numbers among the target age group compared to projected levels [Citation69].
MenACWY-CRM is currently authorized for use from 2 months of age in several countries including the US, Australia, Saudi Arabia, and Argentina, and from 2 years of age in Europe and many other countries worldwide. MenACWY-CRM will continue to contribute to IMD control worldwide and is well positioned to play a greater role in the response to the evolving epidemiology of IMD in the twenty-first century.
6. Five-year view
The epidemiology of IMD is in continuous and dynamic evolution, with emergence of cases caused by MenY and by hypervirulent MenC and MenW strains [Citation9,Citation70]. The current epidemiology of IMD caused by MenB and non-MenB serogroups is already having an impact on meningococcal vaccination programs, prompting reconsideration of ACWY in UMV in different geographical areas across the globe, especially in adolescents and infants, where the medical need is highest.
Additional effectiveness data in children are expected in the next few years from Argentina, which was the first country to introduce MenACWY-CRM as a 2 + 1 dose schedule with a booster at age 11 years in response to rising MenW IMD [Citation71]. However future estimations of effectiveness may be more challenging because 4CMenB has been implemented for infant vaccination in some countries, which may provide some cross-protection against serogroups other than MenB, as already seen [Citation22]. Early investigations in university students suggest a significant impact of MenACWY-CRM on nasopharyngeal carriage of meningococci [Citation55], which could translate to herd immune effects in UMV settings with high coverage.
The long-term goal for IMD control is the development of larger combination vaccines such as a pentavalent ABCWY to provide even broader protection against IMD. Investigation of a combined MenABCWY-CRM-conjugated vaccine has been started in adolescents and adults.
Key issues
MenACWY-CRM has contributed to control of invasive meningococcal disease since 2010.
When used in recommended schedules, MenACWY-CRM induced seroprotective hSBA titers in the majority of vaccinated subjects. Coadministration studies in infants, adolescents, and adults did not suggest any clinically relevant vaccine interactions between MenACWY-CRM and routine vaccines in any age group.
MenACWY-CRM provides flexibility for public health policy, allowing it to be incorporated in all parts of the vaccination schedule from infancy thorough adulthood.
Data collected after adoption of MenACWY-CRM into the field since its first launch in 2010, and evidence from a large clinical and epidemiological database of subjects exposed to MenACWY-CRM, confirm its tolerability and safety profile.
Authors’ Contributions
All authors participated in the development and the review of the manuscript and approved the final submitted version. The corresponding author had final responsibility to submit for publication. Drafts were developed by a professional publication writer according to the recommendations, documentation, and outline provided by the lead author.
Declaration of interest
All authors are employees of the GSK group of companies. PK, MP, MN hold shares in the GSK group of companies. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Trademarks
Menveo and Bexsero are trademarks of the GSK group of companies. Nimenrix and Trumenba are trademarks of Pfizer. Menactra is a trademark of Sanofi Pasteur.
Acknowledgments
Writing assistance was provided by Joanne Wolter (Independent medical writer c/o GSK) and editorial and coordination assistance was provided by Olivier Box (XPE Pharma & Science c/o GSK).
Additional information
Funding
References
- Baccarini C, Ternouth A, Wieffer H, et al. The changing epidemiology of meningococcal disease in North America 1945–2010. Hum Vacc Immunother. 2013;9(1):162–171.
- Vyse A, Anonychuk A, Jäkel A, et al. The burden and impact of severe and long-term sequelae of meningococcal disease. Expert Rev Anti Infec Ther. 2013;11(6):597–604.
- Pollard AJ, Ochnio J, Ho M, et al. Disease susceptibility to ST11 complex meningococci bearing serogroup C or W135 polysaccharide capsules, North America. Emerg Infect Dis. 2004;10(10):1812–1815.
- CDC – ABCs. Surveillance reports main page - active bacterial core surveillance [internet]. [updated 2016 Jun 27]. Available from: http://www.cdc.gov/abcs/reports-findings/surv-reports.html.
- Whittaker R, Dias JG, Ramliden M, et al. The epidemiology of invasive meningococcal disease in EU/EEA countries, 2004–2014. Vaccine. 2017;35(16):2034–2041.
- Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27 Suppl 2(Suppl 2):B51–B63.
- Pelton SI. The global evolution of meningococcal epidemiology following the introduction of meningococcal vaccines. J Adolesc Health. 2016;59(2 Suppl):S3–S11.
- Halperin SA, Bettinger JA, Greenwood B, et al. The changing and dynamic epidemiology of meningococcal disease. Vaccine. 2012;30(Suppl 2):B26–B36.
- Borrow R, Alarcon P, Carlos J, et al. The global meningococcal initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines. 2017;16(4):313–328.
- Miller E, Salisbury D, Ramsay M. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine. 2001;20(Suppl 1):S58–S67.
- Hellenbrand W, Elias J, Wichmann O, et al. Epidemiology of invasive meningococcal disease in Germany, 2002–2010, and impact of vaccination with meningococcal C conjugate vaccine. J Infect. 2013;66(1):48–56.
- Garrido-Estepa M, León-Gómez I, Herruzo R, et al. Changes in meningococcal C epidemiology and vaccine effectiveness after vaccine introduction and schedule modification. Vaccine. 2014;32(22):2604–2609.
- De Wals P, Deceuninck G, Lefebvre B, et al. Effectiveness of serogroup C meningococcal conjugate vaccine: a 7-year follow-up in Quebec, Canada. Pediatr Infect Dis J. 2011;30(7):566–569.
- European Center for Disease Prevention and Control. Surveillance Report. Surveillance of invasive bacterial diseases in Europe. 2012. Available from: www.ecdc.europa.eu
- Ladhani SN, Beebeejaun K, Lucidarme J, et al. Increase in endemic Neisseria meningitidis capsular group W sequence type 11 complex associated with severe invasive disease in England and Wales. Clin Infect Dis. 2015;60(4):578–585.
- Campbell H, Saliba V, Borrow R, et al. Targeted vaccination of teenagers following continued rapid endemic expansion of a single meningococcal group W clone (sequence type 11 clonal complex), United Kingdom 2015. Euro Surveill. 2015;20:28.
- Abad R, López EL, Debbag R, et al. Serogroup W meningococcal disease: global spread and current affect on the Southern Cone in Latin America. Epidemiol Infect. 2014;142(12):2461–2470.
- Lahra MM, Enriquez RP; National Neisseria Network. Australian meningococcal surveillance programme annual report, 2015. Commun Dis Intell Q Rep. 2016;40(4):E503–E511.
- Dutch National Immunisation Programme. Meningococcal vaccine changed [internet]. [cited 2018 May 17]. Available from: https://rijksvaccinatieprogramma.nl/professionals/menacwy-vaccinatie
- Cooper B, DeTora L, Stoddard J. Menveo®: a novel quadrivalent meningococcal CRM197 conjugate vaccine against serogroups A, C, W-135 and Y. Expert Rev Vaccines. 2011;10(1):21–33.
- Broker M, Cooper B, Detora LM, et al. Critical appraisal of a quadrivalent CRM(197) conjugate vaccine against meningococcal serogroups A, C, W-135 and Y (Menveo) in the context of treatment and prevention of invasive disease. Infect Drug Resist. 2011;4:137–147.
- Findlow J, Bai X, Findlow H, et al. Safety and immunogenicity of a four-component meningococcal group B vaccine (4CMenB) and a quadrivalent meningococcal group ACWY conjugate vaccine administered concomitantly in healthy laboratory workers. Vaccine. 2015;33(29):3322–3330.
- Kim HW, Park IH, You S, et al. Immunogenicity of menACWY-CRM in Korean military recruits: influence of tetanus-diphtheria toxoid vaccination on the vaccine response to menACWY-CRM. Yonsei Med J. 2016;57(6):1511–1516.
- Ishola DA, Andrews N, Waight P, et al. Randomized trial to compare the immunogenicity and safety of a CRM or TT conjugated quadrivalent meningococcal vaccine in teenagers who received a CRM or TT conjugated serogroup C vaccine at preschool age. Pediatr Infect Dis J. 2015;34(8):865–874.
- Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The Role of Humoral Antibodies. J Exp Med. 1969;129(6):1307–1326.
- Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection–serum bactericidal antibody activity. Vaccine. 2005;23(17–18):2222–2227.
- Tregnaghi M, Lopez P, Stamboulian D, et al. Immunogenicity and safety of a quadrivalent meningococcal polysaccharide CRM conjugate vaccine in infants and toddlers. Int J Infect Dis. 2014;26:22–30.
- Nolan TM, Nissen MD, Naz A, et al. Immunogenicity and safety of a CRM-conjugated meningococcal ACWY vaccine administered concomitantly with routine vaccines starting at 2 months of age. Hum Vaccin Immunother. 2014;10(2):280–289.
- Block SL, Shepard J, Garfield H, et al. Immunogenicity and safety of a 3- and 4-dose vaccination series of a meningococcal ACWY conjugate vaccine in infants: results of a phase 3b, randomized, open-label trial. Pediatr Infect Dis J. 2016;35(2):e48–e59.
- Klein NP, Reisinger KS, Johnston W, et al. Safety and immunogenicity of a novel quadrivalent meningococcal CRM-conjugate vaccine given concomitantly with routine vaccinations in infants. Pediatr Infect Dis J. 2012;31(1):64–71.
- Klein N, Block S, Johnston W, et al. Persistence of meningococcal bactericidal antibodies and booster response at 60-months of age in children who received infant or toddler doses of MenACWY-CRM conjugate vaccine. Poster session presented at: IDWeek; 2014 Oct 8–12; Philadelphia, PA.
- Baxter R, Keshavan P, Welsch JA, et al. Persistence of the immune response after MenACWY-CRM vaccination and response to a booster dose, in adolescents, children and infants. Hum Vaccin Immunother. 2016;12(5):1300–1310.
- Klein NP, Shepard J, Bedell L, et al. Immunogenicity and safety of a quadrivalent meningococcal conjugate vaccine administered concomitantly with measles, mumps, rubella, varicella vaccine in healthy toddlers. Vaccine. 2012;30(26):3929–3936.
- Abdelnour A, Silas PE, Lamas MR, et al. Safety of a quadrivalent meningococcal serogroups A, C, W and Y conjugate vaccine (MenACWY-CRM) administered with routine infant vaccinations: results of an open-label, randomized, phase 3b controlled study in healthy infants. Vaccine. 2014;32(8):965–972.
- Block SL, Christensen S, Verma B, et al. Antibody persistence 5 years after vaccination at 2 to 10 years of age with quadrivalent MenACWY-CRM conjugate vaccine, and responses to a booster vaccination. Vaccine. 2015;33(18):2175–2182.
- Gasparini R, Tregnaghi M, Keshavan P, et al. Safety and Immunogenicity of a quadrivalent meningococcal conjugate vaccine and commonly administered vaccines after coadministration. Pediatr Infect Dis J. 2016;35(1):81–93.
- Blanchard-Rohner G, Snape MD, Kelly DF, et al. The B-cell response to a primary and booster course of MenACWY-CRM197 vaccine administered at 2, 4 and 12 months of age. Vaccine. 2013;31(20):2441–2448.
- Blanchard-Rohner G, Snape MD, Kelly DF, et al. Seroprevalence and placental transmission of maternal antibodies specific for Neisseria meningitidis Serogroups A, C, Y and W135 and influence of maternal antibodies on the immune response to a primary course of MenACWY-CRM vaccine in the United Kingdom. Pediatr Infect Dis J. 2013;32(()7):768–776.
- Huang LM, Chiu NC, Yeh SJ, et al. Immunogenicity and safety of a single dose of a CRM-conjugated meningococcal ACWY vaccine in children and adolescents aged 2–18 years in Taiwan: results of an open label study. Vaccine. 2014;32(40):5177–5184.
- Ilyina N, Kharit S, Namazova-Baranova L, et al. Safety and immunogenicity of meningococcal ACWY CRM197-conjugate vaccine in children, adolescents and adults in Russia. Hum Vaccin Immunother. 2014;10(8):2471–2481.
- Johnston W, Essink B, Kirstein J, et al. Comparative assessment of a single dose and a 2-dose vaccination series of a quadrivalent meningococcal CRM-conjugate vaccine (MenACWY-CRM) in children 2–10 years of age. Pediatr Infect Dis J. 2016;35(1):e19–e27.
- Lalwani S, Agarkhedkar S, Gogtay N, et al. Safety and immunogenicity of an investigational meningococcal ACWY conjugate vaccine (MenACWY-CRM) in healthy Indian subjects aged 2 to 75 years. Int J Infect Dis. 2015;38:36–42.
- MENVEO® Prescribing Information. [cited 2018 Aug 23]. Available from: https://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm201349.pdf.
- Halperin SA, Gupta A, Jeanfreau R, et al. Comparison of the safety and immunogenicity of an investigational and a licensed quadrivalent meningococcal conjugate vaccine in children 2–10 years of age. Vaccine. 2010;28(50):7865–7872.
- European Medicines Agency. Menveo product information [internet]. [upated 2018 Apr 05; cited 2018 Apr 24]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001095/human_med_001323.jsp&mid=WC0b01ac058001d124.
- Lee HJ, Chung MH, Kim WJ, et al. Immunogenicity and safety of a novel quadrivalent meningococcal conjugate vaccine (MenACWY-CRM) in healthy Korean adolescents and adults. Int J Infect Dis. 2014;28:204–210.
- Baxter R, Reisinger K, Block SL, et al. Antibody persistence and booster response of a quadrivalent meningococcal conjugate vaccine in adolescents. J Pediatr. 2014;164(6):1409–1415 e1404.
- Baxter R, Reisinger K, Block SL, et al. Antibody persistence after primary and booster doses of a quadrivalent meningococcal conjugate vaccine in adolescents. Pediatr Infect Dis J. 2014;33(11):1169–1176.
- Jacobson RM, Jackson LA, Reisinger K, et al. Antibody persistence and response to a booster dose of a quadrivalent conjugate vaccine for meningococcal disease in adolescents. Pediatr Infect Dis J. 2013;32(4):e170–e177.
- Gill CJ, Baxter R, Anemona A, et al. Persistence of immune responses after a single dose of Novartis meningococcal serogroup A, C, W-135 and Y CRM-197 conjugate vaccine (Menveo®) or Menactra® among healthy adolescents. Hum Vaccin. 2010;6(11):881–887.
- Alberer M, Burchard G, Jelinek T, et al. Safety and immunogenicity of typhoid fever and yellow fever vaccines when administered concomitantly with quadrivalent meningococcal ACWY glycoconjugate vaccine in healthy adults. J Travel Med. 2015;22(1):48–56.
- Alberer M, Burchard G, Jelinek T, et al. Co-administration of a meningococcal glycoconjugate ACWY vaccine with travel vaccines: a randomized, open-label, multi-center study. Travel Med Infect Dis. 2014;12(5):485–493.
- Alberer M, Burchard G, Jelinek T, et al. Immunogenicity and safety of concomitant administration of a combined hepatitis A/B vaccine and a quadrivalent meningococcal conjugate vaccine in healthy adults. J Travel Med. 2015;22(2):105–114.
- Kimura A, Toneatto D, Kleinschmidt A, et al. Immunogenicity and safety of a multicomponent meningococcal serogroup B vaccine and a quadrivalent meningococcal CRM197 conjugate vaccine against serogroups A, C, W-135, and Y in adults who are at increased risk for occupational exposure to meningococcal isolates. Clin Vaccine Immunol. 2011;18(3):483–486.
- Read RC, Baxter D, Chadwick DR, et al. Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. Lancet. 2014;384(9960):2123–2131.
- Read RC, Dull P, Bai X, et al. A phase III observer-blind randomized, controlled study to evaluate the immune response and the correlation with nasopharyngeal carriage after immunization of university students with a quadrivalent meningococcal ACWY glycoconjugate or serogroup B meningococcal vaccine. Vaccine. 2017;35(3):427–434.
- Mayet A, Duron S, Meynard JB, et al. Surveillance of adverse events following vaccination in the French armed forces, 2011–2012. Public Health. 2015;129(6):763–768.
- Tartof SY, Sy LS, Ackerson BK, et al. Safety of quadrivalent meningococcal conjugate vaccine in children 2–10 years. Pediatr Infect Dis J. 2017;36(11):1087–1092.
- Tseng HF, Sy LS, Ackerson BK, et al. Safety of quadrivalent meningococcal conjugate vaccine in 11- to 21-year-olds. Pediatrics. 2017;139:1.
- Myers TR, McNeil MM, Ng CS, et al. Adverse events following quadrivalent meningococcal CRM-conjugate vaccine (Menveo®) reported to the Vaccine Adverse Event Reporting system (VAERS), 2010–2015. Vaccine. 2017;35(14):1758–1763.
- Muse D, Christensen S, Bhuyan P, et al. A phase 2, randomized, active-controlled, observer-blinded study to assess the immunogenicity, tolerability and safety of bivalent rLP2086, a meningococcal serogroup B vaccine, coadministered with tetanus, diphtheria and acellular pertussis vaccine and serogroup A, C, Y and W-135 meningococcal conjugate vaccine in healthy US adolescents. Pediatr Infect Dis J. 2016;35(6):673–682.
- Jackson LA, Jacobson RM, Reisinger KS, et al. A randomized trial to determine the tolerability and immunogenicity of a quadrivalent meningococcal glycoconjugate vaccine in healthy adolescents. Pediatr Infect Dis J. 2009;28(2):86–91.
- Vesikari T, Forsten A, Bianco V, et al. Immunogenicity, safety and antibody persistence of a booster dose of quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine compared with monovalent meningococcal serogroup C vaccine administered four years after primary vaccination using the same vaccines. Pediatr Infect Dis J. 2015;34(12):e298–e307.
- Daugla DM, Gami JP, Gamougam K, et al. Effect of a serogroup A meningococcal conjugate vaccine (PsA-TT) on serogroup A meningococcal meningitis and carriage in Chad: a community study [corrected]. Lancet. 2014;383(9911):40–47.
- Kristiansen PA, Ba AK, Ouedraogo AS, et al. Persistent low carriage of serogroup A Neisseria meningitidis two years after mass vaccination with the meningococcal conjugate vaccine, MenAfriVac. BMC Infect Dis. 2014;14:663.
- Price G, Plikaytis B, Hollander A, et al. Comparison of different serogroup A immunoassays following a single dose of either MenAfriVac or quadrivalent polysaccharide vaccine in healthy Africans 2- to 29- years of age. Presented at International Pathogenic Neisseria Conferences, 2014 Asheville NC, USA. [cited 2016 Feb 9]. Available from: http://neisseria.org/ipnc/2014/IPNC_2014_abstracts.pdf.
- Borrow R, Abad R, Trotter C, et al. Effectiveness of meningococcal serogroup C vaccine programmes. Vaccine. 2013;31(41):4477–4486.
- Macneil JR, Cohn AC, Zell ER, et al. Early estimate of the effectiveness of quadrivalent meningococcal conjugate vaccine. Pediatr Infect Dis J. 2011;30(6):451–455.
- Joint Committee on Vaccination and Immunisation. Minute of the meeting on 01 February 2017 [internet]. [cited 2017 Aug 07]. Available from: http://www.nitag-resource.org/uploads/media/default/0001/04/3d9769b85796db91fb8ec14df1c0f790ca08fc76.pdf
- Weidlich L, Baethgen LF, Mayer LW, et al. High prevalence of Neisseria meningitidis hypervirulent lineages and emergence of W135: P1.5,2:ST-11clone in Southern Brazil. J Infect. 2008;57(4):324–331.
- Ministerio de Salud (MinSalud). Fundamentos de la introducción de la vacuna tetravelente (ACYW) conjugada contra meningococo al Calendario Nacional de Inmunizaciones. Argentina; 2016. Available from: http://www.msal.gob.ar/images/stories/bes/graficos/0000000927cnt-2016-12_lineamientos-meningo.pdf
- Khatami A, Snape MD, Davis E, et al. Persistence of the immune response at 5 years of age following infant immunisation with investigational quadrivalent MenACWY conjugate vaccine formulations. Vaccine. 2012;30(18):2831–2838.
- Safety and Immunogenicity of Novartis Meningococcal ACWY Conjugate Vaccine in Healthy Children Aged 12–59 Months [internet]. [updated 2015 Nov 6; cited 2017 Nov 26]. Available from: https://clinicaltrials.gov/ct2/show/results/NCT00310817.
- Ramasamy MN, Clutterbuck EA, Haworth K, et al. Randomized clinical trial to evaluate the immunogenicity of quadrivalent meningococcal conjugate and polysaccharide vaccines in adults in the United kingdom. Clin Vaccine Immunol. 2014;21(8):1164–1168.
- Gasparini R, Conversano M, Bona G, et al. Randomized trial on the safety, tolerability, and immunogenicity of MenACWY-CRM, an investigational quadrivalent meningococcal glycoconjugate vaccine, administered concomitantly with a combined tetanus, reduced diphtheria, and acellular pertussis vaccine in adolescents and young adults. Clin Vaccine Immunol. 2010;17(4):537–544.