ABSTRACT
Introduction
The World Health Organization recommends vaccination against hepatitis A virus (HAV) for children aged 1 year and older in areas where endemicity has shifted from high to intermediate. There are no recent comprehensive reviews of the epidemiology of HAV infection in Latin America, but seroprevalence and socioeconomic data suggest that, with improved clean water and sanitation systems, countries are transitioning to intermediate endemicity.
Areas covered
We conducted a systematic literature review of the epidemiology of HAV infection in 25 countries in the Latin American region, which included gray literature. We compiled data on HAV incidence and prevalence, including the identification of epidemiological changes observed in countries that established pediatric HAV vaccination programs.
Expert opinion
We identified 59 relevant articles, including 34 peer-reviewed seroprevalence studies (12 recent studies from Brazil), three incidence studies, and six vaccine impact studies (three from Argentina). Based on the estimated age at midpoint of population immunity in each country, most have a high-intermediate, intermediate, or low-intermediate level of HAV endemicity, suggesting that national childhood immunization may be an appropriate disease prevention strategy. However, recent data were lacking for most countries. Improved data quality and continued epidemiological surveillance are required for this region.
1. Introduction
Around 1.5 million cases of hepatitis A virus (HAV) infection are reported each year worldwide but, after accounting for the large number of asymptomatic infections in young children and high rates of underreporting, the actual number of infections is likely to be about 100–120 million annually [Citation1]. Since the virus is transmitted through person-to-person contact and via contaminated food or water, the highest rates of infection are in areas with poor sanitary conditions and a lack of reliable access to clean water [Citation2]. In these locations, young children commonly play a key role in propagating the infection within their communities. While children younger than 6 years often have asymptomatic infections, in older children, adolescents, and adults, most infections lead to clinically overt acute hepatitis, with jaundice present in over 70% of cases [Citation3]. Prolonged or relapsing disease occurs in up to 20% of symptomatic persons [Citation1]. Other than supportive care, no specific treatment is available for hepatitis A [Citation3].
IgG antibodies to HAV appear early in the course of infection and provide long-term (typically thought to be life-long) protection against the disease [Citation3]. Consequently, in areas with high HAV transmission rates, rates of symptomatic hepatitis A disease are low because adults are likely to have been exposed to HAV as children [Citation2]. Epidemiologic shifts from high to intermediate HAV transmission rates, typically resulting from improvements in sanitation and hygiene, are paradoxically associated with an increase in symptomatic cases. This is due to an increase in the number of older children and adults who are susceptible because they have not been exposed to the virus [Citation1,Citation3]. Increasing age at infection is therefore a risk factor for clinical hepatitis A disease.
In countries with intermediate HAV endemicity, the World Health Organization (WHO) recommends integration of HAV vaccination into the national immunization program for children aged 1 year and older when several considerations are met, including ones pertaining to cost-effectiveness and the incidence of acute hepatitis A disease [Citation4]. In low endemicity settings, targeted vaccination of high-risk groups is advised. However, incidence data for hepatitis A are often incomplete, especially in regions with high endemicity where most cases of infection are asymptomatic and remain undetected [Citation2]. Also, the capacity and resources available for hepatitis A surveillance and reporting systems vary, based on the public health priorities of each country [Citation5,Citation6]. HAV serological data derived from anti-HAV immunoglobulin M tests that detect recent infections (incident cases) and anti-HAV immunoglobulin G (IgG) tests that identify the proportion of the population with immunity due to prior infection are often used to complement or estimate measures of incidence [Citation7]. The age at midpoint of population immunity (AMPI) is the youngest age group for which at least half of the population in that age group has anti-HAV IgG antibodies, indicating past exposure to the virus [Citation8]. Because the average age at infection relates directly to the burden of disease in a population, the AMPI to HAV is recommended as a standard indicator of endemicity [Citation8].
In Latin America, socioeconomic indicators and surveillance data suggest that many countries have transitioned or may be transitioning from high to intermediate or even low HAV endemicity levels [Citation9–11], but no recent comprehensive reviews of hepatitis A epidemiology have been conducted in this region. This systematic literature review therefore aimed to compile and analyze recent data on the epidemiology of HAV infection in Latin American countries in order to estimate the current and predicted burden of HAV infection and disease in this area. Additionally, our findings provide a baseline for future studies of HAV endemicity in the region.
2. Methodology
2.1. Search strategy
Data on the epidemiology of HAV infection in Latin American countries were acquired by performing a systematic literature review that captured all available information from both peer-reviewed publications and the gray literature (). The objectives were to describe the current epidemiology of HAV infection in each country in terms of seroprevalence of anti-HAV IgG antibodies by age group and the incidence of hepatitis A disease. The geographical scope of the review was 25 countries: 20 in Latin America (World Bank categorization: Argentina, Belize, Bolivia, Brazil, Chile, Colombia, Costa Rica, Ecuador, El Salvador, Guatemala, Guyana, Honduras, Mexico, Nicaragua, Panama, Paraguay, Peru, Suriname, Uruguay, and Venezuela) and four in the Caribbean (Cuba, Dominican Republic, Haiti, and Jamaica). Caribbean nations with smaller populations and dependent territories were excluded. French Guiana (a region of France, but considered in this review as a country) was included to capture all of continental South America. At the time of this systematic literature review, pediatric national immunization programs for HAV had been established in Argentina (since 2005), Brazil (2014), Chile (in two regions, Tarapacá and Bíobío, since 2014, then nationwide since 2018), Colombia (2013), Panama (2007), Paraguay (2013), and Uruguay (2008).
Figure 1. The three-stage search strategy for articles that was followed for each country
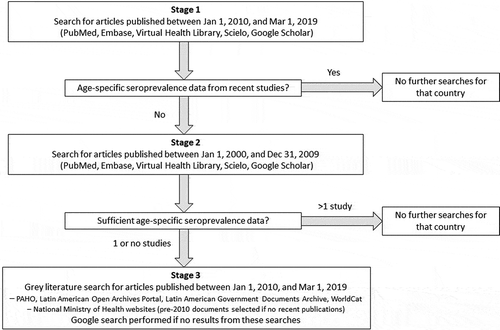
A systematic literature search was conducted according to PRISMA guidelines [Citation12], using Medline accessed through PubMed, Embase, the Virtual Health Library (VHL), and Scielo (search terms are provided in Supplement 1). An additional search was performed using Google Scholar. Articles in English, Spanish, and Portuguese were eligible for inclusion, as well as articles in French for Haiti. Since our goal was to understand current epidemiology, we implemented a three-stage search process that prioritized recent publications and used older resources only when recent data were not available (). In the first stage, we searched for articles published between 1 January 2010, and 1 March 2019, which presented recent age-specific seroprevalence data. For countries without recent age-specific seroprevalence data or with only seroprevalence data that was not representative of the national population (such as data from only one region or a sub-population), a second stage was completed. At stage 2, we searched for articles that were published between 1 January 2000, and 31 December 2009, prioritizing studies that gathered data from 2000 onwards. For any country for which one or no seroprevalence peer-reviewed publication was identified, another search (stage 3) was conducted for relevant gray literature published between 1 January 2010, and 1 March 2019, on the websites of the Pan American Health Organization, Latin American Open Archives Portal, the Latin American Government Documents Archive, and WorldCat, using the search terms ‘hepatitis A’, ‘hepatite A’ or ‘hépatite A’ in combination with the name of the country of interest. The gray literature search also included national Ministry of Health websites of specific countries; data were extracted from documents published before 2010 if no recent data were available. If built-in search functions of gray literature websites did not yield documents, a Google search was performed, using the same search terms as described above.
2.2. Selection of relevant studies and data extraction
Articles retrieved from the PubMed, Embase, VHL, Scielo, and Google Scholar searches were screened against prespecified inclusion and exclusion criteria before a full text review to assess eligibility. Titles and abstracts were screened to identify articles on hepatitis A incidence, (sero)prevalence, morbidity, mortality, hospitalizations, or sequalae in the general population and on the impact of HAV vaccination on hepatitis A incidence. The full text of selected articles was then screened before further examination during the data extraction phase. Letters, editorials, commentaries, and narrative reviews were excluded as well as modeling studies that did not provide original data, laboratory studies of HAV in water or food, outbreak studies, and vaccine effectiveness data (except for hepatitis A incidence data, which were included) or cost-effectiveness data. To ensure completeness of the review, reference lists of excluded systematic reviews were screened and any possibly relevant articles not already identified were screened for eligibility.
Information was extracted from each selected study on the study location, year of publication and data collection, study design, population age group, and age-specific seroprevalence and incidence rates. Seroprevalence rates were identified for non-vaccinated individuals, to focus only on naturally acquired immunity rather than immunity resulting from immunization. The methodological quality of eligible studies was critically appraised using a quality assessment tool for case detection and data collection methods (see Supplement 2), scoring papers as having strong, moderate, or weak quality or, in the case of missing information, having an undetermined quality level. In general, quality was assigned according to the study’s geographical range and its population sampling method [Citation13]. An article was categorized as being of strong quality if it presented seroprevalence data from a study of randomized nationally representative samples that was population-based rather than relying on convenience samples from specific groups, such as visitors to healthcare clinics. Studies of lower quality, such as those reporting data from a single town or using a non-randomized sampling methodology, were also included in the review. Limited methodological information prevented a quality assessment of gray literature.
2.3. Definition of endemicity levels
For each study reporting recent age-specific seroprevalence rates for a suitable set of age groups, we calculated the estimated AMPI in the population by: plotting the midpoint age of each age group on the x-axis against the age-specific anti-HAV IgG seroprevalence rate for the group on the y-axis; fitting logarithmic, exponential, and polynomial curves to the data points to find the best-fit curve with the highest r2 value; and using the equation for the best-fit curve to calculate the youngest age at which 50% of the population is estimated to have immunity to HAV [Citation8,Citation13,Citation14]. For countries with multiple relevant articles, the AMPI was calculated from the study of highest quality. When multiple studies were of high quality, the AMPI was calculated for each and the mean AMPI computed.
We defined high endemicity as an AMPI <5 years of age, high-intermediate as 5–9 years, intermediate as 10–14 years, low-intermediate as 15–19 years, low as 20–29 years, and very low as ≥30 years of age. These six age categories were chosen to allow the epidemiological gradients to be mapped precisely across the region. To enable comparisons of likely endemicity rates across the region, we assigned each country an estimated AMPI value in 2020, using AMPI values calculated from earlier studies and projecting a gradual increase over time to 2020 in each population. The estimated rate of increase took into account the availability of an HAV national immunization program, with the assumption that AMPI would increase to adulthood at a quicker rate in countries with a program than in those without HAV immunization programs [Citation15].
For countries without recent age-specific HAV seroprevalence data, for which AMPI could not be calculated, the estimated AMPI range and HAV endemicity level in 2020 were imputed based on socioeconomic indicators [Citation13,Citation14]. We used the proportion of the population with access to safe water and sanitation services in 2017 [Citation16], the gross national income (GNI) per capita in 2018 [Citation17], and the World Bank country income level classification for 2020 [Citation18]. We assumed that countries with similar socioenvironmental characteristics would have similar endemicity levels. For example, recent seroprevalence data were not available from Panama, but we assumed that Panama has the same low HAV endemicity level as the two other countries classified by the World Bank as high income (Chile and Uruguay) for which HAV data are available. Similarly, because several lower-middle-income countries had seroprevalence data indicating high-intermediate HAV endemicity in 2020, we assigned that endemicity to all countries with GNI per capita less than US 4500 USD in 2018. For countries with GNIs between US 4500 USD and 10,000, USD sanitation access rates below 90% were assumed to correlate with intermediate endemicity and higher rates with low-intermediate endemicity, based on the classifications of countries with available seroprevalence data. Brazil was categorized as low-intermediate based on extensive evidence and the inclusion of hepatitis A vaccination in the national child immunization program since 2014 [Citation19]. Venezuela was assumed to have intermediate rather than low-intermediate endemicity based on its worsening economic status in recent years.
3. Results
Numbers of identified, screened, and selected articles are shown in Supplement 3. In total, 59 epidemiological articles were included (). An additional article was found that presented data on hepatitis A mortality [Citation20]. Since hepatitis A infection is rarely fatal and its burden is usually related to its morbidity [Citation21], this article was excluded from further analysis. Thirty-seven peer-reviewed publications and 16 gray literature references were found describing the epidemiology of HAV (). A further six peer-reviewed publications described the impact of HAV national immunization programs on hepatitis A incidence.
Table 1. Overview of the identified articles for each country stratified by outcome data
The first stage of the article search identified recent (between 2010 and 2019), age-specific seroprevalence studies from three countries: Argentina, Brazil, and Mexico (Supplements 4 and 5). The second stage of the search process (search for articles published between 2000 and 2009) was conducted for all other countries. A search of gray literature and government websites (stage 3) was required for 19 countries (all except Argentina, Bolivia, Brazil, Mexico, Peru, and Uruguay). For Chile, three seroprevalence studies were identified at stages 1 and 2 (Supplement 5) but all reported data for the capital city, Santiago. A gray literature search was therefore conducted in an attempt to find a nationwide study. For Argentina, vaccination against HAV is mandatory and only one suitable age-specific seroprevalence study of non-vaccinated children was identified at stage 1 (Supplement 5) but a gray literature search was not conducted because the national immunization program has been in place since 2005.
With the completion of all search stages, no references were found for Haiti, French Guiana, Guyana, and Suriname. For the Belize, Dominican Republic, El Salvador, Honduras, Jamaica, and Paraguay, only one reference per country met the inclusion criteria.
3.1. HAV seroprevalence
Of the 34 studies that described seroprevalence data (), most originated from Brazil (12 studies), Bolivia, Chile, and Peru (three studies each). For Argentina, Costa Rica, Cuba, the Dominican Republic, Ecuador, Guatemala, Jamaica, Nicaragua, and Venezuela, one seroprevalence study from each country was identified from peer-reviewed articles, but only the study from Costa Rica was based on a national sample. For these eight countries, one gray literature article on incidence was identified for each, except the Dominican Republic and Jamaica, for which no gray literature articles were found. The age-specific HAV seroprevalence rates show considerable variability between and within countries (Supplements 4 and 5). For example, in Brazil, a study conducted in the city of Porto Alegre showed an HAV antibody prevalence rate of 26% among children of low socioeconomic status aged 5–9 years [Citation66], while in a separate survey in rural Amazonia, prevalence was 46% among 5–10 year-olds [Citation67].
No seroprevalence data were available for nine countries – Belize, El Salvador, French Guiana, Guyana, Haiti, Honduras, Panama, Paraguay, and Suriname – in any publication year or language.
3.2. HAV endemicity assignments
We assigned levels of HAV endemicity based on the estimated AMPI in 2020. Fourteen countries had at least one study with age-stratified HAV seroprevalence data for this estimation, as listed in Supplement 4. For the remaining 11 countries, endemicity classifications were imputed based on income level, water access, and sanitation access (), as described in the Methods.
Table 2. Socioeconomic indicator data and assigned HAV endemicity level for each country
shows the overall results of the HAV endemicity level estimations for 2020. Our estimations showed no countries with high (AMPI <5 years of age) or very low (≥30 years) HAV endemicity. Most countries (19 of 25) are estimated to have an endemicity level between high-intermediate and low-intermediate, while six countries are estimated to have low endemicity, including four countries where pediatric HAV national immunization programs have been introduced (Argentina, Chile, Panama, and Uruguay). The other countries with established pediatric HAV national immunization programs (Brazil, Colombia, and Paraguay) are estimated to have low-intermediate endemicity.
Figure 2. Estimated endemicity level for hepatitis A virus infection in 2020 in each country included in the systematic literature review. See also [ https://helac2020.gsk.com/ ]
![Figure 2. Estimated endemicity level for hepatitis A virus infection in 2020 in each country included in the systematic literature review. See also [ https://helac2020.gsk.com/ ]](/cms/asset/bedeeb04-ed7a-49ec-9a12-3869a6fafdff/ierv_a_1813575_f0002_oc.jpg)
3.3. Incidence of hepatitis A
Incidence data were available from 15 countries (; Supplement 6). Most of the recent incidence results came from the gray literature, primarily Ministry of Health documents presenting mandatory report data (). The lowest incidences of hepatitis A were reported in Brazil, Colombia, Panama, and Paraguay (<1 case per 100,000 inhabitants), where pediatric HAV national immunization programs have been introduced, and highest rates in Belize, Ecuador, and Nicaragua (>20 cases per 100,000 inhabitants). However, the data from Belize were from a single district and were for 1994–1995 [Citation68]. Further assessment of the data from Ecuador [Citation69] and Nicaragua [Citation70] was limited because the incidence rates were not stratified completely by age group. For Cuba, three incidence rate studies were identified [Citation71–73], but two comprised a specific city/municipality and the third reported combined surveillance data from 1992 to 2002 (Supplement 6).
Table 3. Nationwide incidence rates of hepatitis A infection per 100,000 inhabitants: the most recent published data that were identified per country are shown
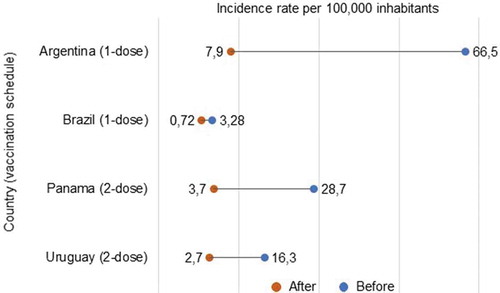
Data on the effect of pediatric immunization programs on the incidence of hepatitis A were available from four countries: Argentina [Citation74], Brazil [Citation19], Panama [Citation75], and Uruguay [Citation76] (). No data were found for Chile, Colombia, or Paraguay. In the post-vaccination years assessed, HAV vaccination coverage rates were 96.8% for Argentina (2006–2011), 97.1% for Brazil (2017), and 70.7% for Panama (2010). The coverage rate for Uruguay in 2010 is not documented; in 2014, it was reported to be 86% [Citation77]. All studies showed a decrease in hepatitis A incidence for each population (all ages, including adults) after the introduction of HAV vaccination ().
Figure 3. Incidence rates of hepatitis A before and after the introduction of hepatitis A virus vaccination in the national immunization program
4. Discussion
This systematic review provides a comprehensive assessment of the current epidemiology of hepatitis A in 25 countries with significant socioeconomic and environmental diversity in the Latin American and Caribbean region. Our estimates show most countries have AMPIs in late childhood or adolescence, although there are a range of profiles within this region, spanning from low- to high-intermediate endemicities. Some countries, including several that implemented national childhood immunization programs more than a decade ago (Argentina, Panama, and Uruguay), now have AMPIs in early adulthood. However, there is a lack of recent seroprevalence data from many countries, and the burden of symptomatic hepatitis A disease is also not well documented, with recent incidence data (2016–2019) available from only nine of 25 countries.
Of all countries, Brazil had the highest number of seroprevalence studies published in 2010–2019. The 12 studies identified were diverse, including data from urban and rural areas as well as populations with different socioeconomic profiles (see Supplement 5). These data indicate HAV endemicity within Brazil is heterogeneous and ranges from low to high endemicity in different populations, but with a general movement toward lower endemicity levels. This led to an estimated endemicity level of low-intermediate overall, which is supported by studies suggesting a decrease in endemicity from high to intermediate since the 1980s [Citation78]. Our included studies suggest a continuing decline, with evidence of low endemicity in certain urban regions [Citation79,Citation80]. For countries with fewer data available, it is more difficult to identify trends. Data are particularly limited for countries in the Caribbean region, with only three seroprevalence studies and two incidence studies identified. Although the AMPI is estimated to have decreased since the 1990s in the Caribbean as a whole [Citation81], there may be considerable differences in endemicity levels between island nations because of socioenvironmental differences [Citation14], but this needs to be confirmed with better hepatitis A surveillance [Citation2].
We also identified peer-reviewed publications that report decreases in disease incidence in countries where childhood HAV immunization programs have already been introduced (Argentina, Brazil, Panama, and Uruguay) [Citation19,Citation74–76]. This is in line with other studies of HAV national immunization programs in areas of intermediate endemicity, demonstrating decreases in the incidence of hepatitis A in vaccinated and non-vaccinated age groups [Citation15].
A notable limitation of our endemicity estimations stems from a gap in the literature for most of the 25 countries included. Our aim was to base this review and analysis on HAV seroprevalence data from the last 10 years but, for many countries, estimations were based on older seroprevalence data and socioeconomic indicators due to the complete absence of recent data.
5. Conclusions
This systematic literature review has established that Latin America is a region with heterogeneous levels of HAV endemicity, mostly ranging from high-intermediate to low-intermediate, supporting the introduction of disease prevention strategies, as recommended by the WHO. Some countries have low endemicity, such as those that already have HAV national immunization programs. However, there is a lack of recent anti-HAV IgG seroprevalence, hepatitis A disease incidence, and HAV vaccination data in many countries in the region. The results of our analysis therefore highlight the need for up-to-date national data on the incidence of HAV infection and symptomatic disease and the age-specific seroprevalence of anti-HAV IgG antibodies from nearly all countries in the region. Long-term (>10 years) follow-up data is also needed from pediatric HAV immunization programs in terms of vaccine coverage and the impact on disease incidence in all age groups. Nevertheless, this is the most comprehensive search of literature conducted to date on the epidemiology of HAV infection in the Latin American region. The available data suggest that most of this region has transitioned or is transitioning to an intermediate level of endemicity.
6. Expert opinion
Like many other largely middle-income regions, most countries in Latin America are undergoing socioeconomic changes, including increased levels of urbanization and greater numbers of people with access to better sanitation services and clean water [Citation16,Citation82]. Paradoxically, decreasing HAV infection rates due to socioeconomic improvements may increase the population-level burden of disease because of a concurrent increase in the proportion of adolescents and adults who remain susceptible to severe disease if they contract the infection.
Groups such as the Global Burden of Disease (GBD) collaboration, hosted by the Institutes for Health Metrics and Evaluation, are generating disease prevalence and incidence estimates that are intended to enable countries to make evidence-informed decisions about health policy [Citation83]. However, the validity and utility of the model outputs generated by the GBD Study are dependent on the quality and quantity of the real-world data used as inputs for the models. Our study shows that many countries in Latin America and the Caribbean have few or no recent data on the incidence or prevalence of hepatitis A and HAV infection. This gap in knowledge impairs the quality of model estimates from the GBD Study [Citation83], and therefore diminishes the ability of governments to make sound preventive healthcare policy recommendations. The best way to overcome this limitation is for countries to collect and share more robust incidence and prevalence data [Citation6], with consistent and timely collection of nationally representative data.
In terms of the epidemiology of HAV infection in Latin America, most countries in this region appear to be in the process of transitioning to a lower endemicity level. Worldwide, many countries have already shifted to low-intermediate levels of endemicity and are trending toward low levels, while some countries continue to have high rates of transmission [Citation2,Citation13,Citation14,Citation84,Citation85]. With decreasing incidences of HAV infection in early life, an increasing number of adolescents and adults are susceptible to infection from a variety of factors such as contaminated food, contact with an infectious person, poor hygiene, water treatment system failures, travel to rural areas in the same country, and travel to other countries with higher endemicity levels [Citation2]. Older individuals who contract HAV usually develop hepatitis symptoms such as fever, fatigue, abdominal pain, nausea, vomiting, and jaundice, and they may experience severe complications, with some developing fulminant hepatitis [Citation21]. Countries with a rising number of symptomatic hepatitis A cases incur significant healthcare system and societal costs, and there are also personal costs due to medical bills, lost wages, and other direct and indirect financial losses. Confirming the endemicity level in each country will enable national policies to align with current epidemiological profiles. In countries where that endemicity level is now intermediate, an informed decision can be made to increase their focus on hepatitis A control and take steps to reduce the anticipated future burden of this disease.
The national prevention and control strategy for viral hepatitis may include the introduction of hepatitis A vaccination for infants (ideally in a national immunization program to ensure high coverage rates), as per WHO recommendations [Citation4]. With the wealth of data on the effectiveness of hepatitis A vaccines, including long-term data from countries such as Israel and the USA [Citation15], an increasing number of countries are establishing pediatric HAV national immunization programs. In Latin America, the hepatitis A vaccine has now been introduced in nine countries, most recently Honduras in January 2020 [Citation86]. However, there are challenges to implementing vaccination against hepatitis A, including competing priorities for new national vaccine introductions [Citation6]. Another perceived challenge is the cost to governments. Although health economic models have established the cost-effectiveness of this intervention in middle-income countries [Citation87], the cost of outbreaks and treatment of cases (in general, as well as during an outbreak) are not well documented in most Latin American countries. It is important to consider the vaccine’s long-term (>10 years) cost-effectiveness, taking both direct and indirect costs into consideration, in addition to monitoring the success of immunization programs in preventing disease in the target population and inducing herd protection. National hepatitis A surveillance programs are particularly important in the context of anticipated increases in global trade, including food supplies, and international travel and migration [Citation2].
The findings of our review underline the need for each country to be aware of its own hepatitis A disease risk profile so that informed, evidence-based decisions can be made about interventions and prevention strategies, with the ability to adapt these strategies according to emerging data. In the next five to 10 years, additional results from epidemiological, cost-effectiveness, and health outcome studies are likely to support the implementation and scaling up of HAV vaccination programs and other prevention strategies, including health education and improved sanitation. Together with information on healthcare utilization and societal costs associated with HAV infection and its prevention, this will improve the accuracy of predicted changes in endemicity and projected impact of those changes in the coming decades.
Article highlights
It is important for countries to monitor the level of HAV endemicity, so that a transition from high to intermediate endemicity can be identified and addressed with disease prevention strategies to reduce excess morbidity and mortality.
We conducted a systematic literature review to examine the epidemiology of HAV infection in Latin America, a region where changes in the socioeconomic status of many countries suggests the likelihood of a transition from high to intermediate endemicity.
The age at midpoint of population immunity was estimated for each of the 25 countries assessed, based on socioeconomic indicators and existing data on the seroprevalence of anti-HAV IgG antibodies.
We established that Latin America is generally a region with intermediate levels of HAV endemicity, ranging from high-intermediate to low-intermediate. (See Figure 2 – Interactive map available securely on the following link: https://helac2020.gsk.com/)
Critical gaps in the literature were identified, indicating the need for more recent data on the national incidence rates of HAV infection and symptomatic disease and on the age-specific seroprevalence of anti-HAV IgG antibodies.
Long-term (>10 years) follow-up data from pediatric HAV vaccination programs are also needed, including vaccination coverage rates and the impact on disease incidence in all age groups.
Author contribution statement
All authors participated in the design, implementation or analysis, and interpretation of the study, as well as the development of this manuscript and the related digital enhancements. All authors had full access to the data and granted their final approval of the paper and the related digital enhancements before submission.
Declaration of interest
A Andani, C Marano and F Salgado are employees of the GSK group of companies. A Andani and C Marano have shares or stock options in the GSK group of companies. K H Jacobsen reports personal fees from the GSK group of companies during the conduct of the study. E M Bunge and T M van Elten report grants from the GSK group of companies during the conduct of the study. Writing assistance was utilized in the production of this manuscript: Editing and publication coordinating services were provided by G Chiapparo (Modis) and writing support services were provided by Joanne Knowles (independent medical writer for Modis), funded by GSK. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download Zip (561.9 KB)Acknowledgments
The authors would like to thank: Bernard Hoet (formerly of GSK, now of Bavarian Nordic) for reviewing and providing his guidance during the development of the manuscript; and Stephane Lorenc (GSK) for the involvement in the development of the manuscript’s interactive map. The authors also thank the Modis platform for editorial assistance and manuscript coordination, on behalf of GSK. Editing and publication coordinating services were provided by Giuseppe Chiapparo (Modis). Writing support services were provided by Joanne Knowles (independent medical writer for Modis).
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- World Health Organization . The immunological basis for immunization series: module 18: hepatitis A. Geneva, Switzerland: World Health Organization; 2019. [accessed 2020 Jan 09]. Available from: https://www.who.int/immunization/documents/ISBN_978-92-51632-7/en/.
- Jacobsen KH. Globalization and the changing epidemiology of hepatitis A virus. Cold Spring Harb Perspect Med. 2018;8(10):pii:a031716.
- Ciocca M. Clinical course and consequences of hepatitis A infection. Vaccine. 2000;18(Suppl 1):S71–S74.
- World Health Organization . WHO position paper on hepatitis A vaccines - June 2012. Wkly Epidemiol Rec. 2012;87(28/29):261–276.
- Stanaway JD , Flaxman AD , Naghavi M , et al. The global burden of viral hepatitis from 1990 to 2013: findings from the global burden of disease study 2013. Lancet. 2016;388(10049):1081–1088.
- Ozawa S , Privor-Dumm LA , Nanni A , et al. Evidence-to-policy gap on hepatitis A vaccine adoption in 6 countries: literature vs. policymakers’ beliefs. Vaccine. 2014;32(32):4089–4096.
- Stapleton JT . Host immune response to hepatitis A virus. J Infect Dis. 1995;171(Suppl 1):S9–S14.
- Mohd Hanafiah K , Jacobsen KH , Wiersma ST . Challenges to mapping the health risk of hepatitis A virus infection. Int J Health Geogr. 2011;10:57.
- Tanaka J . Hepatitis A shifting epidemiology in Latin America. Vaccine. 2000;18(Suppl 1):S57–S60.
- Van Effelterre T , Guignard A , Marano C , et al. Modeling the hepatitis A epidemiological transition in Brazil and Mexico. Hum Vaccin Immunother. 2017;13(8):1942–1951.
- Atun R , de Andrade LO , Almeida G , et al. Health-system reform and universal health coverage in Latin America. Lancet. 2015;385(9974):1230–1247.
- Liberati A , Altman DG , Tetzlaff J , et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
- Itani T , Jacobsen KH , Nguyen T , et al. A new method for imputing country-level estimates of hepatitis A virus endemicity levels in the Eastern Mediterranean region. Vaccine. 2014;32(46):6067–6074.
- Koroglu M , Jacobsen KH , Demiray T , et al. Socioeconomic indicators are strong predictors of hepatitis A seroprevalence rates in the Middle East and North Africa. J Infect Public Health. 2017;10(5):513–517.
- Stuurman AL , Marano C , Bunge EM , et al. Impact of universal mass vaccination with monovalent inactivated hepatitis A vaccines - A systematic review. Hum Vaccin Immunother. 2017;13(3):724–736.
- United Nations Children’s Fund (UNICEF) and World Health Organization . Progress on drinking water, sanitation and hygiene 2000–2017. Special focus on inequalities. New York, USA: United Nations Children’s Fund (UNICEF) and World Health Organization; 2019. [accessed 2020 Jan 09]. Available from: https://www.who.int/water_sanitation_health/publications/jmp-report-2019/en/.
- The World Bank . GNI per capita ranking, Atlas method and PPP based [Internet]. [2019; cited 2020 Jan 09]. Available from: https://datacatalog.worldbank.org/dataset/gni-capita-ranking-atlas-method-and-ppp-based
- The World Bank . World Bank country and lending groups [Internet]. [2020; cited 2020 Jan 09]. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- Souto FJD , de Brito WI , Fontes CJF . Impact of the single-dose universal mass vaccination strategy against hepatitis A in Brazil. Vaccine. 2019;37(6):771–775.
- Perazzo H , Pacheco AG , Luz PM , et al. Age-standardized mortality rates related to viral hepatitis in Brazil. BMC Infect Dis. 2017;17(1):527.
- Linder KA , Malani PN . Hepatitis A. JAMA. 2017;318(23):2393.
- Yanez LA , Lucero NS , Barril PA , et al. Evidence of hepatitis A virus circulation in central Argentina: seroprevalence and environmental surveillance. J Clin Virol. 2014;59(1):38–43.
- Cervio G , Trentadue J , D’Agostino D , et al. Decline in HAV-associated fulminant hepatic failure and liver transplant in children in Argentina after the introduction of a universal hepatitis A vaccination program. Hepat Med. 2011;3:99–106.
- Vizzotti C , Gonzalez J , Rearte A , et al. Single-dose universal hepatitis A immunization in Argentina: low viral circulation and high persistence of protective antibodies up to 4 years. J Pediatric Infect Dis Soc. 2015;4(4):e62–e67.
- Campolmi I , Spinicci M , Mayaregua DR , et al. Seroprevalence of hepatitis A virus, hepatitis E virus, and Helicobacter pylori in rural communities of the Bolivian Chaco, 2013. Am J Trop Med Hyg. 2018;98(5):1275–1280.
- Masuet-Aumatell C , Ma Ramon-Torrell J , Casanova-Rituerto A , et al. Prevalence of hepatitis A antibodies in Eastern Bolivia: a population-based study. J Med Virol. 2013;85(10):1692–1697.
- Gandolfo GM , Ferri GM , Conti L , et al. Prevalencia de las infecciones de los virus A, B, C y E de la hepatitis en dos grupos de niños de nivel socioeconómico distinto de Santa Cruz, Bolivia [Prevalence of infections by hepatitis A, B, C and E viruses in two different socioeconomic groups of children from Santa Cruz, Bolivia]. Med Clin (Barc). 2003;120(19):725–727. Spanish.
- Ciaccia MC , Moreira RC , Ferraro AA , et al. Epidemiological and serological aspects of hepatitis A among children and teenagers in the city of Santos: a cross-sectional study. Sao Paulo Med J. 2012;130(4):230–235.
- Gomes MA , Ferreira Ade S , da Silva AA , et al. Hepatitis A: seroprevalence and associated factors among schoolchildren of Sao Luis (MA), Brazil. Rev Bras Epidemiol. 2011;14(4):548–555.
- Mantovani SA , Delfino BM , Martins AC , et al. Socioeconomic inequities and hepatitis A virus infection in Western Brazilian Amazonian children: spatial distribution and associated factors. BMC Infect Dis. 2015;15:428.
- Nunes HM , Do Carmo Pereira Soares M , Sarmento VP , et al. Soroprevalência da infecção pelos vírus das hepatites A, B, C, D e E em município da região oeste do Estado do Pará, Brasil [Seroprevalence of hepatitis A, B, C, D and E virus infection in a municipality in the western region of the State of Pará, Brazil]. Rev Pan-Amaz Saude. 2016;7(1):55–62. Portugese.
- Nunes HM , Sarmento VP , Malheiros AP , et al. As hepatites virais: aspectos epidemiológicos, clínicos e de prevenção em municípios da Microrregião de Parauapebas, sudeste do estado do Pará, Brasil [Viral hepatitis: epidemiological, clinical and prevention aspects in municipalities of the Parauapebas Microregion, southeastern Pará, Brazil]. Rev Pan-Amaz Saude. 2017;8(2):31–37. Portugese.
- Pereira TM , Mantovani SA , Branco FL , et al. Hepatitis A seroprevalence in preschool children in Assis Brazil, Acre, Brazil, in 2003 and 2010. Int Health. 2016;8(2):132–141.
- Pinheiro RS , Araujo LA , Caetano KA , et al. Intermediate endemicity of hepatitis A virus infection in rural settlement projects of southwest Goiás, Brazil. Arq Gastroenterol. 2015;52(3):200–203.
- Vitral CL , Ospina FL , Artimos S , et al. Declining prevalence of hepatitis A virus antibodies among children from low socioeconomic groups reinforces the need for the implementation of hepatitis A vaccination in Brazil. Mem Inst Oswaldo Cruz. 2012;107(5):652–658.
- Miranda J , Valenzuela M , Hurtado C , et al. Decrease in seroprevalence of hepatitis A virus in Santiago, Chile: A decade-long comparison. Gastroenterol Hepatol. 2019;42(4):248–249.
- Fix AD , Martin OS , Gallicchio L , et al. Age-specific prevalence of antibodies to hepatitis A in Santiago, Chile: risk factors and shift in age of infection among children and young adults. Am J Trop Med Hyg. 2002;66(5):628–632.
- Ministerio de Salud (Chile) . Informe de situación epidemiológica de hepatitis A y viral sin especificación (CIE 10: B15 y B19). Semana Epidemiológica 1— 39 (01 de enero al 29 de septiembre). 2018. Spanish. [accessed 2020 Jan 09]. Available from: http://epi.minsal.cl/wp-content/uploads/2018/10/BET_HEPATITIS_OCTUBRE_2018.pdf
- Rubio MDP , Castro J , Guz E , et al. Seroprevalencia de la Hepatitis A y la Varicela en Bogotá, Colombia [Seroprevalence of Hepatitis A and Varicella in Bogota, Colombia]. Revista Panamericana de Infectología. 2006;3. Spanish. Available from: https://encolombia.com/medicina/revistas-medicas/infectologia/voli-41/revistapanadeinfev4-1-investigaseroprevaha/.
- Vega M , Alvarez C , Arango AE , et al. Frecuencia y distribución de anticuerpos positivos contra la hepatitis A [Frequency and distribution of positive antibodies against hepatitis A]. Acta Med Colomb. 2003;28(2):71–75. Spanish. Available from: http://www.actamedicacolombiana.com/anexo/articulos/02-2003-04.pdf.
- Instituto Nacional de Salud (Colombia) . Boletín Epidemiológico Semanal. 2019. 1–3. Spanish. Available from: http://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2019%20Bolet%C3%ADn%20epidemiol%C3%B3gico%20semana%207.pdf
- Taylor ML , García Z , Holst I , et al. Seroprevalencia de los virus de la Hepatitis A y B en grupos etarios de Costa Rica [Seroprevalence of Hepatitis A and B viruses in Costa Rican age groups]. Acta Med Costarric. 2001;43(4):153–158. Spanish. Available from: http://www.scielo.sa.cr/scielo.php?script=sci_arttext&pid=S0001-60022001000400001&lang=en.
- Ministerio de Salud (Costa Rica) . Análisis de Situación de Salud Costa Rica. 2014. p. 73. Spanish. Available from: https://www.ministeriodesalud.go.cr/index.php/vigilancia-de-la-salud/analisis-de-situacion-de-salud/2618-analisis-de-situacion-de-salud-en-costa-rica/file
- Quintana A , Sanchez L , Larralde O , et al. Prevalence of antibodies to hepatitis E virus in residents of a district in Havana, Cuba. J Med Virol. 2005;76(1):69–70.
- Torres C , Ulloa B , Pabón L , et al. Elevada sero-prevalencia de hepatitis A en estudiantes escolares de Quito [High sero-prevalence of hepatitis A in school students in Quito]. Rev Fac Cien Med (Quito). 2016;41(1):49–56. Spanish.
- Lozano Puertas SV , Miñaca Rea DE Seroprevalenciade hepatitis viral tipo a en alumnos entre 16 y 18 años del Colegio “Capitàn Edmundo Chiriboga G.” de la ciudad de Riobamba, Provincia de Chimborazo en el periodo lectivo 2009 2010” [Seroprevalence of viral hepatitis in students between 16 and 18 years of age at the College “Capita Edmundo Chiriboga G.” in the city of Riobamba, Province of Chimborazo in the school period 2009 2010” - Thesis]. Ecuador: Universidad Nacional de Chimborazo; 2010. Spanish.
- Alexandra LTP Determinación de niveles de inmunoglobulinas IgG contra hepatitis A en escolares menores de 15 años en el Colegio Josè Joaquìn Olmedo del cantón Cayambe diagnosticado por el método de ELISA, en el laboratorio de genètica de la facultad de ciencias médicas periodo 2014 [Determination of immunoglobulin IgG levels against hepatitis A in schoolchildren under the age of 15 at the Josè Joaquìn Olmedo College of the canton Cayambe diagnosed by the ELISA method, in the genetic laboratory of the faculty of medical sciences, period 2014 - Thesis]. Ecuador: Universidad Central del Ecuador; 2015. Spanish.
- Guamán Zhunio D Determinación de hepatitis “A” IgM en niños de séptimo año de educación básica, de la escuela “Ciudad de Huaquillas” e identificación de factores de riesgo [Determination of hepatitis “A” IgM in seventh-year primary education children, in the school “Ciudad de Huaquillas” and identification of risk factors - Thesis]: Universidad Nacional de Loja, Ecuador; 2012. Spanish.
- Chamba Salinas EF Hepatitis A y su relación con factores de riesgo, en niños de 8 a 10 años, en la Parroquia Nambacola [Hepatitis A and its relationship to risk factors, in children aged 8 to 10 years, in Nambacola Parish - Thesis]. Universidad Nacional de Loja, Ecuador; 2015. Spanish.
- Ministerio de Salud (El Salvador) . Datos Epidemiológicos consolidados por grupos de edad y sexo. Semana 14-2019. 2019. Spanish. [accessed 2020 Jan 09]. Available from: http://www.salud.gob.sv/archivos/vigi_epide2018/edad_consolidado402018.pdf
- Steinberg EB , Mendoza CE , Glass R , et al. Prevalence of infection with waterborne pathogens: a seroepidemiologic study in children 6–36 months old in San Juan Sacatepequez, Guatemala. Am J Trop Med Hyg. 2004;70(1):83–88.
- Ministerio de Salud Pública y Asistencia Social (Guatemala) . Semepi. Boletín de la Semana Epideiológica. 2019. p. 6–7. Spanish. Available from: http://epidemiologia.mspas.gob.gt/files/Publicaciones%202019/Boletines%202019/BOLETIN_SEMEPI%20_11.pdf
- Secretariat de Salud (Honduras) . Boletín Epidemiológico República de Honduras. 2019. p. 2 & 19. Available from: https://portalunico.iaip.gob.hn/portal/ver_documento.php?uid=NTA5NjY3ODkzNDc2MzQ4NzEyNDYxOTg3MjM0Mg
- Brown MG , Lindo JF , King SD . Investigations of the epidemiology of infections with hepatitis A virus in Jamaica. Ann Trop Med Parasitol. 2000;94(5):497–502.
- Lazcano-Ponce E , Conde-Gonzalez C , Rojas R , et al. Seroprevalence of hepatitis A virus in a cross-sectional study in Mexico: implications for hepatitis A vaccination. Hum Vaccin Immunother. 2013;9(2):375–381.
- Lopez-Gatell H , Garcia-Garcia L , Echaniz-Aviles G , et al. Hepatitis A seroprevalence in adolescents and young adults in Mexico: A 2012 national health and nutrition survey analysis. Vaccine. 2018;36(52):8094–8099.
- Mayorga Perez O , Brinkhof MW , Egger M , et al. Decreasing risk of hepatitis A infection in Leon, Nicaragua: evidence from cross-sectional and longitudinal seroepidemiology studies. PLoS One. 2014;9(2):e87643.
- Ministerio de Salud (Panama) . Boletin epidemiologico semanal de eventos de notificación obligatoria. 2016. p. 3. Spanish. Available from: http://www.minsa.gob.pa/sites/default/files/publicacion-general/boletin_epidemiologico-_se_52_.pdf
- Organización Panamericana de la Salud, Organización Mundial de la Salud, Pronasida Paraguay, et al . Plan estratégico nacional para la prevención y el control de las hepatitis virales [National strategic plan for the prevention and control of viral hepatitis]. 2018. p. 9. Spanish. Available from: https://www.paho.org/par/index.php?option=com_docman&view=download&category_slug=publicaciones-con-contrapartes&alias=585-plan-estrategico-nacional-para-la-prevencion-y-el-control-de-las-hepatitis-virales-2018-2022&Itemid=253
- Hernández R , Chaparro E , Diaz C , et al. Frecuencia de hepatitis a en niños y adolescentes de cinco ciudades del Perú [Frequency of hepatitis A in children and adolescents from five cities of Peru]. Rev Peru Med Exp Salud Publica. 2015;32(3):499–503. Spanish.
- Heriberto Hidalgo C , Reátegui MG , Rada LA . Prevalencia de hepatitis viral A y B y factores de riesgo asociados a su infección en la población escolar de un distrito de Huánuco - Perú [Prevalence of viral hepatitis A and B and risk factors associated with infection in the school population of a district of Huánuco - Peru]. Rev Peru Med Exp Salud Publica. 2002;19(1):5–9. Spanish.
- Vildósola H , Colichón A , Rubio MP , et al. Prevalencia de anticuerpos contra hepatitis A (anti-HVA IgG) en una población de 01 a 39 años en Lima [Anti-HAV IgG prevalence in a population between 1 and 39 years old in Lima]. Rev Gastroenterol Peru. 2000;20(2):141–145. Spanish.
- Montano Lotito AA , Barañano R , Lageard B , et al. Prevalencia de hepatitis A en niños de 2 a 14 años y en población laboral de 18 a 49 años en Montevideo, Uruguay [Prevalence of hepatitis A in children aged 2 to 14 years and in working populations 18 to 49 years old in Montevideo, Uruguay]. Rev Méd Urug. 2001;17(2):84–98. Spanish.
- Quian J , Rüttimann R , Matrai L . Prevalencia de anticuerpos contra hepatitis A en una población de Montevideo, Uruguay [Prevalence of hepatitis A antibodies in a population in Montevideo, Uruguay]. Arch Pediatr Urug. 2005;76(3):222–227. Spanish.
- Ministerio del Poder Popular para la Salud (Venezuela) . Boletín Epidemiológico, Resumen de la Situación Epidemiológica Nacional. 2016. p. 15. Spanish. Available from: https://www.ovsalud.org/descargas/publicaciones/documentos-oficiales/Boletin-Epidemiologico-2016.pdf
- Krebs LS , Ranieri TM , Kieling CO , et al. Shifting susceptibility to hepatitis A among children and adolescents over the past decade. J Pediatr (Rio J). 2011;87(3):213–218.
- Vitral CL , da Silva-nunes M , Pinto MA , et al. Hepatitis A and E seroprevalence and associated risk factors: a community-based cross-sectional survey in rural Amazonia. BMC Infect Dis. 2014;14:458.
- Bryan JP , Reyes L , Hakre S , et al. Epidemiology of acute hepatitis in the Stann Creek District of Belize, Central America. Am J Trop Med Hyg. 2001;65(4):318–324.
- Ministerio de Salud Publica (Ecuador) . Gaceta Epidemiológica Semanal No. 52. 2018. 13. Spanish. Available from: https://www.salud.gob.ec/wp-content/uploads/2013/02/GACETA-GENERAL-S52.pdf
- Organización Panamericana de la Salud (Nicaragua) . Prevención y Control de Enfermedades. Análisis de Salud. Día Mundial contra la Hepatitis [Prevention and Control of Diseases. Health Analysis. World Day against Hepatitis]. p. 2–4. Spanish. Available from: https://www.paho.org/nic/index.php?option=com_docman&view=download&category_slug=datos-y-estadisticas&alias=651-boletin-informativo-sobre-hepatitis&Itemid=235
- Berdasquera Corcho D , Galindo Santana BM , Gala González Á . Hepatitis viral A: seis años de vigilancia en Guanajay [Viral hepatitis A: six years of surveillance in Guanajay]. Rev Cubana Med Gen Integr. 2006;22(3). Spanish. Available from: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0864-21252006000300001.
- Blanco Hernández N , Julia García Milián A , Coutín Marie G . Hepatitis A en el área de salud; Mártires de Calabazar; 1989–2006 [Hepatitis A in a healthcare region; Mártires de Calabazar; 1989–2006]. Rev Cubana Hig Epidemiol. 2009;47(2). Spanish. Available from: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1561-30032009000200007&lang=en.
- Aguiar P . Reporte Técnico de Vigilancia. Comportamiento Epidemiológico de la Hepatitis A en Cuba [Technical Surveillance Report. Epidemiological Behavior of Hepatitis A in Cuba]. 2004. Spanish. Available from: http://www.sld.cu/galerias/pdf/sitios/vigilancia/rtv0304.pdf
- Vizzotti C , Gonzalez J , Gentile A , et al. Impact of the single-dose immunization strategy against hepatitis A in Argentina. Pediatr Infect Dis J. 2014;33(1):84–88.
- Estripeaut D , Contreras R , Tinajeros O , et al. Impact of Hepatitis A vaccination with a two-dose schedule in Panama: results of epidemiological surveillance and time trend analysis. Vaccine. 2015;33(28):3200–3207.
- Romero C , Perdomo V , Chamorro F , et al. Prevención de hepatitis A mediante vacunación en Uruguay (2005–2010) [Hepatitis A prevention through vaccination in Uruguay (2005–2010)]. Rev Méd Urug. 2012;28(2):115–122. Spanish.
- Catalina Pírez M Logros del Programa Nacional de Inmunización de Uruguay [Achievements of Uruguay’s National Immunization Program] [Internet]. [2015; cited 2020 Jan 09]. Available from: http://www.chlaep.org.uy/descargas/jornadas/1.pdf
- Vitral CL , Gaspar AM , Souto FJ . Epidemiological pattern and mortality rates for hepatitis A in Brazil, 1980–2002–a review. Mem Inst Oswaldo Cruz. 2006;101(2):119–127.
- Kury CM , Pinto MA , Silva JP , et al. Hepatitis A seroprevalence in public school children in Campos dos Goytacazes, Rio de Janeiro State, Brazil, prior to the introduction of the hepatitis A universal childhood vaccination. Cad Saude Publica. 2016;32(11):e00175614.
- Markus JR , Cruz CR , Maluf EM , et al. Seroprevalence of hepatitis A in children and adolescents. J Pediatr (Rio J). 2011;87(5):419–424.
- Jacobsen KH , Wiersma ST . Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine. 2010;28(41):6653–6657.
- United Nations . World urbanization prospects: The 2018 revision (ST/ESA/SER.A/420). New York: United Nations; 2019. [accessed 2020 Jan 09]. Available from: https://population.un.org/wup/Publications/Files/WUP2018-Report.pdf.
- GBD 2017 SDG Collaborators . Measuring progress from 1990 to 2017 and projecting attainment to 2030 of the health-related Sustainable Development Goals for 195 countries and territories: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):2091–2138.
- Patterson J , Abdullahi L , Hussey GD , et al. A systematic review of the epidemiology of hepatitis A in Africa. BMC Infect Dis. 2019;19(1):651.
- Gripenberg M , Aloysia D’Cor N , L’Azou M , et al. Changing sero-epidemiology of hepatitis A in Asia Pacific countries: A systematic review. Int J Infect Dis. 2018;68:13–17.
- World Health Organization . WHO vaccine-preventable diseases: monitoring system. 2019 global summary [Internet]. [2019; cited 2019 Dec 11]. Available from: http://apps.who.int/immunization_monitoring/globalsummary/schedules
- Suwantika AA , Yegenoglu S , Riewpaiboon A , et al. Economic evaluations of hepatitis A vaccination in middle-income countries. Expert Rev Vaccines. 2013;12(12):1479–1494.
