ABSTRACT
Introduction
Despite high vaccination coverage among children and adolescents, pertussis remains a public health problem, with large outbreaks occurring periodically in the US and other developed countries.
Areas covered
We examine lessons learned more than 20 years after implementation of programs which use only acellular pertussis vaccines and propose avenues for possible effective use of acellular pertussis vaccine to prevent large outbreaks.
Expert Opinion
Acellular pertussis vaccines were introduced more than 20 years ago, yet the incidence of pertussis has been increasing over the past decade, with periodic large outbreaks marked by notable shifts in disease burden from infants and young children toward fully vaccinated adolescents and young adults. This age shift is mainly driven by the waning of vaccine immunity. To better protect adolescents against pertussis, modification of the current acellular pertussis vaccination schedule or adoption of new vaccination strategies should be considered. For infants not yet eligible to be vaccinated, maternal vaccination against pertussis during pregnancy is an effective way to protect infants from infection, severe disease and death. Implementation of maternal vaccination programs should be encouraged in countries without one or efforts to improve coverage should be supported in countries with existing program.
1. Introduction
Pertussis, also known as whooping cough, is a highly contagious respiratory disease caused by the bacterium Bordetella pertussis. Before the availability of vaccines, pertussis was a frequent cause of morbidity and mortality in infants and young children [Citation1–3].
In the 1940s, the development and introduction of a combined diphtheria–tetanus–whole-cell pertussis (DTwP) vaccine into childhood immunization programs led to drastic decrease in pertussis incidence worldwide. In the United States (US), yearly incidence decreased from 157/100,000 population in the early 1940s to less than 1/100,000 in 1973 [Citation3,Citation4]. DTwP was effective against pertussis; however, its reactogenicity and unsubstantiated concerns about risks of encephalopathy and death led to parental resistance to its use [Citation5,Citation6]. DTwP was ultimately replaced by less reactogenic acellular pertussis vaccines (diphtheria, tetanus, acellular pertussis vaccines [DTaP]) in the US and most industrialized countries [Citation7–10].
In the US, acellular pertussis vaccines use has evolved since the US Advisory Committee on Immunization Practice (ACIP) began recommending DTaP for infants and young children in the late 1990s [Citation11], with the current schedule consisting of five DTaP doses administered to children between 2 months and 6 years of age. In 2006, in response to pertussis outbreaks in 2004 and 2005 in which 11–18 year-olds accounted for 60% of the cases, the ACIP recommended that 11 and 12 year-olds receive a booster dose of tetanus, reduced antigen acellular pertussis, diphtheria (Tdap) vaccine [Citation12,Citation13]. In 2011, the ACIP expanded their Tdap recommendations to include a single dose administered to pregnant women. This recommendation followed infant deaths due to pertussis in 2010 with the goal of protecting infants through passive transfer of maternal antibodies prior to DTaP administration at 2 months of age. ACIP further expanded this recommendation in 2012 to administer Tdap during every pregnancy [Citation14].
Worldwide, the proportion of infants receiving three doses of diphtheria-tetanus-pertussis vaccine has increased over the years, reaching about 85% in 2019 [Citation15]. In the US, infant coverage with three doses has been above 90% since DTaP was implemented [Citation16–19]. Recent data also suggest high Tdap coverage among adolescents [Citation20].
Despite high acellular pertussis vaccine coverage among children and adolescents, pertussis outbreaks have continued to occur periodically in the US and other developed countries and pertussis remains a public health problem [Citation3,Citation21–26]. In this review, we will examine lessons learned after exclusively using acellular pertussis vaccines for more than 20 years and propose avenues to prevent large outbreaks.
2. Pertussis outbreaks continue to occur and are larger in size
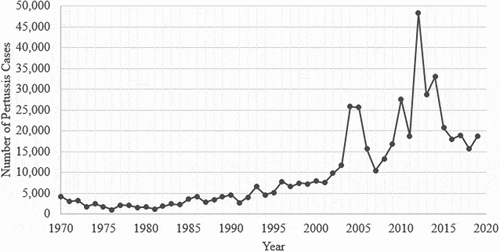
Pertussis outbreaks continue to occur in the era of acellular pertussis vaccines and have been marked by the large size and notable shifts in disease burden from infants and children toward adolescents and young adults [Citation13,Citation26–33]. In the US, pertussis incidence has significantly increased from 2000 to 2016. During this period, outbreaks occurred cyclically with incidence ranging from a low of 2.7 cases/100,000 persons in 2001 to a high of 15.4/100,000 in 2012 [Citation32], corresponding to 25,000 to more than 48,000 reported cases (). In 2012 alone, 48,277 cases were reported, the most since the mid-1950s [Citation3].
Figure 1. Number of pertussis cases reported to the US centers for diseases control and prevention 1980–2018(Adapted from CDC National notifiable diseases surveillance system and supplemental pertussis surveillance system https://www.cdc.dov/pertussis/survreporting.html).
3. Disease burden shifted from infants to adolescents
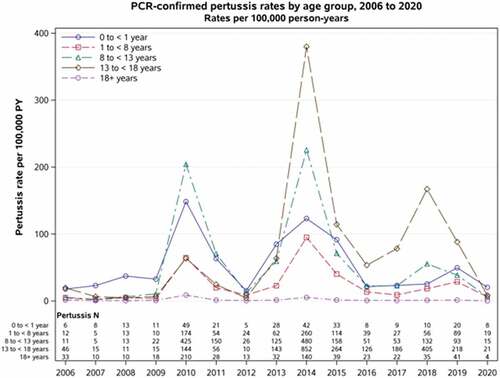
In the US and other countries, incidence of pertussis is highest among infants <1 year of age [Citation32,Citation34]. However, since the widespread use of acellular pertussis vaccines, incidence in the US has risen fastest among 11–18 years old. Between 2000 and 2006, this group had the second highest annual incidence, ranging from 6 cases/100,000 population in 2001 to 26 cases/100,000 population in 2004. Beginning in 2007 and continuing through 2014, the second-highest annual incidence was among children aged 7–10 years. In 2015 and 2016, adolescent ages 11–18 years pertussis rates once again surpassed rates in children aged 7–10 years [Citation13,Citation32]. From 2000 to 2016, adolescents accounted for >25% of all pertussis cases reported in the US [Citation32]. At Kaiser Permanente Northern California (KPNC), an integrated health-care organization with more than 4.5 million members, we found that adolescents, most of whom were vaccinated, accounted for most pertussis cases during the 2014 pertussis outbreak [Citation29]. Since then, this group has continued to have the highest incidence of disease, including during a ‘mini outbreak’ in 2018 (). Although rates of pertussis decreased for all age groups from 2018 to 2020, reaching a nadir in 2020, this decline likely reflected the public health measures put in place in 2020 to mitigate the spread of coronavirus disease 2019 (COVID-19) [Citation35,Citation36]. In addition to the US, other industrialized countries using acellular pertussis vaccines have seen increased incidence of pertussis among adolescents and young adults [Citation33,Citation37].
Figure 2. Rates of confirmed pertussis cases by age groups: Kaiser Permanente Northern California 2006–2020.
Pertussis in adolescents and adults is usually not associated with severe disease or mortality [Citation32,Citation38]. However, it is associated with morbidity, including apnea, insomnia, pneumonia, weight loss, urinary incontinence, syncope, or rib fractures, as well as significant economic costs [Citation39,Citation40]. This group may also pose a transmission risk to their susceptible peers and infants who are too young to be vaccinated [Citation41,Citation42].
4. Factors contributing to increased incidence and shift in disease burden
There is no consensus regarding the reasons for increases in overall incidence of pertussis over time [Citation43–45]. Multiple factors might play a role, including waning vaccine effectiveness, inability of vaccines to prevent colonization and mutation in circulating pertussis strains.
4.1. Acellular pertussis vaccines effective initially but immunity wanes
Most pertussis cases during recent outbreaks occurred among highly vaccinated children and adolescents, suggesting a waning of vaccine effectiveness. We and others have demonstrated that immunity after acellular pertussis vaccines wane over a short period of time [Citation29,Citation46–51]. Following the 2010 California pertussis outbreak, we conducted a case-control study among a highly vaccinated cohort of children 8–12 years of age within KPNC who had only received DTaP vaccines and found that polymerase chain reaction (PCR) test-positive pertussis cases were more likely to have received their 5th DTaP dose earlier than were controls. Compared with PCR-negative controls, the odds of acquiring pertussis increased by 42% on average each year after the 5th DTaP dose (OR 1.42, 95% CI 1.21–1.66) [Citation52], results which were similar to another case-control study also conducted in California [Citation48]. Tartof and colleagues also reported a steady increase in the risk of pertussis each year after vaccination in a study among children born from 1998 to 2003 in Oregon and Minnesota [Citation53]. In Minnesota, the relative risk of pertussis increased from 1.9 (95% CI 1.3–2.9) a year after the fifth dose to 8.9 (95% CI 6.0–13.0) 6 years after. In Australia, Quin and colleagues reported that DTaP vaccine protection wanes between doses. They found that the effectiveness of three doses of DTaP against all reported pertussis confirmed by polymerase chain reaction or isolation by culture or serology test was 83.5% (95% CI, 79.1–87.8%) between 6 and 11 months, declining to 70.7% (95% CI, 64.5–75.8%) between 2 and 3 years of age and 59.2% (95% CI, 51.0–66.0%) between 3 and 4 years of age [Citation46]. These studies provide compelling evidence that DTaP provides reasonable protection to infants and school-age children, but that protection wanes rapidly.
More recently, we conducted another study among 469,982 children 7–11 years old within KPNC to assess the contribution of waning and undervaccination to pertussis risk. This study found that the 5th dose of DTaP was 88.7% (95% CI 80.0–93.6) effective against PCR test-positive pertussis [Citation50]. Pertussis risk was also 5 times higher (aHR = 5.04; 95% CI 1.84–13.80) ≥3 years after vaccination, 2 times higher (aHR = 2.01; 95% CI 1.05–3.85) 2 to <3 years after vaccination and 40% higher (aHR = 1.40; 95% CI 0.86–2.27) 1 to <2 years vs. <1 year after vaccination among children ages 19 to <84 months. Risk was 2 times higher (aHR = 2.32; 95% CI 0.97–5.59) ≥6 years or 5 – <6 years after vaccination (aHR = 2.03, 95% CI 0.92–4.52), 37% and 55% higher 4 to <5 years and 3 to <4 years, respectively, vs. <3 years after vaccination in children ages 84–132 months [Citation50]. Children in this study who were unvaccinated had 13 times higher risk of pertussis than did vaccinated children; however, unvaccinated children represented only 13% of the cases in the study [Citation50]. This study indicates that waning immunity, more than undervaccination, plays a key role in pertussis outbreaks.
Similarly, Tdap vaccine is effective against pertussis, but short-lived [Citation29,Citation30,Citation54,Citation55]. We evaluated a cohort of Tdap-vaccinated adolescents who had only received DTaP vaccines during infancy and childhood and found that while Tdap provided moderate protection of 69% (95% CI: 59.7–75.9) against disease after the first year, protection declined to less than 9% (95% CI: −30.6–36.4) after 4 years [Citation29]. Acosta and colleagues similarly found that among adolescents born 1993–2000 who had only previously received DTaP vaccines, Tdap vaccine effectiveness decreased from 73% within a year of vaccination to 34% two or more years after vaccination [Citation54]. Additional recent studies also suggest that Tdap vaccines are effective but short-lived. Comparative effectiveness studies of brand-specific acellular pertussis vaccines suggest no difference in effectiveness and waning immunity over time [Citation55,Citation56].
Taken together, these studies demonstrate that increasing time since vaccination with acellular pertussis is strongly associated with increased risk of pertussis. As children and adolescents age, DTaP and Tdap provide them with less protection, and they become more susceptible to pertussis.
4.2. Acellular pertussis vaccines are unable to prevent colonization
Acellular pertussis vaccines induce an immune response that is different from immunity after natural infection and whole-cell pertussis vaccines. While both natural infection and to some extent whole-cell pertussis vaccines induce both mucosal and systemic immune responses with strong T helper 17 (Th17) and Th1 memories, acellular pertussis vaccines induce only a systemic immune response with a strong Th1/Th2 response [Citation57,Citation58]. This type of immunity only prevents the development of signs and symptoms of pertussis but does not prevent colonization [Citation57]. Thus, vaccinated people can carry pertussis asymptomatically or have a mild form of the disease and transmit the bacterium to unvaccinated susceptible individuals, which complicates the achievement of heard immunity [Citation59].
4.3. Acellular pertussis vaccines may not protect against new strains of Bordetella Pertussis
Vaccine-induced selection pressure may have led to new Bordetella pertussis strains lacking the antigens included in current acellular vaccines [Citation60–64]. Whether the mismatch between vaccine antigens and the new strains has a significant impact on vaccine effectiveness leading to increases in diseases over time is unclear [Citation65,Citation66].
5. Current vaccination strategies to reduce pertussis disease
Considering the resurgence of pertussis, and in the absence of an alternative less reactogenic vaccine, several strategies have been implemented to reduce the burden of disease. These strategies have mainly focused on adolescents and infants too young to be fully vaccinated, with varying degrees of success.
5.1. Strategies with less successful results: adolescent booster dose and cocooning
To reduce disease in adolescents, a Tdap booster was recommended by the ACIP for adolescent ages 11–12 years [Citation67]. The booster dose initially led to a decrease in the incidence of disease among adolescents, but it does not prevent outbreaks in adolescents, nor does it provide much protection to infants.
Another less successful vaccination strategy was cocooning. This strategy involved administering Tdap to all persons in close contact with newborns and infants, including parents, grandparents, caregivers and older siblings. While the idea seemed to have merit, studies found that it resulted in minimal reductions in infant cases [Citation68,Citation69]. In 2011, the ACIP concluded that cocooning alone is an insufficient strategy to prevent pertussis morbidity and mortality in newborn [Citation70].
5.2. Strategies with successful results: Maternal Tdap vaccination during pregnancy
In October 2011, in an effort to further reduce the burden of pertussis in infants, the ACIP recommended that unvaccinated pregnant women receive a dose of Tdap [Citation70]. Tdap given to pregnant women boosts maternal anti-pertussis antibodies, which are then passed to the fetus via the placenta, providing the newborn with passive protection against pertussis in early life until they are old enough to receive DTaP vaccination.
In February 2013, the ACIP extended their Tdap recommendation to administer the vaccine during every pregnancy regardless of previous Tdap vaccination. They stated it could be given any time during pregnancy but recommended it preferably be given between 27- and 36-weeks’ gestation to maximize antibody transfer [Citation71]. Several other countries have adopted similar recommendations regarding Tdap vaccination of pregnant women to provide protection to newborns [Citation72–77].
Prior to implementing maternal Tdap programs, several countries had high pertussis incidence rates among infants aged <2–3 months, which exceeded 1,000 cases per 100,000 population during outbreaks [Citation77]. After instituting maternal Tdap vaccinations, many countries saw significant decreases in pertussis incidence among infants <1 years of age [Citation3,Citation74,Citation77–80]. Argentina observed a relative reduction of 44% in the incidence of pertussis among children 2–6 months of age after it began vaccinating pregnant with Tdap in 2012 [Citation81]. In the UK, the incidence of pertussis in infants <3 months of age declined overall from 234 per 100,000 in 2012 to 52 per 100,000 in 2019 [Citation82]. At KPNC, incidence rates of pertussis among infants <1 year during 2010 to 2018 declined from more than 148/100,000 person-years to 25/100,000 person-years (). In the US, although pertussis incidence remains highest among infants, KPNC’s high rate of maternal Tdap vaccination has resulted in such substantial decreases in infant disease that pertussis incidence in this younger group is no longer the highest in KPNC ().
Maternal Tdap vaccination programs have also been associated with significant decreases in rates of infant hospitalization due to pertussis. A recent US study reported that, after adjusting for time, rates of hospitalization among infants due to pertussis declined 48% from 2009–2012 to 2013–2017 (8.4 per 100 000 vs. 3.3 per 100 000 infants) [Citation83].
The observed substantial decrease in incidence rates of disease among infants following maternal Tdap vaccination demonstrates that the vaccine is effective at protecting infants. Several studies have demonstrated that Tdap vaccination during pregnancy is 69–94% effective at protecting infants against disease and hospitalization in the first few months of life [Citation77,Citation84,Citation85]. We estimated that Tdap during pregnancy was 69.0% (95% CI, 43.6–82.9) effective at protecting infants against pertussis during the entire first year of life [Citation86].
6. Conclusion
Of the strategies in place to prevent pertussis, maternal Tdap vaccination has been the most successful in reducing pertussis disease, hospitalization, and death in infants. In contrast, current pertussis prevention strategies have not been very successful for adolescents and young adults. New approaches need to be considered to reduce disease burden in this group.
7. Expert opinion
Based on their strength and weakness, we need to consider new strategies to better use acellular pertussis vaccines. The goals of the proposed strategies using current vaccines are to reduce disease burden among infants and adolescents and reduce hospitalization and deaths among infants. Since these strategies will not lead to disease eradication, there is a need for a new generation of pertussis vaccines.
7.1. Need to increase maternal vaccination during pregnancy
There is strong evidence that maternal Tdap vaccination is effective against pertussis among infants. However, vaccine uptake among pregnant women in the US and other countries has been suboptimal. A recent survey among women in the US who were pregnant in 2018–2019 and delivered live-born infants reported that only 56% received a Tdap vaccine [Citation87]. In the UK, in 2020, the average vaccine uptake was 70.5% [Citation82]. We need strategies to increase vaccine uptake.
In 2012, at KPNC, Tdap coverage among pregnant women was only 17%. To increase coverage, the institution implemented an alert system in the electronic medical record to remind clinicians to administer Tdap to pregnant women starting at 27 weeks of gestation [Citation88]. This strategy resulted in an increase in coverage to 87% in 2015 [Citation86]. Similar approaches should be encouraged in other settings to increase coverage. In addition to reminders, health-care professionals should be trained to systematically educate pregnant women about the benefits of immunizations in pregnancy for their unborn child and vaccines need to be made readily available and accessible [Citation89,Citation90].
Some studies have reported reduced levels of antibodies following pertussis vaccination in infants exposed to Tdap during pregnancy compared with those not exposed [Citation91–94]. Although the clinical significance of the reduced immune response among exposed children is unclear, ongoing studies will need to ensure that there are no serious unintended consequences associated with expanded maternal Tdap programs.
7.2. Efficient use of acellular pertussis vaccines to prevent large pertussis outbreaks among adolescents and young adults
As discussed earlier, acellular pertussis vaccines are unable to prevent infection. To account for the cyclical nature of pertussis outbreaks, we previously proposed utilizing a ‘timed’ Tdap vaccination approach rather than an age-based Tdap vaccination strategy for adolescents [Citation30]. As previously described, the goal would be to ensure maximal disease protection for adolescents during periods when there is a greater risk of being exposed to pertussis. One of the previous challenges of this proposed strategy was that it required timely and reliable pertussis surveillance, both on a regional and national level. However, multiple surveillance systems have become adept at tracking and monitoring the spread of COVID-19 in the US and throughout the world and such systems could now be adapted or improved to create real-time surveillance and disease modeling for pertussis.
Alternatively, the current length of time between the 5th DTaP dose and Tdap vaccines (i.e. 5–7 years) puts adolescents at high risk of diseases. Thus, a slight modification to the current U.S. vaccination schedule to administer Tdap to children aged 8–9 years could conceivably reduce disease burden among adolescents and young adults by providing protection before their protection from DTaP wanes completely. Further, adolescents would receive a 2nd Tdap dose at the same time as they receive their recommended 2nd dose of quadrivalent meningococcal conjugate vaccine at 16 years of age [Citation95,Citation96]. This Tdap dose will reduce disease burden among young adults.
The proposed modification generally could be feasible to implement, although it will require a visit between ages 8–9 years, which could pose a burden to parents and providers, but would otherwise only modestly impact the current immunization schedule [Citation95,Citation96]. In addition, most states in the US require that adolescents receive Tdap before entering middle schools (usually ages 11–12 years) [Citation97]. These laws would not need to be modified since children would have been vaccinated earlier.
The cost-effectiveness associated with the proposed modifications and the addition of a second Tdap dose are unknown. Although a previous cost analysis did not find a second dose Tdap to be cost-effective, this proposal differs from the previous analysis because that study proposed to add a second dose at ages 16 years or 21 years [Citation98]. Based on the rate of waning of acellular vaccine immunity, we believe that administering the second dose at age 16 or 21 years after the first dose at 11 or 12 years was too late to have an impact on pertussis cases. It is also possible that previous analyses may have underestimated pertussis disease among adolescents and young adults. Studies are needed to evaluate the cost-effectiveness of our proposal.
7.3. Develop new pertussis vaccines applying new technology used for COVID-19 vaccines
Developing strategies for the best use of current pertussis vaccines is key to preventing and mitigating outbreaks, however the ideal long-term goal is to have pertussis vaccines that protect all vaccinated individuals for a long period of time, possibly leading to disease eradication. In the pursuit of new pertussis vaccines, developers should consider taking advantage of the new platforms used to develop COVID-19 vaccines such as mRNA vaccines, which induce broad immune response and appear to be highly effective against COVID-19 [Citation99–101]. Although the duration of protection following primary doses of mRNA COVID-19 vaccines seems to be short [Citation102], additional booster might provide long-term immunity. If needed, the use of mRNA platforms would also allow for rapid development of vaccines against new Bordetella pertussis strains. We hope that mRNA vaccines can prevent colonization in order to prevent bacterial strain selection pressure as seen with acellular pertussis vaccines. In addition, other subunits and viral vector platforms used to develop COVID-19 vaccines should also be explored as the basis for new pertussis vaccines.
Previously, others have suggested that vaccine developers replace the adjuvant in the current vaccines with another capable of inducing a more robust response [Citation103]. This suggestion can be challenging as it is unknown whether changes in the adjuvant will impact other antigens in the combined pertussis vaccines. Furthermore, as we have seen in the case of whole-cell pertussis vaccines, a reactogenic new adjuvant could lead to parental resistance toward vaccinating their children.
Finally, because Bordetella pertussis targets respiratory epithelia, mucosal immunity may be a key factor for disease prevention. Results of early trials studies using live-attenuated intranasal vaccines able to induce mucosal immunity have been encouraging [Citation104,Citation105]. There are also ongoing efforts to find additional antigens that could be included in the next generation of acellular pertussis vaccines [Citation64].
7.4. Five-year view
We expect to continue seeing a reduction in the number of pertussis cases among infants if maternal Tdap coverage continue to increase. However, if no new strategies are undertaken for older children or adolescents, we will have larger pools of young adults susceptible to pertussis, ultimately leading to periodic pertussis outbreaks among older age groups. Whether the recent ACIP recommendation to replace Td with Tdap for the 10 years tetanus booster will impact the risk of pertussis among young adults remains to be seen. We hope that next generation of pertussis vaccines will provide better protection to all vaccinated people.
Article highlights
Despite high acellular pertussis vaccine (DTaP and Tdap) coverage, the US and other developed countries have had large pertussis outbreaks with disease burden in adolescents and young adults.
Waning of vaccine-induced immunity is mostly the main driver of the shift in disease burden from children to adolescents and young adults.
Alternate approaches to using Tdap in children/adolescents are needed.
Tdap vaccination during pregnancy is an effective strategy to prevent pertussis in infants. Strategies should seek to expand and increase Tdap vaccine coverage among pregnant women.
New pertussis vaccines are needed. Next generation of pertussis vaccines should explore new vaccine platforms, including mRNA vaccines.
Financial and competing interest disclosure
Nicola P. Klein received research support from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Merck &Co. and Protein Science (now Sanofi Pasteur). Ousseny Zerbo received a career development grant from the National Institute of Allergy and Infectious Diseases. Grant # K01AI139275
Author contributions
All authors substantially contributed to the conception and design of the review article and interpreting the relevant literature and have been involved in writing the review article and revising it for intellectual content.
Additional information
Funding
References
- Kilgore PE, Salim AM, Zervos MJ, et al. Pertussis: microbiology, disease, treatment, and prevention. Clin Microbiol Rev. 2016;29(3):449–486.
- Nieves DJ, Heininger U. Bordetella pertussis. Microbiol Spectr. 2016 Jun;4(3). doi:10.1128/microbiolspec.EI10-0008-2015. PMID: 27337481
- CDC. Centers for diseases control and prevention: pertussis (Whooping Cough) surveillance and reporting. (Ed.^(Eds) (2019)
- Cherry JD. Epidemic pertussis in 2012–the resurgence of a vaccine-preventable disease. N Engl J Med. 2012;367(9):785–787.
- Gale JL, Thapa PB, Wassilak SG, et al. Risk of serious acute neurological illness after immunization with diphtheria-tetanus-pertussis vaccine. A population-based case-control study. JAMA. 1994;271(1):37–41.
- Ray P, Hayward J, Michelson D, et al. Encephalopathy after whole-cell pertussis or measles vaccination: lack of evidence for a causal association in a retrospective case-control study. Pediatr Infect Dis J. 2006;25(9):768–773.
- Sato Y, Kimura M, Fukumi H. Development of a pertussis component vaccine in Japan. Lancet. 1984;1(8369):122–126.
- Klein NP. Licensed pertussis vaccines in the United States. History and current state. Hum Vaccin Immunother. 2014;10(9):2684–2690.
- Latasa P, Garcia-Comas L, Gil De Miguel A, et al. Effectiveness of acellular pertussis vaccine and evolution of pertussis incidence in the community of Madrid from 1998 to 2015. Vaccine. 2018;36(12):1643–1649.
- Bell CA, Russell ML, Drews SJ, et al. Acellular pertussis vaccine effectiveness and waning immunity in Alberta, Canada: 2010-2015, a Canadian Immunization Research Network (CIRN) study. Vaccine. 2019;37(30):4140–4146.
- Guris D, Strebel PM, Jafari H, et al. Pertussis vaccination: use of acellular pertussis vaccines among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1997;46(RR–7):1–25.
- Broder KR, Cortese MM, Iskander JK, et al. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55(RR–3):1–34.
- Skoff TH, Martin SW. Impact of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccinations on reported pertussis cases among those 11 to 18 years of age in an era of waning pertussis immunity: a follow-up analysis. JAMA Pediatr. 2016;170(5):453–458.
- Liang JL, Tiwari T, Moro P, et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67(2):1–44.
- World, Health, Organization. Global Immunization 1980–2019: global coverage from 3 doses of DTP containing vaccine. Accessed April 2021. (Ed.^(Eds))
- Data Management Division, National Immunization Program, Centers for Disease Control and Prevention. National, state, and urban area vaccination coverage levels among children aged 19-35 months–United States, 2000. MMWR Morb Mortal Wkly Rep. 2001;50(30):637–641.
- Hill HA, Elam-Evans LD, Yankey D, et al. National, state, and selected local area vaccination coverage among children aged 19-35 months - United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(33):889–896.
- Hill HA, Yankey D, Elam-Evans LD, et al. Vaccination coverage by age 24 months among children born in 2016 and 2017 - National Immunization Survey-Child, United States, 2017-2019. MMWR Morb Mortal Wkly Rep. 2020;69(42):1505–1511.
- Krishnarajah G, Malangone-Monaco E, Palmer L, et al. Age-appropriate compliance and completion of up to five doses of pertussis vaccine in US children. Hum Vaccin Immunother. 2018;14(12):2932–2939.
- Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(33):718–723.
- Choi YH, Campbell H, Amirthalingam G, et al. Investigating the pertussis resurgence in England and Wales, and options for future control. BMC Med. 2016;14(1):121.
- Amirthalingam G. Strategies to control pertussis in infants. Arch Dis Child. 2013;98(7):552–555.
- Khetsuriani N, Bisgard K, Prevots DR, et al. Pertussis outbreak in an elementary school with high vaccination coverage. Pediatr Infect Dis J. 2001;20(12):1108–1112.
- Matthias J, Pritchard PS, Martin SW, et al. Sustained transmission of pertussis in vaccinated, 1-5-year-old children in a preschool, Florida, USA. Emerg Infect Dis. 2016;22(2):242–246.
- Esposito S, Principi N. Prevention of pertussis: an unresolved problem. Hum Vaccin Immunother. 2018;14(10):2452–2459.
- Esposito S, Stefanelli P, Fry NK, et al. Pertussis prevention: reasons for resurgence, and differences in the current acellular pertussis vaccines. Front Immunol. 2019;10:1344.
- Winter K, Zipprich J, Harriman K. Pertussis in California: a Tale of 2 Epidemics. Pediatr Infect Dis J. 2018;37(4):324–328.
- Mink CM, Yeh SH. Pertussis in adolescents and its prevention using Tdap vaccination. Adolesc Med State Art Rev. 2010;21(2):220–235, viii.
- Klein NP, Bartlett J, Fireman B, et al. Waning Tdap effectiveness in adolescents. Pediatrics. 2016;137(3):e20153326.
- Klein NP, Zerbo O. Use of acellular pertussis vaccines in the United States: can we do better? Expert Rev Vaccines. 2017;16(12):1175–1179.
- Pillsbury A, Quinn HE, McIntyre PB. Australian vaccine preventable disease epidemiological review series: pertussis, 2006-2012. Commun Dis Intell Q Rep. 2014;38(3):E179–194.
- Skoff TH, Hadler S, Hariri S. The epidemiology of Nationally reported pertussis in the United States, 2000-2016. Clin Infect Dis. 2019;68(10):1634–1640.
- European Centers for Disease Control. Pertussis Annual Epidemiological Report for 2018. cited 2021 Aug 31. https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2018_pertussis.pdf
- Yeung KHT, Duclos P, Nelson EAS, et al. An update of the global burden of pertussis in children younger than 5 years: a modelling study. Lancet Infect Dis. 2017;17(9):974–980.
- Redlberger-Fritz M, Kundi M, Aberle SW, et al. Significant impact of nationwide SARS-CoV-2 lockdown measures on the circulation of other respiratory virus infections in Austria. J Clin Virol. 2021 Apr;137:104795.
- Kuitunen I, Artama M, Makela L, et al. Effect of social distancing due to the COVID-19 pandemic on the incidence of viral respiratory tract infections in children in Finland during early 2020. Pediatr Infect Dis J. 2020;39(12):e423–e427.
- Macina D, Evans KE. Bordetella pertussis in school-age children, adolescents and adults: a systematic review of epidemiology and mortality in Europe. Infect Dis Ther. 2021 Aug 26:1–48.
- Tan T, Dalby T, Forsyth K, et al. Pertussis across the globe: recent epidemiologic trends from 2000 to 2013. Pediatr Infect Dis J. 2015;34(9):e222–232.
- Lee GM, Lett S, Schauer S, et al. Societal costs and morbidity of pertussis in adolescents and adults. Clin Infect Dis. 2004;39(11):1572–1580.
- Varan AK, Harriman KH, Winter K, et al. Economic and social impact of pertussis among adolescents in San Diego County. J Adolesc Health. 2016;58(2):241–244.
- Wendelboe AM, Njamkepo E, Bourillon A, et al. Transmission of Bordetella pertussis to young infants. Pediatr Infect Dis J. 2007;26(4):293–299.
- Skoff TH, Kenyon C, Cocoros N, et al. Sources of infant pertussis infection in the United States. Pediatrics. 2015;136(4):635–641.
- Damron FH, Barbier M, Dubey P, et al. Overcoming waning immunity in pertussis vaccines: workshop of the national institute of allergy and infectious diseases. J Immunol. 2020;205(4):877–882.
- Cherry JD. The 112-year odyssey of pertussis and pertussis vaccines-mistakes made and implications for the future. J Pediatric Infect Dis Soc. 2019;8(4):334–341.
- Burns DL, Meade BD, Messionnier NE. Pertussis resurgence: perspectives from the working group meeting on pertussis on the causes, possible paths forward, and gaps in our knowledge. J Infect Dis. 2014;209(Suppl 1):S32–35.
- Quinn HE, Snelling TL, Macartney KK, et al. Duration of protection after first dose of acellular pertussis vaccine in infants. Pediatrics. 2014;133(3):e513–519.
- Klein NP, Bartlett J, Fireman B, et al. Waning protection following 5 doses of a 3-component diphtheria, tetanus, and acellular pertussis vaccine. Vaccine. 2017;35(26):3395–3400.
- Misegades LK, Winter K, Harriman K, et al. Association of childhood pertussis with receipt of 5 doses of pertussis vaccine by time since last vaccine dose, California, 2010. JAMA. 2012;308(20):2126–2132.
- Mansour-Ghanaei R, Moradi-Lakeh M, Shakerian S, et al. Acellular pertussis vaccine efficacy: an updated systematic review and meta -analysis. Med J Islam Repub Iran. 2016;30:451.
- Zerbo O, Bartlett J, Goddard K, et al. Acellular pertussis vaccine effectiveness over time. Pediatrics. 2019;144(1):e20183466.
- Chit A, Zivaripiran H, Shin T, et al. Acellular pertussis vaccines effectiveness over time: a systematic review, meta-analysis and modeling study. PLoS One. 2018;13(6):e0197970.
- Klein NP, Bartlett J, Rowhani-Rahbar A, et al. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med. 2012;367(11):1012–1019.
- Tartof SY, Lewis M, Kenyon C, et al. Waning immunity to pertussis following 5 doses of DTaP. Pediatrics. 2013;131(4):e1047–1052.
- Acosta AM, DeBolt C, Tasslimi A, et al. Tdap vaccine effectiveness in adolescents during the 2012 Washington State pertussis epidemic. Pediatrics. 2015;135(6):981–989.
- Koepke R, Eickhoff JC, Ayele RA, et al. Estimating the effectiveness of tetanus-diphtheria-acellular pertussis vaccine (Tdap) for preventing pertussis: evidence of rapidly waning immunity and difference in effectiveness by Tdap brand. J Infect Dis. 2014;210(6):942–953.
- Conway JH, Davis JP, Eickhoff JC, et al. Brand-specific rates of pertussis disease among Wisconsin children given 1-4 doses of pertussis Vaccine, 2010-2014. Vaccine. 2020;38(45):7063–7069.
- Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci U S A. 2014;111(2):787–792.
- Esposito S, Agliardi T, Giammanco A, et al. Long-term pertussis-specific immunity after primary vaccination with a combined diphtheria, tetanus, tricomponent acellular pertussis, and hepatitis B vaccine in comparison with that after natural infection. Infect Immun. 2001;69(7):4516–4520.
- Althouse BM, Scarpino SV. Asymptomatic transmission and the resurgence of Bordetella pertussis. BMC Med. 2015;13:146.
- Williams MM, Sen K, Weigand MR, et al. Bordetella pertussis strain lacking pertactin and pertussis toxin. Emerg Infect Dis. 2016;22(2):319–322.
- Van Gent M, Heuvelman CJ, van der Heide HG, et al. Analysis of Bordetella pertussis clinical isolates circulating in European countries during the period 1998-2012. Eur J Clin Microbiol Infect Dis. 2015;34(4):821–830.
- Bouchez V, Hegerle N, Strati F, et al. new data on vaccine antigen deficient Bordetella pertussis isolates. Vaccines (Basel). 2015;3(3):751–770.
- Cabal A, Schmid D, Hell M, et al. Isolate-based surveillance of Bordetella pertussis, Austria, 2018-2020. Emerg Infect Dis. 2021;27(3):862–871.
- Dewan KK, Linz B, DeRocco SE, et al. Acellular pertussis vaccine components: today and tomorrow. Vaccines (Basel). 2020;8(2):217.
- Breakwell L, Kelso P, Finley C, et al. pertussis vaccine effectiveness in the setting of pertactin-deficient pertussis. Pediatrics. 2016;137(5):e20153973.
- Mooi FR, Van Der Maas NA, De Melker HE. Pertussis resurgence: waning immunity and pathogen adaptation - two sides of the same coin. Epidemiol Infect. 2014;142(4):685–694.
- America Academy of Pediatrics Commitee on Infectious D. Prevention of pertussis among adolescents: recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine. Pediatrics. 2006;117(3):965–978.
- Healy CM, Rench MA, Wootton SH, et al. Evaluation of the impact of a pertussis cocooning program on infant pertussis infection. Pediatr Infect Dis J. 2015;34(1):22–26.
- Castagnini LA, Healy CM, Rench MA, et al. Impact of maternal postpartum tetanus and diphtheria toxoids and acellular pertussis immunization on infant pertussis infection. Clin Infect Dis. 2012;54(1):78–84.
- Centers for Disease Control and Prevention, Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months — advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(41):1424–1426.
- Centers for Disease Control and Prevention, Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women–Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep. 2013;62(7):131–135.
- Mazzilli S, Tavoschi L, Lopalco PL. Tdap vaccination during pregnancy to protect newborns from pertussis infection. Ann Ig. 2018;30(4):346–363.
- Brousseau N, Gagnon D, Vivion M, et al. Expected challenges of implementing universal pertussis vaccination during pregnancy in Quebec: a cross-sectional survey. CMAJ Open. 2018;6(3):E391–E397.
- Campbell H, Gupta S, Dolan GP, et al. Review of vaccination in pregnancy to prevent pertussis in early infancy. J Med Microbiol. 2018;67(10):1426–1456.
- Kong KL, Krishnaswamy S, Giles ML. Maternal vaccinations. Aust J Gen Pract. 2020;49(10):630–635.
- Vizzotti C, Neyro S, Katz N, et al. Maternal immunization in Argentina: a storyline from the prospective of a middle income country. Vaccine. 2015;33(47):6413–6419.
- Kandeil W, van den Ende C, Bunge EM, et al. A systematic review of the burden of pertussis disease in infants and the effectiveness of maternal immunization against pertussis. Expert Rev Vaccines. 2020;19(7):621–638.
- Winter K, Nickell S, Powell M, et al. Effectiveness of prenatal versus postpartum tetanus, diphtheria, and acellular pertussis vaccination in preventing infant pertussis. Clin Infect Dis. 2017;64(1):3–8.
- Langsam D, Anis E, Haas EJ, et al. Tdap vaccination during pregnancy interrupts a twenty-year increase in the incidence of pertussis. Vaccine. 2020;38(12):2700–2706.
- Carrasquilla G, Porras A, Martinez S, et al. Incidence and mortality of pertussis disease in infants <12 months of age following introduction of pertussis maternal universal mass vaccination in Bogota, Colombia. Vaccine. 2020;38(46):7384–7392.
- Vizzotti C, Juarez MV, Bergel E, et al. Impact of a maternal immunization program against pertussis in a developing country. Vaccine. 2016;34(50):6223–6228.
- Sebghati M, Khalil A. Uptake of vaccination in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2021 Apr 6:S1521-6934(21)00046-8. doi:10.1016/j.bpobgyn.2021.03.007. Epub ahead of print. PMID: 33965331; PMCID: PMC8021457
- Boulet SL, Chamberlain AT, Biswas HH, et al. Trends in Infant Pertussis Hospitalizations in the United States, 2009-2017. JAMA. 2019;322(21):2134–2136.
- Fernandes EG, Sato APS, Vaz-de-Lima LRA, et al. The effectiveness of maternal pertussis vaccination in protecting newborn infants in Brazil: a case-control study. Vaccine. 2019;37(36):5481–5484.
- Romanin V, Acosta AM, Juarez MDV, et al. Maternal vaccination in Argentina: tetanus, diphtheria, and acellular pertussis vaccine effectiveness during pregnancy in preventing pertussis in infants <2 months of age. Clin Infect Dis. 2020;70(3):380–387.
- Baxter R, Bartlett J, Fireman B, et al. Effectiveness of vaccination during pregnancy to prevent infant pertussis. Pediatrics. 2017;139(5):e20164091.
- Razzaghi H, Kahn KE, Black CL, et al. Influenza and Tdap vaccination coverage among pregnant women - United States, April 2020. MMWR Morb Mortal Wkly Rep. 2020;69(39):1391–1397.
- Greenfield I, Wu D, Anderson M, et al. Electronic medical record intervention to improve adherence to prenatal vaccination recommendation [12F]. Obstetrics Gynecol. 2019;133:65S.
- Wong VW, Lok KY, Tarrant M. Interventions to increase the uptake of seasonal influenza vaccination among pregnant women: a systematic review. Vaccine. 2016;34(1):20–32.
- Bisset KA, Paterson P. Strategies for increasing uptake of vaccination in pregnancy in high-income countries: a systematic review. Vaccine. 2018;36(20):2751–2759.
- Martinon-Torres F, Halperin SA, Nolan T, et al. Impact of maternal diphtheria-tetanus-acellular pertussis vaccination on pertussis booster immune responses in toddlers: follow-up of a randomized trial. Vaccine. 2021;39(11):1598–1608.
- Perrett KP, Halperin SA, Nolan T, et al. Impact of tetanus-diphtheria-acellular pertussis immunization during pregnancy on subsequent infant immunization seroresponses: follow-up from a large randomized placebo-controlled trial. Vaccine. 2020;38(8):2105–2114.
- Vaz-de-Lima LRA, Sato APS, Pawloski LC, et al. Effect of maternal Tdap on infant antibody response to a primary vaccination series with whole cell pertussis vaccine in Sao Paulo, Brazil. Vaccine. 2021;7:100087.
- Wanlapakorn N, Maertens K, Vongpunsawad S, et al. Quantity and quality of antibodies after acellular versus whole-cell pertussis vaccines in infants born to mothers who received tetanus, diphtheria, and acellular pertussis vaccine during pregnancy: a randomized trial. Clin Infect Dis. 2020;71(1):72–80.
- Wodi AP, Ault K, Hunter P, et al. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger - United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(6):189–192.
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization --- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011 Jan 28;60(2):1-64. Erratum in: MMWR Recomm Rep. 2011 Jul 29;60:993. PMID: 21293327.
- Bugenske E, Stokley S, Kennedy A, et al. Middle school vaccination requirements and adolescent vaccination coverage. Pediatrics. 2012;129(6):1056–1063.
- Kamiya H, Cho BH, Messonnier ML, et al. Impact and cost-effectiveness of a second tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine dose to prevent pertussis in the United States. Vaccine. 2016;34(15):1832–1838.
- Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416.
- Abbasi J. COVID-19 and mRNA vaccines-first large test for a new approach. JAMA. 2020;324(12):1125–1127.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–2615.
- Israel A, Merzon E, Schäffer AA, et al. Elapsed time since BNT162b2 vaccine and risk of SARS-CoV-2 infection in a large cohort. medRxiv. 2021:2021.08.03.21261496. doi:10.1101/2021.08.03.21261496
- Dorji D, Mooi F, Yantorno O, et al. Bordetella Pertussis virulence factors in the continuing evolution of whooping cough vaccines for improved performance. Med Microbiol Immunol. 2018;207(1):3–26.
- Jahnmatz M, Richert L, Al-Tawil N, et al. Safety and immunogenicity of the live attenuated intranasal pertussis vaccine BPZE1: a phase 1b, double-blind, randomised, placebo-controlled dose-escalation study. Lancet Infect Dis. 2020;20(11):1290–1301.
- Thorstensson R, Trollfors B, Al-Tawil N, et al. A phase I clinical study of a live attenuated Bordetella pertussis vaccine–BPZE1; a single centre, double-blind, placebo-controlled, dose-escalating study of BPZE1 given intranasally to healthy adult male volunteers. PLoS One. 2014;9(1):e83449.
