ABSTRACT
Introduction: Hepatitis A, caused by hepatitis A virus (HAV), is primarily transmitted via the fecal/oral route either through ingestion of contaminated food and water or through direct contact with an infectious person. Prevalence of hepatitis A is strongly correlated with socioeconomic factors, decreasing with increased socio-economic development, access to clean water and sanitation. Vaccination against HAV should be part of a comprehensive plan for the prevention and control of viral hepatitis, either as part of regular childhood immunization programs or with other recommended vaccines for travelers.
Areas covered: We present here evidence for the immunogenicity and safety of an inactivated HAV pediatric vaccine (Avaxim® 80U Pediatric, Sanofi Pasteur), indicated for use in children aged 12 months to 15 years. Data evaluated are from trials undertaken during the clinical development of this vaccine, a systematic literature review and post-market pharmacovigilance.
Expert opinion: The pediatric HAV vaccine is highly immunogenic and generates long-lasting protection against hepatitis A disease in children. The safety and immunogenicity data presented in this review suggest that the pediatric HAV vaccine is a valuable option in the prevention of HAV infection in children in many areas of the world where the disease remains a healthcare issue.
1. Introduction
Hepatitis A is one of the most common viral infections worldwide but can be largely controlled by appropriate vaccination strategies. The inactivated hepatitis A vaccine, Avaxim® 160U (Sanofi Pasteur), was approved in 1996 for immunization of adults and children 2 years and over based on safety and immunogenicity assessments undertaken in nine studies involving >3,700 adults and three studies with >500 children aged 2–17 years, undertaken as part of its clinical development [Citation1]. Vaccine efficacy was inferred through a comparison of antibody concentrations with other hepatitis A vaccines demonstrated to provide protective efficacy, with seroprotection defined as antibody levels against hepatitis A virus (HAV) of ≥20 mIU/mL. Substantial antibodies levels far higher than those required for seroprotection could be achieved in children with the approved formulation. Subsequently, a lower dose formulation containing half the antigen units (80U) was chosen for further development specifically for use in children aged 12 months to 15 years inclusive. This pediatric HAV vaccine (Avaxim® 80U Pediatric, Sanofi Pasteur) was first approved in Canada and France in 2001 based on a clinical development program of eight clinical trials and >3,500 participants. Since then, an additional 13 studies have been conducted.
We briefly summarize the epidemiology of hepatitis A as well as diagnosis, treatment and prevention strategies, before reviewing the clinical experience with the pediatric HAV vaccine. Three sources of data were considered in order to provide the most comprehensive overview to date of this pediatric HAV vaccine: an integrated analysis of the clinical trial databases; a review of the pharmacovigilance database; and a systematic review of published studies.
1.1. Epidemiology
Hepatitis A is an acute, inflammatory infection of the liver caused by HAV, genus hepatovirus of the picornavirus family [Citation2]. Infection with HAV is an important public health problem in some parts of the world [Citation3,Citation4], where prevalence of anti-HAV antibodies in the population may exceed 90% by age 10 years. In endemic countries, exposure to HAV usually occurs before the age of 5 years when infections are often asymptomatic [Citation2,Citation5]. The severity of illness tends to increase with age. Prevalence rates vary considerably by country, region, and shift over time [Citation6]. Sporadic outbreaks can occur in otherwise low endemicity countries [Citation7]. Globally, >100 million HAV infections and 15,000–30,000 associated deaths occur annually [Citation8]. The global burden of HAV in 2013 was estimated to be 1.2 million disability-adjusted life years (DALYs), 198,000 years lived with disability (YLDs), and 1.0 million years of life lost (YLLs) [Citation9]. In middle-income countries, routine vaccination of children is cost-effective, even cost saving in some instances [Citation10], and can be a sound economic strategy even in low HAV endemic countries [Citation11].
The virus is spread via the fecal-oral route and is associated with inadequate clean water supply, poor sanitation and hygiene [Citation12]. Prevalence, therefore, varies with the level of socio-economic development, and most cases occur in less developed, low-income countries. As socioeconomic conditions improve, HAV endemicity may decrease with reduced exposure during early childhood, which leads to increased susceptibility among adolescents and adults [Citation13]. This shift in the age at exposure, together with the increasing severity of disease with age, has led to an increase in symptomatic cases and in severe clinical outcomes including liver failure in some developing countries [Citation14,Citation15]. The age-related hepatitis A case-fatality rate varies from 0.1% among children aged <15 years to 0.3% among those aged 15–39 years, and 2.1% among adults aged ≥40 years [Citation5].
Developed, high-come countries have very low HAV endemicity levels (<50% seroprevalence by age 30 years) as a result of stringent public health standards [Citation4,Citation16], and infections tend to occur in specific groups such as travelers to endemic countries, intravenous drug users, and men who have sex with men [Citation14,Citation17]. In the USA, the recent sustained increases in the number of cases reported have been attributed to direct person-to-person transmission of HAV particularly among drug users and the homeless [Citation18,Citation19]. Routine childhood hepatitis A vaccination introduced in 1996 has provided immunity and protection in younger populations; however, older adults remain susceptible and at risk of developing severe disease.
1.2. Diagnosis and treatment
The incubation period of acute hepatitis A is usually 14–28 (up to 50) days. The clinical outcome is strongly correlated with age; young children usually experience asymptomatic infection or mild nonspecific viral-like illness, while adults who have not been infected when young commonly experience symptomatic icteric infection [Citation2,Citation5]. Hepatitis A cases are not clinically distinguishable from other types of acute viral hepatitis [Citation12]. Symptoms in young children include malaise, nausea, fever, and diarrhea in approximately 50% of cases; joint pain, abdominal pain, or vomiting in 20–30% of cases, and jaundice in up to 10% [Citation2]. Liver enzyme levels may be raised; dark urine and clay-colored stools along with jaundice are all possible characteristic manifestations [Citation5]. Specific diagnosis is primarily through detection of anti-HAV immunoglobulin M (IgM) antibodies, which gradually disappear over 6 months post-infection [Citation20]. Additional tests include reverse transcriptase polymerase chain reaction (RT-PCR) to detect for HAV RNA [Citation12], but these are mainly used for research purposes. There is no specific anti-viral treatment for hepatitis A [Citation12] and supportive care remains the mainstay of treatment [Citation21].
1.3. Prevention
Improved sanitation, food safety, and vaccination are the most effective ways to prevent HAV infections. There are a number of inactivated HAV vaccine formulations available from several manufacturers differing in strains and adjuvant used, and antigen dose [Citation22]. In addition, live attenuated HAV vaccines are also available in China and India [Citation23]. In general, these vaccines are licensed for two intramuscular injections 6 months apart, which may be extended to 18–36 months, and are available in pediatric (age >1 year) and adult formulations [Citation23]. Although interrupting the vaccination schedule beyond the recommended interval is not recommended, there is evidence to suggest that a second dose administered at longer intervals still result in boosting the immune response [Citation24,Citation25]. Protective efficacy against disease has been reported to be >90% in susceptible individuals, with a risk of local and systemic adverse events comparable to placebo for the inactivated HAV vaccines [Citation26]. Protection persists beyond 10 years after a full primary vaccination course and may possibly be life-long [Citation27]. Although anti-HAV IgG is also highly efficacious in the short-term for pre- and post-exposure prophylaxis, its use has greatly declined for a variety of reasons including high costs, limited duration of protection and limited availability [Citation23]. Moreover, HAV vaccines may also be considered valid alternatives to anti-HAV IgG for post-exposure prophylaxis [Citation28].
1.4. Avaxim® 80U Pediatric (inactivated HAV vaccine)
The pediatric HAV vaccine is currently available in approximately 80 countries. The vaccine is presented as a sterile suspension for intramuscular use in pre-filled, 0.5 mL syringes or 5 mL multidose vials containing purified, formaldehyde-inactivated HAV obtained from the GBM strain cultured on Medical Research Council cell strain 5 (MRC-5) human diploid cells [Citation29]. Each dose of the vaccine contains 80 antigen units of the active substance, aluminum hydroxide (expressed as aluminum) adjuvant, and 2-phenoxyethanol and formaldehyde preservatives in Medium 199 Hanks water for injection supplemented with polysorbate 80 [Citation30]. The vaccine is indicated for active immunization against HAV infection in children aged 12 months to 15 years, who are at increased risk of infection or more severe disease if infection occurs, and for post-exposure prophylaxis for those in close contact with proven or suspected cases. The vaccination schedule consists of two vaccine doses preferably 6 months to 36 months apart but can be given up to 7 years apart.
2. Clinical experience: methodology
Three approaches were undertaken to review the clinical experience. First, using databases from clinical trials of this vaccine conducted by Sanofi Pasteur, we performed a pooled analysis of safety and an integrated analysis of immunogenicity (pooled analysis between studies that used the same assessment method). Second, we performed a systematic review of published studies in children. Third, we reviewed spontaneous adverse event reports in the Sanofi Pasteur Global Pharmacovigilance database. The following sections describe these approaches and provide a narrative summary of the clinical profile of the vaccine.
2.1. Pooled clinical trial safety data
A total of 21 prospective clinical and observational studies sponsored by Sanofi Pasteur have been conducted with the pediatric inactivated HAV vaccine to document safety. Six studies were excluded from the analysis as either the databases were unavailable, safety was assessed during a limited 3-day period only (duration considered too short and not appropriate for pooling with other data), or the age of the participants was not in the indicated range. The 15 studies retained for pooled analysis evaluated the safety profile of the pediatric inactivated HAV vaccine in participants aged 12 months to 15 years for at least 7 days after the first or second injection (first injection generally followed by a second dose about 6 months later). The study designs and populations assessed are summarized in Table S1.
Characterizing the safety profile of the vaccine was a main objective in 12 studies undertaken from 1996 to 2014: HAF11 [Citation31], HAF17, HAF19, HAF20 [Citation32], HAF22, HAF25 [Citation33], HAF29, HAF36, HAF65 [Citation34], HAF77, HAF78 [Citation35], and HAF87. The vaccine was used as a control in the remaining three studies (CYD08 [Citation36], JEC01 [Citation37] and JEC02 [Citation38]). Safety data were collected using similar methodology and over similar time scales after vaccination to allow for a pooled analysis. Safety analyses were conducted on the safety analysis set of the individual studies, defined as the subset of participants who received at least one dose of pediatric HAV vaccine and analyzed according to the vaccination received. For the analysis at ‘any vaccination’, participants were analyzed according to the first vaccine received. Safety data were more often presented descriptively, except in studies HAF17, HAF19, and HAF20 where logistic regression analysis was used to identify factors that may influence safety. No critical safety issue was identified, and as such, only a descriptive summary (point estimates and 95% confidence intervals [CI]) of the pooled safety data is presented.
2.2. Pooled clinical trial immunogenicity analysis
Immunogenicity was assessed in 11 Sanofi Pasteur sponsored studies. Nine studies for which databases were available were included in the integrated analysis presented here (Table S1): HAF78 [Citation35]; HAF65 [Citation34]; HAF36; HAF29; HAF25 [Citation33]; HAF22; HAF20 [Citation32]; HAF11 [Citation31]; HAF82 (although this study is listed as part of the integrated analysis the data are presented along with the systematic review data below to enable the reader to interpret the continuous long term data available as the data have been published in two reports [Citation39,Citation40]); and HAF57 [Citation41]. Due to difference in study designs and in antibody titer assessment methods as well as time points for blood sampling, the immunogenicity results are presented individually for a number of studies. However, the same titration method (modified radioimmunoassay [RIA]) was used in five studies (HAF11 [Citation31], HAF20 [Citation32], HAF22, HAF25 [Citation33] and HAF29), and as such, data from the full analysis set of these studies (participants who received at least one HAV vaccine injection) were pooled to provide a group point estimate and associated 95% CIs.
2.3. Pharmacovigilance database
We reviewed adverse event reports in the Sanofi Pasteur Global Pharmacovigilance database for the inactivated HAV pediatric vaccine from 2001 when the vaccine was first approved to May 2017. The database contains spontaneously reported safety information directly from clinical practice. It includes reports from various sources, regardless of severity or causality, including health-care professionals, health authorities, and patients. The cumulative data gathered through continuous pharmacovigilance can help identify trends that may be indicative of safety signals in practice. Relative event rates can then be determined from information regarding the worldwide use of the pediatric HAV vaccine from the number of doses distributed.
2.4. Systematic literature search
A systematic review of the literature was undertaken to supplement the available data obtained from the Sanofi Pasteur’s clinical development program and pharmacovigilance database. Such an approach allows for the identification of other relevant studies undertaken by independent investigators and ensures that a wider and more robust assessment of the clinical profile of the vaccine can be made, and a complete view of the clinical experience can be presented.
The PubMed and EMBASE databases were systematically searched on 28–30 June 2017 to identify publications related to the pediatric HAV vaccine using the following terms: ‘(hepatitis A vaccine AND inactivated) OR avaxim’ as free text terms or main phrases. Studies were identified based on the relevance of their title or abstract related to the objectives of this review. Only full published English- or French-language articles of prospective studies that assessed the use of the pediatric HAV vaccine in infants, children and adolescents were eligible for inclusion. Studies were excluded if they assessed pediatric HAV vaccine administered concomitantly with immunoglobulins. Studies identified that were already included in the pooled analyses described above were excluded here to avoid duplication. In addition, meeting abstracts, letters, editorials, and reviews were excluded; however, the reference lists of the later articles were assessed to identify additional studies not captured by database searches.
3. Clinical experience: results
3.1. Disposition and participant characteristics of pooled analyses
3.1.1. Pooled safety analysis
Data from 5458 participants who received at least one injection of inactivated HAV vaccine between 1996 and 2014 were included. Among these, 4777 (87.5%) participants received a second injection. The median age of the participants was 4.3 years (range 11.8 months to 16.0 years) at the time of the first injection, and 5.7 years (range 17.2 months to 16.5 years) at the second injection. The proportion of male and female participants was balanced (51.4% male [n = 2807]), with participants from 12 countries in Europe, Asia, and Latin America.
3.1.2. Pooled immunology analysis
Data from 1806 participants were included in the pooled analysis. Data analyzed were from the full analysis sets, and summarized before and 2–4 weeks after each vaccine injection. The baseline characteristics of the participants included in the integrated immunogenicity are summarized in Table S2.
3.2. Literature identified
The literature search identified 11 [Citation39,Citation40,Citation42–Citation50] reports for inclusion in this review (Figure S1). Although one article by Fisenka et al. should have been excluded from this review because it assessed the use of three hepatitis A vaccines (i.e. no distinction was made between vaccines), it was felt to be of sufficient importance to be included because it was the only study identified that specifically reported the use of Avaxim 80U in preventing disease. In this study 95% of those vaccinated had received Avaxim 80U or Avaxim 160U following the introduction of routine HAV immunization for 6-year-old children [Citation50].
Table S3 summarizes the information collated from identified reports that forms the basis of the narrative systematic review. Of these, 10 reports included immunogenicity [Citation39,Citation40,Citation42–Citation49] and four also reported safety data [Citation42,Citation45,Citation48,Citation49]. Overall, seven reports were from children in the indicated age group (12 months to 15 years) [Citation39,Citation40,Citation42,Citation43,Citation46–Citation48], two were from infants aged <1 year [Citation44,Citation45] and one was from older children and adolescents aged 13–19 years [Citation49].
3.3. Safety profile from pooled clinical studies and literature review
The pooled safety summary is shown in . No related immediate reaction was reported across the studies. In general, there was reduced reactogenicity post-dose 2 compared to post-dose 1. Injection site pain/tenderness (18.1% [95% CI 17.1–19.2]) and gastrointestinal disorders (16.9% (95% CI 11.9–23.1)) were the most common solicited reactions after any vaccine injection; other frequent solicited reactions reported by ≥10% of participants included malaise, abnormal crying, headache, and irritability/crying. Most local and systemic reactions were of Grade 1 intensity (graded on a 3-point scale of increasing severity, Grades 1–3 [see Table S4 for grading definition]), and short-lived. Grade 3 reactions were reported by 1.8% (95% CI 1.5–2.2) of study participants after any injection. Unsolicited, non-serious AEs within 7 days after any injection were reported by 6.8% (95% CI 6.2–7.5) of participants and were mainly classified as infections and infestations (3.0% [95% CI 2.6–3.5]) using the MedDRA System Organ Class and Preferred Term. There were two Grade 3 unsolicited non-serious AEs within 7 days after any injection (1 vomiting and 1 irritability).
Table 1. Solicited local and systemic reactions within 7 days after each and any injection of Avaxim® 80U Pediatric – Safety Analysis Set.
There were no discontinuations due to AEs reported across the studies. Serious adverse events (SAEs) within 30 days after any injection were reported by 0.6% (95% CI 0.4–0.9) of participants and were mainly classified as infections and infestations (0.4% [95% CI 0.3–0.7%]); none were assessed as related to the vaccine. No participant experienced SAEs assessed as related to the vaccine throughout the studies (study durations ranged from 1 month to 19 months). However, studies HAF17, HAF19, HAF20, and HAF22 were excluded from the pooled analysis of related SAEs because the relationship of the SAEs to the vaccine was not captured in the clinical databases. Three SAEs were reported from these four studies, and assessed by the investigator post-hoc as being related to the vaccine (Crohn’s disease, acute viral gastroenteritis and Shigella gastroenteritis and arthralgia). There was one death which was considered unrelated to the vaccination. The participant died due to complications of pre-existing central neurological disorder 75 days after vaccination.
The safety overview per age group (≤23 months, 2–11 years and 12–15 years, respectively) is shown in . The safety profile of the pediatric HAV vaccine was slightly different according to the age of the participants. Although solicited reactions collected were different by age groups, they tended to be generally more frequently reported in participants aged 12–15 years while unsolicited events including SAEs and unsolicited reactions tended to be more reported in the youngest participants, especially those aged ≤23 months.
Table 2. Safety overview within 7 days per age group after any injection (Safety Analysis Set).
Concomitant HAV vaccine administration with other routine childhood vaccines was also assessed in HAF65 (concomitantly with the MMR vaccine compared with two groups that received the two vaccines 4 weeks apart) [Citation34] and HAF29 (concomitantly with the DTwP//PRP∼T and OPV, or diphtheria, tetanus, acellular pertussis, inactivated polio vaccine reconstituting lyophilized tetanus conjugated Haemophilus influenza type b [DTacP-IPV//PRP∼T]). In HAF65, the reactogenicity rate was slightly higher with MMR concomitant administration, with about 8% more participants reporting a solicited injection site reaction and 10% more reporting a solicited systemic reaction than those receiving the vaccines separately [Citation34]. Similar trends toward increased reactogenicity with concomitant administration of a tetravalent pediatric combination vaccine and oral polio vaccine, or a pentavalent combination vaccine were observed in HAF29 study.
The reactogenicity profile of the pediatric HAV vaccine appears similar than that of other pediatric HAV vaccines in studies that allowed for direct safety comparisons (HAF78 [Citation35] and HAF57 [Citation41]).
From the literature review, we identified four studies that reported safety data [Citation42,Citation45,Citation48,Citation49]. No serious adverse events related to the pediatric HAV vaccine occurred during any of these studies. Soysal et al. did not report the reactogenicity profile of Avaxim 80U specifically but across all three childhood hepatitis A vaccines they assessed (Avaxim 80U, Havrix 720 and Vaqta 25). No immediate reactions were reported, and the rates of local and systemic solicited reactions did not differ between the three vaccines assessed after the first or the second vaccine injection [Citation48]. The most commonly reported adverse event was pain (11.5% of participants). The overall rate of local and systemic events after the first injection of the HAV vaccine was reported by Celebi et al. to be 6.6–9.8% among participants who started their schedule at age 1 or 2 years. No local or systemic events were reported after the second injection in those who started vaccination at age 1 year, but were reported at a similar rate as after the first injection in those who started vaccination at age 2 years [Citation42]. Yoon et al. also reported pain as the most common adverse event in adolescents aged 13 to 19 years (32% received the pediatric HAV vaccine dose) post-dose 1 (22%; 11/51) and post-dose 2 (18%; 9/50) [Citation49]. There was a trend towards fewer local and systemic reactions post-dose 2 compared with post-dose 1. Moreover, the reactogenicity profile of the pediatric HAV vaccine appeared similar to other HAV vaccines assessed in the Yoon et al. study.
Interestingly, Lopez et al. (2007) assessed the reactogenicity of the pediatric HAV vaccine in infants vaccinated at 2, 4, 6, and 15–18 months compared with those who received placebo at 2 and 4 months followed by vaccination at 6 and 15–18 months [Citation45]. The reactogenicity profile of the pediatric HAV vaccine at age 2 and 4 months was similar to placebo with a trend towards fewer local reactions with the vaccine than placebo that was more marked after the second injection, and similar rates of systemic reactions. In addition, there was a trend towards fewer local and systemic reactions with the second and third injection of the vaccine compared with the first. The rate of local reactions after the fourth dose of the vaccine at 15–18 months was similar to that reported after the first vaccine injection. In infants who received the first dose of the pediatric HAV vaccine at 6 months, there was a trend towards more local and systemic reactions with the second dose at 15–18 months.
3.4. Spontaneous reports in pharmacovigilance database
More than 22 million doses of the pediatric HAV vaccine have been distributed since its launch in 2001 through to 31 May 2017; therefore, 11–22 million children may have been exposed to the vaccine worldwide assuming some adherence to the recommended two-dose schedule. Over the 16-year surveillance period, 309 spontaneous adverse event cases were reported and the spontaneous report rate was 1.4 cases per 100,000 doses distributed, or 1.4–2.8 cases per 100,000 children exposed. There were no important risks associated with the vaccine or any other safety concerns identified from post-marketing surveillance. The events most frequently reported were local injection site reactions, followed by fever, vomiting, rash or urticaria. Of the spontaneously reported adverse events, 50 cases (16%) met International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) criteria for SAEs. Of note, 23 (46%) of the cases who experienced serious adverse events were concomitantly vaccinated with other vaccines at the same visit; these included diphtheria, tetanus, acellular pertussis, hepatitis B, poliomyelitis and Haemophilus influenzae type b (Hib) conjugate vaccine (DTaP-IPV-HB-Hib), yellow fever, varicella, measles/mumps/rubella, pneumococcal vaccines. One report out of 50 serious reports involved a fatal outcome in a child who died of pneumonia with sepsis; latency between vaccination and the onset of pneumonia was not reported. Although very limited and medically unconfirmed information was provided on this case, infectious origin of the event makes the causal role of the vaccine unlikely. Fourteen other SAEs included underlying bacterial or viral infections (7 cases) and febrile convulsions (7 cases). Four reports of seizures involved 3 children who were aged less than 3 years and one aged 10 years; the origin of seizures and consequently the causal role of the vaccination could not be established based on the limited available information. It should be noted that in 1 case, the child had episodes of convulsions prior to vaccination. One case reported syncope within a day after vaccination. Syncope with or without convulsions is commonly not related to vaccine but related to fear of the injection. Other SAEs included allergic reactions (10 cases) such as urticaria, rashes, pruritus, and face swelling; of these, 3 were severe and immediate anaphylactic reaction requiring emergency therapy, they involved two 2-year-olds and one 4-year-old, all of whom had personal or family history of asthma, or allergy to drugs or food. Allergic reactions occurred on the same day after vaccination for the majority of cases, however, temporal relationship alone is not sufficient evidence to suggest that these were related to the vaccine. Moreover, five of the 10 children who developed serious allergic reactions were simultaneously administered other vaccines such as yellow fever (1 case), varicella (1 case) or DTaP-IPV-HB-Hib (3 cases), which makes assessment of the causal relationship with a particular vaccine difficult. Seven cases referred to as gastrointestinal disorders were expected after vaccination with hepatitis A vaccine and included vomiting, nausea, diarrhea, lack of appetite and abdominal pain. Serious injection site reactions have also been reported (6 cases). Among these, five cases had vaccination site infection, cellulitis or abscess. There were two cases of idiopathic thrombocytopenic purpura, one case of optic neuritis, and one case of walking abnormalities in a 13-month old child, all poorly documented, and the role of the vaccine could not be assessed.
Two cases (0.6% of 309 cases) were described as vaccine failure, where hepatitis A disease was confirmed 2 and 7 years after complete immunization with two doses. However, the medical history of the two cases was not specified and their immunocompetency at the time of vaccination remains unknown.
The adverse events reporting rate has remained low and stable during the 16-year period with no observed increase in frequency or severity. No safety signal has been identified since the vaccine was first introduced and the reported safety profile of the vaccine remains unchanged.
3.5. Immunogenicity in children aged 12 months to 15 years (indicated age group)
3.5.1. Data from pooled analysis
3.5.1.1. Anti-HAV antibody concentrations
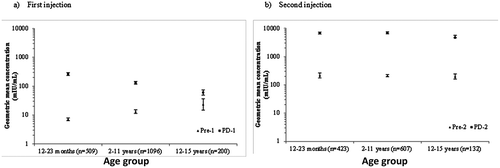
Anti-HAV antibody concentrations pre- and post-dose 1 and 2 are presented in –. summarizes anti-HAV antibody concentrations by age group in participants from the five studies included in the pooled immunogenicity analysis. There was a trend towards increased baseline anti-HAV antibody concentrations with increasing age, and conversely a trend to a lower antibody response to vaccination with increasing age, post-dose 1. However, no age group difference in anti-HAV geometric mean concentrations (GMCs) remained apparent 6 months later, just before the second injection. The participants in the two youngest age groups had similar post-dose 2 HAV GMCs, while GMCs among 12–15 year-olds were approximately about 30% lower. There was no obvious pattern in anti-HAV antibody concentrations achieved in studies undertaken in the different HAV endemicity settings ().
Table 3. Anti-HAV antibody concentrations in all participants (Full Analysis Set).
Table 4. Anti-HAV antibody concentrations in participants aged 12–23 months (Full Analysis Set).
Table 5. Anti-HAV antibody concentrations in participants aged 2–11 years (Full Analysis Set).
Table 6. Anti-HAV antibody concentrations in participants aged 12–15 years (Full Analysis Set).
Table 7. Anti-HAV antibody concentrations in participants by HAV endemicity‡ (Full Analysis Set).
3.5.1.2. Percentage of seroconverted participants
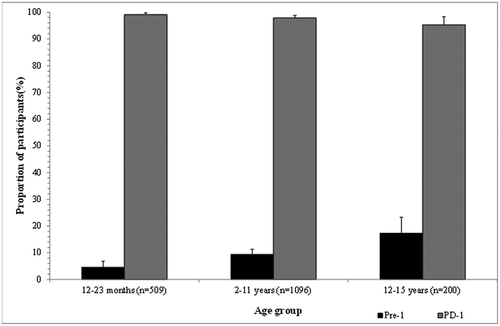
The seroprotection lower limit has been historically defined as 20 mIU/mL with the RIA assays; however, this threshold is not universally accepted, and depending on the assay the 10 mIU/mL threshold may be used to define the seroprotective limit or even the lower limit of quantification (LLOQ). We present proportion of participants with anti-HAV antibody concentrations ≥20 mIU/mL and above the LLOQ (). summarizes the percentage of participants with anti-HAV antibody concentrations ≥20 mIU/mL pre and post-dose 1 in participants included in the pooled immunogenicity analysis. As observed with the anti-HAV antibody concentrations, there was a trend towards increased seroprotection rates with increased age at baseline, but there was a slight decrease in seroprotection rates with increasing age post-dose 1. The proportion of participants with concentrations ≥20 mIU/mL included in the pooled analysis was consistently >95% post-dose 1 irrespective of HAV endemicity and country (Table S5). All participants included in the pooled immunogenicity analysis had concentrations ≥20 mIU/mL before the second dose (data not shown).
Table 8. Seroprotection rates across the studies assessed (Full Analysis Set).
3.5.2. Data from literature review
Of studies that enrolled the indicated age group (12 months to 15 years), only two reported immunogenicity in the short term after two doses of the pediatric HAV vaccine, 6 months apart [Citation42,Citation48], but these used different anti-HAV antibody assays, making comparability of data difficult. Studies that assessed interchangeability of pediatric HAV vaccines, or concomitant administration with other childhood vaccines are summarized below. Celebi et al. [Citation42], in predominantly seronegative children (n = 100) in Turkey, showed that vaccination at age 1 or 2 years achieved similar seroconversion rates (defined as anti-HAV antibodies greater the assay cut-off limit); the seroconversion rates 2 weeks post-dose 1 was 39%, increasing to 89% four weeks after the first dose and reaching 100% post-dose 2 (4 weeks after the second dose). No differences were observed in the 1-year and 2-year age groups, but no quantitative measure of antibody concentrations was performed. Soysal et al. [Citation48], also in Turkish children (n = 137) of whom about 50% were seronegative, reported anti-HAV antibody GMCs of 8628 mIU/mL, 20,502 mIU/mL and 53,506 mIU/mL at post-dose 1 (Week 2 [2 weeks after the first dose]), pre-dose 2 (Week 24) and post-dose 2 (Week 28 [4 weeks after the second dose]), respectively. Seroprotection (≥20 mIU/mL) was achieved in 98.6%, 100%, and 100% of children at the same time points, respectively.
Yoon et al. compared the immunogenicity of Avaxim (n = 53), Epaxal (n = 52) and Havrix (n = 52) in healthy seronegative children and adolescents aged 13 to 19 years in Korea [Citation49]. The participants were randomized in three equal groups to receive two doses of their allocated HAV pediatric or adult vaccine formulation (according to age indication) 6 to 12 months apart. GMCs in those who received Avaxim (32% [17/53] received the pediatric dose) were reported as 736 mIU/mL, 277 mIU/mL and 7208 mIU/mL at post-dose 1, pre-dose 2 and post-dose 2, respectively. The seropositivity rate (≥20 mIU/mL) with Avaxim was 98% 1-month post-dose 1, and increased to 100% at all other time points assessed.
3.5.2.1. Vaccination of infants aged <12 months (presence of maternal antibodies)
Two reports in the literature studied vaccination of infants aged <12 months who were predominantly seropositive (86–88% seropositive; anti-HAV concentration ≥20 mIU/ml) [Citation44,Citation45]. Both studies utilized the same anti-HAV antibody assay which allows comparability between the two reports. Lagos et al. randomized 6-month-old infants (n = 131) in Chile to receive the HAV vaccine either concomitantly with (Group 2) or 2 weeks after the third dose of routine diphtheria-tetanus-whole cell pertussis reconstituting lyophilized tetanus conjugated Haemophilus influenza type b (DTwP//PRP∼T) vaccine and oral poliomyelitis vaccine (OPV) (Group 1), with the second dose 6 months later concomitantly with the MMR vaccine [Citation44]. Although GMCs decreased from post-dose 1 levels of 292 and 278 mIU/ml to 77.6 and 76.0 mIU/ml at 6 months in Groups 1 and 2, respectively, there was a clear anamnestic response post-dose 2 in both groups, with GMCs of 1731 and 1866 mIU/ml. Seropositivity was achieved in all infants. In the second study [Citation45], Lopez et al. randomized infants in Argentina to receive either three HAV vaccine doses (at 2, 4, 6 months of age [Group A]) or one dose (at 6 months of age [Group B]), with a subsequent dose at 15–18 months. Other childhood vaccines during the study period were administered a minimum of 10 days apart from each study injection. GMCs were 2989 and 3637 mIU/mL at enrolment in Groups A and B, but these progressively waned following the initial three doses or one dose to 279 and 52 mIU/mL before the subsequent dose at 15–18 months for both groups, respectively. A clear anamnestic response was also observed after the subsequent dose at 15–18 months in both groups with GMCs of 8236 and 1687 mIU/mL in Groups A and B, respectively. In Group B, 91% of the infants had seroprotective antibody concentrations before the booster dose at 15–18 months unlike Group A who remained seropositive throughout after vaccination.
3.5.2.2. Comparison with other pediatric HAV vaccines
In the Yoon et al. study of healthy seronegative children and adolescents aged 13 to 19 years in Korea comparing the immunogenicity of two doses of Avaxim, Epaxal or Havrix [Citation49], anti-HAV GMCs were approximately 3–4-fold higher post-dose 1 and 2 with Avaxim than those achieved with the other two HAV vaccines. The anamnestic response post-dose 2 with Avaxim was also more pronounced than that with the other two HAV vaccines. Similarly, anti-HAV GMCs were shown to be significantly higher (1.4–2.0-fold higher post-dose 2) after two doses Avaxim 80U than Havrix 720 in the HAF78 study, across toddlers (12–23 months), children (2–11 years) and adolescents (12–15 years) [Citation35]. Studies assessing the interchangeability of childhood HAV vaccines (see next section), have also shown that anti-HAV GMCs following a single Avaxim 80U pediatrics dose to be 1.6–3.1-fold higher than with Havrix 720 or Vaqta 25 [Citation41,Citation48]. As seen with Avaxim 160 [Citation1,Citation51], the higher anti-HAV GMCs achieved with Avaxim 80U pediatrics are expected to translate into protection at earlier times than for the comparators [Citation49].
3.5.2.3. Interchangeability of childhood HAV pediatric vaccines
The interchangeability of the pediatric HAV vaccine with other childhood HAV vaccines was assessed in two studies; one identified from the literature [Citation48] and one was part of the pooled/integrated analysis included above (HAF57 [Citation41]). Soysal et al. also reported on the interchangeability of the pediatric HAV vaccine as a second dose 6 months following an initial dose with Havrix 720 (n = 131) or Vaqta 25 (n = 135) [Citation48]. Anti-HAV antibody GMCs post-dose 1 (Week 2) were 8628 mIU/mL, 2919 mIU/mL and 4645 mIU/mL with the three pediatric HAV vaccines, respectively, and 98.2% of all participants were seroprotected (≥20 mIU/mL). There were no significant differences in GMCs for those given two doses of the same childhood HAV vaccine or Avaxim 80U as a second dose post-dose 2; the GMCs were 32,115 mIU/mL for two doses of the Havrix 720 (n = 65) compared with 27,848 mIU/mL for those who received Havrix 720 followed Avaxim 80U (n = 66), and 52,409 mIU/mL for those who received two doses of Vaqta 25 (n = 67) compared with 47,954mIU/mL for those who received Vaqta 25 followed Avaxim 80 (n = 68). The seropositive rate was 100% in all vaccine groups just before and after the second dose. Abarca et al. also assessed the interchangeability of the pediatric HAV vaccine as a second dose 6 months following an initial dose with Havrix 720 (n = 166) in seronegative Chilean children aged 1–15 years in study HAF57 [Citation41]. The GMCs post-dose 2 were 4008 mIU/mL for those who received two doses of the Havrix 720 (n = 83) compared with 7144 mIU/mL for those who received Havrix 720 followed Avaxim 80U (n = 80). All participants were seropositive. These results suggest that Avaxim 80U may be used to complete a vaccination series started with the alternate childhood HAV vaccines [Citation41,Citation48].
3.5.2.4. Concomitant vaccine administration
Concomitant administration of the pediatric HAV vaccine with other childhood vaccines was assessed in three studies; one identified from the literature [Citation44] and two were part of the pooled/integrated analysis include above (HAF65 [Citation34] and HAF29). The randomized study in predominantly seropositive Chilean infants by Lagos et al. included a group (Group 2) that received the pediatric HAV vaccine concomitantly with the third dose of routine DTwP//PRP∼T vaccine and OPV versus or 2 weeks later, with second dose 6 months later concomitantly with the MMR vaccine [Citation44] (see section 3.5.2.1). Those infants who received concomitant administration of the pediatric HAV vaccine with DTwP//PRP∼T vaccine, and OPV versus had similar post-dose 1 and post-dose 2 anti-HAV antibody GMCs (278 mIU/ml and 1886 mIU/ml, respectively) to those who received pediatric HAV vaccine 2 weeks later (292 mIU/ml and 1731 mIU/ml, respectively). Interference with the response of the routine vaccines was not assessed. The HAF65 randomized clinical study of 12–13 months old healthy hepatitis A seronegative Turkish children also included a group that received the pediatric HAV vaccine concomitantly with the MMR vaccine compared with two groups that received the two vaccines 4 weeks apart (either the pediatric HAV vaccine or MMR vaccine first) [Citation34]. All participants received a second dose of pediatric HAV vaccine 6 months later. Non-inferiority of concomitant administration with MMR compared to separate administration of both vaccines was demonstrated for seroprotection (≥20 mIU/mL) against hepatitis A at 4 weeks post-dose 1 (100% [95% CI 97.84–100.0] vs 99.39% [95% CI 96.63–99.98]) as determined with the electrochemiluminescence immunoassay (ECLIA) assay, but not with the less sensitive microplate enzyme immunoassay (MEIA) which showed much lower seroprotection rates than expected and did not fit with seroprotection hypothesis taken for sample size calculation. All were seroprotected after the second pediatric HAV vaccine dose with the anamnestic response 4 weeks after the second dose boosting anti-HAV GMCs to 5078 and 3271 mIU/mL in the two groups that received the vaccines separately compared to 4314 mIU/mL in the group that received HAV vaccine concomitantly with the MMR vaccine. Measles (≥120 mIU/mL), mumps (≥10 AU/mL) and rubella (≥10 IU/mL) seroprotection rates were ≥96.5% with both separate and concomitant vaccination. Similarly, the HAF29 randomized clinical study of predominantly seronegative 16–19 months old Pilipino and Vietnamese children also included two groups that received the pediatric HAV vaccine concomitantly with the DTwP//PRP∼T and OPV (group A), or DTacP-IPV//PRP∼T (group B) vaccine compared the HAV vaccine alone (group C). All participants received a second dose of pediatric HAV vaccine 6 months later. The anti-HAV seroprotection rates 4 weeks (≥20 mIU/mL) after the first dose in participants who received the HAV vaccine concomitantly with DTwP//PRP∼T and OPV (99.2% [95% CI, 95.5–100]) or DTacP-IPV//PRP∼T (100% [95% CI 97–100%]) were non-inferior (equivalent) to those obtained in participants who received the pediatric HAV vaccine alone (99.7% [95% CI 98.5–100]). All participants were seroprotected after the second pediatric HAV vaccine dose. Similar anamnestic responses after the second HAV dose were observed across the groups with GMCs 4 weeks after the second dose of 6256, 6506 and 7327 mIU/mL in Groups A, B, and C, respectively. Diphtheria (≥0.01 IU/mL), tetanus (≥0.01 IU/mL), polio (titers ≥5) and polyribosyl ribitol phosphate conjugated to tetanus protein (≥0.15 pg/mL) seroprotection rates were ≥96.7% with concomitant vaccination. For pertussis, at least a four-fold increase in anti-pertussis and anti-filamentous haemagglutinin antigen titers were observed in 82.3–88.7% of participants.
3.5.2.5. Long-term immunogenicity responses
Long term immunogenicity data (>1 year) were presented in five reports [Citation39,Citation40,Citation43,Citation46,Citation47]. Three reports, one by Dagan et al. [Citation43] and two by Lopez et al. [Citation46,Citation47], were a long term follow up of HAF11 [Citation31] and HAF20 [Citation32], respectively, included in section 3.2 and 3.5.1 above. The other two long-term follow-up reports by Espul et al. [Citation39,Citation40] were of the HAF82 study a long-term follow up of Argentinian children after one or two doses of the pediatric HAV vaccine. summarizes the observed anti-HAV GMCs post-dose 2 through long-term follow up for up to 14–15 years across the studies identified [Citation39,Citation40,Citation43,Citation46,Citation47]. Espul et al. also reported long-term follow-up data after only a single dose of the HAV vaccine [Citation39,Citation40]; in general, GMCs after one dose were several-fold lower than those achieved following two doses of the HAV vaccine, but nonetheless seropositivity rates remained high ranging from 98.6% to 100% through to seven years follow-up. After two doses of the vaccine, GMCs remained well above the 10 mIU/mL or 20 mIU/mL seropositivity limit throughout follow-up and most participants remained seropositive.
Table 9. Observed long-term immunogenicity responses after a two-dose schedule.
In the 10 years follow-up study of Argentinian children by Lopez et al. [Citation46], one participant became seronegative (<20 mIU/mL) at year 10 and was revaccinated; the participant achieved a concentration of 1400 mIU/mL, higher than after the first booster dose (1200 mIU/mL at week 27). Moreover, they found no significant difference between anti-HAV concentrations in children who had known close contacts with acute HAV infection (445.6 [±464.4 mIU/mL, range: 108–1860 mIU/mL]) and those not exposed during the 10 years (359.9 [±309.2 mIU/mL, range: 36–1460 mIU/mL]) [Citation46]. Overall, three distinct periods of antibody changes were observed by Lopez et al. through to 14–15 years follow-up: rapid rise from baseline up to peak concentration post-dose 2, (GMC 5920 mIU/mL, 95% CI 4758–7364, among those seronegative at enrollment); relatively rapid decay post-peak to 10 years follow-up (to GMC 261 mIU/mL, 95% CI 199–341), followed by slower decay between the 10-year follow-up and 14–15-year follow-up periods (to GMC 253 mIU/mL, 95% CI 181–353). Modeling analysis extrapolating long-term data up to 14–15 years [Citation47], predicted that 88% of seronegative participants before vaccination would remain seroprotected at 30 years post-dose 2, and seroprotection would be lifelong for those seropositive prior to vaccination [Citation47].
3.5.2.6. Efficacy/effectiveness
Fisenka et al. conducted an analysis of HAV epidemiology in Minsk following the introduction of routine mass vaccination of children aged 6 years against hepatitis A [Citation50]. Since 1950, Minsk has had several periods of very high hepatitis A incidence (up to 48 per 10,000 inhabitants) which decreased to historic lows by the mid-1990s. Since 2000, there was already a gradual annual decrease in the incidence of the disease before the introduction of routine mass vaccination of 6-year-old children in 2003. The vast majority (95%) received Avaxim 80U or Avaxim 160U. Shortly after the introduction of routine mass vaccination, there was a much more rapid decrease in the incidence of hepatitis A and by 2005, no cases were reported in children aged 6–9 years. Overall, there were 12-fold, 13-fold, 7-fold and 4-fold decreases in the incidence of hepatitis A in preschool children, those aged 10–14 years, those aged 15–29 years and adults aged ≥30 years, respectively. Of note, a shift in the age pattern disease burden was observed with the proportion of cases in children under 14 years decreasing from 33–41% in 2000–2002 to 7% in 2005–2006.
4. Discussion
This review of the immunogenicity and safety of Sanofi Pasteur’s pediatric HAV vaccine using data from the clinical development program, pharmacovigilance, and a systematic review of the literature, confirms that the pediatric HAV vaccine is immunogenic and has a good safety profile. Anti-HAV antibody levels ≥20 mIU/mL considered sufficient for seroprotection were achieved in >98% of participants after the first dose in most studies in the pediatric HAV vaccine clinical development program and 100% after the second dose (with exception of one individual in the HAF78 study [Citation35]). No safety signal has been identified with the pediatric HAV vaccine through pharmacovigilance since the product was first introduced on the market. The overall spontaneous reporting of adverse events in the 16 years to 2017 submitted to Sanofi Pasteur was 1.4 cases per 100,000 doses distributed, or between 1.4 and 2.8 cases per 100,000 children exposed. This is similar to the spontaneous reporting rate of adverse events to the Vaccine Adverse Event Reporting System (VAERS) in the USA for the influenza vaccine, estimated at 3 per 100,000 vaccine doses distributed, and much lower than for DTwP (26.2 per 100,000 doses distributed) [Citation53].
Although a number of studies used different assays to determine anti-HAV concentrations, which makes comparisons difficult, the immunogenicity data from the systematic review was consistent with the observed pooled and integrated summary obtained from the pediatric HAV vaccine clinical development program. There is a clear anamnestic response following the second vaccine dose, with antibody levels persisting several-fold higher than those observed at baseline for up to 14–15 years. The three distinct periods of anti-hepatitis A antibody changes observed by Lopez et al. with the pediatric HAV vaccine in children through to 14–15 years follow-up [Citation47] are consistent with those observed after two doses of Havrix in HAV-naïve adults, but the decay post-peak level appeared to occur much faster (within 3–4 years), before slowing or stabilizing [Citation54]. Modeling analysis predicted that seropositive anti-HAV antibodies would persist in ≥95% vaccinated adults after 30 years and ≥90% at 40 years. A modeling analysis predicted that 88% HAV-naïve children will remain seropositive after two doses of the pediatric HAV vaccine at 30 years, and seroprotection would be lifelong for those seropositive prior to vaccination [Citation47]. In general, inactivated HAV vaccines are considered to provide long-lasting or life-long immunity, probably even after single primary dose [Citation55].
In endemic settings, a single HAV vaccine dose induces HAV-specific cellular memory immune responses similar to natural infection that are likely sufficient to ensure protection and natural boosting when exposed to HAV, independently of the circulating antibody levels achieved [Citation56,Citation57]. Indeed, a single dose universal HAV vaccination program for children has been introduced in some countries in Latin America [Citation57]. In Argentina, different vaccines have been used by the government since the program began in 2005, including the pediatric HAV vaccine (Avaxim®80U Pediatric). By 2006, overall vaccine coverage in the country was ≥95% for the single dose in children aged 12 months [Citation58,Citation59]. Hepatitis A disease rates including fulminant hepatic failure sharply declined in the years following the initiation of the universal HAV vaccination program, confirming the positive impact of a single dose childhood HAV vaccine.
The pediatric HAV vaccine may also be used to complete a vaccination series started with the other alternate childhood HAV vaccines. No differences in seropositivity rates observed following two doses of the same vaccine compared to the use of the pediatric HAV vaccine as the second dose in two studies [Citation41,Citation48]. However, more rapid anti-HAV antibody GMCs increase after the first dose have been observed with pediatric HAV vaccine compared than with other childhood HAV vaccines, which may be relevant when rapid immunization is required such as in outbreak control and for last minute travelers [Citation60]. Moreover, the rates of local and systemic solicited reactions do not appear to differ across pediatric HAV vaccines after either the first or the second vaccine injection. The current consensus is that inactivated childhood HAV vaccines produced by different manufacturers, including combined hepatitis A vaccines, are interchangeable [Citation5].
5. Expert opinion
The WHO recommends that vaccination against HAV be integrated into the national immunization schedule for children aged ≥1 year if indicated on the basis of incidence of acute hepatitis A, when the endemicity changes from high to intermediate, and with consideration of cost-effectiveness [Citation5]. Vaccination against hepatitis A should be part of a comprehensive plan for the prevention and control of viral hepatitis, including measures to improve hygiene and sanitation and measures for outbreak control. Countries should collect and review the information needed to estimate their national burden of hepatitis A. In highly endemic countries almost all persons are infected with HAV in childhood, which effectively prevents clinical hepatitis A in adolescents and adults. In these countries, large-scale vaccination programs are not recommended [Citation5]. In transitional countries (countries transitioning from high to intermediate endemicity) vaccination programs need to be accompanied with improvements in overall sanitation levels and hygiene, supplemented with health education. In addition, measures for outbreak control such as the use of single HAV vaccines for post-exposure prophylaxis should also be in place [Citation5]. Individuals traveling from low endemic countries to areas of intermediate or high endemicity represent another population at increased risk of hepatitis A and should be considered for pre-exposure vaccination [Citation5].
Until further experience has been obtained with a single-dose schedule, in individuals at substantial risk of contracting hepatitis A, and in immunocompromised individuals, a two-dose schedule is preferred for inactivated HAV vaccines [Citation5]. Moreover, the WHO states that all inactivated HAV vaccines, including combined hepatitis A vaccines, can be used interchangeably [Citation5]. This is supported by evidence from studies which demonstrate that the pediatric HAV vaccine to be interchangeable with other inactivated childhood HAV vaccines and may be used to complete vaccine schedules started with other childhood HAV vaccines [Citation41,Citation48].
There is currently no absolute protective level of HAV antibodies defined, although for seroprotection after vaccination anti-HAV antibody concentrations of 10 to 20 mIU/mL are generally accepted. The duration of protection post-vaccination has also not been fully defined; ongoing studies will provide greater insight with longer-term follow-up data due to become available in the next five years. Only two cases of vaccine failure have been reported from clinical practice and recorded in the Sanofi Pasteur pharmacovigilance system. Overall, the collective safety and immunogenicity data presented in this current report suggest that the pediatric HAV vaccine is valuable option in the prevention of HAV infection in children in many areas of the world where the disease remains a healthcare issue.
6. Five-year view
The HAF82 study is a long-term follow-up of Argentinian children after one or two doses of the pediatric HAV vaccine with data already available up to 7 years post-vaccination. In 2018, the 10-year follow-up data are expected, and in 2023 the 15-year follow-up data. These data will help better define the immunogenicity following one and two doses of the pediatric HAV vaccine over the longer term. In particular, the study will allow the modeling of the long-term persistence of anti-HAV antibody concentrations after a single dose of the pediatric HAV vaccine using observed data through to 10 years to help predict individuals’ anti-HAV levels and long-term seroprotection rates. In addition, the study may provide an opportunity to further evaluate the anamnestic response in participants who become seronegative over time; few participants, in general, become seronegative irrespective of the HAV vaccine used and as such an analysis in this group is usually limited by sample size.
Some countries, such as Canada, now recommend vaccination of infants as young as 6 months of age at increased risk of infection or severe hepatitis A [Citation61]. In the USA, the Advisory Committee on Immunization Practices (ACIP) has recently voted unanimously to recommend HAV vaccines to be administered to infants aged 6–11 months traveling outside the USA when protection against hepatitis A is recommended [Citation62], and in persons experiencing homelessness [Citation63]. For children aged 6–11 months, although the presence of maternal antibodies may limit vaccine response after the first dose [Citation64], similar seroprotection rates to older age children were observed after the first dose with HAV vaccines, and with booster effect suggesting that infants are primed with the initial dose despite maternal antibody interference [Citation44,Citation45,Citation64,Citation65]. The data regarding the long-term persistence of seroprotection appear conflicting, with one study suggesting that seroprotection persists for at least 10 years regardless of maternal anti-HAV status [Citation66], and other suggesting that seroprotection was less frequent through to age 15–16 years among those starting vaccination at age 6 months and those vaccinated at age 12 months or 15 months with anti-HAV positive mothers [Citation67]. Additional studies may be required to fully resolve these conflicting observations. Nonetheless, the pediatric HAV vaccines appear well tolerated and does not interfere with the immune response to other routine vaccination in this population of young infants [Citation44,Citation64,Citation65].
Article highlights
Hepatitis A virus (HAV) infection remains an important public health problem in both high-endemic countries where exposure usually occurs before the age 5 years and low-endemic countries that experience sporadic outbreaks, resulting in greater than 100 million infections annually.
Improved sanitation, food safety, and vaccination are the most effective ways to prevent HAV infection.
A number of HAV vaccine formulations are available from several manufacturers, including Sanofi Pasteur’s lower dose pediatric HAV vaccine developed for use in children aged 12 months to 15 years inclusive.
No critical safety issues were identified among either pharmacovigilance data collected and analyzed from Sanofi Pasteur-sponsored prospective clinical development program conducted with the lower-dose pediatric HAV vaccine or from post-marketing surveillance.
Integrated analysis and review of immunogenicity data indicated that seroprotective anti-HAV antibody levels were achieved in >98% of participants after the first dose of pediatric HAV vaccine and 100% of participants after the second dose.
HAV vaccines are considered to provide long-lasting immunity, probably even after a single primary dose.
Declaration of interest
C Bravo, L Mege, C Vigne and Y Thollot are employees of Sanofi Pasteur. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they have been a speaker receiving fees from Sanofi. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download Zip (128.9 KB)Acknowledgments
Editorial assistance with the preparation of the manuscript was provided by Rebecca Hornby and Richard Glover, inScience Communications, Springer Healthcare, Chester, UK. Funding for this assistance was provided by Sanofi Pasteur.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- AVAXIM® Hepatitis A vaccine (inactivated, adsorbed). New Zealand data sheet. 2012 [cited 2018 Sep 18]. Available from: http://products.sanofi.com.au/vaccines/AVAXIM_NZ_PI.pdf
- Hadler SC, McFarland L. Hepatitis in day care centers: epidemiology and prevention. Rev Infect Dis. 1986;8:548–557.
- WHO. World Health Organization. Technical considerations and case definitions to improve surveillance for viral hepatitis: technical report. 2016 [cited 2018 Sep 18]. Available from: http://apps.who.int/iris/bitstream/10665/204501/1/9789241549547_eng.pdf
- Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine. 2010;28:6653–6657.
- World Health Organization. WHO position paper on hepatitis A vaccines - June 2012. Wkly Epidemiol Rec. 2012;87:261–276.
- Poovorawan Y, Chatchatee P, Chongsrisawat V. Epidemiology and prophylaxis of viral hepatitis: a global perspective. J Gastroenterol Hepatol. 2002;17 Suppl:S155–S166.
- Gossner CM, Severi E. Three simultaneous, food-borne, multi-country outbreaks of hepatitis A virus infection reported in EPIS-FWD in 2013: what does it mean for the European Union? Euro Surveill. 2014;19:20941.
- Averhoff FM, Khudyakov Y, Nelson NP. Hepatitis A vaccines. In: Plotkin SA, Orenstein WA, Offit PA, et al., editors. Vaccines. 7th ed. Philadelphia (PA): Elsevier; 2018. p. 319–341.
- Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the global burden of disease study 2013. Lancet. 2016;388:1081–1088.
- Suwantika AA, Yegenoglu S, Riewpaiboon A, et al. Economic evaluations of hepatitis A vaccination in middle-income countries. Expert Rev Vaccines. 2013;12:1479–1494.
- Dhankhar P, Nwankwo C, Pillsbury M, et al. Public health impact and cost-effectiveness of hepatitis A vaccination in the United States: a disease transmission dynamic modeling approach. Value Health. 2015;18:358–367.
- World Health Organization. Hepatitis A - fact sheet. 2017 [cited 2018 Sep 18]. Available from: http://www.who.int/mediacentre/factsheets/fs328/en/
- Jacobsen KH. The global prevalence of hepatitis A virus infection and susceptibility: a systematic review. Geneva: WHO; 2009 [cited 2018 Sep 18]. Available from: http://apps.who.int/iris/bitstream/10665/70180/1/WHO_IVB_1001_eng.pdf
- Franco E, Meleleo C, Serino L, et al. Hepatitis A: epidemiology and prevention in developing countries. World J Hepatol. 2012;4:68–73.
- Wright R. Viral hepatitis comparative epidemiology. Br Med Bull. 1990;46:548–558.
- Chen NY, Liu ZH, Shie SS, et al. Clinical characteristics of acute hepatitis A outbreak in Taiwan, 2015–2016: observations from a tertiary medical center. BMC Infect Dis. 2017;17:441.
- Pham B, Duval B, De Serres G, et al. Seroprevalence of hepatitis A infection in a low endemicity country: a systematic review. BMC Infect Dis. 2005;5:56.
- Foster M, Ramachandran S, Myatt K, et al. Hepatitis A virus outbreaks associated with drug use and homelessness - California, Kentucky, Michigan, and Utah, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:1208–1210.
- Centers for Disease Control and Prevention. Frequently asked questions: hepatitis A outbreaks among people who use drugs and/or people who are homeless in multiple states. 2018 [cited 2019 Jan 21]. Available from: https://www.cdc.gov/hepatitis/outbreaks/FAQs-HepAOutbreaks.html
- Wu D, Guo CY. Epidemiology and prevention of hepatitis A in travelers. J Travel Med. 2013;20:394–399.
- Matheny SC, Kingery JE. Hepatitis A. Am Fam Physician. 2012;86:1027–1034.
- Plotkin SA, Orenstein WA, Offit PA. Vaccines. 7th ed. 6th ed. Philadelphia (PA): WB Saunders Company; 2012.
- World Health Organization. The immunological basis for immunization series: module 18: hepatitis A. World Health Organization, Department of Immunization, Vaccines and Biologicals; 2011 [cited 2018 Sep 18]. Available from: http://apps.who.int/iris/bitstream/10665/44570/1/9789241501422_eng.pdf
- Iwarson S, Lindh M, Widerstrom L. Excellent booster response 4 to 8 years after a single primary dose of an inactivated hepatitis A vaccine. J Travel Med. 2004;11:120–121.
- Hatz C, van der Ploeg R, Beck BR, et al. Successful memory response following a booster dose with a virosome-formulated hepatitis a vaccine delayed up to 11 years. Clin Vaccine Immunol. 2011;18:885–887.
- Irving GJ, Holden J, Yang R, et al. Hepatitis A immunisation in persons not previously exposed to hepatitis A. Cochrane Database Syst Rev. 2012;7:CD009051.
- Van Damme P, Banatvala J, Fay O, et al. Hepatitis A booster vaccination: is there a need? Lancet. 2003;362:1065–1071.
- Baker CJ. Another success for hepatitis A vaccine. N Engl J Med. 2007;357:1757–1759.
- Flehmig B, Frank H, Frosner GG, et al. Hepatitis A-virus particles in stools of patients from a natural hepatitis outbreak in Germany. Med Microbiol Immunol. 1977;163:209–214.
- Sanofi Pasteur. Product monograph including patient information - Avaxim® - Pediatric hepatitis A vaccine inactivated. 2015 [cited 2018 Sep 18]. Available from: https://www.vaccineshoppecanada.com/documentcfm?file=avaxim_ped_e.pdf
- Dagan R, Greenberg D, Goldenbertg-Gehtman P, et al. Safety and immunogenicity of a new formulation of an inactivated hepatitis A vaccine. Vaccine. 1999;17:1919–1925.
- Lopez EL, Del Carmen Xifro M, Torrado LE, et al. Safety and immunogenicity of a pediatric formulation of inactivated hepatitis A vaccine in Argentinean children. Pediatr Infect Dis J. 2001;20:48–52.
- Lolekha S, Pratuangtham S, Punpanich W, et al. Immunogenicity and safety of two doses of a paediatric hepatitis A vaccine in thai children: comparison of three vaccination schedules. J Trop Pediatr. 2003;49:333–339.
- Yurdakök K, Bakir M, Ince T, et al. Immunogenicity and safety of an inactivated hepatitis A vaccine given with measles-mumps-rubella vaccine to 12–13 month old Turkish children. J Vaccines Vaccin. 2012;3:1000146.
- Li RC, Li Y, Yi N, et al. An open, prospective, randomized study comparing the immunogenicity and safety of two inactivated hepatitis A pediatric vaccines in toddlers, children and adolescents in China. Pediatr Infect Dis J. 2013;32:e77–e81.
- Crevat D, Brion JD, Gailhardou S, et al. First experience of concomitant vaccination against dengue and MMR in toddlers. Pediatr Infect Dis J. 2015;34:884–892.
- Chokephaibulkit K, Sirivichayakul C, Thisyakorn U, et al. Safety and immunogenicity of a single administration of live-attenuated Japanese encephalitis vaccine in previously primed 2-to 5-year-olds and Naive 12-to 24-month-olds multicenter randomized controlled trial. Pediatr Infect Dis J. 2010;29:1111–1117.
- Feroldi E, Pancharoen C, Kosalaraksa P, et al. Single-dose, live-attenuated Japanese encephalitis vaccine in children aged 12–18 months: randomized, controlled phase 3 immunogenicity and safety trial. Hum Vaccin Immunother. 2012;8:929–937.
- Espu C, Benedetti L, Cuello H, et al. Persistence of immunity from 1 year of age after one or two doses of hepatitis A vaccine given to children in Argentina. Hepat Med. 2012;4:53–60.
- Espul C, Benedetti L, Linares M, et al. Five-year follow-up of immune response after one or two doses of inactivated hepatitis A vaccine given at 1 year of age in the Mendoza Province of Argentina. J Viral Hepat. 2015;22:453–458.
- Abarca K, Ibanez I, Perret C, et al. Immunogenicity, safety, and interchangeability of two inactivated hepatitis A vaccines in Chilean children. Int J Infect Dis. 2008;12:270–277.
- Celebi S, Hacimustafaoglu MK, Albayrak Y, et al. Assessment of immune responses to hepatitis A vaccination in children aged 1 and 2 years. Turk J Med Sci. 2013;43:617–624.
- Dagan R, Greenberg D, Weber F. Immunogenicity of an inactivated hepatitis A pediatric vaccine: three-year post-booster follow-up. Vaccine. 2005;23:5144–5148.
- Lagos R, Munoz A, Dumas R, et al. Immunological priming of one dose of inactivated hepatitis A vaccine given during the first year of life in presence of maternal antibodies. Vaccine. 2003;21:3730–3733.
- Lopez EL, Contrini MM, Xifro MC, et al. Hepatitis A vaccination of Argentinean infants: comparison of two vaccination schedules. Vaccine. 2007;25:102–108.
- Lopez EL, Contrini MM, Mistchenko A, et al. Long-term immunity after two doses of inactivated hepatitis A vaccine, in Argentinean children. Pediatr Infect Dis J. 2010;29:568–570.
- Lopez EL, Contrini MM, Mistchenko A, et al. Modeling the long-term persistence of hepatitis A antibody after a two-dose vaccination schedule in Argentinean children. Pediatr Infect Dis J. 2015;34:417–425.
- Soysal A, Gokce I, Pehlivan T, et al. Interchangeability of a hepatitis A vaccine second dose: Avaxim 80 following a first dose of Vaqta 25 or Havrix 720 in children in Turkey. Eur J Pediatr. 2007;166:533–539.
- Yoon SH, Kim HW, Ahn JG, et al. Reappraisal of the immunogenicity and safety of three hepatitis A vaccines in adolescents. J Korean Med Sci. 2016;31:73–79.
- Fisenka EG, Germanovich FA, Glinskaya IN, et al. Effectiveness of universal hepatitis A immunization of children in Minsk City, Belarus: four-year follow-up. J Viral Hepat. 2008;15:57–61.
- Zuckerman J, Peyron F, Wallon M. Comparison of the safety and immunogenicity of two inactivated hepatitis A. Adv Ther. 1997;14:116–124.
- Espul C, Benedetti L, Linares M, et al. Seven-year follow-up of the immune response after one or 2 doses of inactivated hepatitis A vaccine given at 1 year of age in the Mendoza Province of Argentina. Hum Vaccin Immunother. 2017;13:2707–2712.
- Zhou W, Pool V, Iskander JK, et al. Surveillance for safety after immunization: vaccine adverse event reporting system (VAERS)–United States, 1991–2001. MMWR Surveill Summ. 2003;52:1–24.
- Theeten H, Van Herck K, Van Der Meeren O, et al. Long-term antibody persistence after vaccination with a 2-dose Havrix (inactivated hepatitis A vaccine): 20 years of observed data, and long-term model-based predictions. Vaccine. 2015;33:5723–5727.
- Vidor E, Dumas R, Porteret V, et al. Aventis pasteur vaccines containing inactivated hepatitis A virus: a compilation of immunogenicity data. Eur J Clin Microbiol Infect Dis. 2004;23:300–309.
- Mayorga O, Buhler S, Jaeger VK, et al. Single-dose hepatitis A immunization: 7.5-year observational pilot study in Nicaraguan children to assess protective effectiveness and humoral immune memory response. J Infect Dis. 2016;214:1498–1506.
- Melgaco JG, Morgado LN, Santiago MA, et al. A single dose of inactivated hepatitis A vaccine promotes HAV-specific memory cellular response similar to that induced by a natural infection. Vaccine. 2015;33:3813–3820.
- Cervio G, Trentadue J, D’Agostino D, et al. Decline in HAV-associated fulminant hepatic failure and liver transplant in children in Argentina after the introduction of a universal hepatitis A vaccination program. Hepat Med. 2011;3:99–106.
- Vacchino MN. Incidence of Hepatitis A in Argentina after vaccination. J Viral Hepat. 2008;15 Suppl 2:47–50.
- Connor BA. Hepatitis A vaccine in the last-minute traveler. Am J Med. 2005;118 Suppl 10A:58S–62S.
- National Advisory Committee on Immunization (NACI). Update on the recommended use of hepatitis A vaccine. 2016 [cited 2018 Jul 13]. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/update-recommended-use-hepatitis-vaccine.html
- National Foundation for Infectious Diseases. Meeting archive: updates from February 2018 ACIP meeting. [cited 2018 Jul 13]. Available from: https://cc.readytalk.com/cc/s/meetingArchive?eventId=y8begst0letz
- Centers for Disease Control and Prevention. Advisory committee on immunization practices (ACIP): ACIP recommendations. 2018 [cited 2019 Jan 21]. Available from: https://www.cdc.gov/vaccines/acip/recommendations.html
- Bell BP, Negus S, Fiore AE, et al. Immunogenicity of an inactivated hepatitis A vaccine in infants and young children. Pediatr Infect Dis J. 2007;26:116–122.
- Stojanov S, Liese JG, Belohradsky BH, et al. Administration of hepatitis A vaccine at 6 and 12 months of age concomitantly with hexavalent (DTaP-IPV-PRP approximately T-HBs) combination vaccine. Vaccine. 2007;25:7549–7558.
- Sharapov UM, Bulkow LR, Negus SE, et al. Persistence of hepatitis A vaccine induced seropositivity in infants and young children by maternal antibody status: 10-year follow-up. Hepatology. 2012;56:516–522.
- Spradling PR, Bulkow LR, Negus SE, et al. Persistence of seropositivity among persons vaccinated for hepatitis A during infancy by maternal antibody status: 15-year follow-up. Hepatology. 2016;63:703–711.