ABSTRACT
Introduction
Hepatitis E virus (HEV) is an important cause of enterically transmitted viral hepatitis and a significant contributor to maternal mortality in endemic regions around the world, yet the global response has been limited. HEV is a disease of poverty, and the populations experiencing the greatest burden of HEV-associated illness are not benefitting from existing interventions, including WASH strategies and immunization.
Areas Covered
Though a vaccine exists (HEV 239, Hecolin®, Xiamen Innovax Biotech, China), it is only licensed and available in the private market in China and has yet to be prequalified by the WHO for use in endemic settings and outbreaks. This review of the current state of HEV disease and subsequent recommendations for a coordinated public health response are intended to guide the global health community towards breaking the current ‘vicious cycle,’ in which a lack of data prevents actions that would improve health outcomes.
Expert opinion
Vaccine implementation in future outbreaks, targeted studies assessing vaccine effectiveness and immunogenicity in endemic regions and populations, improved understanding of the global burden, and improvements in diagnostic and epidemiologic tools are urgently needed. Strategies for implementing routine vaccination programs, improving water, sanitation, and hygiene in endemic regions.
1. Introduction
Hepatitis E virus (HEV) is an important cause of enterically transmitted viral hepatitis and contributes to over 50% of the acute viral hepatitis cases in endemic areas [Citation1]. A World Health Organization (WHO)-funded modeling study estimated that 20 million people were infected with HEV in 2005, with estimated 3.3 million clinical cases, up to 70,000 deaths, and 3000 stillbirths, mostly in Asia and Africa [Citation2], causing 3.3% of all deaths from viral hepatitis worldwide [Citation3]. HEV accounted for 1.7% of the total global healthy years of life lost (738,508 disability-adjusted life years) due to hepatitis in 2017 [Citation4]. The most affected populations are among the world’s most vulnerable groups – pregnant women and their neonates, as well as displaced persons and those living in resource-limited settings with poor access to clean water and sanitation. HEV is a significant contributor to global maternal mortality, with reported case-fatality rates (CFRs) of 20–30% in pregnant women with the symptomatic disease [Citation5,Citation6]. In industrialized countries, HEV is a growing concern particularly in immunocompromised individuals and patients with chronic liver disease are also at risk for the severe disease after HEV infection [Citation7,Citation8]. However, given the lack of appropriate surveillance or outbreak investigations, these data are very likely significantly under-reported.
As a consequence of the underestimation of HEV morbidity and mortality, the disease is not treated today as a global health priority, and the populations experiencing the greatest burden of HEV-associated illness in low-income regions are unable to benefit from existing interventions. There is a shortage of available and affordable diagnostic tools as well as treatment options for severe disease, making effective outbreak response challenging. As pointed out by Azman et al. in their case for designating hepatitis E as a neglected tropical disease, the ‘limited tools and knowledge preclude investments’ in further research, surveillance, and treatment [Citation6]. One effective tool for the prevention of HEV infection has been developed – a licensed vaccine [Citation9] – but it is not yet available to the populations most at risk.[7]
2. Overview of Hepatitis E Virus (HEV)
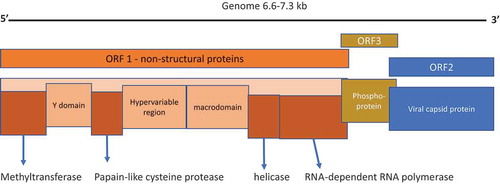
Hepatitis E virus was first identified in 1981 by Russian virologist Mikhail S. Balayan while investigating an outbreak of enterically transmitted non-A, non-B hepatitis in Russian soldiers stationed in Afghanistan. Balayan self-administered stool extract from the affected soldiers and subsequently developed symptoms of hepatitis. The spherical nonenveloped virus particles were identified in his stool samples analyzed by electron microscopy [Citation10]. HEV is a small, single-stranded, plus sense icosahedral RNA virus, within the Hepeviridae family and genus Orthohepevirus A. It contains a genome of ~7.2 kb, organized in three open reading frames, encoding the structural and nonstructural proteins (). HEV is excreted in the feces of infected individuals, is highly infectious, and can persist in the environment. A second, less-infectious, quasi-enveloped form that does not express antigenic proteins is released by host cells into the serum of infected individuals. The capsid and membrane that envelope the viral particles facilitate binding to host cells via normal uptake mechanisms. This envelope is removed in the biliary tract before virus particles are excreted in feces and urine. The nonenveloped particles are 10 times more infectious, which may be due to a different, more efficient host cell entry mechanism [Citation7,Citation11].
HEV is classified into eight genotypes, with genotypes 1–4 being the most common in humans. Genotypes 1 and 2 (HEV1 and HEV2) are found in South, Central, and Southeast Asia, Africa, the Middle East, and Mexico. Genotype 3 (HEV3) is prevalent in industrialized countries worldwide, while genotype 4 (HEV4) is found primarily in Asia [Citation11–14]. HEV1 and HEV2 are obligate human pathogens and are primarily transmitted via the fecal-oral route, commonly causing outbreaks through contaminated water. HEV3 and HEV4 have known animal reservoirs (notably wild and domestic pigs) and cause sporadic zoonotic infection in humans, most often after consumption of undercooked meat [Citation3,Citation7]. HEV can be transmitted vertically from mother to fetus as well as parenterally, and by blood transfusion and transplantation [Citation15–17].
The virus has a long incubation period of 2–8 weeks to onset of symptoms. Viremia persists in acute hepatitis generally for less than a month, while viral RNA is not detectable 3 weeks after the development of symptoms [Citation7]. There is no clear clinical case definition for HEV infection, as it has varying presentations and can be clinically indistinguishable from other forms of viral hepatitis. The nonspecific clinical presentation often leads to misdiagnosis and misattribution in many settings [Citation18].
Most cases of HEV are mild or asymptomatic, but some populations (pregnant women, immunocompromised individuals, and those with chronic liver disease) are more likely to experience severe disease [Citation19]. Certain host characteristics are thought to play a role in HEV virulence, such as pregnancy, and poor nutritional status. Children are much less likely to exhibit clinical disease and seroprevalence peaks between the ages of 15–30 years [Citation20,Citation21]. The difference in susceptibility between age groups may be related to the fact that the duration of immunity after natural infection with HEV1 and HEV2 appears to be limited, although further studies with improved assays are needed [Citation21,Citation22].
A definitive diagnosis of HEV infection requires confirmatory serologic and/or molecular evidence and is costly. There is currently no FDA- or WHO-approved assay available for the detection of an acute or previous HEV infection and HEV3 is the only genotype for which an RNA nucleic acid amplification test standard has been established and approved by WHO [Citation23,Citation24]. A well-established assay for anti-HEV neutralizing antibodies is also lacking [Citation23].
HEV diagnostic methodology has varied historically, and the reliability and comparability of much of the available prevalence data are poor [Citation25,Citation26]. The first generation of in-house laboratory-based assays, including an assay developed by Walter Reed Army Institute of Research (WRAIR), has been replaced with a new group of assays with improved sensitivity and specificity for anti-HEV IgG, such as the commercially available assay Wantai HEV IgG ELISA (Beijing Wantai Biological Pharmacy, Beijing, China) [Citation27]. Recent retrospective studies using these more reliable assays indicate that the seroprevalence of HEV in both endemic and nonendemic regions had been underestimated [Citation7,Citation28,Citation29]. However, the accuracy of the newer assays still varies significantly, which continues to impact disease burden estimates and clinical trials that rely on serological data [Citation25]. ()
Table 1. Diagnosis of acute hepatitis E infection
2.1. HEV Epidemiology
Accurate estimates of HEV disease burden in both endemic and nonendemic regions have been challenging to obtain. In many endemic areas, lack of clinical awareness and lab testing capabilities present significant barriers to diagnosis, and often the only documented cases are those severe enough to require hospitalization. Few low-income countries have hepatitis E surveillance, though some sentinel sites have been established in highly endemic areas such as Bangladesh and Nepal, with support from international funding and resources [Citation35]. Much of the known epidemiologic information about HEV is largely from retrospective analysis and testing of serum from hepatitis patients or affected communities during an outbreak.
Asia and Africa have the most recorded cases of hepatitis E, particularly equatorial Africa and northern India, as these regions are where the enteric genotypes predominate [Citation36,Citation37]. Recent outbreaks have occurred in Chad [Citation38], Central African Republic [Citation39], Namibia [Citation40], and the Ganges River Basin in northern India [Citation41]. Of the estimated 20 million yearly HEV infections worldwide, 60% are thought to occur in East and South Asia [Citation2]. The first large retrospectively identified hepatitis E outbreak occurred in India in the mid-1950s [Citation42], and outbreaks continue to be reported there today, with 27% of the recorded water-borne hepatitis E outbreaks between 1980 and 2017 reported in northern India [Citation37]. Other countries in Asia that have reported outbreaks include Bangladesh, Pakistan, Nepal, and Indonesia [Citation43]. According to a recent geographic modeling analysis, outbreaks in Asia tend to have higher average case numbers (7677 cases/outbreak) than those in Africa (1436 cases/outbreak) [Citation37].
The first well-documented HEV outbreak in Africa occurred in Somalia in 1988–89, with more than 11,000 cases [Citation43]. Large outbreaks have occurred in refugee camps in Sudan, South Sudan, Chad, Darfur, and Kenya [Citation43]. There have been nearly 7000 cases in an ongoing two-year outbreak in Namibia, with 59 deaths as of January 2020 [Citation44]. A recent meta-analysis of 22 pooled studies found an HEV seroprevalence of 29% among pregnant women in Africa [Citation45]. Another meta-analysis of 29 studies measuring HEV prevalence in acute hepatitis patients in Somalia found an overall prevalence rate of 47% [Citation46]., and a recent survey of 1000 adults in Uganda found a similar seroprevalence rate of 47% [Citation47].
The epidemiology of hepatitis E in Egypt is somewhat paradoxical, in that the seroprevalence of HEV1 is very high with a low incidence of symptomatic infection [Citation48]. The HEV seroprevalence in pregnant women is 84%, yet pregnant women in Egypt do not experience the high mortality rates common in other parts of Africa. Egyptian children also have higher rates of acute viral hepatitis caused by HEV compared to other endemic regions where primary infection typically occurs in young adulthood [Citation48].
In industrialized countries, zoonotic HEV3 and HEV4 infections predominate, usually due to consuming undercooked meat, especially pork [Citation7]. A recent meta-analysis of 26 studies from 15 industrialized countries (including Europe, Australia, New Zealand, and North America) found a wide range of HEV seroprevalence rates, ranging from less than 5% to greater than 50% [Citation49]. There is increasing evidence that factors such as viral subtype, infectious dose, food, and host factors such as genetics or nutrition status contribute to the variation in hepatitis E epidemiologic patterns in different regions of the world [Citation21,Citation50].
2.2. Morbidity and mortality
The 2013 Global Burden of Disease Study estimated that hepatitis E accounted for 19.4% of all deaths from acute hepatitis globally in 2013 (7th leading cause of death worldwide) [Citation28].
Hepatitis E infection in immunocompromised individuals is associated with increased mortality [Citation51], and in pregnant women, HEV infection often leads to fulminant hepatic failure, increased risk for peripartum hemorrhage [Citation39], and overall higher fatality rates [Citation52]. A recent systemic review of 23 studies found a median maternal fatality rate of 26% in endemic regions [Citation53]. During a recent outbreak in Niger (2017), 44.7% of the total fatalities were in pregnant women [Citation54]. An analysis of 110,000 pregnancies in rural Bangladesh suggests that hepatitis E may be responsible for 9.8% of all pregnancy-associated deaths [Citation5]. The pathogenesis underlying adverse outcomes in pregnant women and immunocompromised patients is not well understood. There is some evidence that micronutrient deficiencies and immune dysregulation during pregnancy may play a role [Citation43], as well as elevated viral load and prolonged viremia [Citation55,Citation56].
Fetuses and neonates are also at greater risk for mortality. During pregnancy, there is an increased risk of intrauterine fetal demise, premature birth, intrauterine growth restriction, intrapartum stillbirth, and neonatal death [Citation52,Citation57]. A meta-analysis of studies from hepatitis E endemic areas found a median 33% fatality rate in fetuses and an 8% fatality rate in neonates born to HEV-infected mothers [Citation53Citation58].
In children under 15 years of age, HEV attack rates and HEV seroprevalence rates are low compared to older teens and young adults [Citation27]. Children in endemic regions are impacted indirectly by hepatitis E, due to the long-term health implications of maternal death.
HEV infection has also been reported to be associated with a range of severe diseases (reviewed in 53). For example, HEV infection may result in acute chronic liver failure in individuals with underlying liver disease, increasing their risk of death by up to 70%. Immunocompromised individuals, whether due to HIV infection and treatment or organ transplant, are often susceptible to chronic HEV disease which may lead to life-threatening cirrhosis.
2.3. Public health implications
The high maternal death rate associated with hepatitis E exacerbates the negative familial, social, and economic consequences of this disease. A 2010 study of 145 thousand live births in rural Bangladesh found that the cumulative probability of survival to age 10 is only 24% in children whose mothers die, compared with 89% in children whose mothers survive [Citation59]. A study in sub-Saharan African children who lost their mothers before the age of 15 due to HIV/AIDS found long-term negative effects, including significant deficits in height and years of schooling [Citation60]. The impact of an HEV infection and death of the mother impacts not only that pregnancy but the remaining children in that family.
In HEV endemic areas, limited prevention and preparedness leads to costly emergency care, especially in situations of humanitarian emergency when public health services are disrupted. Community-level outbreak response is expensive and may be of long duration. Outbreaks in Uganda, Niger, and South Sudan lasted well over a year each [Citation43,Citation61], and the current outbreak in Namibia has lasted over 2 years to date, in part because of problems with persistent contamination in an underground aquifer that is a primary source of water [Citation62].
In displaced populations, overcrowding and unsanitary conditions are an ideal environment for the spread of waterborne diseases like hepatitis E, cholera and typhoid. Displaced people are likely to experience diminished access to appropriate health care, and these populations often exhibit higher incidences of malnutrition and complications from untreated noncommunicable diseases [Citation63].
2.4. Current strategies for treatment and prevention
There is no specific treatment for hepatitis E. Patients with the uncomplicated infection do not require definitive treatment, with mild cases typically managed through rest and dietary modification. When acute hepatitis leads to fulminant hepatic failure, patients require supportive intensive care and early transplant if available, but the median time between the onset of ALF and death, transplant, or recovery is less than a week [Citation64]. The use of antivirals (ribavirin and alpha-interferon) has been limited to treating chronic HEV3 infections in transplant patients. Recent studies have found some potential drug candidates with antiviral activity against HEV1 in vitro [Citation64,Citation65], however, there is insufficient evidence to support antiviral use for HEV-induced acute hepatitis. Antiviral therapy can be challenging in resource-poor settings due to a lack of generic options and or access to care [Citation66]. Both interferon and ribavirin are also contraindicated during pregnancy, limiting their potential utility for the highest-need populations [Citation64].
Outbreak response typically involves improvements to water, sanitation, and hygiene (WaSH) such as repair of broken water sources and distribution of chlorination products, soap, and water storage containers. These efforts are often complemented with on-the-ground education to improve case recognition and response, including registration of pregnant mothers to expedite case referrals. With the absence of any available intervention or effective treatment, educational efforts often primarily drive patients into hospital settings where they receive reactive and limited management [Citation67].
WASH interventions can be difficult to implement and maintain in outbreak settings. During a 2016 hepatitis E outbreak in Chad, Médecins Sans Frontières found safe levels of free residual chlorine in only 43% of the measured households, even after careful distribution of hygiene kits, education efforts, and achieving wide acceptance of water chlorination by the targeted population [Citation68]. A study of a large outbreak of hepatitis E in a displaced person camp in Darfur found that many people were infected despite obtaining their drinking water from chlorine-treated sources [Citation69].
2.5. Hepatitis E Vaccine
The HEV 239 vaccine (Hecolin®, Xiamen Innovax Biotech, China) is licensed for use in China and has been commercially available in the private market since 2012 () Designed using a HEV1 construct, the vaccine has shown protection against HEV4 in clinical trials [Citation70]. HEV 239 is a recombinant vaccine containing a 239 base pair segment (aa368–606) of the HEV1 ORF2 capsid protein, a major structural protein conserved across the four genotypes [Citation9,Citation70]. The vaccine is given in a three-dose schedule (0, 1, and 6 months) and was found to be 87% effective for up to 4.5 years in a large-randomized controlled trial [Citation71].
Table 2. Summary of HEV 239 Vaccine Information
The use of HEV 239 is limited to the small private market in China. Innovax has expressed an eagerness to expand into other markets, including those in hepatitis E endemic regions, and a willingness to consider expanding manufacturing capacity. The company holds a cGMP (certification for good manufacturing practice) by the Chinese Government Food and Drug Authority (CFDA) [Citation72].
HEV 239 has yet to be prequalified by the WHO for use in endemic settings.
Critically, the WHO HEV Vaccine Position paper specifies that ‘national authorities may decide to use the vaccine based on the local epidemiology’ and that ‘the current WHO position concerning routine programmes should not preclude the use of the vaccine in [special situations such as outbreaks where the risk of hepatitis E or of its complications or mortality is particularly high].’ WHO further states that ‘use of the vaccine to mitigate or prevent outbreaks of hepatitis E should be considered as well as the use of the vaccine to mitigate consequences in high-risk groups such as pregnant women’ [Citation73,Citation74]. Unfortunately, this language seems to have been interpreted by regional WHO offices and relevant Ministries of Health as a recommendation against the use of the vaccine, which has thus far prevented HEV 239 use in outbreak settings despite the existence of willing partners for funding and delivery of the vaccine.
Because the HEV 239 vaccine has not been implemented in an outbreak setting, there is a shortage of much-needed data about how the vaccine might perform. A 2018 transmission model based on data from large hepatitis E outbreaks in displaced population camps in Sudan, Uganda, and South Sudan predicted that preemptive vaccination of nonpregnant adults with two doses of vaccine would have reduced mortality by 35–65%, while initiation of the vaccination program after 50 reported cases would have resulted in a 10–29% reduction in mortality. The authors estimated even lower mortality rates if pregnant women were vaccinated [Citation75]. Another important question is whether a single dose of the vaccine could provide sufficient protection to prevent expansive or prolonged outbreaks. Namibia is considering a pilot implementation of HEV 239 for its population during their ongoing outbreak [Citation76], which may help to answer questions about the vaccine’s effectiveness for outbreak management and prevention.
There is some preliminary evidence of efficacy against other genotypes and with shorter dosing schedules, as well as evidence of safety in pregnant women. In the phase III HEV 239 trial, 37 pregnant women inadvertently received one or more doses of the vaccine, and 33 pregnant women received a placebo. No adverse effects were noted in the women who received the vaccine or their fetuses [Citation9], though it remains unclear if maternal vaccination is protective for the infant. The same trial demonstrated that two vaccine doses provided 5 months of protection, and there was also some evidence for protection against HEV1, which is not surprising as HEV 239 is a genotype 1 recombinant vaccine, although the Phase 3 efficacy study was conducted in a community with circulating HEV4 strains. A subsequent study using a monoclonal competition assay found broadly cross-neutralizing antibodies in serum from patients who had received the HEV 239 vaccine [Citation77,Citation78].
The current commercial presentation of Hecolin is as a single-dose prefilled syringe, without a vaccine vial monitor. This presentation would not generally be considered eligible for WHO prequalification. Programmatic suitable vaccines generally are supplied in a vial presentation (single-dose or multidose with preservative), with stability data generated to support an appropriate vaccine vial monitor and controlled temperature chain guidance.
A second vaccine was under development by GlaxoSmithKline (GSK), but clinical evaluation and subsequent commercial-scale manufacture were discontinued due to lack of public sector funding, insufficient interest from public health ministries in the affected regions, and concerns about market viability for travelers. The GSK vaccine did demonstrate 95.5% efficacy against clinical HEV infection in a phase II trial in 2000 soldiers in Nepal, a highly endemic region where HEV1 is the predominant strain [Citation77].
2.6. Nonvaccine prevention methods
Outside of China, hepatitis E prevention is dependent on costly infrastructural and systematic improvements to water, sanitation, and hygiene. Rates of the disease are significantly lower in populations with adequate nutrition and highly functioning WaSH infrastructure, but this is difficult to sustain in low-resource settings [Citation26] and such interventions have not been effective to date in many HEV-endemic regions including camps for displaced persons. Unfortunately, water, sanitation, and hygiene infrastructure costs are often too expensive to implement in the near term in many of the poor resource settings.
HEV differs from other enteric viruses such as Hepatitis A in that inhabitants of endemic regions do not acquire naturally-induced lifelong protection after their first exposure in childhood. Outbreaks recur in regions where previous epidemics would have been expected to result in sufficient population immunity to prevent further infection. Populations in endemic regions such as South Asia experience large epidemics of hepatitis E almost every year with most cases occurring in adults [Citation26]. There are conflicting reports about antibody persistence after infection, and cases of sero-reversion have also been documented [Citation79]. A study of 100 previously-infected individuals in Bangladesh found that 20% of the subjects had no detectable anti-HEV antibodies 10 years after a documented infection [Citation26]. Unfortunately, there is no data to indicate whether another infection (or vaccine dose) would result in an anamnestic response offering protection against clinical disease.
Other researchers used mixed-effect models to evaluate the seropositivity of subjects in a phase III vaccine trial and predicted a 50% negative seroconversion rate 14.5 years after naturally acquired anti-HEV immunity [Citation80]. The same study predicted that 82–99% of the vaccine recipients would retain anti-HEV IgG for 30 years after vaccination. Current understanding of long-term immunity to HEV calls into question the validity of a passive approach in which populations rely on herd immunity for protection against HEV reinfection. The research to date indicates that vaccination programs in the short-term with WaSH and nutritional improvements over the long-term are key to limiting the continuing morbidity and mortality of hepatitis E.
3. Advancing progress: goals and recommended strategies
The global health community has taken positive steps to combat viral hepatitis in recent decades. WHO joined with the World Hepatitis Alliance in 2012 [Citation81], and in 2015 hepatitis was added to the United Nations Sustainable Development Goals as a focus area of Target 3.3, ‘Good Health and Well-being.’ [Citation82] In 2016, the World Health Assembly adopted the Global Health Sector Strategy (GHSS) to eliminate viral hepatitis by 2030 [Citation83]. The GHSS has led some HEV endemic countries, such as India and Bangladesh, to launch programs to control viral hepatitis at a national level. But more progress is needed to reach the GHSS goal of reducing annual hepatitis deaths from 1.4 million to 500,000 by 2030. Specifically, more funding is needed to help countries with implementation, as only a few have managed to sustain hepatitis treatment and prevention programs [Citation84,Citation85].
In addition, current global efforts focus primarily on hepatitis B and C given the considerable burden attributed to chronic infections [Citation86], but it is also important to address acute hepatitis -including hepatitis E – as it is an under-recognized contributor to the global burden of viral hepatitis. Hepatitis E requires special attention due to its unique epidemiologic patterns, lack of treatment options, impact in low-resource and displaced communities, and disproportionate disease burden in pregnant women [Citation87]. Of note, an initiative to include hepatitis E with other viral hepatitis awareness programs is already evident in Bangladesh; however, so far such activities appear largely driven by independent private-sector entities, such as insurance and telecare companies [Citation88].
3.1. Advancing the vaccine
As discussed above, the WHO position paper on hepatitis E refrained from recommending routine use of the HEV 239 vaccine, and the WHO has not yet prequalified the vaccine (see ). Prequalification enables UN procurement agencies, such as Gavi, The Vaccine Alliance to purchase the vaccine [Citation77]. In 2018, the WHO published technical standards for vaccine production, an important step in the prequalification process [Citation25]. An ongoing HEV 239 vaccine efficacy trial in 19,460 women of childbearing age in Bangladesh (NCT02759991) may produce needed efficacy data about protection against HEV1 as well as safety data in this population, with results expected in late 2020. This phase IV cluster randomized clinical trial (2017–ongoing) with the HEV 239 vaccine has commenced to provide more data on the effectiveness of the vaccine on genotype 1 and the outcome in pregnant women, and on the safety of the vaccine is ongoing in a rural area of Bangladesh [Citation89]. Genotype 1 is the predominant type circulating in the study location, the study protocol recruits women aged 16–39 years, which will provide an evaluation of the effectiveness and immunogenicity of the vaccine among women of child bearing age who become pregnant following vaccination. Importantly the study plans to collect data on women who become pregnant following vaccination, including pregnancy outcome, complications during delivery, and health status of mother and child. This data will be analyzed together with records of eventual HEV disease and type, time, and number of vaccine doses.
Table 3. Data requested by WHO for prequalification [Citation74]
3.2. Considerations for outbreak settings
In addition to such large-scale vaccine trials, targeted vaccine use in outbreaks and endemic settings would also generate needed efficacy data. A few governments have already expressed interest in the potential introduction of hepatitis E vaccination in their public sector programs. However, considering the time and resources required to develop an optimal use case for routine vaccination, the most expedient solution is to enable use in outbreak settings. Organizations such as MSF are willing to fund and deliver the vaccine in outbreaks, as demonstrated by MSF’s attempts to organize vaccine studies during recent outbreaks in Niger, Chad, and Namibia [Citation38,Citation40,Citation54]. To date, the Ministries of Health in affected areas have not been comfortable with vaccine implementation absent a clear WHO recommendation, although the potential pilot program in Namibia could mean that this is starting to change [Citation76].
3.3. Burden understanding and education
More data about HEV disease burden are needed to estimate the amount of vaccine needed for use in outbreaks as well as for routine use. Generation of new evidence can serve to fill in the gaps in our current understanding of the extent of disease burden from hepatitis E, such as where disease concentrates, which populations are at greatest risk, and the degree to which HEV infection contributes to maternal and neonatal deaths and the incidence of acute viral hepatitis. Disease burden data can also help to build the case that the disease is persistently underfunded and under-recognized, as well as help to mobilize the global community, including essential policymakers such as WHO, to act.
3.4. Future considerations
One challenge for vaccine use in endemic settings is the vaccination delivery schedule, of three doses spaced over 7 months, and which population group to target for immunization. Access to routine vaccinations is a significant barrier in these regions, particularly if it is not included in the routine childhood immunization schedule. As noted above and pending data on the potential waning immunity of natural infection or immunization, administering the vaccine as a routine childhood vaccine seems implausible. Similarly, maternal immunization is impractical given the 7-month spacing between the full course of three doses of vaccine. Potential immunization strategies are shown in , including combination vaccines which would require additional development, demanding extra resources and time without a clear market demand. The most attractive option might be the coadministration of the HEV vaccine with HPV to young adolescent girls before they enter their child-bearing years. This would also cater to any suspected waning immunity over the long term.
Table 4. Four potential routine use strategies for hepatitis E vaccine
Our current understanding supports the exploration of an HPV-HEV coadministration vaccine strategy for initial routine use. Additional information is needed such as efficacy and duration of protection afforded by fewer than three doses (to match future HPV delivery schedule), demonstrated efficacy and safety in children less than 16 years of age, and immunogenicity of coadministration with the HPV vaccine. Evidence that transmission in young children is common despite low reported seroprevalence rates [Citation94] could improve the relative attractiveness of an HPV-HEV combination.
Without a shift in global consideration of HEV, outcomes for affected populations are unlikely to improve. Waiting for water, sanitation, and hygiene interventions to be sufficiently effective in HEV-endemic areas are not an option given the significant amount of time and resources required. Even if improvements in water and sanitation ultimately become universal, it is almost certain that another humanitarian crisis or natural disaster will trigger an outbreak by disrupting safe water supplies. Azman et al. points out that because HEV1 and HEV2 do not have animal reservoirs, they are candidates for elimination through public health efforts, one of the criteria for designation as a neglected tropical disease [Citation6], but the elimination of HEV morbidity and mortality will not be possible without the use of a vaccine to protect high-risk populations.
4. Expert opinion
The following recommendations are intended to guide the global health community towards breaking the inertia created by the lack of data on the vaccine and on disease burden preventing necessary actions, such as increased research funding and targeted vaccine use. This would generate important information about HEV as well as improve health outcomes for vulnerable populations.
WHO immunization policies are dependent on vaccine use data obtained from deploying the vaccine in outbreak settings and endemic areas. Efforts should be made to convince the Ministries of Health in affected countries to welcome a vaccine response when an outbreak occurs, even before the HEV 239 vaccine obtains WHO prequalification or a policy recommendation. Targeted trials in endemic areas are needed to demonstrate sufficient effectiveness with reduced or expedited dosing schedules, to establish immunogenicity and safety in pregnant women and immunocompromised patients, and to assess the duration of protection. Researchers should align with the WHO on satisfactory endpoints for prequalification considerations. Vaccine trials and use of the vaccine in outbreak response efforts should target the populations with the greatest need while prioritizing the research questions most relevant to those communities.
A growing body of documented evidence could inform more specific global policy recommendations and help convince the Ministries of Health in affected countries to welcome a vaccination approach when an outbreak occurs, as well as provide evidence to support WHO prequalification. Any use of the vaccine, such as in an outbreak, should be carefully documented to help build an evidence base.
Better understanding and improved awareness of the global HEV burden are essential, including accurate estimates of clinical disease and contribution to hepatitis morbidity/mortality worldwide. More information about disease burden could energize relevant global decision-makers into action, particularly with respect to the high mortality observed in pregnant women and fetal outcomes. Improved surveillance is needed in displaced person camps, where early detection and response can minimize the impact of outbreaks in such settings. Better surveillance can also help identify the highest-risk populations for symptomatic disease in endemic settings.
Experts and organizations should work together to improve standardized diagnostic and epidemiologic tools for HEV detection. Rapid, reliable noninvasive tests, improved assays, and repositories of pooled sera, as well as the ability to test environmental samples, are all needed. Application of the most accurate assays to diagnose acute HEV infections will produce more reliable prevalence estimates from public health surveillance and epidemiological studies and enable more rapid outbreak detection.
Routine vaccination should be implemented in endemic settings in order to prevent outbreaks. It will be necessary to improve delivery strategies for more widespread use, including incorporating the HEV vaccine into feasible vaccination schedules and protecting young women in particular before they reach childbearing age. Efforts to sustainably prevent morbidity and mortality associated with hepatitis E disease by improving sanitation and water safety conditions should continue even when vaccination becomes available.
Researchers, funding organizations, and relief organizations should continue to work to increase investment towards the understanding and prevention of HEV. These goals could be integrated into the existing World Hepatitis Alliance goal of ending viral hepatitis by 2030.
Article highlights
Hepatitis E virus (HEV) is an important cause of enterically transmitted viral hepatitis and a significant contributor to global maternal mortality.
Estimated to be responsible for 3.3 million clinical cases, up to 70,000 deaths, and 3000 stillbirths, which represents 3.3% of all deaths from viral hepatitis worldwide.
Accurate estimates of disease burden, in both endemic and non-endemic regions remain challenging, due to the lack of good diagnostic tools for the serologic and/or nucleic acid-based diagnosis of hepatitis E infection. Currently, no FDA- or WHO-approved assay is available for the detection of an acute or previous infection.
Responsible for endemic disease as well as major disease outbreaks in low-income settings in Asia and Africa.
A vaccine exists - HEV 239 (Hecolin, Xiamen Innovax Biotech, China), which has been shown to provide vaccine efficacy of 87%, with protection up to 4.5 years identified in Phase III studies in China.
The vaccine is only licensed and available in the private market in China and has yet to be prequalified by the WHO for use in endemic settings and outbreaks.
Alternative HEV prevention strategies are dependent on costly infrastructural and systematic improvements to water, sanitation, and hygiene, thus vaccination is the best approach to reduce disease burden.
Studies to support WHO prequalification or a policy recommendation should be prioritized, including vaccine use in a population with the greatest need such as in outbreak and endemic settings.
Improved understanding of the global burden and improvements in diagnostic and epidemiologic tools are urgently needed.
Author contributions
CDK and ADS conceived and initiated the study. The manuscript was drafted by KRD, and ADS and CDK performed manuscript review. All authors approved the manuscript for submission.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Disclaimer
The finding and conclusions in this report are those of the authors and do not necessarily represent the official position of the Bill & Melinda Gates Foundation.
Acknowledgments
The authors thank the numerous experts who provided thoughtful comments on the scientific aspects of HEV, which were extremely helpful in framing the direction for this review.
The authors would like to acknowledge and thank Mrs Marian Blazes for the critical editorial support and for careful review of the manuscript.
Additional information
Funding
References
- Nelson KE, Labrique AB, Kmush BL. Epidemiology of genotype 1 and 2 Hepatitis E virus infections. Cold Spring Harb Perspect Med. 2019;9(6):a031732
- Rein DB, Stevens GA, Theaker J, et al. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55(4):988–997
- World Health Organization. Hepatitis E. cited 2019. [cited 2020 Jul 27]. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e
- IHME Hepatitis Facts: Institute for Health Metrics and Evaluation; [ cited 2019]. Available from:(http://hepatitis.ihme.services/factsheets)
- Labrique AB, Sikder SS, Krain LJ, et al. Hepatitis E, a vaccine - preventable cause of maternal deaths. Emerg Infect Dis. 2012;18(9):1401–1404. .
- Azman AS, Ciglenecki I, Wamala JF, et al., Hepatitis E should be considered a neglected tropical disease. PLoS Negl Trop Dis. 13(7): e0007453. 2019. .
- Webb GW, Dalton HR. Hepatitis E: an underestimated emerging threat. Ther Adv Infect Dis. 2019;6:1–18.
- Capai L, Charrel R, Falchi A. Hepatitis E in high-income countries: what do we know? And what are the knowledge gaps Viruses. 2018;10(6):285.
- Zhu FC, Zhang J, Zhang XF, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376(9744):895–902. .
- Lemon SM, Walker CM. Enterically transmitted non-A, non-B hepatitis and the discovery of Hepatitis E virus. Cold Spring Harb Perspect Med. 2019;9(8):a033449.
- Himmelsbach K, Bender D, Hildt E. Life cycle and morphogenesis of the hepatitis E virus. Emerg Microbes Infect. 2018;7(1):196.
- Lu L, Li C, Hagedorn CH. Phylogenetic analysis of global hepatitis E virus sequences: genetic diversity, subtypes and zoonosis. Rev Med Virol. 2006;16(1):5–36.
- Forni D, Cagliani R, Clerici M, et al. Origin and dispersal of Hepatitis E virus. Emerg Microbes Infect. 2018;7(1):11.
- Nelson KE, Labrique AB, Kmush BL. Epidemiology of Genotype 1 and 2 Hepatitis E Virus Infections Cold Spring Harb Perspect Med. 2019;9(6):a031732.
- Tripathy AS, Sharma M, Deoshatwar AR, et al. Study of a hepatitis E virus outbreak involving drinking water and sewage contamination in Shimla, India, 2015-2016. Trans R Soc Trop Med Hyg. 2019;113(12):789–796.
- Sharma S, Kumar A, Kar P, et al. Risk factors for vertical transmission of hepatitis E virus infection. J Viral Hepat. 2017;24(11):1067–1075. .
- Denner J. Hepatitis E virus (HEV)-The Future. Viruses. 2019;11(3):251.
- Seth A, Sherman KE. Hepatitis E: what We Think We Know. Clin Liver Dis. 2020;15(S1):S37–4419.
- Wu C, Wu X, Xia J. Hepatitis E virus infection during pregnancy. Virol J. 2020;17(1):73.
- Kamar N, Dalton HR, Abravanel F, et al. Hepatitis E virus infection. Clin Microbiol Rev. 2014;27(1):116–138.
- Krain LJ, Nelson KE, Labrique AB. Host immune status and response to Hepatitis E virus infection. Clin Microbiol Rev. 2014;27(1):139–165.
- Kmush BL, Yu H, Huang S, et al. Long-term antibody persistence after Hepatitis E virus infection and vaccination in Dongtai, China. Open Forum Infect Dis. 2019;6(4):ofz144. .
- Expert Committee on Biological Standardization WHO. Human challenge trials for vaccine development: regulatory considerations. 2016.
- Al-Sadeq DW, Majdalawieh AF, Mesleh AG, et al. Laboratory challenges in the diagnosis of hepatitis E virus. J Med Microbiol. 2018;67(4):466–480.
- STANDARDIZATION ECOB. Recommendations to assure the quality, safety and efficacy of 6 recombinant hepatitis E vaccines. World Health Organization, 2018.
- Kmush BL, Zaman K, Yunus M, et al. A ten year immunopersistence study of Hepatitis E antibodies in rural Bangladesh. Am J Epidemiol. 2018;187(7):1501–1510.
- Kmush BL, Labrique AB, Dalton HR, et al. Two generations of “Gold Standards”: the impact of a decade in Hepatitis E virus testing innovation on population seroprevalence. Am J Trop Med Hyg. 2015;93(4):714–717. .
- Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388(10049):1081–1088. .
- Wilhelm B, Waddell L, Greig J, et al. Systematic review and meta-analysis of the seroprevalence of hepatitis E virus in the general population across non-endemic countries. PLoS One. 2019;14(6):e0216826.
- Khudyakov Y, Kamili S. Serological diagnostics of hepatitis E virus infection. Virus Res. 2011;161(1):84–92.
- Hyams C, Mabayoje DA, Copping R, et al. Serological cross reactivity to CMV and EBV causes problems in the diagnosis of acute hepatitis E virus infection. J Med Virol. 2014;86(3):478–483. .
- Kmush BL, Labrique AB, Dalton HR, et al. Two Generations of “Gold Standards”: the Impact of a Decade in Hepatitis E Virus Testing Innovation on Population Seroprevalence. Am J Trop Med Hyg. 2015;93(4):714–717. .
- Vollmer T, Knabbe C, Dreier J. Comparison of Real-Time PCR and antigen assays for detection of hepatitis E virus in blood donors. J Clin Microbiol. 2014;52(6):2150–2156.
- Baylis SA, Blumel J, Mizusawa S, et al. World Health Organization International Standard to harmonize assays for detection of hepatitis E virus RNA. Emerg Infect Dis. 2013;19(5):729–735. .
- Izopet J, Labrique AB, Basnyat B, et al. Hepatitis E virus seroprevalence in three hyperendemic areas: nepal, Bangladesh and southwest France. J Clin Virol. 2015;70:39–42.
- Wu X, Chen P, Lin H, et al. Hepatitis E virus: current epidemiology and vaccine. Hum Vaccin Immunother. 2016;12(10):2603–2610.
- Carratala A, Joost S. Population density and water balance influence the global occurrence of hepatitis E epidemics. Sci Rep. 2019;9(1):10042.
- Spina A, Lenglet A, Beversluis D, et al. A large outbreak of Hepatitis E virus genotype 1 infection in an urban setting in Chad likely linked to household level transmission factors, 2016-2017. PLoS One. 2017;12(11):e0188240. .
- Today ON. Hepatitis E: central African Republic outbreak continues, dozens more cases reported 2018 cited. Available from:http://outbreaknewstoday.com.
- Namibia: Hepatitis E Leading Cause of Mortality Among Pregnant Women: @allafrica; 2019 . https://allafrica.com/stories/201911271003.html.
- Ray K. Hepatitis E outbreak: India among the global hotspots Decan Herald2019. Available From https://www.deccanherald.com/national/hepatitis-e-outbreak-india-among-the-global-hotspots-746742.html.
- Teshale EH, Hu DJ. Hepatitis E: epidemiology and prevention. World J Hepatol. 2011;3(12):285–291.
- Hakim MS, Wang W, Bramer WM, et al. The global burden of hepatitis E outbreaks: a systematic review. Liver Int. 2017;37(1):19–31. .
- Number of hepatitis E infections in Namibia nears 7,000 2020 [ updated 2020 Jan 7]. http://www.xinhuanet.com/english/2020-01/07/c_138685904.htm.
- Dagnew M, Belachew A, Tiruneh M, et al. Hepatitis E virus infection among pregnant women in Africa: systematic review and meta-analysis. BMC Infect Dis. 2019;19(1):519.
- Hassan-Kadle MA, Osman MS, Ogurtsov PP. Epidemiology of viral hepatitis in Somalia: systematic review and meta- analysis study. World J Gastroenterol. 2018;24(34):3927–3957.
- Boon D, Redd AD, Laeyendecker O, et al. Hepatitis E Virus Seroprevalence and Correlates of Anti-HEV IgG Antibodies in the Rakai District, Uganda. J Infect Dis. 2018;217(5):785–789.
- Hasan G, Assiri A, Marzuuk N, et al. Incidence and characteristics of hepatitis E virus infection in children in Assiut, Upper Egypt. J Int Med Res. 2016;44(5):1115–1122.
- Capai L, Falchi A, Charrel R. Meta-Analysis of Human IgG anti-HEV Seroprevalence in Industrialized Countries and a Review of Literature. Viruses. 2019;11(1):84.
- Kamar N, Izopet J, Pavio N, et al. Hepatitis E virus infection. Nat Rev Dis Primers. 2017;3:17086.
- Rivero-Juarez A, Lopez-Lopez P, Frias M, et al. Hepatitis E Infection in HIV-Infected Patients. Front Microbiol. 2019;10:1425.
- Patra S, Kumar A, Trivedi SS, et al. Maternal and fetal outcomes in pregnant women with acute hepatitis E virus infection. Ann Intern Med. 2007;147(1):28–33.
- Berglov A, Hallager S, Weis N. Hepatitis E during pregnancy: maternal and foetal case-fatality rates and adverse outcomes-A systematic review. J Viral Hepat. 2019;26(11):1240–1248.
- Lagare A, Ibrahim A, Ousmane S, et al. Outbreak of Hepatitis E virus infection in displaced persons camps in diffa region, niger, 2017. Am J Trop Med Hyg. 2018;99(4):1055–1057.
- Melgaco JG, Gardinali NR, de Mello VDM, et al. Hepatitis E: update on Prevention and Control. Biomed Res Int. 2018;2018:5769201.
- Kmush BL, Labrique A, Li W, et al. The Association of Cytokines and Micronutrients with Hepatitis E Virus Infection During Pregnancy and the Postpartum Period in Rural Bangladesh. Am J Trop Med Hyg. 2016;94(1):203–211.
- Krain LJ, Atwell JE, Nelson KE, et al. Fetal and Neonatal Health Consequences of Vertically Transmitted Hepatitis E Virus Infection. Am J Trop Med Hyg. 2014;90(2):365–370.
- Horvatits T, Wiesch JS, Lütgehetmann M, et al. The Clinical Perspective on Hepatitis E. Viruses. 2019;11(7):617.
- Ronsmans C, Chowdhury ME, Dasgupta SK, et al. Effect of parent’s death on child survival in rural Bangladesh: a cohort study. Lancet. 2010;375(9730):2024–2031.
- Beegle K, De Weerdt J, Dercon S. The intergenerational impact of the African orphans crisis: a cohort study from an HIV/AIDS affected area. IntJEpidemiol. 2009;38(2):561–568.
- Niger: Hepatitis E Outbreak - Apr 2017. @reliefweb 2019.
- Namibia defends capital’s water safety amid Hepatitis E outbreak 2020 [ updated 2018 Feb 8]. http://www.xinhuanet.com/english/2018-02/08/c_136957174.htm.
- Langlois EV, Haines A, Tomson G, et al. Refugees: towards better access to health-care services. Lancet. 2016;387(10016):319–321.
- Shrestha A, Gupta BP, Lama TK. Current Treatment of Acute and Chronic Hepatitis E Virus Infection: role of Antivirals. Euroasian J Hepatogastroenterol. 2017;7(1):73–77.
- Netzler NE, Enosi Tuipulotu D, Vasudevan SG, et al. Antiviral candidates for treating hepatitis E Virus Infection. Antimicrob Agents Chemother. 2019;63(6):e00003.
- Lemoine M, Nayagam S, Thursz M. Viral hepatitis in resource-limited countries and access to antiviral therapies: current and future challenges. Future Virol. 2013;8(4):371–380.
- Fitzsimons D, Hendrickx G, Hallauer J, et al. Innovative sources for funding of viral hepatitis prevention and treatment in low- and middle-income countries: a roundtable meeting report. Hepatol, Med Policy. 2016;1(1):1–10.
- Spina A, Beversluis D, Irwin A, et al. Learning from water treatment and hygiene interventions in response to a hepatitis E outbreak in an open setting in Chad. J Water Health. 2018;16(2):223–232.
- Guthmann JP, Klovstad H, Boccia D, et al. A large outbreak of hepatitis E among a displaced population in Darfur, Sudan, 2004: the role of water treatment methods. Clin Infect Dis. 2006;42(12):1685–1691.
- Li SW, Zhao Q, Wu T, et al. The development of a recombinant hepatitis E vaccine HEV 239. Hum Vaccin Immunother. 2015;11(4):908–914.
- Zhang J, Zhang XF, Huang SJ, et al., Long-term efficacy of a hepatitis E vaccine. N Engl J Med. 372(10): 914–922. 2015. .
- Nelson KE, Shih JW, Zhang J, et al. Hepatitis e vaccine to prevent morbidity and mortality during epidemics. Open Forum Infect Dis. 2014;1(3):ofu098.
- Safety profile of a recombinant hepatitis E vaccine: Extract from report of GACVS meeting of 2014 Jun 11-12. World Health Organization, 2015; 12:18:13.
- WHO. Hepatitis E vaccine: WHO position paper, May 2015. Wkly Epidemiol Rec. 2015;90(18):185–200.
- Cooper BS, White LJ, Siddiqui R. Reactive and pre-emptive vaccination strategies to control hepatitis E infection in emergency and refugee settings: A modelling study. PLoS Negl Trop Dis. 2018;12(9):e0006807.
- Namibia to acquire Hepatitis E vaccine from China, 2020. [February 2, 2020]. Available from:http://www.xinhuanet.com/english/2020-02/03/c_138752998.htm.
- Innis BL, Lynch JA. Immunization against Hepatitis E. Cold Spring Harb Perspect Med. 2018;8(11):a032573.
- Wu X, Chen P, Lin H, et al. Dynamics of 8G12 competitive antibody in “prime-boost” vaccination of Hepatitis E vaccine. Hum Vaccin Immunother. 2017;13(6):1–6.
- Faber M, Willrich N, Schemmerer M, et al. Hepatitis E virus seroprevalence, seroincidence and seroreversion in the German adult population. J Viral Hepat. 2018;25(6):752–758.
- Su YY, Huang SJ, Guo M, et al. Persistence of antibodies acquired by natural hepatitis E virus infection and effects of vaccination. Clin Microbiol Infect. 2017;23(5):336.e1-.e4.
- World Hepatitis Alliance. Our Story: Milestones on our road to elimination 2019 https://www.worldhepatitisalliance.org/about/our-story.
- World Hepatitis Alliance. Sustainable Development Goals (SDGs) 2019. https://www.worldhepatitisalliance.org/sustainable-development-goals-sdgs.
- World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. 2016.
- Waheed Y, Siddiq M, Jamil Z, et al. Hepatitis elimination by 2030: progress and challenges. World J Gastroenterol. 2018;24(44):4959–4961.
- Gore C, Hicks J, Deelder W. Funding the elimination of viral hepatitis: donors needed. The Lancet Gastroenterol Hepatol. 2017;2(12):843–845.
- World Health Organization. Combating Hepatitis b and C to reach elimination by 2030. 2016.
- Frontieres MS The end of viral hepatitis by 2030 is impossible without tackling hepatitis E 2016 cited Available from: https://www.msf.ch/nos-actualites/articles/end-viral-hepatitis-2030-impossible-without-tackling-hepatitis-e.
- @dailystarnews. WHD 2018: @dailystarnews; 2018 [updated 2018-08-05]. https://www.thedailystar.net/health/whd-2018-1615894.
- Zaman K, Dudman S, Stene-Johansen K, et al. HEV study protocol: design of a cluster-randomised, blinded trial to assess the safety, immunogenicity and effectiveness of the hepatitis E vaccine HEV 239 (Hecolin) in women of childbearing age in rural Bangladesh. BMJ Open. 2020;10(1):e033702.
- Zhang J, Liu CB, Li RC, et al. Randomized-controlled phase II clinical trial of a bacterially expressed recombinant hepatitis E vaccine. Vaccine. 2009;27(12):1869–1874.
- Purcell RH, Nguyen H, Shapiro M, et al. Pre-clinical immunogenicity and efficacy trial of a recombinant hepatitis E vaccine. Vaccine. 2003;21(19–20):2607–2615.
- Chen Z, Lin S, Duan J, et al. Immunogenicity and safety of an accelerated hepatitis E vaccination schedule in healthy adults: a randomized, controlled, open-label, phase IV trial. Clin Microbiol Infect. 2019;25(9):1133–1139.
- Zhang J, Zhang XF, Zhou C, et al. Protection against hepatitis E virus infection by naturally acquired and vaccine-induced immunity. Clin Microbiol Infect. 2014;20(6):O397–405.
- Verghese VP, Robinson JL. A systematic review of hepatitis E virus infection in children. Clin Infect Dis. 2014;59(5):689–697.