ABSTRACT
Introduction
Immunesurveillance is an important tool to monitor the protection of the population against vaccine-preventable diseases, which is currently mostly based on the detection of specific serum antibodies. However, the landscape of immune surveillance is changing, driven by emerging and evolving pathogens, changes in the age distribution of the population and scientific understanding of protective immunity, necessitating a comprehensive review.
Areas covered
To anticipate these changes, reliable and high-throughput detection of antibody levels is desired to enable screening in larger population settings. Antibody levels alone do not always equate with protection and may require additional functional testing of the antibodies or immune cell-based assays. In addition, the location (systemic or locally mucosal) of the infection and whether the antibodies are induced through infection or vaccination have implications for both immune protection and assessing immune status.
Expert commentary
In order to perform multicenter studies on many samples for multiple antigens, more validated reference materials and wider adoption of high-throughput techniques are needed. The field of serosurveillance will also benefit from better correlates of protection and understanding of (local) mechanisms of protection. Here we give an overview of the current state-of-the-art of serosurveillance and how the field could move forward.
1. Introduction
1.1. Why immunesurveillance
Infectious diseases contribute to mortality and morbidity and result in economic costs [Citation1,Citation2]. Some infectious diseases for which treatment or vaccines became available have declined, others are unchanged in incidence and new infectious agents emerge [Citation3,Citation4]. To provide insight into infection risks, pathogen surveillance is needed as well as assessing the protection of the population against infections through immune surveillance () [Citation5,Citation6]. In this review, we focus on immune surveillance by assessing the level of specific antibodies, as this is the most widely applied and cost-effective means to measure immunity in the population.
Table 1. Definitions.
The immune status and vaccine responses may vary between different groups of the population, which could be related to age, sex, race, and environmental factors such as infection history and lifestyle [Citation8–Citation10]. Immune surveillance is a powerful tool aimed at identifying groups at risk of infection in the population. The possible causes of an increased risk can be very diverse and therefore the inclusion of sufficient (e.g. demographic) parameters that may be associated with immune competence should be aimed at, accompanied with sufficient power of the study [Citation11]. Such studies could enhance our understanding of the mechanisms whereby vaccines are effective, or under what conditions vaccines may perform unsatisfactorily. Results of immune surveillance studies can be used to optimize vaccination strategies and programs, and for outbreak management.
1.2. Study designs for immune surveillance
Assessing the immune status of the general population starts with a well-defined aim. This aim is leading in defining the sample population: whom to include and in what numbers, as well as the general design of the study. Results of immune surveillance studies could (and should) impact decision-making, which may require additional epidemiological data. This has been extensively reviewed, so here we only briefly mention the most commonly used approaches [Citation11,Citation12].
To assess the protection of the population, cross-sectional studies are relatively efficient at getting a general overview of the protection of the population, and, depending on what additional data is collected, also allow for more specific analyses of subgroups of the population. Although double-blinded (placebo-) controlled studies are performed to test vaccine efficacy, in the context of immune surveillance these are less often applied [Citation13,Citation14]. The control group can receive a placebo, a different vaccine formula against the same target (e.g. to prove non-inferiority) or an irrelevant vaccine depending on the specific research question. Although double-blinded randomized controlled studies are the gold standard, this design is not always feasible (e.g. for financial or logistic aspects) or not allowed for ethical reasons.
To ultimately prove protection against infection longitudinal cohort studies incorporating pre-outbreak serum samples could provide the means to assess protection against infection [Citation15]. For rare infections, such trials are challenging as large numbers of participants have to be enrolled [Citation16,Citation17]. That longitudinal cohort studies can be demanding and expensive is an important reason that case studies and cross-sectional studies are performed, despite the stronger evidence obtained from randomized and/or longitudinal studies. Also, cohort studies may be performed retrospectively without conducting new epidemiological surveys [Citation16,Citation17]. Both case-control studies and retrospective studies that analyze historic serological and infection data can provide insight into the protection against infections of the general public. Provided reliable and well-defined immune-assays are used, studies can be bridged and compared directly, thereby increasing the sample size or together provide additional insight or save costs.
To establish external validity, investigating the representativeness of the sample population for the target population is crucial. Therefore, the sample population is compared to target population databases to assess whether there may be an enrollment bias that needs to be corrected for in subsequent statistical analyses.
2. Antibodies
2.1. Antibodies and correlates of protection
The primary outcome of immune surveillance studies mostly is the concentration of specific antibodies. Serum is the most widely distributed specimen to detect the presence of specific antibodies following the immunizing event. The predominant antibody analyzed for immune surveillance is IgG, which often is the dominant antibody-type following immunization and can be produced by long-lived B cells. Because the biological half-life of an IgG molecule is limited to 3–4 weeks, IgGs detected months or years after immunization are actively produced by plasma B cells that differentiate from memory B cells (discussed in more detail in the next paragraph). In intervention studies, an increase in antibody levels, seroconversion rates (seroprevalence), or number of memory or effector cells are commonly used outcome measures to provide insight into the immunogenicity of a vaccine and its capacity to induce long-lived protection against infection [Citation18].
Historically, antibodies were discovered to be induced following exposure to pathogens [Citation19,Citation20]. Antibodies were understood as a hallmark of such an exposure in the past and implicated to be important for protective immunity. Detecting antibody levels is an efficient approach as it uses limited amounts of sample and is less laborious and more consistent than cell-based assays. A commonly used procedure is the enzyme-linked immunosorbent assay (ELISA). Multiple ELISAs for different antigens can be performed on a blood sample. A newer generation assay is the fluorescence-based multiplex assays that have an extended dynamic range (limiting the number of dilutions to be tested), increased sensitivity while using less sample and antigen [Citation21]. Internationally, a lot of effort has been dedicated to measuring antibodies and understanding the meaning of the measured concentrations for immune protection against infections. Antibody concentrations as such do not necessarily define protection as protection may occur through different mechanisms, and therefore ideally, a correlate of protection should be defined. A correlate of protection can be a specific antibody level, a cell-based readout or a combination of the two [Citation6,Citation22]. Of note, correlates may differ between vaccinated and infected individuals as the immune response induced often varies between the two, whilst these different immune responses both may confer protection. In addition, certain assays may be better at detecting vaccine-induced rather than infection-induced antibodies. However, even for vaccines that provide protection independent of antibodies, a certain level of specific antibodies may still serve as a surrogate correlate of protective immunity (see ). Alternatively, antibodies and cellular immunity protect at different stages of infection [Citation23,Citation24].
Which concentration of antibodies associates with protection against infection has been indicated for a number of vaccines (), with varying degrees of evidence. However, defining a protective cutoff remains challenging for a number of reasons. One reason is that the number of infected cases from which pre-antibody status has been determined is limited. Sometimes clinical specimens are biobanked prior to particular outbreaks that can be used for such an analysis. For some of the published studies, the investigators were fortunate that participants were sampled prior to a particular outbreak, but for other reasons, or because pre-outbreak serum samples were available for investigation (examples in [Citation25–Citation27]). Such samples may be available when surveillance is being performed for increased risk of infections in occupational settings, such as in a health-care setting, refugee workers or for the military service. Such studies are frequently performed for HIV and hepatitis B in health-care settings [Citation28–Citation30]. Trials designed to identify specific levels of antibodies and then follow the participants longitudinally for an infection to occur is the most straightforward, yet also the most challenging and expensive. Vaccine-efficacy mostly is determined by establishing the reduction of disease cases. The duration of vaccine-mediated immunity can be evaluated by measuring specific antibody levels at several time points after vaccination, in some instances also in conjunction with measurements before vaccination to analyze seroconversion or immunological boosting [Citation31,Citation32]. Measuring antibody concentrations at multiple time points after vaccination (or infection) allows for the estimation of the duration of vaccine-induced immunity by fitting or modeling the decline rate of the antibodies over time. Ideally, longitudinal analysis of the same individuals is the preferred way to do this [Citation33,Citation34], but in practice, this performance characteristic is mostly defined on the basis of a cross-sectional analysis of individuals with different ages and thus different years after vaccination.
Table 2. Immunogenicity correlates of protection for vaccines.
Antibody-based correlates of protection can be defined based on standardized concentrations, on binding strength (avidity), or functional assays such as serum bactericidal assay that involves complement, or cellular assays such as the opsonophagocytosis assay that uses neutrophils or virus neutralization and plaque reduction tests (discussed below).
2.2. The immunobiology behind serosurveys
The majority of readouts for immune surveillance rely on antibodies produced by plasma B cells following infection or vaccination. An exception is maternal antibodies transferred from the mother to the offspring via the placenta or breast milk [Citation50]. Upon initial activation B cells produce IgM and during further differentiation could undergo class switch recombination resulting in the production of IgG or IgA (). Therefore, detecting specific IgM can be an indication of recent exposure to a pathogen or antigen of a pathogen [Citation51,Citation52]. For most vaccines, especially vaccines administered intramuscularly, the majority of B cells will switch toward the production of IgG [Citation53]. Oral vaccines and mucosal infections are typically biased toward the induction of IgA [Citation54,Citation55]. This is caused by the specific stimuli in the local environment of the B cell, such as the draining lymph nodes at the site of vaccination or infection.
Figure 1. Illustration of different stages of B cell differentiation at various stages of infection or vaccination and re-infection or booster vaccination. The initial response is dominated by IgM which following class switching is taken over by IgG. The secondary response following booster vaccination or re-infection is dominated by IgG and is characterized by higher antibody levels. Mucosal vaccination or infection will also induce IgA (not depicted).
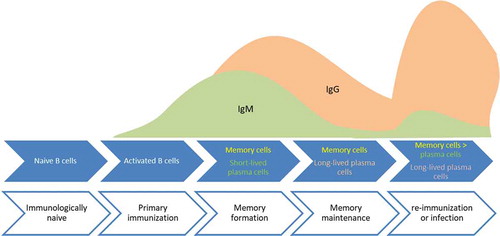
The differentiation of B cells toward memory B cells is crucial to develop a sustained antibody response following infection or vaccination [Citation56]. One of the benchmarks of an effective vaccine is the sustained production of protective antibodies as shown by limited decay in antibody levels [Citation33,Citation34,Citation57,Citation58]. Sustained antibody concentrations present in blood over the years require memory B cells to differentiate into long-lived plasma cells, as antibodies themselves disappear from the circulation, depending on the specific type of antibody, with a half-life of approximately 1 month for IgG, and about 1 week for IgM and IgA [Citation59]. Assessing antibody levels can be complemented with detecting the numbers of memory B cells, the B cells required for persistent antibodies [Citation60]. Memory B cells can rapidly terminally differentiate into antibody-secreting plasma cells in response to re-infection and thereby provide protection. Booster vaccinations typically expand the number of memory B cells and may activate preexisting memory B cells to become plasma cells. In addition to activating cells induced by the primary immunization, new cells are activated as indicated by the arising of antibodies with new specificities. The dynamics of a rapid increase in antibody production and high antibody avidity and level is characteristic for a secondary immune response (). This response is instrumental in a more rapid clearance of an infection, thereby reducing virulence, clinical severity, and possibly also transmission between patients.
3. Mechanisms of protection
3.1. Assessing vaccination versus exposure
Immunity derived from infection often differs from the immune response induced by vaccination, especially when component vaccines are used (). Such differences are also reflected by the induced antibodies, leading to different specificities, affinities, or antibody classes ().
Table 3. Serosurveillance purposes.
Table 4. Type of antibodies related to vaccination and exposure/infection.
3.1.1. Antibody levels and avidity
To establish vaccine immunogenicity often the vaccine components are incorporated in the serological assays [Citation67–Citation69] [Citation70]. In some instances, a quantitative measure may be used to discriminate the vaccine-acquired response from, and infection-mediated response, the latter usually being stronger in avidity or higher in quantitative terms. However, in many cases, this may require assessing another immune discrimination, e.g. local versus systemic antibody levels of IgG and IgA (more about this under 3.2 Systemic versus local responses). Vaccination may not always result in an antibody response (primary vaccine failure), or may induce lower antibody levels, which may wane faster when compared to natural infection [Citation71,Citation72]. However, for HPV for instance, it has been documented that infection induces much lower antibody concentrations compared to vaccination [Citation73].
The ratio in levels of specific IgM and IgG or increased avidity of the antibodies (measure for binding strength) may indicate re-infection, booster vaccination, or repeated re-activation of chronic (latent) infections [Citation52,Citation74]. Using IgM antibody detection in a near – or post-elimination era of measles and rubella indicated that this approach is insufficient to assess new infections [Citation51]. Moreover, the kinetics of IgM induction following exposure to a new pathogen may also change with age, as observed in a novel influenza infection in the USA, where young individuals induced IgM whereas older individuals showed no significant changes in IgM production, probably due to cross-reactive antibodies to previously encountered influenza viruses [Citation75]. Similarly, primary immunization with meningococcal vaccine-induced less protective IgM-mediated immunity in adults aged >50y compared to adolescents [Citation76].
3.1.2. Antigen specificity
When antibody levels, class or avidity measure cannot demonstrate differences between infection or vaccination, assays need to be selective for pathogen antigens that are not incorporated in the vaccine, if possible. Different vaccine formulae are used such as live-attenuated whole viruses that are used for the mumps, measles and rubella vaccines, outer-membrane vesicles from gram-negative bacteria like Neisseria meningitidis, virus-like particles (VLPs) in the case of hepatitis B virus and human papillomavirus, or a selected purified or recombinant protein derived from the microbe, such as for diphtheria and tetanus. The latter type of vaccines can be conjugated and are often adjuvated with particular pharmacological agents such as alum, to enhance the production of antibodies and induce longer-lasting immunity. If selected antigens are used in the vaccine, the potential overlap between the vaccine antigen and the immunodominant antigens of the pathogen can be more distinct. For assays aimed at discriminating exposure to pathogen rather than measuring vaccine-efficacy, preferably antigens should be selected that are not part of the vaccine (also see ).
The majority of vaccines to Streptococcus pneumoniae (pneumococcus), Haemophilus influenzae type b (Hib), and N. meningitidis (meningococcus) are directed against the polysaccharides, the main component of the capsule of these pathogens. The polysaccharides facilitate immune-evasion and are associated with clinical invasiveness of the pathogens. As the vaccines induce antibodies to these polysaccharides, the detection of specific antibodies to proteins of these bacteria indicates recent or previous bacterial infection [Citation77,Citation78]. However, antibodies to such proteins may be cross-reactive and inadvertently being misinterpreted as being generated from infection with a different microbe, but with homologous antigens or epitopes. Another example is the vaccine for Hepatitis B, which targets a surface protein (HbsAg), allowing the detection of antibodies directed against the core proteins to identify hepatitis B infection [Citation79]. Another example of a distinction between vaccine components and pathogenic antigens is the switch from a whole-microbe live-attenuated-based vaccine such as for Bordetella pertussis to an acellular vaccine consisting of 1–5 specific pertussis proteins. Antibodies to non-vaccine proteins could be used to identify infection or previous exposure. However, for live-attenuated or outer-membrane vesicle types there will be too much overlap with the infectious microbe to reliably distinguish between infection and vaccine response [Citation70].
The antigenic differences between the oral poliovirus vaccine (OPV) and the inactivated poliovirus vaccine (IPV) are small, but OPV induces the induction of both IgA and IgG antibodies, also mucosal, whilst IPV mainly induces systemic IgG antibodies (). This is of special interest as the IPV vaccines protect against polio disease but do not provide sterile protection against poliovirus infection. Such an infection may lead to the shedding of poliovirus in the environment, which is considered an important route of transmission and risk of the poliovirus to be transmitted to nonimmune persons [Citation80]. Oral poliovirus vaccines are able to limit this viral shedding, which has been related to the capacity of live poliovirus vaccine to induce mucosal protection [Citation81]. OPV is therefore considered to confer better protection at the population level than IPV, provided sufficient high vaccination coverage can be achieved to block virus transmission. OPV itself is a live virus that can spread in populations with suboptimal vaccine coverage, and an inherent risk of OPV is the (small) possibility of reverting to infectious virus [Citation80,Citation82]. In fact, the detection of IgA in IPV vaccinated persons hallmarks an exposure to live or attenuated poliovirus and can thus be used to identify such infections in IPV vaccinated populations.
Inadequate discrimination between vaccine- and infection-induced antibodies complicates the assessment of the longevity of vaccine responses. Especially for pathogens that are circulating, resulting in exposure and antibody boosting of many individuals in the absence of clinical symptoms, the longevity of the vaccine response may be overestimated. In summary, discriminating vaccination – from infection-induced antibodies can prove challenging; awareness of potentially different origins of the specific antibodies detected can be important for understanding the influence of vaccination and infection on immunity in a population.
3.2. Systemic versus local responses
The majority of the vaccines are administered intramuscularly, efficiently inducing systemic (central) immune responses. The vaccine response is subsequently evaluated routinely in serum, reflecting the amount of circulating antibodies. Systemic or invasive infections that travel through the bloodstream are effectively targeted by such responses. Examples are Hepatitis B virus and measles virus infections, and the prevention of invasive disease by poliovirus, meningococci, pneumococci, and Hib [Citation83,Citation84].
For many pathogens targeted by vaccines, first entry and infection happen at mucosal surfaces in the respiratory tract (e.g. B. pertussis, N. meningitidis, S. pneumoniae, Hib, measles) or the gastrointestinal tract (e.g. poliovirus, rotavirus). Disease symptoms may even be restricted to the respiratory tract, as is the case for whooping cough (B. pertussis) and pneumonia (S. pneumoniae). For these reasons in some studies antibody detection is carried out in oropharyngeal specimens such as saliva (also termed oral fluid), as these may be a better proxy for immune protection of the respiratory tract than antibodies in serum [Citation78,Citation85,Citation86]. However, IgG antibody concentrations in saliva specimens are limited, equal, or less than 1% of what can be detected in serum. The relative proportion of IgA antibodies is higher because mucosal surfaces promote the production of protective IgA that can be secreted and neutralize pathogens before infection can occur [Citation87–Citation90]. Moreover, depending on the specific tissue, efficient transport of dimeric IgA can occur through the polymeric Ig receptor resulting in secretory IgA in the lumen of mucosal surfaces such as the respiratory tract [Citation91,Citation92]. Although memory IgA B cells could be detected 1-year post-MenC vaccination, local IgA antibodies induced by vaccination may decline relatively fast [Citation93,Citation94]. Assessing IgA antibodies after vaccination often proves challenging, because well-defined control sera or serum panels with defined concentrations and general knowledge regarding protective antibody concentrations are lacking.
Studies showing changes in carriage of S. pneumoniae or N. meningitidis under influence of vaccination indicate that systemic immunization has local impact and results in reduced carriage in the population [Citation95,Citation96]. How this is regulated (or controlled) by local antibodies is a challenging subject, but recently a study elucidated that vaccine-induced IgGs are able to agglutinate pneumococci and regulate carriage [Citation97]. This mechanism of agglutination may contribute to herd-immunity.
In contrast to IgG, the ability of IgA to activate complement is limited and the expression of the Fc alpha receptors by phagocytes is not as wide-spread as observed for Fc gamma receptors [Citation98]. Given the poor ability of IgA to activate complement and enhance uptake of pathogens or antigens by phagocytes, the role of IgA on mucosal surface is believed to be immune exclusion through opsonization. Recent work indicates that immune exclusion by mucosal IgA also works at low-density pathogens and isolates the pathogen from the surrounding microbes; a mechanism called enchained growth [Citation89].
Because of the different levels and roles of IgG and IgA antibodies, a different approach is required for the analysis of these antibodies in for instance serum and saliva samples, which represents a proxy for systemic and local immunity, respectively. In humans, the options for blood sampling are sometimes limited for ethical and practical reasons, such as in young children. Salivary samples are noninvasive to obtain and for bronchial and alveolar mucosal antibody levels, salivary samples may be a good proxy. However, this needs further study and validation, especially since data indicate that antibody profiles may be different between systemic and mucosal fluids [Citation86,Citation99]. Although mucosal IgA may be a good marker for local protection, in case this first line of defense is breached, systemic (IgG) antibodies and a solid cellular basis for immunity are required for additional defense and efficient induction of T cell immunity. An important aspect of IgG is the ability to activate receptors on immune cells that increase clearing of infectious microbes and induce an inflammatory response to help clear a pathogen. To our current understanding, the function of IgG and IgA is complementary to each other, and future studies should confirm how IgG and IgA levels in systemic and local fluids may protect against infectious disease, pathogen carriage, or transmission of pathogens.
3.3. Functional antibody and cellular assays
The detection of antigen-specific antibodies has been paramount in the immune surveillance of infectious diseases. While antibody concentration is the most frequently used parameter to define immune protection, the definition of correlates of protection (CoP) based on detected antibody levels alone remains challenging. This can have various reasons, such as which antibodies protect, whether protection is dependent on systemic antibodies, local antibodies, or both, or whether protection is more dependent on cellular mechanisms rather than antibodies [Citation23]. Also, the provided cutoffs should not be regarded as a conservative or fixed value, as there are numerous examples of infections also occurring in persons with pre-exposure antibody levels above the specified cutoff, e.g. in high exposure settings. To answer some of these questions functional assays have been developed to assess the quality of antibodies. At the antibody level, a first indicator for quality is the binding strength of IgG antibodies referred to as antibody avidity. The avidity index determines which proportion of the antibodies is of high affinity, and a better predictor of the antibodies to bind to the pathogen in vivo [Citation74]. The avidity helps in defining the maturation process of the antibody response following antigenic contact by vaccination or infection.
IgG is produced in four different subclasses, with IgG1 being the most abundant. The subclasses IgG1 and IgG3 have the strongest binding ability to complement and Fc gamma receptors [Citation100], while IgG4 has limited functionality in involving complement and Fc gamma receptors. Both IgG1 and IgG3 subclasses hallmark major subclasses involved in common viral infections, the contribution of which changes over time after infection or vaccination. IgG3 for instance often appears early but IgG1 persists the longest, which is at least partly explained by reduced reuse through competition of binding to FcRn with IgG1 [Citation101,Citation102]. Since subclasses have different functional abilities, subclass differentiation may assist to get a better understanding of the type of immune response [Citation103]. In addition to measuring IgG levels, functionality of antibodies to bacteria or viruses is determined [Citation20,Citation104–Citation107]. Serum bactericidal and opsonophagocytosis assays quantify the ability of antibodies to enhance killing of live bacteria by complement or phagocytes, respectively, [Citation20,Citation108,Citation109]. Virus neutralization assays use live virus that is capable of infecting host cells [Citation15,Citation104]. Antibodies that display the ability to neutralize infection of cells either interfere with the binding of virus to host cells or the antibodies directly neutralize the virus, with or without the help of complement. These assays reveal more closely that antibodies directed against particular antigens or epitopes are involved in mechanisms against infection or disease. Antibody-dependent cellular cytotoxicity, opsonophagocytosis, or agglutination assays also aim at measuring the inhibitory or killing effector functions of antibodies [Citation105]. The first two require cells in the assay, whereas the latter does not depend on the presence of immune cells.
Functional assays are more comprehensive and labor-intensive to perform, and sometimes less-well standardized. However, recent developments show that multiplex-based assays may also be modified to serve as proxy for functional assays [Citation110]. Functional assays still need to be compared with in vivo data showing protection by, e.g. preventing disease.
Several studies have indicated that antibodies undergo post-translational modification of the Fc part, thereby changing the binding of antibody, or antibody complexes to complement and Fc receptors expressed on immune cells. Fc parts of antibodies undergo different types of glycosylation; fucosylation of IgG for instance reduces interaction with complement and Fc gamma receptors, which are believed to be important immune mechanisms for the effectivity of vaccine-induced antibodies [Citation111,Citation112].
New developments also facilitate assessing multiple parameters of antibodies in multiplex assays. Such analyses can include assessing FcR binding, complement binding, and identifying glycosylation patterns. These data can be paired with concentration, subclass, and cellular assay data to generate a multiparameter dataset, an approach referred to as systems serology [Citation113].
The long-lasting production of protective antibodies requires long-lived plasma cells, and memory B cells, and for most vaccines, this also requires the help of T cells. Hence, assays that quantify antigen-specific memory T and B cells are both informative [Citation60]. An event of infection or vaccination results in changes in the frequencies of T and B cells locally at the site of vaccination or infection and in peripheral blood [Citation114]. In addition to the number of T cells, their phenotype indicates the way they are able to help or regulate the immune response which can be determined by analyzing the expression or secretion of different cytokines such as IFN-γ for T helper 1 cells, IL-4, IL-5 or IL-13 for T helper 2 cells, or IL-17 for T helper 17 cells. Both ELISPOT and flow cytometric analysis can simultaneously visualize these aspects which provide insight into the characteristics of the T cell response. The same techniques can be applied to quantify the phenotype and frequency of memory B cells by polyclonal stimuli that enable the differentiation of memory B cells into antibody secreting cells. Such assays allow the analysis of their specificity and isotype and reveal the presence of potentially long-lived B cells [Citation115]. Memory B cells and long-lived plasma cells can be further differentiated by flow cytometry. Finally, effector T cells of the CD8+ phenotype can directly clear pathogens in the absence of antibodies and therefore should be considered as another ‘piece of the puzzle.’
4. Changing landscape
4.1. Changes in vaccine uptake and demographics
The success of vaccination programs does not merely depend on the ability of the vaccine to induce protective immunity, but also on the vaccine coverage and acceptance by the general public. Sufficient vaccine coverage is needed to reach the degree of immune protection to acquire herd immunity. Trusted advice from professionals, especially with respect to side-effects of the vaccine, is an important trigger for parents to decide whether or not to have their child vaccinated [Citation116,Citation117]. Serious side-effects of vaccines such as the whole cell B. pertussis vaccines or the oral polio vaccine have been instrumental for replacing these vaccines with ones causing less adverse effects [Citation118]. Replacements of such vaccines may also require adjustments in immune surveillance assay methods to reliably detect vaccine-induced responses. Side effects, convictions of non-existing adverse effects and skepticisms toward certain ingredients (such as adjuvants) in vaccine formulations are currently part of individual decision-making. Reduced vaccine acceptance counteracts the long-term benefits of an established vaccination program, especially with respect to gaining and maintaining herd immunity in the general population [Citation95,Citation118].
Finally, in many countries, the proportion of aging individuals is increasing and birth rates have been decreasing. This demographic change sparked the debate of the need of developing more extensive vaccination interventions for the aging population, as these are increasing in numbers and with aging the risk of various infectious diseases increases. As our understanding of immune mechanisms and insight into groups at increased risk of infection or morbidity following infection develops, increasing attention is given to infection with viruses such as cytomegalovirus (CMV), Epstein Barr virus (EBV), and varicella zoster virus (VZV). These viruses are believed to affect the immune system in the long run, resulting in decreased ability to develop T and/or B cell responses in response to vaccines or other pathogens, especially in the aging population [Citation10,Citation119,Citation120].
Taken together, these various changes in the composition of the population and vaccine uptake warrant continued surveillance and evaluation of the risk and immune status of the population.
4.2. Evolution of the pathogens
Pathogens may evolve independently or as a result of introducing a specific vaccine, leading to an antigenic mismatch between the vaccine strain and the outbreak strain such as happened following the introduction of MenC vaccination which allowed the appearance of MenW of the same clonal complex [Citation121]. For S. pneumoniae studies show that following the introduction of a vaccination program the vaccine-strains decrease in frequency. Yet, the downside is that these strains are being replaced by other strains that are not part of the vaccine [Citation95,Citation122]. For B. pertussis, the emergence of Prn negative strains that are rapidly becoming more frequent and are equally infectious as Prn bearing strains have been reported following a vaccine change [Citation123]. Yet, the Prn protein is an essential part of the a-cellular pertussis vaccine, so the Prn negative strain has become insensitive to these vaccine-induced antibodies [Citation124].
Many pathogens change their genetic composition constantly, sometimes resulting in significant antigenic changes. A well-known example is the influenza virus [Citation125]. Antibodies are crucial in the antiviral defense against influenza A virus. However, due to the rapidly mutating nature of the virus, influenza virus enables specific evasion from these antibodies [Citation126]. This is the main reason why influenza virus vaccines are constantly adapted to new emerging influenza viruses trying to close this immunological gap. In addition, vaccination against the influenza virus alone may not always be sufficient to improve the quality of life as other pathogens also could cause influenza-like illness in an elderly population [Citation127]. Besides these more obvious antigenic changes, resulting in serotype replacements, less-well defined and more diffuse antigenic changes may also change the effectiveness of particular vaccines. This is illustrated by the reemergence of mumps in highly vaccinated populations, indicating waning of the vaccine-acquired immune response and reduced capability to protect against antigenically divergent strains [Citation128,Citation129].
The observation that pathogens escape immunity induced by vaccines only covering part of the antigens of pathogenic strains initiated research efforts to develop vaccines targeted at antigens that do not allow the pathogen to adapt without losing its key mechanism to enter the host. Moreover, such an antigenic target ideally is effective against a broader selection of strains of the pathogen [Citation130,Citation131]. For this purpose, conserved antigens are being identified, with the assumption that conserved regions may be less likely to evolve, and tested as vaccine targets. However, a conserved portion of a protein may not be necessary for the pathogen to maintain viability and virulence and therefore still allow for immune-escape of the pathogen. For serosurveillance purposes, researchers have to assess whether the antigens used to detect antibodies reflect antigens currently circulating to be able to assess the immune status of the population under investigation.
5. Conclusions and future directions
Surveillance of immunological protection against infections is well established as a reliable method to assess the protection of individuals or the general population. Measuring the concentration of specific antibodies in serum is minimally invasive and applied globally which allows for comparison between different studies and countries. When recruiting participants and collecting samples is challenging, researchers could benefit from already existing cohorts the reuse samples and thus avoid the need to collect new samples. Reuse of samples of course still requires ethical clearance which can be tackled up front, and reuse of samples is easier when using assays that require limited sample volume.
Methods of measuring antibody concentrations often vary per laboratory and laboratories may use different quality criteria. Therefore, it is recommended that further exchange of methods and discussion of assay performance continues and more laboratories participate in multi-center and/or ring studies to benchmark assay performance against other laboratories using well-characterized reference serum panels and international standards. Even when quality criteria are met, samples from individuals known to be protected or clinically identified as unprotected by documented infections are limited in availability, necessitating alternative approaches to assess protection of the population. Antibodies can be associated with protection or be functionally involved in protective immunity (). Sometimes low levels of antibodies are still protective [Citation132], but at the same time, relying on a single measurement in time only provides enough information when the kinetics of the response are well defined. Understanding the meaning of an assay result and its implications needs to be subjected to comparison with other data or assays and always requires proper interpretation.
Serological correlates of protection have been identified for some pathogens, based on antibody levels that could be determined before and after infection, but in many cases, this is mostly based on estimates and lacks formal evidence.
That is one of the reasons that functional assays that test the protective ability of serum are used to provide insight into the potential protective role of the antibodies. These are often defined, or considered the gold standard. As normally high concentrations of antibodies will be protective, a subset of samples with moderate or low concentrations and unknown protective ability could be further tested in a functional assay to provide more insight. In addition, new developments to translate functional assays into high-throughput immunoassays could advance extensive characterization of samples (systems serology) [Citation113]. The functionality of antibodies as established in laboratory assays should be evaluated against immune mechanisms that actually provide protection against a pathogen. Functional protection against disease may also be influenced by local protection at the mucosal surfaces. Finally, also little data are available to verify correlates of protection in certain subgroups of the population, for instance, immunocompromised individuals and the aging population [Citation10,Citation54,Citation81,Citation133].
In conclusion, immune surveillance is a highly efficient immunological approach to determine the immune status of the population against infection and to determine subgroups or individuals at risk.
6. Expert opinion
Immune surveillance through measuring specific antibody concentrations is an important tool in monitoring the protection of the general population and the evaluation of vaccine responses in different target populations. Currently, the assays used between laboratories vary from ELISA to functional assays and high-throughput multiplex assays. With the growing need to determine antibody levels for multiple analytes in the same sample the benefits of multiplex assays increases. Apart from differences in efficiency, dynamic range, and sample volume requirements, the concordance between different assay types is reasonably good. A bigger challenge is the lack of international standardized reference and control sera and insufficient efforts to compare lab results between countries. Since pathogens cross country borders, assay results should be comparable between countries.
The expansion of the number of vaccines in vaccination programs including a multitude of vaccine components (e.g. the polysaccharides used in vaccines for meningococcus and especially pneumococcus) results in the need to perform more measurements in samples. Performing large numbers of measurements using ELISA easily forms a bottleneck and therefore the need to implement high-throughput multiplex assays is growing. Such assays also have the benefit of requiring less sample volume and an extended dynamic range, paired with great reproducibility. Several organizations such as the WHO and FDA (Food and Drug Administration, USA) often still prefer data from single ELISA testing, despite that multiplex immune assays (a measles multiplex assay is endorsed by WHO) have already been introduced more than a decade ago and proven to equal, or to excel ELISA testing in comparative studies [Citation21,Citation69,Citation134,Citation135]. We believe that data from laboratories that can prove solid performance in multicentre/ring trials should be accepted, regardless of the platform or technology used. Of course, continuous efforts to develop and implement international reference materials to validate assay results should be encouraged to achieve this goal. In addition, GCLP (good clinical laboratory practice) standards, international reference sera, and comparison of results in multicentre studies should move the quality and ease of comparison of data between labs and countries forward.
In this review a table listing proposed or established correlates of protection is included. Some of these correlates are based on little data and indirect evidence. Investing in defining these correlates will support the evaluation of the protection status of the population. At the same time, whether the correlate (in this case mostly a serological concentration of specific IgG) is actually mechanistically involved in the protection of the host, or a proxy, is not always known. Relying on concentrations alone obviously omits many of the underlying immunological mechanisms needed for protective responses. For this reason, bactericidal or virus neutralization assays are still being conducted as they confer the best functionality in antibody testing, or more functionality is added to enzyme-linked immunoassays or fluorescent multiplex assays by means of, e.g. avidity testing. Functional assays can also be used in addition to assays that measure concentrations to provide input for establishing correlates of protection when such correlates cannot be defined epidemiologically. Which functional assay should be used differs between pathogens as it depends on the mechanisms whereby antibodies contribute to providing protection in vivo. Such protective mechanisms could be prevention of cellular infection, complement-mediated killing or phagocytosis. Some diseases manifest locally, for instance, pneumonia, whereas other infections cause disease in other tissues or fluids then the initial infection as is the case for, e.g. bacteremia or meningitis. For most pathogens discussed here, the respiratory tract is the site of infection. Therefore, understanding how correlates or protection associate with local protection could be an interesting new venue. The advantage of local protection is the control of an infection at the very early stages, even prior to causing any disease. In addition, local control is also more likely to prevent transmission between different individuals and enhance herd immunity. Importantly, protective responses independent of antibodies, such as T cell-responses, may be essential in conferring protection. Consequently, understanding of the mechanism of protection against disease can be essential to define a correlate of protection.
We expect that in the coming 5–10 years the field of immune surveillance will continue to rely on measuring specific IgG concentrations, whilst we also anticipate that using non-serological samples such as saliva and the use of assays to test the functionality of antibodies will gain more attention. The most urgent need, however, is the wider acceptance of modern assays to analyze specific antibodies paired with better standardization of assays and reference materials.
Article Highlights
Measuring infection or vaccine-induced antibody concentrations is an efficient tool to assess the protection of the population to infectious agents.
Although antibody concentrations associate with protection (correlate of protection), and sometimes provide protective cutoffs, other immune mechanisms may be required to protect against disease.
Antibody levels and repertoires induced through vaccination or infection often differ, in addition to the presence and mechanism of protection in different body sites.
Immune surveillance would benefit from more standardization between labs, supply of international reference sera and continuous anticipation of changes in demographics and pathogens.
Author contributions
GdH, FvdK, RvB, GAMB designed the manuscript; GdH wrote the manuscript; FvdK, RvB, GAMB, and AB edited the manuscript.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Lopez AD, Mathers CD, Ezzati M, et al. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006 May 27;367(9524):1747–1757.
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012 Dec 15;380(9859):2095–2128.
- Racaniello VR. Emerging infectious diseases. J Clin Invest. 2004 Mar 15;113(6):796–798.
- Jones KE, Patel NG, Levy MA, et al. Global trends in emerging infectious diseases. Nature. 2008 Feb 21;451(7181):990. online.
- Heymann DL, Rodier GR. Hot spots in a wired world: WHO surveillance of emerging and re-emerging infectious diseases. Lancet Infect Dis. 2001 Dec 01;1(5):345–353.
- Plotkin SA, Plotkin SA. Correlates of vaccine-induced immunity. Clinl Infect Dis. 2008;47(3):401–409.
- Plotkin SA, Gilbert PB. Nomenclature for immune correlates of protection after vaccination. Clinl Infect Dis. 2012;54(11):1615–1617.
- Panda A, Qian F, Mohanty S, et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 2010 Mar 1;184(5):2518–2527.
- Zimmerman RK, Lauderdale DS, Tan SM, et al. Prevalence of high-risk indications for influenza vaccine varies by age, race, and income. Vaccine. 2010 Sep 07;28(39):6470–6477.
- McElhaney JE, Zhou X, Talbot HK, et al. The unmet need in the elderly: how immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine. 2012;30(12):2060–2067.
- Harder T, Takla A, Rehfuess E, et al. Evidence-based decision-making in infectious diseases epidemiology, prevention and control: matching research questions to study designs and quality appraisal tools [journal article]. BMC Med Res Methodol. 2014 May 21;14(1):69.
- Evidence-based methodologies for public health [Technical report]. ECDC; 2011.
- Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6(5):271–278.
- Govaert TM, Thijs C, Masurel N, et al. The efficacy of influenza vaccination in elderly individuals: a randomized double-blind placebo-controlled trial. Jama. 1994;272(21):1661–1665.
- Katzelnick LC, Montoya M, Gresh L, et al. Neutralizing antibody titers against dengue virus correlate with protection from symptomatic infection in a longitudinal cohort. Proc Nat Acad Sci. 2016;113(3):728–733.
- Verstraeten T, Jumaan AO, Mullooly JP, et al. A retrospective cohort study of the association of varicella vaccine failure with asthma, steroid use, age at vaccination, and measles-mumps-rubella vaccination. Pediatrics. 2003;112(2):e98–e103.
- Isacson J, Trollfors B, Taranger J, et al. Safety, immunogenicity and an open, retrospective study of efficacy of a monocomponent pertussis toxoid vaccine in infants. Pediatr Infect Dis J. 1994;13(1):22–27.
- Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis J. 2000;19(3):187–195.
- Brunell PA, Gershon AA, Uduman SA, et al. Varicella-zoster immunoglobulins during varicella, latency, and zoster. J Infect Dis. 1975;132(1):49–54.
- Aftandelians R, Connor JD. Bactericidal antibody in serum during infection with Bordetella pertussis. J Infect Dis. 1973;128(4):555–558.
- van Gageldonk PGM, van Schaijk FG, van der Klis FR, et al. Development and validation of a multiplex immunoassay for the simultaneous determination of serum antibodies to Bordetella pertussis, diphtheria and tetanus. J Immunol Methods. 2008 Jun 01;335(1):79–89.
- World Health Organization. Correlates of vaccine-induced protection: methods and implications. Geneva, Switzerland: World Health Organization; 2013.
- Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccin Immunol. 2010;17(7):1055–1065.
- Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design [Review article]. Nat Rev Immunol. 2008 Apr 01;8(4):247. online.
- Chen RT, Markowitz LE, Albrecht P, et al. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162(5):1036–1042.
- Gouma S, Schurink-van’t Klooster TM, de Melker HE, et al., Mumps serum antibody levels before and after an outbreak to assess infection and immunity in vaccinated students. Open Forum Infect Dis. 2014;1(3):ofu101–ofu101.
- Bolotin S, Hughes SL, Gul N, et al. What is the evidence to support a correlate of protection for measles? A systematic review. J Infect Dis. 2019. DOI:10.1093/infdis/jiz380.
- Baldo V, Floreani A, Vecchio LD, et al. Occupational risk of blood-borne viruses in healthcare workers: a 5-year surveillance program. Infect Control Hosp Epidemiol. 2002;23(6):325–327.
- Tokars JI, Marcus R, Culver DH, et al. Surveillance of HIV infection and zidovudine use among health care workers after occupational exposure to HIV-infected blood. Ann Intern Med. 1993;118(12):913–919.
- Venier AG, Vincent A, L’Hériteau F, et al. Surveillance of occupational blood and body fluid exposures among French healthcare workers in 2004. Infect Control Hosp Epidemiol. 2007;28(10):1196–1201.
- Waaijenborg S, Hahné SJ, Mollema L, et al. Waning of maternal antibodies against measles, mumps, rubella, and varicella in communities with contrasting vaccination coverage. J Infect Dis. 2013;208(1):10–16.
- Borrow R, Andrews N, Findlow H, et al. Kinetics of antibody persistence following administration of a combination meningococcal serogroup C and haemophilus influenzae type b conjugate vaccine in healthy infants in the United Kingdom primed with a monovalent meningococcal serogroup C vaccine. Clin Vaccin Immunol. 2010;17(1):154–159.
- Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357(19):1903–1915.
- Fraser C, Tomassini JE, Xi L, et al. Modeling the long-term antibody response of a human papillomavirus (HPV) virus-like particle (VLP) type 16 prophylactic vaccine. Vaccine. 2007 May 22;25(21):4324–4333.
- Scheifele DW, Ochnio JJ. The immunological basis for immunization series. Module 2: diphtheria. Geneva, Switzerland: World Health Organization; 2009.
- Borrow R, Balmer P, Roper MH, et al. Immunological basis for immunization: module 3: tetanus (update 2006). Geneva, Switzerland: World Health Organization; 2007.
- Long SS, Welkon CJ, Clark JL. Widespread silent transmission of pertussis in families: antibody correlates of infection and symptomatology. J Infect Dis. 1990;161(3):480–486.
- Siber GR, Chang I, Baker S, et al. Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine. 2007;25(19):3816–3826.
- Andrews NJ, Waight PA, Burbidge P, et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis. 2014;14(9):839–846.
- Käyhty R, Peltola H, Karanko V, et al. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1983;147(6):1100.
- Andrews N, Borrow R, Miller E. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin Diagn Lab Immunol. 2003;10(5):780–786.
- Chen RT, Markowitz LE, Albrecht P, et al. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162(5):1036–1042.
- Woudenberg T, van Binnendijk R, Veldhuijzen I, et al. Additional evidence on serological correlates of protection against measles: an observational cohort study among once vaccinated children exposed to measles. Vaccines. 2019;7(4):158.
- Andrews N, Pebody R, Berbers G, et al. The European sero-epidemiology network: standardizing the enzyme immunoassay results for measles, mumps and rubella. Epidemiol Infect Immunity. 2000;125(1):127–143.
- Kaaijk P, Wijmenga-Monsuur AJ, van Houten MA, et al. A third dose of measles-mumps-rubella vaccine to improve immunity against mumps in young adults. J Infect Dis. 2019. DOI:10.1093/infdis/jiz188
- van Lier A, Smits G, Mollema L, et al. Varicella zoster virus infection occurs at a relatively young age in The Netherlands. Vaccine. 2013;31(44):5127–5133.
- Robertson S. The immunological basis for immunization series module 6: poliomyelitis. Geneva, Switzerland: World Health Organization; 1993.
- The immunological basis for immunization series module 22: hepatitis B. World Health Organization; 2012.
- The immunological basis for immunization series: module 18 - hepatitis A. World Health Organization; 2019.
- Sato H, Albrecht P, Reynolds DW, et al. Transfer of measles, mumps, and rubella antibodies from mother to infant: its effect on measles, mumps, and rubella immunization. Am J Dis Children. 1979;133(12):1240–1243.
- Bolotin S, Lim G, Dang V, et al. The utility of measles and rubella IgM serology in an elimination setting, Ontario, Canada, 2009–2014. Plos One. 2017;12(8):e0181172.
- Griffiths PD, Stagno S, Pass RF, et al. Infection with cytomegalovirus during pregnancy: specific IgM antibodies as a marker of recent primary infection. J Infect Dis. 1982;145(5):647–653.
- Haan L, Verweij WR, Holtrop M, et al. Nasal or intramuscular immunization of mice with influenza subunit antigen and the B subunit of Escherichia coli heat-labile toxin induces IgA- or IgG-mediated protective mucosal immunity. Vaccine. 2001 Apr 06;19(20):2898–2907.
- Moldoveanu Z, Clements ML, Prince SJ, et al. Human immune responses to influenza virus vaccines administered by systemic or mucosal routes. Vaccine. 1995 Jan 01;13(11):1006–1012.
- van Splunter M, van Hoffen E, Floris-Vollenbroek EG, et al. Oral cholera vaccination promotes homing of IgA+ memory B cells to the large intestine and the respiratory tract. Mucosal Immunol. 2018 Feb 21;11(4):1254–1264.
- Crotty S, Felgner P, Davies H, et al. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171(10):4969–4973.
- Le T, Cherry JD, Chang S-J, et al. Immune responses and antibody decay after immunization of adolescents and adults with an acellular pertussis vaccine: the APERT study. J Infect Dis. 2004;190(3):535–544.
- Antia A, Ahmed H, Handel A, et al. Heterogeneity and longevity of antibody memory to viruses and vaccines. PLoS Biol. 2018;16(8):e2006601.
- Dixon FJ, Talmage DW, Maurer PH, et al. The half-life of homologous gamma globulin (antibody) in several species. J Exp Med. 1952;96(4):313–318.
- Jahnmatz M, Kesa G, Netterlid E, et al. Optimization of a human IgG B-cell ELISpot assay for the analysis of vaccine-induced B-cell responses. J Immunol Methods. 2013;391(1–2):50–59.
- Schreiber JR, Barrus V, Cates KL, et al. Functional characterization of Human IgG, IgM, and IgA antibody directed to the capsule of Haemophilus influenzae type b. J Infect Dis. 1986;153(1):8–16.
- Pichichero ME, Kaur R, Casey JR, et al. Antibody response to Haemophilus influenzae outer membrane protein D, P6, and OMP26 after nasopharyngeal colonization and acute otitis media in children. Vaccine. 2010;28(44):7184–7192.
- Davidkin I, Jokinen S, Broman M, et al. Persistence of measles, mumps, and rubella antibodies in an MMR-vaccinated cohort: a 20-year follow-up. J Infect Dis. 2008;197(7):950–956.
- Glikmann G, Mordhorst C-H. Secretory and serum immunoglobulin class-specific antibodies to mumps virus after a natural mumps infection. Serodiag Immun Infect Dis. 1987 Aug 01;1(4):275–285.
- Hanson LA, Carlsson B, Jalil F, et al. Different secretory IgA antibody responses after immunization with inactivated and live poliovirus vaccines. Clinl Infect Dis. 1984;6(Supplement_2):S356–S360.
- Chandra RK. Reduced secretory antibody response to live attenuated measles and poliovirus vaccines in malnourished children. BMJ. 1975;2(5971):583–585.
- de Voer RM, Schepp RM, Versteegh FGA, et al. Simultaneous detection of Haemophilus influenzae type b polysaccharide-specific antibodies and Neisseria meningitidis serogroup A, C, Y, and W-135 polysaccharide-specific antibodies in a fluorescent-bead-based multiplex immunoassay. Clin Vaccin Immunol. 2009 March 1;16(3):433–436.
- Scherpenisse M, Schepp RM, Mollers M, et al. Comparison of different assays to assess human papillomavirus (HPV) type 16- and 18-specific antibodies after HPV infection and vaccination. Clin Vaccin Immunol. 2013 August 1;20(8):1329–1332.
- Smits GP, van Gageldonk PG, Schouls LM, et al. Development of a bead-based multiplex immunoassay for simultaneous quantitative detection of IgG serum antibodies against measles, mumps, rubella, and varicella-zoster virus. Clin Vaccin Immunol. 2012;19(3):396–400.
- Thomas MG, Redhead K, Lambert HP. Human serum antibody responses to bordetella pertussis infection and pertussis vaccination. J Infect Dis. 1989;159(2):211–218.
- Johnson PR, Feldman S, Thompson JM, et al. Immunity to influenza A virus infection in young children: a comparison of natural infection, live cold-adapted vaccine, and Inactivated vaccine. J Infect Dis. 1986;154(1):121–127.
- Wendelboe AM, Van Rie A, Salmaso S, et al. Duration of immunity against pertussis after natural infection or vaccination. Pediatr Infect Dis J. 2005;24(5):S58–S61.
- Scherpenisse M, Schepp RM, Mollers M, et al. Characteristics of HPV-specific antibody responses induced by infection and vaccination: cross-reactivity, neutralizing activity, avidity and IgG subclasses. Plos One. 2013;8(9):e74797.
- Grangeot-Keros L, Mayaux MJ, Lebon P, et al. Value of cytomegalovirus (CMV) IgG avidity index for the diagnosis of primary CMV infection in pregnant women. J Infect Dis. 1997;175(4):944–946.
- Li Z-N, Lin S-C, Carney PJ, et al. IgM, IgG, and IgA antibody responses to influenza A(H1N1)pdm09 hemagglutinin in infected persons during the first wave of the 2009 pandemic in the United States. J Clin Vac Immunol. 2014;21(8):1054–1060.
- van der Heiden M, van Ravenhorst MB, Bogaard M, et al. Lower antibody functionality in middle-aged adults compared to adolescents after primary meningococcal vaccination: role of IgM. Exp Gerontol. 2018 May 01;105:101–108.
- Wilson R, Cohen JM, Reglinski M, et al., Naturally acquired human immunity to pneumococcus is dependent on antibody to protein antigens. PLoS Pathog. 2017;13(1):e1006137.
- Horton RE, Stuart J, Christensen H, et al. Influence of age and carriage status on salivary IgA to Neisseria meningitidis. Epidemiol Infect. 2005;133(5):883–889.
- Shepard CW, Simard EP, Finelli L, et al. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev. 2006;28(1):112–125.
- Hird TR, Grassly NC. Systematic review of mucosal immunity induced by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus challenge. PLoS Pathog. 2012;8(4):e1002599.
- Wright PF, Wieland-Alter W, Ilyushina NA, et al. Intestinal immunity is a determinant of clearance of poliovirus after oral vaccination. J Infect Dis. 2014;209(10):1628–1634.
- Kew O, Morris-Glasgow V, Landaverde M, et al. Outbreak of poliomyelitis in hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science. 2002;296(5566):356–359.
- Andrews NJ, Waight PA, George RC, et al. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and wales. Vaccine. 2012 Nov 06;30(48):6802–6808.
- Eskola J, Käyhty H, Takala AK, et al. A randomized, prospective field trial of a conjugate vaccine in the protection of infants and young children against invasive haemophilus influenzae type b disease. N Engl J Med. 1990;323(20):1381–1387.
- Korkeila M, Lehtonen H, Åhman H, et al. Salivary anti-capsular antibodies in infants and children immunised with Streptococcus pneumoniae capsular polysaccharides conjugated to diphtheria or tetanus toxoid. Vaccine. 2000 Jan 21;18(13):1218–1226.
- van Ravenhorst MB, den Hartog G, van der Klis FRM, et al. Induction of salivary antibody levels in Dutch adolescents after immunization with monovalent meningococcal serogroup C or quadrivalent meningococcal serogroup A, C, W and Y conjugate vaccine. Plos One. 2018;13(4):e0191261.
- Macpherson AJ, Gatto D, Sainsbury E, et al. A Primitive T Cell-Independent Mechanism of Intestinal Mucosal IgA Responses to Commensal Bacteria. Science. 2000;288(5474):2222–2226.
- McDermott MR, Bienenstock J. Evidence for a common mucosal immunologic system. I migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979;122(5):1892–1898.
- Moor K, Diard M, Sellin ME, et al. High-avidity IgA protects the intestine by enchaining growing bacteria. Nature. 2017 Apr 12;544(7651):498. online.
- Newcomb RW, Ishizaka K, DeVald BL. Human IgG and IgA diphtheria antitoxins in serum, nasal fluids and saliva. J Immunol. 1969;103(2):215–224.
- Loman S, Radl J, Jansen HM, et al. Vectorial transcytosis of dimeric IgA by the Calu-3 human lung epithelial cell line: upregulation by IFN-gamma. Am J Physiol Lung Cell Mol Physiol. 1997;272(5):L951–L958.
- Kaetzel CS, Mestecky J, Johansen F-E. Two cells, one antibody: the discovery of the cellular origins and transport of secretory IgA [10.4049/jimmunol.1700025]. J Immunol. 2017;198(5):1765.
- Stoof SP, van der Klis FR, van Rooijen DM, et al. Salivary antibody levels in adolescents in response to a meningococcal serogroup C conjugate booster vaccination nine years after priming: systemically induced local immunity and saliva as potential surveillance tool. Vaccine. 2015;33(32):3933–3939.
- Stoof SP, Buisman A-M, van Rooijen DM, et al. Different dynamics for IgG and IgA memory B cells in adolescents following a meningococcal serogroup C tetanus toxoid conjugate booster vaccination nine years after priming: a role for priming age? Plos One. 2015;10(10):e0138665.
- Miller E, Andrews NJ, Waight PA, et al. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and wales: an observational cohort study. Lancet Infect Dis. 2011 Oct 01;11(10):760–768.
- Maiden MCJ, Stuart JM. Carriage of serogroup C meningococci 1 year after meningococcal C conjugate polysaccharide vaccination. Lancet. 2002 May 25;359(9320):1829–1830.
- Mitsi E, Roche AM, Reine J, et al. Agglutination by anti-capsular polysaccharide antibody is associated with protection against experimental human pneumococcal carriage. Mucosal Immunol. 2016 Aug 31;10:385–394. online.
- Takai T. Roles of Fc receptors in autoimmunity [Review #rticle]. Nat Rev Immunol. 2002 Aug 01;2(8):580. online.
- Bemark M, Hazanov H, Strömberg A, et al. Limited clonal relatedness between gut IgA plasma cells and memory B cells after oral immunization [Article]. Nat Commun. 2016 Sep 06;7(1):12698. online.
- Bruhns P, Iannascoli B, England P, et al. Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113(16):3716–3725.
- Stapleton NM, Andersen JT, Stemerding AM, et al. Competition for FcRn-mediated transport gives rise to short half-life of human IgG3 and offers therapeutic potential. Nat Commun. 2011;2:599.
- El Mubarak HS, Ibrahim SA, Vos HW, et al. Measles virus protein-specific IgM, IgA, and IgG subclass responses during the acute and convalescent phase of infection. J Med Virol. 2004;72(2):290–298.
- de Voer RM, van der Klis FRM, Engels CWAM, et al. Development of a fluorescent-bead-based multiplex immunoassay to determine immunoglobulin G subclass responses to Neisseria meningitidis serogroup A and C polysaccharides. Clin Vaccin Immunol. 2008 August 1;15(8):1188–1193.
- Arita M, Iwai M, Wakita T, et al. Development of a poliovirus neutralization test with poliovirus pseudovirus for measurement of neutralizing antibody titer in human serum. Clin Vaccin Immunol. 2011 November 1;18(11):1889–1894.
- Henckaerts I, Durant N, De Grave D, et al. Validation of a routine opsonophagocytosis assay to predict invasive pneumococcal disease efficacy of conjugate vaccine in children. Vaccine. 2007;25(13):2518–2527.
- Sowers SB, Rota JS, Hickman CJ, et al. High concentrations of measles neutralizing antibodies and high-avidity measles IgG accurately identify measles reinfection cases. Clin vaccin immunol. 2016;23(8):707–716.
- Hahne SJ, Nic Lochlainn LM, van Burgel ND, et al. Measles outbreak among previously immunized healthcare workers, the Netherlands, 2014. J Infect Dis. 2016 Dec 15;214(12):1980–1986.
- Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection—serum bactericidal antibody activity. Vaccine. 2005;23(17–18):2222–2227.
- Schlesinger Y, Granoff DM, Murphy TV, et al. Avidity and bactericidal activity of antibody elicited by different haemophilus influenzae type b conjugate vaccines. JAMA. 1992;267(11):1489–1494.
- Brown EP, Weiner JA, Lin S, et al. Optimization and qualification of an Fc Array assay for assessments of antibodies against HIV-1/SIV. J Immunol Methods. 2018 Apr 01;455:24–33.
- Vidarsson G, Dekkers G, Rispens T. IgG Subclasses and allotypes: from structure to effector functions [Review]. Front Immunol. 2014 October 20;5(520). DOI:10.3389/fimmu.2014.00520
- Dekkers G, Treffers L, Plomp R, et al. Decoding the human immunoglobulin G-glycan repertoire reveals a spectrum of Fc-receptor- and complement-mediated-effector activities. Front Immunol. 2017;8:877.
- Brown EP, Dowell KG, Boesch AW, et al. Multiplexed Fc array for evaluation of antigen-specific antibody effector profiles. J Immunol Methods. 2017 Apr 01;443:33–44.
- Christensen D, Mortensen R, Rosenkrands I, et al. Vaccine-induced Th17 cells are established as resident memory cells in the lung and promote local IgA responses [Article]. Mucosal Immunol. 2017 Jan;10(1):260–270 print.
- Pinna D, Corti D, Jarrossay D, et al. Clonal dissection of the human memory B-cell repertoire following infection and vaccination. Eur J Immunol. 2009 May;39(5):1260–1270.
- Omer SB, Salmon DA, Orenstein WA, et al. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. N Engl J Med. 2009;360(19):1981–1988.
- Gust DA, Darling N, Kennedy A, et al. Parents with doubts about vaccines: which vaccines and reasons why. Pediatrics. 2008;122(4):718–725.
- David S, Vermeer-de Bondt PE, van der Maas NAT. Reactogenicity of infant whole cell pertussis combination vaccine compared with acellular pertussis vaccines with or without simultaneous pneumococcal vaccine in the Netherlands. Vaccine. 2008 Oct 29;26(46):5883–5887.
- Arvin A. Aging, immunity, and the varicella–zoster virus. N Engl J Med. 2005;352(22):2266–2267.
- Wang C, Liu Y, Xu LT, et al. Effects of aging, cytomegalovirus infection, and EBV infection on human B cell repertoires. J Immunol. 2013 Jan 15;192(2):603–611.
- Lucidarme J, Scott K, Ure R, et al. An international invasive meningococcal disease outbreak due to a novel and rapidly expanding serogroup W strain, Scotland and Sweden, july to august 2015 [Research]. Eurosurveillance. 2016 November 10;21(15). DOI:10.2807/1560-7917.ES.2016.21.45.30395.
- Elberse KEM, van der Heide HGJ, Witteveen S, et al. Changes in the composition of the pneumococcal population and in IPD incidence in The Netherlands after the implementation of the 7-valent pneumococcal conjugate vaccine. Vaccine. 2012 Dec 14;30(52):7644–7651.
- Barkoff A-M, Mertsola J, Pierard D, et al. Pertactin-deficient Bordetella pertussis isolates: evidence of increased circulation in Europe, 1998 to 2015. Euro Surveill. 2019;24(7):1700832.
- Bodilis H, Guiso N. Virulence of pertactin-negative bordetella pertussis isolates from infants, France. Emerg Infect Dis. 2013;19(3):471–474.
- Smith DJ, Lapedes AS, de Jong JC, et al. Mapping the antigenic and genetic evolution of influenza virus. Science. 2004;305(5682):371–376.
- Nachbagauer R, Choi A, Hirsh A, et al. Defining the antibody cross-reactome directed against the influenza virus surface glycoproteins [Research]. Nat Immunol. 2017 Feb 13;18(4):464–473. online;advance online publication.
- van Beek J, Veenhoven RH, Bruin JP, et al. Influenza-like illness incidence is not reduced by influenza vaccination in a cohort of older adults, despite effectively reducing laboratory-confirmed influenza virus infections. J Infect Dis. 2017;216(4):415–424.
- Homan EJ, Bremel RD. Are cases of mumps in vaccinated patients attributable to mismatches in both vaccine T-cell and B-cell epitopes?: An immunoinformatic analysis. Hum Vaccin Immunother. 2014;10(2):290–300.
- May M, Rieder CA, Rowe RJ. Emergent lineages of mumps virus suggest the need for a polyvalent vaccine. Inter J Infect Dis. 2018 Jan 01;66:1–4.
- Epstein SL, Tumpey TM, Misplon JA, et al. DNA vaccine expressing conserved influenza virus proteins protective against H5N1 challenge infection in mice. Emerg Infect Dis. 2002;8(8):796.
- Pizza M, Scarlato V, Masignani V, et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science. 2000;287(5459):1816–1820.
- Hessell AJ, Poignard P, Hunter M, et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009;15(8):951.
- van der Heiden M, van Zelm MC, Bartol SJ, et al. Differential effects of Cytomegalovirus carriage on the immune phenotype of middle-aged males and females. Sci Rep. 2016;6(1):26892.
- Tcherniaeva I, den Hartog G, Berbers G, et al. The development of a bead-based multiplex immunoassay for the detection of IgG antibodies to CMV and EBV. J Immunol Methods. 2018;462:1–8.
- Meek B, Ekström N, Kantsø B, et al. Multilaboratory comparison of pneumococcal multiplex immunoassays used in immunosurveillance of streptococcus pneumoniae across Europe. mSphere. 2019;4(6):e00455–19.
