ABSTRACT
Introduction: In Asia Pacific, most countries recommend a monovalent hepatitis B virus (HBV) vaccine dose at birth followed by primary vaccination series including three or four doses of combination vaccines against diphtheria, tetanus, and pertussis, with or without Haemophilus influenzae type b (Hib), HBV or poliomyelitis antigens. If hexavalent conjugate vaccines against diphtheria-tetanus-acellular pertussis-HBV-inactivated poliovirus-Hib (DTPa-HBV-IPV/Hib) replace the vaccines included in the primary vaccination series, co-administration of lower-valent vaccines would be avoided but infants would receive ≥4 doses of HBV-containing vaccines before the age of 2 years.
Areas covered: We searched for clinical trials conducted in the South-East Asia and Western Pacific Regions (World Health Organization geographic definition), investigating vaccination regimens with >3 doses of HBV-containing vaccines in infants, including a monovalent HBV vaccine birth dose and ≥1 dose of GSK’s hexavalent DTPa-HBV-IPV/Hib vaccine.
Expert opinion: The six clinical trials included in this review showed that infants who received the monovalent HBV vaccine at birth and three or four doses of DTPa-HBV-IPV/Hib vaccine achieved protective immunogenic titers with a clinically acceptable safety profile. Our results support the integration of hexavalent DTPa-HBV-IPV/Hib vaccine within existing national recommendations in the Asia Pacific region to reduce the number of injections during infancy.
1. Introduction
An increasing number of vaccines against common childhood infectious diseases are becoming available, and their inclusion in universal vaccination programs has significantly contributed to the decline in childhood mortality [Citation1]. To simplify the complex immunization schedules during infancy, pediatric combination vaccines, allowing simultaneous vaccination against several diseases with a single injection, have been developed [Citation2]. Several studies have shown that combination vaccines are effective in increasing vaccination rates as well as timeliness [Citation3–Citation6]. Moreover, their use reduces direct and indirect costs associated with separate vaccine administration and decreases the stress on parents and children caused by multiple injections [Citation7–Citation9]. GSK’s hexavalent diphtheria, tetanus, acellular pertussis (DTPa), hepatitis B virus (HBV), inactivated poliomyelitis (IPV) and Haemophilus influenzae type b (Hib) conjugate vaccine (DTPa-HBV-IPV/Hib, Infanrix hexa, GSK) is currently licensed in more than 90 countries worldwide, and is administered to infants and toddlers according to 3 + 1, 3 + 0 or 2 + 1 primary and booster vaccination schedules [Citation10,Citation11]. DTPa-HBV-IPV/Hib vaccine has been shown to be highly immunogenic and to have comparable safety and immunogenicity profiles when compared to those of DTPa-based pentavalent vaccines co-administered with monovalent HBV or Hib vaccines [Citation6,Citation10,Citation12–Citation14].
HBV infection remains an important health concern in Asia Pacific, with an estimated 100 million people living with chronic HBV disease in the South-East Asia Region (SEAR) in 2015 [Citation15] and 115 million in the West Pacific Region (WPR) in 2016 [Citation16]. In 2005, the prevalence of HBV surface antigen [HBsAg], a marker of HBV infection, among women of childbearing age was approximately 5% in the SEAR, 4% in high-income Asian Pacific countries, and 2.5% in Oceania [Citation17]. Perinatal exposure, either in utero or during delivery, is an important mode of HBV transmission, and approximately 90% of HBV-infected infants will develop chronic infection [Citation18]. Therefore, almost all countries in Asia Pacific recommend a dose of monovalent vaccine against HBV at birth to protect infants born to mothers with HBV infection [Citation15,Citation19]. In this region, the routine infant immunization schedules include three or four doses of vaccines against diphtheria, tetanus, and pertussis, either combined or not with antigens for Hib, HBV and/or poliomyelitis [Citation19]. Higher-valent combination vaccines, like hexavalent DTPa-HBV-IPV/Hib vaccine, could be used in routine infant immunization to simplify the vaccination schedules and increase vaccination coverage [Citation20–Citation22]. Since the number of required antigens differs across scheduled vaccination visits, DTPa-HBV-IPV/Hib vaccine can be used either in mixed schedules incorporating lower-valent combination vaccines or fully replace the primary series [Citation22]. In the latter case, three or four doses of DTPa-HBV-IPV/Hib vaccine can be administered after the birth dose of monovalent vaccine against HBV, resulting in infants receiving 4 to 5 doses of HBV-containing vaccine within the first 2 years of life. According to the World Health Organization (WHO) recommendations, primary vaccination series in infants who received an HBV birth dose should include either two or three additional doses of HBV-containing vaccine, but there is no evidence to support the need for a booster dose of HBV vaccine [Citation17]. Nevertheless, a previous study has shown that higher seroprotection rates were induced by four doses of DTPa-HBV-IPV/Hib vaccine in children who had received HBV at birth compared with children who did not receive the HBV birth dose [Citation23].
The objective of this review was to assess the potential impact of the increased number of HBV-containing vaccine doses administered during infancy on the immune response to all vaccine antigens included in DTPa-HBV-IPV/Hib vaccine and its safety in Asia Pacific. Therefore, we conducted a systematic review of studies, in which at least one group received more than three doses of HBV-containing vaccine due to the integration of DTPa-HBV-IPV/Hib vaccine alongside existing neonate HBV vaccination strategies. We included countries in the SEAR and WPR as per WHO classification in this systematic review to clearly define the geographic remit of Asia Pacific.
2. Literature search
2.1. Search strategy
We performed a systematic literature search using the PubMed database to identify studies published between 1 January 1990 and 19 March 2018 that investigated vaccination regimens with more than three doses of an HBV-containing vaccine, where at least one dose of hexavalent DTPa-HBV-IPV/Hib vaccine was used. Outcomes of interest included the immune responses induced by the different vaccination regimens for all vaccine antigens in terms of seroprotection/seropositivity rates and geometric mean antibody concentrations (GMCs)/titers (GMTs) at 1–2 month(s) after primary and booster vaccination. Safety data were assessed for all studies.
The search strategy used the following Medical Subject Heading (Mesh) terms: ‘Hepatitis B Vaccines/administration and dosage’ OR ‘Hepatitis B Vaccines/adverse effects’ OR ‘Hepatitis B Vaccines/analysis’ OR ‘Hepatitis B Vaccines/immunology’ OR ‘Hepatitis B Vaccines/therapeutic use’ OR ‘Hepatitis B Vaccines/toxicity’, and the following filters: Clinical Study; Clinical Trial; Congresses; Controlled Clinical Trial; Meta-Analysis; Multicenter Study; Observational Study; Randomized Controlled Trial; Research Support; Non-U.S. Gov’t; Systematic Reviews; Abstract, English.
Eligible studies in the initial literature search were conducted in the WHO-defined Eastern Mediterranean, Americas, WPR and SEAR regions. Since the present article focuses on the combination of birth doses of HBV vaccine, it was decided to limit the results to the WPR and SEAR regions where Hepatitis B remains a dominant concern. The countries included in the SEAR and WPR are listed in Supplement 1. Studies were excluded if they were conducted outside of the regions of interest, were not clinical trials, did not report safety or immunogenicity outcomes of interest, did not investigate regimens using more than three doses of an HBV-containing vaccine, did not include hexavalent DTPa-HBV-IPV/Hib vaccine, evaluated monovalent HBV vaccines only, were conducted in special populations (e.g. non-responders), or were booster studies where more than 2 years had elapsed since primary vaccination. Our search was limited to publications written in English.
All retrieved publication abstracts were screened against the pre-specified inclusion and exclusion criteria; for those meeting the inclusion criteria and those for which the eligibility could not be ascertained, the full text was reviewed to assess eligibility. The list of publications that were eligible for full-text analysis was cross-checked by another reviewer. Furthermore, publications were excluded if administration of a birth dose of HBV vaccine was not specified in the Methods, irrespective of the country vaccination recommendations at the time of the study. Then, data were extracted from the selected publications.
2.2. Selection of relevant studies
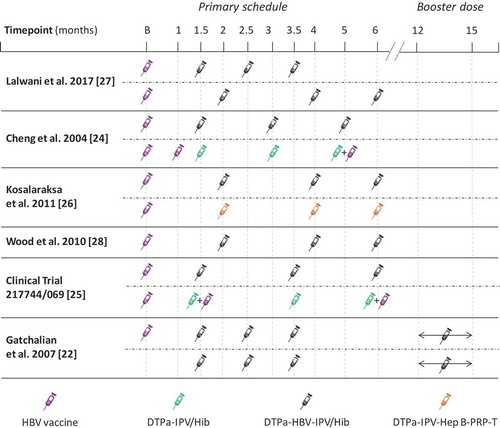
A total of 2 608 publication abstracts were retrieved and screened (). Of these, 2 550 papers were excluded during abstract screening (exclusion criteria are fully listed in Supplement 2) and 44 papers were excluded during full-text review. Besides the 14 publications from the literature search that met the inclusion criteria, two additional publications were added to the list of relevant articles [Citation23,Citation24]. Of these, nine publications presented studies conducted in the WPR and SEAR. Three publications were excluded since they did not include administration of DTPa-HBV-IPV/Hib vaccines. As a result, a total of six publications were included in this review, of which one reported the results of a study conducted in SEAR and five the results of studies conducted in WPR () [Citation23,Citation25–Citation29]. In five studies, the infants received three primary doses of DTPa-HBV-IPV/Hib vaccine according to various routine vaccination schedules () [Citation25–Citation29]. Infants in the last study received three primary doses of DTPa-HBV-IPV/Hib vaccine at 6, 10 and 14 weeks of age followed by a booster dose in the second year of life, according to the Expanded Program for Immunization (EPI) schedule [Citation23].
Table 1. Summary of the characteristics of the six studies analyzed.
Figure 1. PRISMA flow diagram showing publications identified, screened, selected and analyzed from 1 January 1990 to 19 March 2018.
n, number of publications; SEAR, South-East Asia Region; WPR, West Pacific Region; AMR, Region of the Americas; EMR, Eastern Mediterranean Region; WHO, World Health Organization; HBV, hepatitis B virus; DTPa-HBV-IPV/Hib, GSK’s hexavalent diphtheria, tetanus, acellular pertussis, hepatitis B virus, inactivated poliovirus and Haemophilus influenzae type b conjugate vaccine (Infanrix hexa).*Publication by Gatchalian et al. 2007 [Citation23] was known to the authors and also included, but not retrieved during the search due to articles in the Philippine Journal of Pediatrics not being routinely indexed on PubMed.†One further study by Shao et al. 2011 [Citation24] was identified for inclusion. Unpublished data for the group that received an HBV birth dose and a three-dose primary vaccination schedule with DTPa-HBV-IPV/Hib in this study are available online from the GSK clinical study register report and included in this report.**Publications reporting studies that were conducted in regions out of scope of this systematic review.
![Figure 1. PRISMA flow diagram showing publications identified, screened, selected and analyzed from 1 January 1990 to 19 March 2018.n, number of publications; SEAR, South-East Asia Region; WPR, West Pacific Region; AMR, Region of the Americas; EMR, Eastern Mediterranean Region; WHO, World Health Organization; HBV, hepatitis B virus; DTPa-HBV-IPV/Hib, GSK’s hexavalent diphtheria, tetanus, acellular pertussis, hepatitis B virus, inactivated poliovirus and Haemophilus influenzae type b conjugate vaccine (Infanrix hexa).*Publication by Gatchalian et al. 2007 [Citation23] was known to the authors and also included, but not retrieved during the search due to articles in the Philippine Journal of Pediatrics not being routinely indexed on PubMed.†One further study by Shao et al. 2011 [Citation24] was identified for inclusion. Unpublished data for the group that received an HBV birth dose and a three-dose primary vaccination schedule with DTPa-HBV-IPV/Hib in this study are available online from the GSK clinical study register report and included in this report.**Publications reporting studies that were conducted in regions out of scope of this systematic review.](/cms/asset/0ccefdf0-b533-446c-9502-7a298d343866/ierv_a_1646643_f0001_oc.jpg)
Figure 2. Dosing schedules used in analyzed studies from the South-East Asia and West Pacific regions.
B, birth; DTPa, diphtheria-tetanus-acellular pertussis; HBV, hepatitis B virus; Hib, Haemophilus influenzae type b; IPV, inactivated poliovirus vaccine; PRP, polyribosylribitol phosphate from H. influenzae type b; T, tetanus; DTPa-HBV-IPV/Hib, Infanrix hexa (GSK); DTPa-IPV/Hib, Infanrix-IPV/Hib (GSK); DTPa–IPV–Hep B–PRP-T, batch number S4106, Hexaxim (Sanofi Pasteur).
3. Methods to evaluate immunogenicity and safety
3.1. Immunogenicity assessments
Percentages of seroprotected participants were presented for diphtheria (anti-D antibody concentration ≥0.1 International Unit (IU)/mL), tetanus (anti-T antibody concentration ≥0.1 IU/mL), poliovirus types 1, 2 and 3 (anti-poliovirus types 1, 2 and 3 antibody titers ≥1:8), Hib (anti-polyribosylribitol phosphate [PRP] antibody concentration ≥0.15 ug/mL), and HBV (anti-HBV surface antigen [anti-HBs] antibody concentration ≥10 IU/mL).
Seropositivity rates (antibody concentrations ≥5 ELISA units (EU)/ml), vaccine response rates, or seroconversion rates were presented for antibodies against pertussis toxin (PT), filamentous hemagglutinin (FHA) and pertactin (PRN) from pertussis. In the study conducted in Singapore, vaccine response to the three pertussis antigens was defined as the appearance of antibodies in initially seronegative infants, or by the presence of post-vaccination antibody concentrations at least twice as high as the initial pre-vaccination concentrations [Citation25]. In the study conducted in Thailand, seroconversion rates for PT and FHA antigens were pre-defined as a ≥ fourfold increase from baseline in anti-PT and anti-FHA antibody titers [Citation27]. In the study in Australia, vaccine response to the three pertussis antigens was defined as a fourfold increase from the pre-vaccination antibody titers [Citation29]. In the study in Taiwan and India, vaccine response to all pertussis antigens was defined as the appearance of antibodies in initially seronegative infants or post-vaccination antibody concentrations above pre-vaccination concentrations in initially seropositive participants [Citation26,Citation28]. In the Filipino study, a vaccine response to PT, FHA, and PRN was defined as the appearance of antibodies in participants who were initially seronegative in the primary study or a twofold increase in antibody concentrations or titers after the booster dose in participants seropositive before booster [Citation23].
In the studies conducted in Singapore, Australia, and Taiwan, antibodies against HBs were measured by an ELISA assay using an assay cut-off of 10 mIU/ml [Citation25,Citation26,Citation29]. Seroprotection was defined as an anti-HBs antibody concentration ≥10 mIU/ml.
In the studies conducted in India and Thailand, anti-HBs antibody concentrations were measured using a commercial chemiluminescence assay (Indian study: ADIVA CentaurTM Anti-HBs, Siemens Healthcare, Marburg, Germany [cut-off: 6.2 mIU/mL]; Taiwanese study: VITROS anti-HBs assay, Ortho-Clinical Diagnostics, Inc, Rochester, NY USA). Seroprotection was defined as an anti-HBs antibody concentration ≥10 mIU/ml [Citation27,Citation28].
In the Filipino study, antibodies to HBs were determined by radioimmunoassay at GSK Biologicals’ laboratory in Rixensart using an assay cut-off of 10 mIU/mL [Citation23]. Antibody concentrations above the assay cut-off were considered seroprotective.
Antibody GMCs/GMTs were presented for all vaccine antigens with available data.
3.2. Safety evaluation
Reactogenicity results were presented in terms of solicited local events at the injection site and solicited general events for a follow-up period ranging from 4 to 8 days post-vaccination. Unsolicited adverse events (AEs) recorded within 30 days post-vaccination were presented. Serious AEs (SAEs) related or not to vaccination and fatalities were presented for the entire study periods.
4. Results
4.1. Immune response to HBV
Overall, in infants who had received an HBV vaccine dose at birth, seroprotection rates against HBV ranged from 98.5% to 100% (), and anti-HBs antibody GMCs ranged between 277.5 and 3314.5 mIU/mL (), at 1 or 2 months after the three-dose primary vaccination schedule with DTPa-HBV-IPV/Hib vaccine () [Citation23,Citation25–Citation29].
Table 2. Immunogenicity of the different vaccination schedules with respect to hepatitis B virus.
Figure 3. Hepatitis B seroprotection rates post-primary vaccination in the analyzed studies according to vaccination schedule.
HBV seroprotection rate, percentage of study participants with anti-HBs antibodies ≥10 mIU/mL; w, weeks; m, months; HBs, hepatitis B surface antigen; IU, international units. The error bars represent the upper and lower limits of the 95% confidence intervals.
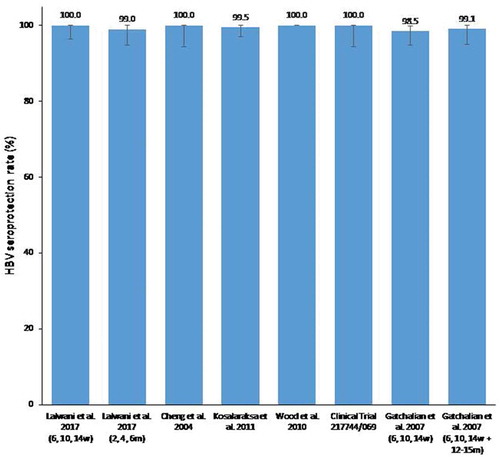
Figure 4. Anti-HBs antibody GMCs post-primary vaccination reported in the analyzed studies according to vaccination schedule.
HBs, hepatitis B surface antigen; GMC, antibody geometric mean concentrations; IU, international units; w, weeks; m, months. The error bars represent the upper and lower limits of the 95% confidence intervals.*No data were available for Cheng et al. 2004 [Citation25].
![Figure 4. Anti-HBs antibody GMCs post-primary vaccination reported in the analyzed studies according to vaccination schedule.HBs, hepatitis B surface antigen; GMC, antibody geometric mean concentrations; IU, international units; w, weeks; m, months. The error bars represent the upper and lower limits of the 95% confidence intervals.*No data were available for Cheng et al. 2004 [Citation25].](/cms/asset/68e4d342-957b-42a5-be5e-7e06ab1b60fa/ierv_a_1646643_f0004_oc.jpg)
In the study conducted in India, HBV seroprotection rates were comparable with both primary vaccination schedules, but anti-HBs antibody GMCs were higher with the less stringent vaccination schedule at 2, 4 and 6 months compared with the vaccination schedule at 6, 10 and 14 weeks () [Citation28]. The study conducted in Singapore showed that all infants who received three primary doses of DTPa-HBV-IPV/Hib vaccine or three primary doses of DTPa-IPV/Hib vaccine and two separate doses of monovalent HBV vaccine were seroprotected against HBV at 1 month post-primary vaccination [Citation25]. In the study in Thailand and Australia, where infants received three primary doses of DTPa-HBV-IPV/Hib vaccine at 2, 4 and 6 months, seroprotection rates were 99.5% and 100%, and anti-HBs antibody GMCs were 2442 and 821.8, respectively [Citation27,Citation29]. In the study conducted in Taiwan, seroprotection rates (100% versus 95.1%) and anti-HBs antibody GMCs (2246.4 versus 928.0) were higher in infants who had received three doses of DTPa-HBV-IPV/Hib vaccine compared to those vaccinated with three doses of DTPa-IPV/Hib vaccine and two separate doses of monovalent HBV vaccine at 1 month post-primary vaccination [Citation26].
In the study in the Philippines, where DTPa-HBV-IPV/Hib vaccine was administered according to the EPI schedule at 6, 10 and 14 weeks of age with a booster dose in the second year of life, HBV seroprotection rates (98.5% versus 77.7%) and anti-HBs antibody GMCs (277.5 versus 54.2) at 1 month post-primary vaccination were higher in infants who received an HBV vaccine dose at birth compared to those who did not receive an HBV vaccine birth dose () [Citation23]. After the booster dose of DTPa-HBV-IPV/Hib vaccine, seroprotective concentrations of anti-HBs antibodies were recorded in 99.1% of participants after the HBV vaccine birth dose, compared with 90% in the group that did not receive an HBV vaccine birth dose. Substantial increase in anti-HBs antibody GMCs was reported following the booster dose administration, with anti-HBs antibody GMCs reaching 2879.9 (95% CI: 1994.1–4159.3) and 387.2 (95% CI: 244.4–613.6) in the children who did or did not receive a birth dose of HBV vaccine, respectively.
4.2. Immune response to diphtheria, tetanus, pertussis, poliovirus types 1, 2 and 3, and hib antigens
In the study conducted in India, 1 month after the third dose of DTPa-HBV-IPV/Hib vaccine administered at either 6, 10 and 14 weeks or 2, 4 and 6 months of age, seroprotection rates for diphtheria, tetanus, poliovirus types 1, 2 and 3, and Hib ranged from 98.6% to 100% () [Citation28]. Of note, children in India received a birth dose of oral poliovirus vaccine (OPV), which was reflected in the percentage of participants who were seroprotected against poliovirus type 1, 2 and 3 before the administration of the first dose of DTPa-HBV-IPV/Hib vaccine (≥68.6%, ≥67.9% and ≥26.1%, respectively). One month post-primary vaccination, all infants were seropositive for anti-PT, -FHA and -PRN antibodies from pertussis. Vaccine responses to the pertussis antigens ranged from 97.0% to 100%.
Table 3. Immunogenicity of the different vaccination schedules with respect to diphtheria, tetanus, pertussis, poliovirus types 1, 2 and 3, and Haemophilus influenzae type b.
In the Singaporean study, 1 month after primary vaccination, 98.4% to 100% of infants who received either DTPa-HBV-IPV/Hib vaccine or DTPa-IPV/Hib vaccine plus monovalent HBV vaccine were seroprotected against diphtheria, tetanus, the three poliovirus types and Hib () [Citation25]. All infants were seropositive for antibodies against, PT, FHA, and PRN from pertussis, with vaccine responses to the three pertussis antigens ranging from 98.4% to 100% in infants who received DTPa-HBV-IPV/Hib vaccine and from 92.2% to 96.9% in infants who received DTPa-IPV/Hib vaccine plus monovalent HBV vaccine.
In the study conducted in Thailand, seroprotection rates for tetanus, poliovirus types 1, 2 and 3, and Hib were high in infants who received DTPa-HBV-IPV/Hib vaccine [Citation27]. However, the percentage of infants with anti-diphtheria antibody concentrations ≥0.1 IU/mL was only 66.3% (95% CI: 59.1–73.0) (). Seroconversion rates were 93.7% for anti-PT antibodies and 95.2% for anti-FHA antibodies at 1 month after the third dose of DTPa-HBV-IPV/Hib vaccine. In this study, PRN was not tested as the investigational vaccine did not contain this pertussis component.
In the Australian study, all participants had protective antibody levels to diphtheria, tetanus, and Hib, and ≥95.0% of participants were seropositive for anti-PT, -FHA and -PRN antibodies at 2 months after completion of the primary schedule () [Citation29]. The antibody response to anti-poliovirus serotypes was not measured.
In the Taiwanese study, 1 month after the third primary dose of DTPa-HBV-IPV/Hib vaccine, all infants had seroprotective antibody concentrations against diphtheria, tetanus, and Hib, and were seropositive for anti-PT, -FHA and -PRN antibodies () [Citation26]. The antibody response to the three poliovirus types could not be determined because the sera showed abnormal toxicity in the micro-neutralization assay.
In the Filipino study, ≥96.8% of infants who did not receive a birth dose of HBV vaccine and ≥94.5% of infants who received a birth dose of HBV vaccine were seroprotected against diphtheria, tetanus, poliovirus serotypes 1, 2 and 3, and Hib at 1 month after the third primary dose of DTPa-HBV-IPV/Hib vaccine () [Citation23]. After the fourth dose of DTPa-HBV-IPV/Hib vaccine administered in the second year of life, these percentages increased to ≥96.8% for infants who did not receive a birth dose of HBV vaccine and ≥99.1% for infants who received a birth dose of HBV vaccine. The percentages of participants with a vaccine response against PT, FHA or PRN ranged from 95.2% to 98.5% and from 94.9% to 98.5% after primary vaccination and from 99.1% to 100% and from 97.9% to 99.0% after the fourth dose of DTPa-HBV-IPV/Hib vaccine in infants/toddlers with and without a history of HBV vaccination at birth.
4.3. Safety
Across all studies and vaccination schedules, vaccines were generally well tolerated [Citation23,Citation25–Citation29]. Overall, reactogenicity, tolerability, and safety profiles were similar in infants receiving DTPa-HBV-IPV/Hib vaccine or DTPa-IPV/Hib vaccine plus monovalent HBV vaccination () [Citation25,Citation26]. In the study from the Philippines, the tolerability and safety of DTPa-HBV-IPV/Hib vaccine were similar in infants who did or did not receive an HBV vaccine dose at birth [Citation23].
Table 4. Safety data recorded in the analyzed studies according to vaccination schedule.
Pain was the most frequently reported solicited local symptom following primary vaccination with DTPa-HBV-IPV/Hib vaccine in the majority of studies, reported by 13.4–80.6% of children during follow-up periods of 4 to 8 days post-vaccination () [Citation23,Citation27,Citation28]. Redness was reported slightly more frequently than pain following primary vaccination with DTPa-HBV-IPV/Hib vaccine in Singapore (17.6% [95% CI: 9.5–28.8] versus 16.2% [95% CI: 8.4–27.1] of children) and Taiwan (73.8% [95% CI: 61.5–84.0] versus 70.8% [95% CI: 58.2–81.4] of children) [Citation25,Citation26]. Pain was also the most frequently reported grade 3 solicited local symptom in all studies, except the ones in Singapore (no grade 3 solicited local symptoms reported) and Taiwan (redness was the most frequently reported grade 3 solicited local symptom) [Citation23,Citation27,Citation28]. Following the fourth dose of DTPa-HBV-IPV/Hib vaccine in toddlers in the Philippines, the most frequently reported grade 3 solicited local symptom was injection site swelling >50 mm, which occurred in four participants with an HBV vaccine dose at birth and in one participant without HBV vaccination at birth [Citation23].
Fever (axillary temperature ≥37.5°C) was the most frequently reported solicited general symptom in infants who received DTPa-HBV-IPV/Hib vaccine either at 6, 10 and 14 weeks (15.3% of infants), or 2, 4 and 6 months (15.2% of infants) of age in India, and at 1.5, 3 and 5 months of age (50.0% of infants) in Singapore () [Citation25,Citation28]. Grade 3 fever was reported in one infant in the study conducted in India and one infant in Singapore. Irritability (77.2% of infants) and crying (74.3% of infants) were the most frequent solicited general symptoms, and fever the most frequent grade 3 solicited general symptom (3.4% of infants), in children who received three-dose primary vaccination with DTPa-HBV-IPV/Hib vaccine co-administered with PCV7 in Thailand [Citation27]. In Taiwan, drowsiness was the most frequent solicited general symptom (84.6% of infants) and irritability the most frequent grade 3 solicited general symptom (7.7% of infants) [Citation26]. In the Philippines, the most frequent general solicited symptom was irritability following primary vaccination, and fever and irritability after the booster dose of DTPa-HBV-IPV/Hib vaccine [Citation23]. There were no significant differences in the incidence of grade 3 general solicited events between participants with or without monovalent HBV vaccination at birth.
In India, during the 31-day follow-up period, at least one unsolicited AE was reported for 35.7% and 22.3% of infants in the 6, 10, 14 weeks and 2, 4, 6 months groups, respectively [Citation28]. Upper respiratory tract infection was the most frequently reported unsolicited AE. None of the participants reported grade 3 unsolicited AEs. In Singapore, unsolicited AEs were reported for 40.8% of infants who received DTPa-IPV/Hib vaccine plus monovalent HBV vaccine, and for 23.5% of infants who received DTPa-HBV-IPV/Hib vaccine during the 30-day follow-up periods after each dose [Citation25]. In Thailand, the frequency of unsolicited AEs was 62.1% in infants who received DTPa-HBV-IPV/Hib vaccine [Citation27]. In Taiwan, 27.1% of infants who received DTPa-HBV-IPV/Hib vaccine reported unsolicited symptoms after each dose in the two groups [Citation26]. Following primary vaccination in the Philippines, unsolicited AEs were observed after 16.3% of doses (evenly spread over infants with and without HBV vaccination at birth); most of them were cough and respiratory system infections [Citation23]. No differences in unsolicited AEs following booster administration were observed between infants with and without HBV vaccination at birth. The percentage of infants with unsolicited AEs assessed by the respective study investigators as causally related to DTPa-HBV-IPV/Hib vaccine ranged from 0% to 11.4% after each dose [Citation23,Citation26–Citation28].
Two SAEs considered probably related to vaccination were observed after the booster dose administration in the Philippines (gastroenteritis/dehydration and vomiting/dehydration) in the group who had received the birth dose. Both SAEs were resolved within 2 weeks and 2 days, respectively, without lasting sequelae () [Citation23]. No SAEs considered causally related to vaccination with DTPa-HBV-IPV/Hib vaccine were reported in the studies in Taiwan [Citation26], India [Citation28], Singapore [Citation25], Australia (association between SAEs and vaccination was not reported in this study) [Citation29] or Thailand [Citation27]. A more detailed overview of SAEs can be found in .
5. Discussion
In this literature review, we present the results of six clinical trials conducted in Asia Pacific (SEAR and WPR) in infants who had received a birth dose of monovalent HBV vaccine. In all studies, three primary doses of hexavalent DTPa-HBV-IPV/Hib vaccine administered during the first 6 months of life according to the local national immunization schedules induced a robust immune response to all vaccine antigens and had a clinically acceptable safety profile [Citation23,Citation25–Citation29]. In one study, a fourth dose of DTPa-HBV-IPV/Hib vaccine administered in the second year of life induced a further increase in antibody levels against all vaccine antigens and was well tolerated, suggesting that up to five doses of HBV-containing vaccine can be administered to infants/toddlers [Citation23].
In the six studies included in this review, the majority of infants (98.5% to 100%) were seroprotected against HBV at 1 month after the third primary dose of DTPa-HBV-IPV/Hib vaccine [Citation23,Citation25–Citation29]. This matches current guidelines made by the Centers for Disease Control and Prevention (CDC) in the United States, the WHO, as well as the Australian health department, recommending the administration of a birth dose of monovalent vaccine against HBV followed by the usual childhood schedule of three doses of either a monovalent vaccine against HBV or a HBV-containing combination vaccine [Citation31–Citation33]. Gatchalian et al. demonstrated that the administration of a fourth dose of DTPa-HBV-IPV/Hib vaccine during the second year of life induced a further increase of the anti-HBs antibody GMCs [Citation23], which may result in long-term antibody persistence against HBV [Citation34].
High immune responses to the other vaccine antigens included in DTPa-HBV-IPV/Hib vaccine were observed in all studies included in this review [Citation23,Citation25–Citation29]. Seroprotection rates against diphtheria, tetanus, and Hib were generally high (≥98.5%) in the six studies included in our review, except against diphtheria in the study in Thailand, for which no interpretation was provided by the authors [Citation23,Citation25–Citation29]. Immune responses to all vaccine antigens induced by DTPa-HBV-IPV/Hib vaccine were high regardless of the applied schedule, which is in line with a previous review [Citation6].
The three primary doses of DTPa-HBV-IPV/Hib vaccine given to children having received an HBV dose at birth in the six studies included in this review and the booster dose given in the second year of life in the study in the Philippines were well tolerated, with most solicited local and general symptoms being mild to moderate in intensity [Citation23,Citation25–Citation29]. Our results also showed that DTPa-HBV-IPV/Hib vaccine was associated with low incidences of unsolicited AEs and very rare SAEs. In one of the studies included in our review, authors concluded that the safety profile of DTPa-HBV-IPV/Hib vaccine was similar in infants with or without the HBV vaccine birth dose [Citation23]. Our results were similar to those observed in other studies using different vaccination schedules with or without an HBV vaccine birth dose [Citation6,Citation35]. The study results match the recommendations from countries in Europe (e.g., Germany, Belgium, the Netherlands), where a 3 + 1 national immunization schedule is recommended in combination with an HBV vaccine birth dose in case of maternal HBV infection [Citation36,Citation37].
The strengths of this review included its systematic nature and the 18-year experience covered by the literature search. The review was the first to evaluate the use of DTPa-HBV-IPV/Hib vaccine in the Asia Pacific region. However, the review has some limitations. Due to the differences among studies in terms of methodology for the reporting of AEs and the evaluation of immunological endpoints (e.g., definition of vaccine response against pertussis, anti-HBs assays used), direct comparisons were not feasible and the results were reported descriptively. Furthermore, only a small number of infants received five doses of HBV-containing vaccine, limiting the comparison with other schedules applied in the SEAR and WPR. Other limitations were the facts that publications not indexed in PubMed, written in another language than English or reporting results of seroprevalence studies may have been missed during the screening phase since the search was only performed in PubMed and was limited to clinical trials written in English. Another limitation was the potential underrepresentation of countries within the geographical area of interest (SEAR and WPR) having a health infrastructure not compatible with the conduct of clinical studies. Moreover, the review did not include data on long-term antibody persistence. However, Behre et al. previously demonstrated persistence of anti-HBs antibodies up to 10 years following administration of three or four doses of HBV-containing vaccines in European infants who did not receive a birth dose of HBV vaccine [Citation38], and a recent study by Schwartz et al. demonstrated persistence up to 14 to 15 years of age in children who received 3 + 1 doses of DTPa-HBV-IPV/Hib vaccine and no birth dose of HBV vaccine in Germany [Citation34]. These studies indicate that anti-HBs antibodies induced by DTPa-HBV-IPV/Hib vaccine may also persist for at least 15 years in the Asia Pacific region.
6. Conclusion
In conclusion, the six studies from the SEAR and WPR regions included in this systematic review showed that in countries where the burden of HBV is high, infants who had received a dose of HBV vaccine at birth can receive up to four doses of hexavalent DTPa-HBV-IPV/Hib vaccine during the first 2 years of life [Citation23,Citation25–Citation29]. The use of combination vaccines has the advantages to reduce the number of injections, to simplify vaccine schedules, and to enhance compliance by parents and health-care professionals, leading to better vaccine coverage and improvement of global health.
7. Expert opinion
The implementation of a universal birth dose of HBV vaccine has been a successful strategy in reducing the Hepatitis B burden in young children. This has been evidenced by the low prevalence of HBsAg in children under 5 years of age worldwide; dropping from 4.3% in the pre-vaccination era to 1.3% in 2015. In contrast, prevalence across all ages after vaccine introduction was only reduced by less than 1% (from 4.3% to 3.5%), indicating that a birth dose of HBV vaccine is a highly effective strategy to lower HBV prevalence across all ages in the long term.
However, there are gaps remaining in HBV vaccine birth dose coverage in several countries in the Asia Pacific region. In the Philippines for instance, up to 38% of births occur outside of a health-care facility and in the absence of a health-care professional. Consequently, these children are not vaccinated within this vital 24-h window which could prevent transmission of HBV from mother to child. Moreover, a lack of awareness in private health-care clinic staff, who are not usually involved in vaccination procedures, further reduces the number of children vaccinated in these countries. Several studies have shown that these scenarios are not unique to the Philippines. In fact, one of the most common reasons for missing the birth dose in many countries was the lack of attendance of a trained health-care professional at birth. Therefore, further efforts in educating health-care professionals in the obstetric field as well as outreach programs and investing in alternative vaccine delivery routes may further increase coverage.
As the burden of HBV is reducing in Asia Pacific, and a universal HBV vaccine birth dose may fail to meet cost-effectiveness expectation in some countries, a generalized testing of pregnant woman for HBV seroprevalence may be an alternative route to plan for and deliver targeted vaccination schedules to newborns at risk. This approach would be similar to non-endemic countries, where currently the HBV vaccine birth dose is only given to newborns at risk.
While an HBV vaccine birth dose is essential to prevent transmission from mother to child as well as infection at a young age, three additional doses of HBV-containing vaccine are essential to develop full protection. The high rates of three-dose DTPa vaccine coverage in WPR (97%) and SEAR (88%) show that a high vaccination coverage is achievable. A hexavalent DTPa-HBV-IPV/Hib vaccine allows the simultaneous vaccination against HBV alongside DTPa immunization. Moreover, it increases the coverage for vaccines with low coverage rates in some regions, such as Hib (28% in WPR).
The combination of universal HBV vaccine birth doses and three doses of hexavalent DTPa-HBV-IPV/Hib may, therefore, be instrumental in reaching the WHO target of less than 1% prevalence of HBsAg in children under the age of 5 years in all endemic countries.
Given the high endemicity of HBV in the Asia Pacific region, it is to be expected that current vaccination strategies will be continued for the foreseeable future.
Additionally, infants are receiving an increasing number of vaccines during early childhood worldwide. The number of injections, as well as the differing schedules for each vaccine, can potentially interfere with the adherence to the recommended vaccination programs.
Recognizing these issues, an increasing number of countries are moving towards recommending combination vaccines which have been shown to improve compliance and reduce complexity of vaccine delivery.
The shift in the WHO recommendation from oral poliovirus vaccine (OPV) to IPV in national programs further supports the introduction of multivalent vaccines including IPV.
While the use of these combination vaccines leads to an increase in HBV vaccine dosages, they are instrumental in ensuring compliance and are therefore expected to ultimately result in better vaccination coverage.
8. Trademarks
Infanrix hexa, Infanrix-IPV/Hib, Engerix-B trademarks are owned by or licensed to the GSK group of companies. Hexaxim is a trademark of Sanofi Pasteur. Prevenar/Prevnar is a trademark of Pfizer, Inc.
Article highlights
In Asia Pacific, the burden of HBV infection is high and hence a birth dose of HBV vaccine is recommended in most countries. Additionally, the routine infant immunization schedules include three or four doses of combination vaccines against diphtheria, tetanus, and pertussis with or without antigens of Hib, HBV, and/or poliomyelitis.
The integration of hexavalent vaccines alongside existing newborn HBV vaccination strategies simplifies the complex immunization schedules but results in infants receiving four to five doses of HBV-containing vaccine within the first 2 years of life.
We selected studies in which at least one group of infants received more than three doses of HBV-containing vaccines. The aim was to assess the immunogenicity and safety of hexavalent DTPa-HBV-IPV/Hib vaccine when administered following a monovalent HBV vaccine birth dose according to existing schedules.
The reviewed studies showed that hexavalent DTPa-HBV-IPV/Hib vaccination regimens resulted in similar immunogenicity and safety compared with pentavalent DTPa-IPV/Hib vaccination regimens.
The results of this review support the use of hexavalent DTPa-HBV-IPV/Hib vaccine in combination with a monovalent HBV vaccine birth dose. This vaccination strategy is likely to improve the compliance with infant vaccination programs in HBV endemic countries.
Declaration of interest
J Dolhain was a full-time employee of the GSK group of companies at the time of the study and is now an employee of Danone Benelux. V Dindore, W Janssen, P Mukherjee, and WY Sohn are full-time employees of the GSK group of companies. W Janssen and P Mukherjee hold shares in the GSK group of companies. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they are an employee of Merck & Co., Inc. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Supplemental Material
Download Zip (20.9 KB)Acknowledgments
Writing support services were provided by Claire Verbelen (Modis c/o GSK). Editing and publication coordinating services were provided by Michaela Conrad (Modis c/o GSK).
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Pollard AJ. Childhood immunisation: what is the future? Arch Dis Child. 2007;92(5):426–433.
- Skibinski DA, Baudner BC, Singh M, et al. Combination vaccines. J Glob Infect Dis. 2011;3(1):63–72.
- Happe LE, Lunacsek OE, Kruzikas DT, et al. Impact of a pentavalent combination vaccine on immunization timeliness in a state medicaid population. Pediatr Infect Dis J. 2009;28(2):98–101.
- Kalies H, Grote V, Verstraeten T, et al. The use of combination vaccines has improved timeliness of vaccination in children. Pediatr Infect Dis J. 2006;25(6):507–512.
- Marshall GS, Happe LE, Lunacsek OE, et al. Use of combination vaccines is associated with improved coverage rates. Pediatr Infect Dis J. 2007;26(6):496–500.
- Zepp F, Schmitt HJ, Cleerbout J, et al. Review of 8 years of experience with infanrix hexa (DTPa-HBV-IPV/Hib hexavalent vaccine). Expert Rev Vaccines. 2009;8(6):663–678.
- Esposito S, Principi N, Cornaglia G, et al. Barriers to the vaccination of children and adolescents and possible solutions. Clin Microbiol Infect. 2014;20(Suppl 5):25–31.
- Kurosky SK, Davis KL, Krishnarajah G. Effect of combination vaccines on completion and compliance of childhood vaccinations in the United States. Hum Vaccin Immunother. 2017;13(11):2494–2502.
- Gust DA, Strine TW, Maurice E, et al. Underimmunization among children: effects of vaccine safety concerns on immunization status. Pediatrics. 2004;114(1):e16–22.
- Dhillon S. Spotlight on DTPa-HBV-IPV/Hib Vaccine (Infanrix hexa). BioDrugs. 2010;24(5):299–302.
- Omenaca F, Vazquez L, Garcia-Corbeira P, et al. Immunization of preterm infants with GSK’s hexavalent combined diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus-Haemophilus influenzae type b conjugate vaccine: A review of safety and immunogenicity. Vaccine. 2018;36(7):986–996.
- Avdicova M, Prikazsky V, Hudeckova H, et al. Immunogenicity and reactogenicity of a novel hexavalent DTPa-HBV-IPV/Hib vaccine compared to separate concomitant injections of DTPa-IPV/Hib and HBV vaccines, when administered according to a 3, 5 and 11 month vaccination schedule. Eur J Pediatr. 2002;161(11):581–587.
- Gabutti G, Zepp F, Schuerman L, et al. Evaluation of the immunogenicity and reactogenicity of a DTPa-HBV-IPV Combination vaccine co-administered with a Hib conjugate vaccine either as a single injection of a hexavalent combination or as two separate injections at 3, 5 and 11 months of age. Scand J Infect Dis. 2004;36(8):585–592.
- Van Der Meeren O, Kuriyakose S, Kolhe D, et al. Immunogenicity of Infanrix hexa administered at 3, 5 and 11 months of age. Vaccine. 2012;30(17):2710–2714.
- World Health Organization. South-East Asia regional vaccine action plan 2016-2020. [citied 2018 Oct 11]. Available from: http://apps.who.int/iris/bitstream/handle/10665/272397/9789290225812-eng.pdf?sequence=1&isAllowed=y
- World Health Organization, Western Pacific Resion. Hepatitis. [cited 2018 Oct 11]. Available at: http://www.wpro.who.int/hepatitis/en/
- Ott JJ, Stevens GA, Groeger J, et al. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30(12):2212–2219.
- Schillie S, Walker T, Veselsky S, et al. Outcomes of infants born to women infected with hepatitis B. Pediatrics. 2015;135(5):e1141–1147.
- World Health Organization. Regional office for South-East Asia. Expanded Programme on Immunization (EPI) - Regional fact sheet 2017. [Accessed on 2018 Oct 11]. Available at: http://www.searo.who.int/immunization/data/sear_2017.pdf
- World Health Organization. Western Pacific region, health information and intelligence platform, HepB3 Immunization coverage 2016; WHO Press. [cited 2018 Sept 5]. Available from: http://hiip.wpro.who.int/portal/Dashboards/Immunization/Immunizationdashboards/TabId/169/ArtMID/905/ArticleID/76/Default
- World Health Organization. Western pacific region, health information and intelligence platform, DTP3 immunization coverage 2016. WHO Press. [cited 2018 Sept 5]. Available from: http://hiip.wpro.who.int/portal/Dashboards/Immunization/Immunizationdashboards/TabId/169/ArtMID/905/ArticleID/76/Default
- Dolhain J, Janssens W, Mesaros N, et al. Hexavalent vaccines: increasing options for policy-makers and providers. A review of the data supporting interchangeability (substitution with vaccines containing fewer antigens) and mixed schedules from the same manufacturer. Expert Rev Vaccines. 2018;17(6):513–524.
- Gatchalian SBL, Cadrona-Carlos J, Espos R, et al. A hexavalent DTPa-HBV-IPV/Hib vaccine administered to Filipino infants at 6, 10 and 14 weeks and 12-15 months of age; importance of the birth dose of HBV. Philipp J Pediatr. 2007;56(3): 153–161.
- Shao PL, Lu CY, Hsieh YC, et al. Immunogenicity and reactogenicity of DTPa-IPV/Hib vaccine co-administered with hepatitis B vaccine for primary and booster vaccination of Taiwanese infants. J Formos Med Assoc. 2011;110(6):415–422.
- Cheng HK, Rajadurai VS, Amin Z, et al. Immunogenicity and reactogenicity of two regimens of diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated polio and Haemophilus influenzae type b vaccines administered to infants primed at birth with hepatitis B vaccine. Southeast Asian J Trop Med Public Health. 2004;35(3):685–692.
- GlaxoSmithKline. Clinical study report. Study 217744/069 (DTPa-HBV-IPV-069). 29 May 2018. [cited 2016 Aug 24]. Available from: https://www.gsk-clinicalstudyregister.com/study/217744/069.
- Kosalaraksa P, Thisyakorn U, Benjaponpitak S, et al. Immunogenicity and safety study of a new DTaP-IPV-Hep B-PRP-T combined vaccine compared to a licensed DTaP-IPV-Hep B//PRP-T comparator, both concomitantly administered with a 7-valent pneumococcal conjugate vaccine at 2, 4, and 6 months of age in Thai infants. Int J Infect Dis. 2011;15(4):e249–256.
- Lalwani SK, Agarkhedkar S, Sundaram B, et al. Immunogenicity and safety of 3-dose primary vaccination with combined DTPa-HBV-IPV/Hib in Indian infants. Hum Vaccin Immunother. 2017;13(1):120–127.
- Wood N, McIntyre P, Marshall H, et al. Acellular pertussis vaccine at birth and one month induces antibody responses by two months of age. Pediatr Infect Dis J. 2010;29(3):209–215.
- Pichichero ME, Blatter MM, Reisinger KS, et al. Impact of a birth dose of hepatitis B vaccine on the reactogenicity and immunogenicity of diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus-haemophilus influenzae type b combination vaccination. Pediatr Infect Dis J. 2002;21(9):854–859.
- Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B Virus Infection in the United States: recommendations of the advisory committee on Immunization Practices. MMWR Recomm Rep. 2018;67(1):1–31.
- World Health Organization. Hepatitis B vaccines: WHO position paper–recommendations. Vaccine. 2010;28(3):589–590.
- National Centre for Immunisation Research and Surveillance. Immunisation recommendations for infants, children and adolescents in Australia. [cited 2019 Jan 28]. Available from: http://www.ncirs.org.au/sites/default/files/2018-11/Childhood-schedule-table_September_2018_to%20insert%20HB%20link.pdf
- Schwarz TF, Behre U, Adelt T, et al. Long-term antibody persistence against hepatitis B in adolescents 14-15-years of age vaccinated with 4 doses of hexavalent DTPa-HBV-IPV/Hib vaccine in infancy. Hum Vaccin Immunother. 2019;15(1):235–241.
- Dhillon S. DTPa-HBV-IPV/Hib vaccine (Infanrix hexa): a review of its use as primary and booster vaccination. Drugs. 2010;70(8):1021–1058.
- European Centre for Disease Prevention and Control (ECDC). Hepatitis B: recommended vaccinations. [Accessed on 2019 Jan 28]. Available at: https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=6&SelectedCountryIdByDisease=−1
- Lernout T, Hendrickx G, Vorsters A, et al. A cohesive European policy for hepatitis B vaccination, are we there yet? Clin Microbiol Infect. 2014;20(Suppl 5):19–24.
- Behre U, Bleckmann G, Crasta PD, et al. Long-term anti-HBs antibody persistence and immune memory in children and adolescents who received routine childhood hepatitis B vaccination. Hum Vaccin Immunother. 2012;8(6):813–818.
