ABSTRACT
Introduction
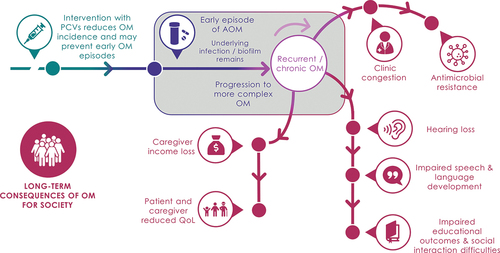
Otitis media (OM) is a common childhood infection. Pneumococcal conjugate vaccines (PCVs) prevent OM episodes, thereby reducing short- and long-term clinical, economic, humanistic, and societal consequences. Most economic evaluations of PCVs focus on direct health gains and cost savings from prevented acute episodes but do not fully account for the broader societal impacts of OM prevention.
Areas covered
This review explores the broader burden of OM on children, caregivers, and society to better inform future economic evaluations of PCVs.
Expert opinion
OM causes a substantial burden to society through long-term sequelae, productivity losses, reduced quality of life for children and caregivers, and contribution to antimicrobial resistance from inappropriate antibiotic use. The effect of PCVs on acute OM has been recognized globally, yet the broader impact has not been consistently quantified, studied, or communicated. Economic evaluations of PCVs must evolve to include broader effects for patients, caregivers, and society from OM prevention. Future PCVs with broader coverage may further reduce OM incidence and antimicrobial resistance, but optimal uptake will depend on increasing the recognition and use of novel frameworks that include broader benefits. Communicating the full value of PCVs to decision makers may result in wider access and positive societal returns.
1. Introduction
1.1. Otitis media
Otitis media (OM), or inflammation of the middle ear, is one of the most common diseases in young children worldwide [Citation1,Citation2]. OM is caused by viral or bacterial infection, most notably by four major bacterial pathogens, Streptococcus pneumoniae, non-typeable Haemophilus influenzae (NTHi), Moraxella catarrhalis, and Streptococcus pyogenes, which are part of the normal flora of the upper respiratory tract. OM is classified into several clinical subtypes, including acute otitis media (AOM), an acute inflammation of the middle ear with effusion and signs and symptoms of acute infection; otitis media with effusion (OME), a chronic yet asymptomatic inflammation of the middle ear with effusion; and chronic suppurative otitis media (CSOM), a chronic infection of the middle ear and mastoid cells with chronic perforation of the eardrum and otorrhea [Citation1]. OM is a leading cause of physician visits and antibiotic use in young children and places considerable strain on health systems globally [Citation3,Citation4]. Compared with other clinical subtypes, AOM is most frequently observed by healthcare professionals, with an estimated 709 million cases per year globally (global incidence rate, 10.9% or 10.9 new episodes per hundred people per year) [Citation5], half of which occur among children under 5 years of age. Incidence varies by region, ranging from 3.6 to 43.3 episodes per 100 population per year in Central Europe to Sub-Saharan Africa, respectively [Citation5].
Most cases of OM resolve spontaneously, but some children experience recurrent OM and CSOM, which can cause ongoing episodes of pain and fever as well as long-term distress to children and their parents [Citation1,Citation2]. These complicated forms are relatively prevalent; before the era of pneumococcal vaccination, approximately 20% of children experienced recurrent OM in their first year of life [Citation6,Citation7], and this may still be the case in countries with limited health resources. The incidence of CSOM varies from 1.7 to 9.4 cases per 1,000 people across the 21 World Health Organization (WHO) regional areas [Citation5]. Recurrent OM, CSOM, and OM-related tympanic membrane rupture can lead to temporary or permanent hearing loss, which can affect social interactions and developmental outcomes in childhood [Citation8–10]. In rare cases, the infection can lead to serious complications such as meningitis, mastoiditis, and intracranial and extracranial abscesses. In 2005, an estimated 21,000 individuals died from OM complications globally, with most of these deaths occurring in low-income countries [Citation5].
Current clinical practice guidelines in the United States (US) recommend using antibiotics to treat AOM with severe symptoms or otorrhea and, for milder cases, analgesics and watchful waiting (i.e. delaying medical therapy or intervention in hope of spontaneous resolution) [Citation11]. Antibiotics are not recommended for treating OME; persistent OME may be treated by inserting ear tubes to keep the middle ear aerated (i.e. tympanostomy) [Citation12]. Eradication of CSOM often requires surgical procedures, namely mastoidectomy, tympanoplasty, or both.
1.2. Pneumococcal conjugate vaccines and their efficacy/effectiveness in preventing OM
Pneumococcal conjugate vaccines (PCV) have been shown to be clinically effective at reducing the burden of OM [Citation13–18]. PCVs contain purified capsular polysaccharides of pneumococcal serotypes conjugated to a carrier protein. The selection of serotypes for the first PCVs was based on those which were the most common and invasive at the time (currently there are ~100 immunologically distinct S. pneumoniae serotypes). The first was a seven-valent PCV (PCV7) containing polysaccharides of seven serotypes () conjugated to diphtheria CRM197 protein, which became available first in the US in 2000. Efficacy of PCV7 against invasive pneumococcal disease [Citation19], pneumonia [Citation20], and OM [Citation21] in children was demonstrated in randomized controlled trials (RCTs). By 2008, PCV7 was licensed in approximately 90 of 193 WHO member states [Citation22].
Figure 1. Current and investigational pneumococcal conjugate vaccines (not exhaustive). *For Synflorix® (10-valent PCV), serotype 18C is conjugated to tetanus toxoid and serotype 19F is conjugated to diphtheria toxoid; the remaining eight serotypes are conjugated to protein D from H. influenzae. Abbreviation: PCV, pneumococcal conjugate vaccine.
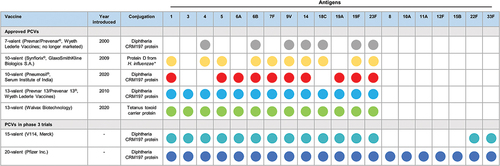
In 2010, to provide expanded S. pneumoniae serotype coverage, PCV7 was replaced by a 13-valent PCV (PCV13)Footnote1 using the same carrier protein (). PCV13 was licensed based on immunobridging to PCV7, which showed that the vaccine elicited a non-inferior immune response to PCV7 for the seven common serotypes, and based on a robust immune response to the six additional serotypes (1, 3, 5, 6A, 7 F, 19A) [Citation23,Citation24]. Because there is no correlate of protection for PCVs against OM, PCV13 is indicated in some countries (including the US) for preventing OM caused by the seven original serotypes in PCV7 (i.e. based on results from a PCV7 efficacy trial [Citation25]). The clinical impact of PCV13 on OM has been established through post-licensure data and effectiveness in real-world settings [Citation15,Citation16,Citation26].
A 10-valent PCV (PCV10), which uses the carrier protein H. influenzae protein D, has also been used in several countries since 2009 for infant vaccination. PCV10 was licensed based on non-inferior immunogenicity against PCV7, and its efficacy against AOM was confirmed in post-licensure RCTs [Citation27].
Most recently, in late 2019, a CRM197-conjugated 10-valent PCV, manufactured by Serum Institute of India, achieved prequalification by the WHO following clinical trials conducted in India and The Gambia [Citation28–31]. An alternative 13-valent PCV, manufactured by Walvax Biotechnology, is also now licensed in China (). While these two new PCVs showed non-inferior immunogenicity to existing products [Citation28,Citation32], their efficacy and effectiveness against OM and other pneumococcal disease is yet to be determined.
PCV7, PCV10, and PCV13 have reduced circulation of both invasive and noninvasive disease-causing serotypes since their introduction into pediatric national immunization programs (NIPs) [Citation33–35], which has resulted in clinically relevant reductions in OM [Citation13,Citation14,Citation16,Citation36–39]. Both PCV10 and PCV13 have demonstrated an incrementally higher impact on OM than PCV7 due to their broader serotype coverage [Citation13–18,Citation40]. Since its introduction, PCV13 has considerably reduced the incidence of OM episodes [Citation13,Citation14] and has been linked with reduced carriage of vaccine serotypes [Citation34,Citation41–43], including two of the most serious disease-causing serotypes, 19A and 7 F [Citation33,Citation34,Citation43]. Consistent with this, the transition from PCV7 to PCV13 is associated with decreased OM-related ambulatory and emergency department visits [Citation44,Citation45], decreased rates of tympanostomy among children [Citation17,Citation46], and decreased rates of antibiotic prescription for AOM [Citation14,Citation47,Citation48] and antibiotic-resistant OM cases [Citation18,Citation49–53]. Similarly, PCV10 has been associated with delayed first OM episode in children [Citation54] and lower outpatient antibiotic use [Citation55] than in the PCV7 era.
PCV13 may also provide protection against OM caused by non-vaccine serotype pneumococci and other otopathogens. Protection from PCV13 may limit mucosal damage inflicted by early episodes of OM, which may in turn reduce biofilm formation and OM recurrence [Citation36,Citation37]. Specifically, some countries have found parallel decreases in pneumococcal and non-pneumococcal OM episodes, such as those caused by NTHi, Moraxella catarrhalis, and Streptococcus pyogenes [Citation36,Citation37]. Similarly, an early formulation of PCV10 was shown in an RCT to reduce AOM episodes with NTHi isolated as the offending pathogen [Citation56]; however, real-world [Citation57,Citation58] and post-licensure clinical trial data [Citation59–61] have not shown effectiveness with this vaccine against OM due to NTHi.
1.3. Limitations of current PCV cost-effectiveness analysis for OM
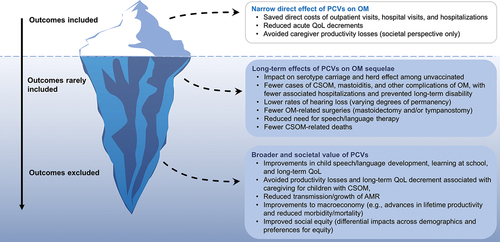
Cost-effectiveness analyses (CEAs) of PCVs have traditionally evaluated only direct health gains, healthcare costs, prevented productivity losses among vaccine recipients, and prevented productivity losses in the community () [Citation62–65]. For OM, most CEAs of PCVs have estimated AOM cases prevented and direct health system costs avoided, based on assumptions of PCV effectiveness against vaccine- and non-vaccine serotypes or against all-cause AOM [Citation63,Citation64,Citation66–68]. Furthermore, most studies have not accounted for PCV effects on severe OM or its sequelae. The few CEAs that included long-term consequences of OM as a model input only considered hearing loss, captured as a quality-adjusted life year decrement [Citation69–71]. Finally, many CEAs adopt a healthcare payer, rather than a societal perspective, and omit longer-term improvements in child health, child and caregiver quality of life (QoL), educational outcomes, and the impact of reduced antimicrobial resistance (AMR) from PCV use [Citation63–65].
Figure 2. Only the tip of the iceberg is considered in CEAs of PCV impact on OM. Abbreviations: AMR, antimicrobial resistance; CSOM, chronic suppurative otitis media; OM, otitis media; PCV, pneumococcal conjugate vaccine; QoL, quality of life.
Although PCVs have reduced mortality and morbidity globally, the overall level of PCV uptake and access could be improved when benchmarked against other pediatric vaccines [Citation72]. Revealing their full value, by including both narrow and broader benefits in CEAs, may help address this. Guidelines from the International Society for Pharmacoeconomics and Outcomes Research (ISPOR), WHO, and the European Vaccine Economics Community all advocate that broader benefits now be included when performing CEAs of vaccines, where appropriate, and that this should consider narrow and broad cost offsets resulting from reduced healthcare utilization [Citation73–75]. Given these guideline updates, this review explores the broader burden of OM on children, caregivers, and society to better inform future economic evaluations of PCVs and to help convey the full value of PCVs to decision makers.
2. Health-related outcomes and costs of OM in children
2.1. Hearing loss and child development
Frequent or severe attacks of OM increase the risk of hearing loss in children. Children with OM tend to have average hearing thresholds of more than 20 dB [Citation76–80], suggesting at least mild hearing loss by conventional classifications [Citation81]. More severe hearing loss is associated with recurrent OM and CSOM [Citation8,Citation10,Citation82–84], cholesteatoma (a benign skin growth behind the ear drum usually caused by recurrent OM) [Citation80,Citation85], and OM in children with cleft lip and palate [Citation86]. In a cross-sectional study of Nigerian schoolchildren, mild-to-moderate conductive hearing loss was detected in around two-thirds of CSOM cases, with children from lower socioeconomic backgrounds being most vulnerable [Citation10]. Hearing loss may last for several weeks or months before an OM episode resolves; occasionally, hearing loss can continue into adulthood [Citation76,Citation77,Citation87].
Few studies have determined the prevalence of permanent hearing loss caused by OM. One estimate suggests OM-associated permanent hearing impairment (defined as permanent hearing loss for best ear >25 dB) had a global prevalence of 30.8 per 10,000 in 2005 [Citation5], the prevalence being relatively low in higher-income countries (typically <4 per 10,000) but much higher in some lower-income regions, for example South Asia (97.0 per 10,000) and Sub-Saharan Africa (30–35 per 10,000). In South Asia, by 5 years of age, six out of 1,000 children were estimated to have permanent hearing impairment caused by sequelae of OM, whereas this occurred in less than one out of 10,000 children in higher-income regions of North America, Europe, and Australasia.
OM-related hearing loss can affect speech and language development, leading to impaired social interaction and learning in children [Citation10,Citation82,Citation88,Citation89]. Even temporary hearing loss during a critical period of a child’s learning may have long-lasting effects on the development of the brain’s speech and language centers [Citation90–92]. OM can cause children to have considerable difficulties perceiving teachers and hearing peers in noisy classrooms, which can cause fatigue from the increased effort needed to listen [Citation93]. Together, impaired hearing and communication can impede learning and necessitate extra educational support and healthcare resources, such as speech therapy. If left untreated, these long-term sequelae of persistent or severe OM might ultimately limit future career prospects [Citation72].
2.2. Quality of life in children
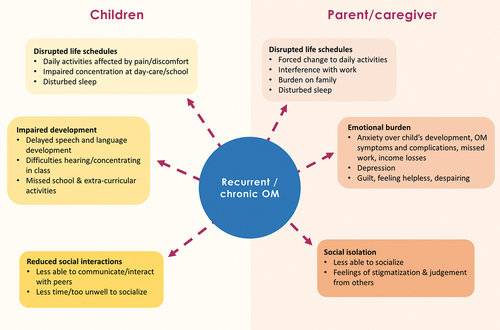
While OM-related reduction in QoL has been included in CEAs, it is usually captured as a quality-adjusted life year decrement [Citation66,Citation94–97]. Other types of patient burden are excluded, such as absenteeism from school or nursery and the long-term impact on QoL in adults who suffered from severe or recurrent OM as children. Evidence suggests that recurrent and severe OM can harm children’s QoL by negatively affecting auditory processing and communication, school readiness, social competence, psychosocial wellbeing, movement and balance, and sleep () [Citation98–105]. Health-related QoL in children with OM is usually evaluated using the Otitis Media-6 (OM-6) questionnaire, which includes six questions that are completed by caregivers. Studies using the OM-6 have shown that OM moderately reduces overall QoL [Citation99–102], and one prospective cross-sectional study reported a significantly higher OM-6 score (i.e. lower QoL) in children with recurrent or chronic OM than in healthy age-matched controls [Citation100]. Studies using the OM-6 also suggest that OM affects short-term aspects of QoL to a greater extent than long-term aspects [Citation103–105]. Studies aimed at assessing the burden of OM on QoL may, however, be biased toward the immediate effects of AOM over longer-term effects like speech impairment, hearing loss, and emotional distress.
Figure 3. Impacts of recurrent and chronic otitis media (OM) on children’s and parent/caregivers’ quality of life.
Longer-term effects might progress even after resolution of OM and may not be captured during the study period. A recent systematic review found that few studies have examined the impact of OM on QoL in children and that their methodological quality and outcome measurements vary [Citation98]. Additionally, a 2019 World Development Report estimated that over 2.4 million disability-adjusted life-years were lost due to otitis media [Citation106]. Given both long-term sequelae and death are more frequent with CSOM than AOM, most life-years lost and life-years with a disability result from chronic infections.
2.3. Healthcare costs and resource utilization
There is substantial health resource utilization associated with more complicated OM cases, which is rarely taken into account by CEAs of PCVs. A cost-of-illness study conducted in Belgium found that public healthcare payer costs for long-term complications and sequelae of AOM ranged from €119 to €7,957 per patient per year [Citation107]. For the 25 patients included, the total cost amounted to €250,000 (third-party plus patient perspective costs) over an 11-year follow-up period after the first AOM event. In addition, OM is a leading reason for pediatric ambulatory visits. In 1997–1999, just before the introduction of PCV7, OM-related visits accounted for one in every 10 ambulatory visits in children in the US [Citation44]. Patients with chronic OM also have a higher risk of developing comorbidities that deplete additional health system resources, such as sudden sensorineural hearing loss [Citation108], and nosocomial or other infections, for example following a tympanostomy [Citation109].
Evidence of healthcare resource utilization from low-to-middle-income countries (LMICs) is less available. Generally, CSOM cases are less likely to be seen in these countries due to limited access to health services and scarcity of ear, nose, and throat specialists [Citation110,Citation111].
3. Health-related outcomes and costs of infant OM for caregivers
3.1. Quality of life in caregivers
Recurrent and severe OM affect not only a child’s QoL, but also that of their parents and caregivers () [Citation112,Citation113]. Caregiver QoL is rarely included in CEAs of PCVs. Episodes of OM in children often force changes in their parents’ daily activities by adding medical responsibilities, for example, attending clinical appointments and managing the episode at home [Citation99,Citation103,Citation114–117]. Associated absenteeism from work can lead to lost earnings, amplifying their distress [Citation118]. OM and its sequelae can also interfere with parents’ sleep and create emotional distress, especially anxiety [Citation115,Citation116]. For example, beyond the immediate distressing symptoms of OM, parents may be concerned about their child’s behavior and learning. Further, chronic OM can lead parents to doubt treatment efficacy and their own competency to manage the illness, experience feelings of stigmatization and social isolation, and become weary in coping with illness management and treatment [Citation116]. Because parents are often poorly informed about OM, they may also feel uncertain about how best to help their child [Citation119].
3.2. Societal productivity loss
OM, especially chronic and recurrent OM, can result in negative long-term consequences for society and the economy (). Caring for a child with OM may result in missed workdays, lower productivity, and income loss. The high incidence of OM translates this into a large societal burden through lost productivity and decreased QoL [Citation120]. In an online interview study conducted in 12 countries with parents whose children experienced an AOM episode (N = 1438), 73% reported absence from work or having to rearrange working hours to care for their child [Citation113]. Of those who took leave, 67% stayed at home for 2–7 days. A US model-based analysis estimated the indirect cost per AOM episode in the 3 months from diagnosis was approximately $1,200 (in 1999 USD) due to caregiver work loss and transportation [Citation118]. These indirect costs accounted for nearly 90% of the 3-month total cost of AOM treatment. In a retrospective analysis of the Canadian pediatric PCV program, $990 million (in Canadian dollars) of indirect costs were saved over a 10-year period alone, with these indirect cost savings attributed to saved hours of lost productivity among caregivers [Citation121]. In addition to these substantial societal benefits, the study estimated approximately 3.7 million AOM episodes were averted and $627 million of direct OM-related costs were saved. The societal burden is typically higher in LMICs than in developed countries [Citation5,Citation122]. For example, in Malaysia, caregivers missed an average of 21 hours from work to care for their child with AOM and experienced reduced productivity at work [Citation103]. Associated income losses may cause particular hardship in LMICs, where absenteeism, consultations, and treatment expenses can take up a considerable proportion of household income [Citation103,Citation111].
4. Community or health system externalities of OM prevention
4.1. Antimicrobial resistance
As the most common condition leading to the prescription of antibacterial agents, AOM is a key contributor to global AMR [Citation2,Citation11,Citation123]. Clinicians in many countries continue to prescribe broad-spectrum antibiotics to manage mild, initial AOM episodes, even though most clinical practice guidelines recommend watchful waiting [Citation11]. AMR may be of particular concern in developing countries, where there are often no guidelines for antibacterial treatment of OM and antibiotics may be easily purchased without mandatory medical prescriptions [Citation124,Citation125].
Studies show that pneumococci, especially serotypes 19A, 15A, and 35B, have become increasingly resistant to antibiotics [Citation18,Citation42]. A systematic review and meta-analysis by Mather et al. [Citation126] found that 15% of AOM cultures were resistant to amoxicillin, an antibiotic commonly used to treat OM. If these trends are compounded over time and geographic regions, OM could become increasingly resistant to antibiotics, leaving fewer treatment options [Citation18,Citation127].
Pneumococcal vaccination early in life helps reduce AMR by preventing initial OM episodes, in turn reducing cases of chronic and recurrent OM that require antibiotic treatment [Citation37] (). Real-world studies show reduced rates of antimicrobial-resistant vaccine-type pneumococcal infections and lower rates of antibiotic prescription since the introduction of PCV7, PCV10, and PCV13 [Citation14,Citation18,Citation47–49,Citation127], yet these impacts are consistently excluded from CEAs.
4.2. Macroeconomics
CEAs of PCVs and other vaccines in pediatric immunization programs rarely consider positive impacts from vaccination on the macroeconomy. By directly improving child health and providing indirect economic benefits, these vaccines help stimulate changes in household behavior (e.g. through increasing household savings), allow public sector budgets to be allocated elsewhere, and increase gross domestic product, especially in LMICs [Citation72,Citation128]. Improvements in population health through vaccination increase foreign investment and can improve workforce productivity [Citation129]. For example, in China, vaccinations given before 15 years of age have been shown to provide long-term benefits on non-health outcomes such as education and cognitive skills, with vaccinated individuals enjoying around one more year of schooling, and performing substantially better on several cognitive tests later in life [Citation130].
4.3. Social equity
For children living in impoverished communities and countries, there is often an excess of vaccine-preventable mortality and morbidity [Citation131]. Widely used vaccines, including PCVs, may improve social equity (e.g. fairness in the distribution of health within a population) provided that vaccination coverage among the poorest of the population is sufficient and vaccines are accessible [Citation132,Citation133]. The use of PCVs in New Zealand has been associated with reduced ethnic and socioeconomic disparities in OM hospitalizations [Citation134]. Similarly, in a US study, socioeconomic and racial disparities in the incidence of invasive pneumococcal disease were found to be lower after the introduction of PCV13 than during the pre-PCV7 era [Citation133]. To continue improving social equity, attention is needed to ensure that PCV programs target disadvantaged individuals and communities, and that these programs are distributed to children of all socioeconomic backgrounds equally.
5. Conclusion
National governments and decision makers worldwide face a common dilemma regarding the efficient appropriation of limited healthcare resources for the maximal benefit of the population. Interventions that target disease prevention, especially vaccination, are preferred to waiting until a disease manifests and treatment is needed. Given the high prevalence of OM, PCVs have substantial positive externalities compared with other health technologies because the benefits are realized across the entire population.
OM causes substantial morbidity, economic losses, and harm to QoL globally, yet many broader impacts of OM on children, their families, and society are overlooked, some of which last far beyond the acute disease phase. Recurrent and chronic forms of OM can result in long-lasting hearing loss, which impairs children at a crucial age in their speech and language development and impacts their QoL and educational achievement. Caregivers also suffer due to emotional distress, income loss, and weariness from managing and treating the illness. Because it is so prevalent, OM additionally burdens society through absenteeism and productivity losses, and can contribute to increased rates of AMR due to inappropriate or excessive antibiotic use – leaving physicians with fewer treatment options for bacterial infections. These broader impacts are especially prominent in developing countries, which have higher prevalence of recurrent OM, CSOM, and resulting sequelae, and fewer health resources to manage them.
Infant immunization with PCVs helps prevent initial episodes of OM and, therefore, breaks the chain of events leading to long-term economic, clinical, humanistic, and societal consequences of chronic and recurrent OM. Conventional CEAs of PCVs have not fully accounted for this effect, meaning that the true value of PCVs to society has been underestimated. Decision makers, therefore, may not be informed on the vaccine’s broader societal benefits, which can lead to undervaluation of PCV programs, suboptimal allocation of resources to PCVs in NIPs, exclusion of PCVs from NIPs, and low PCV uptake rates [Citation62,Citation128,Citation135,Citation136]. Understanding and incorporating broader effects of OM as an outcome of economic evaluations, and highlighting the need for capturing these outcomes, will be important for improving access to PCVs. In parallel, efforts are needed, especially in LMICs, to collect more local country health data on broader societal impacts, as well as sero-epidemiologic data, to optimize future economic analyses.
6. Expert opinion
To help inform future health technology decisions, PCV economic evaluations must evolve to include broader value elements, including societal health impacts and costs of OM among patients, caregivers, and the larger population. Guidelines from ISPOR for the economic evaluation of vaccines recommend that CEAs capture a disease’s long-term effects [Citation73], which is especially relevant for modeling the impact of PCVs on OM. Recent guidelines also advise using a societal perspective as the preferred base case in CEAs [Citation74,Citation75], or reporting outcomes from both payer and societal perspectives [Citation73]. Within the societal perspective, estimating effects on productivity is recommended to account for improvements in children’s future capabilities (e.g. development, education, and work), reductions in AMR, and averted productivity loss of caregivers, to name a few. Additionally, the guidelines recognize that macroeconomic effects of vaccines can be analyzed, although it is likely to require alternative or complementary methodologies to those used in traditional health technology assessments. For example, fiscal health modeling could be used to estimate how a vaccine relates to changes in tax revenues and transfer costs attributable to morbidity and mortality [Citation73].
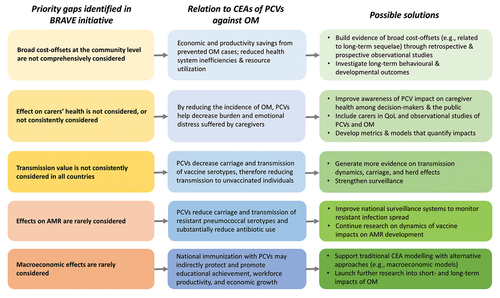
More recently, the Office of Health Economics published a narrative for Broad Recognition of Value in Vaccines Engagement (BRAVE) [Citation137] in which five priority gaps were identified as being either excluded or not consistently considered in vaccine health technology assessments: broad cost-offsets at the community-level; effects on carers’ health; transmission value; effects on AMR; and macroeconomic effects (). For evaluations of PCVs against OM, bridging these priority gaps will require decision makers to recognize the importance of broader vaccine impacts, along with the development of improved metrics, models, and case studies for quantifying the burden of OM on child development, carers’ health, and the macroeconomy. Additionally, the ongoing coronavirus disease 2019 (COVID-19) pandemic provides further learnings on the broader societal effects of vaccination [Citation137,Citation138] that may encourage policymakers to incorporate the broader long-term and societal impacts of PCVs in economic evaluations in the future. Nonetheless, benefits from PCVs depend on other aspects, such as local epidemiological, socioeconomic, environmental, and health system factors, which may ultimately require additional investment in the health sector (e.g. investment into other public health interventions and vaccines) and other sectors (e.g. education and infrastructure) [Citation136]. Therefore, policymakers will need to ensure that resource allocation is based on an intervention’s full value, including broader societal benefits, and the opportunity costs that exist for society by including the new intervention. As argued by others [Citation136], economic evaluations of vaccination should be considered alongside these measures rather than individually.
Figure 5. Applying the five BRAVE priority gaps to recognizing the broad value of PCVs against OM. Abbreviations: AMR, antimicrobial resistance; CEA, cost-effectiveness analysis; OM, otitis media; PCV, pneumococcal conjugate vaccine; QoL, quality of life.
Currently, there are data limitations for including broader societal impacts associated with OM prevention in CEAs of PCVs because these impacts have rarely been quantified. More country-level evidence on the broader impacts of OM and vaccination is especially needed in LMICs to defend future investments in vaccines and immunization programs [Citation135]. A challenge in building this evidence will be the collection of broad cost-offsets and population health data. However, innovative analyses of historical observational datasets could provide evidence to inform modeling assumptions – for example, data on the national incidence and prevalence of recurrent OM and CSOM over time. Studies linking changes in health to long-term behavioral and developmental outcomes have been suggested, although such studies should incorporate analysis techniques that reduce potential biases [Citation135]. Prospective follow-up studies are also needed to measure broader impacts of OM that go beyond the initial infection, which may provide valuable missing long-term clinical and economic data from patients and caregivers to inform CEAs. Moreover, web-based surveys could be conducted to examine how OM affects parents/caregivers QoL, out-of-pocket expenses, and productivity losses. Researchers will also need to consider whether ‘double counting’ may arise by including both productivity loss and QoL reduction, given the influence income reduction may have on utility scores. Acknowledging this, although income loss may somewhat affect utility scores, this does not offset the substantial societal cost due to a patient’s or caregiver’s productivity loss. Another priority is to generate high-quality evidence on the magnitude of PCV impact on AMR, which would likely require reinforced national surveillance of resistant infections [Citation137]. In the short-term, economic evaluations should acknowledge these data limitations and make informed assumptions regarding uncertain input parameters. For example, input parameters or ranges recommended by an expert panel could be used. Finally, the information needs of stakeholders from different sectors (including health and finance sectors, and external donors) should be collected to guide the incorporation of broader benefits into future economic evaluations and assist with their effective communication to decision makers.
Another challenge is that there are few active laboratory-based and population-based surveillance systems for OM. Consequently, the causal pathogen(s) and/or specific strain causing most OM cases is often unknown at a population level and incidence can vary across studies within a nation or region. Many economic analyses use modeling assumptions to overcome these data barriers. For example, studies will assume that a proportion of all OM is due to pneumococcus or that the underlying pneumococcal serotypes causing OM are the same as the invasive pneumococcal disease serotype distribution. Additional clinical studies and surveillance activities are needed to inform these assumptions and model the full burden of OM more accurately.
In the absence of data on vaccine effectiveness against broader OM outcomes, any estimates of these outcomes must also consider confounding factors. For example, various other factors may affect child speech and language development, AMR, and workforce productivity. Similarly, other interventions beside PCVs, such as influenza vaccines [Citation139], prevent a small proportion of OM outcomes and should be separated in model-based economic evaluations.
Both PCV13 and PCV10 have shown greater reductions than PCV7 in vaccine-type pneumococcal carriage, OM episodes, and OM-associated healthcare utilization through broader serotype coverage [Citation13–18,Citation40–42,Citation44,Citation45,Citation54,Citation55]. However, countries with established PCV NIPs have observed increased carriage and disease from non-vaccine serotypes that were initially less prevalent across all age groups [Citation18,Citation35,Citation140], including some serotypes with reduced antimicrobial susceptibility [Citation127,Citation141]. The potential licensure of new higher valent PCVs in the next few years will extend coverage to additional serotypes that have begun to emerge globally () [Citation142]. Specifically, a 15-valent PCV (PCV15; Merck) that includes serotypes 22F and 33F is expected to become available. However, as a new vaccine, its impact on OM will need to be ascertained through clinical trial and real-world studies. Additionally, a next-generation 20-valent PCV (PCV20; Pfizer) is anticipated to be licensed for pediatric use to offer even broader coverage of pneumococcal serotypes by extending PCV13 coverage to serotypes 8, 10A, 11A, 12F, 15B, 22F, and 33F. Future economic evaluations of these and other higher-valent vaccines will need to incorporate learnings from established PCV NIPs, for example national age-specific serotype trends for PCV and non-PCV serotypes. Higher-valent PCVs are expected to continue the decline in pneumococcal carriage, disease, death, and AMR. However, the magnitude of the decline will depend on their uptake in NIPs, which will in turn depend on providing decision makers with economic evaluations that communicate the full value of the vaccine program to society.
Declaration of interest
All authors are employees of and may hold stock or stock options in Pfizer Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Author contributions
All authors substantially contributed to the conception and design of the review article and interpreting the relevant literature, and all were involved in writing the review article or revised it for intellectual content.
Article highlights
Otitis media (OM) is one of the most common diseases in young children worldwide and a leading reason for physician visits and antibiotic use.
Most cases of OM resolve spontaneously, but recurrent and severe forms can have substantial long-term impacts such as hearing loss, impaired language and educational development, and lower patient and caregiver quality of life.
Pneumococcal conjugate vaccines (PCVs) have contributed to a decline in OM incidence and antimicrobial-resistant vaccine serotypes.
Economic evaluations of PCV pediatric national immunization programs traditionally measure only the direct, short-term health benefits and healthcare cost savings associated with acute OM, and therefore undervalue the full benefits of PCVs.
Several health and economic bodies have called for the broader impact of vaccines to be recognized and incorporated into cost-effectiveness analyses.
Including the full burden of OM in economic evaluations is important to communicate the full value of PCVs for informed decision making.
Reviewer disclosures
A reviewer has disclosed that they have previously received a grant from MSD to support analysis of our data on pneumococcal serotypes. Peer reviewers on this manuscript have no other relevant financial or other relationships to disclose.
Acknowledgments
Medical writing was provided by Dr. Jonathan Pitt (Evidera, Paris, France) and funded by Pfizer Inc.
Additional information
Funding
Notes
1. Though other manufacturers are developing PCV13 and PCV10 vaccines, in this article, PCV13 refers to the 13-valent PCV (Prevnar/Prevenar 13®) marketed by Pfizer, and PCV10 refers to the 10-valent PCV (Synflorix®) marketed by GlaxoSmithKline, unless stated otherwise.
References
- Schilder AG, Chonmaitree T, Cripps AW, et al. Otitis media. Nat Rev Dis Primers. 2016;2(1):16063.
- Rovers MM, Schilder AG, Zielhuis GA, et al. Otitis media. Lancet. 2004;363(9407):465–473.
- Arguedas A, Kvaerner K, Liese J, et al. Otitis media across nine countries: disease burden and management. Int J Pediatr Otorhinolaryngol. 2010;74(12):1419–1424.
- Tong S, Amand C, Kieffer A, et al. Trends in healthcare utilization and costs associated with acute otitis media in the United States during 2008-2014. BMC Health Serv Res. 2018;18(1):318.
- Monasta L, Ronfani L, Marchetti F, et al. Burden of disease caused by otitis media: systematic review and global estimates. PLoS One. 2012;7(4):e36226.
- Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160(1):83–94.
- Daly KA, Brown JE, Lindgren BR, et al. Epidemiology of otitis media onset by six months of age. Pediatrics. 1999;103(6):1158–1166.
- Lasisi AO, Sulaiman OA, Afolabi OA. Socio-economic status and hearing loss in chronic suppurative otitis media in Nigeria. Ann Trop Paediatr. 2007;27(4):291–296.
- Niclasen J, Obel C, Homøe P, et al. Associations between otitis media and child behavioural and learning difficulties: results from a Danish cohort. Int J Pediatr Otorhinolaryngol. 2016;84:12–20.
- Olatoke F, Ologe FE, Nwawolo CC, et al. The prevalence of hearing loss among schoolchildren with chronic suppurative otitis media in Nigeria, and its effect on academic performance. Ear Nose Throat J. 2008;87(12):E19.
- Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131(3):e964–999.
- Rosenfeld RM, Shin JJ, Schwartz SR, et al. Clinical practice guideline: otitis media with effusion executive summary (update). Otolaryngol Head Neck Surg. 2016;154(2):201–214.
- Marom T, Tan A, Wilkinson GS, et al. Trends in otitis media-related health care use in the United States, 2001-2011. JAMA Pediatr. 2014;168(1):68–75.
- Lau WC, Murray M, El-Turki A, et al. Impact of pneumococcal conjugate vaccines on childhood otitis media in the United Kingdom. Vaccine. 2015;33(39):5072–5079.
- Ben-Shimol S, Givon-Lavi N, Leibovitz E, et al. Near-elimination of otitis media caused by 13-valent pneumococcal conjugate vaccine (PCV) serotypes in southern Israel shortly after sequential introduction of 7-valent/13-valent PCV. Clin Infect Dis. 2014;59(12):1724–1732.
- Dagan R, Van Der Beek BA, Ben-Shimol S, et al. Effectiveness of the 7- and 13-valent pneumococcal conjugate vaccines against vaccine-serotype otitis media. Clin Infect Dis. 2021;73(4):650–658.
- Gisselsson-Solen M. Trends in otitis media incidence after conjugate pneumococcal vaccination: a national observational study. Pediatr Infect Dis J. 2017;36(11):1027–1031.
- Tin Tin Htar M, van Den Biggelaar AHJ, Sings H, et al. The impact of routine childhood immunization with higher-valent pneumococcal conjugate vaccines on antimicrobial-resistant pneumococcal diseases and carriage: a systematic literature review. Expert Rev Vaccines. 2019;18(10):1069–1089.
- Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. northern california kaiser permanente vaccine study center group. Pediatr Infect Dis J. 2000;19(3):187–195.
- Black SB, Shinefield HR, Ling S, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J. 2002;21(9):810–815.
- Fireman B, Black SB, Shinefield HR, et al. Impact of the pneumococcal conjugate vaccine on otitis media. Pediatr Infect Dis J. 2003;22(1):10–16.
- Centers for Disease Control and Prevention (CDC). Progress in introduction of pneumococcal conjugate vaccine–worldwide, 2000-2008. MMWR Morb Mortal Wkly Rep. 2008;57(42):1148–1151.
- Snape MD, Klinger CL, Daniels ED, et al. Immunogenicity and reactogenicity of a 13-valent-pneumococcal conjugate vaccine administered at 2, 4, and 12 months of age: a double-blind randomized active-controlled trial. Pediatr Infect Dis J. 2010;29(12):e80–90.
- Kieninger DM, Kueper K, Steul K, et al. Safety, tolerability, and immunologic noninferiority of a 13-valent pneumococcal conjugate vaccine compared to a 7-valent pneumococcal conjugate vaccine given with routine pediatric vaccinations in Germany. Vaccine. 2010;28(25):4192–4203.
- Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344(6):403–409.
- Zhao AS, Boyle S, Butrymowicz A, et al. Impact of 13-valent pneumococcal conjugate vaccine on otitis media bacteriology. Int J Pediatr Otorhinolaryngol. 2014;78(3):499–503.
- Lecrenier N, Marijam A, Olbrecht J, et al. Ten years of experience with the pneumococcal non-typeable Haemophilus influenzae protein D-conjugate vaccine (Synflorix) in children. Expert Rev Vaccines. 2020;19(3):247–265.
- Clarke E, Bashorun A, Adigweme I, et al. Immunogenicity and safety of a novel ten-valent pneumococcal conjugate vaccine in healthy infants in The Gambia: a phase 3, randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2021;21(6):834–846.
- Clarke E, Bashorun AO, Okoye M, et al. Safety and immunogenicity of a novel 10-valent pneumococcal conjugate vaccine candidate in adults, toddlers, and infants in The Gambia-Results of a phase 1/2 randomized, double-blinded, controlled trial. Vaccine. 2020;38(2):399–410.
- New pneumococcal vaccine from Serum Institute of India achieves WHO prequalification [Internet]. PATH; 2019. [cited 2020 Nov 19]. Available from: https://www.path.org/media-center/new-pneumococcal-vaccine-serum-institute-india-achieves-who-prequalification/
- Alderson MR, Sethna V, Newhouse LC, et al. Development strategy and lessons learned for a 10-valent pneumococcal conjugate vaccine (PNEUMOSIL(R)). Hum Vaccin Immunother. 2021;17(8):2670–2677.
- Chen JJ, Yuan L, Huang Z, et al. Safety and immunogenicity of a new 13-valent pneumococcal conjugate vaccine versus a licensed 7-valent pneumococcal conjugate vaccine: a study protocol of a randomised non-inferiority trial in China. BMJ Open. 2016;6(10):e012488.
- Guevara M, Ezpeleta C, Gil-Setas A, et al. Reduced incidence of invasive pneumococcal disease after introduction of the 13-valent conjugate vaccine in Navarre, Spain, 2001-2013. Vaccine. 2014;32(22):2553–2562.
- Loughlin AM, Hsu K, Silverio AL, et al. Direct and indirect effects of PCV13 on nasopharyngeal carriage of PCV13 unique pneumococcal serotypes in Massachusetts’ children. Pediatr Infect Dis J. 2014;33(5):504–510.
- Savulescu C, Krizova P, Lepoutre A, et al. Effect of high-valency pneumococcal conjugate vaccines on invasive pneumococcal disease in children in SpIDnet countries: an observational multicentre study. Lancet Respir Med. 2017;5(8):648–656.
- Ben-Shimol S, Givon-Lavi N, Leibovitz E, et al. Impact of widespread introduction of pneumococcal conjugate vaccines on pneumococcal and nonpneumococcal otitis media. Clin Infect Dis. 2016;63(5):611–618.
- Dagan R, Pelton S, Bakaletz L, et al. Prevention of early episodes of otitis media by pneumococcal vaccines might reduce progression to complex disease. Lancet Infect Dis. 2016;16(4):480–492.
- Kaplan SL, Center KJ, Barson WJ, et al. Multicenter surveillance of Streptococcus pneumoniae isolates from middle ear and mastoid cultures in the 13-valent pneumococcal conjugate vaccine era. Clin Infect Dis. 2015;60(9):1339–1345.
- Kaur R, Morris M, Pichichero ME. Epidemiology of acute otitis media in the postpneumococcal conjugate vaccine Era. Pediatrics. 2017;140(3):e20170181.
- Pichichero M, Kaur R, Scott DA, et al. Effectiveness of 13-valent pneumococcal conjugate vaccination for protection against acute otitis media caused by Streptococcus pneumoniae in healthy young children: a prospective observational study. Lancet Child Adolesc Health. 2018;2(8):561–568.
- Lewnard JA, Givon-Lavi N, Dagan R. Dose-specific Effectiveness of 7- and 13-Valent Pneumococcal Conjugate Vaccines Against Vaccine-serotype Streptococcus pneumoniae Colonization in Children. Clin Infect Dis. 2020;71(8):e289–e300.
- Ubukata K, Morozumi M, Sakuma M, et al. Etiology of acute otitis media and characterization of pneumococcal isolates after introduction of 13-valent pneumococcal conjugate vaccine in japanese children. Pediatr Infect Dis J. 2018;37(6):598–604.
- Tin Tin Htar M, Sings HL, Syrochkina M, et al. The impact of pneumococcal conjugate vaccines on serotype 19A nasopharyngeal carriage. Expert Rev Vaccines. 2019;18(12):1243–1270.
- Kawai K, Adil EA, Barrett D, et al. Ambulatory visits for otitis media before and after the introduction of pneumococcal conjugate vaccination. J Pediatr. 2018;201:122–127 e121.
- Zhou X, de Luise C, Gaffney M, et al. National impact of 13-valent pneumococcal conjugate vaccine on ambulatory care visits for otitis media in children under 5years in the United States. Int J Pediatr Otorhinolaryngol. 2019;119:96–102.
- Wiese AD, Huang X, Yu C, et al. Changes in otitis media episodes and pressure equalization tube insertions among young children following introduction of the 13-Valent pneumococcal conjugate vaccine: a birth cohort-based study. Clin Infect Dis. 2019;69(12):2162–2169.
- Suaya JA, Gessner BD, Fung S, et al. Acute otitis media, antimicrobial prescriptions, and medical expenses among children in the United States during 2011-2016. Vaccine. 2018;36(49):7479–7486.
- Howitz MF, Harboe ZB, Ingels H, et al. A nationwide study on the impact of pneumococcal conjugate vaccination on antibiotic use and ventilation tube insertion in Denmark 2000-2014. Vaccine. 2017;35(43):5858–5863.
- Dagan R, Juergens C, Trammel J, et al. Efficacy of 13-valent pneumococcal conjugate vaccine (PCV13) versus that of 7-valent PCV (PCV7) against nasopharyngeal colonization of antibiotic-nonsusceptible Streptococcus pneumoniae. J Infect Dis. 2015;211(7):1144–1153.
- Angoulvant F, Cohen R, Doit C, et al. Trends in antibiotic resistance of Streptococcus pneumoniae and Haemophilus influenzae isolated from nasopharyngeal flora in children with acute otitis media in France before and after 13 valent pneumococcal conjugate vaccine introduction. BMC Infect Dis. 2015;15:236.
- Hays C, Vermee Q, Agathine A, et al. Demonstration of the herd effect in adults after the implementation of pneumococcal vaccination with PCV13 in children. Eur J Clin Microbiol Infect Dis. 2017;36(5):831–838.
- Kempf M, Varon E, Lepoutre A, et al. Decline in antibiotic resistance and changes in the serotype distribution of Streptococcus pneumoniae isolates from children with acute otitis media; a 2001-2011 survey by the French Pneumococcal Network. Clin Microbiol Infect. 2015;21(1):35–42.
- Ben-Shimol S, Givon-Lavi N, Greenberg D, et al. Substantial reduction of antibiotic-non-susceptible pneumococcal otitis media following PCV7/PCV13 sequential introduction. J Antimicrob Chemother. 2020;75(10):3038–3045.
- Fortanier AC, Venekamp RP, Hoes AW, et al. Does pneumococcal conjugate vaccination affect onset and risk of first acute otitis media and recurrences? A primary care-based cohort study. Vaccine. 2019;37(11):1528–1532.
- Fortanier AC, Venekamp RP, Stellato RK, et al. Outpatient antibiotic use in Dutch infants after 10-valent pneumococcal vaccine introduction: a time-series analysis. BMJ Open. 2018;8(6):e020619.
- Prymula R, Peeters P, Chrobok V, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet. 2006;367(9512):740–748.
- Beissbarth J, Smith-Vaughan HC, Harris TM, et al. Use of the 10-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) in an Australian Indigenous paediatric population does not alter the prevalence of nontypeable Haemophilus influenzae without the protein D gene. Vaccine. 2019;37(30):4089–4093.
- Best EJ, Walls T, Souter M, et al. Pneumococcal vaccine impact on otitis media microbiology: a New Zealand cohort study before and after the introduction of PHiD-CV10 vaccine. Vaccine. 2016;34(33):3840–3847.
- Vesikari T, Forsten A, Seppa I, et al. Effectiveness of the 10-Valent pneumococcal nontypeable haemophilus influenzae protein D-Conjugated Vaccine (PHiD-CV) against carriage and acute otitis Media-A Double-Blind randomized clinical trial in Finland. J Pediatric Infect Dis Soc. 2016;5(3):237–248.
- van den Bergh MR, Spijkerman J, Swinnen KM, et al. Effects of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D-conjugate vaccine on nasopharyngeal bacterial colonization in young children: a randomized controlled trial. Clin Infect Dis. 2013;56(3):e30–39.
- Tregnaghi MW, Saez-Llorens X, Lopez P, et al. Efficacy of pneumococcal nontypable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in young Latin American children: a double-blind randomized controlled trial. PLoS Med. 2014;11(6):e1001657.
- Bärnighausen T, Bloom DE, Cafiero-Fonseca ET, et al. Valuing vaccination. Proc Natl Acad Sci U S A. 2014;111(34):12313–12319.
- Farkouh RA, Klok RM, Postma MJ, et al. Cost-effectiveness models of pneumococcal conjugate vaccines: variability and impact of modeling assumptions. Expert Rev Vaccines. 2012;11(10):1235–1247.
- Wasserman M, Sings HL, Jones D, et al. Review of vaccine effectiveness assumptions used in economic evaluations of infant pneumococcal conjugate vaccine. Expert Rev Vaccines. 2018;17(1):71–78.
- Wu DB-C, Chaiyakunapruk N, Chong H-Y, et al. Choosing between 7-, 10- and 13-valent pneumococcal conjugate vaccines in childhood: a review of economic evaluations (2006–2014). Vaccine. 2015;33(14):1633–1658.
- Bergman A, Hjelmgren J, Ortqvist Å, et al. Cost-effectiveness analysis of a universal vaccination programme with the 7-valent pneumococcal conjugate vaccine (PCV-7) in Sweden. Scand J Infect Dis. 2008;40(9):721–729.
- Chapman R, Sutton K, Dillon-Murphy D, et al. Ten year public health impact of 13-valent pneumococcal conjugate vaccination in infants: a modelling analysis. Vaccine. 2020;38(45):7138–7145.
- Chen C, Cervero Liceras F, Flasche S, et al. Effect and cost-effectiveness of pneumococcal conjugate vaccination: a global modelling analysis. Lancet Glob Health. 2019;7(1):e58–e67.
- Kulpeng W, Leelahavarong P, Rattanavipapong W, et al. Cost-utility analysis of 10- and 13-valent pneumococcal conjugate vaccines: protection at what price in the Thai context? Vaccine. 2013;31(26):2839–2847.
- Haasis MA, Ceria JA, Kulpeng W, et al. Do pneumococcal conjugate vaccines represent good value for money in a lower-middle Income Country? A cost-utility analysis in the Philippines. PLoS One. 2015;10(7):e0131156.
- Castiglia P, Pradelli L, Castagna S, et al. Overall effectiveness of pneumococcal conjugate vaccines: an economic analysis of PHiD-CV and PCV-13 in the immunization of infants in Italy. Hum Vaccin Immunother. 2017;13(10):2307–2315.
- Bloom DE, Kirby PN, Pugh S, et al. Commentary: why has uptake of pneumococcal vaccines for children been so slow? The perils of undervaluation. Pediatr Infect Dis J. 2020;39(2):145–156.
- Mauskopf J, Standaert B, Connolly MP, et al. Economic analysis of vaccination programs: an ispor good practices for outcomes research task force report. Value Health. 2018;21(10):1133–1149.
- WHO. guide for standardization of economic evaluations of immunization programmes. Edition II [Internet]. World Health Organization; 2019. [cited 2021 Jan 21]. Available from: https://apps.who.int/iris/bitstream/handle/10665/329389/WHO-IVB-19.10-eng.pdf?ua=1
- Ultsch B, Damm O, Beutels P, et al. Methods for health economic evaluation of vaccines and immunization decision frameworks: a consensus framework from a european vaccine economics community. Pharmacoeconomics. 2016;34(3):227–244.
- Aarhus L, Tambs K, Kvestad E, et al. Childhood otitis media: a cohort study with 30-year follow-up of hearing (The HUNT Study). Ear Hear. 2015;36(3):302–308.
- Krakau M, Dagoo BR, Hellstrom S, et al. Long-term hearing outcomes after recurrent acute otitis media during early childhood. Acta Otolaryngol. 2017;137(12):1238–1243.
- Avnstorp MB, Homoe P, Bjerregaard P, et al. Chronic suppurative otitis media, middle ear pathology and corresponding hearing loss in a cohort of Greenlandic children. Int J Pediatr Otorhinolaryngol. 2016;83:148–153.
- Jensen RG, Koch A, Homoe P. The risk of hearing loss in a population with a high prevalence of chronic suppurative otitis media. Int J Pediatr Otorhinolaryngol. 2013;77(9):1530–1535.
- da Costa SS, Rosito LP, Dornelles C. Sensorineural hearing loss in patients with chronic otitis media. Eur Arch Otorhinolaryngol. 2009;266(2):221–224.
- Olusanya BO, Somefun AO, Swanepoel de W. The need for standardization of methods for worldwide infant hearing screening: a systematic review. Laryngoscope. 2008;118(10):1830–1836.
- Elemraid MA, Brabin BJ, Fraser WD, et al. Characteristics of hearing impairment in Yemeni children with chronic suppurative otitis media: a case-control study. Int J Pediatr Otorhinolaryngol. 2010;74(3):283–286.
- Sanyelbhaa Talaat H, Hasn Kabel A, Qatanani E. Paediatric speech intelligibility (PSI) in normal hearing children with history of recurrent otitis media with effusion (OME). Audiol Med. 2009;7(2):112–119.
- Norowitz HL, Morello T, Kupfer HM, et al. Association between otitis media infection and failed hearing screenings in children. PLoS One. 2019;14(2):e0212777.
- Silveira Netto LF, da Costa SS, Sleifer P, et al. The impact of chronic suppurative otitis media on children’s and teenagers’ hearing. Int J Pediatr Otorhinolaryngol. 2009;73(12):1751–1756.
- Flynn T, Moller C, Jonsson R, et al. The high prevalence of otitis media with effusion in children with cleft lip and palate as compared to children without clefts. Int J Pediatr Otorhinolaryngol. 2009;73(10):1441–1446.
- Aarhus L, Homøe P, Engdahl B. Otitis media in childhood and disease in adulthood: a 40-year follow-up study. Ear Hear. 2020;41(1):67–71.
- Tierney S, O’Brien K, Harman NL, et al. Otitis media with effusion: experiences of children with cleft palate and their parents. Cleft Palate Craniofac J. 2015;52(1):23–30.
- Su JY, Guthridge S, He VY, et al. Impact of hearing impairment on early childhood development in Australian Aboriginal children a data linkage study. J Paediatr Child Health. 2020;56(10):1597–1606.
- Eapen RJ, Buss E, Grose JH, et al. The development of frequency weighting for speech in children with a history of otitis media with effusion. Ear Hear. 2008;29(5):718–724.
- Haapala S, Niemitalo-Haapola E, Raappana A, et al. Long-term influence of recurrent acute otitis media on neural involuntary attention switching in 2-year-old children. Behav Brain Funct. 2016;12(1):1.
- Shetty HN, Koonoor V. Sensory deprivation due to otitis media episodes in early childhood and its effect at later age: a psychoacoustic and speech perception measure. Int J Pediatr Otorhinolaryngol. 2016;90:181–187.
- Cai T, McPherson B. Hearing loss in children with otitis media with effusion: a systematic review. Int J Audiol. 2017;56(2):65–76.
- Talbird SE, Taylor TN, Knoll S, et al. Outcomes and costs associated with PHiD-CV, a new protein D conjugate pneumococcal vaccine, in four countries. Vaccine. 2010;28(6):G23–29.
- Strutton DR, Farkouh RA, Earnshaw SR, et al. Cost-effectiveness of 13-valent pneumococcal conjugate vaccine: Germany, Greece, and The Netherlands. J Infect. 2012;64(1):54–67.
- Delgleize E, Leeuwenkamp O, Theodorou E, et al. Cost-effectiveness analysis of routine pneumococcal vaccination in the UK: a comparison of the PHiD-CV vaccine and the PCV-13 vaccine using a Markov model. BMJ Open. 2016;6(11):e010776.
- Wu DB, Roberts C, Lee VW, et al. Cost-effectiveness analysis of infant universal routine pneumococcal vaccination in Malaysia and Hong Kong. Hum Vaccin Immunother. 2016;12(2):403–416.
- Homoe P, Heidemann CH, Damoiseaux RA, et al. Panel 5: impact of otitis media on quality of life and development. Int J Pediatr Otorhinolaryngol. 2020;1(30 Suppl 1(Suppl 1)):109837.
- Boruk M, Lee P, Faynzilbert Y, et al. Caregiver well-being and child quality of life. Otolaryngol Head Neck Surg. 2007;136(2):159–168.
- Grindler DJ, Blank SJ, Schulz KA, et al. Impact of otitis media severity on children’s quality of life. Otolaryngol Head Neck Surg. 2014;151(2):333–340.
- Indius JH, Alqaderi SK, Kjeldsen AD, et al. Middle ear disease in Danish toddlers attending nursery day-care - Applicability of OM-6, disease specific quality of life and predictors for middle ear symptoms. Int J Pediatr Otorhinolaryngol. 2018;110:130–134.
- Tao J, Schulz K, Jeffe DB, et al. Validations of the OM-6 Parent-Proxy Survey for Infants/Toddlers with Otitis Media. Otolaryngol Head Neck Surg. 2018;158(5):934–941.
- Crawford B, Hashim SS, Prepageran N, et al. Impact of pediatric acute otitis media on child and parental quality of life and associated productivity loss in malaysia: a prospective observational study. Drugs Real World Outcomes. 2017;4(1):21–31.
- Ryborg CT, Søndergaard J, Lous J, et al. Quality of life in children with otitis media–a cohort study. Fam Pract. 2014;31(1):30–37.
- Chow Y, Wabnitz DA, Ling J. Quality of life outcomes after ventilating tube insertion for otitis media in an Australian population. Int J Pediatr Otorhinolaryngol. 2007;71(10):1543–1547.
- Global Health Estimates. Disease burden by cause, age, sex, by country and by region, 2000-2019 [Internet]. World Health Organization; 2020. cited 2021 Dec 30 Available from: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/global-health-estimates-leading-causes-of-dalys
- Strens D, Knerer G, Van Vlaenderen I, et al. A pilot cost-of-illness study on long-term complications/sequelae of AOM. B-ent. 2012;8(3):153–165.
- Yen YC, Lin C, Weng SF, et al. Higher risk of developing sudden sensorineural hearing loss in patients with chronic otitis media. JAMA Otolaryngol Head Neck Surg. 2015;141(5):429–435.
- Jung H, Lee SK, Cha SH, et al. Current bacteriology of chronic otitis media with effusion: high rate of nosocomial infection and decreased antibiotic sensitivity. J Infect. 2009;59(5):308–316.
- Orji F. A survey of the burden of management of chronic suppurative otitis media in a developing country. Ann Med Health Sci Res. 2013;3(4):598–601.
- Pérez-Herrera LC, Peñaranda D, Moreno-López S, et al. Associated factors, health-related quality of life, and reported costs of chronic otitis media in adults at two otologic referral centers in a middle-income country. PLoS One. 2020;15(12):e0244797.
- Lee J, Witsell DL, Dolor RJ, et al. Quality of life of patients with otitis media and caregivers: a multicenter study. Laryngoscope. 2006;116(10):1798–1804.
- Barber C, Ille S, Vergison A, et al. Acute otitis media in young children - what do parents say? Int J Pediatr Otorhinolaryngol. 2014;78(2):300–306.
- Lappin O, Sibbring GC, Palin H-J, et al. Caregiver burden posed by paediatric acute otitis media: sub-set analysis of data from a wider literature review. 36th Annual Meeting of ESPID 2018; [2018 May 28 – June 2]; Malmö, Sweden.
- Blank SJ, Grindler DJ, Schulz KA, et al. Caregiver quality of life is related to severity of otitis media in children. Otolaryngol Head Neck Surg. 2014;151(2):348–353.
- Chando S, Young C, Craig JC, et al. Parental views on otitis media: systematic review of qualitative studies. Eur J Pediatr. 2016;175(10):1295–1305.
- Holl K, Rosenlund M, Giaquinto C, et al. The impact of childhood acute otitis media on parental quality of life in a prospective observational cohort study. Clin Drug Investig. 2015;35(10):613–624.
- Alsarraf R, Jung CJ, Perkins J, et al. Measuring the indirect and direct costs of acute otitis media. Arch Otolaryngol Head Neck Surg. 1999;125(1):12–18.
- Meherali S, Hartling L, Campbell A, et al. Parent information needs and experience regarding acute otitis media in children: a systematic review. Patient Educ Couns. 2021;104(3):554–562.
- Wolleswinkel-van den Bosch JH, Stolk EA, Francois M, et al. The health care burden and societal impact of acute otitis media in seven European countries: results of an Internet survey. Vaccine. 2010;28(6):G39–52.
- Wilson MR, Wasserman MD, Breton MC, et al. Health and economic impact of routine pediatric pneumococcal immunization programs in canada: a retrospective analysis. Infect Dis Ther. 2020;9(2):341–353.
- Berman S. Otitis media in developing countries. Pediatrics. 1995;96(1 Pt 1):126–131.
- American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics. 2004;113(5):1451.
- Okeke IN, Laxminarayan R, Bhutta ZA, et al. Antimicrobial resistance in developing countries. Part I: recent trends and current status. Lancet Infect Dis. 2005;5(8):481–493.
- DeAntonio R, Yarzabal JP, Cruz JP, et al. Epidemiology of otitis media in children from developing countries: a systematic review. Int J Pediatr Otorhinolaryngol. 2016;85:65–74.
- Mather MW, Drinnan M, Perry JD, et al. A systematic review and meta-analysis of antimicrobial resistance in paediatric acute otitis media. Int J Pediatr Otorhinolaryngol. 2019;123:102–109.
- Kaur R, Pham M, Koa Y, et al. Rising pneumococcal antibiotic resistance in the post-13-Valent pneumococcal conjugate vaccine era in pediatric isolates from a primary care setting. Clin Infect Dis. 2021;72(5):797–805.
- Bloom DE, Brenzel L, Cadarette D, et al. Moving beyond traditional valuation of vaccination: needs and opportunities. Vaccine. 2017;35(1):A29–A35.
- Alsan M, Bloom DE, Canning D. The effect of population health on foreign direct investment inflows to low- and middle-income countries. World Dev. 2006;34(4):613–630.
- Oskorouchi HR, Sousa-Poza AS, Bloom DE. The long-term cognitive and schooling effects of childhood vaccinations in China. NBER Working Paper Series: Working Paper 27217. Cambridge (MA): National Bureau of Economic Research (US); 2020.
- Global vaccine action plan. Monitoring, evaluation & accountability. Secretariat annual report 2017 [Internet]. World Health Organization; 2017. cited 2021 Nov 19 Available from: http://www.who.int/immunization/global_vaccine_action_plan/web_gvap_secretariat_report_2017.pdf?ua=1
- Bishai D, Koenig M, Ali Khan M. Measles vaccination improves the equity of health outcomes: evidence from Bangladesh. Health Economics. 2003;12(5):415–419.
- Raman R, Brennan J, Ndi D, et al. Marked reduction of socioeconomic and racial disparities in invasive pneumococcal disease associated with conjugate pneumococcal vaccines. J Infect Dis. 2021;223(7):1250–1259.
- Petousis-Harris H, Howe AS, Paynter J, et al. Pneumococcal conjugate vaccines turning the tide on inequity: a retrospective cohort study of new zealand children born 2006–2015. Clin Infect Dis. 2019;68(5):818–826.
- Deogaonkar R, Hutubessy R, van der Putten I, et al. Systematic review of studies evaluating the broader economic impact of vaccination in low and middle income countries. BMC Public Health. 2012;12(1):878.
- Jit M, Hutubessy R, Png ME, et al. The broader economic impact of vaccination: reviewing and appraising the strength of evidence. BMC Med. 2015;13(1):209.
- Bell E, Neri M, and Steuten L. THE BRAVE INITIATIVE. The BRAVE narrative for broad recognition of value in vaccines engagement. OHE Research Paper. London (UK): Office of Health Economics (UK); 2020.
- Bloom DE, Cadarette D, and Ferranna M. The Societal Value of Vaccination in the Age of COVID-19. Am J Public Health. 2021;111(6):1049–1054.
- Norhayati MN, Ho JJ, Azman MY. Influenza vaccines for preventing acute otitis media in infants and children. Cochrane Database Syst Rev. 2017;10(10):Cd010089.
- Tin Tin Htar M, Christopoulou D, Schmitt HJ. Pneumococcal serotype evolution in Western Europe. BMC Infect Dis. 2015;15:419.
- Rybak A, Levy C, Bonacorsi S, et al. Antibiotic resistance of potential otopathogens isolated from nasopharyngeal flora of children with acute otitis media before, during and after pneumococcal conjugate vaccines implementation. Pediatr Infect Dis J. 2018;37(3):e72–e78.
- Masomian M, Ahmad Z, Gew LT, et al. Development of next generation streptococcus pneumoniae vaccines conferring broad protection. Vaccines (Basel). 2020;8(1):132.