ABSTRACT
Background
To estimate the health economic consequences of the recently introduced PPSV23 vaccination programme for persons aged 65+ in Denmark and of a potential extension of the programme to include persons aged 60–64 years.
Research design and methods
A Markov model was adapted to the Danish healthcare setting to simulate the epidemiological and economic burden of invasive pneumococcal disease and non-bacteremic pneumococcal pneumonia using information from published sources and Danish databases.
Results
We found that the recent introduction of an age-based vaccination programme offering PPSV23 vaccination to the population of persons aged 65+ in Denmark will lead to a societal gain of EUR 72.0 million and prevent 19,707 cases of pneumococcal disease and 1,308 deaths per 1 million persons during the five-year study period.
Similarly, we estimate that extending the programme to include persons aged 60–64 will lead to a gain of EUR 14.6 million per 1 million persons and prevent an additional 6,223 cases of pneumococcal disease and 185 deaths.
Conclusion
The recent introduction of the age-based vaccination programme offering PPSV23 vaccination to all persons aged 65+ in Denmark is cost-effective. This is also the case if the programme is extended to include persons aged 60–64.
1. Introduction
Streptococcus pneumoniae is a gram-positive, alpha-hemolytic and aerotolerant bacterium of the streptococcus family. The bacteria are normally deposited in pairs, also called diplococci. The species is the etiological cause of several diseases in humans, especially respiratory tract infections, i.e. non-bacteremic pneumococcal pneumonia (NBPP). The most severe diseases are pneumococcal meningitis and pneumococcal septicemia (blood poisoning), which in Denmark are collectively referred to as invasive pneumococcal disease (IPD) as defined by Statens Serum Institut (SSI). These two manifestations of IPD can appear individually or concomitantly and are frequently associated with invasion of bacteria from the respiratory tract and the progression of pneumococcal pneumonia [Citation1].
Some persons are at an increased risk of IPD, including children below the age of two, older adults and individuals of any age with underlying medical conditions [Citation2]. Moreover, pneumococci is the most frequent cause of community-acquired pneumonia for persons in all age groups [Citation3].
On 31 March 2020, in the beginning of the COVID-19 pandemic, the Danish Parliament decided to offer free vaccination with PPSV23 to all persons aged 65 and above and to other individuals at an increased risk of IPD, aiming for a vaccine coverage rate of 75% [Citation4]. The PPSV23 programme marks the second age-based vaccination programme for older adults in Denmark, the first being the influenza vaccination programme implemented since 2002.
PPSV23 is a polysaccharide pneumococcal vaccine that contains capsule material from 23 pneumococcus types, including serotype 8, 12 F, 22 F and 9 N, which are the most prevalent IPD-causing serotypes in adults aged 65 and above in Denmark [Citation5]. PPSV23 is not approved for children under the age of two, but is in Denmark recommended for persons over two years of age who are at an increased risk of IPD and to all healthy persons above the age of 65 due to age being a specific risk factor for IPD [Citation6]. In Denmark, it is recommended that healthy persons aged 65 and above are revaccinated six years later at the earliest, while it is recommended that persons across all age groups who are at an especially high risk may be vaccinated at a shorter interval, depending on the specific reason for the increased risk and if serological testing shows that they are unprotected [Citation5].
The incidence of IPD among adults increases with age [Citation7]. As a result, it has been the standard to study IPD in the population of persons aged 65 and above. However, Danish age-level data on IPD incidence suggest that persons in the age range 60–64 experience close to the same IPD risk as persons in the age group 65–69 [Citation8]. For that reason, it becomes relevant to study the cost-effectiveness of offering vaccination to younger age groups. Findings from such a cost-effectiveness analysis can help uncover the pneumococcal disease burden and the potential benefits of extending the current age-based programme.
In this study, we estimated the societal costs as well as the health gains associated with an age-based PPSV23-based vaccination programme against pneumococcal disease to evaluate the cost-effectiveness of the recent introduction of such a programme in Denmark for persons aged 65 and above. At the same time, we estimated the health economic effects of extending the programme to include all persons in the age group 60–64. We compared both scenarios with a scenario where vaccination is not offered to persons in the relevant age groups, since the historical vaccination coverage is considered close to zeroFootnote1.
This study adds to the existing literature on health economic analysis of pneumococcal vaccination by relying on estimates of the cost associated with IPD and NBPP from Danish register studies and previous publications, which enables us to include social care costs and thereby broaden the societal perspective of the analysis.
In addition, this study aims to provide national data on the cost and potential impact on disease burden of introducing a PPSV23-based vaccination programme. This becomes relevant since both the incidence of pneumococcal disease and the serotype distribution vary between countries.
2. Methods
2.1. Model structure
The applied model is a state transition Markov model for pneumococcal disease adapted to the Danish healthcare setting. The model approach follows earlier work in this area, including published evaluations of pneumococcal vaccinations of the elderly in Germany and the UK [Citation9,Citation10].
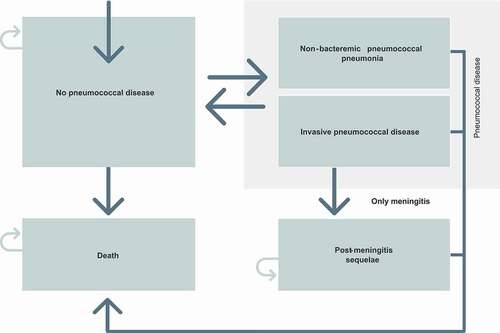
The model follows individuals during the study period or until death with a model cycle of one year. The model divides the population into five health states:
no pneumococcal disease;
IPD;
NBPP;
post-meningitis sequelae (PMS); and
death.
The model continues in the same pattern for all future time periods. The model structure is graphically presented in .
Figure 1. Model structure
2.2. Time horizon and cycle length
Each period (cycle) in the model has a duration of one year, allowing for the model to account for the annual cycles of the incidence of pneumococcus-related diseases.
As the vaccine efficacy for PPSV23 decreases over time, it is currently recommended that healthy individuals are revaccinated with PPSV23 after a minimum of five years. For that reason, we use a time horizon of five years in our analyses [Citation2,Citation11].
2.3. Model parameters and input
2.3.1. Population
We report the estimated results per 1,000,000 individuals. The age distribution and the general mortality is based on the population figures and life tables from Statistics Denmark for individuals born between 1920–1954 [Citation12,Citation13].
2.3.2. Vaccination coverage
We assume that 75% of the target population will receive PPSV23 in both the scenario analyzing the effects of vaccination in persons aged 65 and above and in the age group 60–64 years. This reflects the Danish Ministry of Health’s ambition for the vaccine coverage rate for both the pneumococcal and influenza vaccination programme, as well as WHO’s recommendation for the influenza vaccine for persons aged 65 and above [Citation4].
2.3.3. Invasive pneumococcal disease
The introduction of a vaccine against pneumococcal disease in the Danish childhood vaccination programme in 2007 led to a decrease in the incidence of IPD in all age groups. At the same time, it became mandatory to report incidences of IPD and to submit pneumococcal isolates to SSI, which is the national reference laboratory [Citation5]. Based on the reported data, we set the incidence of IPD to 39.43 per 100,000 person years for persons in the age group 60–64 [Citation8] and 48 per 100,000 person years for persons aged 65 and above [Citation14].
Based on the results from Harboe et al. [Citation15], we assume that 7.55% and 4.27% of the IPD cases are meningitis among persons in the age group 60–64 and persons aged 65 and above, respectively. We assume, based on Korsholm et al. [Citation16], that 40% of cases of pneumococcal meningitis will lead to PMS.
2.3.4. Pneumococcal pneumonia
The incidence of NBPP in the age groups 60–64 years, 65–74 years, 75–84 years and 85 years and above is estimated on the basis of data from Statistics Denmark on the number of patients hospitalized with pneumonia per year from 2010 to 2018 [Citation17]. Based on the findings of Woodhead et al. [Citation18], we adjust for the share of patients who are not hospitalized as a result of the disease. Lastly, we rely on the study from Niederman et al., which reports that up to 30% of all cases of community-acquired pneumonia are caused by the Streptococcus pneumoniae bacterium, to calculate the incidence of NBPP [Citation19].
We have estimated the mortality associated with NBPP on the basis of numbers from the Danish Cause of Death Register [Citation20]. In the period 2010–2018, pneumonia has caused 1,737 annual deaths among persons aged 65 and above and 37 annual deaths among persons in the age group 60–64. Based on the estimated incidence, this is an average case fatality ratio associated with NBPP of 2.05% and 0.67%, respectively.
2.3.5. Vaccine efficacy and waning
We have based PPSV23 efficacy for the prevention of IPD on a study by Falkenhorst et al., who found in a meta-analysis of 4 randomized clinical trials that the vaccine efficacy against all serotypes was 73% (95% CI: 10–92%) [Citation21].
Furthermore, we based PPSV23 efficacy against pneumococcal pneumonia on a prospective multicentre study by Suzuki et al., who found that the vaccine efficacy against vaccine-type pneumococcal pneumonia was 33% in a group of 2,621 patients [Citation22]. In the sensitivity analyses, we show the effect of a reduced vaccine efficacy against NBPP by 25% and 50%, implying a vaccine efficacy of 24.75% and 16.5% against vaccine-type pneumococcal pneumonia.
Finally, we assume that the vaccine efficacy declines linearly over ten years. We base this assumption on a British case-control test-negative study by Lawrence et al. [Citation23], which estimated PPSV23 effectiveness against PPSV23 serotype pneumonia to be 24%, in a group of 717 PPSV23 vaccinated patients aged >16 years with an average follow-up of 10 years since PPSV23 vaccination.
2.3.6. Serotype distribution and the herd immunity effect of the childhood vaccination programme
SSI reports that in 2017, the share of serotypes that are covered by PPSV23 made up 73% of IPD cases among persons aged 65 and above, and that the share of serotypes that are covered by PCV13 made up 12% of the IPD cases in the same age group [Citation24].
In contrast to PPSV23, PCV13 is a pneumococcal conjugate vaccine that is considered to induce immunological memory and reduce carriage (and therefore transmission). The introduction of the PCV13 vaccine in the childhood vaccination programme for the prevention of pneumococcal disease has meant that the prevalence of IPD has decreased by 71% in children below two years of age and by 20% in persons in the age group 50–64 and persons aged 65 and above [Citation5,Citation6]. To account for this reduction in PCV13-specific serotypes causing IPD in the applied model, we lower the number of IPD and NBPP incidences caused by serotypes included in PCV13 by 4.1% per year, which is based on an analysis conducted by the CDC and presented to the ACIP and at the 2018 ISPPD conference [Citation25].
To calculate the long-term effect of the introduction of PPSV23, it is therefore relevant to estimate the share of IPD incidences that are caused by serotypes included in both PPSV23 and in PCV13 (serotypes 1, 3, 4, 5, 6B, 7 F, 9 V, 14, 18 C, 19A, 19 F, 23 F). For this, we use the serotype distribution for cases of pneumococcal meningitis among persons above four years of age in the years 2007–2020, which is published by the SSI [Citation26]. We estimate the share of IPD-causing serotypes that overlap between PCV13 and PPSV23 to be 11.8%. The number of IPD and NBPP cases caused by serotypes that are both included in PCV13 and in PPSV23 are similarly reduced by 4.1% per year.
Finally, we assume that serotype distribution for NBPP and pneumococcal septicemia are the same as the ones reported in for pneumococcal meningitis.
Table 1. Model inputs
2.4. Unit costs
The second part of presents the unit costs that are applied in the model. The applied price of the PPSV23 vaccine is set to the lowest pharmacy purchase price among the suppliers of Pneumovax® (PPSV23).
As no separate tariff for reimbursing general practitioners for vaccinating with PPSV23 in the general population existed, we applied the equivalent tariff for vaccination of patients with elevated risk of IPD against pneumococcal disease as the administration costs for vaccination, which amounts to EUR 19.25.
As an estimate for the treatment costs associated with IPD and PMS, we used results from Gustafsson et al. [Citation30]. The study estimated the societal costs related to meningococcal disease in Denmark among patients over 25 years of age. Although meningococcal disease is primarily found among younger people, the treatments for the two diseases are relatively similar. Specifically, we assume that the costs of treating IPD correspond to the attributable costs in year 0 and year 1, excluding the productivity loss for patients with meningococcal disease. We assume that the yearly treatment costs for PMS equal the long-term treatment costs reported by Gustafsson and coauthors. Specifically, we set the treatment cost of PMS to be the average attributable cost per year for treatment of patients with meningococcal disease in years 2–5 excluding productivity loss.
The applied cost of treatment for the incidence of NBPP that requires hospitalization follows Brogaard et al., who estimated the total healthcare and social costs associated with a hospitalization with pneumonia in three Danish municipalities [Citation31].
Based on expert opinion from the author with extensive clinical experience, the costs associated with the treatment of pneumonia that does not require hospitalization are set to the price corresponding to three consultations at the general practitioner.
Finally, we assume that a sick day will lead to a productivity loss of EUR 101 and EUR 10 per day among persons in the age group 60–64 and persons aged 65 and above, respectively. This is based on data from Statistics Denmark on the earnings of persons aged 60 and above in Denmark [Citation32]. Additionally, we assume that a course of illness with IPD, NBPP or PMS leads to 28, 14 and 365.25 sick days a year on average, respectively.
All cost estimates are presented undiscounted as well as discounted using a discount rate of 4% for all costs which are defrayed after year 1, cf. Amgros’ method guidelines [Citation33].
3. Results
This section presents the results of the analysis on the societal costs and the health effects from 1) the introduction of the Danish age-based vaccination programme against pneumococcal disease for persons aged 65 and above and 2) the effects of extending this programme to all individuals in the age group 60–64. Both scenarios are compared with a scenario in which the analyzed age group is not vaccinated.
shows that in the scenario where the age-based vaccination programme had not been introduced to persons aged 65 and above, we estimated the incidence of IPD to be 2,119 and the incidence of NBPP to be 135,707 per 1 million individuals during the study period of five years. Similarly, we estimate the incidence of IPD to be 2,046 and the incidence of NBPP to be 41,224 per 1 million persons in the age group 60–64 in the scenario where persons in this age group are not offered PPSV23 vaccination.
Table 2. Comparison of incidence and deaths caused by IPD and pneumonia for the two vaccination strategies compared to no vaccination
In addition to this, the table shows that offering PPSV23 to persons aged 65 and above will prevent 679 cases of IPD and 19,028 cases of NBPP per 1 million individuals over five years. Similarly, we estimate that the extension of the age-based vaccination programme to include persons in the age group 60–64 will prevent an additional 646 cases of IPD and 5,577 cases of NBPP per 1 million individuals.
Finally, the analysis showed that the introduction of the age-based vaccination programme to persons aged 65 and above will entail 141 fewer deaths as a result of IPD as well as 1,167 fewer NBPP deaths per 1 million persons aged 65 and above over the five-year period. Moreover, we estimated that the extension of the age-based vaccination programme to include all persons aged 60–64 will prevent an additional 114 IPD deaths and 71 additional NBPP deaths per 1 million individuals in this age-group.
present the model simulations of the yearly incidence of IPD and NBPP, as well as the annual number of deaths caused by pneumococcal diseases, for persons in the age group 60–64 and for persons aged 65 and above. For persons starting in the age-group 60–64, the model simulates an increase in the incidence and mortality over time, which is caused by the increase in the risk of pneumococcal disease as the cohort ages.
Figure 2. Yearly incidence of pneumococcal diseases and expected number of annual deaths caused by IPD and NBPP for persons in the age group 60–64
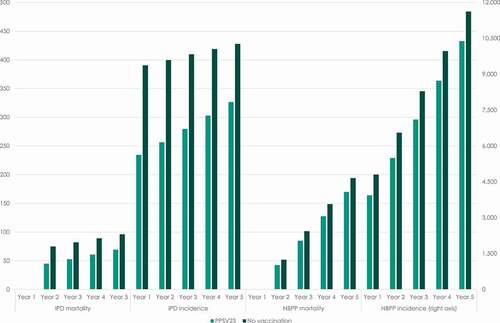
Figure 3. Yearly incidence of pneumococcal diseases and expected number of annual deaths caused by IPD and NBPP for persons aged 65 years and above
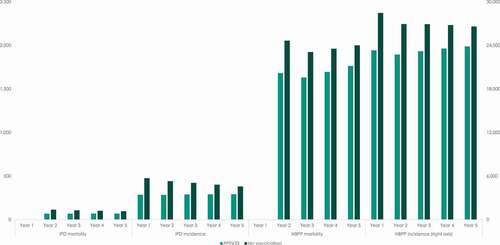
For persons starting in the model at 65 years and above, the model simulates a slight decrease in the incidence of IPD and NBPP over time in the scenario where no vaccination is offered to this age group. Since the incidence rate of IPD is assumed to be constant for all persons aged 65 and above, this is caused by people exiting the model due to general – as well as pneumococcal – mortality. For persons aged 65 and above, the model assumes a stepwise increasing NBPP incidence. The simulations of the model show that this effect is offset by mortality in the population, which explains the decreasing total incidence of NBPP over time.
In contrast, the model simulates a rebound in the incidence of IPD and NBPP over time in the scenario where people in this age group are offered free vaccination with PPSV23. This is caused by the waning of the vaccine efficacy in the study period.
presents the total costs associated with the two analyzed vaccination strategies. In the model, we assume that all expenses of vaccination are upheld in the first year. That entails costs of vaccination of EUR 15.9 million and administration costs of EUR 14.4 million per 1 million individuals offered vaccination, assuming a vaccination coverage of 75%.
Table 3. Societal costs associated with the two vaccination strategies compared to no vaccination (EUR 1,000 per 1 million persons)
If the PPSV23-based vaccination programme had not been introduced to persons aged 65 and above, we estimate the total healthcare costs over the five-year period, including the cost associated with treatment of IPD, NBPP and PMS, to be EUR 642.6 million (discounted: EUR 595.8 million) per 1 million persons in this age group. In addition to this, we estimated the productivity loss associated with sick leave caused by IPD, NBPP and PMS to be EUR 20.0 million (discounted: EUR 18.5 million).
Our model simulations showed that the introduction of an age-based vaccination programme offering PPSV23 vaccination to all individuals aged 65 and above caused a reduction in the healthcare costs associated with treatment of IPD, NBPP and PMS of EUR 99.4 million (discounted: EUR 93.2 million), and moreover that the prevented cases of IPD, NBPP and PMS will cause a reduction in the productivity loss associated with sick leave of EUR 3.0 million (discounted: EUR 2.8 million) per 1 million persons in the age group.
In total, we estimated that the introduction of the age-based vaccination programme to the population of persons aged 65 and above would entail a socioeconomic gain of EUR 72.0 million (discounted: EUR 65.6 million) per 1 million over the five-year study period.
Similarly, we estimate the total healthcare costs of the current vaccination strategy of not offering PPSV23 vaccination to persons in the age group 60–64 to be EUR 229.4 million (discounted: EUR 209.7 million) per 1 million persons in this age group. In addition to this, we estimated the productivity loss associated to be EUR 25.4 million (discounted: EUR 23.8 million).
Our results showed that extending the current age-based vaccination programme to include all persons in the age group 60–64 would cause a reduction in the healthcare costs of EUR 40.1 million (discounted: EUR 37.1 million) per 1 million individuals during the time horizon of five years. In addition to this, we estimated that the extension of the age-based vaccination programme would reduce the productivity loss with EUR 4.9 million (discounted: EUR 4.6 million).
This implies a total socioeconomic gain of EUR 14.6 million (discounted: EUR 11.3 million) per 1 million persons over the five-year study period from extending the current age-based vaccination programme to include all persons in the age group 60–64.
3.1. Sensitivity analyses
In and 5, we present the results of a number of one-way sensitivity analyses. shows the estimates of the incremental incidences of pneumococcal diseases and incremental deaths caused by NBPP and IPD, as well as the incremental disease-free life years from each of the undertaken sensitivity analyses. shows the estimates of the undiscounted incremental costs, along with the percentage change in the undiscounted incremental costs relative to the total cost in the base case scenario.
Table 4. Sensitivity analyses: incremental disease cases, deaths, and disease-free life years
Table 5. Sensitivity analyses incremental costs associated with age-based vaccination programme
The first row in and 5 summarizes the results of the base case analysis. The remaining six rows in and 14 rows in show the results of different sensitivity analyses. In addition to this, graphically presents the changes in the undiscounted incremental costs caused by changes in key model parameters.
Figure 4. Change in undiscounted incremental costs from changes in key model parameters (EUR 1,000 per 1 million persons)
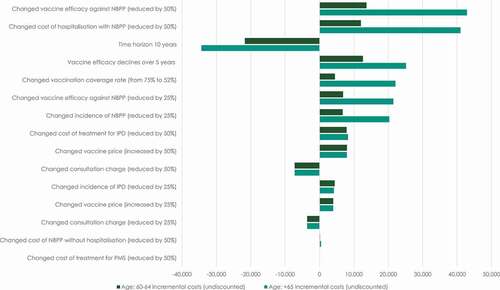
Generally, the sensitivity analyses show that the results of the base case analysis are robust to changes in the main assumptions of the model. The sensitivity analyses show that the introduction of a vaccination strategy in which PPSV23 is offered to all persons aged 65 and above will be cost saving in all scenarios ().
Despite the fact that the results are only impacted by changes in the core assumptions of the model to a limited degree, the sensitivity analyses show that the results are generally most sensitive to changes in: vaccination price, vaccine efficacy against NBPP, costs of treatment for persons hospitalized with NBPP, and the assumption of the degree of the declining vaccine efficacy.
4. Discussion
The findings from this study shows that the introduction of the age-based vaccination programme for persons aged 65 and above as well as an extension of this programme to include persons in the age group 60–64 in Denmark leads to health gains, reduced mortality, and reductions in healthcare resource utilization. The analysis identified the reduction in incidences of NBPP to be a key driver of the cost savings, accounting for 81% and 54% of the total reduction in societal costs in the two age groups, respectively.
To generalize the results, this study presents the expected health gains and socioeconomic costs per 1 million persons in the Danish population. In 2019, the Danish population of persons in the age group 60–64 and persons aged 65 and above constituted 347,194 and 1,200,840 individuals, respectively.
This implies that the introduction of an age-based programme offering PPSV23 vaccination to all persons aged 65 and above in Denmark would result in a societal gain of EUR 86.5 million (discounted: EUR 78.8 million) in addition to preventing 816 IPD cases, 22,849 NBPP cases and 1,572 deaths over the study period of five years.
Similarly, extending the Danish vaccination programme to include the 347,194 persons in the age group 60–64 would imply a societal gain of EUR 3.4 million (discounted: EUR 2,3) and prevent 2,161 incidences of pneumococcal disease and 64 deaths over the study period of five years.
Our findings stand in contrast to the results presented in Wolff et al. [2020, Citation34], who investigated the cost-effectiveness of PPSV23 vaccination in elderly in Sweden. Despite the authors’ conclusion that a vaccination programme using PPSV23 would reduce the burden of pneumococcus-related disease significantly, the authors estimated the cost per QALY to be EUR 94,0000 for vaccinating 65-year-olds. The differences in the results can mainly be explained by the differences in incidence rates of NBPP and the fact that Wolff et al. (2020) only consider short term direct healthcare costs associated with treatment and therefore omits social care costs and productivity loss.
5. Expert Opinion
In Denmark, persons with an elevated risk of contracting IPD have historically been recommended vaccination with PCV13 and/or PPSV23 with partial reimbursement. This vaccination strategy has proven unsuccessful as vaccination coverage among eligible patients has been very low.
By November 2020, less than eight months after the introduction of the universal age-based vaccination programme for persons aged 65 and above, the vaccination coverage in this age group reached 59% [Citation35]. This highlights the importance of easily administrable eligibility criteria, the opportunity for receiving PPSV23 vaccination concomitant with influenza vaccination for persons aged 65 and above, and an increasing public focus on preventing respiratory diseases by vaccination during the COVID-19 pandemic.
Danish data on IPD incidence shows that the increased risk of contracting pneumococcal diseases extends to people younger than 65 year of age. This, combined with an increased productivity loss among people of working age, means that the introduction of a simplified age-based vaccination programme with a vaccination coverage of 75% can be cost-effective, even when extending the age-based vaccination programme to include persons in the age group 60–64. Future studies could investigate the potential health gains and health economic consequences of extending an age-based pneumococcal vaccination programme to an even lower age, e.g. the 50–59 age group, as a considerable IPD and NBPP disease burden also exists in this age group [Citation8].
The COVID-19 pandemic has emphasized the importance of preventing avoidable hospital contacts. The decision by the Danish Government to offer a free vaccination with PPSV23 against pneumococcus to persons aged 65 and above and to adults below 65 who are at an increased risk of pneumococcal disease was introduced as an initiative to free up hospital beds at intensive care units and infectious disease departments for the expected inflow of patients with COVID-19.
As for several common infectious diseases, the incidence of NBPP and IPD have significantly declined during the COVID-19 pandemic as a result of implemented preventive measures. The aim of this study was to estimate the health gains and societal cost of introducing an age-based PPSV23 vaccination programme to persons aged 65 and above and to persons in the age group 60–64 in a stable disease period. The results will therefore extend to a post-COVID-19 period where the incidence of NBPP and IPD have returned to pre-COVID-19 levels.
Going forward, vaccination and disease prevention will be of equal importance as a means of relieving an increasing pressure on the healthcare sector in the period after the COVID-19 pandemic by minimizing preventable hospital contacts. Specifically, for pneumococcal disease, carrier stage in children and transmission to older adults and risk groups is expected to rebound after the COVID-19 pandemic when social distancing is reduced. At the same time, our health economic analysis shows that implementing a PPSV23-based pneumococcal vaccination programme for all older adults irrespective of additional risk factors is a dominant cost-effective strategy.
6. Limitations
Naturally, this model-based study has limitations. In the model simulations, individuals who developed IPD or NBPP in the first model cycle are at an increased risk of death during cycle two. This implies that no individuals transition to death caused by pneumococcal diseases in the first model cycle but are instead included in the results for year two. More importantly, this also implies that the results presented in this study omit the number of prevented deaths from pneumococcal diseases in the last model cycle. Based on the difference in incidences with and without vaccination in year 5 ( and 3, respectively), it can be estimated that the number of avoided deaths is underestimated with 72 and 16, respectively. The study is also limited from not having access to specific input data on NBPP serotype distribution or the percentage of community-acquired pneumonia caused by Streptococcus pneumoniae.
In estimating the prevalence of NBPP from information on pneumonia-related hospitalizations, we relied on the findings of Woodhead et al. [Citation18] to assess the distribution of outpatient and inpatient management of these patients. We acknowledge that the study by Woodhead is out of date, and therefore may not be fully representative of the distribution of Danish patients between primary care management and hospitalization. To the authors’ knowledge, the study by Woodhead represents the best available data.
In addition to this, we rely on the study from Niederman et al., which reports that up to 30% of all cases of community-acquired pneumonia are caused by the Streptococcus pneumoniae bacterium, to calculate the incidence of NBPP [Citation19]. It may be reasonable to assume that the share of cases of community-acquired pneumonia caused by the Streptococcus pneumoniae bacterium varies with age, which would impact our estimates of age-specific incidence rates. To the authors’ knowledge, no evidence on age-specific data on the etiologic distribution exists, and for that reason, we were not able to account for this.
Apart from the presented sensitivity analyses, we have made certain assumptions about model inputs that are likely to change over time and thereby can impact the expected future societal gains of introducing an age-based vaccination programme.
For example, the results in the analysis do not account for the gradual rise in the retirement age. Presumably, this implies an increase in the labor market attachment among persons aged 65 and above in the future and an ensuing greater loss of productivity from pneumococcus-related disease. The prevented incidences of pneumococcal disease associated with the introduction of a vaccination programme will therefore expectedly entail increased societal gains in the future.
Some assumptions in this study have been made prior to the roll-out of the Danish vaccination programme in April 2020. For example, after the start of the vaccination programme the PPSV23 vaccine price changed slightly and a separate tariff for reimbursement of general practitioners for vaccinating with PPSV23 has been established. This is expected to have a minimal effect on the results of the analysis and is covered in the sensitivity analysis of vaccine price and administration costs.
7. Conclusion
This study shows that the recent introduction of a universal age-based vaccination programme in Denmark offering PPSV23 vaccination to the population of persons aged 65 and above is cost-effective. We estimate that over a five-year time horizon, this will lead to a socioeconomic gain of EUR 72.0 million (discounted: EUR 65.6 million) per 1 million persons in addition to preventing a total of 19,707 cases of pneumococcal disease and, most importantly, 1,308 deaths.
Furthermore, our results show that extending the programme to include the population of persons in the age group 60–64 is also both cost-effective and leads to an additional socioeconomic gain of EUR 14.6 million (discounted: EUR 11.3 million) per 1 million persons in addition to preventing an additional 6,223 cases of pneumococcal disease and 185 deaths.
Declaration of interest
Anders Muusfeldt Birck and Liv Nordin Christensen are employees of MSD Denmark ApS, Copenhagen. Mikkel H. Pedersen and Jens Olsen are employees of Incentive, which is a paid vendor of MSD Denmark. Kelly D. Johnson is employed by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, Nj, USA. Goran Bencina is employed by MSD, Madrid, Spain. Thomas Holtkøtter Clausen and Carsten Schade Larsen received an honorarium for their clinical contributions to the modelling work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from Taylor & Frnaics for their review work. Peer reviewers on this manuscript have no other relevant financial or other relationships to disclose.
Authors contributions
Anders Muusfeldt Birck, Liv Nordin Christensen, Jens Olsen, and Mikkel H. Pedersen contributed to the study design, development of the economic model, the interpretation of the results and the drafting of the manuscript. Kelly D. Johnson and Goran Bencina contributed to the interpretation of the results and revision of the manuscript. Thomas Holtkøtter Clausen and Carsten Schade Larsen contributed to the study design by providing clinical expert input and in reviewing the manuscript. All authors have agreed on submitting the article to Expert review of Vaccines, have approved the final version of the manuscript to be published and agree to be accountable for all aspects of the work.
Acknowledgments
We would like to acknowledge Thomas LeFevre, Stefanie Jolak and Bodil Elbrønd for their valuable comments and suggestions throughout the research and writing of this paper.
An earlier version of this paper was accepted for a poster presentation at the annual ISPOR (International Society for Pharmacoeconomics and Outcomes Research) conference in 2020. In connection with that conference, an earlier version of the abstract is published in the abstract supplement of the May issue of Value in Health, 2020.
Additional information
Funding
Notes
1. Individuals in Denmark with an elevated risk of IPD have historically been recommended a sequential vaccination regimen including PCV13 and PPSV23 with partial reimbursement. This includes patients with cardiac failure and chronic cardiac disorders, diabetes mellitus, chronic obstructive pulmonary disease, chronic liver disease, moderate to severe asthma, chronic bronchitis and chronic kidney diseases. There is no official registration of how many Danish citizens are entitled to this reimbursement, but the vaccination coverage in these patient groups were assessed to be close to zero prior to the introduction of the universal vaccination programme.
References
- Gierke R, Wodi AP, Kobayashi M. Epidemiology and prevention of vaccine-preventable diseases: chapter 17, pneumococcal disease. 13th ed. United states: National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention; 2015.
- Kantsø B, Jørgensen CS, Hoffmann S, et al. Pneumokokvaccination uden for børnevaccinationsprogrammet i Danmark. Denmark: Staten Serum Institut; 2019.
- Mongardon N, Max A, Bouglé A, et al. Epidemiology and outcome of severe pneumococcal pneumonia admitted to intensive care unit: a multicenter study. Crit Care. 2012;16(4):R155.
- Sundhedsministeriet. Regering og folketing udvider vaccinationsprogram [Internet]. 2020 [cited 2021 Feb 3]. Available from: https://sum.dk/nyheder/2020/marts/regering-og-folketing-udvider-vaccinationsprogram-
- Statens Serum Institut. Invasiv pneumokoksygdom - opgørelse over sygdomsforekomst 2017 [Internet]. Denmark: Statens Serum Institut; 2018. cited 2020 Jan 4. Available from https://www.ssi.dk/sygdomme-beredskab-og-forskning/sygdomsovervaagning/i/invasiv-pneumokoksygdom-opgoerelse-over-sygdomsforekomst-2017
- Larsen CS Pneumokok [Internet]. Pro.medicin. 2019 [cited 2020 Feb 6]. Available from: https://pro.medicin.dk/Sygdomme/Sygdom/318662
- Lexau CA, Lynfield R, Danila R, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. J Am Med Assoc. 2005;294(16):2043–2051.
- Statens Serum Institut. EPI-NYT - uge 40/ 41-2018: invasiv pneumokoksygdom og tilslutning til pneumokokvaccination i børnevaccinationsprogrammet 2017 [Internet]. Denmark: Staten Serum Institut; 2018. cited 2020 Jan 13. Available from https://www.ssi.dk/aktuelt/nyhedsbreve/epi-nyt/2018/uge-40-41-2018
- Jiang Y, Gauthier A, Keeping S, et al. Cost–effectiveness of vaccinating the elderly and at-risk adults with the 23-valent pneumococcal polysaccharide vaccine or 13-valent pneumococcal conjugate vaccine in the UK. Expert Rev Pharmacoecon Outcomes Res. 2014;14(6):913–927.
- Jiang Y, Gauthier A, Annemans L, et al. Cost–effectiveness of vaccinating adults with the 23-valent pneumococcal polysaccharide vaccine (PPV23) in Germany. Expert Rev Pharmacoecon Outcomes Res. 2012;12(5):645–660.
- Statens Serum Institut. Personer med særlig risiko for invasiv pneumokoksygdom [Internet]. 2018. [cited 2020 Apr 1]. Available from: https://www.ssi.dk/vaccinationer/risikogrupper/invasiv-pneumokoksygdom
- FOLK2: Folketal 1. januar efter køn, alder, herkomst, oprindelsesland og statsborgerskab [Internet]. Statikbanken. 2020 [cited 2020 Feb 6]. Available from: https://statistikbanken.dk/statbank5a/default.asp?w=1920
- HISB8. Dødelighedstavle (2-års tavler) efter køn, alder og dødelighedstavle [Internet]. Statikbanken. 2020 [cited 2020 Feb 6]. Available from: https://statistikbanken.dk/statbank5a/default.asp?w=1920
- Statens Serum Institut. EPI-NYT: nyhedsbrev uge 40-2016 [Internet]. 2016 [cited 2020 Feb 6]. Available from: https://www.ssi.dk/aktuelt/nyhedsbreve/epi-nyt/2016/uge-40—2016
- Harboe ZB, Vallentiner-Branth P, Benfeldt TL, et al. Estimated effect of pneumococcal conjugate vaccination on invasive pneumococcal disease and associated mortality, Denmark 2000–2005. Vaccine. 2008;26(29–30):3765–3771.
- Korsholm J, Kristensen RN, Heslop A, et al. Sequelae og død efter pneumokokmeningitis. Ugeskr Laeger. 2009;171(18):1481–1485.
- IND04: Indlæggelser. sengedage og indlagte patienter efter område, diagnose (99 gruppering), alder og køn. [Internet]. Statikbanken. 2020 [cited 2020 Feb 6]. Available from: https://statistikbanken.dk/statbank5a/default.asp?w=1920
- Woodhead MA, MacFarlane JT, McCracken JS, et al. Prospective study of the aetiology and outcome of pneumonia in the cummunity. Lancet. 1987;329(8534):671–674.
- Niederman MS, Luna CM Community-acquired pneumonia guidelines: aglobal perspective. Seminars in respiratory and critical care medicine. 2012;33(3):298-310.
- FOD507. Døde efter region, køn, alder og dødsårsag. [Internet]. Statikbanken. 2019 [cited 2020 Feb 6]. Available from: https://statistikbanken.dk/statbank5a/default.asp?w=1920
- Falkenhorst G, Remschmidt C, Harder T, et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine (PPV23) against pneumococcal disease in the elderly: systematic review and meta-analysis. PLoS One. 2017;12(1):1.
- Suzuki M, Dhoubhadel BG, Ishifuji T, et al. Serotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older. a multicentre, prospective, test-negative design study. Lancet Infect Dis. 2017;17(3):313–321.
- Lawrence H, Pick H, Baskaran V, et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against vaccine serotype pneumococcal pneumonia in adults: a case-control test-negative design study. PLoS Med. 2020;17(10):e1003326.
- Statens Serum Institut. EPI-NYT 14- 16/20Emne: vaccinationsprogram mod pneumokoksygdom til personer der er fyldt 65 år og til risikogrupper. 2020.
- Pilishvili T, Gierke R, Xing W, et al. Impact of 13-valent Pneumococcal Conjugate Vaccine (PCV13) use on Invasive Pneumococcal Disease (IPD) among adults in the United States. In 2018.
- Overvågning i tal, grafer og kort. Pneumokok meningitis, Individuelle anmeldelsespligtige sygdomme. [Internet]. ssi.dk. 2020 [cited 2020 Feb 6]. Available from: https://statistik.ssi.dk/sygdomsdata#!/?sygdomskode=PNEU&aldersgruppe=9&xaxis=Aar&show=Graph&datatype=Individual
- Andrews NJ, Waight PA, George RC, et al. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine. 2012;30(48):6802–6808.
- Pneumovax [Internet]. Medicinpriser.dk. 2019 [cited 2020 Feb 6]. Available from: https://medicinpriser.dk/Default.aspx?id=15&vnr=373611
- Honorartabel D Overenskomst om almen praksis: 1, oktober 2019 til 31. marts 2020. leager.dk. 2019.
- Gustafsson N, Stallknecht SE, Skovdal M, et al. societal costs due to meningococcal disease: a national registry-based study. Clinicoecon Outcomes Res. 2018;10:563–572.
- Brogaard S, Nielsen MB, Nielsen LU, et al. Health care and social care costs of pneumonia in Denmark: a register-based study of all citizens and patients with COPD in three municipalities. Int J COPD. 15AD. 2015;10:2303–2309.
- INDKP111. Indkomster for personer over 14 år efter region/landsdel, enhed, køn, alder og indkomsttype [Internet]. Statikbanken. [cited 2020 Feb 6]. Available from: https://statistikbanken.dk/statbank5a/default.asp?w=1920
- Amgros. Metodevejledning for omkostningsanalyser af nye lægemidler og indikationer i hospitalssektoren. 2019. Report No.: Version 1.4.
- Wolff E, Storsaeter J, Å Ö, et al. Cost-effectiveness of pneumococcal vaccination for elderly in Sweden. Vaccine. 2020 Jul;38(32):4988–4995.
- Valentiner-Branth P. Status på influenza- og pneumokok-vaccination [Internet]. Denmark: Staten Serum Institut; 2020. Available from https://www.ssi.dk/aktuelt/nyheder/2020/status-pa-influenza–og-pneumokok-vaccination
