ABSTRACT
Introduction
Italy (in pilot regions) and Germany (nationwide) were the first European countries to introduce universal varicella vaccination (UVV) programs.
Areas covered
A systematic review was performed to assess varicella epidemiology before UVV programs and the impact of 1-dose and 2-dose UVV programs in Italy and Germany.
Expert opinion
Italy implemented 1- or 2-dose UVV programs successively in eight pilot regions between 2003 and 2011 and nationwide in 2017. Germany implemented 1- and 2-dose UVV programs in 2004 and 2009, respectively. While Italy had two nationwide surveillance systems in place for varicella in the pre-vaccination era, in Germany, a mandatory notification system for varicella was only introduced in the New Federal States 2 years before the 1-dose UVV implementation. Substantial reductions in moderate/severe varicella and varicella-related hospitalization incidence occurred during the 1-dose era. Further reductions were reported in Italy and Germany after the recommendation of a second dose in a long or short schedule, respectively. Different benefit-risk evaluations of a tetravalent varicella-containing vaccine (MMRV) used as a first dose led to different recommendations (MMRV versus MMR+V) in these countries. Vaccination strategies in both countries tailored to country-specific needs and goals led to a reduction in varicella-related health care hospitalization costs.
1. Introduction
Varicella, also known as chickenpox, is a highly contagious disease resulting from primary infection with the varicella-zoster virus (VZV), usually during childhood [Citation1]. Although generally benign and self-limiting, acute varicella can be complicated by secondary bacterial infection of the skin, pneumonia, encephalitis, cerebellar ataxia, arthritis, appendicitis, hepatitis, glomerulonephritis, pericarditis, and orchitis [Citation2]. Most epidemiological studies show that, in temperate climates, more than 90% of adolescents or young adults are seropositive for VZV [Citation3]. VZV remains latent in sensory ganglia, and its reactivation results in herpes zoster (HZ; shingles) [Citation4,Citation5]. Both varicella and HZ are associated with a significant medical, social, and economic burden on the healthcare system [Citation6–11].
Varicella is a vaccine-preventable disease. The Japanese OKA-strain, an attenuated strain of VZV, is used in the production of varicella vaccines licensed in many countries worldwide. The first formulation of an OKA-strain vaccine was developed in 1974 and subsequently used for research purposes in Japan until its local licensure in 1986 [Citation12]. This vaccine was licensed for high-risk children in several European countries since 1984, and its use was later extended to all children [Citation13]. In the European Union (EU) or its member states, two monovalent varicella live-attenuated vaccines (Varilrix [OKA/RIT; GSK] and Varivax [OKA/Merck; Merck Sharp & Dohme Corp.]) and two combined measles, mumps, rubella, and varicella (MMRV) live-attenuated vaccines (Priorix-Tetra [GSK] and ProQuad [Merck Sharp & Dohme Corp.]) are currently licensed [Citation14]. The frozen formulation of Varilrix was first licensed in 1984 for use in potentially immunocompromised children and their healthy contacts, while its current refrigerator-stable formulation was first licensed in 1994 in Sweden and Germany for use in all children from the age of 9 months [Citation15]. Varivax was first licensed in 1995 in the United States (US) in its frozen formulation [Citation16,Citation17], while its refrigerator-stable formulation was approved in Europe in 2003 for use in individuals aged ≥12 months or, in special circumstances, ≥9 months [Citation18,Citation19]. Priorix-Tetra and ProQuad are licensed for use in individuals from 11 months and ≥12 months of age, respectively. In special circumstances, both can be administered from 9 months of age [Citation20,Citation21]. ProQuad has been approved in the EU in 2006 [Citation22] and Priorix-Tetra in several EU countries starting in 2006 [Citation20].
Universal routine vaccination with a live-attenuated varicella vaccine was first implemented in the US in 1995, resulting in substantial decrease in the varicella incidence and in varicella-related hospitalizations and deaths [Citation17,Citation23,Citation24]. Nonetheless, the effectiveness of the 1-dose varicella vaccination later revealed its shortcoming, as breakthrough disease has been documented for a significant proportion of vaccinated children [Citation24,Citation25]. A recent study showed that in 10 European countries, where even though VZV is endemic, 2 doses of varicella-containing vaccine were highly efficacious against all severities of varicella disease within 10 years from vaccination [Citation26].
The World Health Organization (WHO) first published recommendations on routine immunization with varicella vaccines in 1998 [Citation27], which were updated in 2014 [Citation1]. The WHO recommends inclusion of a varicella vaccine in the universal routine vaccination programs of countries if a coverage of ≥80% can be sustained [Citation1]. The number of doses depends on the aim of each vaccination program. While a 1-dose schedule is adequate to reduce varicella incidence and consequently morbidity and mortality, addition of a second dose to the schedule further decreases incidence, prevents virus circulation and consequently the occurrence of outbreaks, and decreases incidence and severity of breakthrough cases [Citation1,Citation28].
In Europe, universal varicella vaccination (UVV) was first introduced in Italy’s region Sicily in 2003, followed by Germany (nationwide) in 2004 [Citation29–31]. By 2018, 12 European countries, including Italy, published national-level universal vaccination recommendations, among which 6 had publicly funded varicella vaccination programs [Citation28]. Nevertheless, in some European countries, implementation of UVV programs is still hampered by concerns regarding safety, sub-optimal coverage (which could lead to an increase in varicella incidence in older persons, who are at risk of more severe complications), the quality of surveillance systems, the impact of varicella vaccination on the incidence of HZ, and the impact of numerous other vaccines administered at the age when the first varicella vaccine dose should be given. Other barriers include cost-effectiveness and the lack of public health priority of varicella vaccination programs due to the alleged low disease burden [Citation28].
The current review aims to describe the success story of varicella vaccination in the first two European countries that implemented pediatric UVV programs, i.e., Italy and Germany. The review also highlighs the different approaches and evolution of varicella vaccination strategies and their health impact. Although challenges faced during the implementation process were fairly different in the two countries, a positive outcome of the UVV program may help decision-makers define key factors that could affect the impact of varicella vaccination. This may give insight into what might be the best implementation strategy for varicella vaccination in similar settings.
2. Methods
2.1. Scope of the review
The aim of this systematic review was to determine the coverage, effectiveness, safety, and cost-effectiveness of the 1-dose and short and long schedule (<1 year and ≥4 years between doses, respectively) 2-dose pediatric varicella vaccination programs implemented in Germany and Italy.
2.2. PICOS
As recommended for qualitative systematic reviews [Citation32], the PICOS (Population, Interventions, Comparators, Outcomes, Study design) tool was used to outline the inclusion criteria of the publications that were included in the review. In brief:
Population: persons who received varicella vaccination as part of a pediatric varicella vaccination program in Italy (from 2003 onwards, depending on region) or Germany (from 2004 onwards).
Interventions: 1-dose and 2-dose varicella vaccinations in Italy or Germany following implementation of UVV, and MMRV vaccination versus MMR+V in Italy or Germany.
Comparators: no varicella vaccination; 2-dose schedule versus the 1-dose schedule; short versus long 2-dose schedules; and safety of first-dose MMRV versus first-dose MMR+V, with a focus on febrile convulsions.
Outcomes: incidence, prevalence, and morbidity associated with varicella in each country before and after implementation of a vaccination program, as well as vaccine coverage, effectiveness, and impact (on incidence, hospitalizations, complications, breakthrough), health economics (cost-effectiveness), and safety outcomes of vaccinations.
Study design: studies were considered relevant if they assessed varicella infection and vaccination at a regional and/or national level in Italy or Germany.
The full details of each parameter can be found in the protocol (Supplement 1), which has been registered with the International Prospective Register of Systematic Reviews (PROSPERO, registration ID126775). Relevant time periods for assessment of each of the interventions are defined in more detail in the protocol (Supplement 1), but only data for confirmed relevant national or regional varicella vaccination programs were included.
2.3. Search strategy and selection criteria
Literature search strategies were developed using medical subject headings (MeSH) and text words relating to varicella infection and varicella vaccination in Italy and Germany. The full list of terms and details of the search strategies are provided in Supplement 2.
To minimize publication bias, both qualitative and quantitative studies were included, and no study design limits were imposed on the search. Conference databases, clinical trial databases, Internet sources, and the ‘gray’ literature were included in searches, as well as PubMed, Embase, and the Cochrane Library, to ensure that the review included relevant studies regardless of publication type or status. Furthermore, to minimize language bias, searches of PubMed, Embase, and government reports were not restricted to English language papers. Literature in Italian and German (the official languages of the countries of interest) that was deemed relevant based on abstracts was translated and included.
Each potentially relevant reference identified by the searches was assigned a unique identifying number and duplicate citations were removed prior to screening. To minimize bias, studies were assessed for inclusion using selection criteria defined by the Center for Research and Dissemination, University of York, United Kingdom [Citation33]. A two-stage screening process was employed: initial screening of titles and abstracts, followed by retrieval and screening of full papers that were identified as possibly relevant.
2.4. Data extraction and analysis
Combined data extraction and data entry were performed using an electronic form, which facilitated data analysis and generating the tables [Citation33]. To maintain the quality and integrity of the review, each potential study was assessed for quality of the study/publication and risk of bias (e.g., type of study) [Citation33]. To reflect the epidemiological/observational nature of public health studies, the Cochrane grading system for bias was employed and studies were assigned a judgment of ‘Low risk,’ ‘High risk,’ or ‘Unclear risk’ of bias [Citation34,Citation35]. The heterogeneity of the studies identified by this search allowed for a narrative synthesis that explores the relationship and findings both within and between the included studies, in line with the guidance from the Economic and Social Research Council Methods Programme and accepted by the Center for Reviews and Dissemination [Citation33].
2.5. Additional sources
In addition to the articles identified using the aforementioned search protocol, we also retrieved additional publications from the reference lists of articles identified by the systematic review as well as articles containing information on national and regional recommendations and surveillance systems used in Italy and Germany for varicella disease.
3. Results
The initial literature, congress, and website searches identified 3,059 potentially relevant records . Following removal of duplicates and screening of titles/abstracts and full-text articles (where appropriate), 123 records were identified as providing data relevant to the scope of the review (Supplement 3, Table S1) [Citation6,Citation8,Citation29,Citation31,Citation36–154].
Figure 1. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram of eligible studies from initial identification to inclusion in qualitative data synthesis
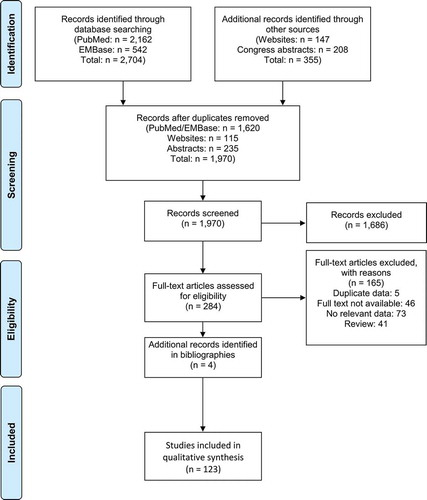
Only few articles focusing on febrile convulsions after MMRV vaccination were identified by our systematic literature review. All of these relied on modeling rather than real-world data and did not allow a robust interpretation of this topic. As the present report is aimed to review real-world safety and effectiveness data, related modeling studies were not included. and Supplementary tables S1–S4 present an overview of the data retrieved by the systematic literature review. The analysis focused primarily on publications with a low risk of bias and those that provided information collected in a consistent way both before and after the introduction of UVV in Italy and Germany.
Table 1. Seroprevalence of varicella infection before and after universal varicella vaccination
3.1. Pre-vaccination varicella epidemiology
3.1.1. Italy
Prior to the introduction of UVV, seroprevalence increased with age, from 20% to 40% in children 0.5–4 years to >80% by the age of 18 years () [Citation36,Citation38,Citation79,Citation91,Citation98]. Of 11 European countries evaluated between 1996 and 2003, Italy was the only one in which less than half (38%) of children aged 5 years were seropositive for VZV. Italy also presented the lowest seroprevalence rate at 15 years of age (78%) and the largest proportion of seronegative 20–29-year-olds (11%), child-bearing females 15–39 years old (13%), and female teenagers (18%) [Citation38]. Varicella was subject to mandatory notification before UVV implementation commenced. Italy’s National Institute of Statistics (ISTAT) and its regional departments collected reports of varicella cases based on a case definition [Citation132]. Due to a certain degree of underreporting in this system [Citation155], a sentinel surveillance system was also established (Italy’s Pediatric Sentinel Surveillance System of Vaccine-Preventable Diseases [SPES]), which included a sample of pediatricians [Citation132]. Before 2003, the annual incidence of varicella was generally greatest in children <5 years of age: 8,020 per 100,000 () [Citation6,Citation36]. SPES data indicated regional variability. Similar or higher incidences were reported in Veneto compared to the national-level, higher in Sicily, and lower in Tuscany [Citation98,Citation112,Citation118,Citation127,Citation132].
Table 2. Varicella incidence before and after universal varicella vaccination
The pre-UVV annual national varicella-related hospitalization rate was 37.5 per 100,000 children <2 years of age () [Citation110-111]. Regional data showed a wide variation in pre-UVV hospitalization rates. Among children ≤17 years of age, hospitalization was required for 16.8% of those reaching to an emergency department with varicella in Florence and 1.2% of notified cases in Tuscany [Citation123-128]. The median age of hospitalized persons was 5 years before the regional immunization program in Sicily [Citation29]. In 1997–1998, a large national epidemiological study showed that among 3,000 children aged <14 years with varicella, 126 (4.8%) had complications [Citation103]. A high percentage (up to 38%) of varicella-related complications were reported to be neurological [Citation120]. In the Tuscan pediatric population, the most frequent complications of hospitalized cases were of respiratory nature [Citation127]. According to ISTAT data, a mean of 5.5 varicella-related deaths occurred annually between 1991 and 2002 [Citation98].
Two large nationwide epidemiological studies estimated the cost of varicella in ≤14-year-olds at ~€130 per patient, with 70% of that being attributed to indirect costs, mainly due to absence from work [Citation102,Citation103].
Table 3. Varicella-related hospitalizations, complications, and vaccine effectiveness before and after universal varicella vaccination
3.1.2. Germany
The seroprevalence rate of VZV-specific antibodies in the pre-UVV period was ≤12% at the end of first year of life, increased to about 65% in 5-year-olds, and reached 95% by the age of 15–19 years [Citation38,Citation53,Citation88]. The immunity gap in women at childbearing age (18–39 years) was 3%–4% [Citation88]. An analysis based on serum samples collected between 1996 and 2003 showed that the proportion of VZV-seronegative persons was among the lowest of the 11 European countries included in the study, reaching 2.3% in 20–29-year-olds [Citation38].
Varicella became a notifiable disease in two German federal states between 2002 and 2003 [Citation48]. Systematic reviews showed that before 2004 the annual varicella incidence was generally greatest in children <5 years of age: 11,884 per 100,000 population () [Citation6,Citation36].
Robust epidemiological and surveillance reports showed annual hospitalization rates due to varicella of 42.6–96.5 per 100,000 for children <1 year, 21.8–47.6 per 100,000 for children of 1–4 years, and 6.1–14.3 per 100,000 in children aged 5 to 9–10 years; rates in older children and adolescents were 1–2 per 100,000 () [Citation39,Citation69]. A large epidemiological study calculated that among children with a mean age of 7.4 years with varicella, 5.7% (95% confidence interval [CI]: 4.5–6.9) had complications [Citation86]. Federal epidemiological data indicated that VZV-associated annual mortality rates were between 0 and 4 per 100,000 between 1994 and 2004 [Citation70,Citation71].
A large epidemiological study estimated the cost of varicella at €150 million/year, with an average of 0.7 and 5.9 working days lost due to uncomplicated varicella in children and adults, respectively [Citation86].
3.2. The 1-dose era
3.2.1. Recommendations and coverage
3.2.1.1. Italy
A 1-dose UVV program was introduced in Sicily and Puglia in 2003 and 2006, respectively, for all children aged approximately 15 months [Citation118,Citation133,Citation156] and for all susceptible adolescents at 12 years of age in Sicily [Citation156]. Until 2017, varicella vaccination was recommended at a regional level only and therefore varicella immunization policies differed widely between regions. Vaccine coverage for the single-dose reached >75% in Veneto and >90% in Puglia [Citation132,Citation133]. In addition to Sicily and Puglia, Basilicata, Calabria, Friuli Venezia Giulia, Sardinia, Tuscany, and Veneto also implemented UVV programs starting from 2005 with a 2-dose schedule. In 2012, the coverage of 1 dose across these 8 Italian regions reached 84–95% in 24-month-olds [Citation125]. Additional details are provided in Supplement 3 (Table S2).
3.2.1.2. Germany
Following the recommendations of the Standing Committee on Vaccination at the Robert Koch Institut (STIKO), Germany implemented national-level routine varicella vaccination in 2004 for all children aged 11–14 months along with a catch-up program for all susceptible children and adolescents, consisting of 2 doses for children over 13 years of age [Citation30]. To evaluate vaccine coverage and the epidemiological impact of the vaccination program, a nationwide sentinel surveillance system was initiated among primary care pediatricians and general practitioners in 2005 [Citation31,Citation157]. A mandatory notification system was introduced in the New Federal States between 2002 and 2009 and nationwide in 2013 [Citation48]. After the introduction of the 1-dose varicella UVV program, coverage rates generally increased year by year up to 65% (Supplement 3, Table S2) [Citation44,Citation61-65,Citation66]. During the single-dose UVV era, coverage of a second varicella vaccine dose (not publicly funded) increased between 2004 and 2009 [Citation31,Citation61,Citation73]. As of 2006, when the MMRV vaccine was licensed, more doses of the combined MMRV vaccine were administered compared to the monovalent varicella vaccine [Citation31].
3.2.2. Vaccine impact
3.2.2.1. Italy
Surveillance studies in regions that implemented a UVV program showed that 1 dose significantly decreased the incidence of varicella infection by 30%–80% (depending on vaccine coverage rate) () [Citation112,Citation113,Citation125,Citation131,Citation133]. Reductions in varicella infections of ≥89% were reported for Sicily and Puglia [Citation29,Citation134]. A nationwide surveillance study also indicated that there was a significant decrease in incidence from 164 cases in 2006 to 101 cases per 100,000 in 2009 (p < 0.01) [Citation95]. In Puglia, Sicily, and Veneto, significant reductions in varicella-related hospitalizations were observed as soon as 4 years after the introduction of UVV (p = 0.0004 for Puglia and p < 0.0001 for both Veneto and Sicily) [Citation134]. This study also showed that compared with non-UVV regions, the varicella incidence and varicella-related hospitalization rates declined more rapidly in UVV regions (p = 0.0428 and 0.0427, respectively). This was consistent with other data from Tuscany as well as from Puglia, which also showed a significant decrease in hospitalization rates within 4 years following UVV introduction (44% in Tuscany, 26% in Puglia) [Citation114,Citation133]. In Sicily, there was a decrease in the number of complications from 57 cases in 2002 to 14 in 2007 () [Citation115].
3.2.2.2. Germany
Surveillance studies demonstrated that the 1-dose UVV program decreased the varicella incidence by approximately 63–75% in children ≤4 years of age () [Citation31,Citation41]. At a regional level, significant decreases in the varicella incidence were also reported in Munich, Bavaria [Citation65]. One dose was shown to be highly effective in 1–2-, ≤4-, and ≤16-year-olds [Citation51,Citation56,Citation57]. A report, based on a surveillance study of outbreaks in day-care centers, showed vaccine effectiveness in 2008–2009 of 72% (95% CI: 59–81, p < 0.001), with no difference by age, day-care center, or gender [Citation44].
Varicella-related hospitalizations decreased from 13.3 (95% CI: 11.7–15.1) per 100,000 people in 2005 to 4.8 (95% CI: 3.6–6.3) per 100,000 in 2011, which equates to an ~65% decrease over the initial period of UVV () [Citation42]. Decreases of a similar and significant magnitude were observed when data on hospitalizations from 2005 to 2012 were compared with data from the pre-UVV era (p < 0.05) [Citation39]. As expected, these reductions were greatest in the <1 and 1–4 years age groups, at 61.3% and 62.6%, respectively. A single study on varicella-related hospitalizations between 2004 and 2010 demonstrated that a two-fold increase in vaccine coverage was associated with a two-fold decrease in hospitalizations [Citation41]. A robust epidemiological study showed that the percentage of varicella-related complications across all ages was 0.4% in 2005/2006 (first season of UVV) and 0.2% by 2008/2009 (fourth UVV season) [Citation31].
VZV-associated annual mortality rates post-UVV introduction were consistently low, at 0–1 per 100,000, based on federal epidemiological data [Citation71]. Even though mortality rates may have decreased following UVV implementation, it is difficult to compare them with pre-UVV data [Citation70], because mortality rates were consistently low.
Herd effects of 1-dose varicella vaccination were shown by relating vaccine coverage to varicella incidence reductions in children from all age groups [Citation56]. As such, varicella-associated hospitalization rates in pediatric oncology patients, who were not eligible for vaccination, showed a decreasing trend in the 2005–2009 period, likely as a result of herd effects [Citation52].
3.2.3. Health economics
3.2.3.1. Italy
Most post-UVV introduction studies focused on reductions in varicella-related hospital costs, with Italian regions calculating a reduction of >70% since the implementation of UVV (Supplement 3, Table S4) [Citation29,Citation114,Citation125]. For example, 1 year after UVV implementation, vaccine coverage was 60% in Friuli Venezia Giulia and a 10% reduction was observed in varicella-related hospitalization costs. In Puglia, 6 years after UVV implementation, coverage was 91% and an 86% reduction was observed [Citation125]. A robust surveillance study in Tuscany of 52,738 varicella cases reported a saving of 43% or €613,121 (€153,280 per year) in the UVV period (2009–2012) compared with the pre-vaccination period (2004–2007) [Citation114]. Transmission models with Italian population data have estimated that varicella vaccination resulted in net savings (indirect and direct costs), mainly from a societal perspective (including reductions in time off work and time caring for sick children), and savings were highest when including the routine vaccination of toddlers [Citation97]. Considering a vaccine coverage of 90%, it was predicted that for every €1 invested in vaccination, a saving of €1.20 from the health-system perspective and of €3.50 from a societal perspective was gained [Citation76].
3.2.3.2. Germany
In 2002, vaccination at the age of 12 months with or without a catch-up program for adolescents aged 11–12 years were anticipated to be the most cost-effective strategies, resulting in reductions in varicella infection of 84% and 83%, respectively, and in net savings of €53,000,000 and €51,300,000, respectively [Citation89] (Supplement 3, Table S4). While vaccination of susceptible 11–12-year-old adolescents was anticipated to have the highest benefits from a societal perspective, vaccination of all children aged approximately 15 months was anticipated to yield the best medical effects [Citation74-75]. A 90% coverage rate was predicted to yield an 87% decrease in varicella-associated deaths, a 61% decrease in societal expenses, and a 51% decrease in third-party payer expenses [Citation77].
3.3. The 2-dose era
3.3.1. Recommendations and coverage
3.3.1.1. Italy
A second varicella vaccine dose in the 5th year of life was added in the regional varicella vaccination program of Sicily in 2010, including the possible use of MMRV vaccine [Citation29]. Similarly, the 1-dose MMR and the monovalent varicella vaccines were replaced in Puglia in 2009 by a first dose of MMRV at the age of 13 months and a second dose of MMRV or MMR+V at 5–6 or 11–12 years of age [Citation133]. In 2005, Veneto introduced UVV for all children at 15 months of age with a second dose at the age of 6 years, and for all susceptible adolescents at the age 12 years [Citation134]. In Tuscany, UVV was implemented in 2008 with 2 doses of MMRV at the ages of 13–15 months and 5–6 years [Citation158]. By 2015, all 8 regions that implemented a regional varicella vaccination program between 2003 and 2013 were using a 2-dose schedule [Citation125]. The trend of increasing vaccine uptake over the years post-UVV implementation was maintained for the 2-dose regimen (Supplement 3, Table S2) [Citation133,Citation134]. However, from the limited data comparing first- and second dose uptake, coverage of the second dose was initially much lower than that of the first dose [Citation133]. Following the introduction of MMRV vaccination, studies indicated that this formulation was preferred, and coverage in Veneto quickly increased up to 79% in the 2008 birth cohort [Citation132]. Vaccination coverage data at 5–6 years of age were not available routinely in Italy before 2016. Starting from the 2014 birth cohort, coverage for the second dose was collected at the national level [Citation159]. One-dose coverage at 24 months of age was on average unsatisfactory in 2015: 30.7% at the national level, and 53–84% across the 8 regions with UVV programs [Citation160]. Nationwide UVV of all children was included in the Italian National Plan for Immunization for 2017–2019 and has been mandatory and reimbursed since 2017. Two doses are recommended at the ages of 13–15 months and 5–6 years [Citation160,Citation161]. For risk groups, varicella vaccination was already included in the 2005–2007 Italian National Plan [Citation162].
3.3.1.2. Germany
The UVV program was amended in 2009 to include a second varicella-containing vaccine dose between 15 and 23 months of age, at no less than 4 weeks after a first dose, which is administered at 11–14 months of age. Vaccination could be either with a monovalent varicella vaccine co-administered with MMR at different injection sites or with MMRV [Citation163,Citation164]. Within 2 years following the introduction of the MMRV vaccination, studies indicated that the MMRV formulation was preferred in ~90% of cases [Citation67]. Due to a slight increase in febrile convulsions after using either of the two available MMRV vaccines as a first dose, separate but concomitant varicella and MMR vaccinations were recommended in 2011 for the first dose instead of MMRV [Citation63,Citation165,Citation166]. Although the use of MMRV as a first dose declined to rates as low as 25% of the initial usage in the year after the recommendation, data from selected centers showed that the overall uptake of the first varicella vaccine dose did not decrease (Supplement 3, Table S2) [Citation67]. Another study identified regional declines in first varicella vaccine dose uptake in Munich (12%) and, to a lesser extent in Würzburg (4%) [Citation85]. A study evaluating administration of the short 2-dose regimen in five states to children born in 2009 (the year of this regimen’s implementation) showed that vaccine coverage was as high as 87% for the first dose. Furthermore, 64% of children also received a second dose [Citation41]. A national surveillance study showed that there was a twofold increase in the coverage of two doses between 2009 and 2014 (35% versus 68%, respectively) [Citation51]. By 2015, the first-dose coverage increased to 80–90% nationwide [Citation50,Citation73], and only <4% of the children who received a first dose did not receive their second dose [Citation73].
3.3.2. Vaccine impact
3.3.2.1. Italy
In Sicily, varicella notifications decreased by >95% between 2003 and 2012 () [Citation29]. In Puglia, Sicily, and Tuscany, the varicella incidence fell below 0.5 cases per 1000 person years by the fourth year after UVV commenced; in Veneto, this decline occurred by the sixth year [Citation125]. In Puglia, a robust study showed that between 2006 and 2012, breakthrough accounted for 3.4% of varicella cases in children aged <72 months and 2.9% of cases in children hospitalized due to varicella [Citation133]. A second study showed that while breakthrough accounted for >25% of cases of varicella infection in Tuscany between 2010 and 2013, this proportion decreased from 40.5% in the 2008 birth cohort to 4.5% in the 2011 birth cohort, which coincided with increasing vaccine effectiveness [Citation131]. This second study included children who had received two doses of varicella vaccine, but calculated the proportion of breakthrough in children who had received at least 1 dose. Of the vaccinated children from the 2008–2011 birth cohorts, only 0.3% experienced breakthrough varicella [Citation131].
A study of nationwide hospital databases revealed a decrease in hospitalization rates from 4.2 per 100,000 inhabitants in 2002 and 2004 to 1.9 per 100,000 in 2013 and 2014 () [Citation91-92]. This coincided with the introduction of regional UVV from 2003 to 2013. Pooled data from all eight regions that had implemented regional UVV programs since 2003 showed a substantial reduction of varicella cases and hospitalizations by 2012 [Citation125]. More exactly, in Sicily and Puglia, the incidence of varicella-related hospitalizations dropped to 0.8 and 1.1 per 100,000 person years by 2012 and 2009–2012, respectively [Citation29,Citation133].
A robust epidemiological study across three regions in Italy implementing UVV indicated that the rate of HZ-related hospitalizations in adult patients reported was consistently lower in these regions since UVV began (Supplement 3, Table S3) [Citation94].
3.3.2.2. Germany
Two national surveillance studies of insurance claims demonstrated that vaccine effectiveness was higher after two doses than after one dose () [Citation50,Citation51]. One of these studies looked at the 2009–2014 period and showed that vaccine effectiveness after 1 dose of varicella vaccine was 86.6% (95% CI: 85.2–87.9) compared with 97.3% (95% CI: 97.0–97.6) after 2 doses [Citation51]. The other study showed that 2-dose effectiveness for all combinations of varicella and MMRV vaccines ranged between 94.3% (95% CI: 93.9–94.8) and 95.0% (95% CI: 94.3–95.5) suggesting that the type of vaccination administered and the order do not influence effectiveness. In addition, an interval of 28 days to 3 years between vaccinations had no effect on vaccine effectiveness [Citation50]. Furthermore, an epidemiological prospective, matched case–control study in Bavaria showed that age at vaccination of <15 vs ≥15 months did not influence vaccine effectiveness [Citation57]. The overall picture indicates 3- to 4-fold decreases in the incidence of varicella infection with the introduction of the 2-dose UVV program. It should be noted that the UVV program has not eradicated varicella infection, with coverage rates in the range of 70%–90% depending on the study, year, and region [Citation31,Citation39,Citation41,Citation50,Citation85]. A sentinel network surveillance study of varicella cases that occurred between April 2005 and March 2014 identified 111,456 cases, of which 4,357 were breakthrough cases [Citation40]. Of these, 80% were in children who had received 1 dose of vaccine and 20% in those who had received 2 doses [Citation40]. However, the absolute number of breakthrough cases appeared to increase over time, which possibly reflects increasing rates of immunization [Citation31]. Some studies provided data suggesting that breakthrough varicella was associated with a lower risk of fever and with moderate disease, and that two doses of vaccine were associated with a lower risk of breakthrough than a single dose [Citation44].
According to national hospital discharge data, the mean age-adjusted incidence of varicella-related hospitalizations decreased from 3.3 to 1.9 per 100,000 person years after UVV introduction (2005–2012 period), with the highest declines observed in regions with the highest vaccination coverage () [Citation39].
A surveillance study concluded that childhood varicella vaccination has not affected the incidence of HZ in older age groups (Supplement 3, Table S3) [Citation39].
3.3.3. Health economics
3.3.3.1. Italy
Using an Economic Varicella Vaccination Tool for Analysis model, the 2-dose vaccination of toddlers aged 1–1.5 years, of adolescents aged 13 years, or of toddlers and adolescents through a catch-up program were anticipated to be highly effective in reducing the disease burden of varicella. These three strategies were also anticipated to result in significant net savings from a societal perspective but to higher costs as compared to return of investment from a National Health Service perspective (Supplement 3, Table S4) [Citation97]. In Sicily, direct varicella-related hospitalization costs decreased by 80% between 2003 (€485,000) and 2012 (€82,000) [Citation29]. In Puglia, varicella-related hospitalization costs decreased from €319,000/year during the pre-vaccination era (2003–2005) to €267,000 per year during the 1-dose era (2006–2008), and to €106,000 per year in the 2-dose era (2009–2012) [Citation133].
A pooled analysis of six Italian regions, namely Basilicata, Friuli Venezia Giulia, Puglia, Sicily, Tuscany, and Veneto, revealed a reduction in healthcare expenses of the Regional Health Service by >50% as compared to 2004, settling at under €1,000,000 in 2012. Annual varicella-related hospitalization costs decreased by 60% during the same period, with higher reductions observed in Puglia (86%), Sicily (83%), Tuscany (77%), Veneto (75%), and Basilicata (71%), whereas in Friuli Venezia Giulia, the latest to implement UVV, this reduction was 10% [Citation125].
3.3.3.2. Germany
Several modeling studies have estimated that varicella vaccination results in net savings despite an increase in direct costs (Supplement 3, Table S4). Of note, a number of these models used scenarios in which only seronegative toddlers and/or adolescents were vaccinated. However, models using routine vaccination of toddlers have estimated total costs savings from the societal perspective of up to 61% and a decrease of 59% in complications compared to no vaccination [Citation77].
3.4. Predictions on the impact of UVV programs on epidemiology of HZ
Predictions using modeling studies including population data show that the HZ incidence would increase in the 50 years post-UVV introduction as a natural boom in both Italy and Germany (Supplement 3, Table S3) [Citation49,Citation80-84,Citation96]. However, after this time, the predicted subsequent impact of long-term UVV (up to 100 years) on the incidence of HZ becomes country-specific. In Italy, the increase in incidence of vaccination-related HZ is predicted to be sustained with long-term UVV [Citation80]. In contrast, in Germany, the initial increase in incidence will not be sustained, with a subsequent decrease of >50% estimated [Citation49]. Nevertheless, these modeling predictions have not been confirmed by real-world evidence [Citation49].
4. Conclusion/summary
Although the UVV strategies adopted in Italy and Germany are significantly different, each met the respective country’s epidemiological situation and objectives and was successful. In pilot regions of Italy and in Germany, 1-dose UVV was effective, especially against disease of moderate/severe intensity and varicella-related hospitalizations. As a result of the age-based epidemiology data generated by each country’s surveillance system, the timing chosen for the second dose differed between Italy and Germany, which adopted a long and a short schedule, respectively. The addition of the second dose to the UVV programs led to further reductions in the incidence of varicella of any severity, with incremental effectiveness of the second dose demonstrated in Germany. Although concomitant MMR+V vaccination reduces the risk of febrile convulsions as compared to MMRV when administered as a first dose, it was also shown to temporarily reduce varicella vaccine uptake. Both the long and the short schedule led to a reduction in healthcare and hospitalization costs.
5. Expert opinion
Before implementation of UVV, the disease burden of varicella in Italy and Germany was very high. Cost-effectiveness studies anticipated that UVV programs would substantially reduce this burden and be cost-effective, especially in susceptible children and adolescents. Nevertheless, some key barriers needed to be overcome in these countries, which will also likely be faced by newly implementing countries.
First of all, varicella is generally perceived to be a mild disease. Nevertheless, when taking into account its burden from all perspectives (e.g., in addition to treatment, the need for an adult caregiver for the afflicted child; expenses from both societal and healthcare perspectives), vaccination was shown to be, as predicted, a cost-effective means to reduce this burden in both Italy and Germany. Particularly, varicella-related hospitalization costs decreased remarkably (by up to ~90%) within a few years after implementation of a UVV program with either 1 or 2 doses. This translates to substantial savings, given that a high proportion of varicella patients with complications end up being hospitalized. Another major benefit of UVV programs is that these also ensure indirect protection of unvaccinated immunocompromised individuals against varicella and of adults against severe varicella through herd effect.
Other barriers include concerns about the shift of varicella to older age groups and concerns regarding potential increases in HZ incidence. In Italy and Germany, no shift to older ages occurred in varicella incidence; reductions occurred in all age groups, suggestive of a herd effect. In contrast with model predictions of HZ incidence within the first 50 years of UVV in Italy and Germany, worldwide evidence gathered to date provides ambiguous results that do not support a vaccination-associated increase in HZ incidence in older populations [Citation5,Citation167,Citation168,Citation169,Citation170,Citation171,Citation172,Citation173]. Moreover, vaccination even prevents HZ in children [Citation174,Citation175]. Although the failure of previous models to predict HZ epidemiology in the context of varicella vaccination is multifactorial, a drawback of these models is that they did not account for endogenous boosting, which has been recently shown to play a role as important as exogenous boosting in protection against HZ [Citation176]. The role of exogeneous boosting in contacts (i.e., parents, grandparents) of varicella-infected children is well established in protection against HZ [Citation177,Citation178]. In real-world settings, the role of endogenous boosting in protecting against HZ has been confirmed in members of contemplative monastic orders, in whom the lack of exposure to VZV did not increase the risk of HZ or result in earlier HZ onset compared to the general population [Citation179]. Of note, as the HZ risk can be substantially reduced by vaccination, any future changes in HZ epidemiology in countries implementing UVV programs need to be interpreted considering vaccination strategies against HZ. In Germany, the STIKO recently issued recommendations on the use of the highly effective adjuvanted recombinant zoster vaccine [Citation180].
Finally, alleged safety concerns perpetuated via the internet and social media may result in the hesitancy of parents to have their children vaccinated. Public health stakeholders of newly implementing countries need to improve communication, so that caregivers are informed about the individual and population-level benefits of UVV programs. Caregivers should also be informed about the vaccines’ safety using evidenced-based information and about the absence of a negative impact on HZ epidemiology. In Italy and Germany, the main drivers behind the change toward UVV implementation were pediatricians, public health physicians, and health economists. It is therefore important to increase awareness of the benefits and well-established safety profile of varicella vaccination among pediatricians, as they are the prime contacts with children’s caregivers and are paramount in the successful implementation of UVV strategies. Evidence-based information regarding vaccination should also be disseminated in a transparent manner via contemporary means of communication (i.e., social media).
To assess the burden of varicella and the impact of UVV programs on varicella and HZ epidemiology, adequate surveillance systems are needed, which can reliably capture the incidence of these diseases. In Italy, varicella was a notifiable disease in the pre-vaccination era and the difference between the incidence data captured by the active and passive surveillance systems allowed for an estimation of the underreporting, which was also highlighted by the large differences observed between regions. In Germany, a mandatory notification system was established between 2002 and 2009 in the New Federal States (only in 2 states before UVV initiation) and in 2013 nationwide [Citation48]. A national sentinel system was also introduced in 2005, 1 year after 1-dose UVV initiation. In-depth epidemiology data by age group help to select the most appropriate vaccination strategy, including the number and timing of doses.
To choose the best vaccination strategy, additional variables need to be considered. The number and timing of doses also depends on the aims of the UVV and its economic considerations, while the choice of the vaccine (whether monovalent or quadrivalent) depends on variables such as availability in the implementing country, the number of doses, the age at which the first dose is recommended, the national immunization scheme, and the subjective different perception about the benefit/risk profile of the quadrivalent varicella vaccine compared to monovalent when used as first dose. When the national UVV programs were implemented, Varivax and Varilrix were licensed and available in Germany, and Varivax, Varilrix, Priorix-Tetra, and ProQuad in Italy. In Italy, Priorix-Tetra and ProQuad were licensed in 2008 and 2006, respectively, just after implementation of the 2-dose UVV programs began in the pilot regions but before the implementation of the national UVV program [Citation181,Citation182]. In Germany, Priorix-Tetra and ProQuad were licensed before the implementation of the 2-dose UVV program. In both Italy and Germany, varicella vaccination was recommended in at-risk populations before the implementation of the nationwide UVV program. In the two Italian pilot regions which implemented 1-dose UVV, vaccination was undertaken from 13 to 15 months of age, when the first MMR dose was recommended in Italy. In Germany, during the 1-dose era, the varicella vaccine was also recommended at the same age as the first MMR dose, at 11–14 months of age [Citation183]. The different age indications of the two monovalent varicella vaccines (minimum age 9 months for Varilrix, 12 months for Varivax) influenced the vaccine choice in Germany, where vaccines were reimbursed, but the lower age limit for the varicella vaccine recommendation was 11 months. Six of the eight Italian pilot regions, as well as all remaining 12 Italian regions, initiated UVV programs with a 2-dose schedule. In the regions which already had 1-dose UVVs (Sicily and Puglia), as well as in Germany, the recommendations were amended to include a second dose. In both countries, the timing of the first dose was maintained. The recommended dosing interval was ≥4 years in Italy (long schedule) and <1 year in Germany (short schedule). This choice was driven by the epidemiology of varicella in each country, showing earlier contact with VZV in Germany than in Italy. The choice of the dosing interval was also driven by the need to keep the already existing planned vaccination visits. The possible use of the tetravalent vaccine as either of the doses was also included in the recommendations in both countries, with the monovalent varicella vaccine preferred in catch-up campaigns of both countries. In Germany, the STIKO recommended concomitant MMR+V administration as first dose in place of MMRV 2 years after implementation of the 2-dose schedule, due to an increased risk of febrile convulsions. Nonetheless, the overall uptake of first varicella vaccine dose did not decrease. After the licensure and availability on the Italian market of the quadrivalent MMRV vaccine Priorix-Tetra, its use was preferred over to the monovalent vaccines in the UVV pilot regions. Some of these regions, such as Tuscany, exploited the drag effect of the already high vaccination coverage for MMR to obtain a high varicella vaccine coverage too. In contrast to Germany, MMR+V as a first dose is the vaccine to be exclusively used for persons with a history of febrile convulsions. Otherwise, MMRV should be preferentially used for both doses, although parents are informed of the slightly increased risk of febrile convulsions after MMRV use as first dose, and are given the opportunity to choose for MMR+V simultaneous administration.
The alleged safety concerns, mostly related to vaccination in general, may result in vaccine hesitancy and could be a possible obstacle in achieving the >80% coverage recommended by the WHO [Citation1]. Coverage rates in 24-month-olds exceeded this threshold in all eight Italian pilot regions in 2012 and also in Germany for the 2007–2009 birth cohorts. Dispelling population concerns using data from trustworthy pharmacovigilance systems paired with an efficient health communication on vaccine-preventable infectious diseases might be a possible solution to reverse vaccine hesitancy and to consequently improve coverage [Citation167].
In sum, even though varicella was generally perceived as a mild disease and UVV programs were associated with potential concerns, disease surveillace systems in Italy and Germany have shown that the actual burden of varicella was high in the pre-vaccination era and that it was significantly reduced after UVV implementation, with none of the concerns being confirmed. In each of these countries, the success of the UVV program relied on the identification of an adapted vaccination strategy, which resulted in high coverage rates within a few years from implementation. With the increasing number of countries that are implementing varicella UVV programs, including Italy and Germany, doubts about effectiveness, safety, and economic impact are considerably decreasing, making varicella vaccination one of the immunization strategies with the highest public health impact.
In the coming years, further consolidation of vaccination against varicella in Italy and Germany as a public health approach will contribute to high and stable coverage and provide the opportunity to generate additional evidence of the safety and cost-effectiveness of this preventive strategy. This especially applies to Italy, where varicella vaccination was only recently moved from a regional to a national context, and has been reimbursed since the introduction in the National Immunization Plan in 2017 and mandatory since shortly after, when the law requiring immunization for school entry was issued in July 2017. Due to the success of varicella vaccination in Italy and Germany, together with other countries such as Spain and Greece, an increasing number of (European) countries may attempt to better estimate the economical and societal impact of varicella and its complications on the healthcare system, and to more thoroughly evaluate the cost/benefit profile of routine pediatric varicella vaccination. Additionally, the recent real-world evidence on the overestimated impact of UVV on the adult HZ incidence will further improve the cost-effectiveness and/or cost-saving potential of UVV. Finally, the recent recommendations by the STIKO in Germany on the use of the adjuvanted recombinant zoster vaccine will provide insights into the possibility of a successful co-existence of protective agents for the pediatric and adult forms of VZV disease. Availability of the recently licensed adjuvanted recombinant zoster vaccine would allow policymakers to recommend UVV in order to prevent morbidity in children and, in the same time, to mitigate the risk of HZ incidence increase in adults, if any [Citation184].
Article highlights
Italy implemented 1-dose universal varicella vaccination in two pilot regions from 2003 onwards.
2-dose universal varicella vaccination was later implemented in six other Italian pilot regions through 2013 and in 2017 nationwide.
Germany implemented universal varicella vaccination with 1 dose in 2004 and 2 doses in 2009.
Long and short interval 2-dose schedules were used in Italy and Germany (≥4 years and <1 year, respectively).
A tetravalent varicella-containing vaccine is preferred over the combined measles-mumps-rubella vaccine and monovalent varicella vaccine as second dose in both countries.
Both countries’ vaccination strategies led to a reduction in varicella-related health care hospitalization costs.
Author contributions
All authors contributed to study conception, interpretation of the data, and critical review of the paper for important intellectual content. All authors are in agreement with the content of the final article and have approved it for submission and publication.
Declaration of interest
F Kauffmann is an employee of the GSK group of companies. G Casabona is an employee of the GSK group of companies and holds shares in the GSK group of companies. A Bechini has received personal fees for advisory boards and meetings from the GSK group of companies, Sanofi Pasteur, Merck Sharp & Dohme, Pfizer and Seqirus outside the submitted work. P Bonanni has received personal fees for advisory boards and meetings from the GSK group of companies, Sanofi Pasteur, Merck Sharp & Dohme, Pfizer and Seqirus outside the submitted work. P Wutzler has received personal fees for advisory boards from the GSK group of companies. Writing assistance, including writing the protocol, literature searching and screening, data extraction and verification, and development of the report on the systematic literature of the review, provided by Andy Noble & Claire Chinn (BioScript Group, Macclesfield, Cheshire), was utilized in the production of this manuscript and funded by GlaxoSmithKline Biologicals SA. Medical writing support, provided by Botond Nagy & Alpár Pöllnitz (Modis), and manuscript development and editorial support, provided by Julie Mellery (Modis), were utilized in the production of this manuscript and funded by GlaxoSmithKline Biologicals SA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Trademark statement
Varilrix and Priorix-Tetra are trademarks of the GSK group of companies. Varivax and ProQuad are trademarks of Merck Sharp & Dohme Corp.
Supplemental Material
Download MS Word (347.1 KB)Acknowledgement
The authors acknowledge the contribution of Andy Noble and Claire Chinn of Bioscript Group, Macclesfield, Cheshire, to writing the protocol, literature searching and screening, data extraction and verification, and development of the report on the systematic literature review, on behalf of GSK. Authors thank Modis for editorial assistance and manuscript coordination, on behalf of GSK. Botond Nagy and Alpár Pöllnitz provided medical writing support and Julie Mellery coordinated the manuscript development and editorial support.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Varicella and herpes zoster vaccines: WHO position paper, June. 2014. Releve Epidemiologique Hebdomadaire. 2014 Jun 20;89(25):265–287.
- Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5th ed. Philadelphia, PA: Saunders Elsevier; 2008.
- Seward J, Jumaan A VSV: Persistence in the population. In: Arvin A, Campadelli-Fiume G, Mocarski E, et al. editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press; 2007. Chapter 40. Available from: https://www.ncbi.nlm.nih.gov/books/NBK47367/
- Yawn BP, Gilden D. The global epidemiology of herpes zoster. Neurology. 2013 Sep 3;81(10):928–930.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: Towards a global perspective. BMJ Open. 2014 Jun 10;4(6):e004833.
- Riera-Montes M, Bollaerts K, Heininger U, et al. Estimation of the burden of varicella in Europe before the introduction of universal childhood immunization. BMC Infect Dis. 2017 May 18;17(1):353.
- Wolfson LJ, Castillo ME, Giglio N, et al. Varicella healthcare resource utilization in middle income countries: A pooled analysis of the multi-country MARVEL study in Latin America & Europe. Hum Vaccin Immunother. 2019 Jan 25;15(4):932–941.
- Banz K, Wagenpfeil S, Neiss A, et al. The burden of varicella in Germany. Potential risks and economic impact. Eur J Health Econ. 2004 Feb;5(1):46–53.
- Gater A, Uhart M, McCool R, et al. The humanistic, economic and societal burden of herpes zoster in Europe: A critical review. BMC Public Health. 2015 Feb 15:193.
- Friesen KJ, Chateau D, Falk J, et al. Cost of shingles: Population based burden of disease analysis of herpes zoster and postherpetic neuralgia. BMC Infect Dis. 2017 Jan 13;17(1):69.
- Panatto D, Bragazzi NL, Rizzitelli E, et al. Evaluation of the economic burden of Herpes Zoster (HZ) infection. Hum Vaccin Immunother. 2015;11(1):245–262.
- Clements DA. Varicella vaccination in children. Biodrugs. 2000 Jul;14(1):49–60.
- Takahashi M. Current status and prospects of live varicella vaccine. Vaccine. 1992;10(14):1007–1014.
- European Centre for Disease Prevention and Control (ECDC). [cited 2019 Feb 4]. Available at: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/Varicella-Guidance-2015.pdf
- Kreth HW, Lee BW, Kosuwon P, et al. Sixteen years of global experience with the first refrigerator-stable varicella vaccine (Varilrix). Biodrugs. 2008;22(6):387–402.
- Varivax. Prescribing Information. [Cited 2019 Feb 13]. Available at: https://www.merck.com/product/usa/pi_circulars/v/varivax/varivax_pi.pdf
- Prevention of varicella: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Centers for Disease Control and Prevention. MMWR Recomm Rep. 1996 Jul 12;45(RR–11):1–36.
- Ferrera G, Gajdos V, Thomas S, et al. Safety of a refrigerator-stable varicella vaccine (VARIVAX) in healthy 12- to 15-month-old children: A randomized, double-blind, cross-over study. Hum Vaccines. 2009 Jul;5(7):455–460.
- Varivax. Summary of product characteristics. [cited 2019 Feb 13]. Available at: https://www.hpra.ie/docs/default-source/vaccine-pils/varivax.pdf?sfvrsn=22
- Priorix-Tetra. Summary of product characteristics. [cited 2019 Apr 10]. Available at: https://gskpro.com/content/dam/global/hcpportal/en_MT/PDF/Homepage/Products/productlisting/priorix-tetra/Priorix_Tetra_PI_II_078_18_Apr_2017.pdf
- ProQuad. Summary of product characteristics. [cited 2019 Apr 10]. Available at: https://www.ema.europa.eu/en/documents/product-information/proquad-epar-product-information_en.pdf
- ProQuad. EPAR. [cited 2019 Feb 13]. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/proquad#authorisation-details-section
- Lopez AS, Zhang J, Brown C, et al. Varicella-related hospitalizations in the United States, 2000–2006: The 1-dose varicella vaccination era. Pediatrics. 2011 Feb;127(2):238–245.
- Marin M, Meer HC, Seward JF. Varicella prevention in the United States: A review of successes and challenges. Pediatrics. 2008 Sep;122(3):e744–51.
- Lee BR, Feaver SL, Miller CA, et al. An elementary school outbreak of varicella attributed to vaccine failure: Policy implications. J Infect Dis. 2004 Aug 1;190(3):477–483.
- Povey M, Henry O, Riise Bergsaker MA, et al. Protection against varicella with two doses of combined measles-mumps-rubella-varicella vaccine or one dose of monovalent varicella vaccine: 10-year follow-up of a phase 3 multicentre, observer-blind, randomised, controlled trial. Lancet Infect Dis. 2019 Mar;19(3):287–297.
- Varicella vaccines. WHO position paper. Releve Epidemiologique Hebdomadaire. 1998 Aug 7;73(32):241–248.
- Spoulou V, Alain S, Gabutti G, et al. Implementing universal varicella vaccination in Europe: The path forward. Pediatr Infect Dis J. 2019 Feb;38(2):181–188.
- Amodio E, Tramuto F, Cracchiolo M, et al. The impact of ten years of infant universal varicella vaccination in Sicily, Italy (2003–2012). Hum Vaccin Immunother. 2015;11(1):236–239.
- Empfehlungen der Ständigen Impfkommission am Robert-Koch-Institut (STIKO). Epidemiol Bull 2004; 49: 421–432. [cited 2019 Feb 4]. Available at: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2004/Ausgabenlinks/49_04.pdf%3F__blob%3DpublicationFile
- Siedler A, Arndt U. Impact of the routine varicella vaccination programme on varicella epidemiology in Germany. Euro Surveill. 2010 Apr 1;15(13):19530.
- Methley AM, Campbell S, Chew-Graham C, et al. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014 Nov;21(14):579.
- Centre for Reviews and Dissemination. Systematic Reviews. CRD’s guidance for undertaking reviews in health care. 2009 [cited 2019 Aug 19]. Available at: https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf
- Harder T, Takla A, Eckmanns T, et al. PRECEPT: An evidence assessment framework for infectious disease epidemiology, prevention and control. Euro Surveill. 2017 Oct;22(40). doi:10.2807/1560-7917.ES.2017.22.40.16-00620.
- The Cochrane Collaboration: The Cochrane Collaboration’s tool for assessing risk of bias. Cochrane handbook for systematic reviews of interventions. 2011 [cited 2019 Nov 1]. Available at: http://handbook-5-1.cochrane.org/.
- Bollaerts K, Riera-Montes M, Heininger U, et al. A systematic review of varicella seroprevalence in European countries before universal childhood immunization: Deriving incidence from seroprevalence data. Epidemiol Infect. 2017 Oct;145(13):2666–2677.
- Horn J, Damm O, Greiner W, et al. Influence of demographic changes on the impact of vaccination against varicella and herpes zoster in Germany - a mathematical modelling study. BMC Med. 2018 Jan 9;16(1):3.
- Nardone A, de Ory F, Carton M, et al. The comparative sero-epidemiology of varicella zoster virus in 11 countries in the European region. Vaccine. 2007 Nov 7;25(45):7866–7872.
- Siedler A, Dettmann M. Hospitalization with varicella and shingles before and after introduction of childhood varicella vaccination in Germany. Hum Vaccin Immunother. 2014;10(12):3594–3600.
- Siedler A, Dettmann M, Tolksdorf K, et al. Laboratory investigations of vaccinated patients with varicella. Vaccine. 2015 Apr 15;33(16):1968–1973.
- Siedler A, Hecht J, Rieck T, et al. Varicella vaccination in Germany. A provisional appraisal in the context of MMR vaccination. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013 Sep;56(9):1313–1320. German
- Streng A, Grote V, Rack-Hoch A, et al. Decline of neurologic varicella complications in children during the first seven years after introduction of universal varicella vaccination in Germany, 2005–2011. Pediatr Infect Dis J. 2017 Jan;36(1):79–86.
- Zoch-Lesniak B, Tolksdorf K, Siedler A. Trends in herpes zoster epidemiology in Germany based on primary care sentinel surveillance data, 2005–2016. Hum Vaccin Immunother. 2018 Jul 3;14(7):1807–1814.
- Spackova M, Wiese-Posselt M, Dehnert M, et al. Comparative varicella vaccine effectiveness during outbreaks in day-care centres. Vaccine. 2010 Jan 8;28(3):686–691.
- Gvozdenovic E, Vetter V, Willame C, et al. Impact of history of febrile convulsions on the risk difference of febrile convulsions with the tetravalent measles-mumps-rubella-varicella vaccine: Post-hoc exploratory analysis of results from a matched-cohort study. Vaccine. 2018 Sep 18;36(39):5803–5806.
- Hagemann C, Seeger K, Kramer A, et al. [Varicella vaccination coverage and possible factors influencing parental vaccination decisions in Munich area 2009–2011 after introduction of routine varicella vaccination]. Gesundheitswesen. 2017 Apr;79(4):286–295. German
- Hagemann C, Streng A, Kraemer A, et al. Heterogeneity in coverage for measles and varicella vaccination in toddlers - analysis of factors influencing parental acceptance. BMC Public Health. 2017 Sep 19;17(1):724.
- Hecht J, Siedler A. The epidemiology of varicella disease in Germany after introduction of a vaccination recommendation: Analysis of mandatory and sentinel data between 2002 and 2014. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2017 Jan;60(1):118–126. German
- Horn J, Karch A, Damm O, et al. Current and future effects of varicella and herpes zoster vaccination in Germany - Insights from a mathematical model in a country with universal varicella vaccination. Hum Vaccin Immunother. 2016 Jul 2;12(7):1766–1776.
- Rieck T, Feig M, Ander Heiden M, et al. Assessing varicella vaccine effectiveness and its influencing factors using health insurance claims data, Germany, 2006 to 2015. Euro Surveill. 2017 Apr 27;22(17). DOI:10.2807/1560-7917.ES.2017.22.17.30521.
- Siedler A, Rieck T, Tolksdorf K. Strong additional effect of a second varicella vaccine dose in children in Germany, 2009–2014. J Pediatr. 2016 Jun;173:202–206e2.
- Streng A, Wiegering V, Liese JG. Varicella in pediatric oncology patients in the post-vaccine era-Analysis of routine hospital data from Bavaria (Germany), 2005–2011. Pediatr Hematol Oncol. 2016 Oct - Nov;33(7–8):468–479.
- Wiese-Posselt M, Siedler A, Mankertz A, et al. Varicella-zoster virus seroprevalence in children and adolescents in the pre-varicella vaccine era, Germany. BMC Infect Dis. 2017 May 19;17(1):356.
- Bauchau V, Van Holle L, Cohen C. Modelling hospitalisation ratios for febrile convulsions and severe varicella under combined measles, mumps, rubella, and varicella (MMRV-Priorix-TetraTM) compared to separate MMR + V vaccination. Drug Saf. 2015 Nov;38(11):1095–1102.
- Grote V, von Kries R, Springer W, et al. Varicella-related deaths in children and adolescents–Germany 2003–2004. Acta Paediatr. 2008 Feb;97(2):187–192.
- Hohle M, Siedler A, Bader HM, et al. Assessment of varicella vaccine effectiveness in Germany: A time-series approach. Epidemiol Infect. 2011 Nov;139(11):1710–1719.
- Liese JG, Cohen C, Rack A, et al. The effectiveness of varicella vaccination in children in Germany: A case-control study. Pediatr Infect Dis J. 2013 Sep;32(9):998–1004.
- Liese JG, Grote V, Rosenfeld E, et al. The burden of varicella complications before the introduction of routine varicella vaccination in Germany. Pediatr Infect Dis J. 2008 Feb;27(2):119–124.
- Neuhauser H, Poethko-Muller C. KIGGS Study Group. [Chronic and vaccine-preventable diseases in children and adolescents in Germany: Results of the KiGGS study: First follow up (KiGGS wave 1)]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2014 Jul;57(7):779–788. German
- Rack AL, Grote V, Streng A, et al. Neurologic varicella complications before routine immunization in Germany. Pediatr Neurol. 2010 Jan;42(1):40–48.
- Reuss AM, Feig M, Kappelmayer L, et al. Varicella vaccination coverage of children under two years of age in Germany. BMC Public Health. 2010 Aug 19;10:502.
- Rieck T, Feig M, Eckmanns T, et al. Vaccination coverage among children in Germany estimated by analysis of health insurance claims data. Hum Vaccin Immunother. 2014;10(2):476–484.
- Schink T, Holstiege J, Kowalzik F, et al. Risk of febrile convulsions after MMRV vaccination in comparison to MMR or MMR+V vaccination. Vaccine. 2014 Feb 3;32(6):645–650.
- Spackova M, Muehlen M, Siedler A. Complications of varicella after implementation of routine childhood varicella vaccination in Germany. Pediatr Infect Dis J. 2010 Sep;29(9):884–886.
- Streng A, Grote V, Carr D, et al. Varicella routine vaccination and the effects on varicella epidemiology - results from the bavarian varicella surveillance project (BaVariPro), 2006–2011. BMC Infect Dis. 2013 Jul 2;13(1):303.
- Streng A, Seeger K, Grote V, et al. Varicella vaccination coverage in Bavaria (Germany) after general vaccine recommendation in 2004. Vaccine. 2010 Aug 9;28(35):5738–5745.
- Sanftenberg L, Schrörs H, Schelling J: Estimation of the vaccination coverage before and after the warning about a quadrivalent vaccine against mumps, measles, rubella and varicella. Presented at the ECCMID. 2016 [cited 2019 Nov 1]. #EP0253, Available at https://www.escmid.org/escmid_publications/escmid_elibrary/.
- Federal Statistical Office: Notifiable diseases, 2017. [cited 2019 Aug 19]. Available at: http://www.gbe-bund.de/gbe10/abrechnung.prc_abr_test_logon?p_uid=gast&p_aid=14709305&p_knoten=VR&p_sprache=E&p_suchstring=varicella
- Liese J, von Kries R, Grote V, et al.: varicella zoster virus infection hospitalisations and complications in children and adolescents: Germany, 2003–2004 – final Report. Presented at the ESPID, 2006.
- Federal Statistical Office: diagnostic data of the hospitals for Germany (1994–1999), 2000. [cited 2019 Aug 19]. Available at: http://www.gbe-bund.de/gbe10/abrechnung.prc_abr_test_logon?p_uid=gast&p_aid=14709305&p_knoten=VR&p_sprache=E&p_suchstring=varicella
- Federal Statistical Office: Deaths, Mortality figures (from 1998), 2016. [cited 2019 Aug 19]. Available at: http://www.gbe-bund.de/gbe10/abrechnung.prc_abr_test_logon?p_uid=gast&p_aid=14709305&p_knoten=VR&p_sprache=E&p_suchstring=varicella
- Federal Statistical Office: Diagnostic data of the prevention and rehabilitation facilities with more than 100 beds, 2017. [cited 2019 Aug 19]. Available at: http://www.gbe-bund.de/gbe10/abrechnung.prc_abr_test_logon?p_uid=gast&p_aid=14709305&p_knoten=VR&p_sprache=E&p_suchstring=varicella
- Federal Statistical Office: Vaccine coverage of children starting school, 2017. [cited 2019 Aug 19]. Available at: http://www.gbe-bund.de/gbe10/abrechnung.prc_abr_test_logon?p_uid=gast&p_aid=14709305&p_knoten=VR&p_sprache=E&p_suchstring=varicella
- Banz K, Wagenpfeil S, Neiss A, et al. The cost-effectiveness of routine childhood varicella vaccination in Germany. Vaccine. 2003 Mar 7;21(11–12):1256–1267.
- Beutels P, Clara R, Tormans G, et al. Costs and benefits of routine varicella vaccination in German children. J Infect Dis. 1996 Nov;174(Suppl 3):S335–41.
- Coudeville L, Brunot A, Giaquinto C, et al. Varicella vaccination in Italy: An economic evaluation of different scenarios. Pharmacoeconomics. 2004;22(13):839–855.
- Coudeville L, Brunot A, Szucs TD, et al. The economic value of childhood varicella vaccination in France and Germany. Value Health. 2005 May-Jun;8(3):209–222.
- Gabutti G, Azzari C. [Priorix Tetra: A new combined vaccine against measles, rubella, mumps and varicella]. Minerva Pediatr. 2008 Aug;60(4):429–441. Italian
- Gabutti G, Penna C, Rossi M, et al. The seroepidemiology of varicella in Italy. Epidemiol Infect. 2001 Jun;126(3):433–440.
- Guzzetta G, Poletti P, Merler S, et al. The epidemiology of herpes zoster after varicella immunization under different biological hypotheses: Perspectives from mathematical modeling. Am J Epidemiol. 2016 Apr 15;183(8):765–773.
- Hammerschmidt T, Bisanz H, Wutzler P. Universal mass vaccination against varicella in Germany using an MMRV combination vaccine with a two-dose schedule: An economic analysis. Vaccine. 2007 Oct 16;25(42):7307–7312.
- Knuf M, Neiss A, Wutzler P. [Impact of universal varicella vaccination in Germany: An epidemiological and economic analysis]. Klin Padiatr. 2006 Jul-Aug;218(4):203–212. German
- Nicolosi A, Sturkenboom M, Mannino S, et al. The incidence of varicella: Correction of a common error. Epidemiology. 2003 Jan;14(1):99–102.
- Poletti P, Melegaro A, Ajelli M, et al. Perspectives on the impact of varicella immunization on herpes zoster. A model-based evaluation from three European countries. PLoS One. 2013;8(4):e60732.
- Streng A, Liese JG. Decline of varicella vaccination in German surveillance regions after recommendation of separate first-dose vaccination for varicella and measles-mumps-rubella. Vaccine. 2014 Feb 12;32(8):897–900.
- Wagenpfeil S, Neiss A, Banz K, et al. Empirical data on the varicella situation in Germany for vaccination decisions. Clin Microbiol Infect. 2004 May;10(5):425–430.
- Weisser K, Meyer C, Petzold D, et al. [Adverse drug reactions following immunization in Germany pursuant to the German infection protection act and the german medicinal products act from January 1, 2004 to December 31, 2005]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2007 Nov;50(11):1404–1417. German
- Wutzler P, Farber I, Wagenpfeil S, et al. Seroprevalence of varicella-zoster virus in the German population. Vaccine. 2001 Oct 12;20(1–2):121–124.
- Wutzler P, Neiss A, Banz K, et al. Can varicella be eliminated by vaccination? Potential clinical and economic effects of universal childhood varicella immunisation in Germany. Med Microbiol Immunol. 2002 Oct;191(2):89–96.
- Ziebold C, von Kries R, Lang R, et al. Severe complications of varicella in previously healthy children in Germany: A 1-year survey. Pediatrics. 2001 Nov;108(5):E79.
- De Donno A, Kuhdari P, Guido M, et al. Has VZV epidemiology changed in Italy? Results of a seroprevalence study. Hum Vaccin Immunother. 2017 Feb;13(2):385–390.
- Melegaro A, Marziano V, Del Fava E, et al. The impact of demographic changes, exogenous boosting and new vaccination policies on varicella and herpes zoster in Italy: A modelling and cost-effectiveness study. BMC Med. 2018 Jul 17;16(1):117.
- Pezzotti P, Bellino S, Prestinaci F, et al. The impact of immunization programs on 10 vaccine preventable diseases in Italy: 1900–2015. Vaccine. 2018 Mar 7;36(11):1435–1443.
- Valente N, Cocchio S, Stefanati A, et al. Temporal trends in herpes zoster-related hospitalizations in Italy, 2001–2013: Differences between regions that have or have not implemented varicella vaccination. Aging Clin Exp Res. 2017 Aug;29(4):771–779.
- Alfonsi V, D’Ancona F, Giambi C, et al. Current immunization policies for pneumococcal, meningococcal C, varicella and rotavirus vaccinations in Italy. Health Policy. 2011 Dec;103(2–3):176–183.
- Betta M, Laurino M, Pugliese A, et al. Perspectives on optimal control of varicella and herpes zoster by mass routine varicella vaccination. Proc Biol Sci. 2016 Mar 16;283(1826):20160054.
- Bonanni P, Boccalini S, Bechini A, et al. Economic evaluation of varicella vaccination in Italian children and adolescents according to different intervention strategies: The burden of uncomplicated hospitalised cases. Vaccine. 2008 Oct 16;26(44):5619–5626.
- Gabutti G, Rota MC, Guido M, et al. The epidemiology of Varicella Zoster virus infection in Italy. BMC Public Health. 2008;8(1):372.
- Holl K, Sauboin C, Amodio E, et al. Coverage, efficacy or dosing interval: Which factor predominantly influences the impact of routine childhood vaccination for the prevention of varicella? A model-based study for Italy. BMC Public Health. 2016 Oct 21;16(1):1103.
- Ciofi Degli Atti ML, Rota MC, Mandolini D, et al. Assessment of varicella underreporting in Italy. Epidemiol Infect. 2002 Jun;128(3):479–484.
- Ciofi Degli Atti ML, Salmaso S, Bella A, et al. Pediatric sentinel surveillance of vaccine-preventable diseases in Italy. Pediatr Infect Dis J. 2002 Aug;21(8):763–768.
- Fornaro P, Gandini F, Marin M, et al. Epidemiology and cost analysis of varicella in Italy: Results of a sentinel study in the pediatric practice. Italian sentinel group on pediatric infectious diseases. Pediatr Infect Dis J. 1999 May;18(5):414–419.
- Giaquinto C, Sturkenboom M, Mannino S, et al. [Epidemiology and outcomes of varicella in Italy: Results of a prospective study of children (0–14 years old) followed up by pediatricians (Pedianet study)]. Ann Ig. 2002 Jul-Aug;14(4Suppl 6):21–27. Italian
- Salmaso S, Mandolini D, Scalia Tomba G, et al. [Prevention of varicella in Italy: Vaccination strategies]. Ann Ig. 2002 Jul-Aug;14(4Suppl 6):35–44. Italian
- Salmaso S, Tomba GS, Mandolini D, et al. [Assessment of the potential impact in Italy of extensive varicella vaccination programs based on a mathematical model]. Epidemiol Prev. 2003 May-Jun;27(3):154–160. Italian
- Santuccio C, Trotta F, Felicetti P, et al.: Post-marketing surveillance on vaccines in Italy 2013, AIFA. 2013 [cited 2019 Nov 1]. Available at: https://www.aifa.gov.it/documents/20142/516919/Rapporto_vaccini_2013_acc_0.pdf/b3e3bd1e-020a-1118-bb48-d6723fd578d6.
- EUVAC.NET. Varicella surveillance report 2000–2007, [cited 2019 Aug 19]. Available at: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/varicella_report_2000_2007_euvacnet.pdf
- EUVAC.NET. Varicella surveillance report 2008–2009, [cited 2019 Aug 19]. Available at: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/varicella_report_2008_2009_euvacnet.pdf
- EUVAC.NET. Varicella surveillance report 2010, [cited 2019 Aug 19]. Available at: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/varicella_report_2010_euvacnet.pdf
- Sbarbati M, Di Pietro AC: Hospitalisations for varicella and measles in children younger than 2 years in 2006–2011 in Italy: A cross sectional study utilising national hospital discharge database. Presented at the ECCMID. 2014 [cited 2019 Nov 1]. #P1862, Available at https://www.escmid.org/escmid_publications/escmid_elibrary/.
- Signorelli C: Andamento delle coperture vaccinali in Italia. 2016 [cited 2019 Nov 1]. Available at http://www.igienistionline.it/docs/2016/37sigministero.pdf.
- Baldo V, Baldovin T, Russo F, et al. Varicella: Epidemiological aspects and vaccination coverage in the Veneto Region. BMC Infect Dis. 2009 Sep 8;9:150.
- Bechini A, Chellini M, Pellegrino E, et al. Impact of vaccination programs against measles, varicella and meningococcus C in Italy and in Tuscany and public health policies in the last decades. J Prev Med Hyg. 2018 Jun;59(2):E120–E127.
- Boccalini S, Bonanni P, Bechini A. Preparing to introduce the varicella vaccine into the Italian immunisation programme: Varicella-related hospitalisations in Tuscany, 2004–2012. Euro Surveill. 2016 Jun 16;21(24).
- Cuccia M, Addario PS, Cernigliaro A, et al. [Varicella vaccine in the Sicilian region: Impact on hospitalizations and notifications]. Medico e Bambino pagine elettroniche. 2010;13(4): Italian.
- Gabutti G, Rota MC, De Donno A, et al. [Sero-epidemiology of VZV infection in Italy: Impact evaluation of extensive vaccination]. Epidemiol Prev. 2014 Nov-Dec;38(6Suppl 2):57–61. Italian
- Gialloreti LE, Divizia M, Pica F, et al. Analysis of the cost-effectiveness of varicella vaccine programmes based on an observational survey in the Latium region of Italy. Herpes:J IHMF. 2005 Oct;12(2):33–37.
- Giammanco G, Ciriminna S, Barberi I, et al. Universal varicella vaccination in the Sicilian paediatric population: Rapid uptake of the vaccination programme and morbidity trends over five years. Euro Surveill. 2009 Sep 3;14(35):19321.
- Giaquinto C, Gabutti G, Baldo V, et al. Impact of a vaccination programme in children vaccinated with ProQuad, and ProQuad-specific effectiveness against varicella in the Veneto region of Italy. BMC Infect Dis. 2018 Mar 5;18(1):103.
- Marchetto S, de Benedictis FM, de Martino M, et al. Epidemiology of hospital admissions for chickenpox in children: An Italian multicentre study in the pre-vaccine era. Acta Paediatr. 2007 Oct;96(10):1490–1493.
- Tafuri S, Gallone MS, Cappelli MG, et al. A seroprevalence survey on varicella among adults in the vaccination era in Apulia (Italy). Vaccine. 2014 Nov 12;32(48):6544–6547.
- Tafuri S, Martinelli D, Prato R, et al. Vaccine effectiveness evaluation during a varicella outbreak among children of primary schools and day-care centers in a region which adopted UMV. Hum Vaccin Immunother. 2013 Jan;9(1):184–188.
- Azzari C, Massai C, Poggiolesi C, et al. Cost of varicella-related hospitalisations in an Italian paediatric hospital: Comparison with possible vaccination expenses. Curr Med Res Opin. 2007 Dec;23(12):2945–2954.
- Baldo V, Ferro A, Napoletano G, et al. Universal varicella vaccination in the Veneto Region, Italy: Launch of a programme targeting all children aged 14 months and susceptible adolescents. Euro Surveill. 2007 Nov 1;12(11):E071101.3.
- Bechini A, Boccalini S, Baldo V, et al. Impact of universal vaccination against varicella in Italy. Hum Vaccin Immunother. 2015;11(1): 63–71.
- Bonanni P, Ferro A, Guerra R, et al. Vaccine coverage in Italy and assessment of the 2012–2014 National Immunization Prevention Plan. Epidemiol Prev. 2015;Jul-Aug;39(4 Suppl 1):146–158.
- Bonsignori F, Chiappini E, Frenos S, et al. Hospitalization rates for complicated and uncomplicated chickenpox in a poorly vaccined pediatric population. Infection. 2007 Dec;35(6):444–450.
- Cocchio S, Zanoni G, Opri R, et al. A postmarket safety comparison of 2 vaccination strategies for measles, mumps, rubella and varicella in Italy. Hum Vaccin Immunother. 2016 Mar 3;12(3):651–654.
- Frenos S, Galli L, Chiappini E, et al. An increasing incidence of chickenpox central nervous system complications in children: What’s happening in Tuscany? J Clin Virol. 2007 Apr;38(4):358–361.
- Moretti F, Chellini E, Baretti S, et al. [Estimate of underreporting of infectious diseases through a sentinel network of pediatricians in the area of local health unit of Florence]. Epidemiol Prev. 2000 Sep-Oct;24(5):224–227. Italian
- Pieri L, Porchia BR, Pieralli F, et al. Assessment of the effectiveness of the universal varicella vaccination program in Toscana (Italy), in the period 2010–2013. Epidemiol Prev. 2015 Jul-Aug;39(4 Suppl 1):119–123.
- Pozza F, Piovesan C, Russo F, et al. Impact of universal vaccination on the epidemiology of varicella in Veneto, Italy. Vaccine. 2011 Nov 28;29(51):9480–9487.
- Tafuri S, Fortunato F, Cappelli MG, et al. Effectiveness of vaccination against varicella in children under 5 years in Puglia, Italy 2006–2012. Hum Vaccin Immunother. 2015;11(1):214–219.
- Trucchi C, Gabutti G, Rota MC, et al. Burden of varicella in Italy, 2001–2010: Analysis of data from multiple sources and assessment of universal vaccination impact in three pilot regions. J Med Microbiol. 2015 Nov;64(11):1387–1394.
- Zotti CM, Maggiorotto G, Migliardi A. [Costs of varicella]. Ann Ig. 2002 Jul-Aug;14(4Suppl 6):29–33. Italian
- Gatti MG, Grandori L, Poggioli P, et al. Monitoring of adverse events in vaccines given at age 0–17, reported in the Province of Modena from 2002 to 2005. Available at: https://www.epicentro.iss.it/vaccini/pdf/Reavac_2002-2005_MO.pdf cited 2019 Sept 03
- Servi Sociali Sanita: Official bulletin of the Lombardy region: Suppl. Straordinario al n. 4–24 gennaio 2006, 2006. [cited 2019 Sept 03]. Available at: https://www.epicentro.iss.it/vaccini/pdf/Normative/Lombardia_%20Dicembre%202012/DGR%20n%C2%B0%208-1587%20del%2022.12.2005.pdf
- Russo F, Piovesan C, Pozza F, et al.: Special objectives of infectious disease 2010, Region of Veneto, 2011. [cited 2019 Sept 03]. Available at: https://www.epicentro.iss.it/territorio/veneto/pdf/RappMI2010.pdf
- Various: Vaccinations in Piedmont, 2018. [cited 2019 Sept 03]. Available at: https://www.epicentro.iss.it/territorio/piemonte/documentazione
- Gatti MG, Pascucci MG, Carati D, et al. Surveillance of adverse events to vaccines given at age 0–17 years reported in Emilia-Romagna period 2006–2007, 2008. [cited 2019 Sept 03]. Available at: https://www.epicentro.iss.it/territorio/emilia-romagna/pdf/eventi_avversi_E-R_2006-07.pdf
- Russo F, Da Re F, Zanella F, et al.: Special objectives of infectious disease 2012–2013, Region of Veneto, 2013. [cited 2019 Sept 03]. Available at: https://www.epicentro.iss.it/territorio/veneto/pdf/REPORT%20MI6_def.pdf
- Gatti MG, Floramo M, Soncini F, et al. Surveillance of adverse events to vaccines reported in Emilia-Romagna in pediatric age (2006–2011) and adult (2010–2011), 2013. [cited 2019 Sept 03]. Available at: https://www.epicentro.iss.it/vaccini/pdf/Vaccini_reazioni_avverse_pediatrico_e_adulti_novembre2013.pdf
- Various: Infectious diseases: 2016 archives, 2016. [Cited 2019 Sept 03]. Available at: https://www.epicentro.iss.it/infettive/archivio2016
- Assessorato Dell’igiene E Sanita’ E Dell’assistenza Sociale Servizio Prevenzione: Regional plan for prevention 2010 – 2012: Regione Sardegna. [Cited 2019 Aug 19]. Available at: https://www.epicentro.iss.it/vaccini/pdf/Normative/Sardegna_Dicembre%202010/PRP%202010_12%20DGR%20n%C2%B0%2047-24%20del%2030.12.10/PRP%202010-12%20quadro-strategico.pdf
- Dipartimento di Prevenzione ASLdA-B: Bollettino Epidemiologico Anno 2011, Region Piedmont, 2011. [Cited 2019 Aug 19]. Available at: https://www.epicentro.iss.it/territorio/piemonte/pdf/Bollettino%20epidemiologico%202011.pdf
- Dipartimento di Prevenzione ASLdP: Bollettino Epidemiologico Anno 2005, Region Piedmont, 2005. [Cited 2019 Aug 19]. Available at: https://www.epicentro.iss.it/territorio/piemonte/pdf/Asl18_Bollettino2005.PDF
- Borello D: Bollettino Epidemiologico Anno 2014, Region Piedmont, ASL di Alba-Bra, 2015. [Cited 2019 Aug 19]. Available at: https://www.epicentro.iss.it/territorio/piemonte/pdf/Bollettino%20Epidemiologico%202014.pdf
- Marinaro L: Bollettino Epidemiologico Anno 2003, Region Piedmont, ASL di Alba-Bra, 2003. [Cited 2019 Aug 19]. Available at: https://www.epicentro.iss.it/territorio/piemonte/pdf/Bra2003%20.pdf
- Agnelli I: Bollettino Epidemiologico Anno 2006, Region Piedmont, ASL di Alba-Bra, 2006. [Cited 2019 Aug 19]. Available at: https://www.epicentro.iss.it/territorio/piemonte/pdf/epid_asl18_2006.pdf
- Epicentro.iss: Incidence per 100.000 children 0–14 years of age for vaccine-preventable diseases, by geographic region and data source, Italy, 2000. [Cited 2019 Aug 19]. Available at: http://www.epicentro.iss.it/ben/2001/maggio/tab2_eng.pdf
- Dipartimento di Prevenzione ASLdA-B: Bollettino Epidemiologico Anno 2012, Region Piedmont, ASL di Alba-Bra, 2012. [Cited 2019 Sept 03]. Available at: https://www.epicentro.iss.it/territorio/piemonte/pdf/Bollettino%20Epidemiologico%202012.pdf
- Dipartimento di Prevenzione ASLdA-B: Bollettino Epidemiologico Anno 2013, Region Piedmont, ASL di Alba-Bra, 2013. [Cited 2019 Sept 03]. Available at: https://www.epicentro.iss.it/territorio/piemonte/pdf/Bollettino%20Epidemiologico%20ASLCN2%20Anno%202013.pdf
- Marchione P, Menniti-Ippolito F, Felicetti P, et al. Rapporto vaccini 2017. 2017 [cited 2019 Nov 1]. Available at: http://www.agenziafarmaco.gov.it//sites/default/files/Rapp_Vaccini_2017.pdf.
- Tafuri S, Guerra R, Cappelli MG, et al. Determinants of varicella breakthrough: Results of a 2012 case-control study. Hum Vaccin Immunother. 2014;10(3):667–670.
- Ciofi Degli Atti ML, Rota MC, Mandolini D, et al. Assessment of varicella underreporting in Italy. Epidemiol Infect. 2002;128(3):479–484.
- Gazzetta ufficiale della regione Siciliana. Palermo - Venerdì 16 Agosto 2002 - n. 38. Vaccinazione anti-varicella nella Regione siciliana. [Cited 2019 Feb 13]. Available at: http://www.gurs.regione.sicilia.it/Gazzette/g02-38.HTM#24.
- Robert-Koch-Institut. Epidemiol Bull 2005; 13: S111–112. [Cited 2019 Feb 4]. Available at: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2005/Ausgabenlinks/13_05.pdf?__blob=publicationFile
- Regione Toscana [Tuscany Region]. Aggiornamento direttive regionali in materia di vaccinazioni [Updating the regional guidelines on vaccinations]. Revoca delibere n. 1249 del 24/ 11/2003,n.379 del 7/3/2005 e n.1060 del 10/ 10/2000.Modifica delibera n. 1386 del 17/ 12/2001.Deliberazione Giunta Regionale del 27 dicembre 2007, n. 1020, Bollettino Ufficiale Della Regione Toscana, N. 2 del 9.1.2008. Italian. [Cited 2019 Feb 4]. Available at: http://www.fimptoscana.org/system/files/delibera%20calendario%20regionale%2027-12-2007_0.pdf
- Ministero della Salute. Vaccinazioni dell’età pediatrica e dell’adolescente - Coperture vaccinali. [Cited 2019 Feb 13]. Available at: http://www.salute.gov.it/portale/documentazione/p6_2_8_3_1.jsp?lingua=italiano&id=20
- Ministero della Salute. Piano nazionale prevenzione vaccinale 2017–2019. [Cited 2019 Apr 10]. Available at: https://www.gazzettaufficiale.it/eli/gu/2017/02/18/41/sg/pdf
- Ministero della Salute. Circolare recante prime indicazioni operative per l’attuazione del decreto-legge 7 giugno 2017, n.73, recante “Disposizioni urgenti in materia di prevenzione vaccinale”. 2017. [Cited 2019 Feb 13]. Available at: http://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2017&codLeg=60282&parte=1%20&serie=null
- Ministero della Salute. Piano nazionale prevenzione vaccinale 2005–2007. [Cited 2019 Apr 10]. Available at: http://www.salute.gov.it/imgs/C_17_pubblicazioni_543_allegato.pdf
- Empfehlungen der Ständigen Impfkommission am Robert-Koch-Institut (STIKO). Epidemiol bull 2009; 32: 319–338. [Cited 2019 Feb 4]. Available at: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2009/Ausgaben/32_09.pdf%3F__blob%3DpublicationFile
- Wiese-Posselt M, Hellenbrand W. Changes to the varicella and pertussis immunisation schedule in Germany 2009: Background, rationale and implementation. Euro Surveill. 2010 Apr 22;15:16.
- Jacobsen SJ, Ackerson BK, Sy LS, et al. Observational safety study of febrile convulsion following first dose MMRV vaccination in a managed care setting. Vaccine. 2009 Jul 23;27(34):4656–4661.
- Klein NP, Fireman B, Yih WK, et al. Measles-mumps-rubella-varicella combination vaccine and the risk of febrile seizures. Pediatrics. 2010 Jul;126(1):e1–8.
- Wutzler P, Casabona G, Cnops J, et al. Herpes zoster in the context of varicella vaccination - An equation with several variables. Vaccine. 2018 Nov 12;36(46):7072–7082.
- Brisson M, Edmunds WJ, Gay NJ. Varicella vaccination: Impact of vaccine efficacy on the epidemiology of VZV. J Med Virol. 2003;70(Suppl S1):S31–7.
- Leung J, Harpaz R, Molinari NA, et al. Herpes zoster incidence among insured persons in the United States, 1993–2006: Evaluation of impact of varicella vaccination. Clin Infect Dis. 2011 Feb 1;52(3):332–340.
- Harpaz R, van Hoek AJ. Point-counterpoint: The Hope-simpson hypothesis and its implications regarding an effect of routine varicella vaccination on herpes zoster incidence. J Infect Dis. 2018 Sep 22;218(suppl_2):S57–S62.
- Harpaz R. Do varicella vaccination programs change the epidemiology of herpes zoster? A comprehensive review, with focus on the United States. Expert Rev Vaccines. 2019 Aug;18(8):793–811.
- Forbes H, Douglas I, Finn A, et al. Risk of herpes zoster after exposure to varicella to explore the exogenous boosting hypothesis: Self controlled case series study using UK electronic healthcare data. BMJ. 2020 Jan 22:368:l6987. doi10.1136/bmj.l6987.
- Wolfson LJ, Daniels VJ, Altland A, et al. The impact of varicella vaccination on the incidence of varicella and herpes zoster in the United States: Updated evidence from observational databases, 1991–2016. Clin Infect Dis. 2020 Mar 3;70(6):995–1002.
- Weinmann S, Naleway AL, Koppolu P, et al. Incidence of Herpes Zoster Among Children: 2003–2014. Pediatrics. 2019 Jul;144(1):e20182917.
- Harpaz R, Leung JW. The epidemiology of herpes zoster in the United States during the era of varicella and herpes zoster vaccines: Changing patterns among children. Clin Infect Dis. 2019 Jul 2;69(2):345–347.
- Sauboin C, Holl K, Bonanni P, et al. The impact of childhood varicella vaccination on the incidence of herpes zoster in the general population: Modelling the effect of exogenous and endogenous varicella-zoster virus immunity boosting. BMC Infect Dis. 2019 Feb 6;19(1):126.
- Ogunjimi B, Smits E, Hens N, et al. Exploring the impact of exposure to primary varicella in children on varicella-zoster virus immunity of parents. Viral Immunol. 2011 Apr;24(2):151–157.
- Ogunjimi B, Van den Bergh J, Meysman P, et al. Multidisciplinary study of the secondary immune response in grandparents re-exposed to chickenpox. Sci Rep. 2017 Apr 24;7(1):1077.
- Gaillat J, Gajdos V, Launay O, et al. Does monastic life predispose to the risk of Saint Anthony’s fire (herpes zoster)? Clin Infect Dis. 2011 Sep;53(5):405–410.
- Robert-Koch-Institut. Epidemiol Bull 2018; 50: 541–570. [Cited 2019 Feb 4]. Available at: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2018/Ausgaben/50_18.pdf?__blob=publicationFile
- Priorix Tetra. Summary of product characteristics. [Cited 2019 Aug 26]. Available at: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000200_038200_RCP.pdf&retry=0&sys=m0b1l3
- ProQuad. Summary of product characteristics. [Cited 2019 Aug 26]. Available at: https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_000737_036893_RCP.pdf&retry=0&sys=m0b1l3
- Empfehlungen der Ständigen Impfkommission am Robert-Koch-Institut (STIKO). Epidemiol Bull. 2005. 30:257–272. [Cited 2019 Feb 4]. Available at: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2005/Ausgabenlinks/30_05.pdf?__blob=publicationFile.
- Finn A. The hope-simpson hypothesis, varicella vaccination and herpes zoster control. J Infect Dis. 2019;219(10):1681.
