ABSTRACT
Background
Cervical cancer is the third most frequent cancer in Chinese women aged 15–44 years old. Human papillomavirus (HPV) is recognized as the main etiologic agent of cervical carcinomas. This study aims to better understand the natural history of HPV infections in Chinese women aged 18–25 years.
Research Design and Methods
Data from 3,025 control arm women (AS04-HPV-16/18 vaccine trial) were analyzed to assess the probability of progression or clearance from a 6-month persistent infection (6MPI) to a cervical intraepithelial neoplasia grade 2 or greater (CIN2+), and the association with clinical determinants. Data were analyzed using univariate and multivariable Cox models.
Results
A total of 1,324 women with 3,814 HPV infections were included, and 65.7% of the women had at least one 6MPI. Among those 6MPI, 5.0% progressed to CIN2+, while 61.0% cleared within 6 months. The risk of progression from 6MPI to CIN2+ was substantially higher for oncogenic versus non-oncogenic HPV types.
Conclusions
Oncogenic HPV infections showed lower clearance and higher risk to progress to CIN2 +. These findings observed in a population of Chinese women, confirmed previous findings from multinational studies.
Trial Registration
The PATRICIA and AS04-HPV-16/18 vaccine trials are registered at ClinicalTrials.gov (NCT00779766 and NCT00122681).
KEYWORDS:
1. Introduction
Cervical cancer is the third most common cancer among women worldwide, with nearly 570,000 new cases and more than 310,000 deaths per year [Citation1]. Development of cervical intraepithelial neoplasia (CIN) and cervical cancer is almost always preceded by a persistent oncogenic human papillomavirus (HPV) infection, with approximately 70% of invasive cervical cancer cases attributable to high-risk HPV types 16 and 18 (HPV-16, HPV-18) [Citation1–3].
In China, over 106,000 new cases of cervical cancer and more than 47,000 deaths are reported each year making cervical cancer the third most frequent cancer among women aged 15 to 44 years. The prevalence of HPV types in China varies among women with and without lesions or with a cervical cancer. A study of cervical adenocarcinoma found that HPV-16 or HPV-18 were the HPV types identified in 88.2% of HPV-positive cases [Citation4]. In normal cytology and low-grade lesions, the most common HPV types are HPV-52 (2.8% and 16.0%, respectively), HPV-16 (2.7% and 15.9%, respectively), and HPV-58 (1.7% and 12.6%, respectively) [Citation5]. While in high-grade lesions the most prevalent HPV types are HPV-16 (37.1%), HPV-52 (17.6%), and HPV-58 (15.7%), and in cervical cancer the most common types are HPV-16 (59.5%), HPV-18 (9.6%), and HPV-58 (8.2%) [Citation5].
It is well established that persistence of an HPV infection is a prerequisite for progression to precancerous lesions, carcinoma in situ, and eventually cancer cell invasion [Citation6]. Provided that the latter step has not yet occurred, this process is reversible by the clearance of HPV infection and regression of precancer by treatment of precancerous lesions, which happen in many women who have ever experienced HPV infection. While most HPV infections are transient and resolve on their own, some persistent high-risk HPV infections are known to have a risk for progress to CIN and ultimately to cervical cancer, with other risk factors for progression playing a part [Citation7–10]. Cervical lesions are categorized into low-grade squamous intraepithelial lesions (LSIL) including atypical squamous cells of undetermined significance (ASC-US) and CIN grade 1 (CIN1), and high-grade intraepithelial lesions (HSIL) including CIN grade 2 (CIN2), and CIN grade 3 (CIN3). CIN3 and cervical carcinoma in situ are considered the immediate precursors to invasive cervical cancer [Citation11].
Aside from clinical determinants, such as the type of HPV infection, individual immune response, and infection by other sexually transmitted pathogens, behavioral risk factors have also been found to affect the persistence of HPV infections and their progression to precancerous lesions [Citation6]. For example, tobacco exposure [Citation7,Citation12] and increased duration of oral contraception use [Citation12,Citation13] are associated with an increased risk of progression to cancer.
Analysis of the women from the control arm of the large multinational PATRICIA (Papilloma TRIal against Cancer In young Adults, NCT00122681) efficacy trial of AS04-HPV-16/18 vaccine (Cervarix, GSK) in women aged 15 to 25 years, confirmed a significantly increased risk of progression to CIN2 or greater (CIN2+) following infection with oncogenic HPV types and previous or concomitant infection CIN1 or greater (CIN1+) [Citation9]. Infections with high-risk HPV types, HPV-16 and HPV-33, were found to have a significantly low likelihood of clearance [Citation9]. Similar risk factors for progression to CIN2+ were found in the analysis of control subjects from the multinational VIVIANE (NCT00294047) trial in women over 25 years old [Citation10].
Our research aimed to study the natural history of HPV infection in Chinese women to assess if the most common clinical determinants of progression observed elsewhere in the world held true in China. We analyzed the risk of progression from cervical HPV infection to CIN lesion and the clearance of infection, in Chinese women aged 18–25 years from the control arm of the large AS04-HPV-16/18 efficacy trial in China (NCT00779766) [Citation14]. In addition, we analyzed pooled data from the study in China and the large PATRICIA trial.
2. Methods
The main analyses were performed using the data obtained from the control arm of phase II/III, double-blind, randomized controlled clinical trial in China (NCT00779766) [Citation15,Citation16]. As a secondary analysis, the data from the subjects in the Chinese study were pooled with the data of the multi-country PATRICIA trial (phase III, double-blind, randomized controlled trial; NCT00122681) [Citation17]. Data were analyzed with the aim to investigate the time from HPV infection (through HPV genotyping) to CIN lesion associated with the same HPV type, and to evaluate HPV infection determinants associated with disease progression or clearance.
2.1. Study population and design
The Chinese study assessed efficacy, immunogenicity, and safety of the AS04-HPV-16/18 vaccine in healthy women aged 18–25 years in the Jiangsu Province, China, followed up to 72 months, between 2008 and 2016. The control arm group (N = 3,025) was included in our study [Citation15,Citation16].
Similarly, the PATRICIA trial assessed the efficacy of the AS04-HPV-16/18 vaccine compared to the hepatitis A vaccine in healthy women aged 15–25 years from multiple countries, followed up to 48 months, between 2004 and 2009. The control group (N = 9,325) was included in our analysis [Citation17].
Study procedures from both studies included a gynecologic examination (at baseline and every 6 or 12 months in the Chinese study or PATRICIA trial, respectively), and a HPV DNA typing by polymerase chain reaction (PCR) performed on cervical liquid-based cytology samples collected at baseline and every 6–12 months.
Subjects included in our study met all the following criteria:
HPV DNA negative (by PCR) at baseline.
Normal or low-grade cytology (negative or atypical squamous cells – undetermined significance or low-grade squamous intraepithelial lesion) at baseline.
At least one HPV infection during the follow-up period and before the last visit.
2.2. Endpoint and determinants definitions
High-risk (oncogenic) HPV types were defined as types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68. Low-risk (non-oncogenic) HPV types were defined as types 6, 11, 34, 40, 42, 43, 44, 53, 54, 70, and 74.
Histopathologically confirmed CIN lesions were detected in the biopsy sample after colposcopy or in the excision specimen after treatment. CIN1+ was defined as CIN1, CIN2, CIN3, adenocarcinoma in situ, or invasive cervical cancer. CIN2+ was defined as CIN2, CIN3, adenocarcinoma in situ, or invasive cervical cancer.
HPV infections were classified as: incident, transient, and 6-month persistent infection (6MPI).
An incident cervical infection was defined as a new detection of a given HPV type (by PCR) following a negative PCR sample. A transient cervical infection was defined as the detection of an HPV type (by PCR) followed by a negative sample for that HPV type at the next 6-month evaluation. A 6MPI infection was defined as the detection of HPV (by PCR) at two consecutive 6-month evaluations.
Co-infection (or concomitant infection) was defined as any HPV infection that occurred at the time of the onset or during the reference HPV infection.
Clearance was defined as one HPV negative sample (by PCR) (or two consecutive negative samples for confirmed clearance) at the next 6-month evaluation after infection was detected.
The effect of clinical determinants (i.e. high-risk oncogenic or low-risk HPV type, previous infection with high-risk or low-risk cervical HPV, previous or concomitant CIN1+ with high-risk or low-risk HPV, and concomitant cervical infection with other high-risk or low-risk HPV types) and other determinants (i.e. original trial, age, and geographical region) were also assessed.
2.3. Objectives
The primary objective was to assess the risk of progression from a 6MPI to CIN2+ associated with the same HPV type, and to evaluate the effect of clinical determinants in Chinese women from the control arm of the efficacy trial conducted in China and in pooled subjects from the Chinese study and PATRICIA trial.
Secondary objectives were to assess the risk of progression in subjects from the control arm of the Chinese study and in pooled subjects from the Chinese study and PATRICIA trial: i) from a 6MPI to CIN1+ associated with the same HPV type; ii) from any cervical HPV infection to CIN1+ or CIN2+ associated with the same HPV type; and iii) to assess the rate of clearance of persistent cervical HPV infections.
2.4. Statistical Methods
Demographic and baseline characteristics were summarized by descriptive statistics. Categorical variables were summarized by frequency and percentage of each category. Statistics were presented for continuous variables: number of non-missing observations; mean; standard deviation (SD); and median and range (minimum and maximum).
Primary and secondary endpoint analyses were performed using univariate and multivariable Cox models and Kaplan–Meier analysis. To account for correlation at subject level, the robust estimation method derived as an extension of the sandwich estimator in Cox regression model was used [Citation18,Citation19].
Analyses were performed using Statistical Analysis Software (SAS) Version 9.4 (SAS Institute Inc., Cary, NC, USA).
2.5. Ethical considerations
This study used the existing datasets from the Chinese study and PATRICIA trial, both of which were locally reviewed, approved, and conducted in accordance with ethical principles including the Declaration of Helsinki, Good Clinical Practice, and all applicable regulatory requirements. Analyses were performed by GSK in line with informed consent of the participants forms from both studies. There was no need for additional ethics review and approval.
3. Results
3.1. Demographic characteristics
Among 3,025 women from the Chinese study, 1,324 (43.7%) had at least one HPV infection and were included in the analysis. In total, 3,814 cervical HPV infections were detected, 53.8% transient and 37.5% 6MPIs ( and Table S1). The women’s mean age at study entry was 22.9 years.
Figure 1. General disposition of cervical HPV infections in the Chinese study. 6MPI: 6-month persistent infection; I: total number of HPV infections; N: total number of subjects with at least one cervical HPV infection.
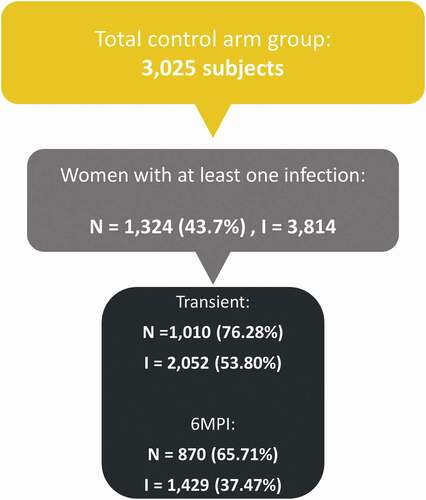
In total, 6,293 subjects (1,324 women from the Chinese study and 4,969 women from the PATRICIA trial) were included in the pooled analysis (Table S1).
3.2. Progression of HPV infection to CIN2+ and CIN1+ lesion
Risk of progression from 6MPI to CIN2+
Among the 870 subjects from the Chinese study in which 1,429 6MPIs were detected, 60.0% reported one single infection while 24.0%, 11.0%, and 5.0% of subjects reported two, three, and more than three infections (consecutive or not), respectively. In total, 72 6MPIs (5.0%) progressed to CIN2+ after a mean (SD) time of 15.5 (12.77) months. Similar to the findings from the PATRICIA trial, highest risks were observed for HPV types HPV-16 (hazard ratio [HR] = 21.96, 95% CI: 7.48–64.42) and HPV-31 (HR = 15.09, 95% CI: 4.49–50.70) ( & Table S2). In China HPV-52 (HR = 3.94, 95% CI: 1.28–12.11) and HPV-58 (HR = 9.97, 95% CI: 3.23–30.79) were also associated with substantial risk of progression from 6MPIs to CIN2+ (). Other risk factors that tended to increase the risk of progression were previous CIN1+ lesion and co-infection with at least one high-risk HPV type ().
Table 1. Risk of progression from 6MPI to CIN1+ or CIN2+ lesion of the same HPV type – Cox model multivariable analysis
Risk of progression from 6MPI to CIN1+
In the Chinese study, 135 out of the 1,429 6MPI (9.5%) progressed to CIN1+ after an average of 13.5 (11.8) months. The highest risk of progression from HPV 6MPI to CIN1+ were observed for the HPV types HPV-16 (HR = 15.43, 95% CI: 6.61–36.02), HPV-31 (HR = 13.06, 95% CI: 4.95–34.49), HPV-58 (HR = 11.83, 95% CI: 4.67–29.97), HPV-18 (HR = 9.31, 95% CI: 3.25–26.72), HPV-52 (HR = 6.91, 95% CI: 2.86–16.70), and HPV-33 (HR = 6.12, 95% CI: 1.80–20.84) (). Other determinants significantly associated with risk of progression were previous CIN1+ and co-infections with at least one high-risk HPV infection ().
Risk of progression from any HPV infection to CIN2+ and CIN1+
After exclusion of cervical HPV infections occurring at the last known visit, a total of 1,307 subjects from the Chinese study had any incident cervical HPV infection, among which 504 (38.6%), 283 (21.7%), 196 (15.0%), 114 (8.7%), and 210 (16.1%) of women reported one, two, three, four, and five or more infections, respectively (Table S3). Of their 3,481 cervical HPV infections, 181 (5.2%) infections progressed to CIN1+ and 94 (2.7%) infections progressed to CIN2+ (Table S3). Similar results were observed in the pooled analysis where 6.3% infections progressed to CIN1+ lesion and 3.4% infections progressed to CIN2+ (Table S3).
In the Chinese study, the risk of progression from any HPV infection to CIN2+ was higher for the high-risk HPV types HPV-16 (HR = 19.77, 95% CI: 8.85–44.14), HPV-31 (HR = 11.48, 95% CI: 4.36–30.18), and HPV-58 (HR = 8.85, 95% CI: 3.46–22.60) (Table S4). Other determinant associated with progression to CIN2+ was co-infection with at least one high- or only low-risk HPV (HR = 1.61, 95% CI: 0.95–2.72 and HR = 1.37, 95% CI: 0.62–3.03, respectively). While the risk of progression to CIN1+ was higher for HPV types HPV-16 (HR = 13.87, 95% CI: 7.27–26.47), HPV-58 (HR = 10.43, 95% CI: 4.92–22.13), and HPV-31 (HR = 9.25, 95% CI: 4.25–20.11) (Table S4). Previous CIN1+ lesion and co-infections were also associated with higher risk of progression to CIN1 + .
The pooled analysis showed similar results (Table S5).
3.3. Clearance of HPV infection
Confirmed clearance (two negative HPV DNA sample)
Confirmed clearance was observed for 887 (61.0%) infections in the Chinese study. The mean time between onset of infection and confirmed clearance was 18.5 (SD: 8.8) months. Confirmed clearance depended on HPV type, with HPV-52 (HR = 0.67, 95% CI: 0.54–0.84), HPV-16 (HR = 0.76, 95% CI: 0.59–0.98), and HPV-31 (HR = 0.73, 95% CI: 0.53–1.01) being associated with lower likelihood of confirmed clearance (). Other determinants were duration of HPV infection (6-month infection versus <6-month infection) and previous HPV infection with high-risk HPV. Confirmed clearance decreased with increasing age.
Table 2. Probability of confirmed clearance from any HPV cervical infection – Cox model multivariable analysis
Similar results were observed for the subjects from the pooled analysis set (Table S6).
4. Discussion
The natural history analysis in Chinese women aged 18–25 years revealed that 5.0% of 6MPI progressed to CIN2+ and 9.5% to CIN1 +. On average, the time for progression from 6MPI to CIN2+ was around 16 months, while the time for progression to CIN1+ was 13 months. The risk of progression from 6MPI to a CIN1+/CIN2+ lesion associated with the same HPV type was significantly higher for oncogenic (high-risk HPV) versus non-oncogenic (low-risk HPV) types (). Other risk factors for progression were previous CIN1+ lesions or co-infections with high-risk HPV type. While 61.0% of persistent infections cleared during the follow-up, clearance was less likely for oncogenic HPV types versus low-risk HPV types and for subjects with previous high- or low-risk HPV infections versus no previous infection. Clearance seemed also to decrease with age, by approximately 3.0% per year.
The findings in Chinese women are consistent with those of the PATRICIA global control arm in women aged 15–25 years [Citation9], with comparable point estimates but wider CIs in the Chinese study, due to the smaller sample size. The pooled analysis did not show effect of geographic location, previous infection, age at infection onset, or co-infection with low-risk HPV type(s) on progression (all p-values >0.25). Similar results (although non-significant) were observed for the subjects from the pooled analysis and the PATRICIA trial regarding higher clearance with low-risk co-infections. Likewise, they also found that increasing age at infection onset seemed to be associated with lower clearance (mean age of 19.1 and 19.7 for the PATRICIA trial and the pooled analysis, respectively) (Table S6) [Citation9]. Mean age in the China study (22.9 years) was higher, further corroborating the impact of age on lower clearance probability (Table S6). These results highlight the importance of aging and the importance of vaccinating at a young age.
In the PATRICIA trial, the risk of progression from a 6MPI to CIN2+ was significantly associated with oncogenic HPV types (mainly HPV-16, HPV-33, then HPV-31, HPV-45; p-values <0.0001) versus low-risk HPV types, previous CIN1+ (p = 0.0004), concomitant CIN1+ (p = 0.0002), and co-infection with other high-risk HPV type(s) (p < 0.0001). This study also assessed behavioral factors for progression from a 6MPI to CIN and found a significant association (p < 0.05) between previous pregnancy and smoking (for CIN1+), use of (contraception) hormones (for CIN2+), and age at first sexual intercourse (for CIN3+). Clearance of HPV infections was less likely for high-risk (HPV-16 and HPV-31) infections versus low-risk HPV types, and for previous HPV infections [Citation9]. The Chinese study did not record any behavioral factor that could affect progression. The analysis results for the PATRICIA trial (unadjusted for behavioral factors) are comparable to the adjusted results reported above, suggesting that these factors are associated with the outcome but not with the exposure.
Comparable results were also observed in the control arm subjects of the VIVIANE (human papillomaVIrus: Vaccine Immunogenicity ANd Efficacy) trial in older women (>25 years) from Asia Pacific, Europe, North America, and Latin America [Citation10]. The risk of progression from a 6MPI to CIN2+ was 5.7% (64/1,128) in the 48-month follow up, and oncogenic HPV type was significantly (p-values <0.0001) associated with progression (primarily HPV-33 and HPV-16) versus non-oncogenic types. In addition, co-infection with a different oncogenic HPV type and presence of a concomitant CIN1+ lesion associated with a different HPV type were also associated with higher risk for progression to CIN2 +. This study did not show an effect of previous HPV infections or lesions, and previous pregnancy was associated with a higher risk of CIN2 +. At 48 months, clearance was 89.0% overall, although smokers were significantly less likely to clear an infection compared with nonsmokers [Citation10].
To enable comparison with global data, the current study only included the most common HPV types identified in cervical cancer. However, post-hoc sensitivity analysis including HPV 52 and 58 types (only types other than HPV-16, 18, 31, 33, and 45 for which more than two cases of CIN2+ were identified) was performed and showed no impact on the HRs associated with HPV-16, 18, 31, and 33. Compared to low-risk HPV types, HPV-52 and HPV-58 were observed to increase the risk of progression from 6MPI to CIN2+ with HRs of 3.94 (95% CI: 1.28–12.11) and 9.97 (95% CI: 3.23–30.79), respectively. Those observations are in line with the prevalence found in cervical cancers for HPV-58 and HPV-52 among Chinese women, being 8.2% and 6.5%, respectively [Citation5].
Given the scarcity of data in Chinese women, our study contributes to the understanding of risk factors for progression and clearance of HPV infections in this population and supports the use of 6MPI as a clinical endpoint for vaccine efficacy or effectiveness. One other study was identified in China that assessed the natural history of cervical cancer using a multistate model to 11-year follow-up data (1999–2010) from a screening study in the Shanxi Province (N = 1,997 women aged 35–45 years at enrollment) [Citation20]. This study estimated that there is a higher chance of regression than progression, with increasing clearance rates and decreasing progression rates over time: for example, after 5 years, 53.79% of women with CIN2 will regress to normal/CIN1 and only 19.66% will progress to CIN3 [Citation20]. This study, however, did not assess risk factors associated with progression and regression, or the risks based on type of infection (HPV type or duration of infection) in Chinese women.
Several limitations apply to this analysis, as with the other two natural history studies based on trials [Citation9,Citation10]. HPV infections below the threshold for detection by PCR (false negatives) could have affected the results by underestimating the number of 6MPIs or showing apparent clearance of infection. To reduce the potential impact on clearance outcomes, the definition of confirmed clearance required two consecutive negative PCR results. The duration of follow-up was 6 years for the study in China and 4 years for the PATRICIA trial, however, it is possible that some CIN lesions associated that may not have been detected within this timeframe, resulted in underestimation of progression rates. All subjects in the Chinese study and many subjects in the PATRICIA trial were already sexually active at enrollment. As such, no data were available on HPV infections prior to the study or on age at the start of persistent infections present at baseline. As age at onset of infection is a factor that may affect clearance, these results should be interpreted cautiously. Moreover, the Cox multivariable model characterizes risk based on time to the event, thus, HPV types with fastest progression will be associated with higher risk for progression to CIN2+ [Citation21,Citation22]. Finally, the Chinese study did not record behavioral risk factors for progression.
5. Conclusions
Analysis of the control arm of the Chinese study showed that the risk of developing a CIN2+/CIN1+ associated with the same HPV type as a 6MPI varied by HPV type, and was significantly higher for oncogenic versus non-oncogenic HPV types. Infections with oncogenic types HPV-52, HPV-16, and HPV-31 were least likely to clear and have the highest risk of progression to CIN2 +. Similar findings on risk factors for progression and clearance were seen in the analysis of women in the control arms from the multinational PATRICIA and VIVIANE trials.
Article highlights
There are over 106,000 new cases of cervical cancers and 47,000 related deaths each year in China. Human papillomavirus (HPV) types 16 and 18 are responsible for most cervical cancers.
We aimed to better understand the progression from viral infection to potential precancerous lesions, or to clearance of the infection in the Chinese female population.
We analyzed the data from control subjects of a large clinical trial in China on: information on the type of virus present; the type of lesions; and potential determinants of disease progression.
The risk of infection progress to precancerous lesions was significantly higher for HPV types classified as oncogenic, particularly HPV-16 and HPV-31.
Previous precancerous lesion and infections with other HPV types seemed to increase the risk of progression.
While most infections cleared (61.0%), infections with the oncogenic types HPV-52, HPV-16, and HPV-31 were least likely to clear.
Overall, findings from the Chinese female population were comparable to similar analyses on other populations included in multinational studies, although contributions of HPV-52 and HPV-58 to cervical infections are larger in the Chinese population than in other regions.
Abbreviations
6MPI, 6-month persistent infection
CI, confidence interval
CIN, cervical intraepithelial neoplasia
CIN1, cervical intraepithelial neoplasia grade 1
CIN1+, cervical intraepithelial neoplasia grade 1 or greater
CIN2, cervical intraepithelial neoplasia grade 2
CIN2+, cervical intraepithelial neoplasia grade 2 or greater
CIN3, cervical intraepithelial neoplasia grade 3
GSK, GlaxoSmithKline
HPV, human papillomavirus
HR, hazard ratio
PATRICIA, PApilloma TRIal against Cancer In young Adults
PCR, polymerase chain reaction
SAS, Statistical Analysis Software
SD, standard deviation
VIVIANE, human papillomaVIrus: Vaccine Immunogenicity ANd Efficacy.
Data sharing
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Trademark statement
Cervarix is a trademark owned by or licensed to the GSK group of companies.
Author contributions
Sarah Welby was the coordinating author and wrote the outline of the initial draft. Sarah Welby, Dominique Rosillon, Yang Feng, and Dorota Borys were involved in the conception and study design. Dominique Rosillon and Yang Feng performed the statistical analyses. All authors were involved in the interpretation of the analyses, critically reviewed the manuscript, and gave their final approval on the submitted version.
Declaration of interest
S Welby, D Rosillon, and D Borys were employees of the GSK group of companies. D Borys and S Welby held shares of the GSK group of companies. Y Feng was a consultant at Ningyang Group Co. Ltd. working on behalf of the GSK group of companies during the study conduct. All authors declared no other financial or non-financial relationships or activities.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they were an investigator on the PATRICIA trial described in this study. Peer reviewers on this manuscript have no other relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (53.3 KB)Acknowledgments
The authors would like to thank the study participants, investigators, and contributors involved in the clinical trials HPV-008 (NCT00122681) and HPV-039 (NCT00779766) funded by GlaxoSmithKline Biologicals SA. The authors also thank Sophie Caterina (GSK), Violaine Tuzelet (GSK), and Yang Li (Ningyang Group Co. Ltd, on behalf of GSK, XIAMEN, CHINA) for statistical analysis support. The authors also thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Kavi Littlewood (independent medical writer, Littlewood Writing Solution, on behalf of GSK) provided medical writing support; Lyes Derouiche (Publications Manager, Business & Decision Life Sciences, on behalf of GSK) coordinated manuscript development and editorial support.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Bruni L, Albero G, Serrano B, et al. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World. Summary Report 17 June 2019. [cited 2020 Aug 17].
- de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–1056.
- Koshiol J, Lindsay L, Pimenta JM, et al. Persistent human papillomavirus infection and cervical neoplasia: a systematic review and meta-analysis. Am J Epidemiol. 2008;168(2):123–137.
- Chen W, Molijn A, Enqi W, et al. The variable clinicopathological categories and role of human papillomavirus in cervical adenocarcinoma: a hospital based nation-wide multi-center retrospective study across China. Int J Cancer. 2016;139(12):2687–2697.
- Bruni L, Albero G, Serrano B, et al. Human Papillomavirus and Related Diseases in China. Summary Report 17 June 2019. [cited 17 Aug 2020]. Available from: https://hpvcentre.net/statistics/reports/CHN.pdf
- de Sanjosé S, Brotons M, Pavón MA. The natural history of human papillomavirus infection. Best Pract Res Clin Obstetrics Gynaecol. 2018;47:2–13.
- Appleby P, Beral V, Berrington de Gonzalez A, et al. Carcinoma of the cervix and tobacco smoking: collaborative reanalysis of individual data on 13,541 women with carcinoma of the cervix and 23,017 women without carcinoma of the cervix from 23 epidemiological studies. Int J Cancer. 2006;118(6):1481–1495.
- Smith JS, Munoz N, Herrero R, et al. Evidence for Chlamydia trachomatis as a human papillomavirus cofactor in the etiology of invasive cervical cancer in Brazil and the Philippines. J Infect Dis. 2002;185(3):324–331.
- Jaisamrarn U, Castellsague X, Garland SM, et al. Natural history of progression of HPV infection to cervical lesion or clearance: analysis of the control arm of the large, randomised PATRICIA study. PloS One. 2013;8(11):e79260.
- Skinner SR, Wheeler CM, Romanowski B, et al. Progression of HPV infection to detectable cervical lesions or clearance in adult women: analysis of the control arm of the VIVIANE study. Int J Cancer. 2016;138(10):2428–2438.
- Wentzensen N, Schiffman M, Dunn ST, et al. Grading the severity of cervical neoplasia based on combined histopathology, cytopathology, and HPV genotype distribution among 1,700 women referred to colposcopy in Oklahoma. Int J Cancer. 2009;124(4):964–969.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Comparison of risk factors for invasive squamous cell carcinoma and adenocarcinoma of the cervix: collaborative reanalysis of individual data on 8,097 women with squamous cell carcinoma and 1,374 women with adenocarcinoma from 12 epidemiological studies. Int J Cancer. 2007;120(4):885–891.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical carcinoma and sexual behavior: collaborative reanalysis of individual data on 15,461 women with cervical carcinoma and 29,164 women without cervical carcinoma from 21 epidemiological studies. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1060–1069.
- Zhu FC, Chen W, Hu YM, et al. Efficacy, immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine in healthy Chinese women aged 18-25 years: results from a randomized controlled trial. Int J Cancer. 2014;135(11):2612–2622.
- Zhu F, Li J, Hu Y, et al. Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine in healthy Chinese girls and women aged 9 to 45 years. Hum Vaccin Immunother. 2014;10(7):1795–1806.
- Zhu FC, Hu SY, Hong Y, et al. Efficacy, immunogenicity and safety of the AS04-HPV-16/18 vaccine in Chinese women aged 18-25 years: end-of-study results from a phase II/III, randomised, controlled trial. Cancer Med. 2019;8(14):6195–6211.
- Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–314.
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130.
- Lin D, Wei L. The robust inference for the Cox proportional hazards model. J Amer Statist Assoc. 1989;84:1074–1078.
- Zhang SK, Kang LN, Chang IJ, et al. The natural history of cervical cancer in Chinese women: results from an 11-year follow-up study in China using a multistate model. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1298–1305.
- Donken R, Hoes J, Knol MJ, et al. Measuring vaccine effectiveness against persistent HPV infections: a comparison of different statistical approaches. BMC Infect Dis. 2020;20(1):482.
- Spruance SL, Reid JE, Grace M, et al. Hazard ratio in clinical trials. Antimicrob Agents Chemother. 2004;48(8):2787–2792.
