ABSTRACT
Introduction
Meningococcal disease caused by Neisseria meningitidis is a major cause of meningitis and septicemia with high rates of morbidity and mortality worldwide. MenACWY-TT and MenACWY-CRM197 are meningococcal conjugate vaccines approved for use in children and adults in the UK. The aim of this review was to evaluate and compare antibody responses and persistence in different age groups after MenACWY-TT and MenACWY-CRM197.
Areas covered
Randomized trials showed that MenACWY-TT is immunogenic at all ages. MenACWY-CRM197 is immunogenic for infants and adults, but there is a lack of data for children aged 1 to 2 years. Studies on MenACWY-TT indicated that serum bactericidal antibody (SBA) utilizing baby rabbit complement (rSBA) titers were significantly higher and more stable than SBA using human complement (hSBA) titers, compared with hSBA titers, which were lower and declined more rapidly by 1 year following post-primary MenACWY-TT and MenACWY-CRM197 vaccination, especially for MenA.
Expert opinion
MenACWY-TT and MenACWY-CRM197 are both well tolerated and induce similar antibody persistence and immunogenicity against all four serogroups for individuals more than one year old. rSBA assay is a more robust assay than the hSBA assay when vaccinating with MenACWY-TT, while rSBA and hSBA assays had similar antibody persistence when vaccinating with MenACWY-CRM197.
1. Introduction
Invasive meningococcal disease (IMD) caused by Neisseria meningitidis remains an important cause of meningitis and septicemia globally with significant morbidity and mortality, especially in infants, children under 5 years old and young adults [Citation1]. 1.2 million cases of meningococcal infection occur and 135,000 people die every year [Citation2,Citation3]. Forty percent have meningococcemia and 50–60% develop meningitis [Citation1,Citation2]. The organism is transmitted via respiratory droplets from an infected individual [Citation4]. Approximately 10% of the general population carries N. meningitidis in their nasopharynx, where it is usually asymptomatic [Citation5].
Six serogroups (A, B, C, W, X, and Y) account for the majority of IMD [Citation3,Citation6,Citation7]. Other serogroups (E, H, I, J, L, and Z), although not causing disease in immune-competent hosts, are found in carriers and can cause IMD in particular complement deficient patients [Citation6,Citation8]. IMD has three peaks in age. The incidence of meningococcal disease is highest in young children due to the waning of protective maternal antibodies worldwide, but older children and adolescents have the second highest rate mostly because of their higher carriage rates [Citation3]. The third peak occurs in people over 65 years old due to their weakened immunity [Citation2].
Since the original meningococcal polysaccharide vaccines in the 1970s, there is still no universal vaccine against IMD. Plain polysaccharide vaccines, protein polysaccharide conjugate vaccines, and serogroup B protein-based vaccines are the three meningococcal vaccines currently licensed and in use. The first two contain the capsular polysaccharides of meningococci [Citation9]. Quadrivalent ACWY vaccine (MenACWY-PS) is a typical example of pure polysaccharide vaccine. It was licensed from 1970s and can induce protective immune response in individuals more than 2 years of age. However, the antibody persistence only lasts three to 5 years. Furthermore, pure polysaccharide vaccine cannot induce B-cell memory and herd protection [Citation10,Citation11].
There are two quadrivalent meningococcal capsular groups A, C, W, and Y conjugate vaccines currently licensed in European countries (MenACWY-TT, Nimenrix®; Men ACWY-CRM, Menveo ®) [Citation11]. The T cell-independent polysaccharide vaccines can be transformed into T cell-dependent antigenic vaccines by conjugating meningococcal polysaccharides to immunogenic carrier proteins (conjugate vaccines). These vaccines induce herd protection by stimulating B-cell memory and preventing the colonization of the bacteria in the nasopharynx. They stimulate the immature immune system of the children under two years old and the immune system of older people [Citation11,Citation12].
1.1. MenACWY-TT
MenACWY-TT is a quadrivalent, intramuscular meningococcal conjugate vaccine using tetanus toxoid (TT) as a carrier protein. It was developed by GSK but is now marketed by Pfizer [Citation2]. It consists of 5 µg of each serogroup of purified A, C, W, and Y capsular polysaccharides, coupled with 44 µg of TT carrier protein [Citation13]. Serogroups A and C polysaccharides are indirectly conjugated to TT carrier protein with an adipic dihydrazide (AH) spacer molecule. Serogroups W and Y polysaccharides are conjugated directly to TT carrier protein [Citation2,Citation13]. This was the first quadrivalent conjugate vaccine approved for use as a single dose in infants aged at 12 months or older in European countries [Citation2,Citation14]. It is approved for use as three doses in infants aged from 6 weeks in some countries [Citation13]. In Canada and Australia, Men-ACWY is licensed for use in the 12 months to 55-year-old people [Citation13]. The immunogenicity and safety of ACWY-TT are similar to the previously licensed MenACWY-PS and co-administration with other childhood vaccines does not have additional side effects [Citation11].
1.2. MenACWY-CRM197
MenACWY-CRM197 is a quadrivalent meningococcal conjugate vaccine using mutant diphtheria toxin (cross-reactive material 197 or CRM197). It was developed by Novartis and now marketed by GSK [Citation14]. The quadrivalent meningococcal vaccine MenACWY-CRM197 was first approved for use in the United States in 2010. It is now approved in more than 64 countries in the world. In the USA and European countries, it is approved for use in people aged at 11 to 55 years old [Citation14]. In 2011, the approved population extended to 2 to 55 years old people. In 2013, Advisory Committee on Immunization Practices (ACIP) recommended the age group from 2 months to 23 months to accept this vaccine for protecting IMD [Citation2]. MenACWY-CRM197 has long-term immunogenicity and safety records in all age groups [Citation15]. Several studies have found that SBA titers are higher after vaccination with MenACWY-CRM197 compared to quadrivalent meningococcal conjugate vaccine with diphtheria toxin carrier protein (MenACWY-DT) or meningococcal polysaccharide vaccine (MPSV4) [Citation16].
1.3. MenACWY-DT
MenACWY-DT is a quadrivalent meningococcal conjugate vaccine using diphtheria toxin (DT) as a carrier protein. In 2005, it was approved by Food and Drug Administration (FDA) for individuals aged from 11 to 55 years. In 2007, the approved population extended to 2 to 10 years old children with one dose. Four years later, it was also approved for individuals aged from 9 to 23 months with two doses [Citation2,Citation14]. However, ACWY-DT has not yet been approved in Europe. Some studies compared the immunological response of MenACWY-DT and MPSV4. In a similar population, the four serogroups of MenACWY-DT have higher SBA titers. MenACWY-DT and MPSV4 vaccines have similar safety profiles [Citation16].
1.4. Surrogates of protection
It is important to have laboratory immunological markers that reliably predict clinical protection. A surrogate of protection for IMD is the serum bactericidal antibody (SBA) assay. Since the study of Goldschneider and colleagues in the 1960s, this method has become the gold standard for assessing IMD immunity in vitro [Citation17]. SBA is a functional antibody measurement method because it allows the determination of antibody-mediated complement lysis of meningococcal cells [Citation18]. The early developed SBA assays used human complement lacking anti-meningococcal antibodies as an exogenous source (hSBA). However, obtaining suitable human complement requires screening of many donors or sources. Standardization is difficult and it is not suitable for the general use of a group of meningococcal strains. In the 1970s, rabbit serum was recommended as a source of exogenous complement (rSBA), mainly because it is easier to obtain [Citation19]. hSBA titers ≥4 are deemed to be the protective threshold, whilst for the rSBA titers ≥8 are protective [Citation19].
Comparison of hSBA and rSBA assays indicated that the two assays did not necessarily correlate with one another [Citation20]. Santos et al used rSBA titers ≥128 as reliable predictors of hSBA titers ≥4 when given MenC-CRM197 to toddlers and young children. However, most individuals had MenC hSBA titers ≥4 while MenC rSBA titers ≤128. Gill et al., 2011 showed that the titers are higher when using rSBA assay than hSBA assay. The rSBA titers and hSBA serogroup W and Y titers do not correlate, while serogroup C titers do. Meningococcal factor H binding protein may help to explain the difference [Citation21,Citation22]. Factor H binding protein prevents complement-mediated killing [Citation23].
A series of studies observed that the hSBA but not rSBA titers decreased rapidly post-vaccination, especially serogroup A [Citation24–Citation27], suggesting that hSBA may underestimate immune response to MenACWY-TT [Citation23]. hSBA assays may also suggest lower initial immune response to serogroup W and Y than rSBA assays, especially for toddlers receiving one dose of vaccine [Citation23,Citation26–Citation28]. However, hSBA titers increased at later time points despite no additional booster doses. rSBA titers had the opposite results compared with the result of hSBA titers, as they were high post-primary vaccination and declined over time. Thus, rSBA assays seem to be a more appropriate method to measure antibody persistence and immunogenicity in terms of MenACWY-TT, especially for serogroup A [Citation23,Citation25,Citation26,Citation28,Citation29].
This review aims to evaluate and compare antibody responses and persistence in different age groups after vaccination with either MenACWY-TT or MenACWY-CRM197.
2. Methods
PubMed was searched between 1 January 2009 to 30 June 2019 for English terms containing ‘meningococcal tetanus toxoid,’ ‘quadrivalent meningococcal serogroups,’ ‘meningococcal ACWY vaccine,’ ‘meningococcal conjugate vaccine’ or ‘ACWY-CRM,’ ‘ACWY-TT.’ Articles were selected which have ‘Clinical Trials. gov Identifier’ and use human or rabbit serum bactericidal antibody assay from the above literature and compared all the trials with two literature reviews [Citation14,Citation15].
3. Results
3.1. Meningococcal vaccine responses measured by rSBA and hSBA in infants (0–1-year olds) to ACWY-TT
Merino Arribas et al. [Citation30] studied 2,095 healthy infants aged 6–12 weeks, randomized (1:1:1:1) into 4 groups to receive (i) MenACWY-TT (at 2, 3, 4 and 12 months) (ACWY-TT_3), (ii) MenACWY-TT (ACWY-TT_2), (at 2, 4 and 12 months) (iii) MenC-CRM197, or (iv) MenC-TT (at 2, 4 and 12 months) in a phase III, open labeled trial (NCT01144663) (). This study was performed in Estonia, Germany, and Spain. PHiD-CV and DTaP-HBV-IPV/Hib were also administered to all subjects (at 2, 3, 4, and 12 months). Immunogenicity was measured by rSBA and hSBA pre-vaccination, one-month post-primary vaccination, pre-booster, and one-month post-booster vaccination.
Figure 1. rSBA or hSBA GMTs pre-vaccination, one-month post-primary vaccination, pre-booster and one-month post-booster vaccination.
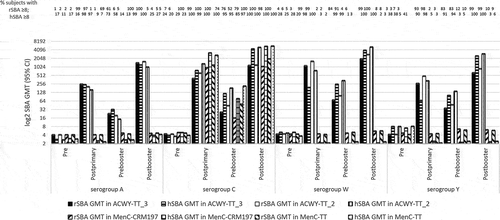
One-month post-primary vaccination, >93% (95% confidence interval [CI]: 90–95) of subjects in the ACWY-TT_3 group and >97% (95% CI 95–99) of subjects in the ACWY-TT_2 group had rSBA titers ≥8 for each serogroup. For the four serogroups, the rSBA GMT in the ACWY-TT_3 and ACWY-TT_2 groups were at least 61X and 49X higher 1 month after primary vaccination than the pre-vaccination, respectively. One-month post-primary vaccination, the percentage of subjects with hSBA titers ≥8 was >90% (95% CI 85–93) and >97% (95% CI 93–99) in the ACWY-TT_3 and ACWY-TT_2 groups for each serogroup, respectively. The rSBA titers ≥8 and hSBA titers ≥8 were 100% in MenC-TT and MenC-CRM197 groups. For the lower limit of the standardized 95% CI for differences between groups, the immunogenicity of two or three doses of MenACWY-TT, MenC-CRM197 and MenC-TT were not significantly different.
One-month post-booster, the percentage of subjects with rSBA titers ≥8 was >99% in both the ACWY-TT_3 (95% CI 98–100 and ACWY-TT_2 (95% CI 98–100) groups for each serogroup. The rSBA GMT in the ACWY-TT_3 and ACWY-TT_2 groups were at least 17X and 18X higher at one-month post-booster than pre-booster, respectively. One-month post-booster, >99% of subjects in the ACWY-TT_3 group (95% CI 97–100) and the ACWY-TT_2 group (95% CI 97–100) had hSBA titers ≥8 for each serogroup. The rSBA titers and hSBA titers ≥8 were 100% in MenC-TT and MenC-CRM197 groups. For the lower limit of the standardized 95% CI for differences between groups, the immunogenicity of two or three doses of MenACWY-TT showed non-inferiority compared to the two doses of MenC-CRM197 and MenC-TT.
Serogroups A, W, and Y reached all pre-specified criteria associated with the immunogenicity of 2 or 3 doses of MenACWY-TT. Compared with the pre-vaccination, the four serogroups also had robust increase in rSBA and hSBA GMTs.
3.2. Meningococcal vaccine responses measured by hSBA in infants (0–1-year olds) to MenACWY-CRM197
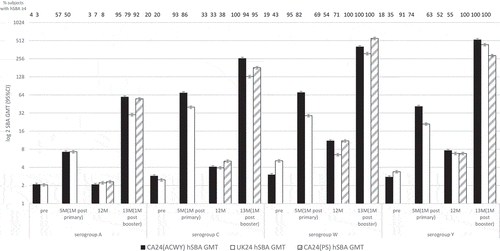
Perrett et al. [Citation31] studied 180 healthy infants from Canada and UK, who were randomized (1:1) to receive two doses of MenACWY-CRM197 (at 2 and 4 months). Then at 12 months of age, the Canadian group (CA24 (ACWY)) received either MenACWY-CRM197 or a licensed meningococcal polysaccharide vaccine (CA24(PS)) and UK group (UK (24)) received a booster dose of MenACWY-CRM197. hSBA were assayed 5, 12, and 13 months of age.
At 5-months old (one-month post-vaccination with MenACWY-CRM197 at 2 and 4 months of age), 57% and 50% of infants against serogroup A in Canada and the UK, respectively, 93% and 86% against serogroup C, 95% and 82% against serogroup W,
and 91% and 74% against serogroup Y (). By 12 months old hSBA GMT had declined in all serogroups. At 13 months of age (one-month post-booster with MenACWY-CRM197), 100% of children had hSBA titers ≥4 in W and Y serogroups in all three groups. For serogroup A, >79% (95% CI 66–88) of children had hSBA titers ≥4, and >94% (95% CI 85–98) for serogroup C. The hSBA GMT in the CA24 (ACWY) and CA24 (PS) groups were at least 28X and 23X higher one-month after booster than the pre-booster, respectively.
Figure 2. hSBA meningococcal serogroup GMT (95% CI) in children pre-vaccination and at 5, 12 and 13 months of age.
3.3. Meningococcal vaccine responses measured by rSBA and hSBA in toddlers (12–23 months old) to ACWY-TT or MenC-CRM197
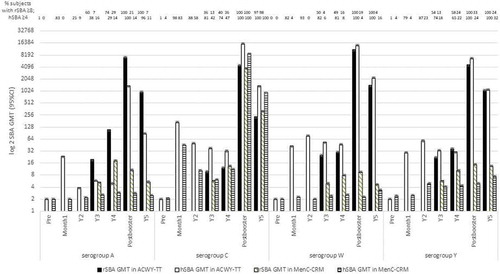
Vesikari et al. [Citation28,Citation29] studied toddlers in Finland aged at 12–23 months, randomized (3:1) to receive one dose of MenACWY-TT or MenC-CRM197. Antibody responses were measured by rSBA and hSBA at 3- and 4-years post-primary, and 1-month and 1-year post-booster vaccination. Boosters were given at four-years post-primary which was at 5 years of age. In the MenACWY-TT group four-years post-primary vaccination, only 29% (95% CI 23–36) of subjects had MenA hSBA titers ≥4 and 65% (95% CI 57–74) of subjects had MenC, W, Y hSBA titers ≥4 (). At the same time point in the MenC-CRM197 group, ≥47% (95% CI 29–65) had MenC hSBA titers ≥4. One-month post-booster, all subjects in the MenACWY-TT group had rSBA titers ≥8 and hSBA titers ≥4 for all four serogroups. All toddlers receiving the MenC-CRM197 booster had MenC rSBA titers ≥8 and hSBA titers ≥4. One-year post-booster, >97% (95% CI 94–99) of toddlers receiving both the MenACWY-TT and MenC-CRM vaccines had MenC rSBA titers ≥8.
Figure 3. rSBA GMT at year 3, 4, post-booster and year 5, and hSBA GMT at pre-vaccination, one-month post-vaccination, year 2, 3, 4, post-booster and year 5.
3.4. Meningococcal vaccine responses measured by rSBA and hSBA in children aged 2–10 years old receiving ACWY-TT or MenC-CRM197
In two studies of Knuf et al. [Citation32,Citation33], children aged at 2 to 10 years in France and Germany were randomized to receive one dose of MenACWY-TT or MenC-CRM197 (NCT00674583). Antibody responses were measured by rSBA and hSBA at 32, 44, 56, and 68 months post-vaccination. A booster with MenACWY-TT was received at 68 months and SBA measured one-month pre- and post-booster.
At 68 months post-primary vaccination, ≥40% (95% CI 33–48) of children in the MenACWY-TT group and ≥62% (95% CI 49–74) in the MenC-CRM197 group had rSBA titers ≥8 for serogroup C. For serogroup A, W and Y, the percentage of subjects with rSBA titers ≥8 was ≥53% (95% CI 45–60) and ≥13% (95% CI 6–24) in the MenACWY-TT and MenC-CRM groups (). One-month post-booster, all children in both groups had rSBA titers ≥8 for the four serogroups.
Figure 4. rSBA GMT at 1-month (M1), 32-months (M32), 44-months (M44), 56-months (M56) and 68-months (M68) post-primary vaccination, and 1-month pre- and post-booster; as well as hSBA GMT at 32-months (M32), 44-months (M44), 56-months (M56) and 68 months (M68) post-primary vaccination.
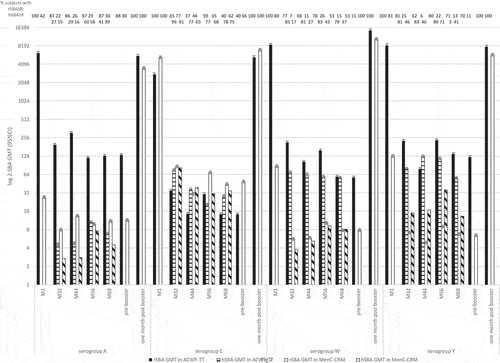
At 68 months post-primary vaccination, ≥78% (95% CI 71–84) of children receiving MenACWY-TT and ≥75% (95% CI 62–86) receiving MenC-CRM197 had MenC hSBA titers ≥4. MenA, W, and Y hSBA titers ≥4 were ≥41% (95% [CI]: 34–49) and ≥37% (95% CI 24–51) in the MenACWY-TT and MenC-CRM197 groups, respectively. One-month post-booster with the ACWY-TT vaccine, rSBA GMT against the four serogroups increased ≥42-fold. One-month post-booster with the MenC-CRM197 vaccine, the MenC rSBA GMT increased 143X compared to 68 months.
3.5. Meningococcal vaccine responses measured by hSBA in children aged 2–10 years old receiving ACWY-CRM197
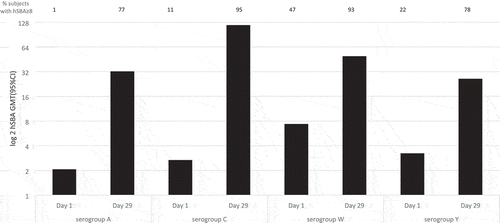
In the study of Huang et al. [Citation34], a total 341 children aged at 2–18 years in Taiwan were randomized to receive one dose of MenACWY-CRM197. Antibody responses were measured by hSBA at baseline (Day 1) and post-MenACWY-CRM197 (Day 29).
We first analyzed the data from children aged 2–10 years. At 29 days post-vaccination, the percentages of children aged 2–10 years old with MenA, C, W, and Y hSBA titers ≥8 were 77% (95% CI 70–83), 95% (95% CI 90–98), 93% (95% CI 88–96) and 78% (95% CI 71–84), respectively (). MenA, C, W, and Y hSBA GMTs increased 16X, 44X, 7X, and 8X compared to day 1 (baseline).
Figure 5. hSBA GMTs at baseline (Day 1) and post-MenACWY-CRM197 (Day 29).
3.6. Meningococcal vaccine responses measured by rSBA or hSBA in adolescents (11–18 years old) receiving ACWY-TT or ACWY-CRM197
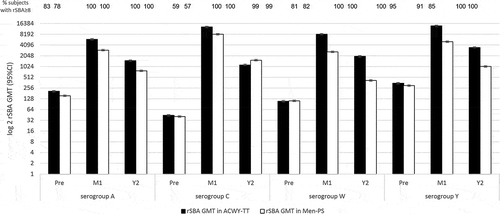
Quiambao et al. [Citation35] studied healthy adolescents aged at 11–18 years, in Philippines, India, and Taiwan, randomized (3:1) to receive one dose of MenACWY-TT or Men-PS (NCT00974363). Antibody responses were measured by rSBA complement 1-month and 2-years post-vaccination. One-month post-vaccination, all subjects in two groups had rSBA titers ≥8. The rSBA GMTs against four serogroups increased at least 28X in the ACWY-TT group, compared with 17X in the Men-PS group (). Two years post-vaccination, the MenA, C, W, and Y rSBA titers were declining but still ≥8 in >95% of both the MenACWY-TT (95% CI 98–100) and Men-PS groups (95% CI 89–98). In conclusion, in adolescent’s rSBA titers remained protective at ≥8 for all serogroups at least 2 years after one dose of MenACWY-TT.
Figure 6. rSBA GMT at baseline, 1-month post-vaccination and 2-years post-vaccination.
Gill et al. [Citation36] studied 597 healthy adolescents (mean 16 (SD ±2) years old) in the USA, randomized to receive one dose of MenACWY-CRM197 or MenACWY-diphtheria-toxoid conjugated vaccine (MCV4). Antibody responses were measured by rSBA and hSBA one-month and 22-months post-vaccination. One-month post-vaccination, ≥95% (95% [CI]: 91–99) receiving either the MenACWY-CRM197 or the MCV4 (95% [CI]: 89–98) vaccine had MenA, C, W, Y rSBA titers ≥8 (). For W and Y serogroups, the rSBA GMT after MenACWY-CRM197 were significantly higher than after the ACWY-DT vaccine (P=0.2 in serogroup A; P=0.05 in serogroup C; P < 0.01 in serogroup W; P < 0.001 in serogroup Y).
Figure 7. rSBA and hSBA GMT at 1-month post- and 22-months post-vaccination.
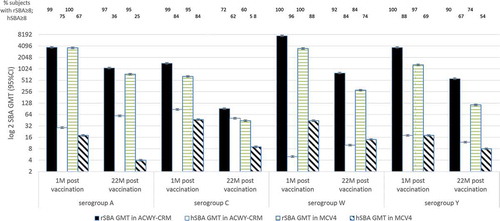
One-month post-vaccination, ≥75% of subjects receiving the MenACWY-CRM197 vaccine and ≥67% of subjects receiving the MenACWY-DT vaccine had MenA, C, W, and Y hSBA titers ≥8. Again, the hSBA GMT after the MenACWY-CRM197 vaccine was significantly higher than after the MenACWY-DT vaccine. 22-months post-vaccination, ≥72% of subjects in the MenACWY-CRM197 group and ≥60% of subjects in the MenACWY-DT group had MenC rSBA titers ≥8. For serogroup A, W and Y, the percentage of subjects with rSBA titers ≥8 was ≥74% and ≥37% in the MenACWY-CRM197 and MenACWY-DT groups, respectively. The rSBA GMT in the MenACWY-CRM197 was significantly higher than the MenACWY-DT group (P=0.2 in serogroup A; P=0.05 in serogroup C; P < 0.01 in serogroup W; P < 0.001 in serogroup Y). 22-months post-vaccination, only ≥36% of subjects in the MenACWY-CRM197 group and ≥25% of subjects in the MenACWY-DT group had MenA hSBA titers ≥8. For serogroup C, W and Y, the percentage of subjects with hSBA titers ≥8 was ≥62% and ≥54% in the MenACWY-CRM197 and MenACWY-DT groups, respectively. The hSBA GMT in the MenACWY-CRM197 was significantly higher than the MenACWY-DT group (P < 0.001 in serogroup A and Y; P=0.1 in serogroup C; P=0.08 in serogroup W). Thus, antibody responses were higher 22-months post-MenACWY-CRM197 than post- MenACWY-DT vaccine.
4. Discussion
Immunogenicity of MenACWY-TT in infancy was excellent 1 month following the primary series for all serogroups, but SBA GMTs declined rapidly during the following 8 months [Citation30]. Interestingly, rSBA and hSBA both gave higher SBA GMTs for serogroups C, Y, and W following the 2 vs 3 primary series. Multivalent conjugate vaccines using high doses of TT as a carrier protein have been found to be associated with reduced responses to polysaccharides [Citation37]. Dagan et al. [Citation38] showed that infants receiving a quadrivalent pneumococcal vaccine conjugated to TT and a diphtheria-tetanus-pertussis-polio-Haemophilus influenzae type b (Hib)-TT vaccine had significantly lower Hib antibody levels than those infants receiving a similar quadrivalent pneumococcal vaccine but conjugated to diphtheria toxoid or a placebo [Citation38]. This may be due to epitopic suppression mediated by the clonal dominance of B cells specific for carrier protein epitopes [Citation37].
Serogroup C SBA titers were higher after monovalent serogroup C conjugates than quadrivalent ACWY conjugates [Citation32,Citation39]. rSBA-MenC GMTs were higher with MenC-CRM197 than MenACWY-TT in 2–10-year-old children, and MenACWY-CRM197 in infants. The difference in immunogenicity of monovalent and quadrivalent vaccines may relate to the different concentrations of MenC polysaccharide in the vaccines. MenACWY-TT and MenACWY-CRM197 both contain 5 μg of serogroup C polysaccharide, which is lower than 10 μg contained in MenC-CRM197. Additional serogroups contained within the quadrivalent vaccine may also result in reduced responses to the other serogroups, when compared to monovalent vaccines. It should also be noted that MenC-CRM197 also contains an aluminum adjuvant, in contrast to MenACWY-TT and MenACWY-CRM197.
MenC hSBA GMTs showed a more significant decline, but of uncertain clinical significance, from post-primary vaccination to booster vaccination with MenACWY-CRM197 than monovalent MenC vaccine [Citation31]. >86% of subjects had MenC hSBA titers ≥4 after two doses of MenACWY-CRM197, and MenC hSBA titers significantly increased post-booster dose. It may be important to receive a booster dose to provide continuous protection to the age of 2 years.
One month after immunization, Canadian infants had significantly higher MenC, MenW, and Y hSBA GMTs than UK infants, the latter who did not receive the PCV vaccine. This suggests that the CRM carrier protein of PCV enhances the meningococcal polysaccharide responses when combined with MenACWY-CRM197.
The study of Snape et al. [Citation40] using a similar CRM-based monovalent serogroup C conjugate meningococcal vaccine (Menjugate) had similar findings. Of note, at 5 months, the diphtheria GMC responses were lower in the UK group (received 3 doses) compared with the Canada group (received 2 doses of diphtheria). This might be explained by carrier protein enhancement or the interval of vaccine doses. When Canada infants completed both primary and booster doses of PCV vaccine, more than 93% of subjects had seroprotective effects against all seven PCV serogroups. The seroprotective rates for diphtheria, hepatitis B, and tetanus successfully achieved the level of protection for all subjects.
In the study of Vesikari et al. [Citation29], the hSBA GMTs of MenACWY-TT it might have been expected that antibody titers would have declined at year 2 when compared to 1-month post-primary vaccination for serogroups W and Y. However, these increased. In two studies of Knuf et al. [Citation32,Citation33], for serogroup C, W, and Y, the rSBA GMTs with MenACWY-TT unexpectedly went up in the 56 months, and the similar trend in the 44 months for serogroup A. These unexpected increases might be explained by the variability of assays, a common observation with functional hSBA assay and rSBA assay. It is also possible that this is due to the different times when samples were tested (44 months (M44) and 56 months (M56)).
The hSBA titers and hSBA GMTs of MenACWY-CRM and MenC-CRM showed comparable persistence for serogroup C [Citation19,Citation41]. In a study of Beernink et al. [Citation42], mice were immunized with Native Outer Membrane Vesicle (NOMV) vaccines with overexpressed FHbp subfamily B (NOMV-FHbp) and measured human complement-mediated SBA responses. They found that the differences between rabbit and human complement. Rabbit factor H does not bind human factor H which is a difference between these two and factor H is a down-regulator of the complement pathway.
In a study of Quiambao et al. [Citation35], the percentage of subjects with rSBA titers ≥8 and also magnitude of the rSBA GMTs suggested that antibody persistence was significantly higher after MenACWY-TT group than Men-PS group for serogroup W and Y. Although rSBA GMTs declined by year 2, it still retained higher than pre-primary vaccination levels. However, the MenC rSBA GMT was higher in the Men-PS group than the MenACWY-TT group in year 2. The study of Borja-Tabora et al. [Citation43] had a similar finding in adolescents and adults. Although the rSBA GMT was lower with MenACWY-TT group, the seroprotection rates of the two groups were similar in the study of Quiambao et al. [Citation35].
In the study of Gill et al. [Citation19], the rSBA titers greatly exceeded hSBA titers for all four serogroups post 1-month and 22-months of primary vaccination. The results of rSBA and hSBA have been shown to lack correlation in previous studies [Citation20] but in this review nothing was found that showed the rSBA results contradicted the hSBA results. No matter which source of complement was used, the subjects with MenACWY-CRM were significantly more likely to obtain seroprotection than MCV4 post 2 years of primary vaccination. Although the proportion of subjects reached MenC rSBA or hSBA titers ≥8 were similar in the two vaccine subjects.
The limitation of this literature review included the variability in time points. For example, one trial had many time points measuring rSBA (3, 4 and 5 years post-primary vaccination), and hSBA (1 month, 2 years, 3 years, 4 years, and 5 years post-primary vaccination) [Citation29]. Another trial only had one time point measuring hSBA (1 month post-primary vaccination) [Citation34]. Only the common time points in these trials can be chosen to compare the immunogenicity and antibody persistence, but this will result in incomplete analysis of the data. Not all trials used both rSBA and hSBA. Some trials used rSBA only as a method to measure antibody persistence, so it can only be compared with the trial that also used rSBA, and hSBA. The review is also limited by lacking external quality assessment control in laboratories between different countries. Trials in different countries will be carried out in different levels of laboratories, the instruments and reagents used in laboratories in different countries may be different, and whether the personnel in laboratories have conducted professional technical training affect the accuracy of the trials, so these laboratories need regular external quality assessment control to ensure that the data results of different laboratorial sources are comparable.
Another potential bias was that this review did not compare the MenACWY-DT vaccine with the MenACWY-TT and MenACWY-CRM197 vaccines. We did not discuss MenACWY-DT because it has not been approved in European countries, but is only licensed in the USA in 2005 and extended the age of using it from 9 months to 55 years old. We cannot compare the non-inferiority of the MenACWY-DT and MenACWY-TT or MenACWY-CRM197. In addition, the current trials were only searched for in PubMed, it only includes articles published in English, but the articles published in other languages are not included, such as Chinese, non-English in African, American, or European countries. For many developing countries, the lacking of studies of protein polysaccharide conjugate vaccines makes it difficult to compare the antibody persistence and immunogenicity in different races. Also, some of the latest research results have not yet been published as an article, so the experimental results collected in this review may have publication bias.
This literature review compares the immunogenicity and antibody persistence in different age groups and doses between the MenACWY-TT and MenACWY-CRM197 on the serogroups A, C, W, and Y, but not serogroup B, the most common serogroup caused IMD in European countries and the most difficult serogroup to design an effective vaccine. In September 2015, the UK National Immunization Schedule introduced the four-component capsular group B meningococcal vaccine (4CMenB) to infants for 3 doses at 2, 4, and 12 months of age [Citation44]. This vaccine against serogroup B IMD has now proved to be effective but antibody persistence studies are required to determine the duration of protection [Citation45]. Serogroup A was the most common serogroup that causes MD in sub-Saharan Africa. MenAfriVac is a serogroup A meningococcal polysaccharide-tetanus toxoid conjugate vaccine, it has been licensed for those aged at 1 to 29 years in countries across the African meningitis belt. Studies have shown that antibody levels assessed by SBA titers were more persistent in people between the ages of 2 and 29 years then those under 1 year of age [Citation46]. The greater the antibody level pre-vaccination, the higher the immunogenicity and antibody persistence after vaccination. In a study of Yaro et al. [Citation47], the relative change in geometric mean antibody level in the 2016 survey was greater among younger groups in the different age groups of 6 to 29 years of age compared to 2011. Since the introduction of MenAfriVac, a significant reduction in serogroup A invasive disease has brought hope for the elimination of MD outbreaks in sub-Saharan Africa.
Immunodeficient children and adults with asplenia or splenic dysfunction or complement deficiencies are at high-risk group of developing invasive meningococcal disease. However, there are few current trials including these individuals. It would be beneficial to include these clinical risk groups into forthcoming trials of meningococcal vaccines. It is also beneficial to study whether extra doses of vaccine are required for risk groups based on age and immunization history.
5. Conclusion
MenACWY-TT and MenACWY-CRM197 are both well tolerated and induce similar antibody persistence and immunogenicity against all four serogroups for individuals more than one year old. rSBA assay is a more appropriate correlate than the hSBA assay with MenACWY-TT while rSBA and hSBA assays had similar antibody persistence with MenACWY-CRM197. rSBA titers are more stable and higher than hSBA titers while hSBA titers declined rapidly post-primary vaccination.
6. Expert opinion
Both MenACWY-TT and MenACWY-CRM197 are induced similar antibody persistence and immunogenicity against all four serogroups of A, C, W, and Y for individuals more than 1 year of age. The rSBA assay is a more appropriate assay than the hSBA assay for investigating antibody persistence.
6.1. Five year view
Antibody persistence has been demonstrated following the currently licensed meningococcal ACWY conjugate vaccines though new quadrivalent vaccines that are undergoing development will be licensed in the future [Citation48]. A further development is likely to see new meningococcal vaccines where the serogroup B protein-based vaccines are incorporated into the quadrivalent ACWY conjugates yielding pentavalent ABCWY vaccines [Citation49].
Declaration of interest
RB performs contract research on behalf of Public Health England for GSK, Pfizer and Sanofi Pasteur. WL and PDA declare no conflicts of interest.
References
- Purmohamad A, Abasi E, Azimi T, et al. Global estimate of Neisseria meningitidis serogroups proportion in invasive meningococcal disease: a systematic review and meta-analysis. Microb Path. 2019;134:103571.
- Assaf-Casals A, Dbaibo G. Meningococcal quadrivalent tetanus toxoid conjugate vaccine (MenACWY-TT, Nimenrix™): A review of its immunogenicity, safety, co-administration, and antibody persistence. Hum Vaccin Immunother. 2016;12(7):1825–1837.
- Rouphael NG, Stephens DS. Neisseria meningitidis: biology, microbiology, and epidemiology. Methods Mol Biol. 2012;799:1–20.
- Borrow R, Alarcón P, Carlos J, et al., The global meningococcal initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines. 16(4): 313–328. 2016.
- Yazdankhah S. Neisseria meningitidis: an overview of the carriage state. J Med Microbiol. 2004;53(9):821–832.
- Li J, Shao Z, Liu G, et al. Meningococcal disease and control in China: findings and updates from the Global Meningococcal Initiative (GMI). J Infect. 2018;76(5):429–437.
- Halperin SA, Bettinger JA, Greenwood B, et al. The changing and dynamic epidemiology of meningococcal disease. Vaccine. 2012;30(Suppl 2):B26–36.
- Ladhani S, Campbell H, Lucidarme J, et al. Invasive meningococcal disease in patients with complement deficiencies: a case series (2008–2017). BMC Infect Dis. 2019;19(1):522.
- Nadel S, Ninis N. Invasive meningococcal disease in the vaccine era. Front Pediatr. 2018;6:321.
- Dellicour S, Greenwood B. Systematic review: impact of meningococcal vaccination on pharyngeal carriage of meningococci. Trop Med Int Health. 2007;12(12):1409–1421.
- Dhillon S, Pace D. Meningococcal quadrivalent tetanus toxoid conjugate vaccine (MenACWY-TT; Nimenrix®): A Review. Drugs. 2017;77(17):1881–1896.
- Pichichero M. Protein carriers of conjugate vaccines: characteristics, development and clinical trials. Hum Vaccin Immunother. 2013;9(12):2505–2523.
- Serra L, York L, Balmer P, et al. Meningococcal group A, C, W, and Y tetanus toxoid conjugate vaccine: a review of clinical data in adolescents. J Adolesc Health. 2018;63(3):269–279.
- Findlow H, Borrow R. Immunogenicity and safety of a meningococcal serogroup A, C, Y and W glycoconjugate vaccine, ACWY-TT. Adv Ther. 2013;30(5):431–458.
- Keshavan P, Pellegrini M, Vadivelu-Pechai K, et al. An update of clinical experience with the quadrivalent meningococcal ACWY-CRM conjugate vaccine. Expert Rev Vaccines. 2018;17(10):865–880.
- Dretler A, Rouphael N, Stephens D. Progress toward the global control of Neisseria meningitidis: 21st century vaccines, current guidelines, and challenges for future vaccine development. Hum Vaccin Immunother. 2018;14(5):1146–1160.
- Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129(6):1307–1326. .
- Balmer P, Borrow R. The immunological basis for immunization series. Module 15: meningococcal disease. World Health Organization; 2010 June. Available from: https://apps.who.int/iris/bitstream/handle/10665/44376/9789241599849_eng.pdf;jsessionid=46EFE2ABBCCE119DF16B5A6568CD8822?sequence=1
- Gill C, Ram S, Welsch J, et al. Correlation between serum bactericidal activity against Neisseria meningitidis serogroups A, C, W-135 and Y measured using human versus rabbit serum as the complement source. Vaccine. 2011;30(1):29–34.
- Santos GF, Deck RR, Donnelly J, et al. Importance of complement source in measuring meningococcal bactericidal titers. Clin Diagn Lab Immunol. 2001;8(3):616–623.
- Schneider MC, Exley RM, Chan H, et al. Functional significance of factor H binding to Neisseria meningitidis. J Immunol. 2006;176(12):7566–7575.
- Granoff DM, Welsch JA, Ram S. Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infect Immun. 2009;77(2):764–769.
- Findlow J, Balmer P, Borrow R. A review of complement sources used in serum bactericidal assays for evaluating immune responses to meningococcal ACWY conjugate vaccines. Hum Vaccin Immunother. 2019;15(10):2491–2500.
- Dbaibo G, Tinoco Favila JC, Traskine M, et al. Immunogenicity and safety of MenACWY-TT, a meningococcal conjugate vaccine, co-administered with routine childhood vaccine in healthy infants: A phase III, randomized study. Vaccine. 2018;36(28):4102–4111.
- Baxter R, Baine Y, Kolhe D, et al. Five-year antibody persistence and booster response to a single dose of meningococcal A, C, W and Y tetanus toxoid conjugate vaccine in adolescents and young adults. Pediatr Infect Dis J. 2015;34(11):1236–1243.
- Klein NP, Baine Y, Bianco V, et al. One or two doses of quadrivalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine is immunogenic in 9- to 12-month-old children. Pediatr Infect Dis J. 2013;32(7):760–767. .
- Vesikari T, Forstén A, Boutriau D, et al. Randomized trial to assess the immunogenicity, safety and antibody persistence up to three years after a single dose of a tetravalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine in toddlers. Hum Vaccin Immunother. 2012;8(12):1892–1903.
- Vesikari T, Forsten A, Bianco V, et al. Antibody persistence to meningococcal serogroups A, C, W and Y in toddlers two years after vaccination with a quadrivalent meningococcal ACWY-tetanus toxoid conjugate (MenACWY-TT) vaccine as measured by bactericidal antibody assays using rabbit or human complement. Trials Vaccinol. 2014;3:121–126.
- Vesikari T, Forsten A, Bianco V, et al. Antibody persistence up to 5 years after vaccination of toddlers and children between 12 months and 10 years of age with a quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine. Hum Vaccin Immunother. 2015;12(1):132–139.
- Merino Arribas JM, Carmona Martínez A, Horn M, et al. Safety and immunogenicity of the quadrivalent meningococcal serogroups A, C, W and Y tetanus toxoid conjugate vaccine coadministered with routine childhood vaccines in European infants. Pediatr Infect Dis J. 2017;36(4):e98–e107.
- Perrett K, Snape M, Ford K, et al. Immunogenicity and immune memory of a nonadjuvanted quadrivalent meningococcal glycoconjugate vaccine in infants. Pediatr Infect Dis J. 2009;28(3):186–193.
- Knuf M, Romain O, Kindler K, et al. Immunogenicity and safety of the quadrivalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine (MenACWY-TT) in 2–10-year-old children: results of an open, randomised, controlled study. Eur J Pediatr. 2013;12(5):601–612.
- Knuf M, Helm K, Kolhe D, et al. Antibody persistence and booster response 68 months after vaccination at 2–10 years of age with one dose of MenACWY-TT conjugate vaccine. Vaccine. 2018;36(23):3286–3295.
- Huang L, Chiu N, Yeh S, et al. Immunogenicity and safety of a single dose of a CRM-conjugated meningococcal ACWY vaccine in children and adolescents aged 2–18 years in Taiwan: results of an open label study. Vaccine. 2014;32(40):5177–5184.
- Quiambao B, Jain H, Bavdekar A, et al. Persistence of the immune response two years after vaccination with quadrivalent meningococcal ACWY-tetanus toxoid conjugate vaccine (MenACWY-TT) in Asian adolescents. Hum Vaccin Immunother. 2016;12(8):2162–2168.
- Gill C, Baxter R, Anemona A, et al. Persistence of immune responses after a single dose of Novartis meningococcal serogroup A, C, W-135 and Y CRM-197 conjugate vaccine (Menveo®) or Menactra® among healthy adolescents. Hum Vaccin. 2010;6(11):881–887.
- Dagan R, Poolman J, Siegrist C. Glycoconjugate vaccines and immune interference: a review. Vaccine. 2010;28(34):5513–5523.
- Dagan R, Eskola J, Leclerc C, et al. Reduced response to multiple vaccines sharing common protein epitopes that are administered simultaneously to infants. Infect Imm. 1998;66(5):2093–2098.
- Snape MD, Perrett KP, Ford KJ, et al. Immunogenicity of a tetravalent meningococcal glycoconjugate vaccine in infants: a randomized controlled trial. JAMA. 2008;299(2):173–184.
- Snape MD, Kelly DF, Salt P, et al. Serogroup C meningococcal glycoconjugate vaccine in adolescents: persistence of bactericidal antibodies and kinetics of the immune response to a booster vaccine more than 3 years after immunization. Clin Infect Dis. 2006;43(11):1387–1394.
- Bona G, Castiglia P, Zoppi G, et al. Safety and immunogenicity of a CRM or TT conjugated meningococcal vaccine in healthy toddlers. Vaccine. 2016;34(29):3363–3370.
- Beernink PT, Ispasanie E, Lewis LA, et al. A meningococcal native outer membrane vesicle vaccine with attenuated endotoxin and overexpressed factor H binding protein elicits gonococcal bactericidal antibodies. J Infect Dis. 2018;219(7):1130–1137.
- Borja-Tabora C, Montalban C, Memish Z, et al. Immune response, antibody persistence, and safety of a single dose of the quadrivalent meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate vaccine in adolescents and adults: results of an open, randomised, controlled study. BMC Infect Dis. 2013;13(1):116. .
- Davis K, Ford K, Craik R, et al. The effect of a single 4CMenB vaccine booster in young people more than ten years after infant immunisation: protocol of an exploratory immunogenicity study. Trials. 2019;20(1):455.
- Ladhani SN, Andrews N, Parikh SR, et al. Vaccination of infants with meningococcal group B vaccine (4CMenB) in England. N Engl J Med. 2020;382(4):309–317.
- White M, Idoko O, Sow S, et al. Antibody kinetics following vaccination with MenAfriVac: an analysis of serological data from randomised trials. Lancet Infect Dis. 2019;19(3):327–336.
- Yaro S, Njanpop Lafourcade B, Ouangraoua S, et al. Antibody persistence at the population level 5 Years after mass vaccination with meningococcal serogroup A conjugate vaccine (PsA-TT) in Burkina Faso: need for a booster campaign? Clin Infect Dis. 2018;68(3):435–443. .
- Peterson J, Hedrick J, Pan J, et al. Immunogenicity and safety of a quadrivalent meningococcal conjugate vaccine (MenACYW-TT) administered in adults 18–55 years of age. Open Forum Infect Dis. 2019;6(Suppl 2):S957–S957.
- Sáez-Llorens X, Beltran-Rodriguez J, Novoa Pizarro JM, et al. Four-year antibody persistence and response to a booster dose of a pentavalent MenABCWY vaccine administered to healthy adolescents and young adults. Hum Vaccin Immunother. 2018;14(5):1161–1174.
