ABSTRACT
Introduction
Species of the genus Neisseria are important global pathogens. Neisseria gonorrhoeae (gonococcus) causes the sexually transmitted disease gonorrhea and Neisseria meningitidis (meningococcus) causes meningitis and sepsis. Liposomes are self-assembled spheres of phospholipid bilayers enclosing a central aqueous space, and they have attracted much interest and use as a delivery vehicle for Neisseria vaccine antigens.
Areas covered
A brief background on Neisseria infections and the success of licensed meningococcal vaccines are provided. The absence of a gonococcal vaccine is highlighted. The use of liposomes for delivering Neisseria antigens and adjuvants, for the purposes of generating specific immune responses, is reviewed. The use of other lipid-based systems for antigen and adjuvant delivery is examined briefly.
Expert opinion
With renewed interest in developing a gonococcal vaccine, liposomes remain an attractive option for delivering antigens. The discipline of nanotechnology provides additional nanoparticle-based options for gonococcal vaccine development. Future work would be needed to tailor the composition of liposomes and other nanoparticles to the specific vaccine antigen(s), in order to generate optimal anti-gonococcal immune responses. The potential use of liposomes and other nanoparticles to deliver anti-gonococcal compounds to treat infections also should be explored further.
1. Introduction
1.1. Neisseria infections
The genus Neisseria contains two human pathogens, Neisseria gonorrhoeae (gonococcus) and Neisseria meningitidis (meningococcus). N. gonorrhoeae is an obligate human pathogen that causes the sexually transmitted disease gonorrhea. N. gonorrhoeae has co-evolved with its human host for millennia [Citation1], but is not part of the normal microbiota of the urogenital mucosa. Worldwide, there are ~87 million cases of gonorrhea reported annually with the highest burden in the least developed and low-to-middle-income countries [Citation2]. However, this figure is certainly an underestimate due to unreported asymptomatic infections. Gonococci colonize primarily the mucosal epithelium of the male urethra, causing urethritis and a painful discharge, and of the female endo/ectocervix, causing a mucopurulent cervicitis. Asymptomatic infections are common in both genders [Citation3], but more serious for women, since in approximately 10–25% of untreated women, gonococci can ascend into the upper reproductive tract and induce a clinical syndrome of Pelvic Inflammatory Disease. This syndrome can leave patients with long-term and/or permanent sequelae such as chronic pelvic pain, Fallopian tube damage, endometritis, ectopic pregnancy and infertility [Citation3,Citation4]. Although gonococcal infections are mainly localized in the genitourinary tract, atypical infections can occur at other anatomical sites, because of disseminated gonococcal infection, or as primary infections due to direct interaction of the pathogen [Citation5]. Co-infection with other sexually transmitted disease pathogens e.g. Human Immunodeficiency Virus (HIV), Treponema pallidum (syphilis), and Chlamydia, is not uncommon. Moreover, gonococcal infection is known to promote HIV transmission and infection [Citation6].
N. meningitidis lives as a commensal of the human nasopharyngeal microbiota, but more rarely can behave as an opportunistic pathogen to cause Systemic (Invasive) Meningococcal Disease (SMD). Meningococcal infection has caused historically significant mortality and morbidity worldwide [Citation7] and SMD can be classified into four clinically distinctive disorders: i) shock without meningitis (fulminant septicemia), ii) shock and meningitis, iii) meningitis without shock and iv) meningococcemia without shock or meningitis (mild SMD, where patients usually present with fever and may also have a petechial rash) [Citation8]. Meningitis is the most common presentation of SMD and shock without meningitis has the highest mortality rate. SMD is a consequence of unrestrained compartmentalized intravascular and intracranial meningococcal growth and host inflammation. Like gonococci, meningococci can also cause atypical infections of other anatomical sites, including the genitourinary tract and eye [Citation5].
Both N. meningitidis and N. gonorrhoeae have evolved a multitude of strategies to avoid the human immune system, including a high degree of genome plasticity, and structural and antigenic and phase variation and molecular mimicry, amongst many others. These strategies are comprehensively reviewed elsewhere and both pathogens share many similar mechanisms, and many that are pathogen-unique, for avoiding innate immune recognition [Citation9–11]. Protection against meningococcal disease is dependent on the generation of long-lived serum IgG antibodies that can induce complement-mediated bactericidal activity, used as the correlate of protection for meningococcal vaccines. In addition, the generation of opsonophagocytic antibodies combined with neutrophil, macrophage and dendritic cell uptake of meningococci, provides protection against disease and aids in recovery. There is also significant evidence of a mucosal T effector/memory cell response, which is age-dependent and may be present without bactericidal antibodies [Citation12]. Understanding natural immunity to meningococcal infection has underpinned the successful development of the meningococcal vaccines described below. By contrast to meningococcal infection, gonorrhea does not confer protective immunity against repeat infection, which is commonly observed [Citation11]. This lack of natural immunity has made identification of potential protective antigens and any correlates of protection extremely difficult. In addition, there are no animal models that can reproduce the true pathology of human gonorrhea, which makes studying immune and vaccine responses also difficult. Gonococci can also subvert/suppress the immune response, and it has been suggested that this pathogen selectively induces Th17-driven innate responses that the gonococcus can itself oppose, and simultaneously subdues Th1- and Th2-driven adaptive immune responses that would eliminate it [Citation13]. Furthermore, antibody and T cell-mediated immune responses to uncomplicated gonorrhea are weak and of short duration, reinforcing the opinion that gonococci probably suppress adaptive immune responses. The natural history of gonococcal infection and the pathogen’s intimate relationship with its obligate human host, are factors that underpin the lack of a vaccine to prevent gonorrhea.
1.2. Neisseria vaccines
Vaccines available for preventing SMD are of two types: 1) the conjugate vaccines containing capsule polysaccharides (CPS) of the major meningococcal (Men) serogroups MenA, MenC, MenY and MenW135, e.g. tetravalent Nimenrix and Menveo vaccines in the UK and Menactra and Menveo vaccines in the US, and 2) protein-based vaccines for MenB based on defined recombinant Outer Membrane (OM) Proteins (OMPs), i.e. Bexsero and Trumenba. Historically, the burden of SMD cases has been caused by MenA infection in the ‘meningitis belt’ countries of Sub-Saharan Africa, with an incidence of ~100 cases/100,000 population, which equated to ~300,000–600,000 cases annually (depending on population estimates). However, introduction of the MenA-CPS conjugate vaccine MenAfriVac has eliminated SMD in those countries with active immunization programs, and data from the recent Global Meningococcal Initiative meeting [Citation14] can be used to estimate crudely the number of cases of SMD worldwide, which now only number ~14,000 [Citation5]. Furthermore, the CPS-conjugate vaccine approach is readily transferable to enable development of vaccines to emerging serogroups causing infection, e.g. MenX.
By contrast to the more facile development of MenACYW CPS-conjugate vaccines, the MenB CPS was excluded from vaccine development, due to its poor immunogenicity, which is probably due to molecular mimicry between the α(2–8) N-acetyl-neuraminic acid linked homopolymer of the B CPS and a modification of mammalian neural cell adhesion molecule [Citation15]. Developing MenB vaccines has necessitated a deeper understanding of the meningococcal proteome and the nature of protective protein antigens in the OM of the organism. MenB OM vesicle (OMV) vaccines have been used to successfully limit clonal outbreaks, and the major immunodominant OMP is PorA (variously known as Class I protein, PI, molecular weight 41–42 kDa). PorA-induced immunity is generally specific to the serosubtype of the protein, which is defined by the hypervariable amino acid sequences to be found at the apices of the OM surface-exposed loops 1 and 4 of the protein [Citation16]. Thus, an OMV vaccine containing any one particular PorA serosubtype will not offer much cross-protection against meningococci expressing other PorA serosubtypes. To overcome this limitation, in silico genome- and proteome-based ‘Reverse Vaccinology’ was developed to identify potential vaccine antigens within the meningococcal OM and this approach led to the Novartis vaccine Bexsero/4CMenB. This vaccine contains Neisseria Heparin Binding Antigen (NHBA) [Citation17], non-lipidated factor H binding protein, (fHbp) [Citation18] and Neisserial adhesin A (NadA) [Citation19]. In addition, proteins GNA1030 and GNA2091, both of which also induced some protection, were fused to NHBA and fHbp to enhance their immunogenicity [Citation20,Citation21]. These recombinant proteins were then mixed with MeNZB, the OMV vaccine that was used to stop a clonal outbreak in New Zealand. Multilocus sequence typing and genome sequencing are used to organize meningococci into clonal complexes [Citation22] and most disease is caused by a limited number of these complexes. Mixing the MeNZB OMV with the recombinant antigens in Bexsero provides broader predicted vaccine coverage and additional protection against strains of the hyperinvasive ST41/44 clonal complex (cc41/44/lineage 3), which have been reported to cause substantial increases in disease incidence [Citation23]. In addition, the OMV provided an immuno-adjuvant effect due to i) the immuno-modulatory properties of the residual lipooligosaccharide (LOS), ii) the presence of other OM components such as porins, and iii) the bilayer structure of the OMV. The adjuvant effect of LOS is probably due to the fact that it is a potent Toll-Like Receptor (TLR)4 agonist, albeit with retained and unfavorable reactogenicity [Citation24]. PorB has been shown to have a potent adjuvant effect that is dependent on its interaction with TLR2 [Citation25]. The bilayer structure of the OMV provides indirect adjuvant effects by enabling the presentation of Pathogen Associated Molecular Patterns (PAMPs) such as LOS and OMPs to Pattern Recognition Receptors (PRRs) such as TLR molecules. Their ability to fuse with eukaryotic cell membranes is particularly useful for antigen delivery to antigen presenting cells (APCs) [Citation26]. Notably, the ability of the OMV bilayer to carry and deliver antigens is important in the context of using liposomes to carry Neisseria antigens, as will be described below.
The other MenB vaccine (Trumenba) was developed by Wyeth, who used more traditional biochemical approaches to study the complex proteome of soluble meningococcal OMPs. The vaccine is a bivalent one containing a lipidated subfamily A fHbp variant A05 protein and a lipidated subfamily B fHbp variant B01 protein, to provide broad strain coverage [Citation27]. Both Bexsero and Trumenba have been shown to reduce MenB infection significantly, in temperate countries where infection with this serogroup is more prevalent [Citation28].
By contrast to the successful near-eradication of SMD, there are no vaccines for preventing N. gonorrhoeae infections. We continue to depend on antibiotics to treat gonorrhea: however, because the gonococcus has developed resistance to every class of antibiotic introduced [Citation29], an acceleration in novel drug discovery and vaccine research is essential. Past clinical trials of a killed whole-cell vaccine, a purified single-antigen pilus-based vaccine, and a Porin B (PorB) protein enriched vaccine (contaminated with LOS, Reduction modifiable protein (Rmp, or Class IV protein) and Opacity (Opa) protein) all largely failed [Citation10]. Several reasons account for the failure of these vaccines. Gonococcal surface antigens, including PorB, pili and Opa protein are highly phase and antigenically variable. The killed whole-cell vaccine was prepared from a single strain of N. gonorrhoeae and used to vaccinate an Inuit population in Canada, and failure is likely due to an inability to provide protection against heterologous circulating gonococcal strains within a population that recorded a high incidence of gonorrhea of 25% [Citation30]. The purified pilus vaccine was given to US military personnel in Korea, and its failure is attributable to the fact that the vaccine did not provide protection against circulating heterologous pilus-expressing strains [Citation31]. It has been hypothesized that the PorB-enriched vaccine failed because of the production of antibodies to other gonococcal antigens that had a negative cumulative effect on the generation of complement-dependent bactericidal activity [Citation10]. Indeed, antibodies generated to Rmp/Protein III [Citation32] can block complement-dependent killing of N. gonorrhoeae by anti-Por and anti-LOS antibodies [Citation33].
1.3. Cross-protection from gonococcal infections conferred by MenB OMV vaccines
Recently, it has been suggested that licensed meningococcal OMV vaccines could be used as one strategy to protect against gonococcal infection. Anecdotal data from Cuba and Norway and a retrospective case-control study in New Zealand suggested a decline in gonorrhea in the period immediately after use of MenB OMV vaccines [Citation34]. After the introduction of MeNZB in New Zealand, there was a reduction in gonorrhea cases, which translated to a vaccine effectiveness estimate of 31% (95% Confidence Intervals 21–39) [Citation35]. Recently, a study in mice has shown that immunization with Bexsero vaccine, which contains the MeNZB OMV component, accelerated the clearance of gonococci from mice in the intravaginal gonorrhea model and that antibodies could recognize several gonococcal proteins [Citation36]. These studies suggest that Bexsero components themselves and the probability of other cross-reactive antigens in the meningococcal OMV may contribute to protection. For example, a rNHBA and rC-fragment of NHBA antigen have been shown to induce murine bactericidal and opsonophagocytic antibodies [Citation37]. Another potential candidate is the Macrophage Infectivity Potentiator (MIP) protein, which is present in both the meningococcal and gonococcal OM. Notably, antibodies raised to a truncated rNm-MIP protein were shown to be bactericidal against gonococci that expressed different Ng-MIP allelic proteins [Citation38].
2. Liposomes in Neisseria vaccine development
Liposomes are spheres of phospholipid bilayers that enclose a central aqueous space. They are formed by self-assembly in water through hydrophobic interactions. They were created by Bangham et al in 1965 [Citation39] and the term liposomes is attributable to Sessa and Weissmann in 1968 [Citation40]. In the context of developing vaccines, the use of liposomes as an antigen delivery vehicle with intrinsic adjuvant properties owes a great deal to the pioneering studies from Gregoriadis and colleagues in the mid-1970s [Citation41]. Since then, the liposome field has expanded enormously and the current literature is vast with many excellent reviews on liposomes as vaccine delivery systems for the interested reader [Citation42–47]. Liposomes have been used in the development of many promising experimental vaccines to prevent viral, bacterial, fungal and parasite infections of animals and humans. However, only 4 human liposomal vaccines are currently licensed, namely the hepatitis A vaccine Epaxal, the influenza vaccine Inflexal, the malaria vaccine Mosquirix and the shingles vaccine Shingrix [Citation47]. By contrast, liposomal-based products have been used extensively and safely in cosmetic products for decades [Citation48]. The emergence of new lipid-based nanoparticle-based technologies for producing licensed vaccines is discussed in the five-year plan below.
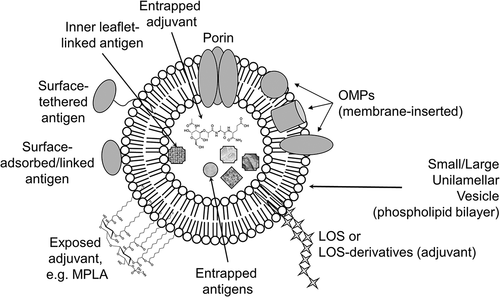
The rationale for this review is to demonstrate that liposomes have many advantages for use in defined antigen-subunit vaccines containing purified or recombinant gonococcal or meningococcal proteins. Liposomes can be produced easily, they are biodegradable and intrinsically safe, as well as detoxifying, i.e. they can reduce antigen toxicity and potential allergic responses, and they can activate both innate and adaptive immune responses [Citation45,Citation46]. Modifications to liposomes are known to influence interactions with immune effector cells, and these have been reviewed extensively elsewhere [Citation44,Citation45]. Liposome size, lamellarity and bilayer fluidity, surface charge and antigen loading are all factors that can influence how vaccine antigens interact with APCs. The fundamental tenet in producing liposome-based vaccines containing recombinant Neisseria OMP(s) is to mimic the ‘native-like’ conformations of the proteins within the bacterial OM, and this can be achieved by refolding/intercalating them within the phospholipid bilayer of the liposome (). Indeed, how antigens are presented within liposomes, especially where this ‘native’ protein conformation is an essential property, is important for interactions with APCs to trigger immune cell maturation, antigen presentation and induce adaptive immunity [Citation44,Citation45]. Incorporating antigens into liposomes can be used also to examine the binding and affinity/avidity of protective antibodies, in order to improve the conformational refolding of proteins and increase immunogenicity. Tailoring liposomes to target different uptake pathways is a possible mechanism for driving particular immune responses. For example, the lipid composition of targeted liposomes has been reported to influence and increase interactions with phagocytic cells and enhance antigen uptake and processing. Such liposomes can be prepared to enhance their own phagocytic uptake by scavenger and innate immune receptors. Multivalent presentation of antigens on their surface can serve to amplify humoral immune responses by promoting the crosslinking of B cell receptors. Immune responses can also be enhanced by the facile phagocytosis of liposomes and the targeted delivery of antigens for processing by APCs. Designing liposomes for delivering antigen contents into the cytoplasm of APCs may also potentially stimulate cytotoxic T-cell responses [Citation47] and this can be done by producing fusogenic liposomes that enable fusion between the liposome and the eukaryotic cell membrane [Citation44]. In addition, antigens that do not require stringent conformational refolding, as well as other adjuvants, can be entrapped within the central aqueous space, which offers the potential to increase antigen/adjuvant load and the possibility of slow-release (). Liposomes can increase the stability of encapsulated antigens and protect them from potentially hostile external environment, e.g. from proteolytic degradation [Citation45,Citation46]. Their surfaces also can be modified with functional moieties and ligands to target specific organs and cells. Co-encapsulation of antigens and adjuvants into liposomes can increase immunogenicity and they can also be tethered directly to the surface of liposomes or adsorbed. One method for adsorbing antigens to liposomes used thiol-maleimide chemistry to covalently ligate model proteins (Cross-Reactive Material 197, NadA and Group B Streptococcus 67 protein) with the TLR9 agonist CpG ODN (DNA-short oligodeoxynucleotides), and the negative charge properties of this conjugate enabled electrostatic binding to cationic liposomes [Citation49]. One method to tether antigens to liposomal surfaces is the use of a novel hydrazine-functionalized poly(ethylene glycol)-phosphatidylethanolamine (PEG-PE)-based amphiphilic polymer to conjugate a variety of ligands, e.g. antibodies and glycoproteins via a reversible, pH-cleavable bond [Citation50]. Liposomes can also be produced that contain a wide variety of PAMPS to stimulate innate immune responses via specific PRRs. Thus, it is clear that a huge variety of different liposomal formulations can be generated to preferentially interact with APCs and induce specific innate and adaptive immune responses and also reduce the possibility of simple clearance, without stimulation, by these cells.
Figure 1. Cartoon of a liposome incorporating Neisseria antigens, with the addition of adjuvants. The cartoon shows the bilayer structure of a unilamellar vesicle containing antigens and adjuvants either intercalated into the membrane itself, or attached to the surface or entrapped. OMPs, Outer Membrane Proteins; LOS, lipooligosaccharide; MPLA, MonoPhosphoryl Lipid A adjuvant
In general, liposomes for the incorporation of Neisseria proteins have been generated using a simple ‘thin-film hydration’ protocol [Citation46]. Initially, varying molar ratios of a selection of lipid molecules are combined in solvent and dried by rotary evaporation under vacuum to produce lipid films. The lipids used can be anionic, cationic or neutral. These are then rehydrated with the solution of protein antigen in buffer containing a detergent, often β-D-Octyl-Glucoside (OG). Subsequent dialysis against buffer or the use of size-exclusion chromatography removes the detergent and induces the formation of protein-lipid vesicles. Various forms of liposomes can be generated depending on need, e.g. i) multilamellar, which are composed of numerous concentric rings, ii) large unilamellar (size between 200 and 500 nm) and small unilamellar (<200 nm), which are single phospholipid bilayers and iii) multi-vesicular liposomes, which consist of a mixture of different sized liposomes and free lipid bilayer within the aqueous cavity of a larger vesicle (). Multilamellar and multivesicular liposomes are particularly useful for sustained antigen release. However, the most frequently used liposomes for delivering Neisseria antigens, and incorporating defined adjuvants, have been the small and/or large unilamellar vesicles (). These are produced from the rehydrated lipid film by sonication, or injection through pore filters or with size-exclusion chromatography. provides brief descriptions of the composition of liposomes used to deliver defined Neisseria vaccine antigens, any alternative and/or variations to the ‘thin-film rehydration’ protocol used, and the nature of the biological responses following immunization.
Figure 2. A general method for preparing Neisseria antigen-containing liposomes. A thin dried lipid film is prepared with phosphatidylcholine and cholesterol and reconstituted with a solution of the detergent octyl-glucoside containing the recombinant Neisseria OMP antigen. Different liposomal structures can be produced to deliver the antigen to the immune system (see text for details)
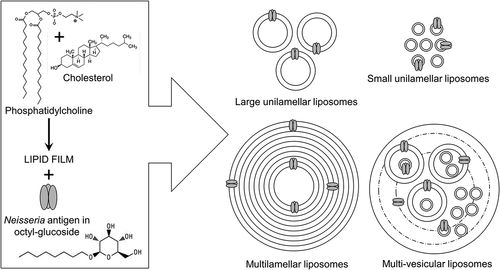
Table 1. Liposomal delivery of candidate Neisseria vaccine antigens
In the following sections, we look at the range of gonococcal and meningococcal vaccine antigens that have been incorporated into liposomes and tested as experimental vaccines. We also look at other non-liposomal delivery vehicles.
2.1. Neisseria gonorrhoeae proteins incorporated into liposomes
Most of the studies using liposomes as a delivery vehicle for gonococcal vaccine antigens have been done with the major OMP porin (variously written as Por, PorB, Protein I or PI, PIA/B, molecular weight between 32 and 39 kDa) (). This porin constitutes 60% of protein in the gonococcal OM and assembles into a trimer with a hydrophilic channel to allow passage of small molecules across the OM. A single por gene encodes Por, and the protein has two major variants called PorB.1A and PorB.1B [Citation79]. Indeed, the earliest report of the preparation of liposomes containing any Neisseria vaccine antigen dates to Young and colleagues in 1983 [Citation80], who described a simple and reproducible protocol for incorporating PI into artificial planar bilayers (). Their protocol was modified from Schindler’s method [Citation81] and used a single detergent-dilution step for both protein-liposome and monolayer formation. During the mid-1980s, Jiskoot and colleagues showed that immunization of mice with PI-liposomes, with and without incorporating additional lipoidal adjuvants, induced anti-PIA IgG antibodies [Citation82]. Contemporaneous studies by Kersten and colleagues showed that the lipid bilayer composition of liposomes incorporating PI influenced the primary murine IgG response, but did not affect the booster memory response [Citation83].
The first demonstration of the ability of PI delivered in liposomes to generate functional immune responses was by Wetzler and colleagues, who showed that rabbit antisera to PIA/PIB-liposomes contained agglutinating, opsonic and bactericidal antibodies [Citation84]. Although known correlates of protection are lacking, the induction of these functional antibody responses are useful markers for vaccine antigen selection. The authors were also the first to hypothesize that functional antibodies are raised to surface-exposed epitopes that are generated by insertion of PI into the liposomal bilayer, which goes toward mimicking the in vivo conformation of the protein in the gonococcal OM. Parmar and colleagues [Citation85] later showed that >80% of PI protein reconstituted into liposomes was orientated facing outwards and presented the same ‘hairpin protein loops’ as found in the native OM. In their study, anti-PI monoclonal antibodies and anti-PI rabbit sera bound to PI-liposomes, but no data were presented for functional antibody activities.
Liposomal incorporation and delivery of PI has not always led to the induction of bactericidal and/or opsonic antibodies. Elkins and colleagues reported that recombinant (r) Por proteins of different gonococci, inserted into liposomes, failed to induce functional antibodies in immunized rabbits, although the sera did show other biologically important functions () [Citation86]. The reasons for lack of functional antibody activity are unclear, but may have included insufficient affinity and/or specificity of antibodies for protective PI epitopes, which probably resulted from non-optimal insertion of PI into the liposome, perhaps due to the method of liposome preparation.
To our knowledge of the published literature, the only other gonococcal antigen that has been reconstituted into liposomes for delivery as an experimental vaccine is a recombinant form of the N. gonorrhoeae Adhesin Complex Protein (Ng-ACP), an inhibitor of human lysozyme. Liposomes containing rNg-ACP induced murine antibodies that were bactericidal for homologous and heterologous antigen expressing strains [Citation87]. Moreover, their immunogenicity was successfully enhanced by incorporation of the TLR4 agonist monophosphoryl lipid A (MPLA), which has been used often with liposome preparations containing meningococcal vaccine antigens (see below).
Gonococcal Protein II, also known as the Opacity (Opa) protein has been considered as a vaccine antigen, with modest levels of strain-specific antibodies detected in sera, seminal fluid and cervical secretions of infected individuals. However, the presence of approximately 11 different opa genes, all subject to phase variation and ‘on-off’ switching of expression, probably excludes Opa as a viable candidate. Opa proteins have been incorporated into liposomes: however, these Opa-containing liposome preparations have only been used for biophysical studies, e.g. on Opa protein-mediated interactions with carcino-embryonic antigen-like cellular adhesion molecule (CEACAM) receptors [Citation88] and subsequent uptake by eukaryotic cells in vitro [Citation89]. Their ability to induce bactericidal and/or opsonic antibodies has not been reported, although Opa-liposomes were shown to bind to human neutrophils and inhibit the adherence of homologous Opa-expressing gonococci [Citation90].
2.2. Neisseria meningitidis PorA porin incorporated into liposomes
Most of the studies using liposomes as a delivery vehicle for meningococcal vaccine antigens have been done with rPorA (), despite the concerns surrounding the serosubtype specificity of the immune response. The rationale for persisting with this antigen was that convalescent sera invariably contained high levels of bactericidal anti-PorA antibodies, and the issue of serosubtype specificity could potentially be obviated by producing vaccines containing multiple rPorA proteins that represented the dominant serosubtypes in the circulating meningococcal population causing SMD. The preparation of liposomes incorporating rPorA protein was first reported in Finnish studies in 1995, using both a dialysis method and a gel filtration method [Citation91–93]. Mice immunized with these rPorA-liposomes produced antibodies that reacted with meningococci in a PorA serosubtype-specific manner, induced complement-mediated killing in vitro and were also protective in the infant rat model of meningococcal meningitis. Thus, production of functional anti-PorA antibodies demonstrated that these simple liposomes enabled protein refolding and presentation of conformational-dependent PorA-specific protective epitopes to the immune system. Moreover, rPorA has been shown to form trimers within the liposomal membrane, thus mimicking the native trimer conformation in the meningococcal OM [Citation94].
Functional activity is also influenced by the structure of the rPorA protein produced. In the early Finnish studies, rPorA was expressed in B. subtilis as a mature protein with a small N-terminal extension of 11 amino acid residues that was necessary for expression in the production system [Citation92]. In a contemporaneous study, rPorA was expressed as a fusion with a bacteriophage T7 gene 10 capsid protein and induced rabbit antisera with weak bactericidal activity after incorporation into liposomes [Citation95]. This low functional activity was attributable to the non-optimal conformation of the large rPorA-fusion protein within the liposome. However, several cloning strategies and vectors are available commercially that have been used to produce mature PorA proteins, and incorporation of these recombinant proteins into liposomes successfully induced high titers of murine bactericidal antibodies [Citation96].
As mentioned above, a PorA-based vaccine would have to contain PorA proteins that are representative of the dominant disease-causing strains circulating in the population, in order to overcome the hyper-variability of the porin loop surface-exposed protective epitopes. This approach could theoretically protect against a broad range of meningococcal strains, and an experimental liposome-based vaccine containing rPorA serosubtype proteins from four different strains has been developed [Citation97]. Each rPorA serosubtype induced high levels of bactericidal activity against the homologous strain. In addition, liposome preparations containing multiple serosubtypes induced high levels of bactericidal activity against each of the four strains, with no evidence of antigenic competition. Significantly, antisera raised against monovalent and multivalent liposomes showed cross-reactive bactericidal activity against heterologous strains [Citation97].
Liposomes have also been used to reconstitute multi-porin complexes consisting of rPorA with rPorB [Citation98] and of rPorA with rPorB and rRmpM (Class 4) proteins [Citation99,Citation100], and the arrangements of porin complexes obtained were similar to those observed in native OMVs. The liposomes contained homocomplexes of each individual porin and rPorA/rPorB, rRmpM/rPorB, and rPorA/rPorB/rRmpM heterocomplexes. These studies confirmed that liposomes can restore the native structure of meningococcal porins as trimers inserted within the membranes and that rRmpM appears to bind only to rPorB. In addition, association of rPorA and rPorB allowed the production of antibodies that recognized conformational epitopes, and these antisera also cross-reacted with epitopes on an unidentified 50 kDa OMP [Citation98].
2.3. Adjuvant strategies to increase immunogenicity of PorA incorporated into liposomes
The physiochemical properties of liposomes such as lipid choice and composition, liposome size and charge, bilayer fluidity/rigidity, method of antigen incorporation and/or attachment, all influence how liposomes interact with the immune system and how they exert an intrinsic immuno-adjuvant effect to stimulate immunity [Citation101]. Functional immune responses to several Neisseria antigens, including OM porins, can be achieved simply by incorporation into liposomes (), and they can be enhanced by manipulating phospholipid formulations, bilayer composition and liposome charge and with the addition of adjuvants. For example, PorA reconstituted into different types of ‘targeted’ liposomes, e.g. by using mannose or phosphatidylserine as targeting moieties, or by using positively charged liposomes, enhanced uptake by murine dendritic cells (DCs) [Citation102]. Immunization with targeted liposomes containing purified PorA enhanced the expression of DC maturation markers CD80, CD86, major histocompatibility complex class II and CD40. In addition, liposome uptake resulted in DC maturation and IL-12 production and increased DC localization in draining lymph nodes. These effects on DCs enhanced the immune response, as the targeted liposomes generated improved bactericidal antibody responses compared to those generated by non-‘targeted’ liposomes [Citation102]. Bilayer composition and the addition of mannosyl moieties have been shown also to influence liposome interactions with DCs and the immune response to meningococcal PorA [Citation103]. Anionic (negatively charged) liposomes with a bilayer composition of DMPC/CHO/DMPG or DMPS interacted with low numbers of DCs. However, addition of mannosylated-DMPE increased the interaction of negatively charged liposomes with DCs through their mannose-receptors. By contrast, cationic (positively charged) liposomes with a bilayer composition of PC/DM-TAP/CHO interacted with very high numbers of DCs and could also be observed intracellularly [Citation103]. Thus, liposome bilayer composition can influence interactions with APCs, which is critical for vaccination success.
Several studies have explored the addition of adjuvants to increase the immunogenicity of rPorA incorporated into liposomes. The most important conclusions from these studies were that i) attempts to increase immunogenicity by the incorporation of adjuvants should not interfere with optimal protein folding [Citation95] and ii) the type of adjuvant used could impact on the ability of porins to induce functional, bactericidal immune responses. In one early study, the effects on immunogenicity of incorporating the adjuvants MPLA or muramyl dipeptide (MDP) into rPorA-liposomes was examined. The rPorA-liposomes alone induced rabbit antisera with weak bactericidal activity, and although addition of the adjuvants stimulated the overall immune response, the resulting antisera were non-bactericidal [Citation95]. Another study compared the immunogenicity of rPorA-liposomes with rPorA-liposomes containing either MPLA or MTP-PE (muramyl tripeptide phosphatidylethanolamine) adjuvant, alongside rPorA administered with other adjuvants, e.g. adsorption to Al(OH)3, or emulsification in Ribi Adjuvant or Pluronic oil [Citation104]. This study found that only the rPorA-liposome preparation induced murine antisera that contained anti-PorA antibodies of high avidity that were bactericidal [Citation104]. Adsorption to Al(OH)3 and emulsification in oil adjuvants appeared to be inappropriate, possibly because they do not maintain native conformation of the protective epitopes at the apices of the surface-exposed loops [Citation104]. Furthermore, it is possible that the addition of adjuvants such as MPLA and MTP-PE into PorA-liposomes disrupts the PorA trimer conformation in the liposome, which may account for the generation of antibodies in mice that failed to recognize native PorA and were non-bactericidal [Citation104]. Other studies support the conclusion that PorA protein should be delivered in liposomes alone (i.e. with no addition of adjuvant) in order to generate functional immune responses. For example, a PorA protein purified from OMVs and depleted of LOS, could not induce bactericidal antibodies on its own, and incorporation into liposomes was essential [Citation94]. However, it is worth noting that the effects of incorporating MPLA into liposomes vary depending on the nature of the meningococcal antigen: thus, for rNm-ACP and rChaperonin60 proteins, the presence of MPLA eliminated the bactericidal response, for rNMB1612 the response remained unaffected, and for the rPorB porin, rOpc and rNm-MIP) proteins, it was augmented [Citation105–110].
One alternative to incorporating MPLA and MTP-PE adjuvants into Neisseria antigen-containing liposomes is to use lpxL1 LOS. The lpxL mutant of N. meningitidis LOS is constructed containing penta- instead of hexa-acylated lipid A, and the advantages of this mutant LOS molecule are that it has similar adjuvant activity as wild-type LOS, but with substantially reduced toxicity [Citation111]. Arigita and colleagues developed PorA-liposomes containing three LOS-like adjuvants: MPLA, toxic galE LOS and the reduced-toxicity lpxL1 LOS mutant, and also adsorbed PorA-liposomes onto AlPO4 [Citation112]. They found that PorA-liposomes containing MPLA or that were AlPO4-adsorbed had a reduced percentage of mouse responders to the vaccines and that the bactericidal responses were not improved. Conversely, all animals immunized with PorA-liposomes containing either galE LOS or lpxL1 LOS responded to these vaccines with increased bactericidal responses. Notably, using lpxL1 LOS adjuvant improved the PorA-specific proliferation of lymph node cells and increased IL-2 production. These observations suggest that the lpxL1 LOS would make a potentially useful adjuvant for general use with liposomes, as long as it does not compromise the conformation of potential vaccine antigens.
Another strategy to attempt to enhance immunogenicity involves fusing rPorA proteins to carrier proteins that might provide the adjuvant effect. Although early studies have suggested that rPorA-fusion proteins are not optimal for generating bactericidal antibodies [Citation95], this may well be dependent on the nature of the fused antigen. Thus, expression of rPorA as a fusion with the P64K protein was reported to induce bactericidal mouse antibodies, although disappointingly, no direct comparison was made with rPorA alone [Citation113].
2.4. Liposomes containing other meningococcal proteins
Liposomes have also been used to incorporate, refold and deliver a wide variety of other purified and/or recombinant meningococcal vaccine antigens (). Many of these liposome-incorporated antigens induced the production of bactericidal antibody responses as a measure of vaccine potential, but of varying potency, thus making it difficult to compare between different studies for the purpose of ranking the antigens for vaccine inclusion.
Liposomes have also been used as carriers for microencapsulated Neisseria antigens and defined adjuvants. For example, microencapsulation of free meningococcal CPS and a recombinant carrier protein from Mycobacterium leprae into liposomes induced a memory antibody response in mice to the CPS molecule [Citation114]. This is a potential process to obviate chemical conjugation of CPS to a carrier protein, which is necessary to induce a T-cell dependent response. For those antigens not tested for their vaccine potential, liposomal incorporation was used to examine other biological/biophysical properties, such as the size, polydispersity and zeta potential of protein-containing liposomes, their bio-distribution in vivo and antigen processing in vitro and in vivo, for structural studies of antibody binding to incorporated antigen(s), and for interactions with metabolic ligands ().
2.5. Other methods for delivering Neisseria vaccine antigens
A brief mention should be made of other methods that have been used in attempts to reconstitute Neisseria antigens to ‘native-like conformations’ for experimental vaccine delivery, and to function also as carriers for antigens from other pathogens (). These include simple detergent micelles, proteosomes, ImmunoStimulatingComplexes (ISCOMS), microspheres, the GMMA technology and OMV genetically engineered to over-express particular antigens.
Table 2. Other lipid-based delivery vehicles for Neisseria vaccine antigens
The simple use of detergents alone has been reported as a method for refolding of OM-located Neisseria vaccine antigens to ‘native-like’ conformation(s). It is probable that detergent solubilization and micelle formation exposes those conformationally-dependent immunodominant epitopes that can induce functional immune responses. However, this is certainly an antigen-dependent process (). Proteosomes are ‘liposome-like’ structures that are produced by treating isolated OM complex vesicles with detergents to produce multi-molecular preparations of lipid membranous and hydrophobic Neisseria OMPs [Citation124,Citation125]. Indeed, the licensed detergent-extracted meningococcal OMV vaccines and experimental gonococcal OMV vaccines [Citation126] are examples of proteosomes. Since these proteosomes are safe for human use, they can be used as general carriers for antigen delivery (), which involves the formation of hydrophobic protein-protein interactions between antigen and proteosome. The proteins within a MenB proteosome also provide the Th cell epitopes that allow the complex to function as a classical antigen carrier. Moreover, the system also makes use of the intrinsic adjuvant properties of the proteosomes to increase antigenicity, e.g. by enabling Th1 differentiation, enhancing T-cell dependent and independent antigen responses and by activating DCs [Citation127,Citation128].
MenB proteosomes have been further developed by transformation in a calcium-environment to produce a proteosome adjuvant called AFCo1 [Citation129,Citation130]. Both the original proteosome (called AFPL1) and derived AFCo1-proteosomes had several key features for an effective adjuvant-delivery system: they could 1) present a multi-protein composition with multiple proteins and PAMPs; 2) induce type I IFNγ and IL-12 cytokines required for the stimulation of human plasmocytoid precursor cells and conventional DCs, respectively; 3) polarize immune responses, e.g. in vivo Th1 with CD4+, CD8 + T cell responses; and 4) function by parenteral and mucosal immunization routes.
However, proteosomes are not always derived from OM complex vesicles and do not always contain lipids. One example is the ‘Por (PI) proteosome’ described by Wetzler and colleagues [Citation131], which was produced by suspending the protein in a detergent and then dialyzing against PBS to reduce the detergent ().
ISCOMS are formed by the inclusion of the saponin QuilA into liposomes [Citation83,Citation132]. Kersten and colleagues [Citation133] proposed a model of the structure of ISCOMs as multi-micellar structures that are shaped and stabilized by hydrophobic interactions, electrostatic repulsion, steric factors and possibly hydrogen bonds. Individual micelles were flat, ring-shaped structures with the central space available for one of the two bulky saponin sugar chains [Citation133]. Despite the increased immunogenicity generated by including QuilA, the use of ISCOMS for Neisseria vaccine antigens has been discontinued and this saponin has been removed as a licensed adjuvant for human vaccines. Recently, however, nontoxic saponin fractions have been identified and these can be potential replacements for QuilA saponin. For example, Novavax’s recently licensed human COVID-19 vaccine contains the company’s patented Matrix-M adjuvant, which is comprised of newly formulated, nontoxic saponin fractions [Citation134].
A slow-release antigen delivery system for PorA-liposomes and OMV has been developed based on microencapsulation in biodegradable dextran- and mannan-based microspheres [Citation135] (). Refinement of this strategy may be necessary for different Neisseria antigens, and would require studying the influence of microsphere size on the immune response, the influence of biodegradation on the rate of antigen release, how microspheres could target APCs, and the possibility of co-encapsulation of adjuvants. By varying the surface and release characteristics of these microspheres, it may be possible to produce ‘single shot’ vaccines that obviate the need for booster vaccinations.
The Generalized Modules for Membrane Antigens (GMMA) technology platform was developed by GlaxoSmithKline. GMMA are OMV that are derived from bacteria genetically engineered to enhance OMV release in culture and that also contain modified LOS with reduced or no endotoxin activity [Citation136]. They offer the opportunity to deliver multiple antigens by using different chemistries to conjugate the purified antigens directly to the GMMA-derivatized surface. It is possible to foresee gonococcal antigens expressed in heterologous GMMA, e.g. from Salmonella typhimurium, S. sonnei or MenB (produced from a ΔsynX, Δctra, Δgna33, ΔlpxL1 N. meningitidis mutant strain). Like liposomes, both GMMA and OMV provide intrinsic adjuvanticity. However, liposomes have the advantage in producing defined antigen vaccines, whereas both GMMA and OMV have considerably more complex antigenic heterogeneity. Moreover, liposome manufacture is arguably more facile than GMMA production, which requires significant chemistry and characterization of the conjugates [Citation136].
3. Expert opinion
Today, meningococcal infections are not considered a serious global problem due to the use of effective CPS-conjugate and protein-based vaccines. However, refinement of the MenB vaccines may be necessary in the future, if antigenic variation leads to reduced vaccine efficacy. Thus, surveillance of the diversity of the Bexsero and Trumenba MenB vaccine antigens amongst circulating meningococci would inform whether the current recombinant proteins would need replacing with new variants. Regardless, replacement of antigens within these vaccines should be facile and not deviate from the licensed process, and any re-formulation to include liposomes is probably unlikely.
In this section, the focus is rather on the utility of liposomes in the development of gonococcal vaccines, which is a considerable challenge due to the difficulties in identifying potential protective antigens and by the lack of known correlates of protection [Citation137]. Liposomes could help to overcome these problems by enabling candidate antigens identified by genomic, proteomic and transcriptomic approaches to be tested for their ability to induce functional, protective immune responses [Citation10]. A case can be made for using liposomes to deliver gonococcal proteins, and this is dependent on the nature of the antigens chosen for inclusion in any new vaccines. The reviews from Rice and colleagues [Citation10] and Russell and colleagues [Citation11] provide comprehensive lists of candidate vaccine antigens that elicit bactericidal antibodies and/or opsonophagocytic antibodies, and in we have added additional information on the nature of the adjuvants used with these antigens. This list of candidate antigens is increasing as new ones are identified from bioinformatic analyses of antigen expression during natural mucosal infection [Citation138], from immuno-proteomics studies of sera from gonococcal-infected patients [Citation139], from quantitative proteomics [Citation140], from classical reverse vaccinology and from a ‘reverse vaccinology 2.0ʹ strategy that uses high throughput discovery of protective human antibodies, sequencing of the B cell repertoire, and structure-based approaches to characterize protective antigens/epitopes [Citation141].
Table 3. The nature of adjuvants used with candidate N. gonorrhoeae vaccine antigens
Crystal structures and inferred topology models are available for many of these candidates. As mentioned above, liposomes have been used to deliver rPorB, rOpa and rNg-ACP proteins, resulting in the induction of bactericidal antibodies. However, bactericidal antibodies could be induced against other candidate antigens using traditional adjuvants, including emulsions such as Freund’s (complete and incomplete) and Titremax, adsorption to aluminum salts (hydroxide, phosphate), and the addition of oft-used adjuvants such as MPLA, QS21 saponin, cholera toxin subunit and CpG ODN (). Another delivery-adjuvant system involved the genetic engineering of Neisseria OMV to over-express particular protective antigens, e.g. LbpA and ZnuD. The advantages of over-expression in OMV are that the proteins are folded correctly in the Neisseria OM and that the OM contains other proteins with adjuvant properties, e.g. PorB and LOS. However, OMV would need to be modified to remove antigens that can induce blocking antibodies, i.e. RmpM, and LOS replaced with nontoxic derivatives. In addition, OMV potentially have disadvantages of batch-to-batch variability in antigen composition, depending on the method of manufacture [Citation161]. Conversely, it is possible to envisage the production of liposome-based vaccines containing multiple defined antigens, either by mixing individual antigen-containing liposomes or incorporating several antigens into a single liposomal formulation [Citation97]. However, the potential for antigen competition within these multi-component vaccines would need to be investigated.
The design and characterization considerations for producing liposome-based gonococcal vaccines should include the choice of lipid, physical morphology of the vesicles (size, lamellar nature, surface charge, bilayer fluidity), the antigen-loading mode (e.g. within the liposomal bilayer for OM-spanning antigens, or the aqueous space or surface tethering), the choice of adjuvants including PRR agonists, and the deposition properties of the liposomes. Other factors that need to be examined would include physiochemical properties such as purity and stability of the vaccine, the possibility of degradation over time and storage, the in vitro release rates of antigen and adjuvant from the vesicles, and the possibility of immune cell clearance rather than activation. Many of these factors could potentially influence immunogenicity and become more complex if liposomal vaccines require multiple antigens to provide cross-protection against circulating gonococci. Furthermore, varying any of all of these factors could be used to engage specific APCs and dictate the direction of the immune response to gonococcal antigen(s) toward mixed Th-1/Th-2 pathways. Subsequently, suitable animal models would be needed for pre-clinical evaluation to examine routes of administration (topical and/or systemic) and dose schedules [Citation46] and the humoral and cell-mediated immune responses to the gonococcal antigen(s) [Citation10,Citation11]. Production of liposome-based gonococcal vaccines would have to meet Good Manufacturing Practice (GMP) and will require industrial processes that are rapid, reproducible and cost-effective. There are a plethora of methods that can be up-scaled for industrial manufacture of liposomes – other than the simple ones that have been used thus far with Neisseria antigens – and these methods should be investigated early in pre-clinical research. GMP compliance will be essential for clinical translation of liposomal vaccines [Citation162].
There is also an enormous literature on the use of liposomes as delivery vehicles for drugs and antimicrobial compounds [Citation163]. The potential use of liposomes and other nanoparticle structures to deliver anti-gonococcal compounds to infected mucosal surfaces should be explored further. Liposomes expressing Neisseria surface adhesion/invasion molecules could also use the principle of antigen-receptor targeting to deliver drugs, antimicrobials and potentially vaccines to intracellular compartments of eukaryotic cells. For example, Opa-containing liposomes that bind cell surface CEACAM and/or heparin sulfate proteoglycan receptors on APCs and mucosal epithelial cells could be used to deliver vaccine antigens and topical drugs or antimicrobials, respectively. Future research on non-systemic methods of delivering liposome-based gonococcal vaccines could also involve the use of patches and vaccination of the reproductive tract. For an example of the potential of patch immunization, mice vaccinated with a microneedle mucosal patch containing two types of liposomes, the mannosylated lipid A-liposomes (200 nm MLLs) and ‘stealth’ lipid A-liposomes (50 nm SLLs), loaded with model antigens ovalbumin or Herpes Simplex Virus (HSV)2 gD protein, and with the addition of NH4HCO3, generated strong antigen-specific humoral and cellular immune responses (mixed Th1/Th2) at the systemic level and, more importantly, especially in the reproductive and intestinal duct mucosae [Citation164]. Notably, the MLL liposomes were processed by mucosal DCs, and the SLL liposomes were processed by macrophages in the draining lymph nodes. Furthermore, vaginal vaccination with the HSV2 gD antigen presented in this microneedle mucosal patch protected mice from live virus challenge [Citation164]. Intravaginal immunization of mice with gonococcal OMV and IL-12 has been shown to generate a Th-1 driven immune response that cleared gonococcal infection [Citation165] and it would be interesting to see if similar protective immune responses can be generated to gonococcal antigens delivered with a mucosal patch-liposome system.
3.1. Five-year view
Past and current extensive studies of the gonococcus have identified many candidate proteins that show real promise for development into vaccines within the next 5 years [Citation10,Citation11]. Decisions will need to be made on how to deliver these antigens to the human immune system, and the many positive features of liposomes make them an attractive vehicle for new defined-subunit gonococcal vaccines (). But they are no longer the only option: we are entering, now, a rapidly expanding era of lipid-based nanoparticle-based nanotechnologies for the potential delivery of drugs and vaccines. The most successful example of the application of these nanotechnologies is the development of the lipid nanoparticles (LNPs) used in mRNA COVID-19 vaccines as delivery vehicles. These LNPs are composed of a neutral phospholipid, cholesterol, a polyethylene-glycol (PEG)-lipid, and an ionizable cationic lipid [Citation166]. Hypothetically, the mRNA- or adenovirus vector-based technologies used in producing COVID-19 vaccines may be applicable also to developing vaccines for bacterial sexually transmitted infections. A comprehensive review of nanotechnology is outside the scope of this current review, and the reader is directed to many articles describing nanoparticle use for drug delivery and for developing vaccines for cancers and infectious diseases [Citation167–169].
Examples of lipid-based nanoparticles abound and include solid lipid nanoparticles (SLNs), nanoemulsions and nanosuspensions, polymer-based nanoformulations, dendrimers, nanocapsules and nanospheres, RNA-lipoplexes and hybrid formulations such as SLNs with oil-in-water emulsions. Like liposomes, these all have large surface areas, high loading capacities and the properties of controlled release and enhanced delivery. Other nanoparticle-based technologies that could be studied for vaccine development include carbon nanoformulations such as carbon nanotubes, graphene oxide nanoparticles and fullerenes and even inorganic nanoparticles (gold, silver, copper, etc). For the latter, interesting and recent examples of experimental bacterial vaccines include gold nanoparticle (AuNP)-protein-LPS conjugates for Burkholderia mallei [Citation170], AuNP-Vibrio cholerae antigens [Citation171], AuNP-oligorhamnosides of Streptococcus agalactiae (group A) [Citation172], AuNP-LomW and EscC antigens from Escherichia coli O157:H7 [Citation173] and zinc oxide nanoparticles used with an antigen ScaA from Orientia tsutsugamushi [Citation174]. Recently, there has also been the emergence of technologies to develop self-assembly protein nanoparticle vaccines [Citation175], including for respiratory syncytial virus [Citation176], rift valley fever virus [Citation177] and SARS-CoV-2 [Citation178]. However, more basic research would be needed, in order to evaluate the potential of such nanoparticle systems to deliver gonococcal vaccine antigens.
During the next 5 years, the efficacy of many of these candidate gonococcal antigens should see testing in the mouse gonorrhea model and potentially move to phase I studies with the experimental male urethral infection model [Citation179]. Successful candidate antigens should be able to generate Th-1/Th-2 adaptive immune responses that can clear gonococcal infection of the lower reproductive tract. The accompanied presence of bactericidal and/or opsonophagocytic antibodies in animal models could potentially be useful immune response markers. It is worth noting that only a few vaccine candidates to date have been reported to successfully accelerate clearance of gonococci in the mouse vaginal colonization gonorrhea model. These were i) the LOS 2C7 epitope; ii) a native OM (LOS-replete) prepared by lithium chloride extraction and centrifugation [Citation180]; and iii) a rPorB-viral replicon particle vaccine [Citation181]. Interestingly, the LOS 2C7 epitope and OM vaccine induced bactericidal antibodies, whereas the viral replicon vaccine did not.
Based on currently available data, one could speculatively propose that a defined recombinant antigen-based vaccine could offer the best option for a new gonococcal vaccine and it might contain any number of different antigens, for example 2C7, TbpA/TbpB, PilQ, MtrE, NHBA, all of which can be argued are ‘front rank’ candidates, based on their ability to meet the criteria described in the previous paragraph. Gonococcal OMV vaccines themselves are an option, with or without manipulation to over-express key antigens, as long as they can be shown to provide broad strain coverage. However, if recombinant protein-based vaccines are the chosen way forward, then liposomes and new nanoparticle delivery systems deserve consideration for inclusion in the development of vaccine formulations.
Box 1. Description and basic properties of the antigen delivery systems mentioned in this review
Article highlights
Neisseria gonorrhoeae (gonococcus) causes the sexually transmitted disease gonorrhoea and Neisseria meningitidis (meningococcus) causes meningitis and sepsis. Effective vaccines have reduced meningococcal infections globally, but there are no licensed vaccines for gonorrhoea.
Liposomes and other lipid-based systems that reconstitute Neisseria antigens to ‘native-like conformations’ should be examined as vaccine delivery vehicles.
Design considerations for developing liposome-based antigen-specific gonococcal vaccines, such as choice of lipid, vesicle size, lamellar nature, surface charge, bilayer fluidity, antigen-loading mode and choice of adjuvant, need to be examined.
The emergence of new nanotechnologies offers additional options for delivering gonococcal vaccines and therapeutics and should be explored.
Author contribution
All authors substantially contributed to the conception and design of the review article and interpreting the relevant literature. M Christodoulides wrote the review and JE Heckels, MV Humbert and M Christodoulides were all involved in editing and revision.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Disclosure statement
M Christodoulides and JE Heckels have previously received funding from GlaxoSmithKline (GSK) for research into Neisseria vaccines and received royalties for a licensed patent. M Christodoulides has received funding from GSK to support a PhD studentship for N. gonorrhoeae pathogenesis and vaccine studies.
Additional information
Funding
References
- Christodoulides M. Preface. In: Christodoulides M, editor. Neisseria gonorrhoeae: methods and protocols. Meth Mol Biol. New York: Humana Press, Springer; 2019. p. v–xii.
- Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019 Aug 1;97(8):548–562.
- Quillin SJ, Seifert HS. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat Rev Microbiol. 2018 Apr;16(4):226–240.
- Stevens JS, Criss AK. Pathogenesis of Neisseria gonorrhoeae in the female reproductive tract: neutrophilic host response, sustained infection, and clinical sequelae. Curr Opin Hematol. 2018 Jan;25(1):13–21.
- Humbert MV, Christodoulides M. Atypical, yet not infrequent, infections with Neisseria species. Pathogens. 2019 Dec 20;9(1):10.
- Guvenc F, Kaul R, Gray-Owen SD. Intimate relations: molecular and immunologic interactions between Neisseria gonorrhoeae and HIV-1. Front Microbiol. 2020;11:1299.
- Schoen C, Blom J, Claus H, et al. Whole-genome comparison of disease and carriage strains provides insights into virulence evolution in Neisseria meningitidis. Proc Natl Acad Sci USA. 2008 Mar 4;105(9):3473–3478.
- Brandtzaeg P, van Deuren M. Classification and pathogenesis of meningococcal infections. In: Christodoulides M, editor. Neisseria meningitidis: advancedmethods and protocols. Methods in Molecular Biology. Vol. 799, New York: Humana Press; 2012. p. 21–35.
- Gasparini R, Panatto D, Bragazzi NL, et al. How the Knowledge of Interactions between Meningococcus and the Human Immune System Has Been Used to Prepare Effective Neisseria meningitidis Vaccines. J Immunol Res. 2015;2015:189153.
- Rice PA, Shafer WM, Ram S, et al. Neisseria gonorrhoeae: drug resistance, mouse models, and vaccine development. Annu Rev Microbiol. 2017 Sep 08;71(1):665–686.
- Russell MW, Jerse AE, Gray-Owen SD. Progress toward a gonococcal vaccine: the way forward. Front Immunol. 2019;10:2417.
- Davenport V, Guthrie T, Findlow J, et al. Evidence for naturally acquired T cell-mediated mucosal immunity to Neisseria meningitidis. J Immunol. 2003;171(8):4263–4270.
- Liu Y, Feinen B, Russell MW. New concepts in immunity to Neisseria gonorrhoeae: innate responses and suppression of adaptive immunity favor the pathogen, not the host. Front Microbiol. 2011;2:52.
- Acevedo R, Bai X, Borrow R, et al. The Global Meningococcal Initiative meeting on prevention of meningococcal disease worldwide: epidemiology, surveillance, hypervirulent strains, antibiotic resistance and high-risk populations. Exp Rev Vacc. 2019 Jan;18(1):15–30.
- Toikka J, Aalto J, Hayrinen J, et al. The polysialic acid units of the neural cell adhesion molecule N-CAM form filament bundle networks. J Biol Chem. 1998 Oct 30;273(44):28557–28559.
- Van Der Ley P, Heckels JE, Virji M, et al. Topology of outer membrane porins in pathogenic Neisseria spp. InfectImmun. 1991;59:2963–2971.
- Serruto D, Spadafina T, Ciucchi L, et al. Neisseria meningitidis GNA2132, a heparin-binding protein that induces protective immunity in humans. Proc Natl Acad Sci U S A. 2010 Feb 23;107(8):3770–3775.
- Madico G, Welsch JA, Lewis LA, et al. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol. 2006 Jul 1;177(1):501–510.
- Comanducci M, Bambini S, Brunelli B, et al. NadA, a novel vaccine candidate of Neisseria meningitidis. J Exp Med. 2002 Jun 03;195(11):1445–1454.
- Giuliani MM, Adu-Bobie J, Comanducci M, et al. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci U S A. 2006 Jul 18;103(29):10834–10839.
- Serruto D, Bottomley MJ, Ram S, et al. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine. 2012 May 30;30(Suppl 2):B87–97.
- Maiden MC, Bygraves JA, Feil E, et al. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci USA. 1998 Mar 17;95(6):3140–3145.
- Elias J, Schouls LM, Van De Pol I, et al. Vaccine preventability of meningococcal clone, Greater Aachen Region, Germany. Emerg Infect Dis. 2010 Mar;16(3):465–472.
- Christodoulides M. Toll-like receptor 4 interactions with Neisseria. In: Rossetti C, Peri F, editors. The role of toll-like receptor 4 in infectious and non infectious inflammation. Prog Inflamm Res. Vol. 87, Cham, Switzerland: Springer; 2020. p. 79–91.
- Massari P, Henneke P, Ho Y, et al. Cutting edge: immune stimulation by neisserial porins is toll- like receptor 2 and MyD88 dependent. J Immunol. 2002;168(4):1533–1537.
- Tan K, Li R, Huang X, et al. Outer membrane vesicles: current status and future direction of these novel vaccine adjuvants. Front Microbiol. 2018;9:783.
- Gandhi A, Balmer P, York LJ. Characteristics of a new meningococcal serogroup B vaccine, bivalent rLP2086 (MenB-FHbp; Trumenba(R)). Postgrad Med. 2016 Aug;128(6):548–556.
- Rivero-Calle I, Raguindin PF, Gómez-Rial J, et al. Meningococcal Group B vaccine for the prevention of invasive meningococcal disease caused by Neisseria meningitidis Serogroup B. Infect Drug Resist. 2019;12:3169–3188.
- Unemo M, Del Rio C, Shafer WM. Antimicrobial resistance expressed by Neisseria gonorrhoeae: a major global public health problem in the 21st century. Microbiol Spectr. 2016 Jun;4(3). DOI:https://doi.org/10.1128/microbiolspec.EI10-0009-2015.
- Greenberg L. Field trials of a gonococcal vaccine. J Reprod Med. 1975;14:34–36.
- Tramont EC, Boslego JW. Pilus vaccines. Vaccine. 1985 Mar;3(1):3–10.
- Virji M, Heckels JE. Location of a blocking epitope on outer membrane protein III of Neisseria gonorrhoeae by synthetic peptide analysis. J Gen Microbiol. 1989;135:1895–1899.
- Rice PA, Vayo HE, Tam MR, et al. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeae by immune serum. J Exp Med. 1986;164(5):1735–1748.
- Petousis-Harris H, Radcliff FJ. Exploitation of Neisseria meningitidis Group B OMV vaccines against N. gonorrhoeae to inform the development and deployment of effective Gonorrhea vaccines. Front Immunol. 2019;10:683.
- Petousis-Harris H, Paynter J, Morgan J, et al. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet. 2017 Sep 30;390(10102):1603–1610.
- Leduc I, Connolly KL, Begum A, et al. The serogroup B meningococcal outer membrane vesicle-based vaccine 4CMenB induces cross-species protection against Neisseria gonorrhoeae. PLoS Pathog. 2020 Dec;16(12):e1008602.
- Semchenko EA, Day CJ, Seib KL. The Neisseria gonorrhoeae vaccine candidate NHBA elicits antibodies that are bactericidal, opsonophagocytic and that reduce gonococcal adherence to epithelial cells. Vaccines (Basel). 2020 May 13;8(2):219.
- Humbert MV, Christodoulides M. Immunization with recombinant truncated Neisseria meningitidis-Macrophage Infectivity Potentiator (rT-Nm-MIP) protein induces murine antibodies that are cross-reactive and bactericidal for Neisseria gonorrhoeae. Vaccine. 2018 May 23;36(27):3926–3936.
- Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965 Aug;13(1):238–252.
- Sessa G, Weissmann G. Phospholipid spherules (liposomes) as a model for biological membranes. J Lipid Res. 1968 May;9(3):310–318.
- Allison AC, Gregoriadis G. Liposomes as immunological adjuvants. Nature. 1974;252(5480):252.
- Schwendener RA. Liposomes as vaccine delivery systems: a review of the recent advances. Ther Adv Vaccines. 2014 Nov;2(6):159–182.
- Bernasconi V, Norling K, Bally M, et al. Mucosal vaccine development based on liposome technology. J Immunol Res. 2016;2016:5482087.
- Perrie Y, Crofts F, Devitt A, et al. Designing liposomal adjuvants for the next generation of vaccines. Adv Drug Deliv Rev. 2016 Apr 1;99(Pt A):85–96.
- De Serrano LO, Burkhart DJ. Liposomal vaccine formulations as prophylactic agents: design considerations for modern vaccines. J Nanobiotech. 2017 Nov 17;15(1):83.
- Ahmed KS, Hussein SA, Ali AH, et al. Liposome: composition, characterisation, preparation, and recent innovation in clinical applications. J Drug Target. 2019 Aug;27(7):742–761.
- Wang N, Chen M, Wang T. Liposomes used as a vaccine adjuvant-delivery system: from basics to clinical immunization. J Control Release. 2019 Jun 10;303:130–150.
- Ahmad U, Ahmad Z, Khan AA, et al. Strategies in development and delivery of nanotechnology based cosmetic products. Drug Res (Stuttg). 2018 Oct;68(10):545–552.
- Chatzikleanthous D, Cunliffe R, Carboni F, et al. Synthesis of protein conjugates adsorbed on cationic liposomes surface. MethodsX. 2020;7:100942.
- Biswas S, Dodwadkar NS, Sawant RR, et al. Development of the novel PEG-PE-based polymer for the reversible attachment of specific ligands to liposomes: synthesis and in vitro characterization. Bioconjug Chem. 2011 Oct 19;22(10):2005–2013.
- Wetzler LM, Blake MS, Gotschlich EC. Protein 1 (Por) of Neisseria gonorrhoeae as an immunogen: liposomes, proteosomes, and the lack of blocking antibodies. Trans Am Assoc Phys. 1989;102:78–90.
- Mimms LT, Zampighi G, Nozaki Y, et al. Phospholipid vesicle formation and transmembrane protein incorporation using octyl glucoside. Biochemistry. 1981 Feb 17;20(4):833–840.
- Olesky M, Zhao S, Rosenberg RL, et al. Porin-mediated antibiotic resistance in Neisseria gonorrhoeae: ion, solute, and antibiotic permeation through PIB proteins with penB mutations. J Bacteriol. 2006 Apr;188(7):2300–2308.
- Zollinger WD, Babcock JG, Moran EE, et al. Phase I study of a Neisseria meningitidis liposomal vaccine containing purified outer membrane proteins and detoxified lipooligosaccharide. Vaccine. 2012 Jan 17;30(4):712–721.
- Tamargo B, Márquez Y, Ramírez W, et al. New proteoliposome vaccine formulation from N. meningitidis serogroup B, without aluminum hydroxide, retains its antimeningococcal protectogenic potential as well as Th-1 adjuvant capacity. BMC Immunol. 2013;14(Suppl1):S12.
- Petrov AB, Semenov BF, Vartanyan YP, et al. Toxicity and immunogenicity of Neisseria meningitidis lipopolysaccharide incorporated into liposomes. Infect Immun. 1992;60(9):3897–3903.
- Petrov AB, Kolenko VM, Koshkina NV, et al. Non-specific modulation of the immune response with liposomal meningococcal lipopolysaccharide: role of different cells and cytokines. Vaccine. 1994;12(12):1064–1070.
- Zakirov MM, Petrov AB, Burkhanov SA, et al. The immunological activity of Neisseria meningitidis lipo-oligosaccharide incorporated into liposomes. Zh Mikrobiol Epidemiol Immunobiol. 1995 Jan-Feb;(1):49–53.
- Mistretta N, Guy B, Berard Y, et al. Improvement of immunogenicity of meningococcal lipooligosaccharide by coformulation with lipidated transferrin-binding protein B in liposomes: implications for vaccine development. Clin Vaccine Immunol. 2012 May;19(5):711–722.
- Minetti CASA, Tai JY, Blake MS, et al. Structural and functional characterization of a recombinant PorB class 2 protein from Neisseria meningitidis - Conformational stability and porin activity. J Biol Chem. 1997;272(16):10710–10720.
- Minetti CA, Blake MS, Remeta DP. Characterization of the structure, function, and conformational stability of PorB class 3 protein from Neisseria meningitidis. A porin with unusual physicochemical properties. J Biol Chem. 1998 Sep 25;273(39):25329–25338.
- Song J, Minetti CA, Blake MS, et al. Successful recovery of the normal electrophysiological properties of PorB (class 3) porin from Neisseria meningitidis after expression in Escherichia coli and renaturation. Biochim Biophys Acta. 1998 Mar 13;1370(2):289–298.
- Jadhav SR, Zheng Y, Michael Garavito R, et al. Functional characterization of PorB class II porin from Neisseria meningitidis using a tethered bilayer lipid membrane. Biosens Bioelectron. 2008 Dec 1;24(4):837–841.
- Tanabe M, Nimigean CM, Iverson TM. Structural basis for solute transport, nucleotide regulation, and immunological recognition of Neisseria meningitidis PorB. Proc Natl Acad Sci U S A. 2010 Apr 13;107(15):6811–6816.
- Rosenqvist E, Hoiby EA, Wedege E, et al. The 5C protein of Neisseria meningitidis is highly immunogenic in humans and induces bactericidal antibodies. J Infect Dis. 1993 May;167(5):1065–1073.
- Carmenate T, Mesa C, Menéndez T, et al. Recombinant Opc protein from Neisseria meningitidis reconstituted into liposomes elicits opsonic antibodies following immunization. Biotechnol Appl Biochem. 2001 Aug;34(1):63–69.
- de Jonge MI, Hamstra HJ, Jiskoot W, et al. Intranasal immunisation of mice with liposomes containing recombinant meningococcal OpaB and OpaJ proteins. Vaccine. 2004 Sep 28;22(29–30):4021–4028.
- Arenas J, van Dijken H, Kuipers B, et al. Coincorporation of LpxL1 and PagL mutant lipopolysaccharides into liposomes with Neisseria meningitidis opacity protein: influence on endotoxic and adjuvant activity. Clin Vaccine Immunol. 2010 Apr;17(4):487–495.
- Jones C, Sadarangani M, Lewis S, et al. Characterisation of the immunomodulatory effects of meningococcal opa proteins on human peripheral blood mononuclear cells and CD4+ T cells. PLoS One. 2016;11(4):e0154153.
- Hou VC, Moe GR, Raad Z, et al. Conformational epitopes recognized by protective anti-neisserial surface protein A antibodies. Infect Immun. 2003 Dec;71(12):6844–6849.
- Stokes RH, Oakhill JS, Joannou CL, et al. Meningococcal transferrin-binding proteins A and B show cooperation in their binding kinetics for human transferrin. Infect Immun. 2005 Feb;73(2):944–952.
- Delgado M, Yero D, Niebla O, et al. Lipoprotein NMB0928 from Neisseria meningitidis serogroup B as a novel vaccine candidate. Vaccine. 2007 Dec 5;25(50):8420–8431.
- Guilvout I, Chami M, Berrier C, et al. In vitro multimerization and membrane insertion of bacterial outer membrane secretin PulD. J Mol Biol. 2008 Sep 26;382(1):13–23.
- Sardinas G, Yero D, Climent Y, et al. Neisseria meningitidis antigen NMB0088: sequence variability, protein topology and vaccine potential. J Med Microbiol. 2009 Feb;58(Pt 2):196–208.
- Bielecka MK, Devos N, Gilbert M, et al. Recombinant protein truncation strategy for inducing bactericidal antibodies to the macrophage infectivity potentiator protein of Neisseria meningitidis and circumventing potential cross-reactivity with human FK506-binding proteins. Infect Immun. 2015 Feb;83(2):730–742.
- Costoya L, Marzoa J, Ferreiros C, et al. Liposomes or traditional adjuvants: induction of bactericidal activity by the macrophage infectivity potentiator protein (Mip) of Neisseria meningitidis. APMIS. 2017 Aug;125(8):725–731.
- Humbert M, Hung M, Phillips R, et al. Vaccine potential and diversity of the putative Cell Binding Factor (CBF, NMB0345/NEIS1825) protein of Neisseria meningitidis. PLoS One. 2016;11(8):e0160403.
- Anderluzzi G, Schmidt ST, Cunliffe R, et al. Rational design of adjuvants for subunit vaccines: the format of cationic adjuvants affects the induction of antigen-specific antibody responses. J Control Release. 2021 Feb 10;330:933-944.
- Shaughnessy J, Ram S, Rice PA. Biology of the Gonococcus: disease and Pathogenesis. In: Christodoulides M, editor. Neisseria gonorrhoeae: methods and protocols. Meth Mol Biol. 2019/05/24ed. New York: Humana Press/Springer; 2019. p. 1–27.
- Young JDE, Blake M, Mauro A, et al. Properties of the major outer membrane protein from Neisseria gonorrhoeae incorporated into model lipid membranes. Proc Natl Acad Sci USA. 1983;80(12):3831–3835.
- Schindler H. Formation of planar bilayers from artificial or native membrane vesicles. FEBS Lett. 1980 Dec 15;122(1):77–79.
- Jiskoot W, Teerlink T, Van Hoof MMM, et al. Immunogenic activity of gonococcal protein I in mice with three different lipoidal adjuvants delivered in liposomes and in complexes. Infect Immun. 1986;54(2):333–338.
- Kersten GFA, Van De Put AM, Teerlink T, et al. Immunogenicity of liposomes and iscoms containing the major outer membrane protein of Neisseria gonorrhoeae: influence of protein content and liposomal bilayer composition. Infect Immun. 1988;56(6):1661–1664.
- Wetzler LM, Blake MS, Gotschlich EC. Characterization and specificity of antibodies to protein I of Neisseria gonorrhoeae produced by injection with various protein I-adjuvant preparations. J Exp Med. 1988;168(5):1883–1897.
- Parmar MM, Blake MS, Madden TD. Biophysical and antigenic characterization of gonococcal protein I incorporated into liposomes. Vaccine. 1997 Oct;15(15):1641–1651.
- Elkins C, Barkley KB, Carbonetti NH, et al. Immunobiology of purified recombinant outer membrane porin protein I of Neisseria gonorrhoeae. Mol Microbiol. 1994 Dec;14(5):1059–1075.
- Almonacid-Mendoza HL, Humbert MV, Dijokaite A, et al. Structure of the Recombinant Neisseria gonorrhoeae Adhesin Complex Protein (rNg-ACP) and generation of murine antibodies with bactericidal activity against Gonococci. mSphere. 2018 Oct 10;3(5). DOI:https://doi.org/10.1128/mSphere.00331-18.
- Martin JN, Ball LM, Solomon TL, et al. Neisserial Opa protein-CEACAM interactions: competition for receptors as a means of bacterial invasion and pathogenesis. Biochem. 2016 Aug 9;55(31):4286–4294.
- Kuhn J, Smirnov A, Criss AK, et al. Quantifying carcinoembryonic antigen-like cell adhesion molecule-targeted liposome delivery using imaging flow cytometry. Mol Pharm. 2019 Jun 3;16(6):2354–2363.
- Naids FL, Belisle B, Lee N, et al. Interactions of Neisseria gonorrhoeae with human neutrophils: studies with purified PII (Opa) outer membrane proteins and synthetic Opa peptides. Infect Immun. 1991;59(12):4628–4635.
- Idanpaan-Heikkila I, Muttilainen S, Wahlstrom E, et al. The antibody response to a prototype liposome vaccine containing Neisseria meningitidis outer membrane protein P1 produced in Bacillus subtilis. Vaccine. 1995 Nov;13(16):1501–1508.
- Muttilainen S, Butcher SJ, Runeberg K, et al. Heterologous production of the P1 porin of Neisseria meningitidis in Bacillus subtilis: the effect of an N-terminal extension on the presentation of native-like epitopes. Microb Path. 1995;18(5):365–371.
- Muttilainen S, Idanpaan-Heikkila I, Wahlstrom E, et al. The Neisseria meningitidis outer membrane protein P1 produced in Bacillus subtilis and reconstituted into phospholipid vesicles elicits antibodies to native P1 epitopes. Microb Path. 1995;18(6):423–436.
- Arigita C, Kersten GF, Hazendonk T, et al. Restored functional immunogenicity of purified meningococcal PorA by incorporation into liposomes. Vaccine. 2003 Feb 14;21(9–10):950–960.
- Ward SJ, Scopes DA, Christodoulides M, et al. Expression of Neisseria meningitidis class 1 porin as a fusion protein in Escherichia coli: the influence of liposomes and adjuvants on the production of a bactericidal immune response. Microb Path. 1996;21(6):499–512.
- Humphries HE, Williams JN, Christodoulides M, et al. Recombinant meningococcal PorA protein, expressed using a vector system with potential for human vaccination, induces a bactericidal immune response. Vaccine. 2004 Mar 29;22(11–12):1564–1569.
- Humphries H, Williams J, Blackstone R, et al. Multivalent liposome-based vaccines containing different serosubtypes of PorA protein induce cross-protective bactericidal immune responses against Neisseria meningitidis. Vaccine. 2006;24(1):35–44.
- Sanchez S, Abel A, Marzoa J, et al. Characterisation and immune responses to meningococcal recombinant porin complexes incorporated into liposomes. Vaccine. 2009 Aug 27;27(39):5338–5343.
- Freixeiro P, Dieguez-Casal E, Costoya L, et al. High resolution clear native electrophoresis (hrCNE) allows a detailed analysis of the heterotrimeric structure of recombinant Neisseria meningitidis porins inserted into liposomes. J Prot Res. 2013 Feb 1;12(2):777–784.
- Freixeiro P, Dieguez-Casal E, Costoya L, et al. Study of the stability of proteoliposomes as vehicles for vaccines against Neisseria meningitidis based on recombinant porin complexes. Int J Pharm. 2013 Feb 25;443(1–2):1–8.
- Watson DS, Endsley AN, Huang L. Design considerations for liposomal vaccines: influence of formulation parameters on antibody and cell-mediated immune responses to liposome associated antigens. Vaccine. 2012 Mar 16;30(13):2256–2272.
- Arigita C, Bevaart L, Everse LA, et al. Liposomal meningococcal B vaccination: role of dendritic cell targeting in the development of a protective immune response. Infect Immun. 2003 Sep;71(9):5210–5218.
- Foged C, Arigita C, Sundblad A, et al. Interaction of dendritic cells with antigen-containing liposomes: effect of bilayer composition. Vaccine. 2004 May 7;22(15–16):1903–1913.
- Christodoulides M, Brooks JL, Rattue E, et al. Immunization with recombinant class I outermembrane protein from Neisseria meningitidis: influence of liposomes and adjuvants on antibody avidity, recognition of native protein and the induction of a bactericidal immune response against meningococci. Microbiology. 1998;144(11):3027–3037.
- Hung MC, Salim O, Williams JN, et al. The Neisseria meningitidis macrophage infectivity potentiator protein induces cross-strain serum bactericidal activity and is a potential serogroup B vaccine candidate. Infect Immun. 2011 Sep;79(9):3784–3791.
- Hung MC, Heckels JE, Christodoulides M. The adhesin complex protein (ACP) of Neisseria meningitidis is a new adhesin with vaccine potential. mBio. 2013 Feb 26;4(2):e00041–13.
- Phillips R, Williams JN, Tan WM, et al. Immunization with recombinant Chaperonin60 (Chp60) outer membrane protein induces a bactericidal antibody response against Neisseria meningitidis. Vaccine. 2013 May 24;31(22):2584–2590.
- Hung M, Humbert M, Laver J, et al. A putative amino acid ABC transporter substrate-binding protein, NMB1612, from Neisseria meningitidis, induces murine bactericidal antibodies against meningococci expressing heterologous NMB1612 proteins. Vaccine. 2015 Aug 26;33(36):4486–4494.
- Wright JC, Williams JN, Christodoulides M, et al. Immunization with the recombinant PorB outer membrane protein induces a bactericidal immune response against Neisseria meningitidis. Infect Immun. 2002;70(8):4028–4034.
- Jolley K, Appleby L, Wright JC, et al. Immunization with recombinant Opc outer membrane protein from Neisseria meningitidis: influence of sequence variation and levels of expression on the bactericidal immune response against Meningococci. Infect Immun. 2001;69(6):3809–3916.
- Van Der Ley P, Steeghs L, Hamstra HJ, et al. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect Immun. 2001 Oct;69(10):5981–5990.
- Arigita C, Luijkx T, Jiskoot W, et al. Well-defined and potent liposomal meningococcal B vaccines adjuvated with LPS derivatives. Vaccine. 2005 Oct 17;23(43):5091–5098.
- Niebla O, Alvarez A, Martin A, et al. Immunogenicity of recombinant class 1 protein from Neisseria meningitidis refolded into phospholipid vesicles and detergent. Vaccine. 2001 May 14;19(25–26):3568–3574.
- Bueno Da Costa MH, Quintilio W, Tanizaki MM, et al. Heat shock protein micro-encapsulation as a double tool for the improvement of new generation vaccines. J Liposome Res. 2002 Feb-May;12(1–2):29–35.
- Livingston PO, Calves MJ, Helling F, et al. GD3/proteosome vaccines induce consistent IgM antibodies against the ganglioside GD3. Vaccine. 1993 Sep;11(12):1199–1204.
- Osorio M, Gracia E, Rodriguez E, et al. Heterophilic NeuGcGM3 ganglioside cancer vaccine in advanced melanoma patients: results of a Phase Ib/IIa study. Cancer Biol Ther. 2008 Apr;7(4):488–495.
- Morera Y, Bequet-Romero M, Ayala M, et al. Immunogenicity and some safety features of a VEGF-based cancer therapeutic vaccine in rats, rabbits and non-human primates. Vaccine. 2010 Apr 26;28(19):3453–3461.
- Hall MA, Stroop SD, Hu MC, et al. Intranasal immunization with multivalent group A streptococcal vaccines protects mice against intranasal challenge infections. Infect Immun. 2004 May;72(5):2507–2512.
- Peeters CC, Claassen IJ, Schuller M, et al. Immunogenicity of various presentation forms of PorA outer membrane protein of Neisseria meningitidis in mice. Vaccine. 1999 Jun 4;17(20–21):2702–2712.
- Idanpaan-Heikkila I, Wahlstrom E, Muttilainen S, et al. Immunization with meningococcal class 1 outer membrane protein produced in Bacillus subtilis and reconstituted in the presence of Zwittergent or Triton X-100. Vaccine. 1996;14(9):886–891.
- Jansen C, Wiese A, Reubsaet L, et al. Biochemical and biophysical characterization of in vitro folded outer membrane porin PorA of Neisseria meningitidis. Biochim Biophys Acta. 2000;1464(2):284–298.
- Jansen C, Kuipers B, van der Biezen J, et al. Immunogenicity of in vitro folded outer membrane protein PorA of Neisseria meningitidis. FEMS Immunol Med Micro. 2000;27(3):227–233.
- Musacchio A, Carmenate T, Delgado M, et al. Recombinant Opc meningococcal protein, folded in vitro, elicits bactericidal antibodies after immunization. Vaccine. 1997 Apr-May;15(6–7):751–758.
- Lowell GH, Smith LF, Seid RC, et al. Peptides bound to proteosomes via hydrophobic feet become highly immunogenic without adjuvants. J Exp Med. 1988;167(2):658–663.
- Lowell GH, Ballou WR, Smith LF, et al. Proteosome-lipopeptide vaccines: enhancement of immunogenicity for malaria CS peptides. Science. 1988;240(4853):800–802.
- Francis IP, Lui X, Wetzler LM. Isolation of naturally released gonococcal outer membrane vesicles as vaccine antigens. In: Christodoulides M, editor. Neisseria gonorrhoeae: methods and protocols. Meth Mol Biol 1997. 2019/05/24ed. New York: Springer/Humana Press; 2019. pp. 121-141.
- Donnelly JJ, Deck RR, Liu MA. Immunogenicity of a Haemophilus influenzae polysaccharide-Neisseria meningitidis outer-membrane protein complex conjugate vaccine. J Immunol. 1990;145:3071–3079.
- Mesa C, De Leon J, Rigley K, et al. Very small size proteoliposomes derived from Neisseria meningitidis: an effective adjuvant for Th1 induction and dendritic cell activation. Vaccine. 2004 Aug 13;22(23–24):3045–3052.
- Perez O, Lastre M, Cabrera O, et al. New vaccines require potent adjuvants like AFPL1 and AFCo1. Scand J Immunol. 2007 Aug-Sep;66(2–3):271–277.
- Del Campo J, Lindqvist M, Cuello M, et al. Intranasal immunization with a proteoliposome-derived cochleate containing recombinant gD protein confers protective immunity against genital herpes in mice. Vaccine. 2010 Feb 3;28(5):1193–1200.
- Wetzler LM, Blake MS, Barry K, et al. Gonococcal porin vaccine evaluation - comparison of por proteosomes, liposomes, and blebs isolated from rmp deletion mutants. J Infect Dis. 1992;166(3):551–555.
- Kersten GFA, Teerlink T, Derks HJGM, et al. Incorporation of the major outer membrane protein of Neisseria gonorrhoeae in saponin-lipid complexes (ISCOMS): chemical analysis, some structural features, and comparison of their immunogenicity with three other antigen delivery systems. Infect Immun. 1988;56(2):432–438.
- Kersten GFA, Spiekstra A, Beuvery EC, et al. On the structure of immune-stimulating saponin-lipid complexes (iscoms). Biochem Biophys Acta. 1991;1062(2):165–171.
- Magnusson SE, Altenburg AF, Bengtsson KL, et al. Matrix-M™ adjuvant enhances immunogenicity of both protein- and modified vaccinia virus Ankara-based influenza vaccines in mice. Immunol Res. 2018;66(2):224–233.
- Arigita C, Van Den Berg J, Wensink K, et al. Immunogenicity of meningococcal PorA formulations encapsulated in biodegradable microspheres. Eur J Pharm Sci. 2004 Feb;21(2–3):131–141.
- Micoli F, Alfini R, Di Benedetto R, et al. GMMA is a versatile platform to design effective multivalent combination vaccines. Vaccines (Basel). 2020 Sep 17;8(3):540.
- Wetzler LM, Feavers IM, Gray-Owen SD, et al. Summary and recommendations from the National Institute of Allergy and Infectious Diseases (NIAID) workshop “Gonorrhea Vaccines: the way forward.” Clin Vacc Immunol. 2016 Aug;23(8):656–663.
- Zhu T, McClure R, Harrison OB, et al. Integrated bioinformatic analyses and immune characterization of new Neisseria gonorrhoeae vaccine antigens expressed during natural mucosal infection. Vaccines (Basel). 2019 Oct 17;7(4):153.
- Dijokaite A, Humbert MV, Skipp P, et al. Pilot study: immuno-proteomics analysis of sera from patients with uncomplicated gonorrhoea informs potential vaccine development. 1st Neisseria gonorrhoeae Research Society Conference; October 21- 24; Multi-national, cross-continent Virtual Conference 2020.
- Zielke RA, Wierzbicki IH, Weber JV, et al. Quantitative proteomics of the Neisseria gonorrhoeae cell envelope and membrane vesicles for the discovery of potential therapeutic targets. Mol Cell Proteomics. 2014 May;13(5):1299–1317.
- Rappuoli R, Bottomley MJ, D’Oro U, et al. Reverse vaccinology 2.0: human immunology instructs vaccine antigen design. J Exp Med. 2016 Apr 4;213(4):469–481.
- Haghi F, Peerayeh SN, Siadat SD, et al. Recombinant outer membrane secretin PilQ(406-770) as a vaccine candidate for serogroup B Neisseria meningitidis. Vaccine. 2012 Feb 21;30(9):1710–1714.
- Callaghan MJ, Lewis S, Sadarangani M, et al. Potential of recombinant opa proteins as vaccine candidates against hyperinvasive meningococci. Infect Immun. 2011 Jul;79(7):2810–2818.
- Cole JG, Jerse AE. Functional characterization of antibodies against Neisseria gonorrhoeae opacity protein loops. PLoS One. 2009 Dec 1;4(12):e8108.
- Keiser PB, Gibbs BT, Coster TS, et al. A phase 1 study of a group B meningococcal native outer membrane vesicle vaccine made from a strain with deleted lpxL2 and synX and stable expression of opcA. Vaccine. 2010 Oct 8;28(43):6970–6976.
- Price GA, Masri HP, Hollander AM, et al. Gonococcal transferrin binding protein chimeras induce bactericidal and growth inhibitory antibodies in mice. Vaccine. 2007 Oct 10;25(41):7247–7260.
- Pettersson A, Kortekaas J, Weynants VE, et al. Vaccine potential of the Neisseria meningitidis lactoferrin-binding proteins LbpA and LbpB. Vaccine. 2006 Apr 24;24(17):3545–3557.
- Stork M, Bos MP, Jongerius I, et al. An outer membrane receptor of Neisseria meningitidis involved in zinc acquisition with vaccine potential. PLoS Pathog. 2010 Jul 01;6(7):e1000969.
- Wang S, Xue J, Lu P, et al. Gonococcal MtrE and its surface-expressed Loop 2 are immunogenic and elicit bactericidal antibodies. J Infect. 2018 Sep;77(3):191–204.
- Shewell LK, Ku SC, Schulz BL, et al. Recombinant truncated AniA of pathogenic Neisseria elicits a non-native immune response and functional blocking antibodies. Biochem Biophys Res Commun. 2013 Feb 8;431(2):215–220.
- Li G, Jiao H, Jiang G, et al. Neisseria gonorrhoeae NspA induces specific bactericidal and opsonic antibodies in mice. Clin Vacc Immunol. 2011 Nov;18(11):1817–1822.
- Zielke RA, Wierzbicki IH, Baarda BI, et al. Proteomics-driven antigen discovery for development of vaccines against Gonorrhea. Mol Cell Proteomics. 2016 Jul;15(7):2338–2355.
- Semchenko EA, Day CJ, Seib KL. MetQ of Neisseria gonorrhoeae Is a surface-expressed antigen that elicits bactericidal and functional blocking antibodies. Infect Immun. 2017 Feb;85(2):e00898–16.
- Sikora AE, Gomez C, Le Van A, et al. A novel gonorrhea vaccine composed of MetQ lipoprotein formulated with CpG shortens experimental murine infection. Vaccine. 2020 Dec 3;38(51):8175–8184.
- Baarda BI, Emerson S, Proteau PJ, et al. Deciphering the function of new gonococcal vaccine antigens using phenotypic microarrays. J Bacteriol. 2017 Sep 1;199(17). DOI:https://doi.org/10.1128/JB.00037-17.
- Zielke RA, Le Van A, Baarda BI, et al. SliC is a surface-displayed lipoprotein that is required for the anti-lysozyme strategy during Neisseria gonorrhoeae infection. PLoS Pathog. 2018 Jul;14(7):e1007081.
- Gulati S, McQuillen DP, Sharon J, et al. Experimental immunization with a monoclonal anti-idiotope antibody that mimics the Neisseria gonorrhoeae lipooligosaccharide epitope 2C7. J Infect Dis. 1996 Dec;174(6):1238–1248.
- Ngampasutadol J, Rice PA, Walsh MT, et al. Characterization of a peptide vaccine candidate mimicking an oligosaccharide epitope of Neisseria gonorrhoeae and resultant immune responses and function. Vaccine. 2006 Jan 12;24(2):157–170.
- Gulati S, Zheng B, Reed GW, et al. Immunization against a saccharide epitope accelerates clearance of experimental gonococcal infection. PLoS Pathog. 2013;9(8):e1003559.
- Parzych EM, Gulati S, Zheng B, et al. Synthetic DNA delivery of an optimized and engineered monoclonal antibody provides rapid and prolonged protection against experimental gonococcal infection. mBio. 2021 Mar 16;12(2). DOI:https://doi.org/10.1128/mBio.00242-21.
- Christodoulides M. Neisseria proteomics for antigen discovery and vaccine development. Exp Rev Prot. 2014 Oct;11(5):573–591.
- Souto EB, Silva GF, Dias-Ferreira J, et al. Nanopharmaceutics: Part I-Clinical trials legislation and Good Manufacturing Practices (GMP) of nanotherapeutics in the EU. Pharmaceutics. 2020 Feb 11;12(2):146.
- Wang DY, van der Mei HC, Ren Y, et al. Lipid-Based antimicrobial delivery-systems for the treatment of bacterial infections. Front Chem. 2019;7:872.
- Wang N, Zhen Y, Jin Y, et al. Combining different types of multifunctional liposomes loaded with ammonium bicarbonate to fabricate microneedle arrays as a vaginal mucosal vaccine adjuvant-dual delivery system (VADDS). J Control Release. 2017 Jan 28;246:12–29.
- Liu Y, Hammer LA, Liu W, et al. Experimental vaccine induces Th1-driven immune responses and resistance to Neisseria gonorrhoeae infection in a murine model. Mucosal Immunol. 2017 Nov;10(6):1594–1608.
- Schoenmaker L, Witzigmann D, Kulkarni JA, et al. mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int J Pharm. 2021 May 15;601:120586.
- Zaidi S, Misba L, Khan AU. Nano-therapeutics: a revolution in infection control in post antibiotic era. Nanomed. 2017 Oct;13(7):2281–2301.
- Fries CN, Curvino EJ, Chen JL, et al. Advances in nanomaterial vaccine strategies to address infectious diseases impacting global health. Nature Nanotechnol. 2021 Apr;16(4):1–14.
- Vahedifard F, Chakravarthy K. Nanomedicine for COVID-19: the role of nanotechnology in the treatment and diagnosis of COVID-19. Emergent Mater. 2021 Feb;13:1–25.
- Tapia D, Sanchez-Villamil JI, Torres AG. Multicomponent gold nano-glycoconjugate as a highly immunogenic and protective platform against Burkholderia mallei. NPJ Vaccines. 2020;5(1):82.
- Dykman LA, Volokh OA, Gromova OV, et al. Obtaining and characteristic of antibodies to Vibrio cholerae protective antigens conjugated with gold nanoparticles. Dokl Biochem Biophys. 2020 Jan;490(1):19–21.
- Pitirollo O, Micoli F, Necchi F, et al. Gold nanoparticles morphology does not affect the multivalent presentation and antibody recognition of Group A Streptococcus synthetic oligorhamnans. Bioorg Chem. 2020 Jun;99:103815.
- Sanchez-Villamil JI, Tapia D, Torres AG. Development of a gold nanoparticle vaccine against enterohemorrhagic Escherichia coli O157:H7. mBio. 2019 Aug 13;10(4). DOI:https://doi.org/10.1128/mBio.01869-19.
- Ha NY, Shin HM, Sharma P, et al. Generation of protective immunity against Orientia tsutsugamushi infection by immunization with a zinc oxide nanoparticle combined with ScaA antigen. J Nanobiotechnol. 2016 Nov 26;14(1):76.
- Pan C, Wu J, Qing S, et al. Biosynthesis of self-assembled proteinaceous nanoparticles for vaccination. Adv Mater. 2020 Oct;32(42):e2002940.
- Marcandalli J, Fiala B, Ols S, et al. Induction of potent neutralizing antibody responses by a designed protein nanoparticle vaccine for respiratory syncytial virus. Cell. 2019 Mar 7;176(6):1420–1431.e17.
- Wichgers Schreur PJ, Tacken M, Gutjahr B, et al. Vaccine efficacy of self-assembled multimeric protein scaffold particles displaying the glycoprotein Gn head domain of rift valley fever virus. Vaccines (Basel). 2021 Mar 23;9(3):301.
- Kang YF, Sun C, Zhuang Z, et al. Rapid development of SARS-CoV-2 spike protein receptor-binding domain self-assembled nanoparticle vaccine candidates. ACS Nano. 2021 Feb 23;15(2):2738–2752.
- Hobbs MM, Sparling PF, Cohen MS, et al. Experimental gonococcal infection in male volunteers: cumulative experience with Neisseria gonorrhoeae strains FA1090 and MS11mkC. Front Microbiol. 2011;2:123.
- Plante M, Jerse A, Hamel J, et al. Intranasal immunization with gonococcal outer membrane preparations reduces the duration of vaginal colonization of mice by Neisseria gonorrhoeae. J Infect Dis. 2000 Sep;182(3):848–855.
- Zhu W, Thomas CE, Chen CJ, et al. Comparison of immune responses to gonococcal PorB delivered as outer membrane vesicles, recombinant protein, or Venezuelan equine encephalitis virus replicon particles. Infect Immun. 2005 Nov;73(11):7558–7568.
