ABSTRACT
Introduction
Modeling analyses have attempted to quantify the global impact of pneumococcal conjugate vaccines (PCVs) on pneumococcal disease (PD), however these pediatric models face several challenges in obtaining comprehensive impact measurements.
Areas Covered
We present several measurement challenges and discuss examples from recently published pediatric modeling evaluations. Challenges include estimating the number of infants fully or partially vaccinated with PCVs, inclusion of indirect effects of vaccination, accounting for various dosing schedules, capturing effect of PCVs on nonspecific, noninvasive PD, and inclusion of adult PCV use.
Expert Opinion
The true impact of PCVs has been consistently underestimated in published analyses due to multiple measurement challenges. Nearly 100 million adults are estimated to have received PCV13 over the last decade globally, potentially preventing up to 662 thousand cases of PD. Approximately 4.1 million cases of invasive PD alone may have been averted through indirect protection. Estimates of PCV impact on noninvasive PD remain a challenge due to altered epidemiology. Program switches, incomplete vaccination, and private market uptake among children also confound PD impact estimates. Taken together, the number of averted PD cases from PCV use in the last ten years may be up to three times higher than estimated in previous studies.
1. Introduction
Pneumococcal disease resulting from the bacterium Streptococcus pneumoniae (S. pneumoniae) is a leading contributor of vaccine-preventable morbidity and mortality worldwide [Citation1]. The bacterium has at least one hundred distinct serotypes, and can cause both invasive pneumococcal disease (IPD), such as meningitis and bacteremia, and noninvasive disease, including pneumonia and otitis media (OM) [Citation2]. Cases of pneumococcal disease most commonly occur among young children, in the elderly, and in adults with certain chronic or immunocompromising conditions [Citation3–5].
Since 2000, pneumococcal conjugate vaccines (PCVs) have been introduced in pediatric national immunization programs (NIPs) to provide infants with protection against the most prevalent and pathogenic serotypes. The first PCV that was licensed was the 7-valent PCV (PCV7; Prevenar, Pfizer Inc), which was replaced beginning in 2009 by two higher valent vaccines covering 10 and 13 serotypes, namely PCV10 (Synflorix, GlaxoSmithKline) and PCV13 (Prevenar 13, Pfizer Inc). As of the end of 2020, PCVs are included in the national schedule in more than 160 countries across the globe, with PCV13 in more than 130 NIPs, and PCV10 in over 30 NIPs [Citation6,Citation7]. Additionally, another 10-valent PCV (Pneumosil, Serum Institute of India) has been licensed in some markets since receiving World Health Organization (WHO) pre-qualification in December 2019. There have been numerous studies globally that have documented reductions in IPD [Citation8–11], OM [Citation12–15], pneumonia [Citation16–19], and antibiotic resistance [Citation20] from the use of PCVs.
In 2018, Wahl et al. estimated the global burden of pneumococcal disease attributable to S. pneumoniae from 2000 to 2015, whereupon authors predicted the number of deaths prevented by PCV use during this period [Citation21]. Applying vaccine coverage, vaccine-type IPD vaccine efficacy, and regional serotype coverage estimates pre-PCV introduction to disease burden results, roughly 250 thousand pneumococcal deaths were estimated to be averted among children under five years of age during this 15-year period. However, calculations considered PCV7, which is no longer in use since second-generation PCVs have been introduced, and did not capture the impact of PCVs in the latter half of the decade during which PCV uptake increased substantially.
Two publications recently attempted to estimate a cumulative multi-country public health impact of these second-generation PCVs since their introduction [Citation6,Citation7]. Chapman et al. (2020) conducted a modeling analysis to calculate the number of pneumococcal disease cases and consequent deaths averted in children less than five years old resulting from PCV13 NIPs between 2010 and 2019 [Citation6]. The paper concludes that in the last ten years, PCV13 NIPs have prevented approximately 175.2 million cases of disease and 625 thousand deaths, underscoring the massive public health impact this vaccine has had globally. With a similar objective, Lecrenier et al. (2020) published an estimation of the number of pneumococcal deaths averted from PCV10 vaccination among children less than five years old over the last decade within a review of its ten-year experience [Citation7]. Authors stated that estimations were based on the number of PCV10 doses distributed globally and PCV10 vaccine efficacy from a randomized controlled trial in Latin America, however details of the methods or the model are not provided. According to their calculations, using a broader outcome definition than Chapman et al. (2020), an estimated 696 thousand deaths have been prevented in the birth cohorts receiving PCV10 between 2009 and 2018. Notably, both studies only measured the impact of pediatric PCV vaccination in children under five, not accounting for the indirect effects of PCVs in unvaccinated individuals.
An additional group, Chen and colleagues (2019), took a more optimistic view of the world and estimated the potential future vaccine preventable disease episodes and deaths from 2015 to 2045 assuming 180 countries provided PCV13 to their infant birth cohort with high uptake comparable to diphtheria-tetanus-pertussis (DTP) vaccine [Citation22]. They estimated that under this optimal scenario, over 54.6 million disease cases and 399 thousand deaths could be averted annually in children less than five years of age.
Although these recently published analyses all suggest a tremendous public health impact of PCVs, either over the past decades or predicted into the future, there are multiple challenges in comprehensively and accurately measuring this impact. The objective of this review is to present the most pertinent challenges in measuring the true public health impact of PCVs: 1) estimating the number of infants vaccinated with PCVs; 2) including indirect effects of vaccination; 3) accounting for alternate dosing schedules; 4) capturing the effect of PCVs on nonspecific, noninvasive pneumococcal disease; and 5) accounting for the impact of adult PCV use. In each section, we explore how each measurement challenge may consequently affect estimates of disease cases and deaths prevented by vaccination.
2. Review of measurement challenges
2.1. Number of infants vaccinated with PCVs
To estimate pneumococcal disease cases and deaths prevented by PCVs, a primary step is to determine the number of children receiving direct protection from vaccination. In Lecrenier et al. [Citation7], the number of children potentially reached with PCV10 is estimated using internal data of the number of vaccine doses shipped to each country. Meanwhile, in Chapman et al. [Citation6] and Wahl et al. [Citation21], the number of children protected with PCVs were derived from country-specific series completion statistics by birth cohort reported by the WHO [Citation23]. Administrative data used to estimate PCV coverage are oftentimes misreported and likely overestimated in lower-middle income countries due to poor vaccine coverage data, although the magnitude is uncertain. Moreover, children who partially completed their PCV vaccination series were not captured in the projected number of vaccine-protected children in either study. When comparing WHO-reported first (PCV1), second (PCV2), and final PCV dose coverage (PCV3) in the latest year of available data by country, on average an estimated 4.0% and 6.8% of children born in countries with PCV NIPs complete their first dose or second dose of the series, respectively (Appendix A1) [Citation23]. As a result, nearly 11% of children who received only one or two doses of PCV (PCV1 or PCV2) are assumed to have no vaccine protection in model calculations. Though some studies have suggested that incompletely vaccinated children may derive some protection, it could be at a lower level than children fully vaccinated with the recommended three or four dose schedule [Citation15,Citation24–26].
In addition, some countries were excluded from the aforementioned PCV13 global impact analysis [Citation6] if they had switched their NIP from PCV13 to the lower-valent PCV10. This switch has been primarily driven by either budget constraints or the success of PCV13 in suppressing the burden of vaccine-type disease [Citation27]. As examples, countries including Belgium, New Zealand, Sweden, and El Salvador have nationally or regionally opted to change the PCV13 infant vaccination program to PCV10 [Citation27]. However, recent experiences of switching from PCV13 to PCV10 vaccination, such as in Belgium, have resulted in an increase in IPD incidence from the reemergence of PCV13 unique serotypes (e.g. 19A), suggesting that maintaining a higher valent PCV in the NIP is necessary even when disease rates for the covered serotypes have fallen at the population level [Citation28]. Countries with vaccination discontinuity were previously omitted [Citation6] due to the challenges in attributing the benefit of either PCV across years or country jurisdictions, further contributing to the underestimation of the total number of infants protected by PCV13 throughout the last decade.
Finally, the estimated number of children vaccinated with PCVs in prior global impact studies’ base case analyses did not include infants vaccinated outside of a country’s NIP [Citation6,Citation21], specifically when parents or caregivers pay for the vaccine out of their own pocket. Many countries in the Eastern Mediterranean, Europe, South-East Asia, and Western Pacific regions have licensed PCV13 but lack a NIP, therefore Chapman et al. conducted a scenario analysis in which 10% of those country-specific birth cohorts each year were assumed to be fully vaccinated with PCV13 through out-of-pocket payments [Citation6]. When considering private PCV13 market uptake in countries besides those with PCV10 NIPs (e.g. China, Indonesia, Egypt, and Iran), there was a 3.3% increase in estimated disease cases averted and a 2.2% increase in estimated deaths averted from vaccination among children under five years old, suggesting that accounting for children who were vaccinated in the non-NIP market can increase the total global public health impact of PCV vaccination [Citation6]. When accounting for private PCV13 vaccination across all countries, including countries with PCV10 NIPs and high private PCV13 uptake that were previously excludedFootnote1 [Citation6], and assuming a conservative private vaccination rate of 5%, we estimate that between 10 to 15 million children have been administered the PCV13 series in the private market between 2010 and 2019 globally (Appendix A2) [Citation29,Citation30].
2.2. Indirect effects of PCV vaccination
Indirect effects are considered as the combined overall effect of herd protection and serotype replacement. Herd protection, where the benefits of a vaccine extend to those who did not receive the vaccine, is a well-documented benefit of PCVs () [Citation31]. In estimating the total public health impact of pediatric PCV vaccination, cases prevented due to herd protection can surpass cases averted through direct protection [Citation8,Citation32]. This occurs because of the disruption of transmission, resulting from and further contributing to a reduction in nasopharyngeal carriage of serotypes contained within the respective vaccine formulations [Citation33–35]. PCV uptake varies on a sub-national level, however overall vaccine coverage of approximately two-thirds of children under five years of age has been found to induce herd protection among unvaccinated individuals.
Figure 1. Example illustration of herd protection among unvaccinated individuals
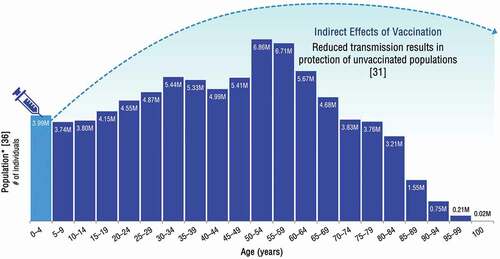
Averted disease from herd protection can be diluted by increases in disease due to non-vaccine serotypes, often referred to as serotype replacement [Citation37]. Although pneumococcal disease incidence varies depending on a country’s local serotype distribution and historical PCV uptake, in countries with good surveillance, generally vaccine-type pneumococcal disease cases immediately decline after the introduction of an infant PCV NIP, followed by a gradual increase in the prevalence of non-vaccine serotypes across the entire population. Nonetheless, pneumococcal disease cases and incidence have exhibited a net decrease as a result of PCV vaccination. Even in rare cases where serotype replacement has almost completely reversed the total decline in pneumococcal disease incidence (e.g. among older adults in the United Kingdom [UK] and Finland) [Citation34,Citation38], the net benefit of vaccination is still positive due to the reduction in disease cases experienced during the intervening period between the onset of herd protection and the emergence of non-PCV serotypes.
In prior analyses that have estimated the public health impact of PCV pediatric vaccination pre-2015 [Citation21], in the last decade [Citation6,Citation7], as well as the future potential impact of PCVs [Citation22], the impact of PCVs was underestimated, given that indirect effects among unvaccinated individuals above five years of age are unaccounted for. Despite the challenge in capturing the global indirect effects of PCV use, we have estimated that the disease cases and consequent deaths averted across all ages from the reduction in nasopharyngeal carriage following infant PCV vaccination is significant. For example, studies in the United States (US) (where PCV13 is used in a 3 + 1 schedule) found that for every one case of IPD averted in vaccinated children, as many as two cases are averted among those unvaccinated [Citation8]. Applying this to Chapman et al.’s estimates [Citation6], an additional 4.1 million cases of IPD alone may have been prevented worldwide from the indirect protection provided by PCV13 vaccination in the last decade.
2.3. PCV dosing schedule variability
In 2000, PCV7 was licensed based on a 3 + 1 schedule, with 3 priming doses in the first year of life followed by a booster dose between 12 and 15 months. While some countries still use this schedule, the majority currently administer PCVs either as 2 + 1 or as 3 + 0 in alignment with the 3-dose schedule most recently recommended by the WHO [Citation3]. Recently, two countries have implemented novel dosing regimens to lower the burden in crowded vaccination schedules or reduce vaccine acquisition costs (the UK and Canada [province of Quebec]) [Citation38–40]. The UK began vaccinating infants born in January 2020 with a 1 + 1 schedule [Citation41]. While this schedule was shown to have comparable immunogenicity to a 2 + 1 schedule following the booster dose, modeling studies have suggested it could result in an increase in disease due to reduced protection in the first year of life [Citation39,Citation42]. In Quebec, the immunization committee implemented a schedule in September 2020 containing two priming doses with PCV10 followed by a PCV13 booster dose [Citation40,Citation43]. The public health impact of these novel dosing schedules will be difficult to assess, considering both were implemented during the coronavirus disease 2019 (COVID-19) pandemic, where poor vaccination adherence, social distancing, and stay-at-home orders have confounded most measures of endemic infectious diseases [Citation44–46].
To date, these nuances of dosing schedule effectiveness in preventing pneumococcal disease have not been reflected in published global impact analyses. In Chapman et al. [Citation6], all vaccinated children were assumed to have vaccine effectiveness commensurate with the three dose PCV13 schedule for simplification purposes. Lecrenier et al. [Citation7] utilized PCV10 efficacy results from the randomized control trial in Latin America (3 + 1 study) and extrapolated to all vaccine schedules. Wahl et al. [Citation21] and Chen et al. [Citation22] also assumed that PCV NIPs would achieve near or full elimination of vaccine-type serotypes irrespective of dosing schedule once overall coverage levels met a determined threshold which differed between the studies. In the future, lower dose and mixed vaccine schedules should be considered in models when evaluating future impact of PCV programs, especially if evidence begins to show that these regimens have variable effectiveness.
2.4. Impact on nonspecific, noninvasive pneumococcal disease
Pneumonia and OM can be caused by various pathogens, one of the most common of which is S.pneumoniae. Within the portion of disease burden caused by S. pneumoniae, vaccine-preventable disease is a function of the serotypes contained within the PCV’s formulation (). There are several challenges associated with measuring the preventable disease burden of pneumonia and OM in both children and adults because in most cases a causative pathogen is neither routinely assessed nor identified. Before PCV use, S. pneumoniae was estimated to cause about one-third of both pneumonia and OM, however this burden was variable across ages and geographies [Citation47–49]. Once PCVs were introduced, this epidemiologic equilibrium was perturbed, leading to further variability across time.
Figure 2. Illustration of vaccine preventable and non-vaccine preventable noninvasive pneumococcal disease (all-cause pneumonia or all-cause otitis media)
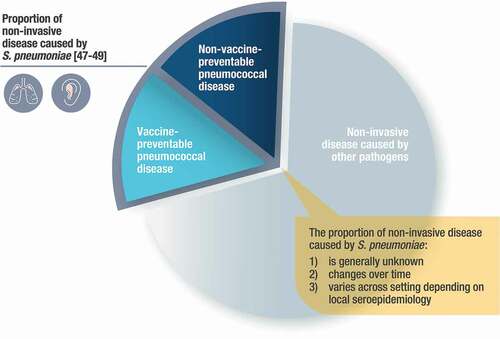
Considering this change in epidemiology and variability, most data quantifying the burden of serotype-specific pneumococcal disease are limited to IPD, as culture methods are less reliable for detecting noninvasive pneumococcal disease. As such, many observations regarding vaccine efficacy, effectiveness, and real-world impact are limited to a nonspecific measurement of all-cause pneumonia and all-cause OM. In the absence of robust diagnostic tools, most predictive models utilize IPD serotype distribution as a proxy for pneumonia and OM burden [Citation50], however vaccine probe studies have confirmed that serotypes have different propensities for disease manifestations [Citation19,Citation51]. Several studies of vaccine effectiveness and impact have even exceeded modeled expectations, further highlighting the challenges in estimating the true impact of PCVs on noninvasive pneumococcal disease.
2.4.1. Pneumonia
When PCV7 was licensed for pediatric use in 2000, randomized controlled trials determining vaccine efficacy against pneumonia found that PCV7 provided approximately 27% protection against all-cause inpatient/hospitalized pneumonia and 6% protection against all-cause outpatient pneumonia in children under two years of age [Citation52–55]. Following widespread use of PCV7 and the introduction of higher valent PCVs, real-world effectiveness studies have continued to show an association between PCV use and declines in nonspecific, all-cause pneumonia incidence in children [Citation56–58]. Depending on the country of study and case definition, all-cause pneumonia hospitalizations have been reduced by 13% to 72% among children under five years of age as a result of PCV vaccination programs, with a significantly sharper decline in cases observed following the replacement of a lower valent PCV with a higher valent PCV [Citation51,Citation56]. The variable impact of PCVs against nonspecific, all-cause pneumonia is a function of the underlying epidemiology at the time PCV vaccination is implemented, as well as the potential impact of PCVs on non-pneumococcal outcomes (discussed further below). More accurate modeling of future pneumonia outcomes will require an estimate of the proportion of disease attributable to vaccine-type serotypes when vaccine effectiveness is measured, as well as the current epidemiologic environment into which the vaccine is introduced.
Using more specific clinical endpoints such as pneumococcal pneumonia would provide a more precise approach to estimating the impact of PCVs, however administrative claims databases have been found to consistently underreport pneumococcal pneumonia as a portion of all-cause pneumonia, primarily due to the absence of a robust diagnostic tool [Citation59,Citation60]. In adults, pathogen identification, when conducted, was typically derived from sputum samples until the availability of urine antigen detection (UAD) assays. A commercially available S. pneumoniae urinary assay (Binax-NOW®) can detect the C-polysaccharide from S. pneumoniae in adults with pneumonia, however it is not serotype-specific [Citation61,Citation62]. A proprietary serotype-specific UAD assay has been developed and validated for use in adult bacteremic pneumonia with high sensitivity and specificity [Citation63], but has uncertain sensitivity for non-bacteremic pneumonia. This diagnostic test is currently being studied for future use in children [Citation64]. Other serotype-specific UADs have been developed, but none are commercially available [Citation65]. Considering the Centers for Disease Control and Prevention (CDC) 2019 evaluation of the PCV recommendation for adults 65 years of age and older following the initial recommendation in 2014, the residual contribution of vaccine-type pneumonia in the US, as measured by serotype-specific UAD, was ~4% of all-cause pneumonia [Citation66]; however, the calculated vaccine efficacy was 6–11.4% against the nonspecific all-cause pneumonia outcome [Citation67], highlighting the challenges in predicting future impact.
Despite the limitations surrounding the identification of specific pneumococcal serotypes’ contribution to pneumonia, previously published global impact analyses discussed here [Citation6,Citation21,Citation22] have attempted to account for pneumococcal pneumonia in their model calculations. In Chapman et al., the public health impact analysis modeled only pneumococcal pneumonia, rather than all-cause, as modeled incidence for this endpoint was available for all countries in the analysis [Citation21]. If all-cause pneumonia had been used instead, the additional global disease cases and consequent deaths prevented by PCV13 vaccination is estimated to be substantially larger. For example, assuming that 34% of all-cause pneumonia cases are pneumococcal as reported by Wahl et al. [Citation21] and applying Kaboré et al.’s [Citation57] estimate that PCV13 vaccination reduces all-cause pneumonia incidence in children by 34% (selected within the range of 13% to 72%), we estimate that approximately 21.1 million cases of all-cause pneumonia may have been prevented in children under five years of age with PCV13, nearly 1.5 times greater than the 14.8 million pneumococcal pneumonia cases previously estimated [Citation6].
2.4.2. Otitis media (OM)
Similar to pneumonia, there is a paucity of evidence summarizing the etiology of OM episodes globally, as the identification of a causative pathogen in OM is typically only obtained in cases when a spontaneously ruptured or a more invasive intentional puncturing of the tympanic membrane provide a specimen for culture, or when a nasopharyngeal carriage isolate accompanies a clinical OM to be used to predict a causative pathogen [Citation68]. There is even less evidence characterizing the serotypes that cause OM cases attributable to S. pneumoniae relative to pneumonia, and as a result, the prevailing measure of PCV impact is all-cause OM.
In the pivotal efficacy study (3 + 1), PCV7 was found to reduce all-cause mild OM by 7% and moderate/severe OM by 14–16% [Citation53,Citation69]. Once PCVs were widely introduced in NIPs, numerous real-world effectiveness studies demonstrated an even broader impact on OM. For example, OM visits in children <2 years of age in the US decreased at a rate of 0.03/child-year annually from 2001 to 2009, corresponding to the period of PCV7 use, followed by a more drastic decrease in 2010–2011 at 0.27/child-year annually after the introduction of PCV13. This translates into an overall reduction in OM visits of 40.6%, much greater than the original efficacy estimates would have predicted [Citation12]. Similarly, in the UK (2 + 1), a significant 21.8% decrease in the monthly incidence of OM was observed in children <2 years of age from the pre-PCV period to the post-PCV7 period. An additional 18.5% decline in OM incidence occurred in the UK following the introduction of PCV13, relative to the PCV7 period [Citation13]. This broad all-cause impact was also observed in Israel (2 + 1), where there was a 60% reduction in all-cause OM rates from the pre-PCV period to the PCV13 period in children <2 years of age [Citation14].
Where serotype-specific OM data have been available, it is evident that there is a direct relationship between the introduction of higher valent PCVs and a reduction in serotype-specific OM. For example, the annual incidence of pneumococcal OM caused by PCV7 plus 6A serotypes in Israel declined a total of 96% post-PCV13 compared to the pre-PCV period. The incidence of OM caused by the five additional serotypes in PCV13 (minus 6A) declined significantly following PCV13 introduction, with an overall reduction of 85% [Citation14].
Beyond having a profound impact on serotype-specific OM, it has also been proposed that by targeting highly pathogenic serotypes, PCVs can reduce mucosal damage inflicted by early onset OM, reducing biofilm formation and recurrent OM episodes later caused by non-vaccine pneumococcal serotypes, as well as other micro-organisms, particularly non-typable Haemophilus influenzae (NTHi) [Citation70]. Notably, a study in southern Israel found that five years after PCV7 and PCV13 were introduced, OM episodes caused by NTHi fell by 66% in children <2 years of age [Citation71]. Furthermore, following the introduction of PCV7 and PCV13 in Israel, rates of progression from carriage acquisition to OM episodes of non-vaccine serotypes fell 43–74% [Citation68]. Based on this evidence, focusing solely on vaccine-type or pneumococcal OM episodes may underestimate the totality of impact that higher-valent PCVs can provide.
2.5. Public health impact of adult PCV vaccination
While previously published modeling analyses have focused on the impact of PCVs in pediatric NIPs, PCV13 has also been licensed for use in adults globally since 2011. Adults who are immunocompromised, have certain comorbidities, and those of advanced age are at higher risk of pneumococcal disease [Citation3]. According to most recent estimates, as of January 2020, PCV13 is recommended for use in adults above a specified age or within a determined risk group in approximately 50 countries, though many of these countries have limited or no adult reimbursementFootnote2 and low compliance [Citation72,Citation73].
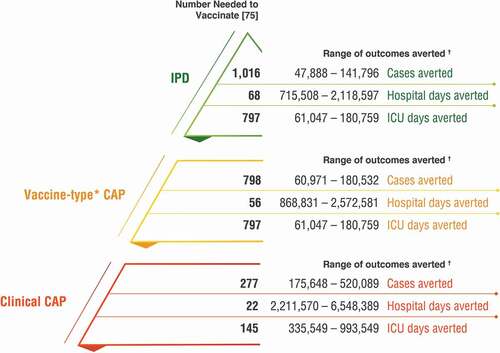
Previous analyses have not accounted for the effect of PCV13 use in adults [Citation6,Citation21,Citation22], primarily because estimates of adult PCV13 coverage are not readily available and surveillance systems for pneumococcal disease in adults are often inadequate or nonexistent in lower-income countries. To estimate the direct impact of PCV13 in adults, we estimated the number of adults vaccinated with PCV13 across the last decade. From 2010 through the end of 2019, 1.3 billion doses of PCV13 have been manufactured and distributed by Pfizer. Taking into consideration doses attributable to PCV13 infant immunization programs (accounting for country-specific vaccination schedules) [Citation6], doses used in children receiving incomplete vaccination series (Appendix A1), doses used for out-of-pocket vaccination among children (Appendix A2), vaccine wastage (5–10% per WHO and Gavi estimates [Citation74]), and current stockpiles (assumed as one-fourth to one-third of annual shipments of approximately 150 million doses), we estimate that approximately 96.4 million doses of PCV13 (midpoint in the range of 48.7 to 144.1 million) were employed to vaccinate adults over the last ten yearsFootnote3. Applying a number needed to vaccinate (NNV) to prevent one case of pneumonia or IPD as previously reported [Citation75], we estimate that between 175.6 thousand and 520.1 thousand clinical community acquired pneumonia (CAP) cases and between 47.9 thousand and 141.8 thousand IPD cases have been averted among adults globally over the last decade as a result of adult PCV13 vaccination (). Assuming the distributed vaccine was administered according to the local recommendation, this suggests that up to 661.9 thousand cases of pneumococcal disease could have been prevented through direct adult protection.
Figure 3. Estimated public health impact of PCV13 vaccination among adults globally, 2010–2019
3. Conclusion
The public health impact of PCV use over the past 20 years has been recognized as one of the greatest public health achievements in the last century [Citation6,Citation76]. However, there are numerous challenges in precisely measuring this impact. Although multi-country analyses have underscored the massive effect PCVs have had in the last decades and will continue to have in years to come, they have captured only part of the total number of pneumococcal disease cases and deaths averted [Citation6,Citation7,Citation21,Citation22]. Both PCVs in current use have exceeded the expectations set forth from the original efficacy studies of PCV7, the first licensed PCV. As this review has demonstrated, earlier models have subsequently underestimated the true impact of PCVs due to the measurement challenges described here. Estimating the exact number of vaccinated individuals, as well as the impact of alternative dosing schedules, indirect effects, PCV use in adults, and the potential impact of PCVs on nonspecific disease outcomes, are among the key challenges in capturing the full public health impact of PCV vaccination.
4. Expert opinion
The first global analysis of the cost-effectiveness of PCVs projected that if future PCV13 coverage rates were comparable to DTP uptake rates, 54.6 million pneumococcal disease episodes and 0.4 million related deaths would be averted in children under five years of age annually [Citation22]. To capture the public health impact of PCVs since their introduction, others calculated the effect of pediatric PCV vaccination over the last ten to 20 years [Citation6,Citation7,Citation21]. These previous estimates have yielded highly different results depending on the data inputs. For example, the estimated annual disease cases averted with PCV13 by Chapman et al. were 12.5%, 35.8%, and 32.4% of those calculated by Chen et al. for IPD, pneumococcal pneumonia, and OM cases averted, respectively, and 15.7% of deaths averted as calculated by Chen et al. Much of this variation can be explained through the number of infants vaccinated in each study, however the residual differences highlight the challenges in determining the true impact of PCVs.
This review has addressed some of the most salient measurement challenges that should be considered in the estimation of PCV’s public health value. For example, when including children incompletely vaccinated or vaccinated in the private market, the indirect effects from infant vaccination, and adult vaccination programs, the number of averted IPD cases from PCV13 use in the last ten years may be three times greater than estimated in Chapman et al.’s base case analysis [Citation6]. Although the number of noninvasive pneumococcal disease cases averted from PCVs is harder to measure, the amount of pneumonia and OM cases prevented from PCV13 is also likely two to three times greater than previously calculated [Citation6].
Nonetheless, there remain broader public health benefits that have not been addressed here. For example, PCVs have been shown to reduce the need for antibiotic prescribing by preventing cases of OM and pneumonia. Reduced transmission of resistant strains also lessens the occurrence of antimicrobial resistance, which is not captured in current models [Citation20,Citation77]. Additionally, the introduction of PCVs has prevented cases of other noninvasive pneumococcal infections such as sinusitis, bronchitis, and conjunctivitis, all of which are manifestations of pneumococcal disease that have not been accounted for in past global or country-specific impact estimates [Citation78]. Long-term impacts of pneumococcal disease on measures like productivity, educational attainment, and caregiver burden have also been ignored, such as ear tube insertions, hearing loss, and sequelae due to meningitis. As healthcare budgets continue to be stretched, it is essential to attribute the full clinical benefit of vaccination when measuring the success of or considering changes to a vaccine program.
Future studies estimating the public health impact of PCVs will need to consider the effect of the COVID-19 pandemic on pneumococcal infections. Although pneumococcal disease incidence declined when social distancing and stay-at-home orders were introduced to prevent the spread of COVID-19, it remains to be seen to what extent and how quickly S. pneumoniae circulation and disease will return to pre-COVID endemic levels once public health measures are lifted, in the context of decreased routine childhood vaccination and reduced person-to-person transmission during the pandemic [Citation79]. Increased interseasonal respiratory syncytial virus (RSV) circulation has been observed since the relaxation of COVID-19 restrictions in countries including the US, Australia, and South Africa, suggesting that other respiratory illnesses like pneumococcal disease may follow a similar trend [Citation80–82]. Morbidity and mortality rates associated with pneumococcal disease may also increase in coming years, as recovered COVID-19 patients who experience long-term lung damage could be at an increased risk of pneumococcal infection [Citation83]. Finally, the pandemic created public awareness of the broad impact infectious diseases have on all sectors of society, which may spur health researchers and policymakers to rethink the way they value and invest in routine immunization programs across all ages, such as for PCVs [Citation84].
4.1. Five-year view
In the coming years, the introduction of new lower-valent PCVs (i.e. 13- and 10-valent vaccines by different manufacturers), expansion of PCV access to children and adults, and licensure of higher-valent PCVs (15- and 20-valent vaccines) will further increase the public health impact of PCV vaccination programs. As PCVs are introduced in more NIPs, including countries with large birth cohorts such as China, India, and Indonesia, and coverage rates increase to levels as seen with other vaccines such as DTP, the global impact of PCVs will continue to increase. Future expanded valency PCVs may address serotype replacement and provide protection against additional pneumococcal disease serotypes, consequently preventing even more morbidity and mortality worldwide. Continuing efforts to increase vaccine access to both infants and adults on a national scale and improve adherence to dosing schedules at an individual level will help to maximize the impact of these public health interventions.
Article highlights
Publications have recently attempted to estimate the cumulative number of pneumococcal disease cases and deaths averted from pediatric pneumococcal conjugate vaccine (PCV) immunization programs, highlighting the tremendous public health impact of PCVs to date and in years to come.
However, there are multiple challenges in comprehensively and accurately measuring the public health impact of PCVs, contributing to the consistent underestimation of pneumococcal disease cases and deaths averted from PCVs in modeling evaluations.
The number of infants receiving direct protection from vaccination has been underestimated because children who received incomplete vaccination series, were vaccinated in countries with national immunization programs that have switched between PCVs, or were vaccinated in the private market have been excluded from global impact analyses.
Because of the challenge in capturing the indirect effects of PCV vaccination, herd protection among unvaccinated individuals has been unaccounted for in model calculations; however, additional disease cases and deaths averted across all ages from the reduction in nasopharyngeal carriage following infant PCV vaccination is estimated to be significant.
The variance in PCV effectiveness in preventing pneumococcal disease across different dosing schedules has not been reflected in global impact analyses, which future assessments may need to consider as novel dosing schedules are implemented.
Due to the paucity of evidence summarizing the etiology and serotype-specific burden of pneumonia and otitis media episodes globally, the true impact of PCVs on noninvasive pneumococcal disease is difficult to determine and has consequently been underestimated in published modeling estimates.
PCV13 vaccination among adults has been increasing in recent years, with an estimated 100 million adults above a specified age or within a determined risk group vaccinated with PCV13 in the last decade; however, model calculations have solely accounted for the public health impact derived from pediatric immunization.
Considering these measurement challenges together, the true global impact of PCVs on pneumococcal disease cases and deaths may in fact be up to three times the impact proposed by previously published modeling analyses.
Abbreviations
CAP, community acquired pneumonia; CDC, Centers for Disease Control and Prevention; COVID-19, coronavirus disease 2019; DTP, diphtheria-tetanus-pertussis; IPD, invasive pneumococcal disease; NIP, national immunization program; NNV, number needed to vaccinate; NTHi, non-typable Haemophilus influenzae; OM, otitis media; PCV, pneumococcal conjugate vaccine; RSV, respiratory syncytial virus; S. pneumoniae, Streptococcus pneumoniae; UAD, urine antigen detection; US, United States; UK, United Kingdom; WHO, World Health Organization.
Declaration of interest
Emily K Horn, Matt D Wasserman, Cassandra Hall-Murray, Heather L Sings, and Raymond A Farkouh are employees of Pfizer and may own stock or stock options. Ruth Chapman has received consulting fees from Pfizer as part of research related to this work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has disclosed investigator-initiated grant funding from Merck Sharpe and Dohme on unrelated projects. Another reviewer has disclosed grant support from Pfizer and Merck, and serving on a technical advisory committee for Merck. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
Emily K Horn, Matt D Wasserman, Cassandra Hall-Murray, Heather L Sings, Ruth Chapman, and Raymond A Farkouh have substantially contributed to the conception and design of the review article and interpreting the relevant literature, and have been involved in writing the review article or revised it for intellectual content.
Supplemental Material
Download MS Word (58.1 KB)Supplementary material
Supplemental data for this article can be accessed https://doi.org/10.1080/14760584.2021.1971521.
Additional information
Funding
Notes
1. Countries considered in this analysis include Belarus, Bosnia and Herzegovina, China, Croatia, Egypt, Estonia, Indonesia, Iran, Jordan, Malaysia, Montenegro, Thailand, and Ukraine (Chapman et al. 2020), as well as Brazil, Nigeria, Pakistan, and Poland (PCV10 NIPs excluded from Chapman et al. 2020). A range of total children privately vaccinated with PCV13 was computed to account for smaller PCV13 private markets not captured in this list of countries.
2. Data on file, Pfizer Inc.
3. Other doses omitted in our calculations include those administered in countries that have switched from PCV13 to PCV10 in their NIP, as well as doses administered during catch-up programs when countries transitioned from PCV10 to PCV13 NIPs. However, the number of doses attributable to either of these scenarios is estimated to be negligible and therefore the calculated range of adults vaccinated with PCV13 is assumed to account for such variation.
References
- Klugman K, Black S, Dagan R, et al. Pneumococcal conjugate vaccine and pneumococcal common protein vaccines. In: Plotkin S, Orenstein W, Offit P, editors. Vaccines. 6th ed. China: Elsevier Saudners; 2013. p. 504–541. Available from: https://www.elsevier.com/books/vaccines/9781455700905.
- Centers for Disease Control and Prevention. Pneumococcal disease. In: Hamborsky J, Kroger A, Wolfe C, editors. Epidemiology and prevention of vaccine-preventable diseases. 13th ed. Washington D.C.: Public Health Foundation; 2021. p. 279–296. Available from: https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/pneumo.pdf.
- World Health Organization. Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper – february 2019. [Internet]. Weekly Epidemiological Record. 2019. Available from: http://www.who.int/immu-%0Ahttps://www.who.int/immunization/policy/position_papers/who_pp_pcv_2019_summary.pdf?ua=1.
- Torres A, Blasi F, Dartois N, et al. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax. 2015;70(10):984–989. Available from https://pubmed.ncbi.nlm.nih.gov/26219979/.
- World Health Organization (WHO). Estimated Hib and pneumococcal deaths for children under 5 years of age, 2000 [Internet]. 2014 [cited 2021 Jan 29]; Available from: https://www.who.int/immunization/monitoring_surveillance/burden/estimates/Pneumo_hib_2000/en/.
- Chapman R, Sutton K, Dillon-Murphy D, et al. Ten year public health impact of 13-valent pneumococcal conjugate vaccination in infants: a modelling analysis. Vaccine. 2020;38(45):7138–7145. Available from: https://pubmed.ncbi.nlm.nih.gov/32912642/ .
- Lecrenier N, Marijam A, Olbrecht J, et al. Ten years of experience with the pneumococcal non-typeable Haemophilus influenzae protein D-conjugate vaccine (Synflorix) in children. Expert Rev Vaccines. 2020;19(3):247–265. Available from https://pubmed.ncbi.nlm.nih.gov/32195602/ .
- Moore MR, Link-Gelles R, Schaffner W, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15(3):301–309. Available from: https://pubmed.ncbi.nlm.nih.gov/25656600/.
- Harboe ZB, Thomsen RW, Riis A, et al. Pneumococcal serotypes and mortality following invasive pneumococcal disease: a population-based cohort study. PLoS Med. 2009;6(5):e1000081. Available from: https://pubmed.ncbi.nlm.nih.gov/19468297/.
- Lepoutre A, Varon E, Georges S, et al. Impact of the pneumococcal conjugate vaccines on invasive pneumococcal disease in France, 2001-2012. Vaccine. 2015;33(2):359–366. Available from: https://pubmed.ncbi.nlm.nih.gov/25448105/.
- Waight Pa, Andrews NJ, Ladhani SN, et al. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15(5):535–543. Available from: https://pubmed.ncbi.nlm.nih.gov/25801458/.
- Marom T, Tan A, Wilkinson GS, et al. Trends in otitis media-related health care use in the United States, 2001-2011. JAMA Pediatr. 2014;168(1):68–75. Available from: https://pubmed.ncbi.nlm.nih.gov/24276262/.
- Lau WCY, Murray M, El-Turki A, et al. Impact of pneumococcal conjugate vaccines on childhood otitis media in the United Kingdom. Vaccine. 2015;33(39):5072–5079. Available from: https://pubmed.ncbi.nlm.nih.gov/26297875/.
- Ben-Shimol S, Givon-Lavi N, Leibovitz E, et al. Near-elimination of otitis media caused by 13-valent pneumococcal conjugate vaccine (PCV) serotypes in southern Israel shortly after sequential introduction of 7-valent/13-valent PCV. Clinl Infect Dis. 2014;59(12):1724–1732. Available from: https://pubmed.ncbi.nlm.nih.gov/25159581/.
- Dagan R, van der Beek BA, Ben-Shimol S, et al. Effectiveness of the seven- and thirteen valent pneumococcal conjugate vaccines against vaccine-serotype otitis media. Clinl Infect Dis. 2021;73(4):650–658. Available from: https://pubmed.ncbi.nlm.nih.gov/33507250/.
- Griffin MR, Zhu Y, Moore MR, et al. U.S. Hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369(2):155–163. Available from: http://www.nejm.org/doi/https://doi.org/10.1056/NEJMoa1209165.
- Becker-Dreps S, Amaya E, Liu L, et al. Changes in childhood pneumonia and infant mortality rates following introduction of the 13-valent pneumococcal conjugate vaccine in Nicaragua. Pediatr Infect Dis J. 2014;33(6):637–642. Available from: https://pubmed.ncbi.nlm.nih.gov/24445827/.
- Angoulvant F, Levy C, Grimprel E, et al. Early impact of 13-valent pneumococcal conjugate vaccine on community-acquired pneumonia in children. Clinl Infect Dis. 2014;58(7):918–924. Available from: https://pubmed.ncbi.nlm.nih.gov/24532543/.
- Greenberg D, Givon-Lavi N, Ben-Shimol S, et al. Impact of PCV7/PCV13 introduction on community-acquired alveolar pneumonia in children <5 years. Vaccine. 2015;33(36):4623–4629. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0264410X15008634.
- Tin Tin Htar M, Van Den Biggelaar AHJ, Sings H, et al. The impact of routine childhood immunization with higher-valent pneumococcal conjugate vaccines on antimicrobial-resistant pneumococcal diseases and carriage: a systematic literature review. Expert Rev Vaccines. 2019;18(10):1069–1089. Available from: https://pubmed.ncbi.nlm.nih.gov/31585049/.
- Wahl B, O’Brien KL, Greenbaum A, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health. 2018;6(7):e744–e757. Available from: https://pubmed.ncbi.nlm.nih.gov/29903376/ .
- Chen C, Cervero Liceras F, Flasche S, et al. Effect and cost-effectiveness of pneumococcal conjugate vaccination: a global modelling analysis. Lancet Glob Health. 2019;7(1):e58–e67. Available from: https://pubmed.ncbi.nlm.nih.gov/30554762/
- World Health Organization. Time series reported estimates of PCV coverage [Internet]. 2020 [cited 2021 Jan 29]; Available from: https://apps.who.int/immunization_monitoring/globalsummary/timeseries/tscoveragepcv3.html.
- Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis J. 2000;19(3):187–195. Available from: https://pubmed.ncbi.nlm.nih.gov/10749457/.
- Lewnard JA, Givon-Lavi N, Dagan R. Dose-specific effectiveness of 7-and 13-Valent pneumococcal conjugate vaccines against vaccine-serotype streptococcus pneumoniae colonization in children. Clinl Infect Dis. 2020;71(8):E289–E300. Available from: https://pubmed.ncbi.nlm.nih.gov/31784753/.
- Andrews N, Kent A, Amin-Chowdhury Z, et al. Effectiveness of the seven-valent and thirteen-valent pneumococcal conjugate vaccines in England: the indirect cohort design, 2006–2018. Vaccine. 2019;37(32):4491–4498. Available from:https://pubmed.ncbi.nlm.nih.gov/31272872/.
- Desmet S, Verhaegen J, Van Ranst M, et al. Switch in a childhood pneumococcal vaccination programme from PCV13 to PCV10: a defendable approach? Lancet Infect Dis. 2018;18(8):830–831. Available from: https://pubmed.ncbi.nlm.nih.gov/30001857/.
- Desmet S, Lagrou K, Wyndham-Thomas C, et al. Dynamic changes in paediatric invasive pneumococcal disease after sequential switches of conjugate vaccine in Belgium: a national retrospective observational study. Lancet Infect Dis. 2021;21(1):127–136. Available from:https://pubmed.ncbi.nlm.nih.gov/32702303/.
- United Nations Population Division. World population prospects 2019. Data Query: number of births, box sexes combined (thousands) [Internet]. 2019 [cited 2021 Jan 29]; Available from: https://population.un.org/wpp/DataQuery/.
- United Nations Statistical Division. Table 9. Live births and crude birth rates, by urban/rural residence. United Nations Demographic Yearbook. 2018. [cited 2021 Jan 29]. Available from: https://unstats.un.org/unsd/demographic-social/products/dyb/dyb_2017/.
- Dagan R. Relationship between immune response to pneumococcal conjugate vaccines in infants and indirect protection after vaccine implementation. Expert Rev Vaccines. 2019;18(6):641–661. Available from: https://pubmed.ncbi.nlm.nih.gov/31230486/.
- Centers for Disease Control and Prevention. Direct and Indirect Effects of Routine Vaccination of Children with 7-Valent Pneumococcal Conjugate Vaccine on Incidence of Invasive Pneumococcal Disease - United States, 1998-2003. Morbidity and Mortality Weekly Report. 2005;893–897. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5436a1.htm.
- Shiri T, Datta S, Madan J, et al. Indirect effects of childhood pneumococcal conjugate vaccination on invasive pneumococcal disease: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(1):e51–e59. Available from:https://pubmed.ncbi.nlm.nih.gov/27955789/.
- Ladhani SN, Collins S, Djennad A, et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: a prospective national observational cohort study. Lancet Infect Dis. 2018;18(4):441–451. Available from: https://pubmed.ncbi.nlm.nih.gov/29395999/.
- Ahmed SS, Pondo T, Xing W, et al. Early impact of 13-valent pneumococcal conjugate vaccine use on invasive pneumococcal disease among adults with and without underlying medical conditions-United States. Clinl Infect Dis. 2020;70(12):2484–2492. Available from: https://pubmed.ncbi.nlm.nih.gov/31402387/.
- PopulationPyramid.net. Germany: 2019 [Internet]. 2019 [cited 2021 Jun 24]; Available from: https://www.populationpyramid.net/germany/2019/.
- Wasserman M, Sings HL, Jones D, et al. Review of vaccine effectiveness assumptions used in economic evaluations of infant pneumococcal conjugate vaccine. Expert Rev Vaccines. 2018;17(1):71–78. Available from: https://pubmed.ncbi.nlm.nih.gov/29164952/.
- Finnish Institute of Health and Welfare. Incidence of invasive pneumococcal disease in Finland [Internet]. 2020 [cited 2021 May 7]; Available from: https://thl.fi/en/web/thlfi-en/research-and-development/research-and-projects/monitoring-the-population-effectiveness-of-pneumococcal-conjugate-vaccination-in-the-finnish-national-vaccination-programme/incidence-of-invasive-pneumococcal-disease-in-finland.
- Choi YH, Andrews N, Miller E. Estimated impact of revising the 13-valent pneumococcal conjugate vaccine schedule from 2+1 to 1+1 in England and Wales: a modelling study. PLoS Med. 2019;16(7):e1002845. Available from: https://pubmed.ncbi.nlm.nih.gov/31269018/.
- Institut National de Santé Publique du Québec (Comité sur l’immunisation du Québec). Scientific advisory on the optimal schedule for childhood immunization against pneumococcal disease in Québec [Internet]. 2017. [cited 2021 Jan 29]. Available from: https://www.inspq.qc.ca/es/node/11372.
- Public Health England. PCV christmas special vaccine update [Internet]. 2019. [cited 2021 Jan 29]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/852623/PHE_1103_vaccine_update_303_PCV_special_edition_December_2019.pdf.
- Wasserman M, Lucas A, Jones D, et al. Dynamic transmission modelling to address infant pneumococcal conjugate vaccine schedule modifications in the UK. Epidemiol Infect. 2018;146(14):1797–1806. Available from: https://pubmed.ncbi.nlm.nih.gov/30012224/.
- Gouvernement du Québec. Pneumococcal vaccination program [Internet]. 2020 [cited 2021 Mar 9]; Available from: https://www.quebec.ca/en/health/advice-and-prevention/vaccination/pneumococcal-vaccination-program/.
- Kitano T, Aoki H. A model for the incremental burden of invasive Haemophilus influenzae type b due to a decline of childhood vaccination during the COVID-19 outbreak: a dynamic transmission model in Japan. Vaccine. 2021;39(2):343–349. Available from: https://pubmed.ncbi.nlm.nih.gov/33280853/.
- Choi YH, Miller E. Potential impact of Covid-19 response measures on invasive pneumococcal disease in England and Wales. medRxiv. 2020. DOI:https://doi.org/10.1101/2020.06.01.20119057.
- Amin-Chowdhury Z, Aiano F, Mensah A, et al. Impact of the Coronavirus Disease 2019 (COVID-19) pandemic on invasive pneumococcal disease and risk of pneumococcal coinfection with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): prospective National Cohort Study, England. Clinl Infect Dis. 2021;72(5):E65–E75. Available from: https://pubmed.ncbi.nlm.nih.gov/33196783/.
- Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67(1):71–79. Available from: https://pubmed.ncbi.nlm.nih.gov/20729232/.
- Luotonen J, Herva E, Karma P, et al. The bacteriology of acute otitis media in children with special reference to streptococcus pneumoniae as studied by bacteriological and antigen detection methods. Scand J Infect Dis. 1981;13(3):177–183. Available from: https://pubmed.ncbi.nlm.nih.gov/7313573/.
- Del Beccaro MA, Mendelman PM, Inglis AF, et al. Bacteriology of acute otitis media: a new perspective. J Pediatr. 1992;120(1):81–84. Available from: https://pubmed.ncbi.nlm.nih.gov/1731029/.
- Farkouh RA, Klok RM, Postma MJ, et al. Cost–effectiveness models of pneumococcal conjugate vaccines: variability and impact of modeling assumptions. Expert Rev Vaccines. 2012;11(10):1235–1247. Available from: https://pubmed.ncbi.nlm.nih.gov/23170992/.
- International Vaccines Access Center (IVAC). The evidence base for Pneumococcal Conjugate Vaccines (PCVs): data for decision-making around PCV use in childhood [Internet]. Baltimore (MD); 2017. [cited 2021 Jan 29]. Available from: https://www.jhsph.edu/ivac/wp-content/uploads/2018/05/PCVEvidenceBase-Jan2017.pdf.
- Lucero MG, Dulalia VE, Nillos LT, et al. Pneumococcal conjugate vaccines for preventing vaccine-type invasive pneumococcal disease and X-ray defined pneumonia in children less than two years of age. Cochrane Database Syst Rev. 2009;2009(4). Available from: https://pubmed.ncbi.nlm.nih.gov/19821336/.
- Ray GT, Whitney CG, Fireman BH, et al. Cost-effectiveness of pneumococcal conjugate vaccine: evidence from the first 5 years of use in the United States incorporating herd effects. Pediatr Infect Dis J. 2006;25(6):494–501. Available from: https://journals.lww.com/00006454-200606000-00006.
- Hansen J, Black S, Shinefield H, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: updated analysis using World Health Organization standardized interpretation of chest radiographs. Pediatr Infect Dis J. 2006;25(9):779–781. Available from: https://pubmed.ncbi.nlm.nih.gov/16940833/.
- Black SB, Shinefield HR, Ling S, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J. 2002;21(9):810–815. Available from: https://pubmed.ncbi.nlm.nih.gov/12352800/.
- Griffin MR, Mitchel E, Moore MR, et al. Declines in pneumonia hospitalizations of children aged <2 years associated with the use of pneumococcal conjugate vaccines - Tennessee, 1998-2012. MMWR Morbidity and mortality weekly report. 2014;63( 44):995–998. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25375070.
- Kaboré L, Ouattara S, Sawadogo F, et al. Impact of 13-valent pneumococcal conjugate vaccine on the incidence of hospitalizations for all-cause pneumonia among children aged less than 5 years in Burkina Faso: an interrupted time-series analysis. Inter J Infect Dis. 2020;96:31–38. Available from: https://pubmed.ncbi.nlm.nih.gov/32234344/.
- Berglund A, Ekelund M, Fletcher MA, et al. All-cause pneumonia hospitalizations in children <2 years old in Sweden, 1998 to 2012: impact of pneumococcal conjugate vaccine introduction. PLoS ONE. 2014;9(11):e112211. Available from:https://pubmed.ncbi.nlm.nih.gov/25379659/. https://pubmed.ncbi.nlm.nih.gov/25379659/.
- Shea KM, Edelsberg J, Weycker D, et al. Rates of pneumococcal disease in adults with chronic medical conditions. Open Forum Infect Dis. 2014;1(1). Available from: https://pubmed.ncbi.nlm.nih.gov/25734097/.
- Pelton SI, Shea KM, Farkouh RA, et al. Rates of pneumonia among children and adults with chronic medical conditions in Germany. BMC Infect Dis. 2015;15(1). Available from: https://pubmed.ncbi.nlm.nih.gov/26515134/.
- Leeming JP, Cartwright K, Morris R, et al. Diagnosis of invasive pneumococcal infection by serotype-specific urinary antigen detection. J Clin Microbiol. 2005;43(10):4972–4976. Available from: https://pubmed.ncbi.nlm.nih.gov/16207950/.
- Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol. 2003;41(7):2810–2813. Available from: https://pubmed.ncbi.nlm.nih.gov/12843005/.
- Pride MW, Huijts SM, Wu K, et al. Validation of an immunodiagnostic assay for detection of 13 Streptococcus pneumoniae serotype-specific polysaccharides in human urine. Clin Vaccine Immunol. 2012;19(8):1131–1141. Available from: https://pubmed.ncbi.nlm.nih.gov/22675155/.
- Bountogo M, Sanogo B, Pride MW, et al. Application of a pneumococcal serotype-specific urinary antigen detection test for identification of pediatric pneumonia in Burkina Faso. Pediatr Infect Dis J. 2021;40(5):418–425. Available from: https://pubmed.ncbi.nlm.nih.gov/33464020/.
- Sheppard CL, Harrison TG, Smith MD, et al. Development of a sensitive, multiplexed immunoassay using xMAP beads for detection of serotype-specific Streptococcus pneumoniae antigen in urine samples. J Med Microbiol. 2011;60(1):49–55. Available from:https://www-microbiologyresearch-org.eu1.proxy.openathens.net/content/journal/jmm/https://doi.org/10.1099/jmm.0.023150-0.
- Department of Health and Human Services, Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices (ACIP) Summary Report [Internet]. Atlanta, Georgia; 2019. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/min-archive/min-2019-06-508.pdf.
- Lessa F. Effectiveness of 13-valent pneumococcal conjugate vaccine in US adults hospitalized with pneumonia, 2014–2017. IDWeek. Washington D.C.; 2019. [cited 2021 Jan 29]. Available from: https://www.eventscribe.com/2019/IDWeek/fsPopup.asp?efp=Q0NRVktHSkw2ODg2&PosterID=229707&rnd=0.479131&mode=posterinfo.
- Lewnard JA, Givon-Lavi N, Weinberger DM, et al. Pan-serotype reduction in progression of streptococcus pneumoniae to Otitis media after rollout of pneumococcal conjugate vaccines. Clin Infect Dis. 2017;65(11):1853–1861. Available from: https://pubmed.ncbi.nlm.nih.gov/29020218/.
- Fireman B, Black SB, Shinefield HR, et al. Impact of the pneumococcal conjugate vaccine on otitis media. Pediatr Infect Dis J. 2003;22(1):10–16. Available from: https://pubmed.ncbi.nlm.nih.gov/12544402/.
- Dagan R, Pelton S, Bakaletz L, et al. Prevention of early episodes of otitis media by pneumococcal vaccines might reduce progression to complex disease. Lancet Infect Dis. 2016;16(4):480–492. Available from: https://pubmed.ncbi.nlm.nih.gov/27036355/.
- Dagan R, Ben-Shimol S, Leibovitz E, et al. Implementation of PCV7/PCV13 in Israel had a significant impact on both pneumococcal and non-pneumococcal complex Otitis Media (OM) rates. IDWeek. Philadelphia, Pennslyvania; 2014. [cited 2021 Jan 29]. Available from: https://idsa.confex.com/idsa/2014/webprogram/Paper45636.html.
- McLaughlin JM, Swerdlow DL, Khan F, et al. Disparities in uptake of 13-valent pneumococcal conjugate vaccine among older adults in the United States. Hum Vaccines Immunother. 2019;15(4):841–849. Available from: https://pubmed.ncbi.nlm.nih.gov/30676236/.
- Vietri J, Harnett J, Emir B, et al. Uptake of 13-Valent pneumococcal conjugate vaccine among US adults aged 19 to 64 years with immunocompromising conditions. Hum Vaccines Immunother. 2020;16(1):161–168. Available from: https://pubmed.ncbi.nlm.nih.gov/31343949/.
- Gavi The Vaccine Alliance. Detailed product profiles [Internet]. 2020 [cited 2021 Jan 20]; Available from: https://www.gavi.org/our-alliance/market-shaping/product-information-vaccines-cold-chain-equipment.
- Gessner BD, Jiang Q, Van Werkhoven CH, et al. A public health evaluation of 13-valent pneumococcal conjugate vaccine impact on adult disease outcomes from a randomized clinical trial in the Netherlands. Vaccine. 2019;37(38):5777–5787. Available from: https://pubmed.ncbi.nlm.nih.gov/29861177/.
- Wasserman M, Chapman R, Lapidot R, et al. Twenty-Year public health impact of 7- and 13-Valent pneumococcal conjugate vaccines in US children. Emerg Infect Dis. 2021;27(6):1627–1636. Available from: https://wwwnc.cdc.gov/eid/article/27/6/20-4238_article.
- Bloom DE, Kirby PN, Pugh S, et al. Commentary: why has uptake of pneumococcal vaccines for children been so slow? The Perils of undervaluation. Pediatr Infect Dis J. 2020;39(2):145–156. Available from: http://journals.lww.com/https://doi.org/10.1097/INF.0000000000002521.
- Dagan R, Ben-Shimol S, Greenberg D, et al. A prospective, population-based study to determine the incidence and bacteriology of bacterial conjunctivitis in children <2 years of age following 7-valent and 13-valent pneumococcal conjugate vaccine sequential implementation. Clinl Infect Dis. 2021;72(7):1200–1207. Available from: https://academic.oup.com/cid/article/72/7/1200/5782465.
- Murthy BP, Zell E, Kirtland K, et al. Impact of the COVID-19 pandemic on administration of selected routine childhood and adolescent vaccinations — 10 U.S. jurisdictions, march–september 2020. MMWR Morbidity and Mortality Weekly Report. 2021;70( 23):840–845. Available from: https://www.cdc.gov/mmwr/volumes/70/wr/mm7023a2.htm.
- Nunes M. RSV - An annual epidemic. European Society for Paediatric Infectious Diseases. 2021. [cited 2021 Aug 06]. Available from: https://2021.espidmeeting.org/webcasted-industry-sessions/#1622188616813-0e9467ae-5dbf.
- Centers for Disease Control and Prevention. Increased interseasonal Respiratory Syncytial Virus (RSV) activity in parts of the Southern United States [Internet]. CDC Health Alert Network (HAN). 2021; [cited 2021 Aug 06]. Available from: https://emergency.cdc.gov/han/2021/han00443.asp.
- Foley DA, Yeoh DK, Minney-Smith CA, et al. The interseasonal resurgence of respiratory syncytial virus in Australian children following the reduction of coronavirus disease 2019-related public health measures. Clinical Infectious Diseases : an Official Publication of the Infectious Diseases Society of America. 2021; [cited 2021 Aug 06]. Available from: https://europepmc.org/articles/PMC7929151.
- Grist JT, Chen M, Collier GJ, et al. Hyperpolarized 129 Xe MRI abnormalities in dyspneic participants 3 months after COVID-19 Pneumonia: preliminary Results. Radiology. 2021:210033. Available from: https://pubs.rsna.org/doi/abs/https://doi.org/10.1148/radiol.2021210033.
- Bloom D, Cadarette D, Ferranna M. The societal value of vaccination in the age of COVID-19. Am J Public Health. 2021;111(6):1049–1054. Available from: https://pubmed.ncbi.nlm.nih.gov/33856880/.
Appendix A1
Pneumococcal conjugate vaccine (PCV) coverage data by country was based on the latest year of complete first dose (PCV1), second dose (PCV2), and final dose (PCV3) data reported by the World Health Organization (WHO). A weight was created in which each country´s birth cohort was divided by the total births. These birth cohort size weights were applied to the calculated percentage of children who received no, one, or two PCV dose(s) in each country. The weighted coverage by country was summed to estimate the global percentage of children who received only one or only two doses of the series.Data of birth cohort estimates available at:United National Statistical Division https://unstats.un.org/unsd/demographic-social/sconcerns/birth_death/#statisticsUnited National Population Division https://population.un.org/wpp/DataQuery/Data of WHO vaccine coverage estimates available at: http://www.who.int/entity/immunization/monitoring_surveillance/data/coverage_estimates_series.xls
