ABSTRACT
Objectives
Understanding public vaccine acceptability is critical to preparing for future pandemics. This research investigated Japanese individuals’ willingness to be vaccinated against a hypothetical infectious disease.
Methods
A conjoint analysis was conducted with a general public panel via an Internet survey agency. Vaccine efficacy, vaccine safety, duration of immunity, and price were chosen as analysis attributes. Each respondent chose from 12 hypothetical scenarios using an online panel.
Results
From the 2,155 complete responses, 51,720 results were extracted and analyzed. Higher efficacy, lifetime immunity duration, and fear of the pandemic positively affected willingness to be vaccinated, while higher vaccination price and higher toxicity had negative effects. The number of infected individuals and deaths had no significant impact. A total of 69.2% of the study population reported being willing to receive a vaccine with 100% efficacy, lifetime immunity, and low toxicity and free of charge.
Conclusions
This study assessed the preferences for vaccines against infectious diseases with the potential to become pandemics during the COVID-19 pandemic in Japan. This result can influence vaccine-related policy and pandemic preparedness and help governments consider public intention to prepare health communication campaigns that encourage vaccination.
1. Introduction
The first coronavirus disease 2019 (COVID-19) case in Japan was confirmed in January 2020, and the virus has continued to spread ever since. Toward the end of June 2021, the cumulative number of infected cases was approximately 820,000, while the cumulative number of deaths was approximately 15,000. In early March 2021, the third wave of COVID-19 appeared to be under control; however, the virus started to spread again, so a state of emergency was declared for the fourth time on 12 July 2021. This new wave showed that the COVID-19 situation is still difficult to predict [Citation1–4].
The first vaccine for COVID-19, COMIRNATY by Pfizer Inc., was approved by the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan on February 2021, and Spikevax by Moderna Inc. followed soon after, on May 2021 [Citation5]. In general, newly approved vaccines in Japan are categorized under ‘voluntary vaccination’ wherein the costs are not publicly funded, while other vaccines could be covered by some regional governments [Citation6,Citation7]. Vaccines need to be nominated under the ‘routine vaccination’ category for them to qualify for nationwide public funding, which is decided by the special committee for vaccination under the Ministry of Health, Labor and Welfare (MHLW) in Japan [Citation8,Citation9]. However, due to the severe COVID-19 situation, two novel vaccines were publicly funded immediately after market approval by establishing a new category: ‘temporal vaccination [Citation10].’ Under this system, all citizens aged 12 and above are eligible for vaccination free of charge [Citation5].
Japan is ranked among the countries with the lowest vaccine confidence in the world [Citation11]. This low confidence seemed to be linked to the human papillomavirus (HPV) vaccine safety scares that started in 2013 and the subsequent decision by the Japanese MHLW to stop proactively recommending the HPV vaccine in June 2013 [Citation11]. As a result of this vaccine safety scare, HPV vaccination coverage decreased from 68.4–74.0% in the 1994–1998 birth cohort to 0.6% in the 2000 birth cohort [Citation12]. The news of Japan suspending its proactive recommendation of the HPV vaccine traveled globally through online media and social media networks and was applauded by anti-vaccination groups but not by the global scientific community [Citation13]. The ban of the measles, mumps, and rubella vaccine in 1993 due to severe adverse reactions, including non-viral meningitis, could be another reason for vaccine scarcities. However, the Japanese government recently approved the 9-valent HPV vaccine [Citation14] and decided to resume recommending HPV vaccination to the target girls and provide catch-up vaccinations for older girls who had missed the opportunity of being vaccinated during the suspension [Citation15,Citation16].
Previous studies have shown that public willingness to receive a COVID-19 vaccine varies worldwide and over time [Citation17–25]. In June 2020, Lazarus et al. found that if the vaccines were proven safe and effective, 75.4% of respondents in the US and 54.8% to 88.6% of respondents worldwide were willing to be vaccinated [Citation17]. In contrast, Sallam [Citation18] reported lower vaccination acceptance rates in the Middle East, US, Russia, Africa, and several European countries. The gap in the willingness to be vaccinated between these studies emphasizes the varied nature of the public perception of vaccination. Studies have found correlations between vaccine hesitancy and skepticism regarding vaccine safety and possible side effects [Citation19]. Research has shown that approximately 66% of Japanese are willing to be vaccinated [Citation23–25]. For instance, Machida et al. [Citation24] revealed that approximately 62% of Japanese residents were willing to be vaccinated against COVID-19. In contrast, after conducting a survey in Japan, Kadoya et al. indicated that 47% of the respondents were willing to be vaccinated once the vaccine was available, 22% unwilling, and 31% indecisive [Citation25]. The survey timing could influence people’s willingness to receive COVID-19 vaccines, and multiple factors could be associated with the general population’s intention. Healthcare policy-makers need to know how vaccine characteristics influence vaccination choice.
Therefore, the study was conducted in Japan to characterize and quantify the factors (i.e. vaccine cost, efficacy, vaccine safety, and prevalence) that contribute to the willingness to be vaccinated of Japan in a hypothetical infectious disease epidemic situation that could occur in the future and consider public policy, public communication, and resource allocation for such a vaccination drive.
2. Methods
2.1. Study participants and survey design
We performed an online survey in November 2020 to assess individual hypothetical vaccine preferences. The survey was conducted by requesting cooperation from randomly selected Internet monitors owned by INTAGE, Inc. The study participants were recruited through convenience sampling; however, the survey platform incorporated quota samples that approximated nationally representative samples in terms of their demographic characteristics. The survey was designed to collect 2,000 responses evenly divided by gender in 10-year age increments. In total, the number of valid responses was 2,155 (response rate: 23.0%). In general, no specific exclusion criteria were established for our analysis. Whether you have had COVID-19 could be an important factor for the respondents. However, due to the restrictions of the survey agency whose services we employed, we could not ask the respondent’s COVID-19 history. Nonetheless, given that the cumulative number of COVID-19 infected individuals in Japan was around 150,000, or 1 out of 800 residents, by the end of November 2020 (when the survey was conducted), we estimated that this factor would not affect the overall results. The study was approved by the ethical committee. In addition, the survey was conducted when the third wave of the COVID-19 pandemic had peaked the interest of the general public in Japan regarding COVID-19. The following is an overview of the survey design.
2.1.1. Survey design
1) Survey area: Japan (47 prefectures nationwide)
2) Target population: Men and women between the ages of 20 and 69
3) Sample size: 2,155 (Response rate: 23.0%)
4) Method of sampling: Random sampling using the monitors for the Internet survey
5) Survey method: Internet survey
6) Survey period: November 19–27, 2020
7) Research organization: INTAGE, Inc.
2.2. Conjoint analysis methodology
The study employed a choice-based conjoint design to identify factors associated with the self-reported likelihood of vaccination. Preferences for attributes can be measured through conjoint analyses, such as discrete choice experiments, including research examining factors associated with public preferences concerning vaccines [Citation26,Citation27]. This approach approximates actual decisions and has been shown to inform how future health behaviors are estimated. In this study, respondents were informed about hypothetical vaccines being developed against infectious viruses. Vaccine attributes used in this analysis were chosen based on the previous studies [Citation18,Citation19], which consisted of five categories in the presented vaccination and pandemic profiles: (1) efficacy, (2) safety, (3) immunity duration, (4) price, and (5) infected patients/deaths (see ). The numbers of categories needed to be limited because asking many questions in various kinds of scenarios for one respondent reduce the response rate of the overall survey. The maximum number of scenarios per one respondent was set to be between 10 and 15, similar to our previous studies and survey experience using conjoint analyses [Citation28]. Although we did not conduct the pilot survey in the same manner, we believe that our approach captures the preferences of the general public. In the questionnaire, the vaccine’s efficacy is indicated by the prevention rate of the viral infection. For example, 100% efficacy means that the vaccine is efficacious for everyone. In contrast, an efficacy of 50% means that it is efficacious only for half of the people receiving the vaccine.
Table 1. The hypothetical vaccine’s characteristics and situation in the questionnaire
Out of 108 patterns, 25 were selected via an orthogonal design to reduce the burden on respondents. These 25 situations were divided into two groups. Each respondent was asked to choose between two scenarios randomly selected from the two blocks. Twelve questions were presented before each respondent.
The levels of each attribute for each vaccine were assigned randomly, and the attribute order was randomized across participants.
2.3. Questionnaire example
Participants evaluated two hypothetical vaccines in each task and were asked whether they would choose vaccine A, vaccine B, or neither of the vaccines. The numbers of infected people and deaths are shown in . A questionnaire example is shown below:
An infectious disease is spreading rapidly in Japan. People’s work and daily lives are being restricted, causing social and emotional burdens with the spread of the infectious virus. Now, two companies, A and B, have developed vaccines to prevent this infectious disease. If each of these vaccines can prevent this infectious disease that has infected 100,000 individuals and caused 5,000 deaths in Japan, which company’s vaccine would you choose, or would you not take either of them?
Vaccine A
Efficacy: efficacious for everyone (100%)
Safety (prevalence rate of side-effects, e.g. skin redness, pain, or fever): Occurs in 1 in 1000 people (0.1%)
Immunity duration: 1 year
Price (out of pocket): JPY 20,000
Vaccine B
Efficacy: efficacious for 1 in 2 persons (50%)
Safety (prevalence rate of side-effects, e.g. skin redness, pain, or fever): Occurs in 1 in 1000 people (0.1%)
Immunity duration: 1 year
Price (Out of pocket): JPY 10,000
2.4. Statistical analysis
The statistical analysis was conducted using the panel-logit model, which allows panel data or one respondent to answer multiple questionnaires but cannot be considered fully independent. Five attributes (price, safety, efficacy, immunity duration, and disease prevalence) and two additional characteristics (fear of COVID-19 and hospital visits) were set as independent variables for this model. The dependent variable – individual attitude – was measured using the following fixed question with a dichotomous answer (‘Yes’ or ‘No’): ‘Would you want to take this vaccine if it brought about the below-mentioned situation?’ Through logistic conversion, this response was converted into a continuous variable. The panel-logit model was applied to calculate the probability of the respondents answering ‘Yes’ for each situation.
Questionnaires were developed via orthogonal methods using Design-Expert v13 (Stat-Ease Inc.). Statistical analysis was performed using STATA MP 15.0 (Stata software).
3. Results
A total of 2,155 respondents were recruited, each of whom was given 12 hypothetical scenarios. Then, 2,155 × 2 × 12 = 51,720 results were extracted and analyzed. The results were doubled because the results for Vaccine A and B could both be obtained through one question. The respondents were the general public panel (N = 2,155), and shows the demographic distributions of the study samples compared to the Japanese population [Citation29]. The average age was 45.0 ± 14.1, and the proportion of men was 49.6%. In terms of the financial impact of COVID-19, 40.0% of the participants answered that they earned less in 2020 than in 2019, while very few respondents (6.4%) stated that their income was higher in 2020. Regarding the fear of an unknown pandemic virus like COVID-19, almost half of the respondents (49.4%) reported being terrified.
Table 2. Demographics of respondents
shows the results of the conjoint analysis using various models. In addition to the four main attributes – price, efficacy (100% vs. 50%), toxicity (1% vs. 0.1%), and immunity duration (lifetime vs. one-year) – four other factors were assessed: the number of infected people (10 million infections, 1 million infections, or 1 hundred thousand infections), baseline income and income decrease in 2020, fear of an unknown pandemic virus like COVID-19, and hospital visits. In general, higher efficacy and lifetime immunity duration positively affected the willingness to be vaccinated, and higher price and toxicity were negative for all models. According to the results from Model 2 (positive coefficient for income), those with higher income were more willing to be vaccinated. The magnitude of fear regarding an unknown pandemic virus like COVID-19 significantly affected willingness to vaccinate. Those who were not scared of such a virus were significantly less willing to be vaccinated; however, such people were few among the whole cohort (16.9%). Those with an ‘Extreme’ (‘very scared,’ 49.4%) and ‘Moderate’ (‘fairly scared, but not so much at present,’ 33.6%) level of fear had a stronger willingness to be vaccinated than others. According to Model D, those who had to visit the hospital were also more willing to be vaccinated (Supplemental Table 1). Notably, according to the results from Model A in Supplemental Table 1, the widespread status of the pandemic or the incidence and mortality rate of the pandemic did not affect the respondents’ willingness to be vaccinated. Two coefficients for incidence and mortality rates were not statistically significant, while positive point estimation values (0.1092 and 0.0387) were observed. In the analysis of the willingness to be vaccinated, to show the relationship between vaccination price and overall willingness (i.e. probability of answering ‘Yes’ to vaccination) in hypothetical situations, and to classify the fear level of an unknown pandemic virus like COVID-19. shows the most optimistic case in terms of vaccine characteristics (100% efficacy, lifetime immunity duration, and low toxicity). depict more conservative situations, under which one of three attitudes is downgraded (1B: year’s immunity duration, 1 C: high toxicity (1%), and 1D: 50% efficacy). Even in the most optimistic case, or with no co-payment, the willingness was only 69.2%.
Figure 1. Relationship between willingness to vaccinate and cost considering different vaccine variables. a. Willingness to receive a vaccine with 100% efficacy, lifetime immunity duration, and low toxicity b. Willingness to receive a vaccine with 100% efficacy, one-year immunity duration, and low toxicity c. Willingness to receive a vaccine with 100% efficacy, lifetime immunity duration, and high toxicity d. Willingness to receive a vaccine with 50% efficacy, lifetime immunity duration, and low toxicity
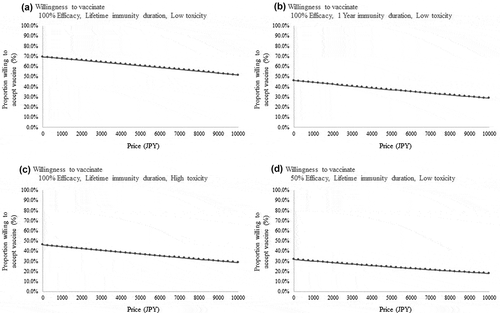
Figure 2. Relationship between willingness to vaccinate and cost considering fear level regarding COVID-19. a. Willingness to receive a vaccine with 100% efficacy, lifetime immunity duration, and low toxicity b. Willingness to receive a vaccine with 100% efficacy, one-year immunity duration, and low toxicity c. Willingness to receive a vaccine with 100% efficacy, lifetime immunity duration, and high toxicity d. Willingness to receive a vaccine with 50% efficacy, lifetime immunity duration, and low toxicity
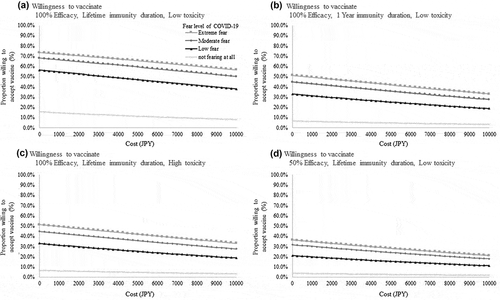
Table 3. Individual factors associated with willingness to get the hypothetical vaccine
4. Discussion
This study provides systematic evidence of the holistic factors associated with individual preferences regarding pandemic vaccination in Japan using a conjoint experiment. Some conjoint experiments related to vaccination have been conducted globally, revealing that vaccine attributes affect people’s willingness to be vaccinated [Citation30–33]. Our analysis builds on one of the first conjoint experiments of factors driving the willingness to be vaccinated against a pandemic in Japan and investigates how the price, efficacy, toxicity, lifetime duration, and fear of the unknown virus affect people’s intention to be vaccinated. The number of infections (10 million infections, 1 million infections, or 1 hundred thousand infections) was not significantly related to the willingness to be vaccinated. One of the reasons may be that the general population could not evaluate the pandemic situation based on the number of infections. A risk analysis revealed that people show disproportional compassionate and affective responses to the scope of human mortality risk, and such result could be related to the result of this research [Citation34]. This finding suggests that public health communication strategies are needed to increase awareness regarding the severity of the pandemic. This study reveals novel results about the willingness to receive the vaccine for pandemic-causing viruses, considering the price of the vaccine. We found that 69.2% of the general population was willing to receive free vaccines with 100% efficacy, lifetime duration, and low toxicity, as shown in . The willingness to be vaccinated was strongly affected by the fear of an unknown pandemic virus like COVID-19. In cases where the respondents did not fear the pandemic virus, as shown in , only 20% reported a willingness to be vaccinated, even if the vaccine was 100% efficacious, had low toxicity, and imparted lifetime immunity. To combat new viruses, we need to achieve herd immunity; to do so, the government needs to focus on people with negative attitudes toward vaccination.
Consistent with previous research, vaccine efficacy and safety were associated with vaccine choice and the likelihood of self-reported willingness to be vaccinated [Citation24,Citation25]. Moreover, regarding the willingness to be vaccinated, the phrasing of the questions and the timing of the research may lead to different results in different surveys because the risk rate of COVID-19 could directly affect the respondents’ preferences and thoughts. However, the results of this report are consistent with those of previous research [Citation24,Citation25] regarding the rate of people who are willing to be vaccinated.
In Japan, COVID-19 vaccines were approved under a special approval system for emergency use by the PMDA [Citation35]. The data relating to vaccination is reviewed post-approval, leading to confirming safety, efficacy, and immunity duration in the real world [Citation10]. As mentioned earlier, currently, COVID-19 vaccines are being provided free of charge [Citation5]. The supplemental budget for the nationwide procurement of COVID vaccines has been secured. While the healthcare budget for COVID-19 should not be restricted during the pandemic phase, as was implied in the recommendations for COVID-19 vaccination by the Advisory Committee on Immunization Practices in the US [Citation36], discussions are needed when considering how to implement booster vaccinations or routine re-vaccinations. Even if the free-of-charge policy continues, decision-makers should know how much citizens ‘value’ a vaccination for protection against a pandemic and how it is associated with various factors to maintain the continuity with other vaccination and prevention policies. Japan ranked among the countries with the lowest vaccine confidence in the world because of HPV vaccination’s issues
[Citation11,Citation12], and this study aimed to investigate the public willingness to be vaccinated for an unknown infectious virus. Our survey was implemented during the third wave of COVID-19 in Japan and while the citizens were experiencing fear of the contagion. If the COVID-19 infectious rate decreases, the willingness to be vaccinated could also drop. This is why political measures to cultivate vaccine confidence and prevention value are necessary to overcome the next unknown infectious disease.
In this research, we assessed the value of the vaccine for unknown infectious diseases by measuring the willingness to be vaccinated. The pandemic created significant economic challenges from early 2020, leading to a steep recession. The pandemic crisis affects not only public health but also contributes to global economic crises. Vaccination imparts hope because of its potential to achieve economic recovery and reduce the infection rate. Multiple aspects of the vaccine’s value must be considered, and the appropriate value assessments discussed when the government, payers, or health technology assessment agencies evaluate a vaccine that can prevent a pandemic like COVID-19.
This study had several limitations. First, the study participants were recruited using convenience sampling; however, the survey platform incorporated quota samples that approximated nationally representative samples in terms of their demographic characteristics. Our sample population did not include those who do not have access to online surveys and people under the age of 20 or over the age of 70. Gaps between the occupation of this study’s respondents and the Japanese population overall were found because of the differences in the sampling age () [Citation29].
Second, the information provided to the study participants was based on hypothetical vaccines; the self-reported responses about vaccine choice and willingness to receive a hypothetical vaccine may not accurately reflect vaccine choices and behaviors related to vaccine acceptance or hesitancy involving actual pandemic vaccines.
Third, the definition of efficacy and toxicity were imprecise in this survey, and, while they were explained in the survey’s questionnaire, an accurate endpoint was not indicated because this might have made it difficult for the general public to understand. In effect, the term ‘vaccine efficacy’ may seem ambiguous and be easily misunderstood. While some might consider it to be related to the preventive effect for infection, others might define it as being related to either exacerbation or COVID-related death. Although we mentioned that the vaccine efficacy was defined as risk reduction of the infection, some people might have still misunderstood the expression. Few people correctly understand that vaccine efficacy is related to relative risk reduction instead of absolute risk reduction. The definition of adverse effects itself has a broader meaning that can be interpreted in different ways by different respondents. Other factors may also be involved, such as how individuals and societies can rejuvenate the economy after vaccination, how the government promotes vaccination, how often respondents should be vaccinated and intervals for multiple vaccinations, and how comprehensively the vaccine is provided. Therefore, we believe that further research is required to facilitate future vaccination campaigns. Meanwhile, there are still some limitations for setting the fixed case fatality rate (1 to 200 patients, 0.5%), so the results should be generalized to other types of vaccines, in particular those for less-fatal diseases, such as seasonal influenza, with caution.
In summary, this study assessed the preferences for vaccines against infectious diseases with the potential to become pandemics during the COVID-19 pandemic in Japan. A future follow-up study is necessary to assess the stability of these preferences and the preference for hypothetical vaccines for hypothetical future pandemics. This result can influence vaccine policy and pandemic preparedness regarding prevention.
Author contributions
A Igarashi supervised this work. All authors set the conceptual framework for this research, contributed to the discussion, and approved the final version of the report.
Supplemental Material
Download JPEG Image (164.3 KB)Acknowledgments
We thank Dr. Rei Goto, Dr. Ichiro Wada, Mr. Masami Morita and Mr. Minoru Ito for their valuable suggestions.
Supplemental data
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Sonoo M, Idogawa M, Kanbayashi T, et al. [COVID-19 in Japan: insights from the epidemiological data]. Brain and Nerve Japanese . 2020;72(10):1023–1030. .
- Ministry of Health, Labour and Welfare. Novel coronavirus (COVID-19) [Internet] [cited 2021 Jul 26]. Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000164708_00079.html2021; 2021.
- Thompson MK, Kalkowska DA, and Badizadegan K. Hypothetical emergence of poliovirus in 2020: part 1. Consequences of policy decisions to respond using nonpharmaceutical interventions. Expert Rev Vaccines 2021;20(4):465–481.
- Thompson MK, Kalkowska DA, and Badizadegan K. Hypothetical emergence of poliovirus in 2020: part 2. exploration of the potential role of vaccines in control and eradication. Expert Rev Vaccines 2021;20(4):449–460.
- Ministry of Health, Labour and Welfare. Coronavirus (COVID-19). vaccinations [Internet]. [cited 2021 Jul 26]. Available from: https://v-sys.mhlw.go.jp/en/8; 2021.
- Saitoh A, Okabe N. Progress and challenges for the Japanese immunization program: beyond the “vaccine gap.” Vaccine. 2018;36(30):4582–4588.
- Nakayama T. Vaccine chronicle in Japan. J Infect Chemother. 2013;19(5):787–798.
- Hoshina T, Kawase M, Watanabe S, et al. Trends in voluntary vaccination coverage in a Japanese city. Pediatr Int. 2021. DOI:https://doi.org/10.1111/ped.14712.
- Saitoh A, Okabe N. Current issues with the immunization program in Japan: can we fill the “vaccine gap”? Vaccine. 2012;30(32):4752–4756.
- Pharmaceutical and Medical Devices Agency. PMDA’s efforts to combat COVID-19 [Internet] [cited 2021 Jul 26]. Available from: https://www.pmda.go.jp/english/about-pmda/0002.html 2021.
- Simms KT, Hanley SJB, Smith MA, et al. Impact of HPV vaccine hesitancy on cervical cancer in Japan: a modelling study. Lancet Public Health. 2020;5(4):e223–e234.
- Hanley SJ, Yoshioka E, Ito Y, et al. HPV vaccination crisis in Japan. Lancet. 2015;385(9987):2571.
- Kawado M, Hashimoto S, Ohta A, et al. Estimating nationwide cases of sexually transmitted diseases in 2015 from sentinel surveillance data in Japan. BMC Infect Dis. 2020;20(1):77.
- Japan approves MSD’s 9-Valent HPV vaccine 5 years after filing 2020 [cited 2021]. Available from: https://pj.jiho.jp/article/242550.
- Ueda Y, Yagi A, Ikeda S, et al. Beyond resumption of the Japanese government’s recommendation of the HPV vaccine. Lancet Oncol. 2018;19(12):1563–1564.
- Japan to resume active promotion of HPV vaccinations. Tokyo: JIJI press: https://jen.jiji.com/jc/eng?g=eco&k=2021111200936; Accessed 12 November 2021 2021
- Lazarus JV, Ratzan SC, Palayew A, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2021;27(2):225–228.
- Sallam M. COVID-19 vaccine hesitancy worldwide: a concise systematic review of vaccine acceptance rates. Vaccines (Basel). 2021;9(2):120.
- Guidry JPD, Laestadius LI, and Vraga EK, et al. Willingness to get the COVID-19 vaccine with and without emergency use authorization. Am J Infect Control. 2021;49(2):137–142.
- Kelly BJ, Southwell BG, and McCormack LA, et al. Predictors of willingness to get a COVID-19 vaccine in the U.S. BMC Infect Dis. 2021;21(1):338.
- Dror AA, Eisenbach N, and Taiber S, et al. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur J Epidemiol. 2020;35(8):775–779.
- Fisher KA, Bloomstone SJ, and Walder J, et al. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of U.S. adults Ann Intern Med. 2020;173(12):964–973.
- Yoda T, Katsuyama H. Willingness to receive COVID-19 vaccination in Japan. Vaccines (Basel). 2021;9(1):48.
- Machida M, Nakamura I, Kojima T, et al. Acceptance of a COVID-19 vaccine in Japan during the COVID-19 pandemic. Vaccines (Basel). 2021;9(3):210.
- Kadoya Y, Watanapongvanich S, Yuktadatta P, et al. Willing or hesitant? A socioeconomic study on the potential acceptance of COVID-19 vaccine in Japan. Int J Environ Res Public Health. 2021;18(9):4864.
- Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health—A checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14(4):403–413.
- Trapero-Bertran M, Rodríguez-Martín B, López-Bastida J. What attributes should be included in a discrete choice experiment related to health technologies? A systematic literature review. PLOS ONE. 2019;14(7):e0219905.
- Igarashi A, Goto R, Yoneyama-Hirozane M. Willingness to pay for QALY: perspectives and contexts in Japan. J Med Econ. 2019 Oct;22(10):1041–1046.
- Portal site of official statistics of Japan. Stat. Jpn. 2021. [cited 2021 Jul 26]. Available from:2021. Available from: https://www.e-stat.go.jp/en/stat-search?page=1&query=population&layout=dataset.
- Kaplan RM, Milstein A. Influence of a COVID-19 vaccine’s effectiveness and safety profile on vaccination acceptance. Proc Natl Acad Sci USA. 2021;118(10):e2021726118.
- Ii M, Ohkusa Y. Nihon Koshu Eisei zasshi. [An empirical research for demand of influenza vaccination]. Nihon Koshu Eisei Zasshi. 2001;48(1):16–27. Japanese.
- Kreps S, Prasad S, Brownstein JS, et al. Factors associated with US adults’ likelihood of accepting COVID-19 vaccination. JAMA Network Open. 2020;3(10):e2025594.
- Kreps SE, Kriner DL. Factors influencing Covid-19 vaccine acceptance across subgroups in the United States: evidence from a conjoint experiment. Vaccine. 2021;39(24):3250–3258.
- Bhatia S, Walasek L, Slovic P, et al. The more who die, the less we care: evidence from natural language analysis of online news articles and social media posts. Risk Anal. 2021;41(1):179–203.
- Maeda H. Japan’s special approval for emergency system during COVID-19 pandemic. Clin Pharmacol Ther. 2021. DOI:https://doi.org/10.1002/cpt.2310
- Centers for Disease Control and Prevention. MMWR Morb. cited 2021 Jul 26]. Available from:2021. Available from Mortal Wkly Rep. 2021;: https://www.cdc.gov/mmwr/volumes/69/wr/mm695152e2.htm
