ABSTRACT
Introduction: Vaccination against varicella rapidly reduces disease incidence, resulting in reductions in both individual burden and societal costs. Despite these benefits, there is no standardization of varicella immunization policies in Europe, including countries in Central and Eastern Europe (CEE).
Areas covered: This systematic literature review identified publications on the epidemiology of varicella, its associated health and economic burden, and vaccination strategies within the CEE region, defined as Albania, Bosnia-Herzegovina, Bulgaria, Croatia, Cyprus, Czech Republic, Estonia, Hungary, Latvia, Lithuania, Poland, Romania, Serbia, Slovakia, and Slovenia. Twenty-six studies were identified from a search of PubMed, Embase®, and MEDLINE® biomedical literature databases, supplemented by gray literature and country-specific/global websites.
Expert commentary: Limited information exists in published studies on the burden of varicella in CEE. The wide variability in incidence rates between countries is likely explained by a lack of consistency in reporting systems. Funded universal varicella vaccination (UVV) in CEE is currently available only in Latvia as a one-dose schedule, but Hungary together with Latvia are introducing a two-dose strategy in 2019. For countries that do not provide UVV, introduction of vaccination is predicted to provide substantial reductions in cases and rates of associated complications, with important economic benefits.
1. Introduction
Varicella is a highly contagious disease. In a country with no vaccination program, the majority of incident cases (52‒78%) occur in children aged under 6 years, rising to 89‒96% in those aged under 12 years [Citation1]. A systematic literature review (SLR) examining varicella disease burden across 31 European countries estimated a total of 5.5 million varicella cases annually before the introduction of childhood immunization, including 3 million cases in children aged under 5 years [Citation2]. In Central and Eastern Europe (CEE) in particular, annual incidences of community varicella in children aged under 5 years ranged from 7108 per 100,000 same-age cohort in Romania to 11,640 per 100,000 in Slovenia [Citation2]. Incidences of varicella-related hospitalizations in CEE were also highest in this age group, ranging from 22–27 per 100,000 children of the same age in Romania to 36–44 per 100,000 in Slovenia [Citation2].
While varicella is usually a mild or moderate illness [Citation1–Citation4], a small proportion of varicella cases are serious, including complications such as superinfection of the skin or soft tissue, respiratory syndromes, and neurological manifestations (e.g. cerebellar ataxia, encephalitis) [Citation1,Citation5]. Risk of serious varicella infection, or even death, is higher in young children, the elderly, immunocompromised individuals, and those with underlying health issues [Citation1]. However, the susceptibility of even healthy children to complications of varicella emphasizes the importance of recognizing the disease as a health priority [Citation6].
Vaccination against varicella reduces the number, size, and duration of varicella outbreaks [Citation1,Citation7] and rapidly reduces disease incidence over time [Citation6], thereby reducing societal and financial costs [Citation5]. Despite these benefits, there is no consensus across Europe on varicella immunization policy [Citation6]. Within CEE, only Cyprus, Czech Republic, and Latvia recommend universal varicella vaccination (UVV) for all children [Citation8] and, of these, only Latvia has a state-provided one-dose varicella immunization program. In addition to Latvia, Hungary recently announced the introduction of a two-dose funded vaccination strategy in 2019 [Citation9]. A number of other CEE countries endorse vaccination only for susceptible (medical or occupational) at-risk groups [Citation1]. Barriers to the adoption of a UVV program among children include the public perception of varicella as a generally mild disease, cost, the risk of an increase in herpes-zoster incidence among those not vaccinated against varicella or herpes zoster predicted in multidisciplinary studies [Citation10–Citation14], and a temporary increase in herpes zoster cases following reduced circulation of varicella zoster virus under the influence of a UVV program (‘exogenous boosting’ hypothesis) [Citation7,Citation15,Citation16]. The lack of availability of a registered varicella vaccine within some CEE countries – Bulgaria, for example – can also act as a barrier.
The burden of varicella on public health has not been well studied in CEE, and gaps in evidence can delay policy decisions on vaccination. This SLR examines the available evidence on the epidemiology of varicella in CEE, its attributable health and economic burden including health care resource utilization, and data pertaining to varicella vaccination programs in CEE, with the objective to improve the understanding, prevention, and management of varicella across the region.
2. Methods
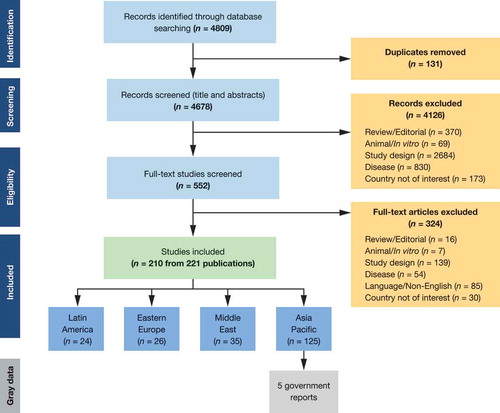
The SLR was performed following guidance issued by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA; www.prisma-statement.org). A search of biomedical literature databases (PubMed, Excerpta Medica Database [Embase®], Medical Literature Analysis, and Retrieval System Online [MEDLINE®]) was conducted from database inception to 1 February 2016 (the date of the literature search) to identify relevant English-language publications ().
Table 1. Search strategy in Embase® and MEDLINE® using Embase.com platform.
The current review focuses on publications from the CEE region: Albania, Bosnia-Herzegovina, Bulgaria, Croatia, Cyprus, Czech Republic, Estonia, Hungary, Latvia, Lithuania, Poland, Romania, Serbia, Slovakia, and Slovenia. This CEE analysis forms part of a wider review that includes regions from Latin America, the Middle East, and Asia-Pacific with the aim to gain an understanding of trends around the world.
A supplementary gray literature search, performed under the guidance of local key opinion leaders, identified published and unpublished data from other sources, including non-English language literature, government reports, PhD theses, European surveillance programs, health bulletins, and the Institute for Health Metrics and Evaluation (IHME) website. Searches were also conducted on country/region-specific websites, including those from the European Centre for Disease Prevention and Control (http://ecdc.europa.eu/), Bulgaria (http://www.ncipd.org/; http://ncpha.government.bg/index.php?option=com_content&view=article&id=519:morbidity-of-population&catid=372&Itemid=656&lang=en), Croatia (https://www.hzjz.hr/en/cat/news/), Czech Republic (http://www.szu.cz/), Hungary (http://www.oek.hu/), Latvia (https://www.spkc.gov.lv/), Lithuania (http://www.ulac.lt), Poland (http://www.gis.gov.pl/), Romania (https://cnscbt.ro), Slovakia (http://www.uvzsr.sk/; http://www.epis.sk/), Slovenia (http://www.nijz.si/), and the World Health Organization (WHO; http://www.who.int/).
2.1. Study selection and data extraction
Studies identified in the literature search were screened for inclusion based on predefined eligibility criteria. Studies were eligible for inclusion if they: (1) included males or females of any age and race who had primary and/or breakthrough varicella or were undergoing serological testing for antibodies to varicella; (2) assessed the epidemiological and/or economic burden of varicella; and (3) were of the following study design or study type: epidemiological, cohort, case-control, cross-sectional, or registry/database. Cost studies/surveys/analyses, budget impact models, database cost studies, resource-use studies, and cost-of-illness studies; cost-effectiveness, cost-utility, cost-benefit, cost-minimization, and cost-consequence analyses; and routine surveillance reports were also included.
Publications were initially screened for inclusion based on title and abstract; full-text copies of potentially eligible studies were subsequently reviewed. First and second screening processes were undertaken by a single reviewer. A second independent reviewer validated a random sample of 20% of studies from both first and second screenings. There were no cases of disagreement in ranking between the first and second reviewers.
Cost data were adjusted to 2017 US dollars (USD) and euros by initially using annual inflation rates to obtain 2017 costs in country-specific currencies and subsequently converting all costs to USD and euros based on 2017-dated exchange rates using country-specific websites. For studies where cost-year was not mentioned, the publication year was considered as the cost-year for all calculations.
3. Results
3.1. Studies included in the analysis
In the wider review of Latin America, the Middle East, CEE, and Asia-Pacific, a total of 4809 records were identified from literature databases. Of these, 210 studies were identified for inclusion in the overarching SLR after screening the records based on eligibility criteria (). The majority of studies provided evidence on Asia-Pacific and the Middle East, while 26 studies were identified as relevant to CEE. Studies described in this review are summarized in .
Table 2. Summary of published studies on varicella in CEE.
3.2. Epidemiology
A total of 17 studies from CEE included data on the epidemiology of varicella. All were observational and reported outcomes including seroprevalence (5 studies) [Citation17–Citation21], incidence (10 studies) [Citation17,Citation20,Citation22–Citation29], complications (7 studies) [Citation17,Citation22,Citation25,Citation26,Citation30–Citation32], and mortality (2 studies) [Citation21,Citation28].
3.2.1. Seroprevalence
Seroprevalence data were provided in studies from Slovenia [Citation19,Citation20], Albania [Citation17], Croatia [Citation21], and Poland [Citation18]. The available seroprevalence data varied substantially across these countries due to different age ranges and assay methods, ranging from 31% in Albania (predominantly among children aged 1–14 years) [Citation17] to 98% in Slovenia (among students aged 18–32 years) [Citation19]. The use of different assay methods may have influenced these outcomes, with the Slovenian and Polish teams using the Enzygnost anti-varicella zoster virus/immunoglobulin G assay (Dade Behring, Marburg, Germany) [Citation18–Citation20], the Croatian team adopting the Virotech assay (Russelsheim, Germany) [Citation21], and the Albanian team using an IgM ELISA [Citation17].
Overall, seroprevalence was age-dependent. For children in the Polish and Slovenian populations, seroprevalence was high during the first 6 months following birth (likely due to maternal antibodies), fell between 6 and 12 months, and increased steadily thereafter [Citation18,Citation20]. Seroprevalence was comparable between males and females [Citation18,Citation20]. Among adults in Croatia, there was a nonlinear increase in seroprevalence with increasing age, from 79% in those aged 16–20 years to 94% in 41–45-year-olds () [Citation21].
Figure 2. Seroprevalence (with 95% confidence intervals) of VZV IgG antibodies* in Croatian women of reproductive age [Citation21].
IgG, Immunoglobulin G; VZV, Varicella zoster virus.*By commercial enzyme-linked immunosorbent assay. n = positive for VZV IgG antibodies; N = total number tested.
![Figure 2. Seroprevalence (with 95% confidence intervals) of VZV IgG antibodies* in Croatian women of reproductive age [Citation21].IgG, Immunoglobulin G; VZV, Varicella zoster virus.*By commercial enzyme-linked immunosorbent assay. n = positive for VZV IgG antibodies; N = total number tested.](/cms/asset/3dca74ed-5e26-4588-839b-c53926cda31f/ierv_a_1573145_f0002_b.gif)
3.2.2. Varicella incidence
All CEE countries that are members of the European Union/European Economic Area (EU/EEA) have implemented mandatory reporting of varicella [Citation24,Citation33–Citation35]. However, there is no standardized case definition of varicella across the EU/EEA [Citation36] and, consequently, obtaining reliable inter-country data on the incidence and burden of varicella is challenging [Citation36].
A 2010 study by the European surveillance network for selected vaccine-preventable diseases (EUVAC; now hosted by the European Centre for Disease Prevention and Control [ECDC]) reported varicella incidence data in countries with mandatory reporting [Citation24]. Poland, Czech Republic, Estonia, and Slovenia had the highest reported incidence rates, at 481, 459, 459, and 444 cases per 100,000 inhabitants, respectively, while Cyprus had the lowest incidence at 9 per 100,000 inhabitants (). However, a study that attempted to estimate incidence rates from extrapolated regional surveillance data indicated that notification efficiency (proportion of all cases reported) could range anywhere from 1% to 50% in CEE [Citation2].
Figure 3. Incidence rate of varicella per 100,000 inhabitants in CEE (2000–2010) [Citation24].
CEE: Central and Eastern Europe.
![Figure 3. Incidence rate of varicella per 100,000 inhabitants in CEE (2000–2010) [Citation24].CEE: Central and Eastern Europe.](/cms/asset/e28664d1-6aa2-484c-9851-21b80df99faf/ierv_a_1573145_f0003_oc.jpg)
Among the publications identified in the current SLR, varicella incidence data were provided from Slovenia [Citation20,Citation28,Citation29], Poland [Citation25,Citation27], Albania [Citation17,Citation26], Croatia [Citation23], and Romania [Citation22]. In Slovenia, the overall incidence of varicella declined during the prevaccination period from 1979 to 2005, from 815.2 per 100,000 inhabitants during 1979–1998 to 458.9 per 100,000 in 2005; the vaccine was launched in 2006 [Citation28,Citation29,Citation35]. In Poland, where the varicella vaccine has been available in the private market since 1999, recommended since 2002, and freely available to immunocompromised individuals since 2009, varicella incidence showed a nonlinear increase from 340.2 per 100,000 inhabitants in 2008 to 448.7 per 100,000 in 2011 [Citation25,Citation27]. The authors attribute this rise to an improvement in surveillance methods in later years [Citation27]. A similar trend was observed in Romania, where the incidence of varicella increased three-fold between 1986 and 2004 (from 110 per 100,000 to 316 per 100,000) [Citation22]. As in the Polish study, the increase in varicella incidence can most likely be ascribed to more effective methods of disease surveillance [Citation22].
In studies across Europe, the incidence of varicella is generally highest in younger children, aged <9 years [Citation17,Citation22,Citation23,Citation26–Citation29]. In Latvia, for example, there were a total of 1564 cases of varicella in 2017, including 1462 in children aged 0–17 years; of these, 837 cases were in children aged 1–6 years [Citation37]. The annual peak incidence occurs during winter (October to January) and spring (March and April) months [Citation20,Citation23,Citation27,Citation28]. shows the seasonality of varicella in Croatia, a trend representative of other studies in the analysis [Citation23].
Figure 4. Seasonality of varicella in Croatia (1977–2012). Reproduced, with permission, from Vjekoslav Bakašun and Đana Pahor, ‘Epidemiological Patterns of Varicella in the Period of 1977 to 2012 in the Rijeka District, Croatia’, Epidemiology Research International, vol. 2014, Article ID 193,678 [Citation23].
![Figure 4. Seasonality of varicella in Croatia (1977–2012). Reproduced, with permission, from Vjekoslav Bakašun and Đana Pahor, ‘Epidemiological Patterns of Varicella in the Period of 1977 to 2012 in the Rijeka District, Croatia’, Epidemiology Research International, vol. 2014, Article ID 193,678 [Citation23].](/cms/asset/044258d6-2a14-49bb-bb1c-b5a24dd53ac6/ierv_a_1573145_f0004_b.gif)
Gray literature sources identified additional cyclical trends in varicella incidence. In Bulgaria and Slovakia, for example, where varicella vaccination is neither mandatory nor publicly funded and coverage is low, there are wide variations in annual peaks of varicella. As an illustration, varicella incidences in Bulgaria in 1980, 1990, 2000, 2010, 2015, and 2016 were 537.2, 418.6, 285.8, 261.8, 345.0, and 455.6 per 100,000 population, respectively [Citation38]. This suggests that a 5-year average of incidences may be a more reliable measure of overall varicella burden (http://www.epis.sk/; http://www.ncipd.org/). Lithuania, which introduced universal varicella vaccination in 2008, reports a range of annual incidences from 331.7 cases per 100,000 in 2010 (lowest) to 760.8 cases per 100,000 in 2014 (highest), with a mean annual incidence of 482.2 cases per 100,000 for the period 2007 to 2016 [Citation39].
3.2.3. Complications/mortality
The EUVAC surveillance study describes varicella complication rates of 0.1% and 0.2% in Slovakia and Slovenia, respectively, during 2008–2010, and 1.0% and 0.1% in Estonia and Hungary, respectively, in 2010 [Citation24].
Varicella-associated complications were explored in seven studies originating from CEE countries, including Albania, Poland, Bosnia-Herzegovina, Bulgaria, and Romania, in addition to two national reports from Slovenia and Lithuania [Citation17,Citation22,Citation25,Citation26,Citation30–Citation32,Citation40,Citation41]. The majority of the studies did not describe the vaccination status of patients; studies that did so were conducted on unvaccinated groups [Citation25,Citation31]. Rates of varicella-associated complications were generally low. The most common complications affected the respiratory system (pneumonia, respiratory tract infection, and cough), skin (skin and soft tissue infections), and hematological and neurological systems (e.g. cerebellitis, encephalitis, status epilepticus). Notably, the overwhelming majority of patients who developed complications were immunocompetent and previously healthy [Citation25,Citation30]. Neither of the two studies with evidence on mortality in the CEE region reported any varicella-related deaths [Citation23,Citation28]. A more recent national report from Lithuania reports one case of death from varicella in 2016 [Citation40].
Primary varicella is frequently more severe in adults than in children [Citation42]. Two studies, from Bosnia-Herzegovina and Romania, respectively, described complications of varicella in both adult and young populations [Citation22,Citation32]. Incidences of specific complications appeared to differ according to the age of the patient. For example, rates of varicella pneumopathy were higher in adults [Citation32], while bacterial skin infections were more common in children [Citation22]. In addition, a study from the central region of Slovenia reported that of the 0.69% of varicella cases that were hospitalized, predominantly due to complications, pneumonia was more common in adults while skin and central nervous system complications prevailed in children [Citation43].
3.3. Economic burden and health care resource utilization
3.3.1. Resource use and hospitalization rates
Reliable information on primary care visits for varicella is not available in all CEE countries. However, data from Hungary for the period 2011–2015 reveal a substantial disease burden related to varicella among children, with 87% of outpatients visiting their doctor at least once and 20% visiting a hospital outpatient clinic [Citation3].
Nine studies provided evidence on resource use and hospitalization rates in Slovenia [Citation20,Citation28,Citation29], Poland [Citation25,Citation27,Citation31], Bulgaria [Citation30], and Albania [Citation26]. The EUVAC surveillance study, mentioned above, described wide variations in hospitalization rates across CEE. Highest hospitalization rates were reported in Latvia, the only CEE country with mandatory universal vaccination () [Citation24]; it is possible that stringent medical practices and prioritization of varicella as a public health concern contribute to the hospitalization rates in Latvia, although interpretation is complex.
Figure 5. Percentage of persons of all ages with varicella who were hospitalized across CEE (2008–2010) [Citation24].
![Figure 5. Percentage of persons of all ages with varicella who were hospitalized across CEE (2008–2010) [Citation24].](/cms/asset/17a3ea7e-7542-4220-a277-731858a5fe50/ierv_a_1573145_f0005_oc.jpg)
In data available from Poland, Albania, and Bulgaria, the mean length of hospital stay ranged from 5 to 7 days [Citation25,Citation26,Citation30,Citation31]. Hospitalization rates in a Polish population over the period 2007–2011 generally remained constant, ranging from 0.51% to 0.59% [Citation25,Citation27]. In Slovenia, the proportion of varicella cases that were hospitalized over the period 1996–2005 were highest in children under 1 year (19.5 per 1000 varicella patients) and adults over 30 years (23.3 per 1000), and were lowest in patients aged 10–14 years (3.3 per 1000) [Citation28]. In a study of children and adolescents in Poland, hospitalization rates were highest during the first year of life, which the authors ascribe to the common use of preschool childcare facilities and early exposure to varicella virus [Citation25].
The use of antibiotics for treating bacterial complications in pediatric varicella patients is being investigated in the multi-country MARVEL study, which reports widespread high rates of inappropriate or unconfirmed antibiotic use [Citation44]. The authors suggest that wider adoption of UVV would reduce both the inappropriate as well as appropriate use of antibiotics in this population.
3.3.2. Economic burden/economic evaluations
Only one study was identified in the SLR that described the economic burden of varicella in CEE [Citation45]. This Hungarian study, utilizing National Health Insurance Fund Administration data, estimated that the direct and indirect costs of varicella-related health care provision in 2012 were almost one billion forint annually (equivalent to approximately 3.8 million USD and 3.4 million euros, 2017-dated).
An economic model using epidemiological data from the Epidemiological Information System in Slovakia, published in 2000, calculated that a varicella vaccination strategy would provide total direct and indirect cost savings of 2,751,270.24 USD (equivalent to 2,902,155.88 USD and 2,546,817.89 euros, 2017-dated), a cost-effectiveness ratio of 432.95 USD, calculated from a numerator of expected changes in the costs of treatment influenced positively by vaccination and a denominator of expected improvement in health achieved by vaccination (456.69 USD and 405.17 euros, 2017-dated), and a benefit-cost ratio of 0.15 [Citation46].
A cost-benefit study from Slovenia, published in 2002, which assumed 95% vaccine coverage and 90% efficacy for a single dose in a hypothetical birth cohort of 5800 children, estimated a cost-benefit ratio of 0.25 using medical costs and 0.89 using both medical and nonmedical costs [Citation47].
Finally, a recently published paper from Poland reports on the economic burden of varicella in children aged 1–12 years [Citation48]. In an analysis of 150 outpatients and 150 inpatients taken from seven selected physician sites, health care resource utilization was as follows: over-the-counter medications, 80.0% outpatients and 81.3% inpatients; prescription medications, 80.0% outpatients and 93.3% inpatients; tests/procedures, 0.0% outpatients and 69.3% inpatients; and allied health professional consults, 0.0% outpatients and 24.0% inpatients. Total (direct and indirect) 2015-dated cost per varicella case was 5013.3 zloties (PLN) (13,33.51 USD and 1183.09 euros, 2017-dated) for inpatients and 1027.2 PLN (273.19 USD and 242.37 euros, 2017-dated) for outpatients, resulting in an estimated overall annual (2015) cost of varicella in Poland of 178,198,320 PLN (47,402,920.06 USD and 42,055,693.9 euros, 2017-dated). The authors conclude that a significant clinical and economic burden is associated with varicella in Poland.
3.4. Vaccination programs and coverage
WHO guidelines recommend routine childhood immunization against varicella where high (>80%) and sustained vaccine coverage can be achieved and where the disease represents an important public health issue in the context of affordability [Citation49]. As noted by the European Centre for Disease Prevention and Control: ‘The experience of outbreaks in vaccinated populations has shown that varicella vaccination decreases the number, size and duration of varicella outbreaks and that decreases were greater with a two-dose schedule’ [Citation1]. In CEE, only Latvia has introduced a funded UVV program, with a single-dose regimen for children aged between 12 and 15 months introduced in 2008 and a two-dose regimen with the second dose at age 7 years commencing in 2019 [Citation24]. A mandatory combined vaccine (measles-mumps-rubella-varicella) is included in the childhood immunization schedule for children aged 12–15 months [Citation8,Citation33]. Varicella immunization coverage in Latvia was 48.1% in 2008, 63.9% in 2009, and 78.9% in 2010 [Citation33]. Recently published data show that varicella coverage in Latvia had increased further to 85.2% in 2017 (www.spkc.gov.lv). Although specific data on the impact of vaccination in Latvia have not been published, the low incidence of varicella recorded in 2010 (second only to Cyprus) appears to confirm the effectiveness of vaccination [Citation24].
Additional CEE countries that have adopted varicella vaccination for susceptible individuals and/or high-risk groups include Poland (mandatory since 2010) [Citation8,Citation24], Cyprus [Citation8,Citation33,Citation50], Estonia [Citation24,Citation33], Lithuania [Citation8,Citation50], and Slovenia [Citation33] ().
Table 3. Recommendations for varicella vaccination in different CEE populations.
In Hungary, varicella vaccination was made available in 2003, but only in the private market. Coverage in Hungary remains poor [Citation51], although this is expected to improve with the introduction of a two-dose, funded vaccination strategy starting in 2019 [Citation9], presumably in accordance with the current recommendation of 15 and 18 months of age, although measles vaccination is given at 15 months and 11 years of age. Varicella vaccination is available in Lithuania only in the private market or in governmental health care institutions where patients are also required to pay for it. In Slovakia, non-specific coverage by health-insurance companies can extend to varicella vaccination. Moreover, physicians can request vaccination coverage for certain patients, such as those with immunodeficiency, before the start of biological therapy.
Recommendations from the Central European Vaccination Awareness Group, published in 2014, aim to raise awareness and formalize guidance on immunization in high-risk pediatric populations across central Europe [Citation52].
4. Conclusion
The impact of varicella and its associated morbidity has, to date, not been well studied in CEE, which has contributed to an under-estimation and under-prioritization of varicella as a public health issue [Citation5,Citation53]. This SLR evaluated published information on the epidemiology and economic burden of varicella in CEE and the existing varicella vaccination programs in this region.
The study data describe a wide variation in the incidence of varicella between CEE countries. Several factors are likely to contribute to this variation, including the lack of a standardized case definition and heterogeneity in the surveillance systems and reporting rates. The mild nature of varicella in most patients means that doctors are often not consulted [Citation1] and the lack of mandatory reporting in some countries, even when medical advice is sought, is likely to contribute to under-reporting of the true incidence [Citation5].
Limited data availability also precludes an analysis of changes in varicella incidence following the introduction of vaccination in CEE. Experience in other geographical regions, such as the United States and other areas of Europe, is that vaccination provides highly effective and long-lasting protection, and that a two-dose strategy is superior to a one-dose approach [Citation54–Citation56]. Latvia is the only CEE country that provides universal vaccination, currently in the form of a single dose, while other countries in CEE offer vaccination limited to at-risk groups or in the private sector. No CEE countries have yet adopted a two-dose vaccination regimen, although Latvia and Hungary are doing so from 2019 [Citation9,Citation24].
In conclusion, this SLR assessed published studies on the incidence and burden of varicella in CEE and summarized the vaccination programs in this region. Despite the dearth of data on varicella in CEE countries, the evidence from this region – similar to that from other areas of the world – suggests that introduction of varicella vaccination programs provides benefit from both patient and public health perspectives. In order to achieve a full herd protection effect, such as a reduction of varicella in the unvaccinated population <12 months of age, high coverage is required [Citation57]. In CEE countries, this is traditionally achieved through state-funded mandatory universal immunization programs.
5. Expert commentary
• What are the key weaknesses in clinical management so far?
• Lack of:
• dedicated studies examining incidence/societal/financial impact
• mandatory, standardized surveillance systems (under-reporting of varicella as an issue)
• European Union case definition
• economic/resource utilization studies
• Inter-country variation in vaccination strategies
• Under-recognition of varicella as a public health priority
• What potential does further research hold? What is the ultimate goal in this field?
• Reduction of the disease burden of varicella through vaccination
• Widespread availability of fully funded, mandatory varicella vaccination programs
• What research or knowledge is needed to achieve this goal and what is the biggest challenge in this goal being achieved?
• Standardization of varicella reporting
• Verification of the effectiveness of the various vaccination strategies (one dose vs. two doses; universal vaccination vs. ‘at-risk’ populations) currently in place across CEE, and consensus regarding the most efficient means of reducing the incidence/impact of varicella
• Better understanding of the impact of regional factors like climate and/or social mixing patterns on seroprevalence/incidence rates
• Is there any particular area of the research you are finding of interest at present?
• Not applicable
6. Expert opinion
The literature on the burden of varicella in CEE that is analyzed in the current systematic review raises a number of interesting issues. Firstly, there is an important lack of dedicated studies that examine the incidence and societal/financial impact of varicella in this region. Mandatory, standardized surveillance systems are lacking, and there is in consequence an under-recognition of varicella as a public health priority. Initiatives from the Central European Vaccination Awareness Group aim to address this deficit. Secondly, verification of the effectiveness of vaccination strategies – as has been demonstrated in other regions of the world – is awaited across CEE. The wider introduction of universal varicella vaccination, in particular the two-dose strategy, is predicted to bring important benefits to CEE from both patient and public health perspectives.
Article highlights
Limited information exists in published studies on the burden of varicella in Central and Eastern Europe.
The wide variability in reported incidence rates between countries is likely explained by a lack of consistency in reporting systems.
Funded universal varicella vaccination (UVV) in the CEE region is currently available only in Latvia as a one-dose schedule, but Hungary together with Latvia are introducing a two-dose strategy in 2019.
For countries that do not provide UVV, the introduction of vaccination is predicted to provide substantial reductions in cases and rates of associated complications, with important economic benefits.
This box summarizes key points contained in the article
Declaration of interest
J. Wysocki received travel grants to attend international scientific conferences and fees for lectures from Pfizer and payment from a grant sponsored by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. I. Ivaskeviciene has received a USA travel grant to attend international scientific meeting, from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth. M. Pokorn has received a research grant from Pfizer and payment for lectures from Pfizer, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and GSK. L. Jancoriene has received travel grants to attend international scientific conferences and fees for lectures from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, AbbVie and Pfizer and payment for a clinical study sponsored by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. J. Pluta and L.J. Wolfson are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and stockholders of Merck & Co., Inc., Kenilworth, NJ, USA.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has declared that they have received grants from Sanofi Pasteur MSD, GSK Biologicals SA, Novartis, Crucell/Janssen, Pfizer, Sanofi Pasteur Italy, MSD Italy, PaxVax and Seqirus for taking part to advisory boards, expert meetings, for acting as speaker and/or organizer of meetings/congresses and as principal investigator and chief of O.U. in RCTs.
Acknowledgments
The authors take full responsibility for the scope, direction, and content of the manuscript, and have approved the submitted manuscript. Medical writing assistance was provided by Eleanor Finn of PAREXEL International and was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. The authors wish to thank the following for contributions in development of the manuscript: Barbara J. Kuter, PhD, MPH, Global Vaccines Medical Affairs, and Tracey J. Weiss, Center for Observational and Real-World Evidence (CORE), Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Additional information
Funding
References
- European Centre for Disease Prevention and Control. ECDC guidance: varicella vaccination in the European Union. Available from: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/Varicella-Guidance-2015.pdf
- Riera-Montes M, Bollaerts K, Heininger U, et al. Estimation of the burden of varicella in Europe before the introduction of universal childhood immunization. BMC Infect Dis. 2017;17:353.
- Meszner Z, Molnar Z, Rampakakis E, et al. Economic burden of varicella in children 1-12 years of age in Hungary, 2011-2015. BMC Infect Dis. 2017;17:495.
- Gabutti G, Franchi M, Maniscalco L, et al. Varicella-zoster virus: pathogenesis, incidence patterns and vaccination programs. Minerva Pediatr. 2016;68:213–225.
- Bonanni P, Breuer J, Gershon A, et al. Varicella vaccination in Europe - taking the practical approach. BMC Med. 2009;7:26.
- Helmuth IG, Poulsen A, Suppli CH, et al. Varicella in Europe - a review of the epidemiology and experience with vaccination. Vaccine. 2015;33:2406–2413.
- Papaloukas O, Giannouli G, Papaevangelou V. Successes and challenges in varicella vaccine. Ther Adv Vaccines. 2014;2:39–55.
- European centre for disease prevention and control. Vaccination scheduler. 2018. Available from: https://ecdc.europa.eu/en/immunisation-vaccines/EU-vaccination-schedules
- ThePharmaLetter. Hungary to provide chickenpox vaccine free of charge from next January. [cited]. Available from: https://www.thepharmaletter.com/article/hungary-to-provide-chickenpox-vaccine-free-of-charge-from-next-january
- Brisson M, Gay NJ, Edmunds WJ, et al. Exposure to varicella boosts immunity to herpes-zoster: implications for mass vaccination against chickenpox. Vaccine. 2002;20:2500–2507.
- Ogunjimi B, Van Damme P, Beutels P. Herpes zoster risk reduction through exposure to chickenpox patients: a systematic multidisciplinary review. PloS One. 2013;8:e66485.
- Ogunjimi B, Van Den Bergh J, Meysman P, et al. Multidisciplinary study of the secondary immune response in grandparents re-exposed to chickenpox. Sci Rep. 2017;7:1077.
- Ogunjimi B, Willem L, Beutels P, et al. Integrating between-host transmission and within-host immunity to analyze the impact of varicella vaccination on zoster. eLife. 2015;4:e07116.
- Guzzetta G, Poletti P, Merler S, et al. The epidemiology of herpes zoster after varicella immunization under different biological hypotheses: perspectives from mathematical modeling. Am J Epidemiol. 2016;183:765–773.
- Betta M, Laurino M, Pugliese A, et al. Perspectives on optimal control of varicella and herpes zoster by mass routine varicella vaccination. Proc Biol Sci. 2016;283:20160054.
- Wutzler P, Bonanni P, Burgess M, et al. Varicella vaccination – the global experience. Expert Rev Vaccines. 2017;16:833–843.
- Robo A. Varicella outbreaks in Albania. Acta Microbiol Hellenica. 2015;60:158.
- Siennicka J, Trzcinska A, Rosinska M, et al. Seroprevalence of varicella-zoster virus in Polish population. Przegl Epidemiol. 2009;63:495–499.
- Socan M, Berginc N. High seroprevalence of varicella, measles, mumps, rubella and pertussis antibodies in first-grade medical students. Wien Klin Wochenschr. 2008;120:422–426.
- Sočan M, Berginc N, Lajovic J. Varicella susceptibility and transmission dynamics in Slovenia. BMC Public Health. 2010;10:360.
- Vilibic-Cavlek T, Ljubin-Sternak S, Kolaric B, et al. Immunity to varicella-zoster virus in Croatian women of reproductive age targeted for serology testing. Arch Gynecol Obstet. 2012;286:901–904.
- Arama V. Varicella in Romania: epidemiological trends, 1986-2004. Euro Surveill. 2005;10:E050811–E050816.
- Bakasun V, Pahor D. Epidemiological patterns of varicella in the period of 1977 to 2012 in the Rijeka district, Croatia. Epidemiol Res Int. 2014. Volume 2014, Article ID 193678, 4 pages. Available from: http://dx.doi.org/10.1155/2014/193678.
- EUVAC.net. Varicella surveillance report 2010. Available from: https://ecdc.europa.eu/en/publications-data/varicella-surveillance-report-2010
- Gowin E, Wysocki J, Michalak M. Don’t forget how severe varicella can be–complications of varicella in children in a defined Polish population. Int J Infect Dis. 2013;17:e485–e489.
- Hoxha H. The study of epidemiological data of varicella and its complications in Albanian children. Int J Infect Dis. 2010;14:e464.
- Lipke M, Paradowska-Stankiewicz I. Chickenpox in Poland in 2011. Przegl Epidemiol. 2013;67:195–197, 317–198.
- Sočan M, Blaško M. Surveillance of varicella and herpes zoster in Slovenia, 1996–2005. Euro Surveill. 2007;12:687.
- Socan M, Kraigher A, Pahor L. Epidemiology of varicella in Slovenia over a 20-year period (1979-98). Epidemiol Infect. 2001;126:279–283.
- Komitova R, Boev I, Kazakova Z, et al. Varicella complications-could we do more? Arch Dis Childhood. 2014;99:A308.
- Kuchar E, Miskiewicz K, Szenborn L, et al. Respiratory complications in children hospitalized with varicella. Adv Exp Med Biol. 2013;788:97–102.
- Mijailovic Z, Canovic P, Todorovic Z, et al. Characteristics of radiological changes in lungs during varicella zoster viral infection. Med Glas (Zenica). 2011;8:280–283.
- VENICE-II Consortium. Varicella and herpes zoster: surveillance and vaccination recommendations 2010-2011. Available from: http://venice.cineca.org/report_final_varicella.pdf
- Croatian National Institute of Public Health. Croatian health service yearbook 2013. Available from: https://www.hzjz.hr/wp-content/uploads/2014/12/Ljetopis_2013__.pdf
- Socan M. Evaluation of mandatory case-based reporting system for varicella in the prevaccine era. Cent Eur J Public Health. 2010;18:99–103.
- Bollaerts K, Riera-Montes M, Heininger U, et al. A systematic review of varicella seroprevalence in European countries before universal childhood immunization: deriving incidence from seroprevalence data. Epidemiol Infect. 2017;145:2666–2677.
- Infekcijas slimības un imunizācija. Slimibu profilakses un kontroles centrs. [Infectious diseases and immunization. Hospital profile and control center]. Available from: https://www.spkc.gov.lv/lv/statistika-un-petijumi/infekcijas-slimibas/valsts-statistikas-parskati/statistikas-parskati Latvian.
- National Center of Public Health and Analyses in Bulgaria. Registered cases of certain infectious disease subject to obligatory reporting. Available from: http://ncpha.government.bg/files/nczi/zdr.statistika/health_BB_1.pdf
- Savickiene E, Caplinskas S, Skrickiene A, et al. Epidemiological situation of varicella (chickenpox) in Lithuania. Poster session presented at: 14th Congress of the BADV. 2nd Vilnius Summit on Communicable Diseases; 2017 Oct 4 –7;Vilnius, Lithuania.
- Sergamumo Užkrečiamosiomis Ligomis Lietuvoje 2016 M. Apžvalga [Incidence of infectious diseases in Lithuania in 2016: overview]. Available from: http://www.ulac.lt/uploads/downloads/en_sergamumo_katalogas/2016_en/Sergamumo_apzvalga_2016.pdf Lithuanian.
- Epidemiološko Spremljanje Nalezljivih Bolezni v Sloveniji v Letu 2016. [Epidemiological monitoring of common diseases in Slovenia in 2016]. [cited Nov 2018]. Available from: http://www.nijz.si/sites/www.nijz.si/files/datoteke/epidemiolosko_spremljanje_nb_slo_2016.pdf Slovenian.
- Leonid I, Evelyn L. Primary varicella in an immunocompetent adult. J Clin Aesthet Dermatol. 2009;2:36–38.
- Ahčan J, Čižman M, Pleterski-Rigler D, et al. [Varicella hospitalization]. Zdrav Vestn. 2002;71: 621–627. Slovenian.
- Wolfson L, Giglio N, Castilo M. The use of antibiotics in the treatment of pediatric varicella patients: evidence from the multi-country MARVEL study. Int J Infect Dis. 2018;73S:139.
- Toth E. Varicella, a cost of illness Hungarian study. Value Health. 2012;15:A390.
- Hudeckova H, Straka S, Rusnakova S. Epidemiological features and economic evaluation of a potential chickenpox vaccination strategy in Slovak Republic. Cent Eur J Public Health. 2000;8:227–228.
- Ahčan J, Čižman M, Pleterski-Rigler D, et al. [Value of universal childhood varicella vaccination in Slovenia]. Zdrav Vestn. 2002;71: 667–672. Slovenian.
- Wysocki J, Malecka I, Stryczynska-Kazubska J, et al. Varicella in Poland: economic burden in children 1-12 years of age in Poland, 2010-2015. BMC Public Health. 2018;18:410.
- World Health Organization (WHO). Systematic review of available evidence on effectiveness and duration of protection of varicella vaccines. Available from: http://www.who.int/immunization/sage/meetings/2014/april/4_Systematic_review_on_effectiveness_and_duration_of_protection_of_varicella_vaccines.pdf
- Maltezou HC, Wicker S, Borg M, et al. Vaccination policies for health-care workers in acute health-care facilities in Europe. Vaccine. 2011;29:9557–9562.
- Szabo LJ, Jackowska T, KaloZ, et al. Varicella vaccination in Hungary and Poland: optimization of public benefits from prophylaxis technologies in the time of austerity. New Med. 2013;3:97–102.
- Richter D, Anca I, Andre FE, et al. Immunization of high-risk paediatric populations: central European vaccination awareness group recommendations. Expert Rev Vaccines. 2014;13:801–815.
- Allaert FA, Blanc A, Megard Y, et al. Parents’ attitudes towards varicella vaccination acceptance in France and Germany: effect of vaccine recommendation and reimbursement (a survey). J Public Health. 2009;17:71–76.
- Kuter B, Matthews H, Shinefield H, et al. Ten year follow-up of healthy children who received one or two injections of varicella vaccine. Pediatr Infect Dis J. 2004;23:132–137.
- Spackova M, Wiese-Posselt M, Dehnert M, et al. Comparative varicella vaccine effectiveness during outbreaks in day-care centres. Vaccine. 2010;28:686–691.
- Thomas CA, Shwe T, Bixler D, et al. Two-dose varicella vaccine effectiveness and rash severity in outbreaks of varicella among public school students. Pediatr Infect Dis J. 2014;33:1164–1168.
- Chaves SS, Lopez AS, Watson TL, et al. Varicella in infants after implementation of the US varicella vaccination program. Pediatrics. 2011;128:1071–1077.