ABSTRACT
Introduction
Adjuvants are critical components of vaccines to improve the quality and durability of immune responses. Molecular adjuvants are a specific subclass of adjuvants where ligands of known immune-modulatory receptors are directly fused to an antigen. Co-stimulation of the B cell receptor (BCR) and immune-modulatory receptors through this strategy can augment downstream signaling to improve antibody titers and/or potency, and survival in challenge models.
Areas covered
C3d has been the most extensively studied molecular adjuvant and shown to improve immune responses to a number of antigens. Similarly, tumor necrosis superfamily ligands, such as BAFF and APRIL, as well as CD40, CD180, and immune complex ligands can also improve humoral immunity as molecular adjuvants.
Expert opinion
However, no single strategy has emerged that improves immune outcomes in all contexts. Thus, systematic exploration of molecular adjuvants that target B cell receptors will be required to realize their full potential as next-generation vaccine technologies.
1. Introduction
Vaccines are invaluable tools to prevent infection and halt the spread of diseases [Citation1]. The availability and fast production of effective and safe vaccines to emerging pathogens have been highlighted in recent months due to the ongoing COVID-19 pandemic. Unfortunately, effective vaccines against many prominent pathogens remain elusive despite researchers’ best efforts. While live attenuated vaccines remain one of the most effective forms of vaccination, concerns of potential virulence in susceptible individuals and reversal of attenuation often preclude their development. As a result, recombinant subunit vaccines have emerged as an alternative due to their greater safety and tolerability, since these rely solely on isolated components of pathogens and are incapable of causing infection in the host [Citation2]. A key advance in the development of subunit vaccines has been the identification of antigens from the sequencing of pathogens, made possible by vast improvements in sequencing technologies. Leveraging this technology, recombinant proteins mimicking antigens of interest in their native form can be produced, aiming to induce long-lived immunological memory primarily through the elicitation of antibodies toward the protective epitopes of the antigen [Citation3,Citation4], as was demonstrated for the recently licensed meningococcus B vaccine [Citation5]. Thus, a new generation of subunit vaccines takes advantage of the identification of key antigenic epitopes from a pathogen.
Importantly, almost all current vaccines rely on the presence of antibodies circulating in serum or on mucosa that block infection or bacteremia/viremia, and these often represent the best correlate of vaccine-induced protection [Citation1,Citation6,Citation7]. Correspondingly, advances in sequencing technologies have also enabled the characterization of antibody responses to many antigens, parsing out the characteristics of desirable immune responses that should be preferentially elicited by immunization. As such, eliciting the highest titer of protective antibodies that focus the response on the most protective epitopes of antigens often constitutes a primary goal of vaccination [Citation8,Citation9]. For instance, one area of high interest is the mapping of potent epitopes of the Plasmodium falciparum circumsporozoite protein (CSP) for the design of next-generation vaccines against malaria. Recent reports have identified the central ‘NANP’ repeat region of CSP and sequence motifs upstream of this repeat as recognized by potent inhibitory antibodies [Citation10–13], and suggested its flanking C- and N-terminal domains may not contain as potent neutralizing epitopes [Citation14,Citation15]. Indeed, understanding non-neutralizing antibody responses can also provide important insights into antigen design: by avoiding such epitopes, the elicitation of antibodies that have poor protective function can be minimized to favor protective antibody responses.
Beyond antigen sequence, the structural requirements underlying antigen recognition by potently protective antibodies has been extensively studied. For instance, the surface-expressed trimeric fusion machinery of multiple viruses have been shown to require stabilization in a pre-fusion state to elicit antibodies that provide potent protection (e.g. Respiratory syncytial virus (RSV), human immunodeficiency virus (HIV), and severe acute respiratory coronavirus 2 (SARS-CoV-2) [Citation15–25]). A third consideration for antigen design against pathogens with vast genetic diversity, such as HIV and influenza, is the elicitation of broadly neutralizing antibodies (bnAbs), which are able to neutralize virus despite sequence heterogeneity between viral strains [Citation26]. This can often be achieved by focusing the response on epitopes conserved across strains and minimizing antibodies against variable regions within the antigen [Citation27–30]. One approach to elicit bnAbs is ‘germline-targeting,’ in which epitopes included in the immunogen are selected to engage specific germline precursor B cells, thus leveraging the naturally occurring process of affinity maturation to shepherd the immune system toward the generation of bnAbs. This strategy is being employed by the HIV vaccine candidate eOD-GT8, where the outer domain of gp120 was engineered to engage the inferred germline precursor of the VRC01 antibody [Citation31]. Here, the modified gp120 led to improvements in the breadth and affinity of the VRC01-like antibody response when used as a prime, and boosted with more native trimeric spikes [Citation31–33]. Thus, as discussed above, the purposeful selection of antigenic epitopes, as well as their mode of presentation, are considerations of high importance to promote protective antibody responses and minimize off-target, non-neutralizing antibodies against challenging pathogens.
Despite improvements in antigen design, recombinant subunit vaccines are notorious for their poor immunogenicity. Strategies to promote and support immune responses elicited by a subunit vaccine are thus a critical consideration in vaccine design. Traditional adjuvants have proven to be excellent tools to enhance the immunogenicity of vaccine candidates by reducing the number of doses required to achieve protection [Citation34], prolonging memory elicited by the vaccine [Citation35,Citation36], and expanding the breadth and potency of elicited antibodies [Citation37]. Currently, several adjuvants are approved for use in humans. These adjuvants vary in composition (e.g. aluminum salts, TLR agonists, emulsions, etc.), and most of them have been shown to engage the innate immune system to ultimately enhance the magnitude of adaptive responses [Citation38–40] through a variety of mechanisms [Citation37,Citation39,Citation41–43]. The choice of adjuvant has been shown to significantly impact immune outcomes resulting from vaccination, which can range from complete protection to poor immunogenicity and no protection for the same antigen [Citation44]. This is exemplified by the RTS,S malaria vaccine, which is based on Plasmodium falciparum CSP: initial studies of this vaccine adjuvanted with an aluminum salt (alum) was successful in eliciting anti-CSP antibodies in the sera, but conferred no protection [Citation45]. When RTS,S was administered together with an oil-in-water emulsion adjuvant (AS02A) or liposomal formulation (AS01B), 30–50% protection was observed [Citation46]. Thus, the choice of a suitable adjuvant for a given vaccine can be pivotal, and has been an area of intense research (reviewed in [Citation47–49]).
Beyond the clear benefits provided by antigen optimization and adjuvants targeting innate immunity to augment protective responses, could the specific targeting of receptors on lymphocytes provide additional adjuvant effects? In this review, we describe recent efforts in the design of molecular adjuvants particularly targeting B cell receptors for the enhancement of immunogenicity in vaccination.
1.1. B Cell Signaling Upon Antigen Encounter
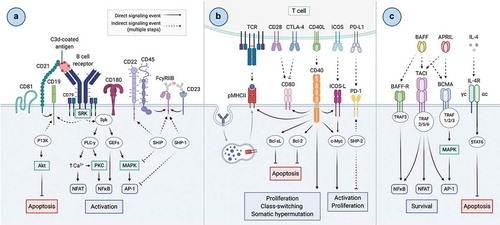
Antibody-mediated immunity orchestrated by B cells plays a vital role in the protection against pathogens. The recognition of antigen by a highly specific B cell receptor (BCR) displayed on the surface of a B cell initiates signaling processes and is termed ‘Signal 1’ (depicted in ). The interaction between antigen and BCR induces receptor micro-clustering on the cell surface, triggering signaling cascades via the phosphorylation of key intermediary kinases (such as PKC, Akt, and MAPK) and influx of calcium, resulting in the transcriptional activation of NFAT and NFκB [Citation50–52]. These signaling processes leading to downstream B cell activation are tightly regulated by antigen specificity and affinity [Citation53,Citation54], as well as by co-receptors clustering with the BCR; these can act in concert with the BCR to either stimulate (e.g. CD19, CD21) or inhibit (e.g. CD22, CD45, FcγRIIb) the activation signal [Citation55–59]. Following Signal 1, B cells process the antigen and present antigenic fragments on Major Histocompatibility Complex (MHC) class II molecules to cognate T cells primed by the same antigen earlier in the response, enabling them to receive additional signals via costimulatory molecules upregulated on both cell types after priming (‘Signal 2’) (e.g. CD80/86, CD40, ICOS-L) () (reviewed in [Citation60]). These signals induce B cells to rapidly proliferate and form structures called germinal centers (GCs) within secondary lymphoid organs (SLOs), where somatic hypermutation (SHM) and class-switch recombination (CSR) take place to yield a higher-affinity, multifunctional antibody response [Citation61]. A third set of signals (‘Signal 3’) provides essential survival and developmental cues to B cells; these are usually maintained by soluble mediators within the cell’s microenvironment, such as BAFF, APRIL and interleukin-4 (IL-4) () [Citation62–65]. Following activation, B cells undergo many cell fate decisions. They can differentiate into plasma cells (PCs) specialized in the production and secretion of antibodies targeting the antigen [Citation66]; most of these are short-lived within the SLO and rapidly undergo apoptosis, but a subset of PCs migrate to the bone marrow, where their longevity enables them to contribute over 80% of the antibody present in serum [Citation67–69]. Alternatively, B cells can differentiate into memory cells, poised to quickly react upon re-exposure to the antigen. In this case, memory B cells can reenter the GC reaction to undergo further SHM, or differentiate into PCs at this later time point [Citation70]. B cell fate is regulated via receptors present on its surface at multiple checkpoints (signals 1, 2 and 3). Thus, targeting B cell co-receptors at each of these signals could be a strategic avenue to modulate immune responses to vaccination. Several receptors on the surface of B cells have been shown to be capable of altering immune responses following antigen interaction with the BCR. Of these, the modulation of B cell signaling via CD21, a co-receptor known to lower the threshold for B cell activation and enhance BCR signaling, has been most extensively studied. The inhibitory co-receptor FcγRIIb has also been studied, conversely known to dampen BCR signaling and cellular activation. Other receptors with more general roles in overall B cell survival and differentiation (e.g. signals 2 and 3), such as the tumor necrosis factor (TNF) superfamily members have also been actively researched. This review explores these receptors for their potential to be targeted by molecular adjuvants with the goal to modulate immune responses and alter cellular outcomes that improve recombinant vaccines.
Figure 1. Overview of B cell signaling. A) Signal 1 – antigen interacts with the B cell receptor (BCR) on the surface of B cells to initiate a signaling cascade leading to the downstream activation of transcription factors NFAT, NFκB and AP-1 for the activation of B cells. The signal initiated by the antigen-BCR interaction is strictly regulated by stimulatory (i.e. CD21, CD19) and inhibitory (i.e. CD22, FcγRIIb) receptors micro-clustering with the BCR upon antigen recognition. B) Signal 2 – antigen is internalized, processed, and presented on the B cell on MHC class II molecules to cognate T cells primed earlier in the response, enabling additional signals from T cells to promote B cell proliferation, CSR and SHM. C) Signal 3 – interactions of TNFSF receptors with soluble mediators in the microenvironment (BAFF, APRIL, IL-4) promote B cell survival and suppress apoptosis. Created with Biorender.com
2. C3d
The complement system is essential for immune homeostasis and plays a critical role to bridge the innate and adaptive arms of the immune system. One critically important member of the complement system is C3d, which is derived from complement component 3 (C3) and results in C3d-tagged micro-organisms or antigens (reviewed in [Citation71,Citation72]) with the capacity to bind to complement receptor 2 (CR2 or CD21) on B cells and follicular dendritic cells for opsonization, as well as to assist in antigen presentation and the maintenance of immunological memory [Citation73]. CD21 is typically found on the surface of B cells in association with CD19 and CD81 [Citation74,Citation75] and plays an integral role in immune regulation (reviewed in [Citation76,Citation77]). While CD21 itself does not have a signaling motif, binding of CD21 to C3d results in downstream signaling through the immunoreceptor tyrosine-based activation motifs (ITAMs) on CD19, resulting in improved B cell survival and proliferation [Citation78–80]. When C3d-tagged pathogens simultaneously interact with CD21 and the BCR, pathway crosstalk decreases the signal threshold of antigen required to trigger B cell activation [Citation81]. This pathway crosstalk is also capable of reducing inhibitory signals and preventing apoptosis, which are important factors for the induction of immunological memory.
There is a long history of using C3d as a molecular adjuvant to boost B cell signaling in response to various antigens in a vaccination setting. First demonstrated by Dempsey et al. in 1996, a 10,000-fold increase in secondary immune responses was obtained for 2–3x murine C3d (mC3d) tagged hen egg-white lysozyme (HEL) in comparison to HEL alone [Citation81]. Since this initial finding, similar results have been achieved for viral, bacterial, parasitic and self-antigens, as summarized in (and reviewed in [Citation72,Citation82]). Fusion of tandem repeats of C3d (three is often sufficient) to antigens and administration through various routes results in improved antibody titers, often also improving neutralization and survival in challenge experiments in mice. Interestingly, C3d fusion seems most frequently to polarize the immune system toward a Th2 response in these vaccinated mice, characterized by IgG1 dominance and the secretion of high levels of IL-4 and IL-5 [Citation83–86], although Th1-dominated responses have also been observed [Citation87].
Table 1. Summary of immunization experiments containing C3d
Table 2. Summary of immunization experiments containing TNFSF ligands. Ligands from rabbit sequences are denoted with an ‘r’ and murine ligands are denoted with an ‘m.’
It is well established that multiple copies of C3d fused to the antigen of interest is required for optimal adjuvant effect. However, in some cases, C3d fusion has been shown to reduce the efficacy of the immunogen [Citation91–93]. One possible explanation for this effect is that C3d fusion to the antigen may block important epitopes on the antigen, making them inaccessible for the development of antibody responses. Indeed, one such example was suggested when the malaria immunogen Plasmodium berghei CSP (PbCSP) was fused to 3x tandem repeats of mC3d at its C-terminus [Citation92]. DNA immunizations in BALB/c mice resulted in a reduction of anti-CSP antibody titers and loss of protective efficacy in a challenge model of infection in comparison to PbCSP alone [Citation92]. This issue was resolved in a follow-up study through the fusion of 3x tandem repeats of the minimal CD21 binding domain of mC3d, termed P28, in place of full-length mC3d. This smaller fragment of mC3d rescued mC3d-mediated enhancement and resulted in improved antibody titers, enhanced immunogenicity, and improved protection in comparison to both CSP alone and CSP-mC3d fusions [Citation83].
This is one example of many where using P28 as a molecular adjuvant has been shown to be similarly robust or even superior to mC3d for CD21-mediated enhancement of immune responses to a given antigen. Recent studies have employed P28 in DNA vaccines targeted toward HIV [Citation94], West Nile [Citation95] and Rabies [Citation96] viruses, as well as in an attenuated Salmonella vaccine candidate that expresses or secretes the Trichinella spiralis antigen Ag30 [Citation84], amongst others [Citation85,Citation97]. These studies report a P28-mediated enhancement of antibody titers and Th2 bias, but was often dependent on the antigen and immunization route employed. For example, in the case of West Nile virus immunogens, high antibody titers were observed upon gene gun administration for all tested antigens (prM/E, EctoE, DIII) regardless of the presence of P28, whereas upon intramuscular (I.M.) administration with the same antigens, higher antibody titers were observed when EctoE or DIII was fused to P28. Interestingly, EctoE-P28 administered via gene gun was the only antigen in this study (other than prM/E – the positive control) that was able to provide complete protection against West Nile challenge [Citation95]. These results further highlight the importance of both immunogen design and the exploration of different administration routes for optimal vaccine efficacy. In a different study, the use of attenuated Salmonella expressing T. spiralis antigen Ag30 was explored either alone or fused to P28 via a C-terminal linker before being attached to the surface of Salmonella (either by a cleavable or non-cleavable linker). The highest antibody titers and neutralization potency were obtained when Ag30-P28 was attached to attenuated Salmonella via a cleavable linker. In fact, the same antigen fused to P28 through a non-cleavable linker behaved poorly in immunizations in comparison to cleavable Ag30-P28, as well as Ag30 alone [Citation84]. This study also suggested that the position of the P28 fusion is critical; an optimal adjuvant effect was only obtained when P28 was located at the C-terminus of the antigen. This observation is consistent with other studies that reported optimal enhancements where C3d or P28 were fused to antigens at the C-terminus [Citation94,Citation97,Citation98].
The wealth of data available for C3d/P28-mediated enhancement of humoral immune responses supports CD21 targeting as an avenue for the improvement of vaccine candidates, although the magnitude of the adjuvant effect appears to depend strongly on the antigen, administration route, animal model, and vaccine type. In addition, our incomplete understanding of the mechanism of action of C3d-linked antigens was highlighted following the results of Haas et al. in 2004, which showed C3d-mediated enhancement of immune responses in CD21-/-/CD35-/- mice [Citation99]. This data raises important questions about C3d specificity and the mechanisms of action of immune modulation via C3d. As a result, alternative mechanisms have been proposed for the observed immune enhancement, including :1) improved antigen half-life through C3d fusion, 2) additional availability of T cell epitopes that may be present on C3d, and 3) alternate C3d receptors that may facilitate immune modulation other than CD21 and CD35 [Citation99]. Further studies looking into the biological impact of C3d fusion at the cellular level and in immune signaling are required to fully understand the effect of C3d fusion in these systems and represent an important avenue to assess whether molecular adjuvants can have an impact on other aspects of a successful vaccine candidate, such as improved memory and long-lived plasma cell (LLPC) populations.
3. Tumor necrosis family (TNF) receptors
The tumor necrosis factor (TNF) superfamily (TNFSF) of ligands and their receptors play critical roles for cellular survival, proliferation, and affinity maturation. The TNFSF is composed of 19 ligands and 29 receptors, which have been reviewed extensively for their roles in cell death, survival, proliferation, and differentiation [Citation100,Citation101]. Of the TNF ligands, B cell Activating Factor (BAFF, also known as BLyS) and A Proliferation Inducing Ligand (APRIL) represent attractive targets for their ability to modulate immune outcomes through B cell targeting, although additional TNFSF members have been explored for their co-stimulatory effects on dendritic cells and T cells as well (reviewed in [Citation102]). BAFF is a critical B cell survival ligand, without which normal B cell maturation cannot occur [Citation103]. Conversely, while APRIL is known to play a key role in the survival of LLPCs in the bone marrow, APRIL-deficient mice do not show any developmental or immune cell defects, suggesting that APRIL is not essential for normal immune development and function [Citation104]. BAFF and APRIL are both type II membrane proteins expressed primarily by T cells, macrophages, dendritic cells, and monocytes. Like many other ligands in the TNFSF, proteolytic cleavage of BAFF and APRIL at their conserved furin cleavage site results in the release of active, soluble ligands. BAFF can be found in its membrane active form, but cleavage to form soluble, extracellular BAFF is essential for B cell homeostasis. Indeed, mutation of the BAFF furin cleavage site results in immune dysfunction and impairment of antibody responses [Citation105]. APRIL, however, is cleaved intracellularly in the Golgi prior to release and is typically only found in its soluble form extracellularly [Citation106]. BAFF and APRIL are most commonly found in circulation as homotrimers [Citation107], however, unlike other TNFSF members, BAFF has also been found as a 60-mer in physiological conditions [Citation108]. Interestingly, APRIL, but not BAFF, is capable of binding heparin sulfate proteoglycans (HSPGs) [Citation109], a function which may assist in the clustering and concentration of APRIL on a local cell surface, similar to the avidity effect that could be obtained by 60-mer BAFF binding to multiple receptors on a single cell [Citation110].
BAFF and APRIL are both capable of interacting specifically and with high affinity with either B-Cell Maturation Antigen (BCMA), or Transmembrane Activator and Cyclophilin ligand Interactor (TACI). BAFF also has additional specificity to BAFF receptor (BAFF-R) and the recently described Nogo-66 receptor (NgR) [Citation111]. BCMA, TACI, and BAFF-R are type I transmembrane proteins that are expressed primarily by B cell lineages at varying levels depending on the stage of B-cell development (summarized in [Citation110]). BAFF-R is expressed on immature B cells following the expression of a functional BCR. The interaction between BAFF and BAFF-R is critical for the development and homeostasis of mature B cells and acts as a primary driving force in B cell survival and maturation. Similar to the phenotypes observed in BAFF-deficient mice, BAFF-R-deficient or -mutated mice show a block in B cell maturation ultimately resulting in autoimmunity and other immune defects [Citation112,Citation113]. Following the initial interactions of BAFF with BAFF-R in early B cell maturation, BAFF and APRIL play key roles in interacting with TACI and BCMA on mature B cell populations. TACI is highly expressed on marginal zone B cells and B1-B cells where it plays key roles in T cell-independent responses, inhibition of B cell expansion [Citation114,Citation115], IgG to IgA class switching, and the differentiation and survival of PCs [Citation116,Citation117]. BCMA expression is restricted to plasmablasts and PCs, and its role is primarily limited to the survival of LLPCs in the bone marrow. BCMA binds with high affinity to APRIL and lower affinity to BAFF, although this low affinity can be modulated through avidity effects, which have been observed via BCMA dimerization [Citation118].
Another member of the TNFSF is CD40L, which is expressed primarily on CD4+ T cells [Citation119]. CD40L, like BAFF and APRIL, can be proteolytically cleaved to create soluble forms of CD40L that retains full biological activity, though CD40L is more commonly considered a membrane-bound ligand [Citation120]. CD40L interacts directly with CD40 on B cells and dendritic cells and plays critical roles in the provision of a T-cell help signal required in GC formation and for antibody isotype switching and affinity maturation [Citation121]. Due to the roles of BAFF-R, TACI, BCMA, and CD40 in B cell differentiation and survival, they present themselves as targets for immune modulation in the context of vaccine design.
BAFF and APRIL homotrimers have both been utilized as molecular adjuvants for the improvement of immune responses toward challenging pathogens, as summarized in . In immunization studies for rabies virus, RABV viral vector immunogens expressing membrane-anchored BAFF have been shown to be capable of inducing faster and higher titers of neutralizing antibodies toward RABV antigens without adversely affecting the longevity of the immune response [Citation122]. Conversely, in a separate study, anchoring of APRIL to RABV viral vectors had no effect on antibody responses, and APRIL co-engagement did not improve the generation of LLPCs [Citation123]. These studies are some of the first to assess whether the addition of molecular adjuvants can affect the longevity of the induced memory response through the generation of LLPCs. This represents an avenue for further research to improve the resulting memory responses through this immunogen design platform and holds the potential to have significant impact for pathogens in which long-term memory remains a significant barrier in vaccine development, such as rabies and malaria. In influenza, WT, BAFF- and APRIL-containing H5N1 VLPs were utilized to immunize BALB/c mice in the presence of either alum or MPL adjuvants [Citation124]. Both BAFF- and APRIL-VLPs showed significantly higher antibody titers in comparison to WT VLPs but only BAFF-VLPs were capable of improving the potency and breadth of the resulting neutralizing antibodies. In all cases, adjuvants were still required to achieve high titers and potency despite the presence of BAFF and APRIL as molecular adjuvants [Citation124]. However, the longevity of the resulting memory responses remains to be assessed.
For HIV immunogen design, trimeric Env was C-terminally fused to either APRIL, BAFF, or CD40 in an attempt to target Env directly to B cells and improve Env-specific antibody responses [Citation125]. For all three immunogens, there was an enhanced expression of Activation-induced cytidine-deaminase (AID) in comparison to controls in vitro upon incubation with antigen-specific B cells, suggesting the potential for an improved level of SHM through TNFSF co-engagement. Env-CD40L was the most effective in boosting AID expression in this system. In all three cases, the immunogens were capable of inducing IgM, IgG, and IgA secretion from human B cells in vitro. However, in an immunization experiment in rabbits, only Env-APRIL was capable of inducing high titers of Env-specific antibody responses with heterologous tier 1 neutralization in vivo [Citation125]. In a more recent study, BALB/c mice were co-immunized with a mixture of DNA plasmids containing Env gp140 together with multi-trimers of BAFF or APRIL (TNFSF trimers multimerized on surfactant protein-D (SP-D) scaffolds) and IL-12, followed by a protein boost with Env. It is important to note that for this study, Env, BAFF/APRIL multi-trimers and IL-4 were all present on different plasmids and were not fused to each other. Despite not being directly fused, this immunization strategy was found to induce neutralizing antibodies against tier 1 and 2 viruses. The APRIL multi-trimer-containing vaccine candidate was particularly effective at generating tier 2 neutralization. Furthermore, both BAFF and APRIL candidates were capable of inducing enhanced GC reactivity, as well as increased anti-gp120 antibody-secreting cells and improved anti-gp120 avidity [Citation126].
These results together show that the use of BAFF, APRIL, and CD40L as molecular adjuvants to specifically target BAFF-R, BCMA, TACI, and CD40 on B cells is a promising approach to improve vaccine outcomes against various pathogens. Interestingly, similar to the responses observed for C3d fusion, careful antigen design and systematic assessment of the different molecular adjuvants seems to be essential for enhancement of immune responses by these TNFSF ligands. For immunization schemes with both rabies and influenza antigens, BAFF-fused antigens showed the best overall immune responses, with increased titers and enhanced neutralization potency by resulting antibody responses, but were unable to improve the generation of effective immunological memory through increased LLPCs. Conversely, for HIV antigens, APRIL-fused Env resulted in the most favorable and reproducible antibody responses across two different studies, and direct fusion of APRIL to Env was not required for the adjuvant effect to be observed. However, the longevity of this response was not assessed. This data highlights the importance of a systematic evaluation of the use of TNF ligands as molecular adjuvants and stresses the value of testing multiple adjuvants in parallel, as a single strategy that works for all antigens hasn't yet been identified.
4. CD180
CD180 is a TLR receptor expressed both on B cells and DCs and plays a critical role in regulating the responses of these cell types to TLR ligands. On B cells, it has been shown that CD180 is important for B cell activation, and that directly targeting this receptor with an anti-CD180 antibody can trigger CD180-induced calcium flux, B cell activation, and proliferation [Citation127,Citation128]. Furthermore, internalization following ligation has been described for CD180, making it an attractive target for immunogen design, as CD180 internalization can assist with antigen processing by DCs and B cells to activate T cells in signal 2 [Citation129]. CD180 is closely related to TLR4 and is an orphan member of the TLR family [Citation130], which are known as effective targets for adjuvants [Citation38,Citation42]. Due to these factors, CD180 represents an intriguing target as a molecular adjuvant to improve immune responses.
In a recent study, authors used an anti-CD180 (αCD180) monoclonal antibody directly fused to the antigen hapten 4-hydroxy-3-nitro-phenacetyl (NP) to act as molecular adjuvant to boost immune responses [Citation129]. In comparison to NP fused to an isotype control and αCD180 alone (without NP antigen), NP-αCD180 induced a 15-fold increase in polyclonal serum IgG titers and strong antigen-specific IgG and IgM immune response in WT, CD40 KO, and T cell-deficient mice. Furthermore, in comparison to NP in alum, NP-αCD180 induced a rapid anti-NP IgG response which peaked 11 days sooner than NP in alum in WT mice. NP-αCD180 was also shown to be effective in inducing GC formation, affinity maturation, and immunological memory. This platform was then expanded to assess the effect of CD180 targeting with the protein antigen ovalbumin (OVA). Strong Ag-specific IgG responses were observed in the OVA-αCD180 group in comparison to OVA in alum controls and OVA-isotype controls. These results were dependent on the direct fusion of αCD180 to the antigen of interest, as co-administration of unfused antigen with αCD180 did not result in a robust immune response [Citation129].
In a follow-up study, the authors showed that CD180 targeting of OVA and HEL antigens induced rapid BCR internalization and that all B cell subsets were subsequently able to process and present the internalized antigens for CD4+ T cell help in WT mice [Citation131]. These results were expanded to explore APC generation in BAFF-R−/- mice, and the authors were able to demonstrate that αCD180, but not αCD40 targeting induced efficient antigen processing and presentation, even in the immature B cell subsets present within these mice. Within 48 h of immunization, αCD180 targeting induced the maturation of T follicular helper (Tfh) cells, suggesting that this targeting strategy is effective for the development of robust T-dependent humoral responses [Citation131].
These studies are the first to specifically target CD180 as a molecular adjuvant and employ a novel technique of using an αCD180-specific monoclonal antibody – as opposed to the natural ligand of the receptor – for co-engagement. Follow-up studies that expand the breadth of antigens tested to include pathogens of high-priority will be required to fully understand the possible utility of CD180 as a molecular adjuvant in vaccination.
5. Immune complexes
There is over a century of history describing the use of immune complexes (ICs) in vaccination [Citation132–134]. The earliest experiments in humans involved complexing live vaccines with anti-sera to improve both the safety and efficacy of the earliest vaccine candidates [Citation132,Citation135]. The generation of ICs involves the complexation of immunogens of interest with specific monoclonal or polyclonal antibodies. These antibodies are then capable of interacting with Fcγ receptors on immune cells (macrophages, monocytes, DCs, B cells, etc.) promoting efficient antigen uptake and processing, providing improved T cell help for the generation of immune memory and antibody responses [Citation136–138].
One implication of IC vaccination is the specific interaction with Fcγ receptors on B cells that may modulate the outcome of B cell responses. A recent study showed that immune responses toward the seasonal trivalent inactivated influenza vaccine (TIV) could be modulated through the complexation of the TIV with purified polyclonal Abs from individuals that had a high sialic acid prevalence in their antibodies [Citation139]. Immunization with these complexes (but not complexes made with asialylated IgGs) showed improved neutralization capacity, despite lower titers. The proposed model to explain these results was that ICs with sialylated IgG bound specifically to CD23 on B cells, resulting in the upregulation of the inhibitory cell-surface Fc receptor FcγRIIb. This upregulation of FcγRIIb would then increase the signal threshold required for B cell activation, thus limiting the cells that are activated to only those that possess high cognate BCR affinity and specificity to the antigen. In turn, this would improve the quality, but not necessarily the magnitude, of the B cell response [Citation139].
This study suggests an intriguing mechanism for modulation of the quality of antibody responses at Signal 1 on B cells. Nonetheless, the specific interaction between sialylated IgGs and CD23 on B cells remains to be fully demonstrated biophysically. A recent study investigated this specificity but was unable to detect binding of IgG to CD23 or DC-SIGN through cellular surface plasmon resonance imaging (cSPRi) or by fluorescence activated cell sorting (FACS) regardless of the glycosylation state of the antibody [Citation140]. These studies represent an interesting avenue of research that requires further investigation to determine the ability of an increased signal threshold on B cells to improve the quality of antibody responses. If successful, this approach could have considerable implications across fields where poor antibody quality and neutralization capacity continue to plague efforts toward an effective vaccine.
6. Expert opinion
Co-engagement of the BCR with immune-modulatory co-receptors is an approach with several successful examples of improving immunization outcomes, including improved antibody titers, potency, breadth and/or improved survival in challenge experiments [Citation81,Citation122,Citation124,Citation139,Citation141]. The majority of these experiments have been performed in mouse models, with a select few in rabbits. Expanding animal models to include those closest to humans (i.e. non-human primates) would provide critical insight into the translatability of these molecular adjuvant approaches across model organisms, toward the goal of being able to use these as vaccination strategies in humans, which has not yet been attempted.
Through the direct fusion of antigens to natural ligands that target either stimulatory (CD21, BCMA, BAFF-R, CD180) or inhibitory (FcγRIIb and TACI) receptors on B cells, the outcome of the immunological responses of vaccination can be altered [Citation84,Citation86]. Unfortunately, there does not seem to be a single molecular adjuvant, vaccine type, or immunization route that is capable of consistently boosting immune responses across all antigens. However, this is not an unfamiliar problem in the vaccine field, as vaccine formulation, adjuvant composition and immunization route often require optimization for the best possible immune outcomes, even in classical vaccine designs [Citation142]. This, however, suggests that despite the promise of molecular adjuvants to modulate immune responses, it is not a‘plug-and-play’ system that can be universally deployed. Thus, optimization is required to identify the most efficacious signaling pathway to target for an antigen of interest. Consequently, it is of critical importance that studies employing these molecular adjuvants continue to do so in a systematic way to properly assess the best strategy for immunogen design, as is common for chimeric immunogens [Citation143]. Assessing best methods for conjugating molecular adjuvants to antigens should also be explored to ensure that the chimeric immunogens are designed in a way that optimizes both protective epitopes on the antigen and functional binding sites for the molecular adjuvant. Indeed, it has been shown in the context of C3d that the terminus to which the adjuvant is fused as well as its size can be critical for the quality of the resulting immune responses [Citation83,Citation84,Citation92]. In addition, expanded studies to look at the different types of molecular adjuvants (i.e. C3d, TNFSF, CD180, and ICs) within the same study would be valuable for the assessment of the best molecular adjuvants for a given antigen.
Some datasets covered in this review contain conflicting results, which could in part be due to the promiscuous nature of ligand binding to B cell co-receptors. Indeed, it is well established that many of the natural ligands used in these studies have targets on B cells, as well as on other cell populations. As such, it becomes difficult to deconvolute with certainty which pathway is associated with the observed modulation of immunity. For C3d, antibody enhancement was evident even in CD21-/- mice, suggesting additional mechanisms of action to the one initially envisioned [Citation78]; immune complexes can bind Fcγ receptors on heterogenous populations of immune cell types [Citation138]; and the family of TNF receptors and their ligands are notoriously promiscuous in their binding capabilities [Citation100,Citation101]. One approach to remove this complexity is to use an alternate, more specific molecule for targeting immune receptors of interest. Antibodies, or antigen-binding fragments (Fabs) of antibodies, serve as an excellent alternative to natural ligands and have been proven to be effective as a molecular adjuvant in CD180-targeting strategies [Citation129,Citation131]. Expanding this platform to include antibodies that are specific to receptors targeted by these molecular adjuvants (CD21, BCMA, BAFF-R, TACI, CD40) could potentially be key to understanding the mechanisms of immune modulation through B cell co-receptor targeting. Indeed, the improved specificity provided by antibodies would ensure that only the receptor of interest is being engaged by a chimeric immunogen.
Receptor clustering has been repeatedly shown to be critical for immunogens employing the use of molecular adjuvants. Targeting of CD21 through C3d requires at least 2–3 repeats of C3d or P28 for enhancement [Citation81,Citation83,Citation94,Citation97,Citation144–146], whereas targeting through the TNFSF requires that ligands are presented in trimeric arrangement for receptor engagement [Citation124–126] or even multimerization into multi-trimers using an SP-D scaffold [Citation126,Citation141]. The use of protein-based nanoparticles for adjuvant multimerization represents an attractive avenue for improved B cell modulation [Citation147]. Naturally-occurring protein-based nanoparticles, such as ferritin [Citation30,Citation148], lumazine synthase [Citation31,Citation33], E2p [Citation149] as well as non-natural two-component protein nanoparticles, such as I53-50 [Citation150], could be utilized to co-display antigens of interest and molecular adjuvants. Using these nanoparticles for this function would ensure avidity effect for the antigen, thus improving BCR clustering, while also allowing for multiple copies of the molecular adjuvant to be presented simultaneously. Investigation of the symmetry features of these protein-based nanoparticles can also guide immunogen design to be able to include higher-order ligands, such a TNFSF homotrimers at three-fold axes within the nanoparticles [Citation30,Citation148–150].
Expanding these experiments will provide valuable insight into the best strategies for immune modulation via molecular adjuvants, and also help uncover previously unknown features of B cell biology. Indeed, these experiments offer the opportunity to closely examine the downstream signaling pathways associated with the targeting receptors () and expand our understanding of how their modulation impacts the expansion of the immune repertoire. Importantly, the design of next-generation vaccines against challenging pathogens will require novel strategies to induce long-lived protective antibodies and potentially the quick re-activation of the memory compartment to outpace pathogens. Whether this can be achieved by the molecular adjuvants presented in this review, among other strategies, remains to be determined. An improved understanding of basic B cell biology at the molecular level will provide promise for the emergence of hypotheses in immune modulation and translate into next-generation adjuvant technologies.
Article Highlights
Molecular adjuvants can act by simultaneously targeting the B cell receptor and immune-modulatory receptors to augment downstream signaling cascades and improve immune responses in immunizations.
Tandem repeats of C3d, the natural ligand of complement receptor 2 (CR2 or CD21) on B cells, and P28, a minimal binding domain of C3d, have been extensively studied as molecular adjuvants.
Members of the tumor necrosis family of ligands (BAFF-R, TACI, BCMA) represent a key class of immune-modulatory receptors that have been targeted by molecular adjuvants through the fusion of their natural ligands with antigens.
CD180 has recently emerged as a target of molecular adjuvants. Targeting CD180 with an αCD180 monoclonal antibody showed enhancements in antigen internalization and processing, as well as antibody titers for select model antigens.
Although the enhancement of immune responses by immune complexes is well established, the exact mechanism of this approach for immune modulation of B cells remains to be fully elucidated.
No single molecular adjuvant has been shown to be superior for all antigens, immunization routes and animal models. Thus, systematic studies continue to be required for each immunogen towards the goal of being able to use these types of molecular adjuvants in a clinical setting.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
The text was written and edited by TS, AK and JPJ. The figure was designed and made by AK.
Acknowledgments
We would like to thank Bebhinn Treanor and Michael Ratcliffe for their input and discussions.
Additional information
Funding
References
- Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17(7):1055–1065.
- Liljeqvist S, Ståhl S. Production of recombinant subunit vaccines: protein immunogens, live delivery systems and nucleic acid vaccines. J Biotechnol. 1999;73(1):1–33.
- Palumbo E, Fiaschi L, Brunelli B, et al. Antigen identification starting from the genome: a “reverse vaccinology” approach applied to MenB. Methods Mol Biol. 2012;799:361–403.
- Serruto D, Bottomley MJ, Ram S, et al. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine. 2012;30:B87–B97.
- O’Ryan M, Stoddard J, Toneatto D, et al. A multi-component meningococcal serogroup B vaccine (4CMenB): the clinical development program. Drugs. 2014;74(1):15–30.
- Plotkin SA. Vaccines: correlates of vaccine‐induced immunity. Clin Infect Dis. 2008;47(3):401–409.
- Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357(19):1903–1915.
- McLeod B, Miura K, Scally SW, et al. Potent antibody lineage against malaria transmission elicited by human vaccination with Pfs25. Nat Commun. 2019;10(1):4328.
- Scally SW, McLeod B, Bosch A, et al. Molecular definition of multiple sites of antibody inhibition of malaria transmission-blocking vaccine antigen Pfs25. Nat Commun. 2017;8(1):1568.
- Oyen D, Torres JL, Wille-Reece U, et al. Structural basis for antibody recognition of the NANP repeats in plasmodium falciparum circumsporozoite protein. Proc Natl Acad Sci. 2017;114(48):E10438–E10445.
- Murugan R, Scally SW, Costa G, et al. Evolution of protective human antibodies against Plasmodium falciparum circumsporozoite protein repeat motifs. Nat Med. 2020;26(7):1135–1145.
- Kisalu NK, Idris AH, Weidle C, et al. A human monoclonal antibody prevents malaria infection by targeting a new site of vulnerability on the parasite. Nat Med. 2018;24(4):408–416.
- Tan J, Sack BK, Oyen D, et al. A public antibody lineage that potently inhibits malaria infection through dual binding to the circumsporozoite protein. Nat Med. 2018;24(4):401–407.
- Scally SW, Murugan R, Bosch A, et al. Rare PfCSP C-terminal antibodies induced by live sporozoite vaccination are ineffective against malaria infection. J Exp Med. 2018;215(1):63–75.
- Thai E, Costa G, Weyrich A, et al. A high-affinity antibody against the CSP N-terminal domain lacks plasmodium falciparum inhibitory activity. J Exp Med. 2020;217(11):e20200061.
- McLellan JS, Chen M, Joyce MG, et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science. 2013;342(6158):592–598.
- McLellan JS, Chen M, Leung S, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340(6136):1113–1117.
- Cai Y, Zhang J, Xiao T, et al. Distinct conformational states of SARS-CoV-2 spike protein. Science. 2020; eabd4251. 10.1126/science.abd4251.
- de Taeye SW, Ozorowski G, Torrents de la Peña A, et al. Immunogenicity of stabilized HIV-1 envelope trimers with reduced exposure of non-neutralizing epitopes. Cell. 2015;163(7):1702–1715.
- Do Kwon Y, Pancera M, Acharya P, et al. Crystal structure, conformational fixation and entry-related interactions of mature ligand-free HIV-1 Env. Nat Struct Mol Biol. 2015;22(7):522–531.
- Rutten L, Lai Y-T, Blokland S, et al. A universal approach to optimize the folding and stability of prefusion-closed HIV-1 Envelope trimers. Cell Rep. 2018;23(2):584–595.
- Torrents de la Peña A, Sanders RW. Stabilizing HIV-1 envelope glycoprotein trimers to induce neutralizing antibodies. Retrovirology. 2018;15(1):63.
- Sanders RW, Derking R, Cupo A, et al. A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog. 2013;9(9):e1003618.
- Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263.
- Walls AC, Park Y-J, Tortorici MA, et al. Structure, function, and antigenicity of the SARS-CoV-2 Spike glycoprotein. Cell. 2020;181(2):e6.
- Shang J, Ye G, Shi K, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224.
- Joyce MG, Georgiev IS, Yang Y, et al. Soluble prefusion closed DS-SOSIP664-Env trimers of diverse HIV-1 strains. Cell Rep. 2017;21(10):2992–3002.
- Steel J, Lowen AC, Wang TT, et al. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. MBio. 2010;1(1):e00018–10.
- Impagliazzo A, Milder F, Kuipers H, et al. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science. 2015;349(6254):1301–1306.
- Yassine HM, Boyington JC, McTamney PM, et al. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat Med. 2015;21(9):1065–1070.
- Jardine JG, Kulp DW, Havenar-Daughton C, et al. HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen. Science. 2016;351(6280):1458–1463.
- Havenar-Daughton C, Sarkar A, Kulp DW, et al. The human naive B cell repertoire contains distinct subclasses for a germline-targeting HIV-1 vaccine immunogen. Sci Transl Med. 2018;10(448):eaat0381.
- Jardine J, Julien J-P, Menis S, et al. Rational HIV immunogen design to target specific germline B cell receptors. Science. 2013;340(6133):711–716.
- Dietrich J, Andreasen LV, Andersen P, et al. Inducing dose sparing with inactivated polio virus formulated in adjuvant CAF01. PLoS One. 2014;9(6):e100879. Rodrigues MM, editor.
- Lindenstrøm T, Agger EM, Korsholm KS, et al. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J Immunol. 2009;182(12):8047–8055.
- Kasturi SP, Rasheed MAU, Havenar-Daughton C, et al. 3M-052, a synthetic TLR-7/8 agonist, induces durable HIV-1 envelope–specific plasma cells and humoral immunity in nonhuman primates. Sci Immunol. 2020;5(48):eabb1025.
- Khurana S, Chearwae W, Castellino F, et al. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Sci Transl Med. 2010;2:15ra5-15ra5.
- McKee AS, Munks MW, Marrack P. How do adjuvants work? Important considerations for new generation adjuvants. Immunity. 2007;27(5):687–690.
- Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33(4):492–503.
- Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327(5963):291–295.
- Mosca F, Tritto E, Muzzi A, et al. Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci. 2008;105(30):10501–10506.
- Mbow ML, De Gregorio E, Valiante NM, et al. New adjuvants for human vaccines. Curr Opin Immunol. 2010;22(3):411–416.
- Gavin AL, Hoebe K, Duong B, et al. Adjuvant-enhanced antibody responses in the absence of Toll-like receptor signaling. Science. 2006;314(5807):1936–1938.
- Knudsen NPH, Olsen A, Buonsanti C, et al. Different human vaccine adjuvants promote distinct antigen-independent immunological signatures tailored to different pathogens. Sci Rep. 2016;6(1):1–13.
- Stoute JA, Slaoui M, Heppner DG, et al. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against plasmodium falciparum malaria. N Engl J Med. 1997;336(2):86–91.
- Kester KE, Cummings JF, Ofori‐Anyinam O, et al. Randomized, double‐blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria‐naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis. 2009;200(3):337–346.
- JDS A, Lunardelli VAS, Coirada FC, et al. Adjuvants: classification, modus operandi, and licensing. J Immunol Res. 2016;2016:1–16.
- Petrovsky N. Comparative safety of vaccine adjuvants: a summary of current evidence and future needs. Drug Saf. 2015;38(11):1059–1074.
- Del Giudice G, Rappuoli R, Didierlaurent AM. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin Immunol. 2018;39:14–21.
- Dolmetsch RE, Lewis RS, Goodnow CC, et al. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386(6627):855–858.
- Hashimoto A, Okada H, Jiang A, et al. Involvement of guanosine triphosphatases and phospholipase C-γ2 in extracellular signal–regulated kinase, c-Jun NH2-terminal kinase, and p38 mitogen-activated protein kinase activation by the B cell antigen receptor. J Exp Med. 1998;188(7):1287–1295.
- Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15(1):707–747.
- Kouskoff V, Famiglietti S, Lacaud G, et al. Antigens varying in affinity for the B cell receptor induce differential B lymphocyte responses. J Exp Med. 1998;188(8):1453–1464.
- Liu W, Meckel T, Tolar P, et al. Antigen affinity discrimination is an intrinsic function of the B cell receptor. J Exp Med. 2010;207(5):1095–1111.
- Carter R, Fearon D. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science. 1992;256(5053):105–107.
- Gasparrini F, Feest C, Bruckbauer A, et al. Nanoscale organization and dynamics of the siglec CD22 cooperate with the cytoskeleton in restraining BCR signalling. Embo J. 2016;35(3):258–280.
- Nitschke L, Carsetti R, Ocker B, et al. CD22 is a negative regulator of B-cell receptor signalling. Curr Biol. 1997;7(2):133–143.
- Giovannone N, Smith LK, Treanor B, et al. Galectin-glycan interactions as regulators of B cell immunity. Front Immunol. 2018;9:2839.
- Wang TT, Maamary J, Tan GS, et al. Anti-HA glycoforms drive B cell affinity selection and determine influenza vaccine efficacy. Cell. 2015;162(1):160–169.
- Petersone L, Edner NM, Ovcinnikovs V, et al. T cell/B cell collaboration and autoimmunity: an intimate relationship. Front Immunol. 2018;9:1941.
- Kwak K, Akkaya M, Pierce SK. B cell signaling in context. Nat Immunol. 2019;20(8):963–969.
- Benson MJ, Elgueta R, Noelle RJ. B cell survival: an unexpected mechanism of lymphocyte vitality. Immunol Cell Biol. 2008;86(6):485–486.
- Mackay F, Schneider P, Rennert P, et al. BAFF AND APRIL: A Tutorial on B Cell Survival. Annu Rev Immunol. 2003;21(1):231–264.
- Smulski CR, Eibel H. BAFF and BAFF-receptor in B cell selection and survival. Front Immunol. 2018;9:2285.
- Wurster AL, Rodgers VL, White MF, et al. Interleukin-4-mediated protection of primary B cells from apoptosis through Stat6-dependent up-regulation of Bcl-xL. J Biol Chem. 2002;277(30):27169–27175.
- Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;12(6):509–517.
- Brynjolfsson SF, Persson Berg L, Olsen Ekerhult T, et al. Long-lived plasma cells in mice and men. Front Immunol. 2018;9:2673.
- Slifka MK, Antia R, Whitmire JK, et al. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8(3):363–372.
- McHeyzer-Williams MG, Ahmed R. B cell memory and the long-lived plasma cell. Curr Opin Immunol. 1999;11(2):172–179.
- Kurosaki T, Kometani K. Ise W. memory B cells. Nat Rev Immunol. 2015;15(3):149–159.
- Ricklin D, Reis ES, Mastellos DC, et al. Complement component C3 - the “swiss army knife” of innate immunity and host defense. Immunol Rev. 2016;274:33–58.
- Bergmann-Leitner ES, Leitner WW, Tsokos GC. Complement 3d: from molecular adjuvant to target of immune escape mechanisms. Clin Immunol. 2006;121(2):177–185.
- Walport MJ. Complement: second of two parts. N Engl J Med. 2001;344:1140–1144.
- Matsumoto AK, Kopicky-Burd J, Carter RH, et al. Intersection of the complement and immune systems: A signal transduction complex of the B lymphocyte-containing complement receptor type 2 and CD19. J Exp Med. 1991. 10.1084/jem.173.1.55
- Bradbury LE, Kansas GS, Levy S, et al. The CD19/CD21 signal transducing complex of human B lymphocytes includes the target of antiproliferative antibody-1 and Leu-13 molecules. J Immunol. 1992;149:2841 LP– 2850.
- Cooper NR, Moore MD, Nemerow GR. Immunobiology of CR2, the B lymphocyte receptor for Epstein-Barr virus and the C3d complement fragment. Annu Rev Immunol. 1988;6(1):85–113.
- Ahearn JM, Fearon DT. Structure and function of the complement receptors, CR1 (CD35) and CR2 (CD21). Adv Immunol. 1989;46:183–219.
- Hasegawa M, Fujimoto M, Poe JC, et al. CD19 can regulate B lymphocyte signal transduction independent of complement activation. J Immunol. 2001;167(6):3190–3200.
- Fearon DT, Carter RH. The CD19/CR2/TAPA-1 complex of B lymphocytes: linking natural to acquired immunity. Annu Rev Immunol. 1995;13(1):127–149.
- Cambier JC, Pleiman CM, Clark MR. Signal transduction by the B cell antigen receptor and its coreceptors. Annu Rev Immunol. 1994;12(1):457–486.
- Dempsey PW, Allison MED, Akkaraju S, et al., C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996; 271(5247): 348–350.
- Toapanta FR, Ross TM. Complement-mediated activation of the adaptive immune responses: role of C3d in linking the innate and adaptive Immunity. Immunol Res. 2006;36(1–3):197–210.
- Bergmann-Leitner ES, Duncan EH, Leitner WW, et al. C3d-defined complement receptor-binding peptide p28 conjugated to circumsporozoite protein provides protection against plasmodium berghei. Vaccine. 2007;25(45):7732–7736.
- Pompa-Mera EN, Arroyo-Matus P, Ocaña-Mondragón A, et al. Protective immunity against enteral stages of trichinella spiralis elicited in mice by live attenuated salmonella vaccine that secretes a 30-mer parasite epitope fused to the molecular adjuvant C3d-P28. Res Vet Sci. 2014;97(3):533–545.
- Movsesyan N, Mkrtichyan M, Petrushina I, et al. DNA epitope vaccine containing complement component C3d enhances anti-amyloid-β antibody production and polarizes the immune response towards a Th2 phenotype. J Neuroimmunol. 2008;205(1–2):57–63.
- Wang XL, Zhao XR, Yu M, et al. Gene conjugation of molecular adjuvant C3d3 to hCGβ increased the anti-hCGβ Th2 and humoral immune response in DNA immunization. J Gene Med. 2006;8(4):498–505.
- Toapanta FR, Ross TM. Mouse strain-dependent differences in enhancement of immune responses by C3d. Vaccine. 2004;22:1773–1781.
- Mitchell J. Induction of heterosubtypic immunity to influenza A virus using a DNA vaccine expressing hemagglutinin-C3d fusion proteins. Vaccine. 2003;21:902–914.
- Green TD, Newton BR, Rota PA, et al. C3d enhancement of neutralizing antibodies to measles hemagglutinin. Vaccine. 2001;20(1–2):242–248.
- Green TD, Montefiori DC, Ross TM. Enhancement of antibodies to the human immunodeficiency virus type 1 envelope by using the molecular adjuvant C3d. J Virol. 2003;77:2046–2055.
- Suradhat S, Braun RP, Lewis PJ, et al. Fusion of C3d molecule with bovine rotavirus VP7 or bovine herpesvirus type 1 glycoprotein D inhibits immune responses following DNA immunization. Vet Immunol Immunopathol. 2001;83(1–2):79–92.
- Bergmann-Leitner ES. C3d binding to the circumsporozoite protein carboxy-terminus deviates immunity against malaria. Int Immunol. 2005;17(3):245–255.
- Gor D, Ding X, Li Q, et al. Genetic fusion of three tandem copies of murine C3d sequences to diphtheria toxin fragment B elicits a decreased fragment B-specific antibody response. Immunol Lett. 2006;102(1):38–49.
- Bower JF, Ross TM. A minimum CR2 binding domain of C3d enhances immunity following vaccination. curr. top. complement. Boston, MA: Springer US; 2006. p. 249–264.
- Dunn MD, Rossi SL, Carter DM, et al. Enhancement of anti-DIII antibodies by the C3d derivative P28 results in lower viral titers and augments protection in mice. Virol J. 2010;7:95.
- Galvez-Romero G, Salas-Rojas M, Pompa-Mera EN, et al. Addition of C3d-P28 adjuvant to a rabies DNA vaccine encoding the G5 linear epitope enhances the humoral immune response and confers protection. Vaccine. 2018;36(2):292–298.
- Zhang D, Xia Q, Wu J, et al. Construction and immunogenicity of DNA vaccines encoding fusion protein of murine complement C3d-p28 and GP5 gene of porcine reproductive and respiratory syndrome virus. Vaccine. 2011;29(4):629–635.
- Weiss R, Gabler M, Jacobs T, et al. Differential effects of C3d on the immunogenicity of gene gun vaccines encoding Plasmodium falciparum and Plasmodium berghei MSP142. Vaccine. 2010;28(28):4515–4522.
- Haas KM, Toapanta FR, Oliver JA, et al., Cutting edge: C3d functions as a molecular adjuvant in the absence of CD21/35 expression. J Immunol. 2004; 172(10): 5833–5837.
- ÉS V, Faustman DL. Structural principles of tumor necrosis factor superfamily signaling. Sci Signal. 2018;11(511):eaao4910.
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756.
- Gupta S, Termini JM, Kanagavelu S, et al. Design of vaccine adjuvants incorporating TNF superfamily ligands and TNF superfamily molecular mimics. Immunol Res. 2013;57:303–310.
- Schneider P, MacKay F, Steiner V, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189(11):1747–1756.
- Varfolomeev E, Kischkel F, Martin F, et al. APRIL-deficient mice have normal immune system development. Mol Cell Biol. 2004;24(3):997–1006.
- Bossen C, Tardivel A, Willen L, et al. Mutation of the BAFF furin cleavage site impairs B-cell homeostasis and antibody responses. Eur J Immunol. 2011;41(3):787–797.
- López‐Fraga M, Fernández R, Albar JP, et al. Biologically active APRIL is secreted following intracellular processing in the Golgi apparatus by furin convertase. EMBO Rep. 2001;2(10):945–951.
- Bossen C, Cachero TG, Tardivel A, et al. TACI, unlike BAFF-R, is solely activated by oligomeric BAFF and APRIL to support survival of activated B cells and plasmablasts. Blood. 2008;111(3):1004–1012.
- Cachero TG, Schwartz IM, Qian F, et al. Formation of virus-like clusters is an intrinsic property of the tumor necrosis factor family member BAFF (B cell activating factor). Biochemistry. 2006;45(7):2006–2013.
- Ingold K, Zumsteg A, Tardivel A, et al. Identification of proteoglycans as the APRIL-specific binding partners. J Exp Med. 2005;201(9):1375–1383.
- Vincent FB, Saulep-Easton D, Figgett WA, et al. The BAFF/APRIL system: emerging functions beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev. 2013;24(3):203–215.
- Zhang L, Zheng S, Wu H, et al. Identification of BLyS (B lymphocyte stimulator), a non-myelin-associated protein, as a functional ligand for Nogo-66 receptor. J Neurosci. 2009;29(19):6348–6352.
- Yan M, Brady JR, Chan B, et al. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr Biol. 2001;11(19):1547–1552.
- Sasaki Y, Casola S, Kutok JL, et al. TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology. J Immunol. 2004;173(4):2245–2252.
- Sakurai D, Kanno Y, Hase H, et al. TACI attenuates antibody production costimulated by BAFF-R and CD40. Eur J Immunol. 2007;37(1):110–118.
- Seshasayee D, Valdez P, Yan M, et al. Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BLyS receptor. Immunity. 2003;18(2):279–288.
- Tsuji S, Cortesão C, Bram RJ, et al. TACI deficiency impairs sustained Blimp-1 expression in B cells decreasing long-lived plasma cells in the bone marrow. Blood. 2011;118:5832–5839.
- Tsuji S, Stein L, Kamada N, et al. TACI deficiency enhances antibody avidity and clearance of an intestinal pathogen. J Clin Invest. 2014;124(11):4857–4866.
- Day ES, Cachero TG, Qian F, et al. Selectivity of BAFF/BLyS and APRIL for binding to the TNF family receptors BAFFR/BR3 and BCMA. Biochemistry. 2005;44(6):1919–1931.
- Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies. Cell. 2001;104(4):487–501.
- Pietravalle F, Lecoanet-Henchoz S, Aubry J-P, et al. Cleavage of membrane-bound CD40 ligand is not required for inducing B cell proliferation and differentiation. Eur J Immunol. 1996;26:725–728.
- Ma DY, Clark EA. The role of CD40 and CD154/CD40L in dendritic cells. Semin Immunol. 2009;21(5):265–272.
- Plummer JR, McGettigan JP. Incorporating B cell activating factor (BAFF) into the membrane of rabies virus (RABV) particles improves the speed and magnitude of vaccine-induced antibody responses. PLoS Negl Trop Dis. 2019;13:e0007800.
- Haley SL, Tzvetkov EP, Lytle AG, et al. APRIL:TACI axis is dispensable for the immune response to rabies vaccination. Antiviral Res. 2017;144:130–137.
- Hong J-Y, Chen T-H, Chen Y-J, et al. Highly immunogenic influenza virus-like particles containing B-cell-activating factor (BAFF) for multi-subtype vaccine development. Antiviral Res. 2019;164:12–22.
- Melchers M, Bontjer I, Tong T, et al. Targeting HIV-1 envelope glycoprotein trimers to B cells using APRIL improves antibody responses. Retrovirology. 2012;9:P300.
- Gupta S, Clark ES, Termini JM, et al. DNA vaccine molecular adjuvants SP-D-BAFF and SP-D-APRIL enhance anti-gp120 immune response and increase HIV-1 neutralizing antibody titers. J Virol. 2015;89:4158–4169.
- Valentine MA, Clark EA, Shu GL, et al. Antibody to a novel 95-kDa surface glycoprotein on human B cells induces calcium mobilization and B cell activation. J Immunol. 1988;140(12):4071–8.
- Miyake K, Yamashita Y, Hitoshi Y, et al. Murine B cell proliferation and protection from apoptosis with an antibody against a 105-kD molecule: unresponsiveness of X-linked immunodeficient B cells. J Exp Med. 1994;180(4):1217–1224.
- Chaplin JW, Chappell CP, Clark EA. Targeting antigens to CD180 rapidly induces antigen-specific IgG, affinity maturation, and immunological memory. J Exp Med. 2013;210:2135–2146.
- Miyake K, Yamashita Y, Ogata M, et al. RP105, a novel B cell surface molecule implicated in B cell activation, is a member of the leucine-rich repeat protein family. J Immunol. 1995;154:3333–3340.
- Roe K, Shu GL, Draves KE, et al., Targeting antigens to CD180 but not CD40 programs immature and mature B cell subsets to become efficient APCs. J Immunol. 2019; 203(7): 1715–1729.
- Copeman SM. Experiences with the Schick test and active immunization against Diphtheria. Proc R Soc Med. 1922;15:41–43.
- Olitzki, L. The antigenic properties of bacteria combined with antibodies. J Immunol. 1935; 29(6):453–465.
- Wen Y, Mu L, Shi Y. Immunoregulatory functions of immune complexes in vaccine and therapy. EMBO Mol Med. 2016;8(10):1120–1133.
- Terres G, Wolins W. Enhanced immunological sensitization of mice by the simultaneous injection of antigen and specific antiserum. J Immunol. 1961;86:361–8.
- Regnault A, Lankar D, Lacabanne V, et al. Fcγ receptor–mediated induction of dendritic cell maturation and major histocompatibility complex class I–restricted antigen presentation after immune complex internalization. J Exp Med. 1999;189(2):371–380.
- van Montfoort N, ’T Hoen P, Mangsbo S, et al. Fcγ receptor IIb strongly regulates Fcγ receptor-facilitated T cell activation by dendritic cells. J Immunol. 2012;189:92–101.
- Schuurhuis DH, Ioan-Facsinay A, Nagelkerken B, et al. Antigen-antibody immune complexes empower dendritic cells to efficiently prime specific CD8 CTL responses in vivo. J Immunol. 2002;168(5):2240–2246.
- Maamary J, Wang TT, Tan GS, et al. Increasing the breadth and potency of response to the seasonal influenza virus vaccine by immune complex immunization. Proc Natl Acad Sci. 2017;114:10172–10177.
- Temming AR, Dekkers G, van de Bovenkamp FS, et al. Human DC-SIGN and CD23 do not interact with human IgG. Sci Rep. 2019;9(1):9995.
- Kanagavelu S, Termini JM, Gupta S, et al. HIV-1 adenoviral vector vaccines expressing multi-trimeric BAFF and 4-1BBL enhance T cell mediated anti-viral immunity. PLoS One. 2014;9:e90100.
- Darrah PA, Zeppa JJ, Maiello P, et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature. 2020;577:95–102.
- Roques E, Girard A, Gagnon CA, et al. Antibody responses induced in mice immunized with recombinant adenovectors expressing chimeric proteins of various porcine pathogens. Vaccine. 2013;31(24):2698–2704.
- Ross TM, Xu Y, Bright RA, et al. C3d enhancement of antibodies to hemagglutinin accelerates protection against influenza virus challenge. Nat Immunol. 2000;1(2):127–131.
- Ross TM, Xu Y, Green TD, et al. Enhanced avidity maturation of antibody to human immunodeficiency virus Envelope: DNA vaccination with gp120–C3d fusion proteins. AIDS Res Hum Retroviruses. 2001;17(9):829–835.
- Musa HH, Zhang WJ, Lv J, et al. The molecular adjuvant mC3d enhances the immunogenicity of FimA from type I fimbriae of Salmonella enterica serovar Enteritidis. J Microbiol Immunol Infect. 2014;47(1):57–62.
- Al-Halifa S, Gauthier L, Arpin D, et al. Nanoparticle-based vaccines against respiratory viruses. Front Immunol. 2019;10:22.
- Sliepen K, Ozorowski G, Burger JA, et al. Presenting native-like HIV-1 envelope trimers on ferritin nanoparticles improves their immunogenicity. Retrovirology. 2015;12:82.
- He L, de Val N, Morris CD, et al. Presenting native-like trimeric HIV-1 antigens with self-assembling nanoparticles. Nat Commun. 2016;7(1):12041.
- Bale JB, Gonen S, Liu Y, et al. Accurate design of megadalton-scale two-component icosahedral protein complexes. Science. 2016;353(6297):389–394.
