ABSTRACT
Introduction
Asthma is one of the most common chronic respiratory conditions worldwide and can be exacerbated by influenza. Findings from early trials demonstrated a higher risk of medically significant wheezing in otherwise healthy young children (aged 6 − 23 months) following administration of the Ann Arbor-backbone live attenuated influenza vaccine (LAIV-AA). In more recent years, several additional studies have investigated the safety of LAIV-AA in older children (2 − 17 years of age) and adults with asthma or prior wheezing, but these findings have not yet been systematically evaluated.
Areas covered
We conducted a systematic literature review to assess and synthesize the evidence from all available studies on the safety of LAIV-AA in people aged 2 − 49 years with a diagnosis of asthma or recurrent wheezing.
Expert opinion
Fourteen studies over 20 years, involving a total of 1.2 million participants, provided evidence that LAIV-AA was well tolerated with no safety concerns in individuals aged 2 − 49 years with a diagnosis of asthma or recurrent wheezing. These data can help inform guidelines for use of LAIV-AA in children and adults with a history of asthma or recurrent wheezing.
1. Introduction
Asthma is one of the most prevalent chronic respiratory conditions worldwide, affecting an estimated 339 million people globally [Citation1]. Among children, asthma is the most common chronic disease, with an estimated 14% of all children globally having been diagnosed with asthma at some point in their lives [Citation2,Citation3]. Influenza can be associated with asthma complications as it can cause further inflammation of the airways and lungs, worsening asthma symptoms, and triggering acute asthma exacerbations [Citation4]. Children with asthma have higher rates of influenza associated hospitalization compared with healthy children/children without asthma [Citation5,Citation6].
Vaccination remains the most effective method of preventing influenza [Citation7,Citation8], with public health and medical experts, along with stakeholder groups such as the United States (US) Advisory Committee on Immunization Practices (ACIP) and Canada’s National Advisory Committee on Immunization recommending that children and adults with asthma receive an annual influenza vaccine [Citation4,Citation9,Citation10]. In the United Kingdom (UK), influenza vaccination is offered to everyone 6 months and older with severe asthma [Citation11]. Influenza vaccination is effective in reducing the number and severity of respiratory infections and can prevent asthma exacerbations. Importantly, influenza vaccination demonstrated vaccine effectiveness of 59–78% for the prevention of asthma attacks leading to emergency visits and/or hospitalizations [Citation12].
Live attenuated influenza vaccine (Ann Arbor-backbone; LAIV-AA), developed using master donor viruses type A and B (A/AA and B/AA), is approved for use in those aged 2–49 years of age in the US and multiple additional countries [Citation13], and those aged 2–17 years of age in Europe [Citation14]. An early pragmatic, randomized, placebo-controlled safety study of children aged 1–17 years found a significant increase in illnesses coded as reactive airway disease in children aged 18–35 months (risk ratio 4.06; 90% confidence interval: 1.29 to 17.86) [Citation15]. Subsequently, in a randomized study of children aged 6–59 months without a recent episode of wheezing or severe asthma, prospectively defined medically significant wheezing was increased in children aged 6–23 months who received LAIV-AA compared with those who received inactivated influenza vaccine (IIV) [Citation13,Citation16]. This study also demonstrated a higher risk of hospitalization in children aged 6–11 months following administration of LAIV-AA [Citation13].
As a result, clinical guidance via prescribing information and vaccination policy guidelines recommended against the use of LAIV-AA in children younger than 2 years of age, as well as individuals 2 years and older with a history of asthma or wheezing. Current US Food and Drug Administration (FDA) label warnings for use of LAIV-AA in the US note that children younger than 5 years of age with recurrent wheezing and persons of any age with asthma may be at increased risk of wheezing following the administration of LAIV-AA [Citation13,Citation17]. In the US ACIP recommendations, LAIV-AA is listed as a contraindication in children aged 2 − 4 years who have received an asthma diagnosis, or who have had a wheezing episode diagnosed by a healthcare provider in the preceding 12 months. Much like the FDA, the ACIP list asthma in persons aged 5 years or over as a precaution for the use of LAIV-AA [Citation9].
Over the 8 years since the US approval, the safety and efficacy of LAIV-AA in children with a history of asthma or recurrent respiratory infections had been demonstrated [Citation18,Citation19]. As a result, children aged 2 − 17 years with a history of mild to moderate asthma or recurrent wheeze are within the EU indication. Use of LAIV-AA in children and adolescents with severe asthma or active wheezing are listed as a special precaution and warning because these individuals have not been adequately studied in clinical trials. Similar to the US, the EMA recommends that LAIV-AA should not be used in infants and toddlers below 24 months of age because of safety concerns regarding increased rates of hospitalization and wheezing in this population [Citation14].
Following the significant use of LAIV-AA in children in recent years, a number of studies have investigated the safety of LAIV-AA in individuals with asthma or prior wheezing. Findings from these studies have not yet been reviewed as a whole. The aim of this systematic literature review was to assess the available evidence regarding the safety of LAIV-AA in individuals aged 2–49 years with a diagnosis of asthma (any severity) or recurrent wheezing.
2. Methods
This systematic literature review evaluated studies relating to the safety of LAIV-AA in children and adults aged 2–49 years. The systematic review has been registered with PROSPERO (https://www.crd.york.ac.uk/prospero/), ID CRD42020171839. shows the full methodology, including the search, screening, data collection, and narrative synthesis processes.
Figure 1. Full methodology of the systematic literature review, including the search, screening, data extraction, and narrative synthesis processes. LAIV, live attenuated influenza vaccine
2.1. Search strategy and eligibility criteria
The systematic literature search was conducted using the electronic databases PubMed and The Cochrane Database of Systematic Reviews. The following search terms were used: (‘Live attenuated influenza vaccine’ OR LAIV) AND ‘safety’ AND ‘asthma’ OR (‘respiratory sounds’ OR ‘respiratory’ AND ‘sounds’ OR ‘respiratory sounds’ OR ‘wheezing’ OR ‘respiratory’). There were no time restrictions applied to the search criteria.
provides an overview of the eligibility criteria for this systematic literature review. Studies were excluded if they did not contain data on Ann Arbor LAIV.
Table 1. Eligibility criteria for studies to be included in the systematic literature review
2.2. Study selection
Initially, study titles and abstracts were screened for eligibility and were excluded if they clearly did not meet the inclusion criteria. Thereafter, full texts of potentially relevant studies were obtained and reviewed independently by two reviewers to determine eligibility for inclusion. Studies containing duplicate information or not meeting the inclusion criteria upon further review were excluded. Ambiguous decisions were resolved after discussion with the authors.
2.3. Data extraction
Data were extracted into an Excel document to collate and summarize the study design and results. Data extracted included study design, setting, time period, participants, LAIV-AA formulation, comparator, outcome, events in the vaccinated and comparator groups, types of event measured, and key results. Any studies that did not contain data meeting the inclusion criteria during data extraction were subsequently excluded. Meta-analysis was not considered appropriate for this collection of studies due to the variability in outcomes measured, which included hazard ratios, relative risk, odds ratios, spirometry values, change in asthma control test scores, incidence of asthma exacerbation, and rates of adverse events. Hence, a narrative synthesis was performed.
2.4. Synthesis
A narrative synthesis was undertaken to synthesize the findings of the included studies. First, the results of each study were assessed systematically, highlighting the important characteristics (outcomes, age groups investigated, comparators etc.) and key results (safety and reactogenicity data). The studies were then grouped by key outcomes and findings in relation to age group and presented in tabular form. This allowed for comparison of studies with similar outcomes measured and to help identify any patterns. Studies with outcomes not repeated or measured in other studies could not be grouped and were assessed separately. The key implications of the studies were then summarized descriptively following the comparison of the study outcomes.
3. Results
The initial literature search identified 84 records, and from these, 28 full-text articles were assessed for eligibility (). Fourteen articles were then excluded as they did not meet the eligibility criteria for the following reasons: no measures of asthma or wheezing (one article), did not report incidence rate/risk measure (four articles), insufficient data regarding safety of LAIV-AA with asthma (one article), duplicate data from other studies (four articles), participants not being within the age range (one article), no comparison for healthy controls (one article), LAIV-AA was not assessed (one article), and no results available as the article was a protocol (one article).
Figure 2. PRISMA flow diagram of systematic literature review search strategy and screening process. LAIV, live attenuated influenza vaccine; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses
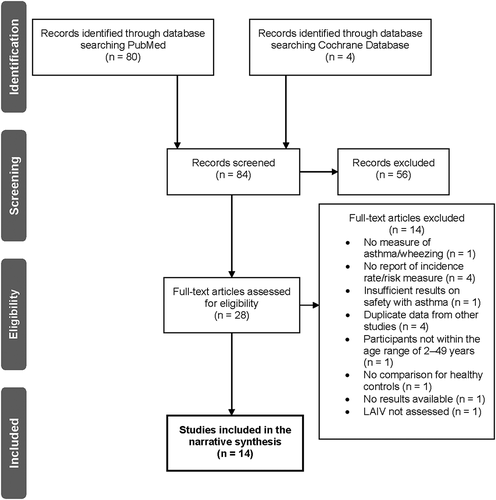
A total of 14 studies were retained for the narrative synthesis of the systematic literature review. Detailed characteristics of the studies included in the review are presented in . Study design was diverse and included: randomized, non-randomized, retrospective, prospective, observational, and non-interventional analysis. The number of subjects with asthma or recurrent wheezing in the studies ranged substantially, from 48 [Citation20] to 166,174 [Citation21]. The included studies were conducted from 1997–1998 [Citation20] to 2016–2017 [Citation22], over 20 influenza seasons.
Table 2. Baseline characteristics and key results for included studies in the systematic literature review
The method of comparison also varied within the studies; nine studies used IIV as a control [Citation18,Citation21,Citation23–29], two used unvaccinated controls [Citation28,Citation29], three used a reference time period self-control (a specific time frame pre-vaccination or post-vaccination) [Citation23,Citation30,Citation31], one used placebo [Citation20], One used a cohort without asthma [Citation32], and one used baseline asthma symptoms as the control [Citation22]. The majority of studies (12; 86%) evaluated the safety of LAIV-AA in children and adolescents (aged 2–18 years), whereas only two were performed in adults aged 18–49 years [Citation28,Citation31].
The key results from the studies in terms of outcomes measured are presented in with the study details. Notably, no studies found an increased risk of significant clinical outcomes measured post-vaccination with LAIV-AA in children and adolescents with a history of wheeze or asthma. Specifically, no significant differences in the risk of asthma exacerbations, wheezing, or healthcare utilization were identified in LAIV-AA recipients compared with the controls. In two studies, there was a higher incidence of a runny or stuffy nose in children following LAIV-AA [Citation18,Citation24]. LAIV-AA was associated with lower odds of inpatient or emergency department visits for asthma exacerbation compared with IIV [Citation26] in one study, and in another, significantly higher incidences of wheezing were noted in those receiving inactivated trivalent influenza vaccine compared with trivalent LAIV-AA [Citation18].
To aid further comparison, study findings are presented by risk ratio in , and by type of outcome measured and age group in . Approximately 79% of studies evaluated medically attended acute/lower respiratory illness, hospitalizations, emergency department visits, and healthcare utilization, which were investigated across a wide range of age groups, from infants to adults. LAIV-AA was not associated with an increased risk for any of the measured outcomes in any age group investigated (). demonstrates the range of findings with no outcomes being statistically increased in LAIV-AA recipients versus controls.
Figure 3. Key findings grouped by study. Studies not reporting outcomes by ratio are not presented here. CI; confidence interval; IIV, inactivated influenza vaccine; ED, emergency department; LAIV, live attenuated influenza vaccine; LRE, lower respiratory events; LRTI, lower respiratory tract infection; MAARI, medically attended acute respiratory illness; SCRI, self-controlled risk interval. *During days 0–14 post-LAIV3. †During days 0–14 in children aged 5–9 years; reference period is before day 0 and after 14 or 42 days post-LAIV. ‡During the 1–42-day risk interval post-vaccination. §Any hospitalization during the 42-day risk interval post-vaccination. ‖Risk ratio/relative risk. ¶Ratio of odds ratios. #Incidence rate ratio. **Adjusted hazard ratio. ††Adjusted ratio of rate ratios. ‡‡Ratio covers different types of statistical analysis. The x-axis scale uses a log10 scale
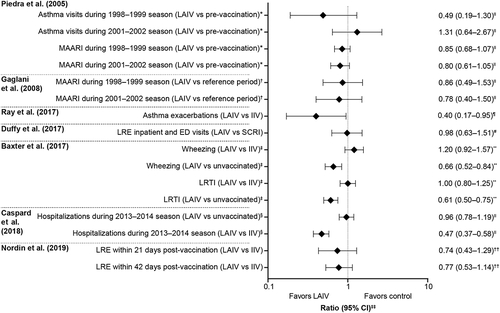
Table 3. Key study findings by outcome measured and age group
There was no difference seen in the risk of an asthma associated event when comparing the outcomes measured in children and adults. In the two studies performed in adults aged 18 − 49 years, the outcomes investigated included medically attended lower respiratory events, hospitalizations [Citation28,Citation31], vaccine safety signals, and wheezing [Citation28]. All other outcomes were only assessed in children aged 2–18 years.
4. Discussion
The results of this systematic literature review demonstrated that LAIV-AA was well tolerated in adults (18–49 years of age) and children (2–17 years of age) with a diagnosis of asthma or a history of recurrent wheezing. No safety concerns were associated with LAIV-AA in children and adolescents with asthma across a wide range of asthma-related outcomes investigated by the studies included in this review. Only runny nose/nasal congestion was associated with LAIV, which is a minor and well-known reactogenicity event associated with the intranasal replication of LAIV-AA [Citation33].A strength of this review was the comprehensive approach, which allowed for a detailed assessment of all studies available to date despite methodological differences. A total of 471,802 participants with asthma or recurrent wheezing from the included studies were evaluated as part of this systematic review (in studies where N is specified for each treatment group; LAIV-AA participants N = 55,849, controls N = 356,753), which adds to the robustness of the findings. There was a wide range in the number of participants across the studies. The largest of which was from a US vertically integrated healthcare system which included 166,174 participants with asthma or recurrent wheezing, of whom 9,278 (6%) received LAIV-AA [Citation21].
The 14 studies evaluated support the safety of LAIV-AA in individuals 2–49 years of age with asthma across a range of outcomes, and when compared with placebo, trivalent or quadrivalent IIV, reference periods, or unvaccinated or intra-patient controls, across 20 influenza seasons. Although two large studies investigated the safety of LAIV-AA in adults (total of 73,801 participants) [Citation28,Citation31], no data are available for adults older than 49 years of age.
As studies were primarily undertaken in persons with mild to moderate asthma or a history of recurrent wheezing (), this analysis provides reassurance of the safety of LAIV-AA in this population. However, given the limited data on the safety of LAIV-AA in children and adults with severe asthma, conclusions on the safety of LAIV-AA in this population will require further investigation before informed guidance for this population can be developed.
A limitation of this systematic review is the different methodologies and different outcomes used in the studies included. As a result, a formal meta-analysis could not be conducted. Treatment administration (LAIV, IIV, or no vaccine) was not controlled in the non-randomized studies, so observed differences between treatment groups may be confounded by differences between the groups at baseline. The included articles studied a range of different LAIV-AA formulations including monovalent, trivalent and quadrivalent. The solicited sides effects of these formulations have a high degree of homogeneity suggesting similar safety profiles regardless of valency. Despite these limitations, the overall results were consistent across studies in this review, providing confidence in the findings.
Several of the included studies recommended that the guidelines (specific to the US) for use of LAIV-AA in children with asthma or wheezing should be reconsidered in the light of the evidence demonstrating no additional risk compared with study controls. Duffy et al [Citation31] stated that the precaution in the US guidance regarding increased risk of wheezing after LAIV-AA in those with intermittent or mild persistent asthma or children 2–4 years old with recurrent wheezing might not be warranted. Similarly, Piedra et al [Citation30] suggested that expanding the current recommendations to include children with mild intermittent asthma should be considered. The UK has retained a recommendation against use of LAIV-AA in those with an acute asthma exacerbation in the previous 72 hours, as there are no available data on the safety of LAIV-AA vaccination in this setting (and such children should be offered IIV [Citation34]). However, the study by Turner et al [Citation22] provided evidence for the revised UK guidance for the 2019/20 influenza season that ‘children with asthma on inhaled corticosteroids may safely be given LAIV, irrespective of the dose prescribed.’
5. Conclusions
The evidence from this systematic literature review demonstrated that LAIV-AA was well tolerated in children and adults aged 2 − 49 years with mild to moderate asthma or recurrent wheeze, with no safety concerns or increased risk identified for any of the respiratory outcomes measured when comparing LAIV-AA with injectable influenza vaccines or non-vaccine controls.
6. Expert opinion
Although randomized controlled trials have demonstrated a higher risk of reactive airway disease and wheezing events in children 6 − 23 months following administration of LAIV-AA [Citation13,Citation15,Citation16], multiple studies in individuals 2 − 49 years of age with asthma or a history of recurrent wheezing have shown no increased risk of significant adverse events following LAIV-AA. Fourteen studies that included a total of 471,802 participants with asthma or recurrent wheezing found no significant safety concerns or increased risk for any asthma-related outcomes measured. The evidence presented in the studies in this review may help inform clinical guidelines for the use of LAIV-AA in children and adults with mild to moderate asthma or recurrent wheeze [Citation30,Citation31]. Due to limited data, the safety of LAIV-AA in individuals with severe asthma or active wheezing has not been established.
When licensed and recommended vaccines already exist, newer vaccines, particularly those with a novel mechanism of action, face a high hurdle to demonstrate safety in specific subpopulations. It is not always possible to conduct the necessary studies prior to approval and thus data obtained post-licensure becomes particularly important. While prospective, double-blind, randomized studies remain the gold standard for evidence, high-quality, non-randomized studies can greatly contribute to our understanding of vaccine safety and effectiveness in the real world. When possible, such studies should be conducted in a consistent manner to enable pooling of data across studies; such harmonization would rely on explicit guidance from vaccine experts and policymakers regarding best practices.
Article highlights
Influenza has been associated with asthma complications and exacerbations; vaccinating against influenza is effective in reducing the number and severity of respiratory infections and can prevent asthma exacerbation.
Findings from early randomized controlled trials suggested a higher risk of wheezing in children aged 6–23 months following administration of LAIV. At that time, limited data were available in older children and adults with a history of asthma or recurrent wheezing. As a result, initial clinical guidance warned against the use of LAIV-AA in individuals with a history of asthma or recurrent wheezing.
Since initial approval, multiple studies have been conducted evaluating the safety of LAIV-AA in individuals aged 2–49 years with a diagnosis of asthma or recurrent wheezing. This systematic literature review assessed and synthesized the available evidence regarding the safety of LAIV-AA in this population.
No studies found an increased risk of significant asthma-related clinical outcomes measured post-vaccination with LAIV; including no significant differences in the risk of asthma exacerbations, wheezing, or hospitalizations in LAIV-AA recipients with a history of mild to moderate asthma or recurrent wheezing compared with controls. The only event increased after LAIV-AA was runny nose/nasal congestion.
Gaps in the literature include a lack of data in individuals with active wheezing or diagnosed with severe asthma.
Declaration of interest
A Bandell and CS Ambrose are employees of and shareholders in AstraZeneca. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgment(s)
India Wright, MSc and Talya Underwood MPhil (Cantab) performed the systematic literature review and provided medical writing support, and editorial support was provided by Rachael Cazaly, BSc, all of Core Medica, London, UK, supported by AstraZeneca according to Good Publication Practice guidelines (Link). The Sponsor was involved in the study design, collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
Additional information
Funding
References
- World Health Organisation. Asthma 2020 [cited 2021 Jun]. Available from: https://www.who.int/news-room/q-a-detail/asthma.
- Pearce N, Ait-Khaled N, Beasley R, et al. Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax. 2007;62(9):758–766.
- Collaborators GBDCRD. Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8(6):585–596.
- CDC. Flu and People with Asthma 2020 [cited 2021 Jun]. Available from: https://www.cdc.gov/flu/highrisk/asthma.htm.
- Homaira N, Briggs N, Jl O, et al. Impact of influenza on hospitalization rates in children with a range of chronic lung diseases. Influenza Other Respir Viruses. 2019;13(3):233–239.
- Miller EK, Griffin MR, Edwards KM, et al. Influenza burden for children with asthma. Pediatrics. 2008;121(1):1–8.
- World Health Organization. Vaccination Last reviewed 2020. Available from: http://www.euro.who.int/en/health-topics/communicable-diseases/influenza/vaccination.
- CDC. Healthy habits to prevent flu 2020 [cited 2021 Jun]. Available from: https://www.cdc.gov/flu/prevent/actions-prevent-flu.htm.
- Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and Control of Seasonal Influenza with Vaccines: recommendations of the Advisory Committee on Immunization Practices - United States, 2020-21 Influenza Season. MMWR Recomm Rep. 2020;69(8):1–24.
- Young K, Gemmill I, Harrison R, Immunization NACo. Summary of the NACI Seasonal Influenza Vaccine Statement for 2020–2021. Canada Communicable Disease Report. 2020;46(5):132–137.
- Public Health England. The national flu immunisation programme 2020 to 2021- update Last reviewed 2020. [cited 2021 Jun]. Available from: https://www.england.nhs.uk/wp-content/uploads/2020/05/Letter_AnnualFlu_2020-21_20200805.pdf.
- Vasileiou E, Sheikh A, Butler C, et al. Effectiveness of influenza vaccines in asthma: a systematic review and meta-analysis. Clin Infect Dis. 2017;65(8):1388–1395.
- MedImmune LLC FLUMIST Quadrivalent. 2020-2021 formula. Highlights of Prescribing Information 2020. [cited 2021 Jun]. Available from: https://www.azpicentral.com/flumistquadrivalent/flumistquadrivalent.pdf.
- AstraZeneca. Fluenz Tetra Summary of Product Characteristics 2019.[cited 2021 Jun]. Available from: https://www.ema.europa.eu/en/documents/product-information/fluenz-tetra-epar-product-information_en.pdf.
- Bergen R, Black S, Shinefield H, et al. Safety of cold-adapted live attenuated influenza vaccine in a large cohort of children and adolescents. Pediatr Infect Dis J. 2004;23(2):138–144.
- Belshe RB, Edwards KM, Vesikari T, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007;356(7):685–696.
- US Food and Drug Administration. FDA FluMist full prescribing information 2020. [cited 2021 Jun]. Available from: https://www.fda.gov/media/120689/download.
- Fleming DM, Crovari P, Wahn U, et al. Comparison of the efficacy and safety of live attenuated cold-adapted influenza vaccine, trivalent, with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J. 2006;25(10):860–869.
- Ashkenazi S, Vertruyen A, Arístegui J, et al. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J. 2006;25(10):870–879.
- Redding G, Re W, Hessel C, et al. Safety and tolerability of cold-adapted influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J. 2002;21(1):44–48.
- Tennis P, Toback SL, Andrews EB, et al. A US postmarketing evaluation of the frequency and safety of live attenuated influenza vaccine use in nonrecommended children younger than 5 years: 2009–2010 season. Vaccine. 2012;30(42):6099–6102.
- Turner PJ, Fleming L, Saglani S, et al. Safety of live attenuated influenza vaccine (LAIV) in children with moderate to severe asthma. J Allergy Clin Immunol. 2020;145(4):1157–64 e6.
- Gaglani MJ, Piedra PA, Riggs M, et al. Safety of the intranasal, trivalent, live attenuated influenza vaccine (LAIV) in children with intermittent wheezing in an open-label field trial. Pediatr Infect Dis J. 2008;27(5):444–452.
- Ambrose CS, Dubovsky F, Yi T, et al. The safety and efficacy of live attenuated influenza vaccine in young children with asthma or prior wheezing. Eur J Clin Microbiol Infect Dis. 2012;31(10):2549–2557.
- Tennis P, Toback SL, Andrews E, et al. A postmarketing evaluation of the frequency of use and safety of live attenuated influenza vaccine use in nonrecommended children younger than 5 years. Vaccine. 2011;29(31):4947–4952.
- Ray GT, Lewis N, Goddard K, et al. Asthma exacerbations among asthmatic children receiving live attenuated versus inactivated influenza vaccines. Vaccine. 2017;35(20):2668–2675.
- Nordin JD, Vazquez-Benitez G, Olsen A, et al. Safety of guidelines recommending live attenuated influenza vaccine for routine use in children and adolescents with asthma. Vaccine. 2019;37(30):4055–4060.
- Baxter R, Eaton A, Hansen J, et al. Safety of quadrivalent live attenuated influenza vaccine in subjects aged 2–49 years. Vaccine. 2017;35(9):1254–1258.
- Caspard H, Steffey A, Mallory RM, et al. Evaluation of the safety of live attenuated influenza vaccine (LAIV) in children and adolescents with asthma and high-risk conditions: a population-based prospective cohort study conducted in England with the Clinical Practice Research Datalink. BMJ Open. 2018;8(12):e023118.
- Piedra PA, Gaglani MJ, Riggs M, et al. Live attenuated influenza vaccine, trivalent, is safe in healthy children 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age in a community-based, nonrandomized, open-label trial. Pediatrics. 2005;116(3):e397–407.
- Duffy J, Lewis M, Harrington T, et al. Live attenuated influenza vaccine use and safety in children and adults with asthma. Ann Allergy Asthma Immunol. 2017;118(4):439–444.
- Turner PJ, Southern J, Andrews NJ, et al. Safety of live attenuated influenza vaccine in atopic children with egg allergy. J Allergy Clin Immunol. 2015;136(2):376–381.
- Herve C, Laupeze B, Del Giudice G, et al. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines. 2019;4(1):39.
- Public Health England. Influenza: the green book, chapter 19 Last reviewed 2018.[cited 2021 Jun]. Available from: https://www.gov.uk/government/publications/influenza-the-green-book-chapter-19.
