ABSTRACT
Introduction: From its earliest days, the US. military has embraced the use of vaccines to fight infectious diseases. The Army Liposome Formulation (ALF) has been a pivotal innovation as a vaccine adjuvant that provides excellent safety and potency and could lead to dual-use military and civilian benefits. For protection of personnel against difficult disease threats found in many areas of the world, Army vaccine scientists have created novel liposome-based vaccine adjuvants.
Areas covered: ALF consists of liposomes containing saturated phospholipids, cholesterol, and monophosphoryl lipid A (MPLA) as an immunostimulant. ALF exhibited safety and strong potency in many vaccine clinical trials. Improvements based on ALF include: ALF adsorbed to aluminum hydroxide (ALFA); ALF containing QS21 saponin (ALFQ); and ALFQ adsorbed to aluminum hydroxide (ALFQA). Preclinical safety and efficacy studies with ALF, LFA, ALFQ, and ALFQA are discussed in preparation for upcoming vaccine trials targeting malaria, HIV-1, bacterial diarrhea, and opioid addiction.
Expert opinion: The introduction of ALF in the 1980s stimulated commercial interest in vaccines to infectious diseases, and therapeutic vaccines to cancer, and Alzheimer’s disease. It is likely that ALF, ALFA, and ALFQ, will provide momentum for new types of modern vaccines with improved efficacy and safety.
1. Background
The history of vaccine development, together with the evolving nature of the structures of vaccines themselves, have been replete with colorful and heroic characters, extraordinary innovation, and most importantly, huge success in preventing pain and suffering in humans. In a detailed analytical review in 2015 of US. Food and Drug Administration (FDA)-approved vaccines and their ‘innovators,’ the US Army was identified as a major historical player [Citation1]. Among the top six ‘innovator’ organizations, the US. Army, with at least 16 different vaccines, was listed as being second only to Merck with 18 vaccines (followed by Wyeth with 9, Pasteur Institute and the US. National Institutes of Health with 8 each, and Connaught with 7). As a health strategy for protection of its personnel, the United States military has played a large role in vaccine development [Citation2–Citation4], and the Army Liposome Formulation adjuvants are placed in that context as providing innovative, potent, and safe innate immunity for vaccines.
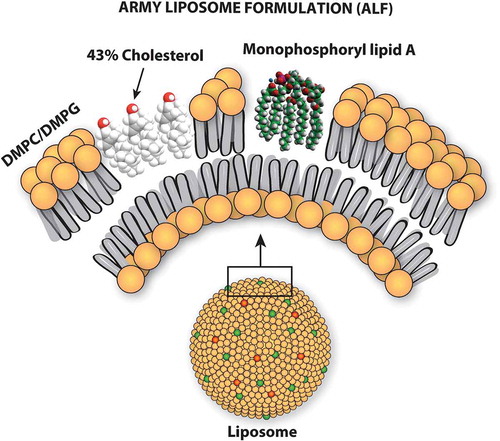
Although the Army has had a history of collaborative adjuvant research using incomplete Freund’s adjuvant as a vaccine adjuvant (mainly for influenza vaccine) [Citation5–Citation7], it was only when adjuvanted liposomes became available in the Army that testing of new types of adjuvants for vaccines commenced [Citation8,Citation9]. Liposomes containing saturated phospholipids, cholesterol, and monophosphoryl lipid A (MPLA) (the lipid component of gram-negative bacterial lipopolysaccharide), which were designed, manufactured, and tested as vaccine adjuvants at the Walter Reed Army Institute of Research (WRAIR) were originally referred to as Walter Reed Liposomes [Citation10,Citation11], but they subsequently became known, and are hereafter referred to, as Army Liposome Formulation (ALF) [Citation12] (). When ALF is adsorbed to aluminum hydroxide gel, the combination is referred to as ALFA; and ALF containing high amounts of cholesterol together with the QS21 (also called QS-21) saponin is referred to as ALFQ. The ALF-type liposomes lacking MPLA had little or no detectable adjuvant activity by themselves, but the same liposomes containing MPLA exhibited considerable adjuvant activity with a variety of antigens [Citation8,Citation13].
Specifically omitted from the above analyses of FDA-approved vaccines were discussions of vaccine adjuvants [Citation1]. The number of FDA-approved vaccines that contain an adjuvant is surprisingly large, with 33 vaccines containing an aluminum salt adjuvant in 2002 [Citation14]; however, only five non-aluminum adjuvants are currently present in five FDA-approved vaccines.Footnote1 Among the latter five vaccines is counted the first FDA-approved liposome-based vaccine, Shingrix® (GlaxoSmithKline, also known as GSK), a vaccine for prevention of shingles which is a painful disorder caused by herpes zoster in those previously infected with varicella (chickenpox) virus [Citation15]. The adjuvant in Shingrix®, which is called ‘Adjuvant System 01B’ (AS01B), is a liposome-based formulation that contains two immunostimulants: MPLA (MPL®) and QS21 (a triterpenoid glycoside saponin derived from the bark of the Quillaja saponaria (soap bark) tree in Chile) [Citation16,Citation17]. Presumably because of the strong potency of AS01B, Shingrix® reportedly exhibited an amazing 98% protective efficacy in individuals aged 70 years or older [Citation18]. Despite the high cost of Shingrix®, and the requirement for a booster immunization, unexpected demand for the vaccine in the United States is so great that it has often surpassed commercial supply [Citation19].
2. Development of ALF
2.1. ALF in early malaria vaccines
Although the AS01 adjuvant system in Shingrix® is the first liposome-based formulation in an FDA-approved vaccine in the US., it is also included in a malaria vaccine that is being tested in sub-Saharan Africa. As background to this, it was in the context of the ongoing WRAIR malaria vaccine research program in 1986 that liposomes containing MPLA (i.e. ALF), which were developed through intramural research at WRAIR, originally caught the attention of the US. Army as a vaccine adjuvant [Citation8]. In the initial clinical studies with ALF (using MPL® from Ribi Immunochem Research, Inc. in Hamilton, Montana – now owned by GSK), two different recombinant proteins were employed as malaria antigens, one (R32NS1) utilizing tetrapeptide repeat sequences from the circumsporozoite (CS) protein of Plasmodium falciparum [Citation20], and the other utilizing a ‘repeatless’ recombinant protein lacking tetrapeptide sequences from CS protein [Citation21]. In each of the above trials, the ALF liposomes that contained encapsulated antigen were adsorbed to aluminum hydroxide, and the combination was found to be safe and strongly potent as an adjuvanted vaccine formulation.
2.2. ALF in an early HIV-1 vaccine
The above studies with ALF in malaria vaccines elicited interest during early stages in the HIV-1 field. In 1992, in a study conducted by the AIDS Vaccine Evaluation Group (AVEG), a phase 1 multi-center randomized, double-blind, placebo-controlled comparison of safety and potency of seven different adjuvants was conducted in HIV-1 uninfected individuals, with each adjuvant using the same recombinant gp120 HIV-1SF-2 protein antigen (AVEG015) (ClinicalTrials.gov NCT00001042) [Citation22]. This led to an important initial regulatory problem for the FDA to determine how seven different vaccine adjuvant products that had been previously tested in humans with different antigens could be incorporated into a single phase 1 trial, a problem that was solved by collaborative agreement between the NIH, the Army, and the FDA [Citation23]. An initial summary evaluation of the AVEG015 trial suggested that, when compared to the other adjuvants, aluminum-adsorbed ALF with 37.5 µg of encapsulated gp120 per dose (compared to 50 µg of gp120 per dose with the other adjuvants), had very strong adjuvant properties with minimal toxicity [Citation24]. An analysis in 2018 of the 1992 AVEG015 study, using more modern techniques, examined archived sera from the ALF-adsorbed-to-aluminum hydroxide group in the AVEG015 study for the ability to cross-react with two different recombinant HIV-1 antigens, gp120IIIB (subtype B) and gp120 A244 (subtype A/E) [Citation25]. Similar subtypes of recombinant HIV-1 envelope proteins had been adsorbed to aluminum hydroxide adjuvant for immunization, together with a canarypox prime immunization, in the RV144 Thai trial (16,402 volunteers) that showed significant protection against HIV-1 infection in 2009 [Citation26]. It was concluded that the 1992 aluminum-adsorbed ALF (containing encapsulated rgp120 in ALF liposomes) induced broadly cross-reacting antibodies to different subtypes of HIV-1 (). Moreover, analysis of the sera from 1992 revealed that the immunizations induced cross-clade binding antibodies to recombinant proteins containing the V1V2 regions of HIV-1; and also induced antibody-dependent cellular cytotoxicity (ADCC); antibody-dependent cellular phagocytosis (ADCP); and antibody-dependent inhibition of the binding of V2 peptides to the α4β7-integrin receptor [Citation25,Citation27]. These new possible adjuvant mechanisms from the 1992 trial in humans now gain further relevance in view of immune correlate analyses, and sieve analysis of breakthrough infection in the RV144 Thai trial that implicated the potential protective importance of the magnitude and duration of cross-clade binding of antibodies to the V2 loop in a vaccine to HIV-1 [Citation28–Citation34]. The data suggest that the ALF adjuvant might provide useful benefits to an HIV-1 vaccine.
Figure 2. AVEG015 study design and antibody responses to gp120 proteins. The vaccination schedule (hollow arrows) and bleeds (bold arrows) are shown. Antigen-specific IgG end point titers to subtype B gp120IIIB (left panel) and subtype A/E gp120 A244 as determined by ELISA are shown. Each dot depicts the arithmetic mean endpoint titer for an individual sample assayed in triplicate. Gray bars represent median endpoint titers and error bars represent interquartile range. The numbers on top represent the median titer for each time point. At week 26 (2 weeks postthird immunization), there was a 32-fold and a fourfold increase (denoted by red arrows) in the gp120IIIB and gp120 A244 titers between the alum and the liposomal groups. Significance between the two arms are noted above the bars (* P ≤.05;** P ≤ .005; ***, P ≤ .0005; 2-tailed Mann-Whitney U test). Reused with permission from [Citation25].
![Figure 2. AVEG015 study design and antibody responses to gp120 proteins. The vaccination schedule (hollow arrows) and bleeds (bold arrows) are shown. Antigen-specific IgG end point titers to subtype B gp120IIIB (left panel) and subtype A/E gp120 A244 as determined by ELISA are shown. Each dot depicts the arithmetic mean endpoint titer for an individual sample assayed in triplicate. Gray bars represent median endpoint titers and error bars represent interquartile range. The numbers on top represent the median titer for each time point. At week 26 (2 weeks postthird immunization), there was a 32-fold and a fourfold increase (denoted by red arrows) in the gp120IIIB and gp120 A244 titers between the alum and the liposomal groups. Significance between the two arms are noted above the bars (* P ≤.05;** P ≤ .005; ***, P ≤ .0005; 2-tailed Mann-Whitney U test). Reused with permission from [Citation25].](/cms/asset/679547e2-ac3f-4584-a358-31cdb5f9855c/ierv_a_1745636_f0002_oc.jpg)
Although the antigens in the above malaria and HIV-1 vaccine studies in 1992 were encapsulated in ALF liposomes, and the liposomes were adsorbed to aluminum hydroxide gel, it was later found (in the absence of aluminum hydroxide) that liposome-encapsulation of another antigen, protective antigen from the anthrax bacillus, was not required in order for the antigen to benefit from the adjuvant properties of ALF, and the ALF liposomes could simply be mixed with the antigen, resulting in equal or greater efficacy [Citation35]. Based on this, all subsequent studies have mixed the respective antigens with the ALF-type adjuvant formulations rather than encapsulation of the antigens in the ALF liposomes.
2.3. Development of ALFQ
After numerous comparative clinical trials, many conducted in collaboration between GSK and the US. Army [Citation36], a successful Plasmodium falciparum malaria sporozoite vaccine (RTS,S/AS01, also known as Mosquirix®) was achieved by using a half-dose of the AS01B adjuvant (AS01E), and a recombinant protein containing repeat sequence tetrapeptides from the CS protein (RTS,S). The RTS,S/AS01 formulation received a positive scientific opinion by the European Medicines Agency exclusively for markets outside the European Union [Citation37], and it is currently being further tested with a four-injection regimen under World Health Organization auspices among hundreds of thousands of children 5 to 9 months of age in settings of moderate-to-high risk of parasite transmission for malaria in three sub-Saharan African countries [Citation38]. However, it should be noted that in a previous phase 3 trial in sub-Saharan Africa, the efficacy of the vaccine ‘provided [only] modest protection against both clinical and severe malaria in young infants’ [Citation39], and it has been suggested that considerable potential challenges could face the implementation of the WHO program [Citation40].
From a military standpoint, a vaccine that benefits both civilians and military personnel represents a dual-use type of product, and the original formulation of liposomes containing MPLA is in the public domain, and is not covered by any current patent protection. However, from a strategic standpoint, it was felt by the Army that a more potent ALF-type adjuvant that could emulate or exceed the potency of the AS01 adjuvant, but which would not infringe the AS01 patent, could be useful. It is within that extended goal that an increased breadth of the ALF family of adjuvants has been developed.
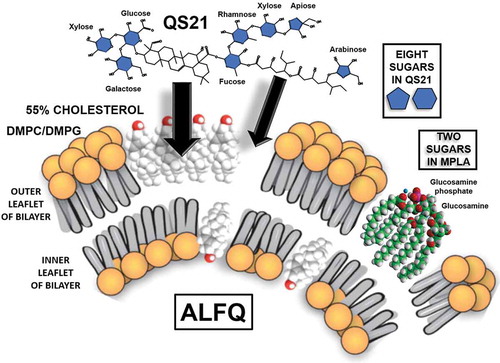
Examination of the US. patent for the AS01 adjuvant revealed many of the fundamental details underlying the intellectual property that support the AS01 adjuvant formulation [Citation17]. With the AS01 parameters having been identified, WRAIR scientists used the ALF structure as a basis to expand the chemical composition of the liposomes and added QS21 as a constituent, to create the formulation which is now referred to as Army Liposome Formulation with QS21 (ALFQ) () [Citation41]. Although ALFQ and AS01 each contains QS21, the liposomes in ALFQ are fundamentally different from those in AS01, both in the phospholipid type (saturated dimyristoyl fatty acids in ALFQ vs. unsaturated dioleoyl fatty acids in AS01) and in the cholesterol content of 55 mol% cholesterol compared to phospholipids in ALFQ vs. 33.7% in AS01. Intramuscular injection of free QS21 results in necrosis at the injection site [Citation16], and it is likely that the toxicity of free QS21 that was observed after injection was caused by the binding of QS21 to cellular cholesterol in the membranes of local cells. QS21 binds irreversibly to cholesterol to form a complex, and when QS21 is exposed to erythrocytes it causes pores to form in the lipid bilayer of the cells, resulting in hemolysis that can be easily measured [Citation42]. When liposomes containing QS21 are added to erythrocytes in vitro, the hemolytic activity of the QS21 is quenched because the liposomal cholesterol acts as a sink for the QS21 that prevents binding to the erythrocytes () [Citation41]. It is worth noting that hemolysis caused by QS21 in ALFQ liposomes containing dimyristoyl phosphatidylcholine (DMPC) was quenched more strongly than with liposomes containing dipalmitoyl phosphatidylcholine (DPPC) or distearoyl phosphatidylcholine (DSPC). The reason for this is not intuitively obvious, and it illustrates some of the complexity of interactions of QS21 with cholesterol that occur in the membrane environments of different types of liposomes.
Figure 3. Differential binding of QS21 to high levels of cholesterol (Chol) in liposomes containing DMPC, DPPC, and DSPC results in differential blocking of QS21-induced hemolysis of adjacent-red blood cells (RBC), as shown. Modified from [Citation12].
![Figure 3. Differential binding of QS21 to high levels of cholesterol (Chol) in liposomes containing DMPC, DPPC, and DSPC results in differential blocking of QS21-induced hemolysis of adjacent-red blood cells (RBC), as shown. Modified from [Citation12].](/cms/asset/dfc4fbc5-727b-4a65-819a-65de112536b2/ierv_a_1745636_f0003_oc.jpg)
Table 1. LIPID COMPOSITIONS OF AS01B AND ALFQ LIPOSOMES.
A fascinating size difference between ALF and ALFQ (and also AS01) occurred during manufacture of ALFQ. When soluble QS21 was added to a suspension of sterile-filtered ALF particles with a high concentration of cholesterol (these were small unilamellar nanovesicles or SUVs), the size of the SUVs suddenly increased up to 600-fold, from 50 to 100 nm to as large as approximately 30,000 nm [Citation43]. To achieve this dramatic size increase, the ALFQ vesicles were formed by cannibalizing the SUVs, resulting in a dramatic increase in the number of large vesicles and a dramatic decrease in the number of small vesicles [Citation44]. Moreover, the large ALFQ particles appeared to be unilamellar or oligolamellar vesicles. Based on solid geometry, it thus appears that the vast majority of the total surface area of the ALFQ vesicles is accounted for by microparticles rather than by nanoparticles. Upon comparison of ALFQ vesicles with those made as described in the AS01B patent, it was evident that the AS01B particles remained as nanoparticles after addition of QS21. It was determined that this difference in size between AS01B particles and ALFQ was due to the dioleoyl phospholipid in AS01B and the dimyristoyl phospholipids in ALFQ [Citation44].
3. Structure-function relationships of MPLA and QS21 in different types of liposomes
3.1. Liposomes containing saturated vs. unsaturated phospholipids
All of the molecules in ALF and ALFQ are amphiphilic in nature; that is, they each contain both hydrophilic and hydrophobic regions. The hydrophobic regions of liposomal phospholipid bilayers are stabilized by van der Waals forces, which are weak short-range forces between individual CH2 groups in adjacent fatty acyl chains [Citation45]. Van der Waals forces decrease greatly with increased distance between interacting CH2 groups, but with diacyl phospholipids, such as DMPC, DMPG, or DOPC, they are additive through the diacyl chain lengths, and they provide cumulative stability to the structures of the hydrophobic regions of the molecules of the lipid bilayer. The cumulative strength through the lengths of adjacent saturated straight fatty acyl chains (as with dimyristoyl groups) is greater than with unsaturated kinked fatty acyl chains (as with dioleoyl groups). Outside access and permeability by enzymes (such as phospholipase A2, or other molecules) to the hydrophobic regions of liposomes containing unsaturated (DOPC) phospholipids is therefore easier than to those containing saturated (DMPC or DMPG) phospholipids.
3.2. Structure-function of liposomal MPLA
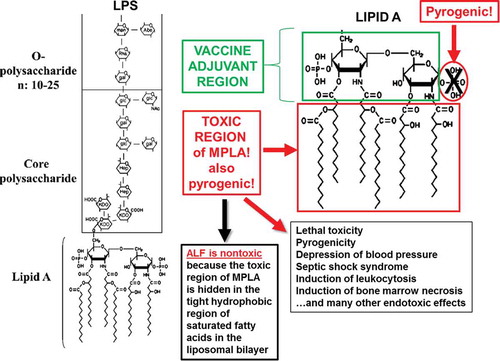
Lipid A has been studied for decades to untangle the structural basis of its numerous endotoxin activities [Citation46,Citation47], and numerous reviews have summarized an amazing history of complex research [Citation48,Citation49]. As pointed out by Beutler and Rietschel ‘… endotoxic activity is not dependent on a single lipid-A constituent (toxophore group), but is dependent on a defined conformation (endotoxic conformation) that is determined by unique features of the primary structure, including steric factors, negative charge and hydrophobic domains’ [Citation50].
With the interactions of MPLA exclusively as a vaccine adjuvant in mind, it seems evident that lipid A, and MPLA, can be divided structurally into two regions with respect to orientation in the liposomal bilayer: the vaccine adjuvant region (the diglucosamine-6ʹ-phosphate polar headgroup), and the toxic (hydrophobic) region (). As described earlier, essentially all of the toxicity of MPLA to humans was eliminated by incorporation into ALF. As a vaccine adjuvant, MPLA retains the ability to bind to the Toll-like receptor 4/myeloid differentiation factor 2 (TLR4/MD-2) receptor complex [Citation49]. Thus, although the toxic region of MPLA is blocked in ALF, based on the previous observations that intraperitoneal injection of ALF induced recruitment of immature macrophages, and that ALF appeared to have ‘intracellular adjuvant activity’ for antigen presentation in vitro in murine bone marrow-derived macrophages [Citation51], the non-toxic region is responsible for all of the adjuvant activity of lipid A. Furthermore, ALF promoted intracellular trafficking patterns that resulted in delivery of the antigen to the Golgi apparatus and presentation via the MHC class I pathway, resulting in the efficient induction of cytotoxic T cell responses [Citation52,Citation53]. Intracellular MPLA is also capable of non-canonical NLRP3 inflammasome activation [Citation49]. From these diverse forms of evidence, it is apparent that multiple cellular mechanisms are in play in the adaptive immune response induced by ALF beyond serving only as a TLR4 agonist.
3.3. Structure-function of liposomal QS21
The exact structure–function relationships of QS21 with liposomes are more difficult to visualize than with MPLA because QS21, which is largely soluble or micellar in water, is integrated into liposomes by binding to cholesterol that is displayed on the outer lamellar surface of the liposomes (). The amount of QS21 bound to the liposomes is proportional to the amount of cholesterol in the liposomes [Citation12]. Based on the similarity of the triterpenoid ring structure of QS21 with the ring structure of cholesterol, it is presumed that this is the major initial binding site of QS21. However, QS21 also has a flexible long-chain fatty acyl chain that displays a sugar at either end (between fucose and arabinose in ). As shown in , it is highly likely that the eight sugars of QS21 are pulled into an orientation on the liposomal surface to form what might be described as a ‘sugar lawn’ covering the polar region of QS21. From this it seems clear that those eight sugars probably act as binding sites for numerous types of lectin receptors in the innate immune system. For example, it has been suggested that the single fucose residue of QS21 binds to DC-SIGN (a C-type lectin) on dendritic cells (DC) [Citation54]. Liposomes, and even pegylated liposomes, cause activation of complement, a process that can occur either because of antibodies to the lipids, or due to spontaneous binding of complement [Citation55–Citation57]. However, it would be expected that complement activation via the lectin pathway, and complement-dependent phagocytosis by antigen-presenting cells, would be greatly enhanced by the complex carbohydrate display on the surface of ALF or ALFQ.
Figure 5. Theoretical formation of a ‘sugar lawn’ of 10 sugars on the surface of ALFQ by interaction of QS21 with ALF liposomes containing Figure 5. Theoretical formation of a ‘sugar lawn’ of 10 sugars on the surface of ALFQ by interaction of QS21 with ALF liposomes containing 55 mol% cholesterol compared to phospholipid.
4. Innate immunity mechanisms of ALF, ALFA, and ALFQ
From an immunity standpoint, upon immunization with recombinant HIV-1 envelope gp140 protein, ALF and ALFQ each induced high fractions of antigen-specific IgG1, lesser fractions of IgG2a, and still lesser fractions of IgG2b subtype antibodies in female BALB/c mice () [Citation43]. After immunization with HIV-1 gp140 together with ALF or ALFQ, each adsorbed to aluminum hydroxide gel, the fractions of IgG1 antibodies increased [Citation58]. This is in keeping with the concept that ALF and ALFQ each induces both Th1 and Th2 types of immunity. However, when analyzed by in vitro secretion of cytokines, ALF and ALFQ each induced equally strong levels of IL-4; in contrast, ALF induced only low levels of IFN-γ, but ALFQ induced high levels of IFN-γ () [Citation43]. Thus, it is concluded that ALF induces Th2-type immunity, with lesser Th1, while ALFQ induces more balanced Th1 and Th2 immunity. This general conclusion was also confirmed with other types of antigens adjuvanted with ALF-family adjuvants [Citation59–Citation62].
Figure 6. IgG subtype analysis of immune sera, and ELISPOT analysis of IL-4-positive and IFN-γ-positive splenic T cells, after immunizing female BALB/c mice with HIV-1 envelope gp140 adjuvanted with ALF, ALFA, ALFQ, ALFQA, or aluminum hydroxide, as shown. The IgG subtype data are from [Citation58]. The cytokine data are from [Citation43]. The latter paper demonstrated that ALF containing an MPLA/phospholipid ratio of 1:5.6 induced higher overall titers of antibodies than a ratio of 1:88, but this was not reflected in the appearance of significant differences in cytokine secretion.
![Figure 6. IgG subtype analysis of immune sera, and ELISPOT analysis of IL-4-positive and IFN-γ-positive splenic T cells, after immunizing female BALB/c mice with HIV-1 envelope gp140 adjuvanted with ALF, ALFA, ALFQ, ALFQA, or aluminum hydroxide, as shown. The IgG subtype data are from [Citation58]. The cytokine data are from [Citation43]. The latter paper demonstrated that ALF containing an MPLA/phospholipid ratio of 1:5.6 induced higher overall titers of antibodies than a ratio of 1:88, but this was not reflected in the appearance of significant differences in cytokine secretion.](/cms/asset/cda9b87c-24df-4884-98a4-80029e11e562/ierv_a_1745636_f0006_oc.jpg)
The precise mechanisms of action of innate immunity induced by the liposomal AS01 formulation containing MPLA and QS21 are still not completely understood; however, complex modeling algorithms are being devised to address those issues [Citation63]. The mechanisms of action of AS01 and ALFQ may differ in certain ways because of the structural differences and compositions of the liposomes: AS01 liposomes have unsaturated phospholipids, and 33.7 mol% cholesterol compared to phospholipid, while ALFQ has saturated phospholipids and 55% cholesterol (). In addition, AS01 is composed of nanoparticles, while ALFQ has a heterogeneous size distribution that is skewed toward microparticles [Citation43,Citation44]. In view of these distinctive differences, further analysis and comparison of the roles of structural compositions of AS01 and ALFQ on their mechanisms of actions as adjuvants could provide useful insights into liposome design as an adjuvant carrier.
5. Safety of ALF
In each of the above malaria and HIV-1 clinical trials [Citation20,Citation21,Citation25], the ALF liposomes, containing encapsulated proteins, were adsorbed to aluminum hydroxide adjuvant. In most cases, the injected liposomes contained a very high dose of MPLA (with multiples of such doses in numerous volunteers) as high as 1100 to 2200 µg of MPLA. To put this dose of MPLA in perspective, in a phase 1 study of intravenously injected free MPLA, it was found that “The major clinical toxicity was fever, chills, and rigor, which occurred in over 50% of the treatments at doses of 250 µg/m2. Two instances of bronchospasm occurred in one patient who received 250 µg/m2.“ [Citation64]. The ‘normal’ body surface area is generally taken to be approximately 1.7 m2, and in the latter study an intravenous dose level of 100 µg/m2 was deemed to be the maximum acceptable level for repeated intravenous doses of MPLA. The maximum individual adult intramuscular dose levels of MPL® used in any FDA-licensed MPL®-adjuvanted GSK vaccine is 50 µg; in contrast, based on previous clinical experiences with malaria and HIV-1 vaccines containing MPL®, the individual intramuscular dose of synthetic MPLA in ALF-type liposomes to be used in several upcoming human clinical trials will be 200 µg ().
5.1. Endotoxin activity of liposomes containing MPLA
It is well-known that the lipid A component of lipopolysaccharide (LPS) on the surface of Gram-negative bacteria is largely, or wholly, responsible for the endotoxin properties of LPS [Citation50,Citation65]. The toxic effect of lipid A is partly reduced by removing the C1 phosphate from the diglucosamine diphosphate headgroup of the lipid A to create MPLA (). However, MPLA still contains reduced levels of endotoxin activity, and these levels are greatly reduced further by incorporating the MPLA in ALF [Citation66]. From a regulatory standpoint, two major types of assays are generally used to detect endotoxin: the Limulus Amebocyte Lysis (LAL) assay (which detects the physical presence of endotoxin in vitro); and the rabbit pyrogenicity assay (which detects endotoxin activity in vivo) [Citation67]. In preclinical studies, the MPLA in ALF was strongly shielded from detection by the LAL assay [Citation68]; and rabbit pyrogenicity of MPL® was also strongly reduced by incorporation into ALF [Citation66].
In the pre-injection analysis for endotoxin activity of the ALF formulations in the above malaria and HIV-1 clinical trials, in which each lot was created under Good Manufacturing Practice (GMP) conditions, at the doses used the ALF adjuvant gave a strong positive signal for the presence of endotoxin when tested by the LAL assay, but each of the GMP-manufactured ALF lots was non-pyrogenic when tested by the rabbit pyrogenicity assay. None of the volunteers who received ALF in any of the malaria or HIV-1 vaccine clinical studies exhibited any injection-related pyrogenicity, thus affirming rabbit pyrogenicity as the gold-standard assay for predicting endotoxin toxicity of ALF in humans. The volunteers in the studies also lacked additional reactions other than those normally associated with the aluminum hydroxide component of the formulation. In each phase 1 study, the aluminum-adsorbed ALF was deemed to be safe as a vaccine adjuvant.
5.2. Preclinical toxicity studies of ALFQ
From an experimental standpoint, when incubated with erythrocytes in vitro, under the conditions illustrated in , the hemolytic activity of QS21 contained in ALFQ was eliminated when compared with free QS21 [Citation12]. Using the standard rabbit pyrogenicity assay, two different lots of GMP-manufactured ALFQ, and four lots of GLP grade ALFQ were tested and found to be non-pyrogenic. Interestingly, raising the mol% of cholesterol to phospholipid of ALF from 33.7% (the level used in the AS01 adjuvant) to 55% (the level used in ALFQ) essentially eliminated the detection of MPLA in the LAL assay [Citation12]. To date, ALFQ has not yet been injected into humans, but upcoming military-associated vaccine phase 1 safety trials with ALFQ as an adjuvant are currently in play (vida infra).
6. Applications of ALF family of adjuvants to military vaccines
Through intramural research at WRAIR, and through interagency collaborations of WRAIR with the Division of AIDS at the National Institute of Allergy and Infectious Diseases at NIH, the National Institute on Drug Abuse at NIH, the Naval Medical Research Center, and through partnerships with private companies, candidate adjuvanted prophylactic vaccines to viral, parasitic, and bacterial diseases have been developed. In addition, because of the perceived need for a potent adjuvant for development of an opioid vaccine, a therapeutic vaccine to opioid addiction is being developed due to a national urgency. All of the above vaccines are in regulatory stages leading to clinical trials. In each case (except the heroin vaccine), a principle rationale underlying the use of ALF, ALFA, ALFQ, or ALFQA lies in the desire to induce not only high levels of antibodies, but also cell-mediated immunity.
6.1. Malaria (Plasmodium falciparum)
The success of the RTS,S/AS01 vaccine for prevention of acquisition of malaria in infants validated the CS protein as a vaccine target, and it also stimulated a search for an improved adjuvanted CS protein vaccine for use in adults. To that end, the WRAIR Malaria Vaccine Branch has produced two new synthetic candidate antigens: Falciparum Malaria Protein-013 (FMP013), a nearly full-length recombinant circumsporozoite protein [Citation59,Citation69]; and FMP014, a self-assembling protein nanoparticle (SAPN) [Citation70]. Interestingly, each of these novel antigens was tested for potential protective efficacy with adjuvants by using a unique mouse intravenous challenge model with an otherwise lethal dose of sporozoites of transgenic P. berghei parasites expressing a functional copy of P. falciparum CS protein ().
Figure 7. Comparative titers of individual mice against full-length CS protein 2-weeks post-third dose; and protection against challenge with otherwise lethal numbers of transgenic P. bergei sporozoites expressing full-length P. falciparum CS protein. Red symbols: protected; black symbols: non-protected mice. Abbreviations: Mont, Montanide ISA 720 VG. The FMP013 cGMP product was stored as frozen bulk protein (B) and in lyophilized form (L). ALFQ formulations with both bulk (ALFQ-B) and lyophilized FMP013 (ALFQ-L) were evaluated while ALF and ALFA were tested only with the lyophilized FMP013. ALFQ-B and ALFQ-L represent ALFQ combined with frozen bulk protein or lyophilized protein. *P < 0.05, **P < 0.01; ***P < 0.0001; ****P < 0.00001. Modified from reference [Citation59].
![Figure 7. Comparative titers of individual mice against full-length CS protein 2-weeks post-third dose; and protection against challenge with otherwise lethal numbers of transgenic P. bergei sporozoites expressing full-length P. falciparum CS protein. Red symbols: protected; black symbols: non-protected mice. Abbreviations: Mont, Montanide ISA 720 VG. The FMP013 cGMP product was stored as frozen bulk protein (B) and in lyophilized form (L). ALFQ formulations with both bulk (ALFQ-B) and lyophilized FMP013 (ALFQ-L) were evaluated while ALF and ALFA were tested only with the lyophilized FMP013. ALFQ-B and ALFQ-L represent ALFQ combined with frozen bulk protein or lyophilized protein. *P < 0.05, **P < 0.01; ***P < 0.0001; ****P < 0.00001. Modified from reference [Citation59].](/cms/asset/eea6070f-015e-4297-a34a-9ab09b0bf630/ierv_a_1745636_f0007_oc.jpg)
The FMP013 antigen is a soluble protein construct which was expressed and purified from E. coli and contains the central repeat (19 NANP+3 NVDP), and the C-terminal and N-terminal regions of the CS protein. In contrast, the RTS,S antigen is ‘hepatitis B virus surface antigen (HBsAg) virus-like particles incorporating a portion of the Plasmodium falciparum-derived circumsporozoite protein genetically fused to HBsAg’ [Citation71]. The differential modest efficacy of the particulate RTS,S/AS01 when compared to the strong efficacy of the soluble shingles/AS01B antigen was part of the rational for selection of a soluble antigen rather than a particulate antigen for upcoming malarial clinical trials. It was noted further that ‘the N-terminal region of CSP [which is present in FMP013 antigen] is not included in RTS,S, although several B and T cell epitopes of CSP have been mapped to this region along with a functional protease cleavage site.’ [Citation59].
The FMP014 antigen, which is an icosahedral shaped nanoparticle, comprises 60 identical monomer chains. Each monomer contains selected P. falciparum CS protein CD4+ and CD8+ epitopes, universal TH epitopes, portions of the α-TSR domain, and six repeats of the NANP motifs of the CS protein [Citation70]. FMP014 has been cGMP manufactured by scientists in the WRAIR Malaria Vaccine Branch in collaboration with a Swiss company (Alpha-O Peptides AG).
For each of the above candidate malaria vaccines, ALFQ was down-selected as an optimal adjuvant, and in each case, volunteers will be enrolled in separate clinical studies; and the first half of each trial will be a phase 1 safety study. In keeping with the concept that small scale human phase 1 trials are more predictive than experiments with rodents for studying potential efficacy [Citation72], the second half of the FMP013 and FMP014 trials will each include a controlled human malaria infection efficacy study in immunized volunteers [Citation73].
6.2. HIV-1
The US. Military HIV Research Program is planning to utilize ALF-family adjuvants in three upcoming human trials at testing sites in Kenya and Thailand. The complexities of the different antigens and strategies are too great to be described in detail here, except with brief descriptions. The study known as RV460, expected to start in Kericho, Kenya in Spring 2020, will examine the feasibility of utilizing various adjuvant strategies to enhance the DNA priming (with DNA encoding HIV-1 gp120 envelope protein), with or without boosting with an adjuvanted recombinant HIV-1 gp145 protein [Citation74]. The adjuvant strategies will include seven arms that will utilize either ALF or ALFA as adjuvants. An additional strategy will utilize transcutaneous immunization enabled by recombinant heat-labile E. coli enterotoxin (dmLT) as an adjuvant, an adjuvanted skin immunization method for human vaccines initiated at WRAIR [Citation9,Citation75].
RV546, a study expected to start in 2020 in Bangkok, Thailand, will boost RV144 volunteers [Citation26] with a gp120 protein adjuvanted with aluminum hydroxide, or with gp120 adjuvanted with ALFQ adsorbed to aluminum hydroxide (ALFQA).
RV509, a study also to be conducted in Thailand, will use a priming immunization with canarypox (ALVAC) and boosting with gp120 adjuvanted with ALFQA in one arm and with full-length single-chain (FLSC) protein adsorbed to aluminum phosphate [Citation76] in the other arm.
6.3. Diarrhea
Among the hazards encountered by military personnel, none is greater than that of diarrhea caused by bacteria, and especially by Campylobacter jejuni, which is among the most common causes of diarrhea [Citation77,Citation78]. The Department of Enteric Diseases at the Naval Medical Research Center has developed a capsule conjugate antigen against C. jejuni strain 81–176 utilizing diphtheria toxin mutant CRM197 as a carrier protein (CPS-CRM). When CPS-CRM adjuvanted with ALFQ was used as a vaccine to immunize non-human primates, it showed the highest protective efficacy, when compared with ALF or aluminum hydroxide adjuvants, against diarrhea following orogastric challenge with C. jejuni [Citation60–Citation62] (). The CPS-CRM antigen adjuvanted with ALFQ will be tested under an FDA-approved investigational new drug application, in collaboration with NIAID/NIH and WRAIR, in a phase 1 trial using ALFQ as an adjuvant.
Table 2. Protective efficacy of immunization with a capsular polysaccharide-CRM (CPS-CRM) conjugate using various adjuvants in a candidate vaccine to prevent diarrhea caused by Campylobacter jejuni in Aotus nancymaae nonhuman primates.
6.4. Heroin and related opioids
The scourge of opioid addition is an international problem that threatens the health of individuals worldwide [Citation79]. Since 2010, in collaboration with the National Institute on Drug Abuse (NIDA/NIH), the WRAIR Laboratory of Adjuvant and Antigen Research has utilized ALF as a potent adjuvant in studies for down-selection of candidate heroin haptens, together with carrier proteins, for development of a therapeutic heroin vaccine [Citation80,Citation81]. The rationale behind this approach is that after intravenous injection of heroin the drug must transit through the blood-brain barrier in order to bind to the mu-receptor in the brain. Because large molecules such as antibodies cannot pass through the blood-brain barrier, antibodies to heroin would serve as a sink to retain the heroin in the blood []. In numerous preclinical studies with rodents, the validity of this strategy was established and optimized. A candidate synthetic hapten (6-AmHap) was conjugated to tetanus toxoid as a carrier protein, and when tested either with ALF or ALFA, high titers of antibodies to heroin and heroin degradation products were achieved. The antibodies also cross-reacted with several other relevant opioids, such as oxycodone and hydrocodone [Citation82]. When challenged intravenously with heroin, the rodent antibodies blocked the characteristic physiological effects of heroin [Citation81]. In collaboration with SUNY Upstate Medical University in Syracuse, a first-in-human clinical trial will be conducted with an estimated start date of Spring 2021 to test this therapeutic heroin vaccine concept.
Figure 8. Schematic illustration of the opioid vaccine and its mechanism. TT-6-AmHap heroin antigen is formulated with the ALFA adjuvant. Upon immunization, the induced antibodies to 6-AmHap react with heroin and its degradation products, and cross-react with other haptenic substances, as shown. The blood-brain barrier is not permeable to large antibody-hapten complexes, and the haptens are thus prevented from binding to the mu-opioid receptor in the brain. Modified from reference [Citation81].
![Figure 8. Schematic illustration of the opioid vaccine and its mechanism. TT-6-AmHap heroin antigen is formulated with the ALFA adjuvant. Upon immunization, the induced antibodies to 6-AmHap react with heroin and its degradation products, and cross-react with other haptenic substances, as shown. The blood-brain barrier is not permeable to large antibody-hapten complexes, and the haptens are thus prevented from binding to the mu-opioid receptor in the brain. Modified from reference [Citation81].](/cms/asset/84af720b-53c9-4300-ba41-aea991e9e8c1/ierv_a_1745636_f0008_oc.jpg)
7. Conclusion
Through a long history of adjuvant development, a group of Army Liposome Formulation constructs has emerged as vaccine adjuvants that exhibit strong potencies for stimulating innate immunity and excellent safety signals. Seven clinical trials are poised for testing of the adjuvants in a variety of vaccines.
8. Expert opinion
Because liposomes can be constructed with a multitude of different physical and chemical characteristics, with differing sizes, surface charges, or membrane flexibilities, they can serve as versatile carriers of different types of antigens or immunostimulants [Citation83]. ALF-based vaccine adjuvants have shown promise for enabling creation of candidate adult vaccines to certain difficult parasitic, bacterial or viral targets, such as P. falciparum malaria, Campylobacter jejuni diarrhea, and HIV-1. Previous clinical studies have also shown that ALF-type adjuvants are effective in candidate vaccines to meningitis caused by group B Neisseria meningitidis [Citation84], and also in a variety of immunotherapeutic cancer vaccines [Citation85–Citation87]; and an ALF-type adjuvant is included in two candidate therapeutic Alzheimer’s disease vaccines (ACI-35 and ACI-24) that are currently in phase 1 or 1/2a testing [Citation88,Citation89]. It seems reasonable to predict that if the results in the upcoming seven military clinical vaccine trials described herein translate into effective results in humans, ALF-based adjuvants could emerge as useful immunostimulants for additional vaccine applications. The success of the commercial AS01B adjuvant in the shingles vaccine has stimulated further promising work toward development of an effective vaccine to tuberculosis [Citation90], and it is possible that this, or other difficult vaccine targets, might also be made feasible with the ALF family of adjuvants.
The emergence of ALF and ALF-based constructs as vaccine adjuvants in military vaccine programs originally occurred because of extensive basic membrane biochemistry research by scientists at WRAIR that led to the understanding of fundamental aspects of the chemical and physical properties of anionic liposome compositions containing cholesterol and either MPLA, or MPLA and QS21 [Citation12,Citation44,Citation91]. The detailed compositions of ALF constructs gradually improved, and will be expected to improve further, because of the focus on basic research, and also because of diverse research and clinical applications of ALF adjuvants. As an example of diverse research, the heroin vaccine program using ALF as an adjuvant originally started in 2010 because of an urgent unmet need by NIDA for access to a potent adjuvant that could provide innate immunity for immunotherapeutic vaccines to drugs of abuse, such as nicotine, cocaine, heroin, oxycodone, and methamphetamine [Citation92]. The introduction of a heroin vaccine could be an additional therapeutic tool to facilitate drug withdrawal by addicted individuals. Because HIV-1 infection is a major problem caused by sharing of dirty needles by injection drug users, the collaboration of WRAIR scientists with NIDA scientists was originally tied to the US Military HIV Research Program mission to develop a prophylactic HIV-1 vaccine, together with the NIDA mission to create an immunotherapeutic heroin vaccine. In response to this, the feasibility of developing a combination ALF-adjuvanted vaccine that targeted both heroin and HIV-1 was demonstrated [Citation93]. As noted earlier, the future in this collaboration lies in the planned first-in-human clinical trial of the jointly developed ALFA-adjuvanted heroin vaccine. The intellectual property covering the ALF-adjuvanted group of novel synthetic heroin haptens was developed as a joint collaboration between WRAIR and NIDA scientists [Citation94].
It is important to remain vigilant to safety issues with liposome-associated adjuvants containing immunostimulants. Harnessing the powers of liposome-associated pathogen-associated molecular patterns (PAMPs) and danger- or damage-associated molecular patterns (DAMPs) to stimulate complex patterns of innate immunity and stimulation of pattern-recognition receptors, requires significant understanding of the molecular architectures of the liposomes themselves that can block the toxic effects while allowing the immunostimulant effects. Balanced against the potency of the shingles/AS01B vaccine formulation, the vaccine is also burdened with increased local reactions and systemic toxicity, when compared with placebo, including local pain, redness, and swelling; and systemic myalgia, fatigue, headache, shivering, fever, and gastrointestinal symptoms [Citation95]. Clearly, these adverse reactions and events, which may be prolonged over several days, did not occur in all individuals and were not so great as to prevent approval for adults aged 50 years and older, but it would be useful to determine the cause of reactogenicity and eliminate it. Post-injection analysis in humans of a hepatitis B vaccine adjuvanted with AS01B (HBsAg-AS01B) revealed that ‘IL-6 and IFN-γ signals were associated with systemic reactogenicity following administration of AS01B-adjuvanted vaccine,’ and based on similar signals found in a hepatitis B vaccine adjuvanted with alum (HBsAg-Alum), it was suggested that ‘… similar innate immune signals may underlie adjuvant reactogenicity and immunogenicity’ [Citation96]. Thus, one proposed mechanism of reactogenicity of an adjuvanted vaccine is that reactogenicity due to inflammatory processes caused by the adjuvant are inherently present as part of the mechanism of potency. While this might or might not always be true, depending on the adjuvant or the antigen, it is clear that pain and reactogenicity after any needle injection can be caused by multiple inciting factors [Citation97]. However, another possibility is that the inherent toxicities of MPLA and/or QS21 are not completely quenched by the particular architecture of AS01B liposomes which contain unsaturated bulk dioleoyl phosphatidylcholine phospholipids. The upcoming clinical safety trials with ALFQ-based liposomes that have only saturated bulk phospholipids might provide some resolution regarding the relationships between reactogenicity and potency of liposomes containing MPLA and QS21. Based on the previous clinical experience with ALF-adjuvanted vaccines, ALFQ might exhibit low reactogenicity but higher potency. This hypothesis will be tested over the next 5 years in the upcoming human trials with ALFQ in which safety and immunogenicity will be major areas of examination. Irrespective of reactogenicity, it is our hypothesis that the adjuvant mechanisms of ALFQ and AS01B are both directly related to the 10 carbohydrate display that forms a ‘sugar lawn’ on the surface of both AS01B and ALFQ liposomes. The future may well lie in a detailed analysis of the roles of those carbohydrates with the thought that liposomes displaying MPLA and QS21 might simply be serving as the carriers of the sugars [Citation98,Citation99].
Article Highlights
The Army Liposome Formulation (ALF) adjuvant was first conceived in the 1980s and designed, manufactured, and tested by the US Army (and collaborators) in human trials of candidate malaria and HIV-1 vaccines in the 1990s.
Safety and immunological effectiveness of ALF containing huge amounts of liposomal monophosphoryl lipid A (>1 mg, or >2 mg) was established in three human safety trials in the 1990s.
A commercial liposomal adjuvant formulation, known as AS01, containing both monophosphoryl lipid A and QS21 saponin as adjuvants, proved successful and was used as the adjuvant for an FDA-licensed shingles vaccine in 2017 in the United States for adults >50 years of age. AS01 was also approved in Europe as the adjuvant for a malaria vaccine for infants in three sub-Saharan countries in Africa.
Inspired by the success of AS01, the US Army created and developed two novel improvements of ALF, starting in 2015: ALF adsorbed to aluminum hydroxide (ALFA); and ALF which contained both monophosphoryl lipid A and QS21 saponin (ALFQ).
ALFQ was strongly potent as a liposomal vaccine adjuvant in rodents and non-human primates; and it was non-pyrogenic and nontoxic in preclinical studies.
Seven upcoming human safety and immunogenicity trials to be conducted by military scientists and collaborators are planned which will employ members of the ALF family of adjuvants in three candidate HIV-1 vaccines, two candidate malaria vaccines, and a candidate bacterial (Campylobacter jejuni) diarrhea vaccine. A seventh human trial is planned with ALFA as the adjuvant in an immunotherapeutic heroin vaccine.
Disclaimer
The views expressed are those of the authors and should not be construed to represent the positions of the US Army or the Department of Defense.
Declaration of Interest
C Alving is a co-inventor on patents for ALFQ, gp145 HIV-1 protein, and heroin vaccine assigned to the US Army. G R Matyas is a co-inventor on patents for gp145 HIV-1 protein, and heroin vaccine assigned to the US Army. M Rao is a co-inventor on patent for gp145 HIV-1 protein assigned to the US Army. Z Beck is co-inventor on patent for ALFQ assigned to the US Army. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
Notes
1. Five non-aluminum adjuvant components in FDA-approved vaccines include: monophosphoryl lipid A (MPL®) (in Cervarix®, a human papillomavirus vaccine; and in Shingrix®, a shingles vaccine); AS03, an oil-in-water emulsion (in stockpiled H5N1 influenza vaccine); MF59, an oil-in-water emulsion (in Fluad®, an influenza vaccine); CpG (in Heplisav-B®, a hepatitis B virus vaccine); and QS21 (in Shingrix®, a shingles vaccine).
References
- Griesenauer RH, Kinch MS. An overview of FDA-approved vaccines & their innovators. Expert Rev Vaccines. 2017 Dec;16(12):1253–1266.
- Artenstein AW, Opal JM, Opal SM, et al. History of U.S. military contributions to the study of vaccines against infectious diseases. Mil Med. 2005 Apr;170(4 Suppl):3–11. .
- Grabenstein JD, Pittman PR, Greenwood JT, et al. Immunization to protect the US Armed Forces: heritage, current practice, and prospects. Epidemiol Rev. 2006;28(1):3–26. .
- Ratto-Kim S, Yoon IK, Paris RM, et al. The US military commitment to vaccine development: a century of successes and challenges. Front Immunol. 2018;9:1397.
- Salk JE, Contakos M, Laurent AM, et al. Use of adjuvants in studies on influenza immunization. III. Degree of persistence of antibody in human subjects two years after vaccination. J Am Med Assoc. 1953 Apr 4;151(14):1169–1175.
- Beebe GW, Simon AH, Vivona S. Follow-up study on army personnel who received adjuvant influenza virus vaccine 1951–1953. Am J Med Sci. 1964 Apr;247:385–406.
- Meiklejohn G. Adjuvant influenza adenovirus vaccine. JAMA. 1962 Feb;24(179):594–597.
- Alving CR, Richards RL, Moss J, et al. Effectiveness of liposomes as potential carriers of vaccines: applications to cholera toxin and human malaria sporozoite antigen. Vaccine. 1986 Sep;4(3):166–172.
- Alving CR, Peachman KK, Rao M, et al. Adjuvants for human vaccines. Curr Opin Immunol. 2012 Jun;24(3):310–315.
- Alving CR, Detrick B, Richards RL, et al. Novel adjuvant strategies for experimental malaria and AIDS vaccines. Ann N Y Acad Sci. 1993 Aug 12;690:265–275.
- Matyas GR, Muderhwa JM, Alving CR. Oil-in-water liposomal emulsions for vaccine delivery. Methods Enzymol. 2003;373:34–50.
- Beck Z, Matyas GR, Alving CR. Detection of liposomal cholesterol and monophosphoryl lipid A by QS-21 saponin and Limulus polyphemus amebocyte lysate. Biochim Biophys Acta. 2015 Mar;1848(3):775–780.
- White WI, Cassatt DR, Madsen J, et al. Antibody and cytotoxic T-lymphocyte responses to a single liposome-associated peptide antigen. Vaccine. 1995 Aug;13(12):1111–1122. .
- Baylor NW, Egan W, Richman P. Aluminum salts in vaccines–US perspective. Vaccine. 2002 May 31;20(Suppl 3):S18–23.
- FDA. 2019 [cited 2019 Aug 10]. Available from: https://www/fda.gov/vaccines-blood-biologics/safety-availability-biologics/common-ingredients-us-licensed-vaccines
- Garcon N, Chomez P, Van Mechelen M. GlaxoSmithKline adjuvant systems in vaccines: concepts, achievements and perspectives. Expert Rev Vaccines. 2007 Oct;6(5):723–739.
- Vandepapeliere P, inventor; Vaccine compositions comprising a saponin adjuvant. patent 10,143,745. 2018.
- Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015 May 28;372(22):2087–2096.
- Association American Pharmacist. 2019 [cited 2019 Oct 16]. Available from: https://www.pharmacist.com/shigles-vaccine-shortage-apha-recommends-patients-take-these-9-steps
- Fries LF, Gordon DM, Richards RL, et al. Liposomal malaria vaccine in humans: a safe and potent adjuvant strategy. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):358–362.
- Heppner DG, Gordon DM, Gross M, et al. Safety, immunogenicity, and efficacy of plasmodium falciparum repeatless circumsporozoite protein vaccine encapsulated in liposomes. J Infect Dis. 1996 Aug;174(2):361–366. .
- Walker MC. Phase I/II preventive vaccine trials: conference summary. AIDS Res Hum Retroviruses. 1995;11(10):1279–1285.
- Goldenthal K, Cavagnaro JA, Alving CR, et al. Safety evaluation of vaccine adjuvant: national cooperative vaccine development meeting working group. AIDS Res Hum Retroviruses. 1993;9(Suppl 1):S47–S51.
- McElrath MJ. Selection of potent immunological adjuvants for vaccine construction. Semin Cancer Biol. 1995 Dec;6(6):375–385.
- Rao M, Onkar S, Peachman KK, et al. Liposome-encapsulated human immunodeficiency virus-1 gp120 induces potent V1V2-specific antibodies in humans. J Infect Dis. 2018 Oct 5;218(10):1541–1550.
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009 Dec 3;361(23):2209–2220.
- Om K, Paquin-Proulx D, Montero M, et al. Adjuvanted HIV-1 vaccine protects against heterologous SHIV challenge and promotes antibody-dependent phagocytic responses. Currently under review. 2020.
- Haynes BF, Gilbert PB, McElrath MJ, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012 Apr 5;366(14):1275–1286.
- Karasavvas N, Billings E, Rao M, et al. The Thai Phase III HIV Type 1 Vaccine trial (RV144) regimen induces antibodies that target conserved regions within the V2 loop of gp120. AIDS Res Hum Retroviruses. 2012 Nov;28(11):1444–1457. .
- Rolland M, Edlefsen PT, Larsen BB, et al. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature. 2012 Oct 18;490(7420):417–420.
- Zolla-Pazner S, deCamp A, Gilbert PB, et al. Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PLoS One. 2014;9(2):e87572. .
- Kim JH, Excler JL, Michael NL. Lessons from the RV144 Thai phase III HIV-1 vaccine trial and the search for correlates of protection. Annu Rev Med. 2015;66(1):423–437.
- Hsu DC, O’Connell RJ. Progress in HIV vaccine development. Hum Vaccin Immunother. 2017 May 4;13(5):1018–1030.
- Duerr R, Gorny MK. V2-Specific Antibodies in HIV-1 vaccbine research and natural infection: controllers or surrogate markers. Vaccines (Basel). 2019 Aug 6;7(3):E82
- Rao M, Peachman KK, Li Q, et al. Highly effective generic adjuvant systems for orphan or poverty-related vaccines. Vaccine. 2011 Jan 29;29(5):873–877.
- Heppner Jr DG, Kester KE, Ockenhouse CF, et al. Towards an RTS,S-based, multi-stage, multi-antigen vaccine against falciparum malaria: progress at the Walter Reed Army Institute of Research. Vaccine. 2005 Mar 18;23(17–18):2243–2250.
- European Medicines Agency. First malaria vaccine receives positive scientific opinion from EMA. 2015 [2019 Oct 10]. Available from: https://www.ema.europa.eu/en/news/first-malaria-vaccine-recieves-positive-scientific-opinion-ema
- World Heatlth Organization. Update on RTS,S Malaria Vaccine Implementation Programme. Geneva, Switzerland 2019 [ cited 2019 Oct 10]. Available from: https://www.who.int/malria/mpac/mpac-april2019-session3-rtss-mvip-update.pdf?ua=1
- Agnandji ST, Lell B, Fernandes JF, et al; RTSS Clinical Trials Partnership. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med. 2012 Dec 13;367(24):2284–2295.
- Dimala CA, Kika BT, Kadia BM, et al. Current challenges and proposed solutions to the effective implementation of the RTS,S/AS01 Malaria Vaccine Program in sub-Saharan Africa: a systematic review. PLoS One. 2018;13(12):e0209744.
- Alving C, Beck Z, inventors; Non-toxic adjuvant formulation comprising a monophosphoryl lipid A (MPLA)-containing liposome composition and a saponin. US patent 10434167. 2019 2019 Oct 8.
- Top H, Sarikahya NB, Nalbantsoy A, et al. Immunomodulatory, hemolytic properties and cytotoxic activity potent of triterpenoid saponins from Cephalaria balansae. Phytochemistry. 2017;137:139–147.
- Beck Z, Matyas GR, Jalah R, et al. Differential immune responses to HIV-1 envelope protein induced by liposomal adjuvant formulations containing monophosphoryl lipid A with or without QS21. Vaccine. 2015 Oct 13;33(42):5578–5587.
- Singh P, Beck Z, Matyas GR, et al. Saturated phospholipids are required for nano- to micron-size transformation of cholesterol-containing liposomes upon QS21 addition. J Liposome Res. 2019 Sep;29(3):247–250.
- Lombardo DCP, Caccamo MT, Magazù S, et al. Colloidal stability of liposomes. AIMS Mat Sci. 2019;6(2):200–213.
- Kotani S, Takada H, Tsujimoto M, et al. Synthetic lipid A with endotoxic and related biological activities comparable to those of a natural lipid A from an Escherichia coli re-mutant. Infect Immun. 1985 Jul;49(1):225–237.
- Homma JY, Matsuura M, Kanegasaki S, et al. Structural requirements of lipid A responsible for the functions: a study with chemically synthesized lipid A and its analogues. J Biochem. 1985 Aug;98(2):395–406.
- Rietschel ET, Kirikae T, Schade FU, et al. Bacterial endotoxin: molecular relationships of structure to activity and function. Faseb J. 1994 Feb;8(2):217–225.
- Xiao X, Sankaranarayanan K, Khosla C. Biosynthesis and structure-activity relationships of the lipid A family of glycolipids. Curr Opin Chem Biol. 2017 Oct;40:127–137.
- Beutler B, Rietschel ET. Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol. 2003 Feb;3(2):169–176.
- Verma JN, Rao M, Amselem S, et al. Adjuvant effects of liposomes containing lipid A: enhancement of liposomal antigen presentation and recruitment of macrophages. Infect Immun. 1992 Jun;60(6):2438–2444.
- Rao M, Alving CR. Delivery of lipids and liposomal proteins to the cytoplasm and Golgi of antigen-presenting cells. Adv Drug Deliv Rev. 2000 Mar 30; 41(2):171–188.
- Alving CR, Rao M, Steers NJ, et al. Liposomes containing lipid A: an effective, safe, generic adjuvant system for synthetic vaccines. Expert Rev Vaccines. 2012 Jun;11(6):733–744.
- Marciani DJ. Is fucose the answer to the immunomodulatory paradox of Quillaja saponins? Int Immunopharmacol. 2015 Dec;29(2):908–913.
- Szebeni J, Baranyi L, Savay S, et al. The interaction of liposomes with the complement system: in vitro and in vivo assays. Methods Enzymol. 2003;373:136–154.
- Wibroe PP, Ahmadvand D, Oghabian MA, et al. An integrated assessment of morphology, size, and complement activation of the PEGylated liposomal doxorubicin products Doxil(R), Caelyx(R), DOXOrubicin, and SinaDoxosome. J Control Release. 2016 Jan;10(221):1–8.
- Ricklin D, Hajishengallis G, Yang K, et al. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010 Sep;11(9):785–797.
- Beck Z, Torres OB, Matyas GR, et al. Immune response to antigen adsorbed to aluminum hydroxide particles: effects of co-adsorption of ALF or ALFQ adjuvant to the aluminum-antigen complex. J Control Release. 2018 Apr;10(275):12–19.
- Genito CJ, Beck Z, Phares TW, et al. Liposomes containing monophosphoryl lipid A and QS-21 serve as an effective adjuvant for soluble circumsporozoite protein malaria vaccine FMP013. Vaccine. 2017 Jul 5;35(31):3865–3874.
- Ramakrishnan A, Schumack NM, Gariepy CL, et al. Enhanced immunogenicity and protective efficacy of a campylobacter jejuni conjugate vaccine coadministered with liposomes containing monophosphoryl lipid A and QS-21. mSphere. 2019 May 1;4:3:e00101-19.
- Ramakrishnan A, Schumack NM, Gariepy CL, et al. Correction for Ramakrishnan et al. Enhanced immunogenicity and protective efficacy of a campylobacter jejuni conjugate vaccine coadministered with liposomes containing monophosphoryl lipid A and QS-21”. mSphere. 2019 May 22;4(3):e00351-19.
- Ramakrishnan A, Schumack NM, Gariepy CL, et al. Erratum for Ramakrishnan et al. Enhanced immunogenicity and protective efficacy of a campylobacter jejuni conjugate vaccine coadministered with liposomes containing monophosphoryl lipid A and QS-21”. mSphere. 2019 Jun 26;4(3):e00440-19.
- Buckley PR, Alden K, Coccia M, et al. Application of modeling approaches to explore vaccine adjuvant mode-of-action. Front Immunol. 2019;10:2150.
- Vosika GJ, Barr C, Gilbertson D. Phase-I study of intravenous modified lipid A. Cancer Immunol Immunother. 1984;18(2):107–112.
- Takada H, Kawabata Y, Tamura M, et al. Cytokine induction by extracellular products of oral viridans group streptococci. Infect Immun. 1993 Dec;61(12):5252–5260.
- Richards RL, Swartz GM, Schultz C, et al. Immunogenicity of liposomal malaria sporozoite antigen in monkeys: adjuvant effects of aluminium hydroxide and non-pyrogenic liposomal lipid A. Vaccine. 1989 Dec;7(6):506–512.
- FDA. 2012 [updated 2018 Mar 22; cited 2019 Oct 20]. Available from: https:www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-pyrogen-and-endotoxins-testing-questions-and-answers#_ftn1
- Richards RL, Aronson J, Schoenbechler M, et al. Antibodies reactive with liposomal phospholipids are produced during experimental Trypanosoma rhodesiense infections in rabbits. J Immunol. 1983 Mar;130(3):1390–1394.
- Cawlfield A, Genito CJ, Beck Z, et al. Safety, toxicity and immunogenicity of a malaria vaccine based on the circumsporozoite protein (FMP013) with the adjuvant army liposome formulation containing QS21 (ALFQ). Vaccine. 2019 Jun 27;37(29):3793–3803.
- Seth L, Bingham Ferlez KM, Kaba SA, et al. Development of a self-assembling protein nanoparticle vaccine targeting Plasmodium falciparum Circumsporozoite Protein delivered in three Army Liposome Formulation adjuvants. Vaccine. 2017 Oct 4;35(41):5448–5454.
- Kaslow DC, Biernaux S. RTS,S: toward a first landmark on the Malaria Vaccine Technology Roadmap. Vaccine. 2015 Dec 22;33(52):7425–7432.
- Alving CR. Design and selection of vaccine adjuvants: animal models and human trials. Vaccine. 2002 May 31;20(Suppl 3):S56–64.
- Regules JA, Cicatelli SB, Bennett JW, et al. Fractional third and fourth dose of RTS,S/AS01 malaria candidate vaccine: a phase 2a controlled human malaria parasite infection and immunogenicity study. J Infect Dis. 2016 Sep 1;214(5):762–771.
- Wieczorek L, Krebs SJ, Kalyanaraman V, et al. Comparable antigenicity and immunogenicity of oligomeric forms of a novel, acute HIV-1 subtype C gp145 envelope for use in preclinical and clinical vaccine research. J Virol. 2015 Aug;89(15):7478–7493.
- Glenn GM, Taylor DN, Li X, et al. Transcutaneous immunization: a human vaccine delivery strategy using a patch. Nat Med. 2000 Dec;6(12):1403–1406.
- Gordon SN, Liyanage NP, Doster MN, et al. Boosting of ALVAC-SIV vaccine-primed macaques with the CD4-SIVgp120 fusion protein elicits antibodies to V2 associated with a decreased risk of SIVmac251 acquisition. J Immunol. 2016 Oct 1;197(7):2726–2737.
- Venkatesan MM. Van de Verg LL. Combination vaccines against diarrheal diseases. Hum Vaccin Immunother. 2015;11(6):1434–1448.
- Agnetti J, Seth-Smith HMB, Ursich S, et al. Clinical impact of the type VI secretion system on virulence of Campylobacter species during infection. BMC Infect Dis. 2019 Mar 7;19(1):237.
- United Nations Office on Drugs and Crime. World Drug Report 2019, Book 1. Executive Summary. 2019. (E.19.XI.8 UNpSN, editor.).
- Matyas GR, Mayorov AV, Rice KC, et al. Liposomes containing monophosphoryl lipid A: a potent adjuvant system for inducing antibodies to heroin hapten analogs. Vaccine. 2013 Jun 10;31(26):2804–2810.
- Sulima A, Jalah R, Antoline JFG, et al. A stable heroin analogue that can serve as a vaccine hapten to induce antibodies that block the effects of heroin and its metabolites in rodents and that cross-react immunologically with related drugs of abuse. J Med Chem. 2018 Jan 11;61(1):329–343.
- Torres OB, Duval AJ, Sulima A, et al. A rapid solution-based method for determining the affinity of heroin hapten-induced antibodies to heroin, its metabolites, and other opioids. Anal Bioanal Chem. 2018 Jun;410(16):3885–3903.
- Wang N, Chen M, Wang T. Liposomes used as a vaccine adjuvant-delivery system: from basics to clinical immunization. J Control Release. 2019 Jun;10(303):130–150.
- Zollinger WD, Babcock JG, Moran EE, et al. Phase I study of a Neisseria meningitidis liposomal vaccine containing purified outer membrane proteins and detoxified lipooligosaccharide. Vaccine. 2012 Jan 17;30(4):712–721.
- Harris DT, Matyas GR, Gomella LG, et al. Immunologic approaches to the treatment of prostate cancer. Semin Oncol. 1999 Aug;26(4):439–447.
- Sangha R, Butts C. L-BLP25: a peptide vaccine strategy in non small cell lung cancer. Clin Cancer Res. 2007 Aug 1;13(15 Pt 2):s4652–4.
- Wrona A. Role of immunotherapy in stage III nonsmall cell lung cancer. Curr Opin Oncol. 2019 Jan;31(1):18–23.
- Theunis C, Crespo-Biel N, Gafner V, et al. Efficacy and safety of a liposome-based vaccine against protein Tau, assessed in tau.P301L mice that model tauopathy. PLoS One. 2013;8(8):e72301.
- AC Immune Pipeline 2019 cited 2019 Nov 11. Available from: https://www.acimmune.com/en/alzheimer-s-disease-ad/
- Van Der Meeren O, Hatherill M, Nduba V, et al. Phase 2b controlled trial of M72/AS01E vaccine to prevent tuberculosis. N Engl J Med. 2018 Oct 25;379(17):1621–1634.
- Banerji B, Alving CR. Lipid A from endotoxin: antigenic activities of purified fractions in liposomes. J Immunol. 1979 Dec;123(6):2558–2562.
- Alving CR, Matyas GR, Torres O, et al. Adjuvants for vaccines to drugs of abuse and addiction. Vaccine. 2014 Sep 22;32(42):5382–5389.
- Torres O, Matyas GR, Rao M, et al. Heroin-HIV-1 (H2) vaccine: induction of dual immunologic effects with a heroin hapten-conjugate and an HIV-1 envelope V2 peptide with liposomal lipid A as an adjuvant. Npj Vaccines. 2017;2(13). DOI:10.1038/s41541-017-0013-9.
- Alving C, Matyas G, Mayorov A, et al., inventors Induction of highly specific antibodies to a hapten but not to a carrier peptide by immunization. US patent 9193739. 2015.
- GlaxoSmithKline package insert for Shingrix. Highlights of prescribing information. 2019. Available from: https://gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Shingrix/pdf/SHINGRIX.PDF
- Burny W, Marchant A, Herve C, et al. Inflammatory parameters associated with systemic reactogenicity following vaccination with adjuvanted hepatitis B vaccines in humans. Vaccine. 2019 Mar 28;37(14):2004–2015.
- Herve C, Laupeze B, Del Giudice G, et al. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines. 2019;4:39.
- Hu J, Qiu L, Wang X, et al. Carbohydrate-based vaccine adjuvants - discovery and development. Expert Opin Drug Discov. 2015 Oct;10(10):1133–1144.
- Tokatlian T, Read BJ, Jones CA, et al. Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. Science. 2019 Feb 8;363(6427):649–654.