ABSTRACT
Objectives: Non-reconstituted, hexavalent vaccines (HV-NRs) can facilitate clinical practice by shortening vaccine preparation and administration time and by reducing the risk of vaccination errors compared to combination vaccines requiring reconstitution. The aim of this study was to determine the budget impact of introducing an HV-NR into the United Kingdom’s (UK) pediatric immunization program, which currently uses a hexavalent vaccine requiring reconstitution (HV-R).
Methods: Abudget impact model covering a 10-year time horizon was developed. The target population constituted closed UK birth cohorts from 2020 to 2029. Total direct costs from the payer’s perspective consisted of four main categories: vaccine acquisition and management, healthcare provider’s service provision, (non-)contaminated needle-stick and sharps injury (NSI), and non-NSI vaccination error costs. The net budget impact was calculated by comparing the costs in two different market share scenarios.
Results: The use of HV-NR instead of HV-R was estimated to save £9,079,927 over a 10-year time horizon (i.e. £907,993 per year). Assuming all other vaccine criteria are equivalent the budget impact was most sensitive to changes in time spent by the healthcare provider and management costs.
Conclusion: Results suggest, introducing an HV-NR into the UK’s pediatric immunization program is potentially cost saving for the healthcare system.
1. Introduction
Immunization has the proven ability to control or even eliminate life-threatening infectious diseases within the general population. It is estimated that immunization averts 2 to 3 million deaths globally each year, making it one of the most cost-effective health investments [Citation1]. This also applies to immunization against diphtheria, tetanus, and pertussis (DTaP), hepatitis B (HepB), poliomyelitis (IPV), and Hemophilus influenzae type b (Hib), particularly considering the high coverage rates of 90–98% seen within the EU5 countries (Germany, France, UK, Italy, and Spain), resulting in remarkably lower numbers of reported incident cases compared to pre-vaccination era figures [Citation2].
Despite the widely accepted benefits of vaccinations, the success of immunization programs and their associated costs still largely depend on the quality of vaccine administration [Citation3]. It has been reported that approximately 27–35% of all vaccinated individuals worldwide experience at least one vaccination error [Citation4]. In the UK, 92% of the vaccination errors are due to administrator mistakes, e.g. administering the wrong vaccine, or at the wrong time, or an incorrect dose, or due to missing a dose [Citation3]. Another important form of vaccination error is (non-)contaminated needle stick and sharp injuries (NSIs) [Citation5]. Healthcare providers (HCPs) are exposed to the risk of (non-)contaminated NSIs whenever they are administering parenteral vaccines. As contaminated NSIs have the potential to transmit pathogens present in human blood, either transiently or persistently, these NSIs pose a risk to the health of the HCP. Moreover, contaminated NSIs also have a significant economic burden. Overall cost estimates for managing a contaminated NSI have been reported to range from £235 to £1,540, depending on their nature [Citation6].
In this economic analysis, the focus lies on the immunization program in the UK. At present, a hexavalent vaccine requiring reconstitution (HV-R) for DT3aP-IPV-HepB/Hib (Infanrix-Hexa®; GlaxoSmithKline) is being used in the UK’s pediatric immunization program. The Hib component is supplied as a monovalent white powder and the DT3aP-IPV-HepB components as a pentavalent turbid white suspension. Therefore, this vaccine requires reconstitution prior to injection. In 2015, a HV-NR for DT5aP-IPV-HepB-Hib (Vaxelis®; MCM Vaccine B.V.) was approved for use in the European Union, including the UK [Citation7].
Combination vaccines allow the delivery of a greater number of antigens in fewer injections. Advantages of non-reconstituted (NR) over reconstituted (R) combination vaccines are that NR products can facilitate clinical practice by shortening the preparation and administration time, have the potential to reduce the risks of (non-)contaminated NSIs and other vaccination errors, and reduce medical wastage [Citation8]. These potential HV-NR advantages might help to reduce the health economic burden in the UK’s vaccination program.
2. Objective
Only two European studies have performed a budget impact analysis (BIA) on vaccinations in the pediatric population [Citation9]. However, none of these studies performed a BIA on introducing an HV-NR into a market that currently uses an HV-R against DTaP, IPV, HepB, and Hib. To gain a better understanding of the budget impact of such an introduction, a budget impact model (BIM) was developed under the assumption of equivalent effectiveness (using immunogenicity as a proxy for effectiveness) [Citation10]. The outcomes of this study can play a role in the decision-making process for local and national payers.
3. Methods
An Excel-based BIM, taking a cost offset approach, was developed to provide evidence-based support for decision-making on either pentavalent (plus monovalent vaccine) and/or hexavalent vaccines against DTaP, IPV, HepB, and Hib. The BIM was designed as a cost-minimization analysis (CMA) [Citation11]. A CMA can be used in comparisons between two interventions that are assumed to be equivalent in terms of their therapeutic outcomes. The interventions used in this BIM were equivalent in terms of effectiveness and thus equivalent in terms of therapeutic outcome according to the study of Orsi et al [Citation10]. A CMA, and therefore this BIM, focusses on comparing the costs and resource use associated with each of the interventions in this model comparing an HV-NR vs. an HV-R.
3.1. Model structure
The core structure of the BIM constitutes four different modules: vaccine costs, HCP costs, NSI costs, and non-NSI vaccination error costs. The vaccine costs consist of the vaccine acquisition cost, spillage costs, and wastage costs (including managing the removal of the disposables). The HCP costs are the costs associated with vaccine administration. The direct NSI-costs can be divided into non-contaminated NSI and contaminated NSI costs. The latter includes testing the source and the exposed HCP, as well as post-exposure medical visits, treatments, and vaccine purchase costs. The non-contaminated NSI costs include the purchase costs of a new (replacement) vaccine. The non-NSI vaccination errors consist of direct (replacement) vaccine purchase costs due to administering a wrong vaccine, an additional or wrong dose, or a recording error. For the purposes of this analysis, the optional module including spillage and wastage costs, integrated by vaccine costs, was included in the main analysis. Two other optional modules can be selected to expand the BIA with indirect costs, consisting of HCP loss of productivity due to contaminated NSIs and parent loss of productivity due to vaccination visits, and exploration of Hib vaccine effectiveness and associated costs. The latter module is, due to data uncertainty and limited applicability to the UK, reviewed in the discussion section. The indirect cost module is explored in a scenario analysis.
Three model inputs (number of vaccinations, number of needle handlings, and number of vaccination visits) provide the basis for the calculations being performed in each of the modules. They have an impact on every downstream calculation, e.g. number of vaccinations is related to the vaccine cost module, number of needle handlings to the cost of (non-)contaminated NSIs, and number of vaccination visits to parent loss of productivity costs.
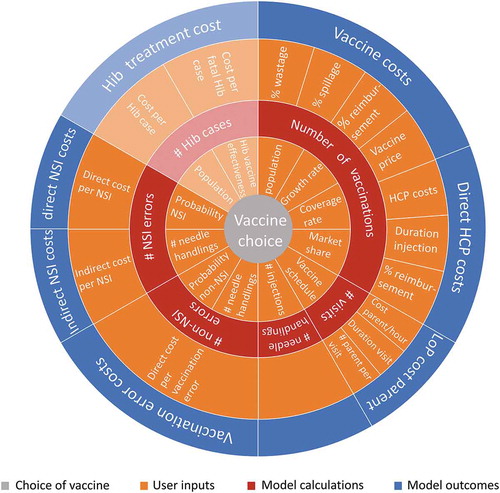
A schematic representation of the model structure () displays how the different inputs relate to the various calculations and their calculated outcomes. The two key assumptions relevant to the UK analysis are: 1) Coverage rates describe the number of children that have completed a full set of vaccinations, i.e. separate coverage rates for individual vaccination rounds (first, second, or third vaccination round) are not considered in the model; and 2) comparators included in the BIM have comparable immunogenicity (as a proxy for effectiveness).
Figure 1. Schematic overview of the budget impact model structure. To provide an overview of the BIM as a whole, Hib treatment cost calculations are shown in grayed tones, because of its inapplicability to the UK setting. (Note: this figure is only used to visualize the connections between user-inputs, model calculations, and model outcomes and that the sizes of the figure parts are not representative for the impact on the model costs)
3.2. Budget impact analysis
This BIA assesses the incremental budget impact between the HV-NR and the HV-R in the UK. Four out of six modules were considered in the main analyses. The impact of the indirect costs was analyzed in a scenario analysis. The UK vaccination market is usually covered by one tender. Therefore, annual budget impact per vaccine was determined for a current market share analysis, in which the HV-NR had zero market shares versus the HV-R, and a future market share analysis, in which the HV-R had zero market shares versus the HV-NR. The incremental budget impact was calculated by subtracting the current market situation from the future market situation.
3.3. Population and model settings
The model population covered closed UK birth cohorts eligible for vaccination against DTaP, IPV, HepB, and Hib. The population size at the outset is projected to be 726,000 in 2020 and is projected to decline by an average of 0.17% per year between 2020 and 2029 [Citation12]. Background mortality was not considered in the model, since all closed cohorts are considered the same for both interventions. The general annual vaccination coverage rate was set at 92.6% [Citation13]. Budget impact calculations were run over a 10-year time horizon from a direct payer’s perspective. This time horizon was used to include the year-on-year changes in population size and several vaccine tender periods in the UK, allowing potential changes in market shares over time. Due to the nature of the outcomes and to know the actual value of the financial (dis)advantages, discounting was not applied to the costs of the BIA. summarizes the model settings and population inputs.
Table 1. Model settings and population inputs
3.4. Resource use and cost inputs
To populate the BIM, resource use and costs for the UK were obtained from existing literature, medical advisors, and assumptions. An overview of the resource use and cost inputs is given in and , respectively. To explicitly determine the budget impact based on HCP administration time, NSI safety, resource use, and ease of use, both vaccinations were assumed to have an equal cost of £20.00 per injection. The extra costs due to non-contaminated NSIs and other vaccination errors (administering wrong vaccine, cost of additional vaccine dose, cost of administrating wrong vaccine dose, and cost of recording error) were assumed to be equivalent to the costs of purchasing one additional vaccine. The remaining cost parameters had the same value for both the HV-NR and HV-R vaccines (). Direct contaminated NSI costs included testing the source and the exposed HCP, as well as post-exposure medical visits, treatments, and vaccine purchase costs. Indirect contaminated NSI costs, determined in a scenario analysis, included the cost of lost productivity for the injured HCP associated with the time required for reporting and receiving initial and follow-up treatment, as well as exposure consequences, overhead, compensation, and litigation. All costs were estimated in 2020 values in Pound Sterling (£), and where necessary, inflated to 2020 according to the consumer price index of the Office for National Statistics [Citation14Citation16Citation17Citation18]. (; )
Table 2. Cost inputs
Table 3. Resource use inputs
In the UK, DTaP, IPV, HepB, and Hib vaccines are administered in the second, third, and fourth month after birth [Citation21]. It was assumed that all immunization tasks were performed by a nurse and are fully reimbursed. A time and motion study by De Coster et al [Citation8]., comparing HV-R versus another HV-NR (Hexyon®, Sanofi Pasteur), assessed their duration of vaccine preparation and administration. Hexyon® (DTaP-IPV-HB-Hib) is a suspension for injection in a pre-filled syringe and is indicated for primary and booster vaccination of infants and toddlers from six weeks of age [Citation22]. Hexyon® is currently not part of the UK immunization program and has a slightly different composition than the HV-NR (Vaxelis®) assessed in this BIA. The preparation and administration times as measured by De Coster et al. [Citation8] were assumed to be applicable to other HV-Rs and HV-NRs as well.
The probability of one of the non-NSI errors has been provided when a non-NSI vaccination error occurred. Although the model allows for non-NSI error analysis, due to poor data availability on non-NSI errors a conservative approach was applied and, therefore, the probability of non-NSI errors was set to zero. Spillage or leakage was assumed to be slightly higher for the HV-R due to the reconstitution step. Probability of (non-)contaminated NSI error was based on the study by Tosini et al [Citation23]. Duration of vaccination visit for both vaccines was assumed to be approximately one hour. This included transport (2x15 min), waiting time (15.5 min) [Citation24], and vaccine administration (8.4 min) [Citation24], and assuming only one guardian would attend all vaccination visits.
3.5. Uncertainty and scenario analysis
To explore the impact of uncertainty around and the relative importance of each key input parameter, which may have been derived from expert opinions, clinical trials, or observational studies, a univariate deterministic sensitivity analysis (DSA) and a probabilistic sensitivity analysis (PSA) were performed. Within the DSA, the lower and upper values of the analysis were assumed to vary with 20% around the point estimate from the base case analysis. Since the PSA analysis is conducted within a BIM and thus no effectiveness results are reported, cost-acceptability curves could not be used to display the results of the PSA; boxplots were used instead.
A separate scenario analysis was performed on including the indirect costs in the main analysis. The indirect costs, consisting of HCP loss of productivity due to contaminated NSIs and parent loss of productivity due to vaccination visits, were examined in a scenario due to the direct payer perspective focus in the UK.
4. Results
4.1. Overview
The model tracked the UK population eligible for DTaP, IPV, HepB, and Hib vaccination over a 10-year time horizon. A total of 6.65 million children are projected to receive these pediatric vaccinations. The base case analysis results include the direct payer costs and additional indirect costs were analyzed in a scenario analysis.
4.2. Budget impact
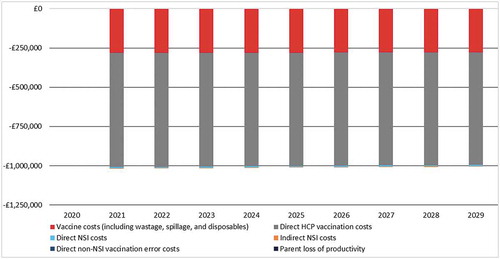
Introducing an HV-NR into the UK market, with a 100% market share led to an average cost-saving of £907,993 per year (). A small constant decrease in population size over time resulted in approximately £1,717 less cost-savings per year. Over a 10-year time horizon, the introduction of the HV-NR led to total cost-savings of £9,079,927. The highest contributors to the cost-savings were vaccine costs (including wastage, spillage, and disposables) with £2,519,701 (27.8%) and direct HCP related costs with £6,495,809 (71.5%). Additionally, smaller savings of £64,417 (0.7%) due to decreased numbers of NSI errors. All costs per category and associated budget impact are provided in the appendix (appendix and ).
4.3. Resource utilization outcomes
In this BIA, it was assumed that the probabilities of contaminated NSIs were equal for both vaccines. However, introducing an HV-NR into the UK market resulted in avoidance of 261 non-contaminated NSIs, leading to an overall cost-saving of £20.00 (vaccine purchase cost) per non-contaminated NSI and £474.40 per contaminated NSI. Furthermore, the HCP vaccine administration time was reduced by 172,480 hours, and 17,997,862 fewer needles were discarded over the 10-year time horizon. This led to a total incremental cost-saving of £6,495,809 and £2,519,701 over a 10-year time horizon, respectively.
4.4. Uncertainty and scenario analyses
The scenario analysis of including the indirect costs in the main analysis resulted in additional cost savings of £29,857 over the 10-year time horizon from a reduction in HCP productivity loss due to contaminated NSI errors.
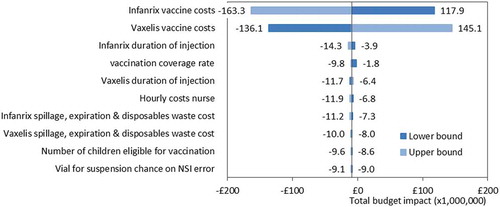
The deterministic sensitivity analysis on total budget impact outcome demonstrates that the vaccine acquisition cost for both HV-R and HV-NR have the most significant impact on the model outcomes (). Another striking result is that, besides the vaccine acquisition cost, the most influential parameters fall below zero for both the upper and lower bound outcomes. Indicating that, despite input parameter uncertainty, the deterministic sensitivity analysis supports the robustness of the conclusion around cost savings after introduction of an HV-NR vaccine.
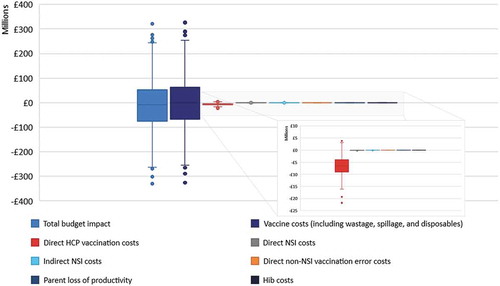
In the probabilistic scenario analysis, 1000 different sets of parameter inputs, drawn by random sampling based on the parameter distributions, show that the median of the total budget impact boxplot is lower than £0. This indicates that 52.5% of the outcomes is in favor of the HV-NR. The most sensitive model parameter according to the uncertainty analysis is the vaccine acquisition cost. The uncertainties around all other cost outcomes and whether they increase or decrease the budget impact are also shown in .
5. Discussion
The current BIM was developed to determine the budget impact over a 10-year time horizon, from a direct payer’s perspective, introducing an HV-NR into the pediatric immunization program of the UK. Between 2020 and 2029, with 100% of the market shares shifting from HV-R in 2017 to HV-NR in 2021 and onwards, the introduction of an HV-NR resulted in a budget impact of -£9,079,927. These costs were saved by a reduction in vaccine costs (£2,519,701), including vaccine purchase costs, wastage, spillage, and disposables, and a reduction in the number of HCP vaccination hours (£6,495,809). A total of 17,997,862 disposed needles would be prevented, 172,480 hours of HCP time reduced, and 261 NSIs avoided. Sensitivity analyses considering uncertainties surrounding input parameters were performed. Including indirect costs resulted in additional cost savings of £29,857 over the 10-year time horizon from a reduction in HCP productivity loss due to contaminated NSI errors. Switching from an HV-R to an HV-NR is likely to be cost-saving and could save, on average, between £1,002,028 and £1,015,761 per year in the UK vaccination program.
5.1. Strengths
According to a recent literature review [Citation9], this is the first BIM focusing on the introduction of an HV-NR in a market that currently uses an HV-R in the pediatric immunization program. Most existing economic evaluations concerning vaccination focused typically on cost-effectiveness and rarely on budget impact [Citation9]. Additionally, only four studies focused on pediatric populations [Citation9]. Hence, this BIM could possibly serve as additional guidance in this field.
The BIM was developed with the perspective of a payer, under the assumption of equivalent vaccine immunogenicity (as a proxy for effectiveness) and safety for the child. However, this BIM allows analysis with a partly societal perspective. Indirect costs of the NSIs and parent loss of productivity can be included in the model, although, other social opportunity costs, such as out-of-pocket direct costs incurred by the patient, were excluded.
Where most BIMs only contain a DSA to address the limitations and model uncertainties, this model also includes a PSA. The PSA demonstrates that the biggest part of the outcome uncertainty comes from the vaccine cost parameters (including vaccine purchase, wastage, spillage, and disposable costs). Unlike the DSA, which strongly supports the cost savings of the HV-NR, the PSA supports cost-savings to a lesser extent. Judging from the results, the difference in support of the cost savings between the DSA and PSA is due to the relative size of the cost savings compared to the overall costs associated with the vaccination program.
This study used UK data whenever available, if UK data were not available other published data were used and validated. Besides the incremental costs, this BIM also calculated the incremental number of (non-)contaminated NSIs, needles saved, and HCP time reduced. Although this model outlines results for the UK, the model has been designed to be flexible enough to be adapted to other European countries and to ensure that it can be updated, should additional clinical data become available in the coming years; this could lead to improvements in the accuracy of the model inputs and subsequently improve the accuracy of the outputs and associated conclusions.
The design of the model () means that it is also able to analyze additional scenarios which may be of interest or relevance in other countries such as those exploring healthcare environments where multiple hexavalent vaccine combinations exist or to explore the effectiveness of the Hib component of the vaccines. The argument for different Hib effectiveness is explained by the fact that post dose 1, the polyribosyl ribitol phosphate (PRP) response percentage differed between recipients of vaccinations with different PRPs as researched by Jackson et al [Citation25]. However, both are not relevant for the UK vaccination program.
5.2. Limitations
The findings from the BIM are subject to several limitations alongside the assumptions made for modeling purposes. A consideration is that the future market shares of the HV-NR will depend on a local tendering process. It is difficult to predict the outcome of a future tender and hence the future market shares.
The model input wastage cost is assumed to include managing the removal of the needles and vaccines. For the HV-R, the extra needle for reconstitution is seen as a second wasted vaccine, which might have led to an overestimation of the waste costs associated with the HV-R.
As reported in an earlier BIA by Fagnani et al [Citation26], the opportunity costs of performing an injection should be included as well and contain at least three categories of indirect costs. These categories are: indirect costs for parental loss of productivity, cost of transportation to the physician’s office, and the indirect cost of pain and emotional distress to the child associated with each injection. In this BIM we only took the costs of parental loss of productivity into account. Due to no differences in number of visits and the time spent on each visit for both hexavalent vaccines, there is no effect on the budget impact. Whilst the UK assessment process tends not to consider indirect costs from a societal perspective, it is worth noting that the total indirect costs for each injection visit is underestimated in this BIM based on Fagnani et al. [Citation26]
6. Conclusion
In conclusion, under the current assumptions and modeling choices, the introduction of an HV-NR for DTaP-IPV-HepB-Hib vaccination into the UK’s childhood immunization program has the potential to generate significant cost-savings over a 10-year time horizon (£9,079,927). Furthermore, according to the most recent review [Citation9], this is the first BIM determining the budget impact of a comparison between two hexavalent vaccines and could possibly serve as a starting point and guidance for future budget impact studies seeking to introduce a new hexavalent vaccine into pediatric immunization programs.
Declaration of interest
This study was commissioned by MCM Vaccine B.V., a partnership between Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and Sanofi Pasteur, Inc., Swiftwater, PA, USA and conducted by Pharmerit International. The authors D.A.R. Mathijssen, S.L. Klijn, and M. Heisen are employed by Pharmerit International, B. van Dijk is a former co-worker at Pharmerit International, all have no additional interest. Lara J. Wolfson is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and may hold stock or stock options in Merck & Co., Inc., Kenilworth, NJ, USA. Xiaoyan Ivy Lu, and Berhanu Alemayehu are former co-workers of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and may hold stock or stock options in Merck & Co., Inc., Kenilworth, NJ, USA. James F. Clark-Wright and Stuart Carrol are employees of Sanofi Pasteur UK & Ireland and may hold stock or stock options in the company. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
D.A.R. Mathijssen: Main developer of the budget impact model and writer of the manuscript.
M. Heisen: Co-developer of the budget impact model and reviewer of the manuscript.
S.L. Klijn: Co-developer of the budget impact model and co-writer of the manuscript.
B. van Dijk: Co-developer of the budget impact model and reviewer of the manuscript.
B. Alemayehu: Project lead, reviewer of the budget impact model and reviewer of the manuscript.
L.J. Wolfson: Responsible for inputs in the model and reviewer of the manuscript.
X. Lu: Reviewer of the manuscript and responsible for model inputs.
J.F. Clarck-wright: Responsible for the inputs of the model, reviewer and co-writer of the manuscript.
S. Carroll: Reviewer of the manuscript and responsible for certain inputs
Additional information
Funding
References
- World Health Organization (WHO). Immunization 2020 [cited 2020 Oct 08]. Available from: https://www.who.int/topics/immunization/en/
- World Health Organization (WHO). Country profiles, data, and statistics on immunization 2020 [cited 2020 Oct 08] 2020]. Available from: https://www.who.int/immunization/monitoring_surveillance/en/
- Lang S, Ford KJ, John T, et al. Immunisation errors reported to a vaccine advice service: intelligence to improve practice. Qual Primary Care. 2014;22(3):139–146. PubMed PMID: 24865341.
- Hamlin JS, Wood D, Pereyra M, et al. Inappropriately timed immunizations: types, causes, and their relationship to record keeping. Am J Public Health. 1996 Dec;86(12):1812–1814. PubMed PMID: 9003145; PubMed Central PMCID: PMCPMC1380741. eng.
- Elseviers MM, Arias-Guillen M, Gorke A, et al. Sharps injuries amongst healthcare workers: review of incidence, transmissions and costs. J Ren Care. 2014 Sep;40(3):150–156. PubMed PMID: 24650088; eng.
- Adams D. Needlestick and sharps injuries: practice update. Nurs Stand (through 2013). 2012;26(37): 49.
- European Medicines Agency. EMA/859285/2015 – Vaxelis; 2015.
- De Coster I, Fournie X, Faure C, et al. Assessment of preparation time with fully-liquid versus non-fully liquid paediatric hexavalent vaccines. A time and motion study. Vaccine. 2015 Jul 31;33(32):3976–3982. PubMed PMID: 26092310.
- Loze PM, Nasciben LB, Sartori AMC, et al. Vaccines are different: A systematic review of budget impact analyses of vaccines. Vaccine. 2017 May 15;35(21):2781–2793. PubMed PMID: 28427846; eng.
- Orsi A, Azzari C, Bozzola E, et al. Hexavalent vaccines: characteristics of available products and practical considerations from a panel of Italian experts. J Prev Med Hyg. 2018;59(2):E107.
- WHO International Working Group for Drug Statistics Methodology, WHO Collaborating Centre for Drug Statistics Methodology, WHO Collaborating Centre for Drug Utilization Research and Clinical Pharmacological Services. Introduction to drug utilization research. World Health Organization; 2003.https://apps.who.int/iris/handle/10665/42627
- Office for National Statistics (ONS). National population projections: 2018-based. In: Nash A, editor. National population projections statistical bulletins. United Kingdom; 2019: 6–7.
- Screening & Immunizations Team (NHS Digital), COVER Team (Public Health England). Childhood Vaccination Coverage Statistics - England (UK) - 2019-2020. In: Jones S, editor. Childhood Vaccination Coverage Statistics; 2020.
- Office for National Statistics (ONS). Consumer price inflation, UK: august 2020. In: Gooding P, editor. Price indices, percentage changes and weights for the different measures of consumer price inflation; 2020.
- Curtis L, Burns A. Unit costs of health and social care 2019. Unit costs of health and social care. Kent, UK: PSSRU; 2019.
- Mannocci A, De Carli G, Di Bari V, et al. How much do needlestick injuries cost? A systematic review of the economic evaluations of needlestick and sharps injuries among healthcare personnel. Infect Control Hosp Epidemiol. 2016;37(6):635–646.
- Leeds City Council. Medical waste 2018 [cited 2018]. Available from: https://www.leeds.gov.uk/residents/bins-and-recycling/medical-waste
- Sharps Compliance Inc. 28-Gallon medical professional sharps recover system with six 2-gallon sharps collection containers; 2018 [cited 2018]. Available from: https://www.sharpsinc.com/store/28-gallon-srs-2g-sharps-container
- OECD. Stat [Internet]. 2020 [cited 2020 Oct 13]. Available from: https://stats.oecd.org/.
- Glazner JE, Beaty B, Berman S. Cost of vaccine administration among pediatric practices. Pediatrics. 2009;124(Supplement 5):S492–S498.
- Public Health England. The complete routine immunisation schedule. Immunisation information for health professionals and immunisation practitioners. Public Health England (UK); 2020.
- European Medicines Agency. Hexyon, summary of product characteristics 2013 [Cited 2019 Jun]. Available from: https://www.ema.europa.eu/documents/product-information/hexyon-epar-product-information_en.pdf
- Tosini W, Ciotti C, Goyer F, et al. Needlestick injury rates according to different types of safety-engineered devices: results of a French multicenter study. Infect Control Hosp Epidemiol. 2010;31(4):402–407.
- Mokiou S, Standaert B, Li X, et al. Measuring the cost of a pediatric vaccine administration in the UK. Vaccine. 2018 Jan 4;36(2):237–242. PubMed PMID: 29208324; eng.
- Jackson C, Mann A, Mangtani P, et al. Effectiveness of Haemophilus influenzae type b vaccines administered according to various schedules: systematic review and meta-analysis of observational data. Pediatr Infect Dis J. 2013;32(11):1261–1269. .
- Fagnani F, Le Fur C, Durand I, et al. Economic evaluation of a combined DTPa, hepatitis B, polio, Hib vaccine. Potential impact of the introduction of Infanrix-Hexa in the French childhood immunisation schedule. Eur J Health Econ. 2004 Jun;5(2):143–149. PubMed PMID: 15452751; eng.
Appendix A
Table A1. Annual vaccine cost per cost-category
Table A2. Annual budget impact per cost-category