ABSTRACT
Introduction
Immunosenescence is a normal biologic process involving deterioration of protective immune responses. Consequently, older adults experience increased risk of infectious diseases, particularly pneumonia, and its leading bacterial cause, Streptococcus pneumoniae. Pneumococcal vaccine recommendations are often limited to adults with specific medical conditions despite similar disease risks among older adults due to immunosenescence.
Areas covered
This article reviews epidemiologic, biologic, and clinical evidence supporting the consideration of older age due to immunosenescence as an immunocompromising condition for the purpose of pneumococcal vaccine policy and the role vaccination can play in healthy aging.
Expert opinion
Epidemiologic and biologic evidence suggest that pneumococcal disease risk increases with age and is comparable for healthy older adults and younger adults with immunocompromising conditions. Because immunocompromising conditions are already indicated for pneumococcal conjugate vaccines (PCVs), a comprehensive public health strategy would also recognize immunosenescence. Moreover, older persons should be vaccinated before reaching the highest risk ages, consistent with the approach for other immunocompromising conditions. To facilitate PCV use among older adults, vaccine technical committees (VTCs) could classify older age as an immunocompromising condition based on the process of immunosenescence. With global aging, VTCs will need to consider immunosenescence and vaccine use during healthy aging.
1. Introduction
Over the past half century, advances in public health and individual health care, development of antimicrobial agents, and improved vaccines have resulted in dramatic increases in life expectancy around the world. The global population of people ≥65 years of age is projected to more than double, from 703 million in 2019 to 1.5 billion in 2050 [Citation1]. There will be one in six of the world’s population ≥65 years of age by 2050, including one in four people living in Europe and North America [Citation2]. Globally, it is projected that the number of adults ≥80 years of age will triple from 143 million in 2019 to 426 million in 2050 [Citation1].
Age-related deterioration of the immune system, termed immunosenescence [Citation3], occurs as a normal part of healthy aging. There is not a defined, universal chronological age at which the deterioration starts to occur, as this process is multi-faceted and happens over a long time span in a highly heterogeneous group experiencing many health states. In older adults, immunosenescence is directly related to the high morbidity and mortality associated with infectious diseases, such as lower respiratory tract infections, including pneumonia. Among pneumonia pathogens, Streptococcus pneumoniae (pneumococcus) is recognized as a major cause [Citation4].
Pneumococcal polysaccharide (PPSV) and conjugate (PCV) vaccines are licensed and recommended for the prevention of bacteremic and bacteremic/non-bacteremic pneumonia, respectively. Of these, non-bacteremic pneumonia is the most common presentation. The recommendations for pneumococcal vaccine use in adults are highly diverse across and within countries (e.g. age vs. risk-based, PPSV + PCV vs. PPSV only vs. PCV only) and within countries in terms of the age groups, comorbid medical conditions included in risk category groupings, and risk categories (e.g. healthy, at-risk based on the presence of a comorbid medical conditions, and high-risk based on the presence of an immunocompromising condition).
Risk-based categories are created to group together conditions based on pneumococcal disease risk, and for purposes of vaccination policy to then prioritize pneumococcal vaccination of those at highest risk. In some countries, age-based pneumococcal vaccination recommendations exist for all persons older than a certain cutoff such as 60 or 65 years. However, there can be a difference in recommendations for persons who are high-risk at any adult age – typically age ≥18 years and who usually receive the highest priority – and older adults. The separation of older adults into a distinct category may occur because older persons are not recognized as being immunocompromised (with respect to pneumococcal disease risk) due to immunosenescence or alternatively, because of lack of awareness that age may confer a pneumococcal disease risk similar to that of some high-risk persons who are already targeted for pneumococcal vaccination.
In this context, the purpose of this review is to provide a rationale and framework for including older age – based on the process of immunosenescence – as an immunocompromising condition for the purposes of pneumococcal vaccine recommendations. In principle, immunosenescence itself could be included as an immunocompromising condition. However, in practice no marker exists currently to identify persons that have reached specific levels of immunosenescence, and consequently we propose that older age be used as a surrogate. In support of this categorization, we will review the biologic evidence pertaining to pneumococcal disease risk in older adults. We then will review the epidemiologic evidence supporting that older age may confer pneumococcal disease risk similar to that of immunocompromising conditions already considered as high-risk for pneumococcal disease. We acknowledge that the epidemiologic evidence presented herein derives from countries that may have a pneumococcal vaccination policy already in place for older adults. However, none have adopted the framework we propose here. Acceptance and application of this framework would demonstrate a consistent approach to pneumococcal vaccine recommendations across the lifespan based on the available evidence for risk of pneumococcal disease. This would thereby facilitate decision-making by national vaccine technical committees (VTCs) and clinicians regardless of the adult pneumococcal vaccination policy that is selected for a particular country.
Classifying older age based on immunosenescence as a high-risk immunocompromising condition for the purposes of adult pneumococcal vaccination policy will facilitate decision-making regardless of whether a country has a robust pediatric national immunization program (NIP) that includes PCV or recommends and funds 23-valent PPSV (PPSV23) for older persons. This is because while pediatric PCV use reduces vaccine serotype disease through indirect protection of unvaccinated age cohorts, and PPSV23 may reduce some vaccine serotype IPD, substantial residual vaccine serotype disease remains among older adults [Citation5–7]. Using 13-valent PCV (PCV13) immunization of older adults as a probe has revealed a large, under recognized burden of vaccine preventable clinical pneumonia [Citation8–11] despite pediatric PCV use and adult PPSV23 use. This residual disease potentially can be addressed by direct immunization of older adults with PCVs, which have demonstrated efficacy and effectiveness in this population against vaccine serotype IPD and pneumonia – including that due to serotype 3 – as well as all-cause or clinical pneumonia (Supplemental Text [Citation5,Citation7–27]). In our arguments regarding immunosenescence, we are not advocating for any particular adult vaccination policy including use of a specific pneumococcal vaccine. Decisions pertaining to the choice of pneumococcal vaccine will be dependent upon numerous local factors that will be weighed by VTCs.
2. Older adult pneumococcal vaccination policy
As will be demonstrated, older adults as a group have an increased risk of pneumococcal disease compared with younger adults as a group. However, these data by themselves do not have direct implications for vaccination policy among older adults because adult pneumococcal vaccine policy is commonly stratified by risk profiles. Specifically, adults are considered to be ‘healthy,’ ‘at-risk,’ or ‘high-risk,’ based on data showing the degree to which underlying or associated conditions increase the risk of pneumococcal disease [Citation28–32]. The specific conditions included in these risk profiles differ by country based on recommendations made by VTCs (). In general, persons classified and hereafter referred to as ‘healthy’ are those who do not have a risk condition included by VTCs in vaccine recommendations. Comorbid medical conditions classified and hereafter referred to as ‘at-risk’ are those that place an individual at some but not substantial increase in risk of pneumococcal disease and may include diseases of the heart, lungs or liver; diabetes mellitus; and alcoholism. Immunocompromising conditions classified and hereafter referred to as ‘high-risk’ are those that substantially increase the risk of pneumococcal disease and may include human immunodeficiency virus (HIV) infection, asplenia, cochlear implant, cerebrospinal fluid leak, organ or bone marrow transplant, sickle cell disease, congenital or acquired immunodeficiencies, hematological cancers, and conditions requiring treatment with immunosuppressive therapy.
Table 1. Medical conditions identified for pneumococcal vaccine recommendations by select countries
Vaccine recommendations and reimbursement policies vary widely across countries. Globally, and in contrast to the relatively standardized recommendations for PCVs in pediatric NIPs, pneumococcal vaccine recommendations for adults are remarkably diverse, reflecting country-based differences in the disease epidemiology, healthcare systems, financing, jurisdictional responsibilities, and vaccine acceptability in the target population [Citation33]. For countries that have a pneumococcal vaccine policy for adults, this diversity is also reflected in the classification of adults into risk categories, the age at which recommendations begin (commonly at age 60 or 65 years), and public reimbursement programs (Supplemental Table 1).
Among countries that have a pneumococcal vaccine recommendation, many of which are high-income, age- and risk-based recommendations are made separately, perhaps based on historic precedence, convenience, or another unstated rationale and using a variety of conditions that are considered to result in an increased risk for pneumococcal disease. Recommendations and reimbursement for adult PCV13 use in most countries subsequently have emphasized vaccinating high-risk but not older adults (usually defined as over 60 or 65 years of age), despite both being at similar risk of pneumococcal disease due to the presence of immunocompromising conditions.
Some jurisdictions target pneumococcal vaccines to persons above an age threshold – such as 60 or 65 years – but as a separate category from at-risk or high-risk groups and without recognizing that older age is a high-risk condition because of the immunocompromising nature of immunosenescence. For example, the US from 2014 through 2019 recommended and funded routine use of PCV13 in high-risk adults of all ages and all adults ≥65 years of age. However, in 2019, the recommendation for older adults was changed to shared clinical decision-making, again creating a different recommendation for the two groups despite similar risk based on both being immunocompromised.
Placing older adults into a separate category from high-risk or immunocompromised may undermine efforts to improve coverage, vaccine acceptance by medical providers, and funding of recommendations since it implies that older adults are in some way at less risk than the high-risk categories and for different reasons. A more evidence-based approach for VTCs globally might be to recognize that older age as a consequence of immunosenescence is the most common high-risk, immunocompromising condition. A practical step toward this recognition would be to classify older age as a high-risk condition for the purposes of pneumococcal vaccine recommendations. The remainder of this manuscript will present the epidemiologic and biologic bases for this recommendation.
3. Pneumococcal disease data by risk and age groupings
3.1. Aging is associated with increased risk and severity of pneumococcal disease
The first issue to address is the degree to which age is associated with an increased risk and severity of pneumococcal disease. Consequently, in this section, we briefly review age-related differences in the risk of all-cause pneumonia, pneumococcal pneumonia (PP), and invasive pneumococcal disease (IPD) and the degree to which age increases adverse outcomes (such as intensive care unit [ICU] admission or mortality) among those with pneumococcal disease. Based on ample evidence from around the globe, it is well accepted that among adults the incidence of pneumonia increases with age and that older adults have the highest incidence. Depending upon the study period, methodology, laboratory detection tools, age groups used for comparison, the incidence of community acquired pneumonia (CAP) ranges from 10-fold higher in Japan to 50-fold higher in the US to >100-fold higher in Europe among older adults compared with younger adults [Citation34–36]. Because S. pneumoniae is among the most common etiologies of pneumonia, age-related trends in PP and IPD, the majority of IPD being bacteremic pneumonia, mirror those of all-cause pneumonia albeit at lower incidence and with wide variability between countries due to study methodology, clinical practice, country notification requirements, and country characteristics [Citation14,Citation16,Citation37–42].
The highest incidence among older adults correlates with the observed high incidence of severe pneumococcal disease and subsequent outcomes in this population. In Switzerland, older age (≥50 and ≥65 years) was associated with increased hospitalization for pneumonia and PP, longer length of stay, and higher mortality [Citation43]. In Canada, mortality rates for pneumococcal CAP (pCAP) increased with age from 3.8% (16–49 years), to 6.3% (50–64 years), and 12.0% (≥65 years) [Citation44]. In the same study, adults ≥65 years of age accounted for 67% of deaths attributed to pCAP, 43% of the ICU admissions, and 43% of patients requiring mechanical ventilation, while these outcome measures (in the same order) were 10%, 20%, and 20% in adults 16–49 years of age [Citation44]. Likewise, among IPD cases in the US in 2018, the mortality rate increased with age from 0.14/100,000 in the youngest adults 18-34 years to 2.3/100,000 among those 65–74 years and 8.4/100,000 among those ≥85 years of age [Citation41]. In the UK, the overall case fatality rate (CFR) of IPD was highest in adults ≥65 years of age [Citation32].
In the short term, patients with pneumonia have a higher risk of exacerbation from comorbid medical conditions such as cardiac events [Citation45–48], and these comorbid conditions are more common among older adults. Although mortality is typically measured during hospitalization or at 30 days as a short-term clinical endpoint, recent studies have found that mortality continues to rise in the long term after hospitalization with pneumonia. Among patients ≥65 years of age who survived an acute episode of pneumonia, 35.8% to 67% died within 5 years [Citation49,Citation50]. In two reviews, age has been identified as a risk factor associated with long-term mortality in patients with pneumonia [Citation51,Citation52].
The effects of immunosenescence on pneumococcal disease risk are compounded by age-associated increases in the prevalence of comorbid medical conditions. A prospective study of hospitalized CAP in the US reported that 44% of older adults had an at-risk condition and an additional 44% had a high-risk condition [Citation13]. Similarly, in older Dutch adults enrolled in the CAPiTA randomized controlled trial (RCT), the percent with a non-immunocompromising at-risk condition was 42% (persons with immunocompromising conditions were excluded from CAPiTA) [Citation23]. Prospective surveillance for hospitalized CAP in Canadian adults showed that about 70% of younger adults 16–49 years of age and about 95% of adults ≥50 years of age had at least one at-risk condition and that the percentage with an immunocompromising condition rose from around 20% in adults aged 16–49 years to around 33% in adults ≥50 years of age [Citation44].
3.2. The risk of pneumococcal disease is higher in older adults than in younger adults with immunocompromising conditions
In Section 3.1 we established that older adults in general are at increased risk of pneumococcal disease compared with younger adults and that many have transitioned to an at-risk or high-risk state. To argue that old age based on immunosenescence should be considered a high-risk category for pneumococcal disease and for designing adult pneumococcal vaccination policy, it also is necessary to show that the disease risk associated with older age is similar to that for conditions already placed in the high-risk category. This can be done most directly by comparing pneumonia and IPD incidence rates among older adults to incidence rates among younger adults with different individual high-risk conditions and who have yet to experience immunosenescence. The rationale is that if the risks are similar in these groups, a harmonized pneumococcal vaccination strategy would treat them the same based on comparable immunocompromised states with respect to pneumococcal disease. Consequently, in this section, we compare the risk of pneumonia, PP, and IPD among healthy and at-risk older adults to the risk of these diseases among younger persons with immunocompromising conditions.
A non-systematic review of PubMed identified publications from the year 2010 onward that reported incidence of all-cause pneumonia, PP, or IPD by risk status (healthy, at-risk, and high-risk) or for specific comorbid medical/immunocompromising conditions and by age strata (at a minimum for younger adults and older adults) among persons ≥16 years of age. Rate ratios and 95% confidence intervals were computed to compare disease incidence of healthy or at-risk older adults ≥65 years of age (or finer age strata of this group) to that of younger adults 18–49 or 19–64 years of age classified as high-risk or having specific immunocompromising conditions. The specific immunocompromising conditions included in the comparisons had the lowest incidence reported in each study, as this was the minimum threshold of risk associated with a pneumococcal vaccine recommendation. The five studies that were identified originated from the US (n = 3) [Citation28–30], Germany (n = 1) [Citation31], and the UK (n = 1) [Citation32]; reported disease incidence for the years of 2007 to 2015; and sourced data from healthcare claims repositories or from cases identified by routine IPD surveillance ().
Table 2. Changes in the adaptive immune system in HIV infection and in older age
The rate ratios of IPD, PP, and all-cause pneumonia in these five studies (Supplemental Table 2), for high-risk younger adults 18–49, 16–64, or 19–64 years of age versus healthy older adults ≥65 years of age, ranged from modestly below to modestly above 1. Specifically, for IPD, the rate ratios ranged from 0.3 to 0.7 for healthy older adults when compared with all high-risk younger adults and from 0.1 to 1.5 when compared with younger adults with the specific immunocompromising conditions of HIV infection, hematologic malignancy, and asplenia/splenic dysfunction (). For PP, the rate ratios ranged from 0.6 to 0.7 for healthy older adults ≥65 years and up to 1.1 for those ≥75 years when compared with all high-risk younger adults and from 0.4 to 0.7 when compared with younger adults with the specific immunocompromising conditions of cochlear implant or presence of immunosuppressive drugs or conditions (). For all-cause pneumonia, the rate ratios ranged from 0.6 to 1.2 for healthy older adults ≥65 years of age and up to 3.8 for those ≥85 years of age when compared with all high-risk younger adults. Similarly, rate ratios ranged from 0.6 to 1.3 for healthy older adults ≥65 years of age and up to 4.7 for those ≥85 years of age when compared with younger adults with the specific immunocompromising conditions of malignant neoplasms, functional/anatomical asplenia, or congenital immunodeficiency. The rate ratios increased in value, often to >1.0, for pneumonia when high-risk younger adults were compared with healthy older adults with increasing age ().
Figure 1. a-c: Comparison of disease incidence in healthy older adults vs high-risk younger adults
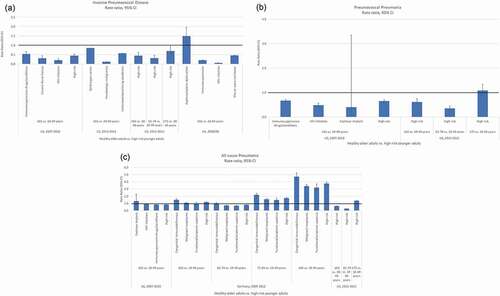
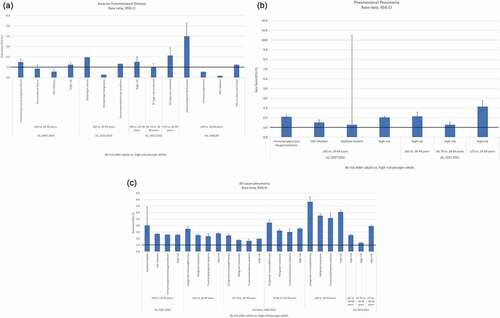
Most older adults transition to having at least one at-risk or high-risk condition. Therefore, we also assessed the incidence rate ratios for at-risk older adults ≥65 years of age compared with younger adults with high-risk conditions. High-risk older and high-risk younger adults were not compared because high-risk adults, regardless of age, typically receive the same pneumococcal vaccination recommendation. At-risk older adults ≥65 years of age had a consistently higher risk of IPD, PP, and all-cause pneumonia compared with all high-risk younger adults 18–49, 16–64, or 19–64 years of age (Supplemental Table 3). For IPD, rate ratios ranged from 1.2 to 1.5 for at-risk older adults ≥65 years of age and up to 2.1 for ≥75 years of age when compared with all high-risk younger adults and from 0.2 to 4.0 when compared with younger adults with the specific immunocompromising conditions of HIV infection or asplenia/splenic dysfunction (). For PP, rate ratios ranged from 2.0 to 2.1 for at-risk older adults ≥65 years of age and up to 3.1 for ≥75 years of age when compared with all high-risk younger adults and from 1.3 to 2.1 when compared with younger adults with the specific immunocompromising conditions of cochlear implant or presence of immunosuppressive drugs or conditions (). For all-cause pneumonia, rate ratios ranged from 2.5 to 2.8 for at-risk older adults ≥65 years of age and up to 6.1 for ≥85 years of age when compared with all high-risk younger adults and from 1.7 to 7.7 when compared with younger adults with the specific immunocompromising conditions of functional/anatomical asplenia or congenital immunodeficiency (). Like healthy older adults, as age increased substantially beyond 65 years, risk ratios became substantially greater than 1.0. The combination of older age and the presence of an at-risk medical condition multiplied the risk of disease for older adults in comparison to high-risk younger adults.
Figure 2. a-c: Comparison of disease incidence in at-risk older adults vs high-risk younger adults
Finally, while we have focused primarily on incidence rate ratios, limited data are available to support the idea that healthy or at-risk older adults have more severe outcomes from IPD or pneumonia than high-risk younger adults. For example, in the UK, the CFR of IPD was higher in adults ≥65 years of age who did not have a risk condition (CFR, 29.1%) compared with adults 16–64 years of age with a high-risk condition (e.g. CFR, 8.5% in HIV positive persons; 15.4% in immunosuppressed persons) [Citation32]. While additional studies are needed, the concept of severity is critical for decision-making. This is because disease burden, which is defined by incidence times severity, is a more relevant metric for vaccination policy than incidence alone.
4. Biologic basis for age-related increase in risk of pneumococcal disease
4.1. Defining key characteristics of immunosenescence
As shown above, there is increasing evidence associating age and the process of immunosenescence with higher risk of pneumococcal disease. Even ‘healthy’ older adults have a greater risk of PP than that of younger adults who are immunocompromised. It is therefore critical to better understand the underlying biologic mechanisms triggering the age-related changes in the immune system or immunosenescence responsible for the progressively increased vulnerability to pneumococcal infection as people age [Citation53]. In the following section, we review the biologic basis of immunosenescence in the innate and adaptive arms of the immune system.
Two hallmark characteristics of immunosenescence are cellular senescence and inflammaging. Cellular senescence is the metabolically altered state of cells that leads to cessation of cell division and cell clearance [Citation54]. Senescent cells secrete pro-inflammatory molecules resulting in a low-grade chronic and systemic inflammatory state, termed inflammaging, which occurs in the absence of detectable infection [Citation55]. As aging, cellular senescence, and inflammaging have different effects on different cell-types, identification of age-related changes in the innate and adaptive immune systems that might contribute to an increased susceptibility to infection is challenging. The evidence for a specific effect on pneumococcal infection is even more limited, and largely based on pneumococcal specific models in older mice [Citation53] (Supplemental Table 4 [Citation55–88]). Here, we summarize current knowledge about the impact of aging, cellular senescence, and inflammaging on host susceptibility to pneumococcal infection.
4.2. Age-related defects in innate responses
With advancing age, cellular senescence of the respiratory tract impairs mechanisms such as mucin production [Citation70] and ciliary beating that are associated with natural clearance of foreign materials, including potential pathogens [Citation71,Citation72,Citation89]. Oropharyngeal dysphagia and impaired cough reflexes among older adults increase the risk of aspiration pneumonia [Citation57], which accounts for 5–15% of cases of CAP in adults ≥65 years of age [Citation90].
The frequency [Citation91] and density [Citation43] of pneumococcal colonization are reported to be reduced in older adults, despite the incidence of pneumococcal CAP and IPD being highest in older age groups. However, recent studies appear to have resolved this seeming paradox, specifically by finding carriage prevalence of older adults approaching that in children when using molecular techniques and testing reservoirs other than the nasopharynx, such as the oropharynx and saliva [Citation53,Citation91,Citation92]. Studies of experimental pneumococcal colonization indicate that colonization can be established in healthy older adults, but it failed to confer serotype-specific immunity [Citation82]. The authors hypothesized that reduced colonization or altered post-colonization immunity in older adults may partially explain their increased susceptibility to pneumococcal infection.
Upon initial entry of pneumococci into the upper airways, neutrophils infiltrate the initial site of infection to form the first line of cellular defense, followed by macrophages if the bacteria persist [Citation60,Citation93]. Although the number of neutrophils is maintained or even increased in older adults, specific functions of neutrophils (chemotaxis, phagocytosis, superoxide burst generation and killing of pneumococci) are impaired [Citation75], which may allow pneumococcal infection to progress unchecked [Citation94]. Despite the presence of chronic low-grade inflammation in the absence of infection, macrophages fail to respond to pneumococcal infection with an adequate acute inflammatory response, exhibiting reduced bactericidal activity, production of pro-inflammatory cytokines and activation of other immune cells [Citation94].
Cellular senescence-related defects in innate immunity also contribute to a higher propensity to PP in older age. The presence of senescent cells and inflammaging increases susceptibility to pneumococcal pneumonia because it increases the expression of host protein (ligands) that facilitate pneumococcal attachment to respiratory epithelial cells [Citation75]. In the absence of infection, plasma levels of proinflammatory cytokines TNFα and IL-6 are 2- to 4-fold higher in older adults compared with middle-aged adults and elevated baseline levels of TNFα and IL-6 were associated with an increased incidence of CAP in otherwise healthy older adults [Citation62]. Inflammaging impairs the development of immunity against pneumococcal colonization. Thus, cellular senescence and inflammaging in the lungs contribute to a higher burden of pneumococcal disease in older adults [Citation95].
4.3. Age-related defects in the adaptive responses
With immune aging, the CD4+ to CD8+ T cell ratio is inverted [Citation96]. The number of naïve CD4+ and CD8+ T cells in the periphery declines [Citation79,Citation80], and naïve T cells are functionally deficient (restricted T cell receptor [TCR] diversity) [Citation97,Citation98]. Memory T cells exhibit impaired cytokine secretion and proliferation upon recall [Citation98]. These shifts in the T cell repertoire may all contribute to the increased risk of PP in older adults. Reduction in the number of circulating naïve CD8+ T cells and accumulation of CD8+ memory and effector T cells occur even earlier [Citation99] and to a greater extent than the decline in naïve CD4+ T cells, being one of the most consistent hallmarks of immune aging in healthy older adults [Citation100].
The key mediators of the adaptive immune response to pneumococcal infection within the respiratory tract are CD4+ T-helper 17 (Th17) cells [Citation101,Citation102]. CD4+ Th17 memory cells produce the pro-inflammatory cytokine IL-17 which recruits macrophages and neutrophils to the site of infection and promotes clearance of pneumococci [Citation81,Citation103]. Th17-mediated responses are antibody independent yet antigen specific, and are considered the primary mechanism of naturally acquired mucosal immunity to S. pneumoniae [Citation102,Citation104]. Anti-pneumococcal capsular polysaccharide antibodies generated during colonization do not have a major role in carriage clearance [Citation104,Citation105], although they may be protective against future colonization with the homologous serotype. However, high levels of anti-capsular antibodies generated following PCV vaccination, do prevent colonization [Citation23,Citation106]. Production of IL-17 by Th17 cells is regulated by regulatory T cells (Tregs). In older adults the number of Th17 cells declines and the number of Treg memory cells increases, which is associated with an increased risk of PP [Citation81]. This decline may also impair the clearance of pneumococcal colonization.
The immunological changes seen in HIV-infected individuals show many similarities to those seen in older adults without HIV infection. It has been suggested that through a process of continuous immune activation, HIV infection causes an acceleration of the adaptive immune aging process, exhausting immune resources prematurely, and thus resulting in immunodeficiency [Citation107] (). It is of note that HIV infection is associated with an increased susceptibility to pneumococcal disease.
Table 3. Characteristics of studies reporting disease incidence by risk group for adults
A major component of naturally acquired immunity to IPD is antibodies to pneumococcal proteins rather than anticapsular antibodies [Citation102,Citation108]. There is an age-related decline in naïve B cells, which show decreased affinity maturation and isotype switching [Citation83,Citation86] and accumulation of senescent antigen-experienced memory B cells (MBCs) [Citation82,Citation83]. With age, MBCs also lose their capacity for differentiation into plasma cells and this is likely to contribute to higher incidence of pneumococcal disease in older adults [Citation83,Citation109]. Humoral responses to plain pneumococcal capsular polysaccharides are T-cell independent (TI) and mediated by B1-B cells, inducing formation of serotype-specific antibodies but without generating high-affinity MBCs [Citation110,Citation111]. Hence, immunization with PPVS23 does not result in replenishment of the MBC pool and repeated doses of PPSV23 may actually induce hypo-responsiveness [Citation112,Citation113] reducing the immune response [Citation114]. Although the total number of B1-B cells expands in older age [Citation115] due to increased levels of IL-6, their number in the periphery declines [Citation83] and many of the B1-B cells generated have auto-antibody reactivity [Citation116–118].
In contrast, the antibody response to the highly immunogenic protein carrier conjugated to polysaccharide in PCVs results in a T-cell dependent anti-capsular response, mediated by B2-B cells, which generate high affinity antibodies with isotype switching and the induction of immunological memory [Citation119,Citation120]. In older age the number of B2-B cells also declines, but the superior quality of the immune response to glycoconjugate confers distinct advantages over a plain polysaccharide underlining the benefits of PCVs in older age [Citation121,Citation122].
Protection against IPD is dependent upon antibody mediated immunity, rather than CD4+ Th17 mediated mechanisms [Citation108]. Although serum immunoglobulin levels are stable, the antibodies generated are of lower avidity because of a shift from IgG to IgM and have reduced opsonic capacity [Citation75]. Less efficient T cells and the altered cytokine environment also contribute to functional defects in antibody production. These defects result in reduced levels of naturally acquired serum IgG, IgM, and IgA against pneumococcal protein and polysaccharide antigens in older adults [Citation75,Citation84]. The reduced frequency of colonization [Citation123] and the decreased level of IgM MBCs [Citation83] may also adversely affect colonization-induced immunity in older people. These changes are likely to contribute to decreased clearance of pneumococci following systemic invasion. Overall, the expansion of antigen-experienced memory T cells and B cells, coupled with a decline in the number of naïve T and B cells, compromises the response to newly encountered pneumococcal antigens, including vaccines.
5. Conclusions: A new approach for pneumococcal vaccination in adults
5.1. Advocacy for healthy aging and a life-course approach to vaccination
The demographic shift in population aging calls for systemic changes that promote healthy aging. The World Health Organization (WHO) defines healthy aging as ‘the process of developing and maintaining the functional ability that enables well-being in older age’ [Citation124]. This definition considers healthy aging to be more than a disease-free state with a focus on the functional ability, autonomy, and quality of life of people as they age. We are living in a time where for the first time in history, the much needed intersecting intergovernmental plans of the Decade of Healthy Aging (2020–2030) [Citation125], the New Global Vaccine Action Plan (GVAP) [Citation126], and the WHO Immunization Agenda 2030 [Citation127] provide a blueprint to consolidate and implement a wide ranging public health program. One of the core elements of the Immunization Agenda 2030 is to expand vaccination programs beyond infancy to other age groups including older adults, and this is aligned with the program for the Decade of Healthy Aging that includes the need of adequate availability of vaccines for older adults [Citation128].
However to date, in contrast to the immense achievements in alleviating the burden of vaccine preventable diseases during early childhood [Citation129], and more recently during adolescence with human papillomavirus and meningococcal conjugate vaccines, vaccination coverage of adults is low and fails to meet established public health standards and goals [Citation130,Citation131], including for pneumococcal vaccination [Citation132,Citation133]. Recently in the context of the COVID-19 pandemic, a framework for prioritizing and delivering adult vaccinations was proposed, highlighting the need for VTCs to recognize the risk and vulnerability of older adults and to provide policy guidance that would catalyze adult vaccination [Citation134]. Only then will demand by clinicians, patients, and purchasers drive uptake and use of vaccines in this population.
Age-related immunosenescence leads to increased risk of infectious diseases with some of the mechanisms and risk differing by etiology. Here we reviewed the association between the increased risk of pneumococcal disease and immunosenescence, which may involve both innate and adaptive immunity. With aging, innate immunity becomes increasingly dysfunctional, through a decreased number and function of immune cells and a state of chronic inflammation. Adaptive immunity also declines with reduced opsonophagocytic antibody activity against pneumococcal antigens.
For pneumococcus, the immunologic changes associated with immunosenescence result in an enormously increased IPD risk, with incidence among healthy and at-risk older adults that is higher than or similar to some high-risk categories of younger adults. Even though the available epidemiologic evidence presented is from high-income countries, the risk relationship between healthy and at-risk older adults and high-risk younger adults is likely to apply to other settings. While data for etiology-specific pneumonia are more difficult to ascertain due to diagnostic limitations for assessing non-bacteremic pneumonia, this same relative increase in risk exists for all-cause pneumonia. Added to immunosenescence, at-risk adults ≥65 years of age may have substantially higher risk than younger high-risk adults. Furthermore, most adults ≥65 years of age have an at-risk or high-risk condition and almost all will eventually transition to these states. For these reasons, classifying older adults as ‘healthy’ may be misleading, particularly with respect to the rationale for pneumococcal vaccine prioritization.
5.2. Older age should be classified as an immunocompromising high-risk condition based on the process of immunosenescence
The evidence presented in this manuscript suggests that one way to communicate the benefits of pneumococcal vaccination for older persons would be to frame vaccination as an intervention designed to address a medical need related to being immunocompromised, similar to conditions already accepted as targets for vaccination. Conceptually, this should be relatively straightforward because in the same way that an HIV positive test is a reliable marker for an increased risk of pneumococcal disease due to HIV infection, aging is a reliable marker for immunosenescence. Practically, in the absence of a definitive, individually administered test for immunosenescence, the precise age marking the onset of immunosenescence at the population level will depend not only on biology, but pneumococcal epidemiology and vaccination program funding.
The US Centers for Disease Prevention and Control and the Infectious Disease Society of America recommend providing indicated vaccines to persons before they enter an immunosuppressed state, e.g. providing pneumococcal vaccination before commencing chemotherapy or bone marrow transplant [Citation135,Citation136]. Because immunosenescence is likely universal and thus predictable, and because this is exacerbated in most older adults through the common transition to an at-risk or high-risk state apart from experiencing immunosenescence, pneumococcal vaccine should be administered before these transitions occur to achieve the greatest public health benefit. This goal should be balanced by vaccinating as close as possible to the transition to a substantially compromised health state to ensure that the risk period is covered for as long as possible assuming a finite vaccine immune duration. Based on age representing the best currently available marker for the transition to a compromised health state, the available epidemiologic data suggest the risk of pneumococcal disease increases around 65 years of age, but this could be an artifact of how the available data are stratified. Consequently, the age when risk increases could be lower; however, additional research to delineate this risk would be advantageous for future vaccine policy [Citation35,Citation37]. Alternatively, additional research on a biological marker of immunosenescence could ultimately provide an individualized assessment of pneumococcal disease risk.
As stated previously, we are not advocating for any particular adult pneumococcal vaccination policy, as this will depend on a) the totality of available data and its interpretation by VTCs related to topics such as efficacy or effectiveness of different vaccines, disease burden, sequelae, reductions in quality of life following pneumococcal disease, exacerbations by pneumococcus of chronic conditions such as chronic lung and cardiovascular disease, and other measures; b) vaccine prices; c) vaccination program and overall health budgets; d) estimated cost-effectiveness; e) competing priorities; and f) cultural values. Regardless of the particular adult pneumococcal vaccination policy selected, the evidence presented herein indicates that older adults experience substantial risk of pneumococcal disease based on the process of immunosenescence and thus that older age should be considered an immunocompromising high-risk condition, rather than separating risk- and age-based recommendations.
Classifying older age as an immunocompromising condition for the purposes of vaccine policy in adults may apply to other vaccines. However, this would need to be supported for each vaccine by biologic, immunologic, and epidemiologic data similar to the intellectual framework we have used for pneumococcal vaccines. Such a change in perspective will make vaccine policy more coherent and evidence-based, and thus may increase the efficient use and coverage of vaccines for older adults. This in turn can improve the quality of life of directly immunized individuals, reduce burden on their families, and provide benefits to society.
6. Expert opinion
PCVs have led to dramatic reductions in disease due to vaccine serotypes among children who are targeted for vaccination, but also among unvaccinated members of the community. While adults have benefited from pediatric PCV use, pneumococcal disease continues to cause significant mortality and morbidity among adults globally. Long-standing recommendations for PPSV23 use in older adults have not diminished the global burden of non-bacteremic pneumonia, the most common type of pneumococcal disease. Besides this, the significant consequences of pneumonia (e.g. cognitive impairment, exacerbation of comorbid medical conditions, poorer quality of life, frailty, among others) are gaining recognition as secondary preventable outcomes of pneumococcal disease. Epidemiologic and biologic evidence suggest that immunosenescence is a key contributor to this persistent and elevated burden of disease later in life.
As the global population ages, public health policies are needed that prevent both the acute and long-term consequences of disease and promote healthy aging. Increasing uptake of vaccines with proven effectiveness for populations at most risk of disease is central to a life course approach to vaccination. However, to achieve this goal, the risk-based rationale must be clearly defined, consistent, and communicated. In the case of older persons, this is represented primarily by the immunocompromising condition of immunosenescence and additionally by the added burden associated with transition to chronic disease states, including other immunocompromising conditions. Consequently, identifying older age as an immunocompromising condition, similar to the approach taken for other immunocompromising conditions may make the rationale clearer for immunizing all adults beyond a certain age. In turn, this may result in improved awareness of who should be vaccinated and dosing compliance among healthcare providers, which could result in higher vaccination coverage among those at greatest risk of disease.
In this review, we have presented data to support consideration of age-related immunosenescence as an immunocompromising and thus high-risk condition for the purposes of pneumococcal vaccine policy. While our review focused on pneumococcal vaccines, other current and future vaccines may benefit from a similar approach including influenza and herpes zoster vaccines as well as potential vaccines targeting respiratory syncytial virus, group B streptococcus, and SARS-CoV-2. Because mechanisms and consequences of immunosenescence may be etiology-specific, an exercise such as we have done should be completed for each of these. Additionally, an etiology-specific biological marker of immunosenescence and evidence of vaccine efficacy or effectiveness across the life course will further guide policies for these other vaccines. Nevertheless, it is likely that age-related immunosenescence will support inclusion of age – at some cutoff point – as an immunocompromising condition for each of these vaccines.
For each vaccine targeting older persons, including pneumococcal vaccines, decision-making will be enhanced with additional research. Data are urgently needed for many outcomes by finer age stratifications than simply 18–49 or 18–64 years and ≥65 years. Additional data are needed for age- and risk-group-related disease burden, age-stratified VE, duration of vaccine-induced immunity, and the potential benefits of booster doses. Research into biologic mechanisms of immunosenescence may aid in the development of adult-specific vaccine designs and novel adjuvants. In countries that include age-related immunosenescence as an immunocompromising condition, evaluations should document the degree to which this influences vaccination policy and coverage for older persons, and consequently the contribution of this policy toward healthier aging.
Article highlights
Immunosenescence, or age-related decline of the immune system, compromises the innate and adaptive immune responses and contributes to the increased susceptibility to pneumococcal infections among older adults.
Risk-based pneumococcal vaccine recommendations generally target individuals with comorbid and immunocompromising conditions associated with an increased risk of pneumococcal disease. However, older age (and the process of immunosenescence) is not specifically named as an immunocompromising condition despite being a condition that confers a similar risk of pneumonia and invasive pneumococcal disease as some other conditions (e.g. asplenia, malignancy, and human immunodeficiency virus infection) in this category.
Available data indicate that a rational approach to risk-based recommendations for pneumococcal vaccination of older adults is for national vaccine technical committees to classify older age as an immunocompromising and thus a high-risk condition, similar to other high-risk conditions currently targeted for prevention by pneumococcal vaccines.
Future studies are needed to identify the appropriate age at which the disease risk increases in older adults as they transition from a healthy to an at-risk or high-risk state and to identify markers of immunosenescence that signal the beginning of the transition to this immunocompromised state.
While we focus on pneumococcal vaccines, the same assessment of epidemiology and biology could be done for other etiologies targeted by adult vaccines to determine if defining immunosenescence as an immunocompromising condition has a broader application.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Declaration of interest
L R Grant, Q Yan, A Cané, L Jodar, R E Isturiz, and B D Gessner are employees of Pfizer, which sponsored the manuscript. M PE Slack has received personal fees from GlaxoSmithKline, Pfizer, Merck, AstraZeneca, and Sanofi Pasteur as a speaker at international meetings and as a member of advisory boards and has undertaken contract work for Pfizer. K Trzciński acknowledges receiving consulting and speaking fees and funds for unrestricted research grants from Pfizer, funds for unrestricted research grants from GlaxoSmithKline, and consulting fees from Merck Sharp & Dohme, all paid directly to his home institution. J Barratt is the Secretary General of International Federation on Ageing; International Federation on Ageing has received unrestricted education grants from Pfizer Global, Pfizer Canada, GSK Global, GSK Canada, Seqirus, and International Federation of Pharmaceutical Manufacturers & Associations, and no monies are received personally for any work related to this paper or any other report. E Sobczyk reports that the Gerontological Society of America receives unrestricted educational grants from several vaccine manufacturers including Pfizer, and she directs educational programs using funding from those grants. J Appleby reports that the Gerontological Society of America receives unrestricted educational grants from several vaccine manufacturers including Pfizer, and the Society staff implements educational programs using funding from those grants. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Supplemental Material
Download MS Word (79 KB)Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- United Nations. World Population Ageing 2019: highlights (ST/ESA/SER.A/430) 2019 [ cited 2020 Jun 29]. Available from: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf.
- United Nations. Ageing [ cited 2020 Aug 10]. Available from: https://www.un.org/en/sections/issues-depth/ageing/.
- Makinodan T. Nature of the decline in antigen-induced humoral immunity with age. Mech Ageing Dev. 1980 Sep-Oct;14(1–2):165–172. PubMed PMID: 7010008.
- Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012 Jan;67(1):71–79. PubMed PMID: 20729232.
- Matanock A. Evidence to recommendations and GRADE for PCV13 use among immunocompetent adults ≥65 years old. Presented to the Advisory Committee on Immunization Practices, February 27-28, 2019 and June 26-27, 2019. [cited 2020 Oct 13]. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/Pneumococcal-5-Matanock-508.pdf.
- Hanquet G, Krizova P, Valentiner-Branth P, et al. Effect of childhood pneumococcal conjugate vaccination on invasive disease in older adults of 10 European countries: implications for adult vaccination. Thorax. 2019 May;74(5):473–482. PubMed PMID: 30355641; PubMed Central PMCID: PMC6484683.
- Djennad A, Ramsay ME, Pebody R, et al. Effectiveness of 23-valent polysaccharide pneumococcal vaccine and changes in invasive pneumococcal disease incidence from 2000 to 2017 in those aged 65 and over in England and wales. EClinicalMedicine. 2018 Dec;6(6):42–50. PubMed PMID: 31193709; PubMed Central PMCID: PMC6537583.
- Lessa F, Spiller M. Effectiveness of PCV13 in adults hospitalized with pneumonia using centers for medicare & medicaid services data, 2014-2017. Presented to the Advisory Committee on Immunization Practices, February 27-28, 2019 and June 26-27, 2019. [ cited 2020 Oct 13]. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2019-02/Pneumococcal-3-Lessa-508.pdf/.
- Lessa FC, Spiller M, Wu X, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine in us adults hospitalized with pneumonia, 2014–2017. Open Forum Infect Dis. 2019 Oct; 6(Suppl 2):S956–S957. PubMed Central PMCID: PMC6809715.
- Kolditz M, Schmitt J, Pletz MW, et al. Impact of the 13-valent pneumococcal conjugate vaccine on the incidence of all-cause pneumonia in adults aged ≥60 years: a population-based, retrospective cohort study. Clin Infect Dis. 2019 May 30;68(12):2117–2119. PubMed PMID: 30462172.
- Gessner BD, Jiang Q, Van Werkhoven CH, et al. A public health evaluation of 13-valent pneumococcal conjugate vaccine impact on adult disease outcomes from a randomized clinical trial in the Netherlands. Vaccine. 2019 Sep 10;37(38):5777–5787. PubMed PMID: 29861177.
- Pick H, Daniel P, Rodrigo C, et al. Pneumococcal serotype trends, surveillance and risk factors in UK adult pneumonia, 2013-18. Thorax. 2020 Jan;75(1):38–49. PubMed PMID: 31594801.
- Isturiz RE, Ramirez J, Self WH, et al. Pneumococcal epidemiology among us adults hospitalized for community-acquired pneumonia. Vaccine. 2019 May 31;37(25):3352–3361. PubMed PMID: 31072732.
- Van Der Linden M, Imohl M, Perniciaro S. Limited indirect effects of an infant pneumococcal vaccination program in an aging population. PLoS One. 2019;14(8):e0220453. PubMed PMID: 31369597; PubMed Central PMCID: PMC6675109.
- McLaughlin JM, Swerdlow DL, Khan F, et al. Disparities in uptake of 13-valent pneumococcal conjugate vaccine among older adults in the United States. Hum Vaccin Immunother. 2019;15(4):841–849. PubMed PMID: 30676236; PubMed Central PMCID: PMC6605819.
- Ladhani SN, Collins S, Djennad A, et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000-17: a prospective national observational cohort study. Lancet Infect Dis. 2018 Apr;18(4):441–451. PubMed PMID: 29395999.
- Forstner C, Kolditz M, Kesselmeier M, et al. Pneumococcal conjugate serotype distribution and predominating role of serotype 3 in German adults with community-acquired pneumonia. Vaccine. 2020 Jan 29;38(5):1129–1136. PubMed PMID: 31761500.
- Diao WQ, Shen N, Yu PX, et al. Efficacy of 23-valent pneumococcal polysaccharide vaccine in preventing community-acquired pneumonia among immunocompetent adults: a systematic review and meta-analysis of randomized trials. Vaccine. 2016 Mar 18;34(13):1496–1503. PubMed PMID: 26899376.
- Moberley S, Holden J, Tatham DP, et al. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013 Jan;31(1):CD000422. PubMed PMID: 23440780; PubMed Central PMCID: PMC7045867.
- Van Werkhoven CH, Huijts SM, Bolkenbaas M, et al. The Impact of age on the efficacy of 13-valent pneumococcal conjugate vaccine in elderly. Clin Infect Dis. 2015 Dec 15;61(12):1835–1838. PubMed PMID: 26265498.
- McLaughlin JM, Jiang Q, Isturiz RE, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine against hospitalization for community-acquired pneumonia in older us adults: a test-negative design. Clin Infect Dis. 2018 Oct 30;67(10):1498–1506. PubMed PMID: 29790925; PubMed Central PMCID: PMC6206101.
- Schmoele-Thoma B, Center KJ, Webber C, et al. Relationship of age to immunogenicity of 13-valent pneumococcal conjugate vaccine (PCV13) from the very young to the elderly. Presented at 10th International Symposium on Pneumococci and Pneumococcal Diseases; 2016 Jun 26-30; Glasgow, Scotland.
- Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015 Mar 19;372(12):1114–1125. PubMed PMID: 25785969.
- Lewis N, Hsiao A, Hansen J, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in older adults. Open Forum Infect Dis. 2019 Oct 23;6(Suppl 2):S953–4. PubMed Central PMCID: PMC6811316.
- Gessner BD, Jiang Q, Van Werkhoven CH, et al. A post-hoc analysis of serotype-specific vaccine efficacy of 13-valent pneumococcal conjugate vaccine against clinical community acquired pneumonia from a randomized clinical trial in the Netherlands. Vaccine. 2019 Jul 9;37(30):4147–4154. PubMed PMID: 31155413.
- McLaughlin JM, Jiang Q, Gessner BD, et al. Pneumococcal conjugate vaccine against serotype 3 pneumococcal pneumonia in adults: a systematic review and pooled analysis. Vaccine. 2019 Oct 8;37(43):6310–6316. PubMed PMID: 31522807.
- Isturiz R, Sings HL, Hilton B, et al. Streptococcus pneumoniae serotype 19A: worldwide epidemiology. Expert Rev Vaccines. 2017 Oct;16(10):1007–1027. PubMed PMID: 28783380.
- Ahmed SS, Pondo T, Xing W, et al. Early Impact of 13-valent pneumococcal conjugate vaccine use on invasive pneumococcal disease among adults with and without underlying medical conditions-united states. Clin Infect Dis. 2020 Jun 10;70(12):2484–2492. PubMed PMID: 31402387.
- Shea KM, Edelsberg J, Weycker D, et al. Rates of pneumococcal disease in adults with chronic medical conditions. Open Forum Infect Dis. 2014 Mar;1(1):ofu024. PubMed PMID: 25734097; PubMed Central PMCID: PMC4324183.
- Pelton SI, Bornheimer R, Doroff R, et al. Decline in pneumococcal disease attenuated in older adults and those with comorbidities following universal childhood PCV13 Immunization. Clin Infect Dis. 2019 May 17;68(11):1831–1838. PubMed PMID: 30239637; PubMed Central PMCID: PMC6522679.
- Pelton SI, Shea KM, Farkouh RA, et al. Rates of pneumonia among children and adults with chronic medical conditions in Germany. BMC Infect Dis. 2015 Oct 30;15(1):470. PubMed PMID: 26515134; PubMed Central PMCID: PMC4627378.
- Van Hoek AJ, Andrews N, Waight PA, et al. The effect of underlying clinical conditions on the risk of developing invasive pneumococcal disease in England. J Infect. 2012 Jul;65(1):17–24. PubMed PMID: 22394683.
- Sheikh S, Biundo E, Courcier S, et al. A report on the status of vaccination in Europe. Vaccine. 2018 Aug 9;36(33):4979–4992. PubMed PMID: 30037416.
- Torres A, Cilloniz C, Blasi F, et al. Burden of pneumococcal community-acquired pneumonia in adults across Europe: a literature review. Respir Med. 2018 Apr;137:6–13. PubMed PMID: 29605214.
- Ramirez JA, Wiemken TL, Peyrani P, et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017 Nov 13;65(11):1806–1812. PubMed PMID: 29020164.
- Morimoto K, Suzuki M, Ishifuji T, et al. The burden and etiology of community-onset pneumonia in the aging Japanese population: a multicenter prospective study. PLoS One. 2015;10(3):e0122247. PubMed PMID: 25822890; PubMed Central PMCID: PMC4378946.
- Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. Adults. N Engl J Med. 2015 Jul 30;373(5):415–427. PubMed PMID: 26172429; PubMed Central PMCID: PMC4728150.
- Ramirez J, Furmanek S, Pena S; et al. Annual number of adults hospitalized with pneumococcal pneumonia in the United States. ISPPD 2020, Abstract #473.
- Vila-Corcoles A, Ochoa-Gondar O, Vila-Rovira A, et al. Incidence and risk of pneumococcal pneumonia in adults with distinct underlying medical conditions: a population-based study. Lung. 2020 Jun;198(3):481–489. PubMed PMID: 32253492.
- Bewick T, Sheppard C, Greenwood S, et al. Serotype prevalence in adults hospitalised with pneumococcal non-invasive community-acquired pneumonia. Thorax. 2012 Jun;67(6):540–545. PubMed PMID: 22374921.
- Centers for Disease Control and Prevention. Active bacterial core surveillance report, emerging infections program network, streptococcus pneumoniae, 2018 [ cited 2020 Aug 10]. Available from: https://www.cdc.gov/abcs/reports-findings/survreports/spneu18.html.
- Meder KN, Jayasinghe S, Beard F, et al. Long-term impact of pneumococcal conjugate vaccines on invasive disease and pneumonia hospitalizations in indigenous and non-indigenous australians. Clin Infect Dis. 2020 Jun 10;70(12):2607–2615. PubMed PMID: 31388670.
- Albrich WC, Rassouli F, Waldeck F, et al. Influence of older age and other risk factors on pneumonia hospitalization in Switzerland in the pneumococcal vaccine era. Front Med (Lausanne). 2019;6:286. PubMed PMID: 31867337; PubMed Central PMCID: PMC6906144.
- LeBlanc J, ElSherif M, Ye L, et al. Age-stratified burden of pneumococcal community acquired pneumonia in hospitalised Canadian adults from 2010 to 2015. BMJ Open Respir Res. 2020 Mar;7(1). PubMed PMID: 32188585; PubMed Central PMCID: PMC7078693.
- Corrales-Medina VF, Musher DM, Shachkina S, et al. Acute pneumonia and the cardiovascular system. Lancet. 2013 Feb 9;381(9865):496–505. PubMed PMID: 23332146.
- O’Meara ES, White M, Siscovick DS, et al. Hospitalization for pneumonia in the Cardiovascular Health Study: incidence, mortality, and influence on longer-term survival. J Am Geriatr Soc. 2005 Jul;53(7):1108–1116. PubMed PMID: 16108926.
- Ramirez J, Aliberti S, Mirsaeidi M, et al. Acute myocardial infarction in hospitalized patients with community-acquired pneumonia. Clin Infect Dis. 2008 Jul 15;47(2):182–187. PubMed PMID: 18533841.
- Bornheimer R, Shea KM, Sato R, et al. Risk of exacerbation following pneumonia in adults with heart failure or chronic obstructive pulmonary disease. PLoS One. 2017;12(10):e0184877. PubMed PMID: 29028810; PubMed Central PMCID: PMC5640217.
- Johnstone J, Eurich DT, Majumdar SR, et al. Long-term morbidity and mortality after hospitalization with community-acquired pneumonia: a population-based cohort study. Medicine (Baltimore). 2008 Nov;87(6):329–334. PubMed PMID: 19011504.
- Yende S, Angus DC, Ali IS, et al. Influence of comorbid conditions on long-term mortality after pneumonia in older people. J Am Geriatr Soc. 2007 Apr;55(4):518–525. PubMed PMID: 17397429.
- Restrepo MI, Faverio P, Anzueto A. Long-term prognosis in community-acquired pneumonia. Curr Opin Infect Dis. 2013 Apr;26(2):151–158. PubMed PMID: 23426328; PubMed Central PMCID: PMC4066634.
- Kolditz M, Braeken D, Ewig S, et al. Severity Assessment and the Immediate and Long-Term Prognosis in Community-Acquired Pneumonia. Semin Respir Crit Care Med. 2016 Dec;37(6):886–896. PubMed PMID: 27960212.
- Krone CL, Van De Groep K, Trzcinski K, et al. Immunosenescence and pneumococcal disease: an imbalance in host-pathogen interactions. Lancet Respir Med. 2014 Feb;2(2):141–153. PubMed PMID: 24503269.
- Uyar B, Palmer D, Kowald A, et al. Single-cell analyses of aging, inflammation and senescence. Ageing Res Rev. 2020 Sep;16(64):101156. PubMed PMID: 32949770; PubMed Central PMCID: PMC7493798.
- Franceschi C, Capri M, Monti D, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007 Jan;128(1):92–105. PubMed ID PMID: 17116321.
- Shivshankar P, Boyd AR, Le Saux CJ, et al. Cellular senescence increases expression of bacterial ligands in the lungs and is positively correlated with increased susceptibility to pneumococcal pneumonia. Aging Cell. 2011 Oct;10(5):798–806. PubMed PMID: 21615674; PubMed Central PMCID: PMC3173515.
- Okazaki T, Ebihara S, Mori T, et al. Association between sarcopenia and pneumonia in older people. Geriatr Gerontol Int. 2020 Jan;20(1):7–13. PubMed PMID: 31808265.
- Hinojosa E, Boyd AR, Orihuela CJ. Age-associated inflammation and toll-like receptor dysfunction prime the lungs for pneumococcal pneumonia. J Infect Dis. 2009 Aug 15;200(4):546–554. PubMed PMID: 19586419; PubMed Central PMCID: PMC3102250.
- Van Der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet. 2009 Oct 31;374(9700):1543–1556. PubMed PMID: 19880020.
- Krone CL, Trzciński K, Zborowski T, et al. Impaired innate mucosal immunity in aged mice permits prolonged Streptococcus pneumoniae colonization. Infect Immun. 2013 Dec;81(12):4615–4625. PubMed PMID: 24082075; PubMed Central PMCID: PMC3837976.
- Cho SJ, Rooney K, Choi AMK, et al. NLRP3 inflammasome activation in aged macrophages is diminished during Streptococcus pneumoniae infection. Am J Physiol Lung Cell Mol Physiol. 2018;314(3):L372–L387. PubMed PMID: 29097427.
- Yende S, Tuomanen EI, Wunderink R, et al. Preinfection systemic inflammatory markers and risk of hospitalization due to pneumonia. Am J Respir Crit Care Med. 2005 Dec 1;172(11):1440–6. PubMed PMID: 16166617; PubMec Central PMCID: PMC2718438.
- Hinojosa CA, Suresh A, Babu R, et al. Elevated A20 contributes to age-dependent macrophage dysfunction in the lungs. Exp Gerontol. 2014 Jun;54:58–66. PubMed PMID: 24440463; PubMed Central PMCID: PMC3989429.
- Boyd AR, Shivshankar P, Jiang S, et al. Age-related defects in TLR2 signaling diminish the cytokine response by alveolar macrophages during murine pneumococcal pneumonia. Exp Gerontol. 2012 Jul;47(7):507–518. PubMed PMID: 22548913; PubMed Central PMCID: PMC3368096.
- Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004 May;395:687–699. PubMed ID PMID: 15130663.
- Spencer NF, Poynter ME, Im SY, et al. Constitutive activation of NF-kappa B in an animal model of aging. Int Immunol. 1997 Oct;9(10):1581–1588. PubMed PMID: 9352364.
- Kreiling JA, Tamamori-Adachi M, Sexton AN, et al. Age-associated increase in heterochromatic marks in murine and primate tissues. Aging Cell. 2011;10(2):292–304. PubMed PMID: 21176091.
- Van Duin D, Mohanty S, Thomas V, et al. Age-associated defect in human TLR-1/2 function. J Immunol. 2007 Jan 15;178(2):970–975. PubMed PMID: 17202359.
- Nyugen J, Agrawal S, Gollapudi S, et al. Impaired functions of peripheral blood monocyte subpopulations in aged humans. J Clin Immunol. 2010 Nov;30(6):806–813. PubMed PMID: 20703784; PubMed Central PMCID: PMC2970801.
- Grubb BR, Livraghi-Butrico A, Rogers TD, et al. Reduced mucociliary clearance in old mice is associated with a decrease in Muc5b mucin. Am J Physiol Lung Cell Mol Physiol. 2016 May 1;310(9):L860–7. PubMed PMID: 26968767; PubMed Central PMCID: PMC4867354.
- Svartengren M, Falk R, Philipson K. Long-term clearance from small airways decreases with age. J European Respiratory Journal. Eur Respir J. 2005;26(4):609–615. PubMed PMID: 16204590.
- Proença De Oliveira-maul J, Barbosa De Carvalho H, Dm G, et al. Aging, diabetes, and hypertension are associated with decreased nasal mucociliary clearance. Chest. 2013 Apr;143(4):1091–1097. PubMed PMID: 23100111.
- Renshaw M, Rockwell J, Engleman C, et al. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002 Nov 1;169(9):4697–4701. PubMed PMID: 12391175.
- Bhalla M, Simmons SR, Abamonte A, et al. Extracellular adenosine signaling reverses the age-driven decline in the ability of neutrophils to kill Streptococcus pneumoniae. Aging Cell. 2020;19(10):e13218. 2020/08/13;n/a(n/a).
- Simell B, Vuorela A, Ekström N, et al. Aging reduces the functionality of anti-pneumococcal antibodies and the killing of Streptococcus pneumoniae by neutrophil phagocytosis. Vaccine. 2011 Feb 24;29(10):1929–1934. PubMed PMID: 21236231.
- Meyer KC, Rosenthal NS, Soergel P, et al. Neutrophils and low-grade inflammation in the seemingly normal aging human lung. Mech Ageing Dev. 1998 Aug 14;104(2):169–181. PubMed PMID: 9792195.
- Mahbub S, Deburghgraeve CR, Kovacs EJ. Advanced age impairs macrophage polarization. J Interferon Cytokine Res. 2012;32(1):18–26. PubMed PMID: 22175541.
- Gupta S. Role of dendritic cells in innate and adaptive immune response in human aging. Exp Gerontol. 2014 Jun;54:47–52. PubMed PMID: 24370374.
- Orsini G, Legitimo A, Failli A, et al. Enumeration of human peripheral blood dendritic cells throughout the life. Int Immunol. 2012 Jun;24(6):347–356. PubMed PMID: 22345276.
- Meyer KC, Ershler W, Rosenthal NS, et al. Immune dysregulation in the aging human lung. Am J Respir Crit Care Med. 1996 Mar;153(3):1072–9. PubMed ID PMID: 8630547.
- Van Der Geest KS, Abdulahad WH, Tete SM, et al. Aging disturbs the balance between effector and regulatory CD4+ T cells. Exp Gerontol. 2014 Dec;60:190–196. PubMed PMID: 25449852.
- Adler H, German EL, Mitsi E, et al. Experimental human pneumococcal colonisation in older adults is feasible and safe, not immunogenic. Am J Respir Crit Care Med. 2021 Mar 1;203(5):604-613. PubMed PMID: 32941735; PubMed Central PMCID: PMC7924584.
- Shi Y, Yamazaki T, Okubo Y, et al. Regulation of aged humoral immune defense against pneumococcal bacteria by IgM memory B cell. J Immunol. 2005 Sep 1;175(5):3262–3267. PubMed PMID: 16116217.
- Simell B, Lahdenkari M, Reunanen A, et al. Effects of ageing and gender on naturally acquired antibodies to pneumococcal capsular polysaccharides and virulence-associated proteins. Clin Vaccine Immunol. 2008 Sep;15(9):1391–1397. PubMed PMID: 18596205; PubMed Central PMCID: PMC2546667.
- Romero-Steiner S, Musher DM, Cetron MS, et al. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin Infect Dis. 1999 Aug;29(2):281–288. PubMed PMID: 10476727.
- Rodriguez-Zhurbenko N, Quach TD, Hopkins TJ, et al. Human B-1 cells and B-1 cell antibodies change with advancing age. Front Immunol. 2019;10:483. PubMed PMID: 30941130; PubMed Central PMCID: PMC6433875.
- Park S, Nahm MH. Older adults have a low capacity to opsonize pneumococci due to low IgM antibody response to pneumococcal vaccinations. Infect Immun. 2011;79(1):314–320. PubMed PMID: 21041499.
- Kolibab K, Smithson SL, Shriner AK, et al. Immune response to pneumococcal polysaccharides 4 and 14 in elderly and young adults. I. Antibody concentrations, avidity and functional activity. Immun Ageing. 2005;2(1):10. PubMed PMID: 15982420.
- Infante AJMJ, Orihuela CJ. Mechanisms of predisposition to pneumonia: infants, the elderly and viral infections. In: Brown JHS, Orihuela CJ, editors. Streptococcus pneumoniae. Amsterdam: Academic Press; 2015. p. 363–382.
- Kikawada M, Iwamoto T, Takasaki M. Aspiration and infection in the elderly: epidemiology, diagnosis and management. Drugs Aging. 2005;22(2):115–130. PubMed PMID: 15733019.
- Arguedas A, Trzciński K, O’Brien KL, et al. Upper respiratory tract colonization with Streptococcus pneumoniae in adults. Expert Rev Vaccines. 2020 Apr;19(4):353–366. PubMed PMID: 32237926.
- Miellet WR, Van Veldhuizen J, Nicolaie MA, et al. Influenza-like Illness exacerbates pneumococcal carriage in older adults. Clin Infect Dis. 2020 Oct 30. ciaa1551. PubMed PMID: 33124669.
- Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest. 2009 Jul;119(7):1899–1909. PubMed PMID: 19509469; PubMed Central PMCID: PMC2701860.
- Goncalves MT, Mitchell TJ, Lord JM. Immune ageing and susceptibility to Streptococcus pneumoniae. Biogerontology. 2016 Jun;17(3):449–465. PubMed PMID: 26472172.
- Boyd AR, Orihuela CJ. Dysregulated inflammation as a risk factor for pneumonia in the elderly. Aging Dis. 2011 Dec;2(6):487–500. PubMed PMID: 22288022; PubMed Central PMCID: PMC3265328.
- Weiskopf D, Weinberger B, Grubeck-Loebenstein B. The aging of the immune system. Transplant international: official journal of the European Society for Organ Transplantation. Transpl Int. 2009 Nov;2211:1041–1050. PMID: 19624493.
- Goronzy JJ, Weyand CM. Mechanisms underlying T cell ageing. Nat Rev Immunol. 2019 Sep;19(9):573–583. PubMed PMID: 31186548.
- Naylor K, Li G, Vallejo AN, et al. The influence of age on T cell generation and TCR diversity. J Immunol. 2005 Jun 1;174(11):7446–7452. PubMed PMID: 15905594.
- Effros RB, Cai Z, Linton PJ. CD8 T cells and aging. Crit Rev Immunol. 2003;23(1–2):45–64. PubMed PMID: 12906259.
- Goronzy JJ, Lee WW, Weyand CM. Aging and T-cell diversity. Exp Gerontol. 2007 May;42(5):400–406. PubMed PMID: 17218073; PubMed Central PMCID: PMC2680153.
- Malley R, Srivastava A, Lipsitch M, et al. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect Immun. 2006 Apr;74(4):2187–2195. PubMed PMID: 16552049; PubMed Central PMCID: PMC1418935.
- Ramos-Sevillano E, Ercoli G, Brown JS. Mechanisms of Naturally Acquired Immunity to Streptococcus pneumoniae. Front Immunol. 2019;10:358. PubMed PMID: 30881363; PubMed Central PMCID: PMC6405633.
- Lu YJ, Gross J, Bogaert D, et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008 Sep 19;4(9):e1000159. PubMed PMID: 18802458; PubMed Central PMCID: PMC2528945.
- Malley R, Trzcinski K, Srivastava A, et al. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci U S A. 2005 Mar 29;102(13):4848–4853. PubMed PMID: 15781870; PubMed Central PMCID: PMC555733.
- Trzcinski K, Thompson C, Malley R, et al. Antibodies to conserved pneumococcal antigens correlate with, but are not required for, protection against pneumococcal colonization induced by prior exposure in a mouse model. Infect Immun. 2005 Oct;73(10):7043–7046. PubMed PMID: 16177389; PubMed Central PMCID: PMC1230924.
- Shinefield HR, Black S. Efficacy of pneumococcal conjugate vaccines in large scale field trials. Pediatr Infect Dis J. 2000 Apr;19(4):394–397. PubMed PMID: 10783042.
- Appay V, Rowland-Jones SL. Premature ageing of the immune system: the cause of AIDS? Trends Immunol. 2002 Dec;23(12):580–585. PubMed PMID: 12464569.
- Wilson R, Cohen JM, Reglinski M, et al. Naturally acquired human immunity to pneumococcus is dependent on antibody to protein antigens. PLoS Pathog. 2017 Jan;13(1):e1006137. PubMed PMID: 28135322; PubMed Central PMCID: PMC5279798.
- Kruetzmann S, Rosado MM, Weber H, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 2003 Apr 7;197(7):939–945. PubMed PMID: 12682112; PubMed Central PMCID: PMC2193885.
- Papadatou I, Tzovara I, Licciardi PV. The role of serotype-specific immunological memory in pneumococcal vaccination: current knowledge and future prospects. Vaccines (Basel). 2019 Jan 29;7(1). PubMed PMID: 30700048; PubMed Central PMCID: PMC6466264.
- Jha V, Janoff EN. Complementary Role of CD4+ T cells in response to pneumococcal polysaccharide vaccines in humans. Vaccines (Basel). 2019 Feb 11;7(1). PubMed PMID: 30754689; PubMed Central PMCID: PMC6466080.
- Poolman J, Borrow R. Hyporesponsiveness and its clinical implications after vaccination with polysaccharide or glycoconjugate vaccines. Expert Rev Vaccines. 2011 Mar;10(3):307–322. PubMed PMID: 21434799.
- Farmaki PF, Chini MC, Mangafas NM, et al. Immunogenicity and immunological memory induced by the 13-valent pneumococcal conjugate followed by the 23-valent polysaccharide vaccine in hiv-infected adults. J Infect Dis. 2018 Jun 5;218(1):26–34. PubMed PMID: 29722823.
- Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat Rev Immunol. 2009 Mar 9;9(3):213–220. PubMed PMID: 19214194.
- Wu YC, Kipling D, Dunn-Walters DK. Age-related changes in human peripheral blood igh repertoire following vaccination. Front Immunol. 2012;3:193. PubMed PMID: 22787463; PubMed Central PMCID: PMC3391689.
- Su I, Tarakhovsky A. B-1 cells: orthodox or conformist? Curr Opin Immunol. 2000 Apr;12(2):191–194. PubMed PMID: 10712945.
- Weksler ME. Changes in the B-cell repertoire with age. Vaccine. 2000 Feb 25;18(16):1624–1628. PubMed PMID: 10689139.
- Weksler ME, Goodhardt M, Szabo P. The effect of age on B cell development and humoral immunity. Springer Semin Immunopathol. 2002;24(1):35–52. PubMed PMID: 11974580.
- Pace D. Glycoconjugate vaccines. Expert Opin Biol Ther. 2013 Jan;13(1):11–33. PubMed PMID: 22992106.
- Hutter J, Lepenies B. Carbohydrate-based vaccines: an overview. Methods Mol Biol. 2015;1331:1–10. PubMed PMID: 26169731.
- Marra F, Vadlamudi NK. Efficacy and safety of the pneumococcal conjugate-13 valent vaccine in adults. Aging Dis. 2019 Apr;10(2):404–418. PubMed PMID: 31011485; PubMed Central PMCID: PMC6457056.
- Vadlamudi NK, Parhar K, Altre Malana KL, et al. Immunogenicity and safety of the 13-valent pneumococcal conjugate vaccine compared to 23-valent pneumococcal polysaccharide in immunocompetent adults: a systematic review and meta-analysis. Vaccine. 2019 Feb 14;37(8):1021–1029. PubMed PMID: 30685252.
- Puchta A, Naidoo A, Verschoor CP, et al. TNF drives monocyte dysfunction with age and results in impaired anti-pneumococcal immunity. PLoS Pathog. 2016 Jan;12(1):e1005368. PubMed PMID: 26766566; PubMed Central PMCID: PMC4713203.
- World Health Organization. World report on ageing and health 2015 [ cited 2020 Aug 10]. Available from: https://www.who.int/ageing/publications/world-report-2015/en/.
- World Health Organization. Decade of Health Ageing 2020-2030. 2020 [ cited 2020 October 10]. Available from: https://www.who.int/initiatives/decade-of-healthy-ageing.
- World Health Organization. Global Vaccine Action Plan 2011-2020 2020 [ cited 2020 October 10]. Available from: https://www.who.int/immunization/global_vaccine_action_plan/GVAP_doc_2011_2020/en/.
- World Health Organization. Immunization Agenda 2030: a global strategy to leave no one behind. 2020 [ cited 2020 October 10]. Available from: https://www.who.int/immunization/immunization_agenda_2030/en/
- Rudnicka E, Napierala P, Podfigurna A, et al. The World Health Organization (WHO) approach to healthy ageing. Maturitas. 2020 Sep;139:6–11. PubMed PMID: 32747042; PubMed Central PMCID: PMC7250103.
- International Federation on Ageing. Adult Immunization Advocacy Summit Report 2015 [ cited 2020 Aug 30]. Available from: https://ifa.ngo/wp-content/uploads/2015/08/IFA-Adult-Immunization-Advocacy-Summit-Report.pdf.
- Doherty TM, Del Giudice G, Maggi S. Adult vaccination as part of a healthy lifestyle: moving from medical intervention to health promotion. Ann Med. 2019 Mar;51(2):128–140. PubMed PMID: 31025882.
- Jorgensen P, Mereckiene J, Cotter S, et al. How close are countries of the WHO European Region to achieving the goal of vaccinating 75% of key risk groups against influenza? Results from national surveys on seasonal influenza vaccination programmes, 2008/2009 to 2014/2015. Vaccine. 2018 Jan 25;36(4):442–452. PubMed PMID: 29287683; PubMed Central PMCID: PMC5777640.
- Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2019 Nov 22;68(46):1069–1075. PubMed PMID: 31751323; PubMed Central PMCID: PMC6871896.
- Healthy People 2020. Immunization and infectious diseases: objectives [ cited 2020 August 15]. Available from: https://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives.
- Privor-Dumm LA, Ga P, Barratt J, et al. A global agenda for older adult immunization in the COVID-19 era: a roadmap for action. Vaccine. 2020 Jul 3:S0264-410X(20)30885-9. PubMed PMID: 32703743; PubMed Central PMCID: PMC7332930.
- Centers for Disease Control and Prevention. Altered Immunocompetence [ cited 2020 October 10]. Available from: https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/immunocompetence.html.
- Rubin LG, Levin MJ, Ljungman P, et al. IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2013 Feb;58(3):e44–100. PubMed PMID: 24311479.
