ABSTRACT
Introduction
Infants too young to be fully immunized are the most vulnerable to severe pertussis disease. To close this susceptibility gap, passive infant immunization through vaccination of pregnant women against pertussis was first introduced in 2011 in the United States and has been extended since then to more than 40 countries.
Areas covered
We conducted two systematic literature searches to describe the worldwide burden of pertussis disease in infants <6 months of age since 2005, and the effectiveness and impact of maternal pertussis vaccination in preventing infant pertussis since 2011.
Expert opinion
Pertussis disease incidence rates in infants aged <2-3 months were substantial in all countries with available data, exceeding 1000 cases per 100,000 population during outbreaks. Virtually all pertussis deaths occurred in this age group. Data from Africa, Eastern Mediterranean, and Asia were limited, but suggest a similar or higher disease burden than in Europe or the Americas. Estimates of effectiveness of second/third trimester pertussis vaccination in preventing pertussis disease in <2-3 months old infants were consistently high (69%–93%) across the observational studies reviewed, conducted in various settings with different designs. Maternal vaccination programs appear to be achieving their goal of reducing the burden of disease in very young infants.
Plain language summary
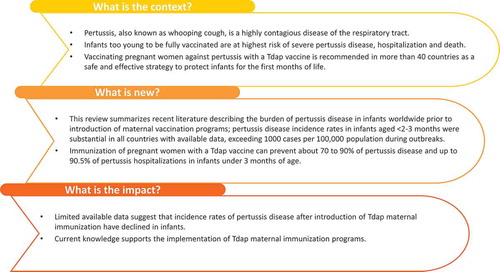
What is the context?
Pertussis, also known as whooping cough, is a highly contagious disease of the respiratory tract.
Infants too young to be fully vaccinated are at the highest risk of severe pertussis disease, hospitalization, and death.
Vaccinating pregnant women against pertussis with a Tdap vaccine is recommended in more than 40 countries as a safe and effective strategy to protect infants for the first months of life.
What is new?
This review summarizes recent literature describing the burden of pertussis disease in infants worldwide prior to the introduction of maternal vaccination programs; pertussis disease incidence rates in infants aged <2-3 months were substantial in all countries with available data, exceeding 1000 cases per 100,000 population during outbreaks.
Immunization of pregnant women with a Tdap vaccine can prevent about 70–90% of pertussis disease and up to 90.5% of pertussis hospitalizations in infants under 3 months of age.
What is the impact?
Limited available data suggest that incidence rates of pertussis disease after the introduction of Tdap maternal immunization have declined in infants.
Current knowledge supports the implementation of Tdap maternal immunization programs.
1. Introduction
Pertussis (whooping cough) is a highly contagious disease of the respiratory tract caused by Bordetella pertussis. Pertussis outbreaks have a cyclical pattern with natural peaks that occur every 3–5 years. Pertussis is endemic worldwide and is still difficult to control, despite decades of universal childhood vaccination [Citation1,Citation2]. Despite high vaccine coverage with either whole-cell (wP) or acellular (aP) pediatric pertussis vaccines in most parts of the world, pertussis continues to cause a substantial disease burden. Pertussis incidence has also been steadily rising in the last two decades in several countries with high vaccination coverage. This resurgence may be explained by multiple factors, such as increased disease awareness and improved diagnosis, faster waning of immunity following vaccination with aP compared to wP vaccines, and/or genetic changes in the bacterium. While approximately 60% of pertussis cases occur in adults and adolescents, the highest incidence of disease and the highest mortality rates occur in infants aged <2 months who are too young to be vaccinated [Citation3,Citation4]. In 2013, the Global Burden of Disease Study estimated mortality due to pertussis in the first year of life to be approximately 400 per million live births, or approximately 56,000 deaths annually [Citation5].
‘Cocooning’ is a strategy that has been implemented to reduce the risk of pertussis transmission from mothers or family members to infants, by vaccinating women immediately postpartum, and all other close contacts with a combined diphtheria-tetanus-aP booster vaccine (Tdap). However, cocooning alone has proven to be insufficient to prevent pertussis morbidity and mortality in newborn infants [Citation6]. In 2011, the United States (US) was the first country to recommend Tdap vaccination during pregnancy to protect infants in the first months of life through the passive transfer of maternal antibodies [Citation7]. Several countries followed suite in 2012, including the United Kingdom (UK) in response to rising infant deaths during a pertussis epidemic in 2012 [Citation8]. Vaccine effectiveness in preventing laboratory-confirmed pertussis in infants <3 months of age was more than 90% in the first 3 years of the UK program [Citation9,Citation10]. This was the first published evidence of the clinical benefit of a Tdap maternal immunization program [Citation11]. Recommendations for maternal Tdap immunization now exist, with some differences in the recommended vaccination window, in over 40 countries () [Citation12–Citation14]. Though evidence of the safety and benefits of maternal Tdap immunization is steadily increasing, many countries still do not have any recommendation concerning Tdap immunization in pregnancy. Where recommendations are in place, information on vaccine coverage is not always available or varies widely, ranging, for example, from 48.8% in the US in 2017 to 93.7% in the Bizkaia province of Spain [Citation15,Citation16].
Table 1. Recommendations for maternal immunization with tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) for each pregnancy in countries with a Tdap maternal immunization program (accurate to our knowledge at the time of writing).
Overall, studies of Tdap immunization of pregnant women showed that vaccination is well tolerated and immunogenic, resulting in transfer of high antibody levels to the fetus ([Citation45–Citation47] and reviewed in [Citation48–Citation50]). Fewer studies have evaluated maternal Tdap vaccine effectiveness in preventing pertussis disease in infants, or the potential impact of the timing of vaccination, or underlying medical conditions such as human immunodeficiency virus (HIV) infection, on effectiveness. In addition, numerous studies describe the burden of disease due to pertussis in infants. However, systematic reviews of the available data are lacking. We conducted two independent systematic literature searches to identify, analyze, and critically appraise the most recent evidence describing the burden of pertussis disease in infants <6 months of age worldwide; Tdap vaccine effectiveness in preventing pertussis in young infants (<2-3 months of age) before commencement of the primary DTaP immunization series; and the impact of Tdap maternal immunization programs on the epidemiology of pertussis in infants up until 1 year of age.
2. Methods
2.1. Objectives
The first study objective was to evaluate the global burden of pertussis disease in infants <6 months of age, stratified by country and by age <3 months and 3–6 months when data allowed. Information on the study design, pertussis surveillance system, the number and incidence of reported/estimated pertussis cases, pertussis-related hospitalizations, deaths, and duration of hospitalization was retrieved.
The second objectives were to summarize worldwide data describing the effectiveness of Tdap immunization during pregnancy at preventing pertussis in young infants (less than 2–3 months of age) before commencement of the primary immunization series; and to summarize the impact of Tdap vaccination during pregnancy on the epidemiology of pertussis disease in infants up to 1 year of age.
The selected literature was restricted to prospective or retrospective observational studies or data from notifiable disease surveillance systems. Information on the other aspects of maternal immunization including safety and immunogenicity was not considered in this review and have been described elsewhere [Citation48–Citation54].
2.2. Article identification, selection and data extraction
The literature search for each objective was carried out according to a pre-established protocol and followed The Cochrane Collaboration guidelines for performing systematic reviews [Citation55]. We searched the PubMed, Embase, and Cochrane Library databases for peer-reviewed articles published between 1 January 2005 and 4 July 2018 for objective 1, and 1 January 2011 and 4 July 2018 for objective 2, using queries specific to each objective. Search queries are provided in Supplementary Table S1 and S2. Grey literature including health authority websites was searched in addition to the peer-reviewed data as described in the on-line supplement. Searches were not limited by language or geography.
Inclusion and exclusion criteria for articles relevant to the study objectives are provided in the online supplement. In brief, articles were selected if they reported outcomes relevant to the study objective. Systematic reviews, meta-analyses, narrative reviews, case reports, and modeling studies were not included. However, systematic reviews and meta-analyses were checked for any potentially overlooked articles.
For each search, titles and abstracts were screened for relevance in duplicate by two researchers, and articles of possible interest underwent full-text screening. The first 10% of articles were appraised in duplicate, with any disagreement between the appraisers being adjudicated by a third researcher. Further scrutiny of the articles was performed during the data-extraction phase. For each literature search, evidence tables were compiled by a senior epidemiologist and reviewed by a second epidemiologist.
The burden of disease was assessed using the following outcomes: (1) number and incidence of reported cases and estimated cases by year, (2) number and incidence of reported and estimated hospitalizations by year, (3) duration of hospitalization, and (4) number and incidence of pertussis-related deaths by year. Denominators of incidence, hospitalization, and mortality rates extracted during the burden of disease assessment referred to the general population (age-specific) unless stated otherwise.
For the second objective, vaccine effectiveness (VE) of Tdap administered during pregnancy against pertussis in infants was determined. If VE was not reported in an article, it was derived from the association between Tdap vaccination during pregnancy and the endpoint of interest (pertussis disease, or pertussis hospitalization, or pertussis death) in infants, measured either as relative risks (RRs), odds ratios (ORs), or hazard ratios (HRs), where VE = (1-RR)x100, (1-OR)x100, or (1-HR)x100. The impact of Tdap vaccination during pregnancy on the epidemiology of pertussis in infants was assessed from studies that reported the following outcomes: (1) (change in) number and incidence of pertussis cases and (2) number and incidence of pertussis-related deaths by year, according to the time point of maternal immunization for each country for which data were available.
2.3. Study quality assessment
Existing quality assessment tools were not suited to many of the study designs reported here. A GRADE analysis (Grading of Recommendations Assessment, Development, and Evaluation) [Citation56] was planned for studies of vaccine effectiveness. However, this proved not possible because the outcomes differed per study and could not be synthesized. Furthermore, data as presented in the included articles on effectiveness needed some recalculation in order to fit a GRADE profile, and we preferred to present the data as in the original articles.
The quality of studies reporting outcomes in terms of population-based rates was therefore evaluated using an in-house quality assessment tool provided in Section 3.0 in the online supplement. According to this tool, any article that contained a ‘fatal error’ (an element that could throw doubt on the main study conclusions) was excluded from the search, while all other articles were included irrespective of the overall rating score (strong, moderate, or weak). Studies describing follow-up of a group of patients were not suited for appraisal with existing checklists or the quality assessment tool mentioned above, and thus were not critically appraised.
3. Results
3.1. Burden of pertussis disease in infants
The search for articles on the burden of pertussis disease yielded 3,359 unique records in the electronic databases, of which 67 articles were included in the final analysis (). Additional information was found in the gray literature for four countries. Many of the articles were conducted in one or several hospitals and reported absolute case numbers, hospitalizations, or deaths. Key characteristics and results of all included studies are provided in Table S3. There were 22 articles that reported population-based incidence rates of pertussis disease, hospitalization, or deaths, and these results are summarized below by WHO region in –. Data are expressed as either per 1000 person-months or per 100,000 population.
Figure 1. PRISMA flow diagrams for the two systematic literature searches performed for this review.
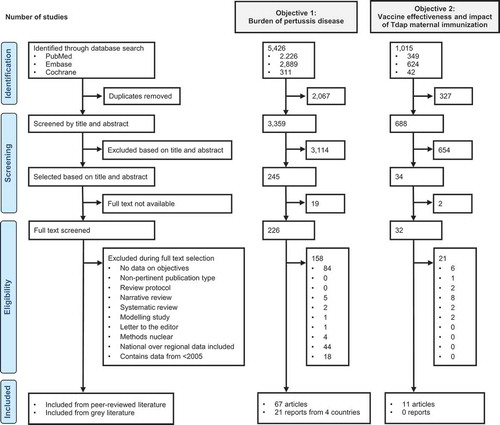
Figure 2. Pertussis disease incidence and hospitalization rates in infants <6 months of age in studies reporting results by person-time [Citation57–Citation59,Citation63,Citation69,Citation78,Citation81,Citation83].
![Figure 2. Pertussis disease incidence and hospitalization rates in infants <6 months of age in studies reporting results by person-time [Citation57–Citation59,Citation63,Citation69,Citation78,Citation81,Citation83].](/cms/asset/152d318b-395c-4c71-aeb3-568e44564110/ierv_a_1791092_f0002_b.gif)
Figure 4. Pertussis-related hospitalizations per 100,000 population in infants <6 months of age [Citation71,Citation72,Citation75,Citation76,Citation86].
![Figure 4. Pertussis-related hospitalizations per 100,000 population in infants <6 months of age [Citation71,Citation72,Citation75,Citation76,Citation86].](/cms/asset/918e88ab-922c-4b47-bed7-f27deb31031c/ierv_a_1791092_f0003_oc.jpg)
Direct comparison of results needs to be made cautiously because of heterogeneity between study designs, data sources, pertussis case definitions, periods analyzed and populations and study settings considered. Several studies were conducted over short time periods or a single season and were unable to take into account the cyclic pattern of pertussis disease outbreaks. Estimates of the disease burden are markedly affected by case detection rates, and the studies we reviewed used clinical symptoms, laboratory-confirmation, epidemiological links, and/or International Classification of Disease (ICD) codes in combination or alone, which may differ in their sensitivity and specificity, as methods of case detection.
3.1.1. Region of Africa
Eight studies describe the burden of pertussis disease data in children <6 months of age from 4 countries in Africa: Algeria, Niger, South Africa (five studies), and Zambia. None of the countries had recommendations for maternal Tdap vaccination in place during the study periods.
Six of the eight studies were hospital-based observational studies and 2 were prospective longitudinal cohort studies; one conducted in mothers previously enrolled in an influenza vaccine trial in South Africa, and one in a cohort of mother-infant pairs from a peri-urban slum in Zambia [Citation57,Citation58]. The studies were conducted between 2008 and 2015 and in 5 studies, the observation period was <1 year.
Population-based estimates of the disease burden were reported in three studies in South Africa and Zambia () [Citation57–Citation59]. The incidence of PCR-positive pertussis per 1,000 person-months ranged from 2.4 to 6.5 in infants aged <3 or <3.5 months, and was 5.5 in infants aged <6 months [Citation57,Citation58]. The incidence of pertussis and pertussis hospitalizations was higher in children who were born to HIV-positive mothers versus HIV-unexposed infants [Citation58,Citation59]. The pertussis death rate in infants aged <6 months was reported in one study (conducted in a single clinic) as 10 deaths (95% confidence of interval (CI) 2–30) per 100,000 infants <6 months of age in the general population [Citation59]. Compared to some other world regions (), the observed pertussis incidence rates and hospitalizations were higher in African countries.
3.1.2. Region of the Americas
Sixteen studies were included from Argentina, Brazil, Colombia, Mexico, Panama, Peru, and the US. Data were also found from the gray literature for the US. Six studies were retrospective cohort studies using national surveillance data or large healthcare claims databases, and 10 studies described clinical features of pertussis cases in hospital or pediatric intensive care unit (PICU) settings. The studies were conducted between 1997 and 2018.
Three studies and one report from the gray literature reported population-based rates in Argentina, Panama, and the US ( and ) [Citation60–Citation63]. The incidence of laboratory-confirmed pertussis in Argentina was assessed using cases notified to the National Health Surveillance System between 2002 and 2011 [Citation60]. The incidence of reported confirmed pertussis was approximately 550 per 100,000 population in infants <2 months of age, 800 per 100,000 population in infants 2-<4 months, and 400 per 100,000 population in infants 4-<6 months [Citation60].
Figure 3. Annual pertussis disease incidence per 100,000 population in infants <6 months of age [Citation60,Citation64–Citation70,Citation72–Citation77,Citation81,Citation84,Citation86].
![Figure 3. Annual pertussis disease incidence per 100,000 population in infants <6 months of age [Citation60,Citation64–Citation70,Citation72–Citation77,Citation81,Citation84,Citation86].](/cms/asset/ef629ff4-d1a5-4ee7-b7e0-bed68e528f15/ierv_a_1791092_f0004_b.gif)
A retrospective hospital-based study in Panama reported the incidence of hospitalizations due to laboratory-confirmed pertussis between 2001 and 2008. The incidence of pertussis hospitalizations per 10,000 all-cause hospitalizations was 29.9 in infants aged <1 month, 88.1 among infants aged 2–3 months, and 28.5 in infants aged 4–6 months [Citation61].
Pertussis is a notifiable disease in the US and the Centers for Disease Control and Prevention report the annual incidence of confirmed and probable cases. Maternal Tdap vaccination was introduced in 2012. The incidence of pertussis in infants <6 months decreased from 169.0 per 100,000 population in 2014 to 57.2 per 100,000 in 2018 (provisional data) () [Citation62]. Between 42.9% and 44.3% of infants were hospitalized each year, and the number of deaths in infants until the age of 1 year decreased from 16 in 2012 (of which 15 were infants <3 months of age) to 4 deaths in 2018 (provisional, age not reported).
A large, retrospective study in the US using claims databases used ICD-Revision 9, Clinical Modification (ICD-9-CM) codes to identify pertussis cases in infants aged <1 year. The incidence of pertussis per 1000 person-months was highest in infants aged <3 months (0.21) and 1-<2 months (0.20) [Citation63].
3.1.3. Eastern Mediterranean region
Seven studies conducted in five countries of the Eastern Mediterranean region were included Iran, Morocco, Oman, Pakistan, and Tunisia. Three studies used surveillance notification data, three were conducted in hospital settings and one was a prospective, active community-based surveillance study conducted in four primary healthcare centers in Pakistan. No reports from the gray literature were found for this region. The studies were conducted between 2008 and 2016.
Population-based rates were reported in three studies from Iran, Oman, and Pakistan ( and ) [Citation67–Citation69]. Two studies in Iran used pertussis surveillance data from the Iranian Center for Diseases Control. The incidence of clinically diagnosed and laboratory-confirmed pertussis in <2 month-old infants between 2008 and 2011 ranged between 132 per 100,000 population (2010) and 463 per 100,000 population (2008) [Citation67]. A study using surveillance data from several government sources in Oman (2011–2015) showed that the incidence of suspected or laboratory-confirmed pertussis ranged between 295 and 1390 per 100,000 infants, peaking during an outbreak in 2013 [Citation68]. In Pakistan, an active community-based surveillance study spanning 14 months reported that the incidence of laboratory-confirmed pertussis in infants aged <2 months was 1.14 per 1,000 person-months (95% CI 0.57–2.28), or 3.96 per 1,000 infants [Citation69].
3.1.4. Region of Europe
Twenty-five studies from 16 European countries were included Bulgaria, Denmark, Finland, France, Germany, Greece, Ireland, Israel, Italy, Norway, Palestine, Spain, Sweden, Switzerland, the Netherlands, and the UK. Data from gray literature was found for France, Spain, and the UK.
Seventeen articles reported data from national pertussis surveillance systems, including one case–control study based on surveillance data. The remainder of the articles described cases in hospital-based studies. The length of periods analyzed ranged from 2 to 21 years, but 15 studies included data covering 10 years or longer. The studies were conducted between 1990 and 2017.
Population-based rates were reported in 10 studies from France, Germany, Israel, Norway, Spain, Sweden, the Netherlands, and the UK, including two reports from France and Spain in the gray literature ( and ) [Citation70–Citation81]. The pertussis incidence rate in the <3 month age-group ranged from 155.2 per 100,000 population (Netherlands 2005–2007) to 1114.3 per 100,000 population in Spain during a pertussis epidemic [Citation75,Citation76]. The incidence of pertussis was higher in preterm than in term infants after the age of 1 month (range 2.99–4.31 versus 1.3–2.3 per 1000 person-months) and was highest in 1- and 2-month-old infants in two of 3 studies with results stratified by month of age ( and ). Compared to very young infants, the incidence of pertussis was lower in the 3–6 months age group, ranging from 130 to 193 per 100,000 population [Citation77].
Hospitalization rates for pertussis in children in the <3 month age group ranged from 103 per 100,000 population (Germany 2013–2015) to 997 per 100,000 population during the 2015 epidemic in Spain. Hospitalization rates were lower in 3–5 months old children, ranging from 45.7 (Netherlands 2011–2012) to 110 per 100,000 population (Spain 2012) ().
The incidence of pertussis-related death was reported using national surveillance data in the UK. The mean number of deaths per 100,000 maternities was 0.708 (95% CI, 0.690–0.751) in infants <6 months old, and 0.601 (95% CI, 0.597–0.613) in infants aged <66 days [Citation79]. The authors of the UK study noted that death certificates may not always mention pertussis as a cause of death in infants, potentially leading to underestimation of the fatality rate [Citation79]. In all other studies reporting pertussis-associated deaths, the majority occurred in children <3 months of age [Citation72,Citation74,Citation76,Citation79].
3.1.5. Region of South-East Asia
Two studies were identified from this region. One was a retrospective observational study at one hospital in Indonesia between 2008 and 2014 [Citation82]. The other was a prospective, population-based active home surveillance study nested within a randomized controlled trial of influenza vaccination during pregnancy in one region of Nepal [Citation83]. A total of 3.483 infants were followed-up over 40 months between 2011 and 2014, and the estimated pertussis incidence rate was 13.3 per 1,000 infant-years [Citation83].
3.1.6. Region of the Western Pacific
Ten studies were included from six countries of the Western Pacific region: Australia, Japan, New Zealand, Philippines, Singapore, and South Korea. For New Zealand, data from the gray literature were also found. Five articles reported data from country-wide notifiable disease surveillance systems, one was an active hospital-based surveillance study, one was a hospital-based study investigating severe pneumonia cases caused by B. pertussis; two analyzed infant pertussis hospitalization either in a nationwide inpatient database or in hospital records; two described pertussis cases reported to an intensive care unit (ICU) admission registry. The study periods encompassed year 1995 to 2018.
Population-based incidence rates in infants <6 months of age were only available for Australia and New Zealand, and varied over time from 137 per 100,000 population (2006–2012) in a region of Australia to 801 per 100,000 population in 2011–2013 in New Zealand during a pertussis epidemic [Citation84–Citation86]. The pertussis hospitalization rate was estimated in one study (Australia 2006–2012) as 257.9 per 100,000 population [Citation85]. The majority of pertussis-related deaths reported in Australia and New Zealand occurred in infants <2 months of age; 10/10 total pertussis deaths between 2006 and 2010 in Australia, and 17/19 total pertussis deaths between 2002 and 2014 in New Zealand [Citation86,Citation87].
3.2. Effectiveness of maternal immunization and impact on infant pertussis disease
The search of articles on the effectiveness and wider impact of maternal immunization on the epidemiology of pertussis disease in infants <12 months of age yielded 688 unique records, of which the full text of 32 articles was assessed and 11 articles included after applying the inclusion and exclusion criteria (). No additional references were included from the gray literature.
Ten out of 11 articles reported on the vaccine effectiveness of maternal Tdap vaccination in preventing pertussis disease in infants [Citation9,Citation10,Citation88–Citation96] and 3 out of 11 provided information on the impact of the maternal immunization program on pertussis disease in infants [Citation9,Citation10,Citation88] ( and ). All of the included studies were observational; six were cohort studies, four were case–control studies, and one was a time-series analysis that compared pertussis incidence between states with a high (>50%) and low (≤50%) Tdap vaccine coverage in Argentina [Citation9,Citation10,Citation88–Citation96]. Case–control studies used surveillance and notification data to identify cases and three of the four studies matched controls for age and place of birth [Citation89–Citation92]. Three cohort studies used large healthcare claims databases or statewide surveillance data and two used the screening method to compare maternal Tdap vaccination coverage in pertussis cases identified through national surveillance, with average national coverage rates [Citation9,Citation10,Citation93–Citation96]. One cohort study recruited mothers from clinics and followed them for 6 months after delivery [Citation96]. Studies of VE were conducted in Argentina, Australia, Brazil, Colombia, Spain, the UK, and the US between 2010 and 2016, and studies of impact were conducted in Argentina and the UK between 2010 and 2015.
Table 2. Key design features and results of studies describing the effectiveness of maternal pertussis immunization.
Table 3. Key design features and results of studies describing the impact of maternal pertussis immunization.
In most studies, VE was adjusted for one or more confounding factors, such as breastfeeding status, household size, and gestational age at birth. As these factors differed between the studies, the adjusted outcomes could not be compared. Unadjusted and adjusted estimates of VE are presented in .
3.2.1. Vaccine effectiveness
3.2.1.1. Infants up to 2 months of age
Estimates of VE (adjusted) in preventing confirmed pertussis disease in infants up to 2 months of age ranged from 77.7% to 93% across studies [Citation9,Citation10,Citation89,Citation91,Citation93]. VE estimates from the three studies conducted in the UK were similar (90%–93%). VE estimates from two US studies were 91.4% and 77.7%, with wide CIs (). In one study, VE against pertussis disease was lower when vaccination occurred in the first or second versus the third trimester of pregnancy (VE 64.3% versus 77.7%) [Citation91].
Figure 5. Effectiveness of maternal Tdap vaccination against pertussis disease and hospitalizations in infants up to 6 months of age [Citation9,Citation10,Citation89–Citation95].
![Figure 5. Effectiveness of maternal Tdap vaccination against pertussis disease and hospitalizations in infants up to 6 months of age [Citation9,Citation10,Citation89–Citation95].](/cms/asset/c4d352a6-66b9-4f10-9958-705b51596577/ierv_a_1791092_f0005_b.gif)
3.2.1.2. Infants up to 3 months of age
Estimates of VE in preventing confirmed pertussis in infants up to 3 months of age were similar to estimates for <2 month-olds in 3 out of 4 studies (range 90.9%–91%) including the two UK studies that used the screening method and a case–control study in Spain [Citation9,Citation10,Citation92]. Saul et al. [Citation90] estimated VE against laboratory-confirmed pertussis at 69% (95% CI, 13–89) in a case–control study in Australia.
VE in preventing pertussis-related deaths was estimated in one UK study [Citation10]. There were 11 deaths among 243 infants with pertussis aged <3 months. The only death attributed to pertussis among the group of maternally immunized infants was in an infant whose mother was vaccinated <10 days before delivery (VE 95%, 95% CI, 79–100).
3.2.1.3. Infants up to 6 months of age
Two studies assessed the VE of maternal Tdap in preventing pertussis infection and pertussis-related hospitalization in infants up to 6 months of age [Citation90,Citation95] (). Estimates of VE against pertussis disease were lower than those in younger infants (39% not significant, and 46% in the respective studies).
A cohort study in Colombia by Hincapie-Palacio et al., followed infants born to vaccinated and non-vaccinated mothers from birth until 6 months of age [Citation96]. The primary study objectives related to immunogenicity. Only two pertussis cases occurred among 1005 enrolled mothers and VE could not be estimated [Citation96].
3.2.1.4. Hospitalization
Winter et al. [Citation94] assessed whether pertussis-infected infants whose mothers received Tdap vaccine during the third trimester of pregnancy had less severe pertussis compared to pertussis-infected infants whose mother did not receive Tdap vaccine (). The crude VE estimate for preventing hospitalization as a measure of severity was 75.4% (95% CI, 49.8–88.0), but was not significant (VE 52.1%, 95% CI, −0.16–80.3) after adjusting for chronological age, gestational age, and pediatric aP vaccines.
Three studies observed that estimates of VE against pertussis disease were lower than VE against hospitalization, suggesting a greater impact of maternal vaccination on severe disease than on milder disease [Citation90,Citation91,Citation95]. Skoff et al. [Citation91] found that VE in preventing pertussis hospitalization was 91.4% (95% CI, 24.8–99.0) when Tdap was administered during the first or second trimesters, and 90.5% (95% CI, 65.2–97.4) when administered during the third trimester. Saul et al. [Citation90] found that the point-estimate for VE was substantially higher in preventing pertussis hospitalization (94%, 95% CI, 59–91 in infants up to 6 months of age) than against laboratory-confirmed pertussis 69% (95% CI, 13–89). Becker Dreps et al. [Citation95] demonstrated significant VE against hospitalization due to pertussis in infants up to 6 months of age (75%) versus 46% against pertussis disease in this age group.
3.2.2. Impact of maternal immunization on pertussis incidence
Three articles reported on the impact of maternal Tdap vaccination on the epidemiology of pertussis disease in infants <12 months of age [Citation9,Citation10,Citation88].
Vizzotti et al. compared the incidence of pertussis disease in Argentina between states with high (>50%) and low (≤50%) maternal Tdap vaccine coverage using data from the Argentinean Health Surveillance System in a time-series model [Citation88]. Low coverage regions were used as a control group, and the time series were compared before and after the implementation of the Tdap program in 2012. Pertussis cases were included if they were confirmed or suspected. There was a relative reduction in pertussis cases of 51% (95% CI 35%–67%) between the high and low vaccine coverage states. In children, 2–6 months of age, the relative reduction in cases was 44% (95% CI 24%–66%). The observed reductions could have been influenced by the cyclic nature of pertussis disease, but the peak in disease amongst <12-month-old in low coverage states in 2012 was not observed in high coverage states.
Country-wide surveillance data from the UK span the period before, during and after the implementation of a nationwide pertussis vaccination campaign in pregnant women [Citation9]. Between 2008 and 2011 the annual number of laboratory-confirmed pertussis cases in infants aged 1 month ranged from 22 to 67. This number increased to 161 in 2012 during a large pertussis epidemic, but reduced to 10 cases in 2013 after implementation and rapid uptake of the maternal vaccination program [Citation9]. The number of hospital admissions also peaked at 209 in 1-month-olds in 2012 and reduced to 68 in 2013.
The greatest reduction in confirmed pertussis cases was observed in infants <3 months (328 cases in 2012 versus 72 cases in 2013, −78%; 95% CI, 72%–83%), although this age group still showed the highest incidence across all age categories. Maternal Tdap coverage in January 2013 was 78%. Further surveillance data showed increasing pertussis disease in all age-groups by 2015, consistent with a cyclical upsurge in disease activity and possibly by changes to pertussis detection methods in some age groups in the UK (PCR testing previously only available for hospitalized infants was extended to all age-groups; oral fluid testing was extended from 8–16 years old to 5–16 years old) [Citation10]. Despite increases in all other age groups, the incidence of pertussis in infants <6 months of age remained lower than incidence rates observed prior to commencement of the maternal vaccination program. Of 16 pertussis-associated deaths in infants between 2013 and 2015, 14 were in infants of mothers who had not received vaccination, and two had mothers who had been vaccinated <10 days prior to delivery [Citation10]. Surveillance data in the UK available since the cutoff date of our review show that the decline in incidence in infants under 3 months of age has continued. The incidence of confirmed pertussis in infants aged <3 months was 30 per 100,000 population in 2018, versus 234 per 100,000 in 2012 [Citation97].
4. Conclusion
Of all age groups, infants bear the greatest pertussis burden in terms of disease incidence, severity, and mortality [Citation4]. The studies we reviewed reported a wide range of disease incidence rates in infants <2-3 months of age that exceeded 1.000 per 100,000 population in some countries during outbreaks, and virtually all pertussis deaths occurred in this age group. We found data from a wide range of low and upper income countries but observed that, in countries where pertussis is not notifiable, hospital-based studies were the main source of information about infant pertussis. These studies often lacked population denominators to estimate the incidence of disease and can only provide a partial view of the disease burden. For countries with existing national surveillance systems, some did not publish data of enough granularity (for example, data for infants all combined as <1 year) to be included in this review. There was a paucity of data for some regions including Africa, the Eastern Mediterranean, and Asia, although the available information point to a disease burden in these countries that is similar or higher than the European region or the Americas.
Maternal immunization aims to prevent pertussis disease in the most vulnerable infants until they are old enough to receive and respond to routine vaccination. We conducted an exhaustive review of the available effectiveness data and found 10 studies, all of which reported significant vaccine effectiveness in preventing pertussis disease and hospitalization in infants <3 months of age. The majority of studies showed that maternal Tdap vaccination was approximately 90% effective in preventing pertussis until the age of 3 months, and reduced thereafter. Maternal Tdap vaccination was effective in preventing between 58.3% and 90.5% of pertussis-associated hospitalizations in 2 months olds, and between 75% and 94% in <6 months old, which suggests that despite waning protection after 3 months of age, maternal vaccination continues to have an impact on severe disease requiring hospitalization until at least 6 months of age. To date, fewer studies have evaluated the impact of maternal Tdap on pertussis epidemiology. Analyses following the introduction of maternal immunization in Argentina and England suggest that maternal vaccination programs are achieving their goal of reducing the burden of disease in young infants before the initiation of primary vaccination ().
Studies published after the date of our review support these conclusions. Burden of disease studies conducted in Brazil and Canada continues to see the highest rates of pertussis disease in infants, with nearly all pertussis-related deaths, hospitalizations, and ICU admissions in children aged <1 year [Citation98,Citation99]. A large health claims database study in the US observed a 48% decrease in infant pertussis hospitalizations; incidence 8.4 per 100,000 (95% CI 7.2–9.7) in 2009–2012 versus 3.3 per 100,000 (95% CI 2.6–4.3) in 2013–2017 [Citation100], coinciding with the 2012 ACIP recommendation for maternal Tdap vaccination. No information on maternal vaccination rates was available in this study.
5. Expert opinion
In the absence of licensed pertussis vaccines for newborns that can be administered in the first days after birth, maternal Tdap vaccination is proving an effective means by which to reduce pertussis disease, hospitalization, and death in infants who are too young to be fully vaccinated. Remarkably, estimates of VE in preventing pertussis disease in <2-3 months old infants were consistently high across the studies we reviewed, despite differences in case detection and confirmation methods, study design, setting, and maternal vaccination program. The results suggest a real benefit of maternal Tdap vaccination for newborns and young infants. Overall, a growing body of publications has found no association between maternal Tdap vaccination and the evaluated adverse pregnancy and/or fetal outcomes [Citation48–Citation54]. An increased risk of chorioamnionitis in women exposed to Tdap during pregnancy was observed in three studies [Citation101–Citation103]. However, these results should be interpreted with caution, especially since no increased risk of preterm births or other adverse neonatal outcomes were observed in the studies. Additionally, other studies by Morgan et al., [Citation104], Berenson et al., [Citation105], and Griffin et al., [Citation106], found no increased risk of chorioamnionitis in women exposed to Tdap during pregnancy. Continued careful evaluation and communication of safety are needed to monitor the benefit/risk of Tdap maternal immunization and maintain confidence amongst mothers and vaccine providers. More data are needed to further characterize the safety of repeated Tdap immunization in successive pregnancies.
Nevertheless, a number of questions remain: What is the optimal timing of Tdap vaccination during pregnancy to maximize protection of the newborn? Vaccine effectiveness estimates by timing of administration included in this review come from only one study which showed a higher point estimate of VE against pertussis disease when Tdap was administered in the third trimester than earlier in pregnancy, but equally high VE point estimates against hospitalization [Citation91]. However, the 95% CI was wide, and no conclusions on the potential difference of effectiveness by the timing of administration can be drawn on the basis of these results. A more recent study conducted in Argentina observed similar VE against confirmed pertussis disease in infants when Tdap was administered during the second or third trimester [Citation107]. The optimal timing for Tdap vaccination during pregnancy needs to balance the desire to achieve the highest vaccine-induced antibody levels during the period of highest disease susceptibility, the need to vaccinate when safest in pregnancy, a desire to minimize any impact on subsequent responses to routine infant vaccination, and the practical consideration of electing a time when vaccine uptake will be maximal [Citation108].
Does maternal Tdap immunization warrant a change in infant vaccination schedules? In a randomized clinical trial, Barug et al. [Citation109] observed the effect of blunting is likely to be higher when starting primary vaccination at age 2 months compared with starting primary vaccinations at 3 months, and concluded that maternal vaccination supports a delay of the first pertussis vaccination in infants until at least age 3 months. In 2018, the Health Council of the Netherlands has recommended that infants of mothers vaccinated in pregnancy receive a 2-dose primary schedule at 3 and 5 months of age, while infants whose mothers were not vaccinated continue to receive the current 3-dose schedule at 2, 3 and 4 months of age [Citation110]. This strategy was also considered as less burdensome for the baby and less expensive [Citation111]. The Netherlands is the first country to adjust the immunization schedule for infants born to women immunized with Tdap during pregnancy. Parallel national schedules for infants may present programmatic challenges in terms of implementation and potentially risk confusion for parents and providers. Furthermore, careful consideration is needed when using a delayed primary immunization schedule given that non-Tdap antigens (hepatitis B, Haemophilus influenzae type band poliomyelitis) are also delayed, and protection by maternal antibodies might not be high enough to prevent disease caused by these pathogens. Ongoing surveillance of pertussis epidemiology might help understand the impact of maternal immunization and modifications to infant schedule on levels of childhood disease.
The burden of pertussis disease in infants is substantial in all parts of the globe but remains poorly documented in some regions where surveillance infrastructure is lacking. Additional work is needed to understand the disease burden in infants in countries where access to healthcare services may be limited. In the future, we can expect that more countries will introduce recommendations for maternal pertussis vaccination, according to national capacity. The first studies in aP-primed mothers may be possible; the optimal timing of maternal vaccination to maximize VE may be defined; and other countries may implement changes to infant pertussis schedules in response to successful maternal vaccination programs. Vaccination of newborns against pertussis has proven problematic in terms of the long-term immune response, and large studies of immunogenicity, safety, and efficacy are currently lacking [Citation112–Citation114]. Maternal vaccination currently stands as the most effective means of protecting newborns and young infants against pertussis disease before starting primary vaccination.
Article highlights
Worldwide, the highest incidence rates of pertussis disease are in infants aged <6 months, with the majority of pertussis-related deaths, hospitalizations, and ICU admissions in this age group.
Infants aged <2-3 months are most at risk. Annual pertussis disease incidence rates in this age group ranged from 132 per 100,000 population to as high as 1390 per 100,000 during outbreaks.
The point estimates of maternal Tdap effectiveness in preventing pertussis in infants below 3 months of age ranged between 69% and 93%.
Maternal Tdap vaccination has been shown to prevent between 58.3% and 94% of pertussis-associated hospitalizations up until the age of 6 months.
Maternal Tdap vaccination is proving an effective means by which to reduce pertussis disease, hospitalization, and death in young infants who are too young to be fully vaccinated.
Author contributions
All authors participated in the design or implementation or analysis, and interpretation of the study. CvdE and EB performed the search strategy, article selection, and data extraction. All authors had full access to the data and participated in the development of the manuscript, revised it critically for important intellectual content, approved the final version before submission, and agreed to be accountable for all aspects of the work.
VJ: contributed to the design of the study, interpretation of the data, and writing the manuscript.
Declaration of interest
V. A. Jenkins, M. A. Ceregido, and A. Guignard are employed by the GlaxoSmithKline group of companies and V. A. Jenkins and A. Guignard hold shares in the GSK group of companies. W. Kandeil was an employee of GlaxoSmithKline at the time of the study. C. van den Ende and E. Bunge are employed by the Pallas company grants from GlaxoSmithKline Vaccines during the conduct of the study; as well as grants from GlaxoSmithKline Vaccines and from Sanofi Pasteur outside the submitted work. The Pallas company received consultancy fees from GlaxoSmithKline for the performed source reports. Writing assistance was utilized in the production of this manuscript: J Fraczek-Goffin and A McGillivray both of GlaxoSmithKline, and J van den Bosch (Pallas, Health Research and Consulting, Rotterdam, The Netherlands), contributed to the source documents (Pallas reports); J Wolter (independent medical writer c/o GlaxoSmithKline) and S Rubio (Modis c/o GlaxoSmithKline) provided writing support services; F Danhier (Modis c/o GlaxoSmithKline) provided publication coordinating services; all services were funded by GlaxoSmithKline. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Trademark statement
Boostrix and Boostrix-IPV are registered trademarks of the GlaxoSmithKline group of companies. Adacel and Repevax are registered trademarks of Sanofi Pasteur.
Supplemental Material
Download MS Word (212.1 KB)Acknowledgments
The authors would like to thank Joanna Fraczek-Goffin, Amanda McGillivray, and Judith van den Bosch for their contribution to the source documents (Pallas reports).
Writing support was provided by Sara Rubio and Joanne Wolter (Modis on behalf of GlaxoSmithKline); editorial support and publication management was provided by Fabienne Danhier (Modis on behalf of GlaxoSmithKline).
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Meade BD, Plotkin SA, Locht C. Possible options for new pertussis vaccines. J Infect Dis. 2014;209(Suppl 1):S24–27.
- Consortium P. PERISCOPE: road towards effective control of pertussis. Lancet Infect Dis. 2019;19(5):e179–e186.
- Healy CM. Pertussis vaccination in pregnancy. Hum Vaccin Immunother. 2016;12(8):1972–1981.
- Tan T, Dalby T, Forsyth K, et al. Pertussis across the globe: recent epidemiologic trends from 2000 to 2013. Pediatr Infect Dis J. 2015;34(9):e222–232.
- Chow MY, Khandaker G, McIntyre P. Global childhood deaths from pertussis: a historical review. Clin Infect Dis. 2016;63(\(suppl 4)):S134–S141. Epub 2016/11/14.
- Centers for Disease C, Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <2 months — advisory committee on immunization practices (acip), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(41):1424–1426.
- Centers for Disease, Control Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women–Advisory Committee on Immunization Practices (ACIP), 2012. MMWR. 2013;62(7):131–135.
- Pregnant women to be offered whooping cough vaccination - News stories. GOV.UK. [cited 2019 Oct 06]. Available from: https://www.gov.uk/government/news/pregnant-women-to-be-offered-whooping-cough-vaccination.
- Amirthalingam G, Andrews N, Campbell H, et al., Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet. 384(9953): 1521–1528. 2014. .
- Amirthalingam G, Campbell H, Ribeiro S, et al. Sustained effectiveness of the maternal pertussis immunization program in England 3 years following introduction. Clin Infect Dis. 2016;63(suppl 4)):S236–S243. Epub 2016/11/14.
- World Health Organization. Pertussis vaccines: WHO position paper - September 2015. Wkly Epidemiol Rec. 2015;90(35):433–458. Epub 2015/09/01.
- Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2018;67(2):1–44.
- Programa Nacional de Control de Enfermedades Inmunoprevenibles/Ministerio de Salud de la Nación Argentina. Fundamentos de la vacunacion de mujeres embarazadas con vacuna triple bacteriana acelular (dTpa). [cited 2019 Oct 06]. Available from: http://www.msal.gob.ar/images/stories/bes/graficos/0000000439cnt-2011-10_lineamientos-vacuna-dTpa-embarazadas.pdf.
- ATAGI. Australian Technical Advisory Group on Immunisation (ATAGI). Australian Immunisation Handbook, Australian Government Department of Health, Canberra, 1 July 2019. 2019.
- Centers for Disease Control and Prevention. Maternal vaccination coverage [Internet] Aug 2017. [cited 2019 Oct 06]. Available from: https://www.cdc.gov/vaccines/pregnancy/hcp-toolkit/maternal-vaccination-coverage.html
- Uriarte PS, Rodriguez SSJ, Sancristobal IG, et al. Effectiveness of dTpa vaccination during pregnancy in preventing whooping cough in infants under 3 months of age. Bizkaia, Basque Country, Spain. Heliyon. 2019;5(2):e01207. Epub 2019/03/01.
- World Health Organization vaccine-preventable disease monitoring system, 2019 global summary. [Internet]. [cited 2020 Apr 06]. Available from: https://apps.who.int/immunization_monitoring/globalsummary/countries?countrycriteria%5Bcountry%5D%5B%5D=ARG&commit=OK.
- Australian Technical Advisory Group on Immunisation (ATAGI). Australian Immunisation Handbook, Australian Government Department of Health. 2018 [cited 2020 Mar]. Canberra. Available from: immunisationhandbook.health.gov.au.
- The Government of The Bahamas Ministry of Health. Immunization schedule. [cited 2020 Apr 06]. Available from: http://www.bahamas.gov.bs/wps/portal/public/Health%20Children/Immunization%20for%20Children/!ut/p/b1/vZTZkqIwFIafpR-AJgEUuGQNKHvY5MYCGdGIsjQC-vSjs9RUTVV338x0cpWq789X55xU6IxO6eySj8cqH47NJa-f52y5ZZFr-won2MKSE4EJQ8dUNY9FLHwAmycAkC1JD8BlwiegwOU6WgBB5f7Ke5L2zLuODy3orvhfefDOksBn-YROww0zq4056Vq8PeQxAXC1PjnINyUeoSBXeTxGQC_xZVpQ446cSsO7og23mZHix22r36mwZoZlfpQCM_TaQZsskW_4VcTOIzKKeCqIf1GTe8pMSuaZu6kaxezm7-3Unrf4POtlNkvV4HmU7s71GCy23bXGQ893vbJbM4Ar4kTfUbHlDwUaFEZzjck1ONkb3jp5enn53cP3i_xkBgmd_UQ-mMIP4IM2O0Zz_kZvHhj_7j0yoEM6BdwWk1tr3k_3gNx9gENzxsSFUAN3B_CJE2o3XObQUbUbvEcAEw2GRHdCEq_ssvDKOIhkSebZsos_FmJr-bVCQYZfLeT_u3DNsA-hxVqOoECM2C8WCv_-0azo7FicX6fd-RW8CiLLiAseCJwAFxBAOiabpag2K0kzg_0hySwmZwfVrI9Nr-vo4kmnYjxtL-ab4fXAJAWa7aDLeq7NelXpGNNNbkpV6-srWlxZtrIc2FfyWgv3UpoockmpMurEDXV6WFKbyKlPFeutnghuK39zwtG0aiAZ3o0N-jpqDwmK9agrrFNjlFbAlF5zSIxrKoYLKvbI_g3jwsRVVjN7OZizMyi0-VqBo0KGyvBxT9JcnKm2N7rrXmCOZ0Bxq2IHZPX5b7TnKBqtZSA4-z_bll6-Aw6QBtw!/dl4/d5/L2dBISEvZ0FBIS9nQSEh/.
- European Centre for Disease Prevention and Control. ECDC Vaccine schedules in all countries of the European Union. [cited 2020 April 06]. Available from: https://vaccine-schedule.ecdc.europa.eu/.
- National Advisory Committee on Immunization. Government of Canada. Update on immunization in pregnancy with tetanus toxoid, reduced diphtheria toxoid and reduced acellular pertussis (Tdap) vaccine. [cited 2020 Apr 06]. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/update-immunization-pregnancy-tdap-vaccine.html.
- Potin M, Fica A, Veliz L, et al. Strategies to protect the newborn and infants under 6 months of age against pertussis: statement of the Advisory Committee for Immunizations of the Chilean Infectious Diseases Society. Rev Chilena Infectol. 2016;33(5):543–546.
- National Immunization Committee. Recommendation for pertussis vaccination in pregnancy for the Czech Republic. Amendment to the National pertussis immunisation strategy. 8 December 2015. [cited 2020 Apr 06]. Available from: http://www.szu.cz/uploads/Epidemiologie/Pertuse/CR_Pertussis_Recommendation_for_pregnant_women.pdf.
- Statens Serum Institut. SSI News. If you are pregnant, you can now be vaccinated against whooping cough free of charge. 20 November 2019. [citned 2020 Apr 03]. Available from: https://en.ssi.dk/news/news/2019/whooping-cough.
- de Salud M. Gobierno de El Salvadore. Esquema Nacional de Vacunación, El Salvador 2020. [cited 2020 April 06]. Available from: https://www.salud.gob.sv/esquema-nacional-de-vacunacion-el-salvador–2020/.
- Public Health England. Pertussis Vaccination Programme for Pregnant Women update: vaccine coverage in England, January to March 2016. Health Prot Rep. 2016;10(18).
- Haute Autorité de santé, 2018. Vaccination contre la coqueluche chez la femme enceinte dans un contexte épidémique à Mayotte. [cited Dec 2019]. Available from: https://www.has-sante.fr/jcms/c_2849533/fr/recommandations-vaccination-coqueluche-femme-enceinte-mayotte-mars2018.
- Hong Kong Centre for Health Protection. Surveillance and epidemiology branch. 4 March 2019. Recommendations on Pertussis Vaccination for Pregnant Women. [cited 2020 Apr 06]. Available from: https://www.chp.gov.hk/files/pdf/letters_to_doctors_20190304.pdf.
- Vashishtha VM, Bansal CP, Gupta SG. Pertussis vaccines: position paper of Indian Academy of Pediatrics (IAP). Indian Pediatr. 2013;50(11):1001–1009.
- HSE National Immunisation Office. Pertussis in pregnancy. [cited 2020 Apr 06]. Available from https: //www.hse.ie/eng/health/immunisation/hcpinfo/othervaccines/pertussis/.
- State of Israel Ministry of Health. Whooping cough vaccination in pregnant women. [cited 2020 Apr 06]. Available from https: //www.health.gov.il/English/Topics/Pregnancy/during/Pages/Vaccination-Whooping_cough.aspx.
- Choi WS, Choi JH, Kwon KT, et al. Revised adult immunization guideline recommended by the korean society of infectious diseases, 2014. Infect Chemother. 2015;47(1):68–79. .
- National Institute for Public Health and the Environment Ministry of Health. Welfare and Sport. 22-week vaccination. [cited 2020 Apr 06]. Available from https://www.rivm.nl/en/22-week-vaccination.
- NewZealand Ministry of Health. Immunisation for pregnant women. [cited 2020 Apr 06]. Available from: https://www.health.govt.nz/your-health/healthy-living/immunisation/immunisation-pregnant-women.
- Ministerio de Salud. Minsa vacunará a gestantes para proteger a futuros bebés contra la tos ferina. [cited 2020 Apr 06]. Available from: https://www.gob.pe/institucion/minsa/noticias/17534-minsa-vacunara-a-gestantes-para-proteger-a-futuros-bebes-contra-la-tos-ferina.
- Schedule for Adult Immunization for Filipinos. 2015. PPD Compendium of Philippine Medicine. 17th ed.
- ECDC European Centre for Disease prevention and Control. Vaccine Schedule 2015 [updated 2015/16/ 2016/11/22/]. [cited 2020 Mar]. Available from: http://vaccine-schedule.ecdc.europa.eu/Pages/Scheduler.aspx.
- Qatar Ministry of Public Health, 2017. Tdap vaccine recommendations for pregnant women.
- Ministry of Health Singapore. MOH establishes national adult immunisation schedule; Extends use of medisave for vaccines under the schedule. [cited 2020 April 06]. Available from: https://www.moh.gov.sg/news-highlights/details/moh-establishes-national-adult-immunisation-schedule-extends-use-of-medisave-for-vaccines-under-the-schedule.
- NIJZ. Navodila za izvajanje. Programa cepljenja in zaščite z zdravili za leto 2017. [cited 2020 Apr 06]. Available from: http://www.nijz.si/sites/www.nijz.si/files/uploaded/navodila_za_izvajanje_ip_2017.pdf.
- Office Fédéral de la Santé Publique. Adaptation des recommandations de vaccination contre la coqueluche: pour les adolescents, les nourrissons fréquentant une structure d’accueil collectif et les femmes enceintes. Bull OFSP. 2013;9:118–123.
- Taiwan Centers for Disease Control. Pertussis [cited 2020 Apr 06]. Available from: https://www.cdc.gov.tw/En/Category/ListContent/bg0g_VU_Ysrgkes_KRUDgQ?uaid=tRFoq7YhMoz6moj0QsJcqA.
- UK Department of Health. Guidance. Whooping Cough Vaccination Programme for Pregnant Women. [cited 2020 Apr 06]. Available from: https://www.gov.uk/government/publications/whooping-cough-vaccination-programme-for-pregnant-women
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep. 2011;60(1):13–15.
- Abu Raya B, Srugo I, Kessel A, et al. The effect of timing of maternal tetanus, diphtheria, and acellular pertussis (Tdap) immunization during pregnancy on newborn pertussis antibody levels - a prospective study. Vaccine. 2014;32(44):5787–5793.
- Munoz FM, Bond NH, Maccato M, et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: a randomized clinical trial. JAMA. 2014;311(17):1760–1769.
- Healy CM, Rench MA, Swaim LS, et al. Association between third-trimester Tdap immunization and neonatal pertussis antibody concentration. JAMA. 2018;320(14):1464–1470.
- D’Heilly C, Switzer C, Macina D. Safety of maternal immunization against pertussis: a systematic review. Infect Dis Ther. 2019. DOI:10.1007/s40121-019-00265-6
- Gkentzi D, Katsakiori P, Marangos M, et al. Maternal vaccination against pertussis: a systematic review of the recent literature. Arch Dis Child Fetal Neonatal Ed. 2017;102(5):F456–F463. .
- Switzer C, D’Heilly C, Macina D. Immunological and clinical benefits of maternal immunization against pertussis: a systematic review. Infect Dis Ther. 2019. DOI:10.1007/s40121-019-00264-7
- Campbell H, Gupta S, Dolan GP, et al. Review of vaccination in pregnancy to prevent pertussis in early infancy. J Med Microbiol. 2018;67(10):1426–1456. Epub 2018/09/18.
- Furuta M, Sin J, Ng ESW, et al. Efficacy and safety of pertussis vaccination for pregnant women - a systematic review of randomised controlled trials and observational studies. BMC Pregnancy Childbirth. 2017;17(1):390. Epub 2017/11/24.
- McMillan M, Clarke M, Parrella A, et al. Safety of tetanus, diphtheria, and pertussis vaccination during pregnancy: a systematic review. Obstet Gynecol. 2017;129(3):560–573. Epub 2017/02/09.
- Brillo E, Tosto V, Giardina I, et al. Maternal tetanus, diphtheria, and acellular pertussis (Tdap) and influenza immunization: an overview. J Matern Fetal Neonatal Med. 2019;1–30.
- The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. Higgins JPT GS, editor: The Cochrane Collaboration, 2011.
- Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394.
- Gill CJ, Mwananyanda L28, MacLeod W, et al. Incidence of severe and nonsevere pertussis among HIV-exposed and -unexposed zambian infants through 14 weeks of age: results from the southern africa mother infant pertussis study (SAMIPS), a longitudinal birth cohort study. Clin Infect Dis. 2016;63(\(suppl 4)):S154–s164. Epub 2016/11/14.
- Nunes MC, Downs S, Jones S, et al. Bordetella pertussis infection in South African HIV-infected and HIV-uninfected mother-infant dyads: a longitudinal cohort study. Clin Infect Dis. 2016;63(\(suppl 4)):S174–s180. Epub 2016/11/14.
- Soofie N, Nunes MC, Kgagudi P, et al. The burden of pertussis hospitalization in HIV-exposed and HIV-unexposed South African infants. Clin Infect Dis. 2016;63(\(suppl 4)):S165–s173. Epub 2016/11/14.
- Romanin V, Agustinho V, Califano G, et al. Epidemiological situation of pertussis and strategies to control it: argentina, 2002–2011. Arch Argent Pediatr. 2014;112(5):413–420. Epub 2014/09/06.
- Nieto Guevara J, Luciani K, Montesdeoca Melian A, et al. Hospital admissions due to whooping cough: experience of the del nino hospital in Panama. Period 2001-2008. An Pediatr (Barc). 2010;72(3):172–178. Epub 2010/02/16.
- Centers for Disease Control and Prevention. Pertussis (Whooping Cough) - Surveillance and Reporting 2017 [2019 July 5]. [cited 2020 Mar]. Available from: https://www.cdc.gov/pertussis/surv-reporting.html
- Masseria C, Martin CK, Krishnarajah G, et al. Incidence and burden of pertussis among infants less than 1 year of age. Pediatr Infect Dis J. 2017;36(3):e54–e61. Epub 2016/12/03.
- Centers for Disease Control and Prevention. Pertussis (Whooping Cough). Surveillance & reporting. [cite 2015 May 26]. Available from: http://www.cdc.gov/pertussis/surv-reporting.html.
- Quinn HE, McIntyre PB. Pertussis epidemiology in Australia over the decade 1995-2005–trends by region and age group. Commun Dis Intell. 2007;31(2):205–215.
- I L-bd B, Baron S, Guiso N, et al., Caro V and Renacoq participants*. Pertussis surveillance in French hospitals: results from a 10 year period. 2007.
- Saffar MJ, Ghorbani G, Hashemi A, et al. Pertussis resurgence in a highly vaccinated population, Mazandaran, North of Iran 2008–2011: an epidemiological analysis. Indian J Pediatr. 2014;81(12):1332–1336. Epub 2014/05/03.
- Al Awaidy ST. Strategic approaches towards pertussis control in Oman. Oman Med J. 2018;33(1):29–36. Epub 2018/02/23.
- Omer SB, Kazi AM, Bednarczyk RA, et al. Epidemiology of pertussis among young Pakistani infants: a community-based prospective surveillance study. Clin Infect Dis. 2016;63(\(suppl 4)):S148–s153. Epub 2016/11/14.
- de Greeff SC, Mooi FR, Schellekens JF, et al. Impact of acellular pertussis preschool booster vaccination on disease burden of pertussis in The Netherlands. Pediatr Infect Dis J. 2008;27(3):218–223. Epub 2008/02/19.
- Schielke A, Takla A, von Kries R, et al. Marked underreporting of pertussis requiring hospitalization in infants as estimated by capture-recapture methodology, Germany, 2013–2015. Pediatr Infect Dis J. 2018;37(2):119–125. Epub 2017/07/21.
- Sizaire V, Garrido-Estepa M, Masa-Calles J, et al. Increase of pertussis incidence in 2010 to 2012 after 12 years of low circulation in Spain. Euro Surveill. 2014;19(32): Epub 2014/08/21. doi:10.2807/1560-7917.ES2014.19.32.20875.
- Stein-Zamir C, Shoob H, Abramson N, et al. The impact of additional pertussis vaccine doses on disease incidence in children and infants. Vaccine. 2010;29(2):207–211. Epub 2010/11/09.
- Tubiana S, Belchior E, Guillot S, et al. Monitoring the impact of vaccination on pertussis in infants using an active hospital-based pediatric surveillance network: results from 17 years’ experience, 1996–2012, France. Pediatr Infect Dis J. 2015;34(8):814–820. Epub 2015/05/09.
- van der Maas NA, Mooi FR, de Greeff SC, et al. Pertussis in the Netherlands, is the current vaccination strategy sufficient to reduce disease burden in young infants? Vaccine. 2013;31(41):4541–4547. Epub 2013/08/13.
- de Viarce Torres de Mier M, López-Perea N, Masa Calles J. Pertussis in Spain, 1998–2016. Preliminary Impact of pertussis vaccination program on pregnant women. Boletín Epidemiológico Semanal. 2018;26(4). Available from: http://revista.isciii.es/index.php/bes/article/view/1060/1302
- Riise OR, Laake I, Vestrheim D, et al. Risk of pertussis in relation to degree of prematurity in children less than 2 years of age. Pediatr Infect Dis J. 2017;36(5):e151–e156.
- Nilsson L, Lepp T, von Segebaden K, et al. Pertussis vaccination in infancy lowers the incidence of pertussis disease and the rate of hospitalisation after one and two doses: analyses of 10 years of pertussis surveillance. Vaccine. 2012;30(21):3239–3247.
- van Hoek AJ, Campbell H, Amirthalingam G, et al. The number of deaths among infants under one year of age in England with pertussis: results of a capture/recapture analysis for the period 2001 to 2011. Euro Surveill. 2013;18(9):20414.
- Bonmarin I, Levy-Bruhl D, Baron S, et al. Pertussis surveillance in French hospitals: results from a 10 year period. Eurosurveillance. 2005;23(1–3):34–38.
- Carlsson RM, von Segebaden K, Bergstrom J, et al. Surveillance of infant pertussis in Sweden 1998-2012; severity of disease in relation to the national vaccination programme. Euro Surveillance. 2015;20(6):12.
- Nataprawira HM, Phangkawira E. A retrospective study of acute pertussis in Hasan Sadikin Hospital-Indonesia. J Acute Dis. 2015;4(2):147–151.
- Hughes MM, Englund JA, Kuypers J, et al. Population-based pertussis incidence and risk factors in infants less than 6 months in Nepal. J Pediatric Infect Dis Soc. 2017;6(1):33–39. Epub 2017/01/12.
- Kiedrzynski T, Bissielo A, Suryaprakash M, et al. Whooping cough-where are we now? A review. N Z Med J. 2015;128(1416):21–27. Epub 2015/06/29.
- Quinn HE, McIntyre PB. Pertussis epidemiology in Australia over the decade 1995-2005–trends by region and age group. Commun Dis Intell Q Rep. 2007;31(2): 205–215. Epub 2007/08/30.
- Pillsbury A, Quinn HE, McIntyre PB. Australian vaccine preventable disease epidemiological review series: pertussis, 2006-2012. Commun Dis Intell Q Rep. 2014;38(3):E179–194.
- Straney L, Schibler A, Ganeshalingham A, et al. Burden and Outcomes of Severe Pertussis Infection in Critically Ill Infants. Pediatr Crit Care Med. 2016;17(8):735–742.
- Vizzotti C, Juarez MV, Bergel E, et al. Impact of a maternal immunization program against pertussis in a developing country. Vaccine. 2016;34(50):6223–6228. Epub 2016/11/17.
- Dabrera G, Amirthalingam G, Andrews N, et al. A case-control study to estimate the effectiveness of maternal pertussis vaccination in protecting newborn infants in England and Wales, 2012-2013. Clin Infect Dis. 2015;60(3):333–337.
- Saul N, Wang K, Bag S, et al. Effectiveness of maternal pertussis vaccination in preventing infection and disease in infants: the NSW Public Health Network case-control study. Vaccine. 2018;36(14):1887–1892. Epub 2018/03/05.
- Skoff TH, Blain AE, Watt J, et al. Impact of the US maternal tetanus, diphtheria, and acellular pertussis vaccination program on preventing pertussis in infants <2 months of age: a case-control evaluation. Clin Infect Dis. 2017;65(12):1977–1983. Epub 2017/10/14.
- Bellido-Blasco J, Guiral-Rodrigo S, Míguez-Santiyán A, et al. A case–control study to assess the effectiveness of pertussis vaccination during pregnancy on newborns, Valencian community, Spain, 1 March 2015 to 29 February 2016. Eurosurveillance. 2017;22(22). DOI:10.2807/1560-7917.ES.2017.22.22.30545.
- Baxter R, Bartlett J, Fireman B, et al. Effectiveness of vaccination during pregnancy to prevent infant pertussis. Pediatrics. 2017;139(5): Epub 2017/05/31. DOI:10.1542/peds.2016-4091.
- Winter K, Cherry JD, Harriman K. Effectiveness of prenatal tetanus, diphtheria, and acellular pertussis vaccination on pertussis severity in infants. Clin Infect Dis. 2017;64(1):9–14. Epub 2016/11/03.
- Becker-Dreps S, Butler AM, McGrath LJ, et al. Effectiveness of prenatal tetanus, diphtheria, acellular pertussis vaccination in the prevention of infant pertussis in the U.S. Am J Prev Med. 2018. Epub 2018/06/19. DOI:10.1016/j.amepre.2018.04.013
- Hincapie-Palacio D, Hoyos MC, Ochoa J, et al. Effect of maternal immunization against pertussis in Medellin and the metropolitan area, Colombia, 2016–2017. Vaccine. 2018;36(27):3984–3991. Epub 2018/05/24.
- Laboratory confirmed cases of pertussis (England): annual report for 2018. Health Protection Report 13(14). [cited 2019 Dec 17]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/797712/hpr1419_prtsss-ann.pdf.
- ENC DB, Nunes AA, Abreu AJL, et al. Pertussis epidemiological pattern and disease burden in Brazil: an analysis of national public health surveillance data. Hum Vaccin Immunother. 2020;16(1):61–69. .
- Abu-Raya B, Bettinger JA, Vanderkooi OG, et al. Burden of children hospitalized with pertussis in Canada in the acellular pertussis vaccine Era, 1999-2015. J Pediatric Infect Dis Soc. 2020 Apr 30;9(2):118–127.
- Boulet SL, Chamberlain AT, Biswas HH, et al. Trends in infant pertussis hospitalizations in the United States, 2009-2017. JAMA. 2019;322(21):2134–2136.
- DeSilva M, Vazquez-Benitez G, Nordin JD, et al. Maternal Tdap vaccination and risk of infant morbidity. Vaccine. 2017;35(29):3655–3660.
- Kharbanda EO, Vazquez-Benitez G, Lipkind HS, et al. Evaluation of the association of maternal pertussis vaccination with obstetric events and birth outcomes. JAMA. 2014;312(18):1897–1904.
- Layton JB, Butler AM, Li D, et al. Prenatal Tdap immunization and risk of maternal and newborn adverse events. Vaccine. 2017;35(33):4072–4078.
- Morgan JL, Baggari SR, McIntire DD, et al. Pregnancy outcomes after antepartum tetanus, diphtheria, and acellular pertussis vaccination. Obstet Gynecol. 2015;125(6):1433–1438.
- Berenson AB, Hirth JM, Rahman M, et al. Maternal and infant outcomes among women vaccinated against pertussis during pregnancy. Hum Vaccin Immunother. 2016;12(8):1965–1971.
- Griffin JB, Yu L, Watson D, et al. Pertussis Immunisation in Pregnancy Safety (PIPS) Study: A retrospective cohort study of safety outcomes in pregnant women vaccinated with Tdap vaccine. Vaccine. 2018;36(34):5173–5179.
- Romanin V, Acosta AM, Juarez MDV, et al. Maternal vaccination in argentina: tetanus, diphtheria, and acellular pertussis vaccine effectiveness during pregnancy in preventing pertussis in infants <2 months of age. Clin Infect Dis. 2020;70(3):380–387.
- Abu-Raya B, Edwards KM. Optimizing the timing of vaccine administration during pregnancy. JAMA. 2019;321(10):935–936.
- Barug D, Pronk I, van Houten MA, et al. Maternal pertussis vaccination and its effects on the immune response of infants aged up to 12 months in the Netherlands: an open-label, parallel, randomised controlled trial. Lancet Infect Dis. 2019;19(4):392–401.
- Health Council of the Netherlands. Vaccination schedule for infants after maternal pertussis vaccination No. 2018/27. The Hague. 2018 Dec 18.
- Rijksinstituut voor Volksgezondheid en Milieu. Kinkhoestvaccinatie van zwangeren en het vaccinatieschema voor hun baby’s. Aanpassing gewenst? Brief rapport 2018–0176. [cited 2020 Jan 27]. Available from: https://www.rivm.nl/publicaties/kinkhoestvaccinatie-van-zwangeren-en-vaccinatieschema-voor-hun-babys-aanpassing-gewenst
- Wood N, Marshall H, White OJ, et al. Antibody and cell-mediated immunity to pertussis 4 years after monovalent acellular pertussis vaccine at birth. Pediatr Infect Dis J. 2014;33(5):511–517.
- Knuf M, Schmitt H-J, Wolter J, et al. Neonatal vaccination with an acellular pertussis vaccine accelerates the acquisition of pertussis antibodies in infants. J Pediatr. 2008;152(5):655–660, 660.e651.
- Knuf M, Schmitt H-J, Jacquet J-M, et al. Booster vaccination after neonatal priming with acellular pertussis vaccine. J Pediatr. 2010;156(4):675–678.