ABSTRACT
Introduction
Rotaviruses (RVs) cause acute gastroenteritis (AGE) in infants and young children worldwide and also in older adults (≥60 years), however the burden among this age group is not well understood. Herd immunity through pediatric RV vaccination may reduce the burden of RVGE across all ages, however the impact of pediatric vaccination on burden in older adults is poorly understood.
Areas covered
This systematic review was undertaken to identify studies related to the following objectives: understand the burden of RV in older adults, RV seroprevalence, and the impact of pediatric vaccination on this burden and highlight evidence gaps to guide future research. Of studies identified, 59 studies from two databases were included in this analysis following a review by two reviewers.
Expert opinion
RV is an understudied disease in older adults. We found that 0–62% of patients with AGE tested positive for RV, with results varying by setting, country, and patient age. Results also suggest that pediatric vaccination benefits older adults through herd protection. Several studies showed a reduction in RV incidence after vaccination. However, there was variety in results and lack of consistency in outcomes reported. Further studies targeting older adults are needed to better characterize RV burden.
1. Introduction
Rotaviruses (RVs), forming a genus of the Reoviridae family, are a major cause of acute gastroenteritis (AGE), mainly characterized by diarrhea, vomiting, and fever [Citation1,Citation2]. If untreated, rotavirus gastroenteritis (RVGE) can be fatal [Citation1]. RVs are ubiquitous; prior to the introduction of RV vaccination, 95% of the children would contract RV and experience symptoms of RVGE by 5 years of age [Citation3]. As a result, RV is recognized as the leading causative agent of acute gastroenteritis (AGE) in infants and young children (<5 years of age) worldwide, responsible for 185 thousand deaths in children <5 globally in 2017 [Citation4–6].
In 2016, approximately 4.48 billion diarrhea episodes and 1.66 million diarrhea-related deaths were recorded worldwide across all age groups [Citation4]. Diarrhea and AGE can be caused by a variety of pathogens, including RVs, noroviruses, sapoviruses, astroviruses, and entero-viruses [Citation7,Citation8]. While the role of RV in causing AGE is well documented in the pediatric population, its role in causing AGE among older adults is less well understood [Citation7]. Since 2006, over 100 countries have introduced RV vaccination into their National Immunization Programs (NIP) [Citation9]. The introduction of RV vaccination has had a sustained impact on RV cases and AGE-related hospitalizations and deaths among children, with a median reduction of 59% in RV hospitalizations in children <5 [Citation9]. While the introduction of pediatric RV vaccination into NIPs has been found to prevent RV disease in older children due to vaccine-induced herd immunity (e.g., indirect protection), the impact in older adults, who are at increased risk of severe outcomes associated with AGE, is not as well established [Citation10,Citation11]. In 2016, RV was the leading etiology for diarrhea mortality among all ages (or for 14% of all diarrhea deaths), and projected to be responsible for up to 8% of diarrhea deaths among those >70 [Citation4,Citation12–14]. Notably, RVGE among older adults typically occurs in community settings, with outbreaks in long-term care facilities associated with substantial morbidity and mortality [Citation15].
This review was undertaken to assess the available evidence relating to the global burden of RVGE in older adults (≥60 years of age), and the impact of pediatric RV vaccination on this burden in older adults. Results will be of interest to public health decision-makers interested in understanding the impact of pediatric vaccination on the burden of RVGE in older adults, and infectious disease modelers trying to quantify RV transmission.
2. Methods
Medline and Embase databases were searched via the OVID platform on 11 March 2021, for surveillance studies and real-world observational research published between January 2000 and March 2021. The search strategy was created in accordance with standard PRISMA and Cochrane guidelines [Citation16,Citation17], and employed a combination of keywords and medical subject heading (MeSH) terms pertaining to RV, RVGE, RV vaccines,and older adults. These terms were combined with Boolean operators to produce a comprehensive search strategy (Supplementary Table 1). To ensure that all relevant information was captured, a search of the following conference proceedings was also conducted from January 2019 to March 2021: International Society for Pharmacoeconomics and Outcomes Research (ISPOR), European Society for Pediatric Infectious Diseases (ESPID), and the International Rotavirus Symposium.
All identified references were reviewed for inclusion at the title and abstract screening stage by two reviewers using the population, comparators, outcomes, and study design (PICOS) framework (Supplementary Table 2). Among other considerations, publications were included if they: (1) were related to RV disease OR RV vaccination; (2) reported results for older adults ≥60 years of age (or where results related to older adults were disaggregated from the wider population); and (3) reported outcomes related to disease burden such as hospitalization or outpatient incidence, impact of vaccination, or prevalence of RV among AGE cases. Studies were excluded if they: (1) contained results related to older adults that were not disaggregated from the general population; (2) reported AGE without an indication of the percentage caused by RVGE; (3) reported on RV strains among animals (RV C); and (4) took the form of case studies, editorials, or reviews. Publications included at this stage underwent full-text screening by two reviewers using the same PICOS criteria. All discrepancies identified were resolved by consensus via a third researcher.
Data were extracted on the epidemiology, clinical, and economic burden of RVGE among older adults before and after the introduction of pediatric vaccination, with no limit on geography. Data from articles included at the full-text screening stage were extracted into a data extraction form in Microsoft Excel by one reviewer and checked for accuracy by a second reviewer. Data were then checked by a senior reviewer.
A quality assessment was conducted on all included publications based on the National Institute for Health and Care Excellence (NICE) quality assessment for qualitative studies, to ensure robustness and relevance to the study objectives [Citation18].
3. Results
3.1. Search results
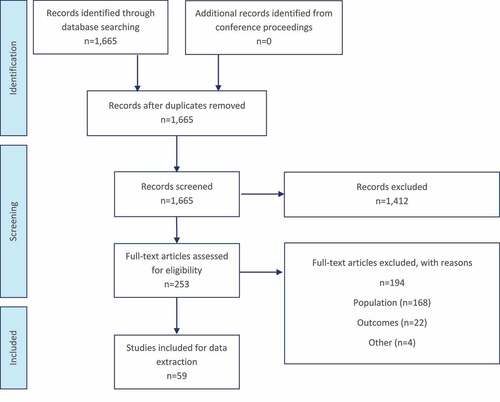
The search, run on 11 March 2021, identified 1,665 articles, of which 253 were taken forward to full-text review. No additional publications were identified from conference proceedings. After screening against the eligibility criteria, 59 publications were included for data extraction and analysis ().
Studies were identified from 26 countries, with most studies conducted in the US (n = 8) [Citation19–26], Australia (n = 6) [Citation27–32], the UK (n = 6) [Citation33–38], and China (n = 5) [Citation39–43]. Most of the studies were database/registry analyses (n = 16) [Citation19,Citation22–25,Citation29,Citation30,Citation34,Citation35,Citation40,Citation44–49], which used a total of 21 databases, including routine surveillance and hospitalization records.
The most commonly reported age groups were ≥60 years of age (n = 18) [Citation43,Citation44,Citation49–64], followed by ≥65 years of age (n = 17) [Citation19–21,Citation23,Citation27,Citation30,Citation34–36,Citation41,Citation46,Citation65–70]. Of note, a total of 12 studies reported the overall sample size but did not clearly report the sample size of subgroups of interest, e.g., those aged ≥60 years [Citation22,Citation26,Citation27,Citation30,Citation32,Citation35,Citation38,Citation45,Citation46,Citation57,Citation61,Citation63,Citation64]. Furthermore, nine publications stratified the sample size into further subgroups, i.e., ≥70 years of age or ≥80 years of age [Citation22,Citation25,Citation26,Citation32,Citation33,Citation38,Citation42,Citation45,Citation48,Citation71].
3.2. Burden of RV and RVGE in older adults (≥60 years of age)
3.2.1. Prevalence of RV among AGE cases
Overall, 31 publications reported the prevalence of RV among AGE cases in older adults (≥60 years of age; Supplementary Table 3) [Citation4,Citation20,Citation25,Citation26,Citation28,Citation31,Citation33,Citation39–43,Citation48–50,Citation52–54,Citation56–59,Citation61,Citation62,Citation66,Citation68,Citation69,Citation72–75]. Prevalence varied between 0% and 62%, with results differing by country. Of these studies, 12 reported the prevalence of RV in adults ≥60 years of age, while others reported prevalence in older age-groups or stratified results by age-group. RV prevalence in those ≥60 years ranged from 0% to 33% [Citation43,Citation49,Citation50,Citation52–54,Citation56–59,Citation61,Citation62]. Only one study by McAuliffe et al. reported a prevalence of 0% [Citation49]. The authors noted that the small number of positive tests was likely explained by the study period, which fell outside the annual peak season of July to September [Citation49].
Two studies reported the prevalence of RV among AGE cases in older adults by age subgroups (e.g., 60–70, 70–80) [Citation26,Citation42]. The prevalence of RV increased slightly with age. Wang et al. reported a prevalence of 6.6% for the age group 60–69 years, and 7.9% for ≥70 years [Citation42]. Similarly, Wilhelm et al. reported a prevalence of 1.0% for the age group 60–69 years, increasing to 3.5% for 70–79 years [Citation26].
One study reported the prevalence of RV among AGE cases for the same age group at different time points [Citation52]. In India, Tatte et al. reported a significant increase for the same age group at different times: from 5.2% between 1993 and 1996 to 17.2% between 2004 and 2007 (P < 0.001) [Citation52].
3.2.2. Clinical burden
Five studies reported RVGE incidence rates for older adults by care setting (hospitalized and outpatient) () [Citation21,Citation25,Citation32,Citation45,Citation55]. Three of these determined the incidence rate through the analysis of stool samples collected from patients hospitalized with AGE [Citation21,Citation25,Citation32]. The incidence rate of RVGE was typically higher in hospitalized older age groups (≥85 years of age) [Citation32,Citation45]. Donato et al. and Morton et al. reported an increase of 53% and 93% in incidence rates for adults ≥85 years of age compared to adults aged <85 years, respectively [Citation32,Citation45]. Furthermore, Cardemil et al. reported that the incidence of RVGE was lower among outpatients than inpatients (6.6 versus 51.7 100,000 person-years) [Citation21].
Table 1. Incidence rates of RVGE in adults ≥60 years of age (n = 5)
3.2.3. Seroprevalence
Two studies reported the seroprevalence of RV serotype A (RV A) among older adults (≥60 years of age; ) [Citation38,Citation51]. Seroprevalence was determined through analysis of serum samples from various populations including outpatients of general hospitals, the general population, and inpatients without AGE.
Table 2. Seroprevalence of antibodies to RV pre-vaccine introduction in adults ≥60 years of age (n = 2)
In a study by Seo et al., RV A IgG seroprevalence was 100% at all timepoints in South Korea from 1989 to 2005 in adults >60 years of age [Citation51]. In contrast, Cox et al. reported that the IgM seroprevalence of RV A varied across age groups in the pre-vaccination period in the UK, at 0.145, 0.190.195, 0.176 among those aged 60–69 years, 70–79 years, and 80–89 years, respectively, with the greatest prevalence reported in 70–79 years (in log-units) [Citation38]. The authors defined seropositivity as >1.0 log antibody units [Citation38].
3.2.4. Healthcare resource utilization
Four studies reported the duration of hospital stay for patients with RVGE [Citation23,Citation44,Citation55,Citation76]. Cardemil et al. reported a median hospital stay of 5.5 days for patients ≥77 years () [Citation76]. The majority of patients in these studies only had RVGE and were noted to have ‘no or few pre-existing health disorders’ [Citation44].
Table 3. Duration of inpatient stay of patients with RVGE aged ≥60 years
Beck-Friis et al. reported the longest median stay of 19 days, which was for hospital acquired RVGE in Sweden [Citation44]. Of note, Beck-Friis estimated hospital acquired RVGE to be associated with a case-fatality rate of 8.6% compared to 0.8% for community acquired RVGE [Citation44]. It was noted that hospital-onset RV infections were typically seen in individuals with multiple comorbidities [Citation44].
Three studies reported on the cost of RVGE hospitalization (Supplementary Table 4) [Citation22,Citation45,Citation77]. Morton et al. reported pooled data from hospitalization records in the pre-vaccination period (2006 to 2011) in Canada and estimated a mean cost of up to $1.8 million per year for RVGE hospitalizations among older adults aged 65–84 years and up to $571,448 per year among those aged ≥85 years [Citation45]. Lopman et al. pooled data in the pre-vaccination and post-vaccination period from the US (1996 to 2007) to estimate a total cost of RVGE hospitalizations per year of up to $33 million (2007 USD) for patients aged 75–84 years [Citation22]. Additionally, Piednoir et al. reported the total direct hospital costs for an RV outbreak at a long-term care facility in France in 2008 to be €17,959 or €285 per patient (Supplementary Table 5) [Citation77].
3.3. RVGE in older adults before and after vaccine introduction
The incidence of RVGE among adults ≥65 years of age before and after vaccine introduction varied across included studies () [Citation34,Citation35,Citation46,Citation60]. One database study reported RVGE outpatient incidence before and after vaccination among adults ≥65 years of age in Germany [Citation46]. One modeling study estimated incidence among adults >60 years of age using a predictive model in Germany [Citation60]. The remaining studies reported RVGE hospitalization incidence before and after vaccine introduction in the UK (n = 2), Canada (n = 2), the US (n = 3), Australia (n = 3), and Ireland (N = 1) [Citation19,Citation23,Citation24,Citation27,Citation29,Citation30,Citation34,Citation35,Citation46,Citation47,Citation67,Citation78].
Table 4. Impact of RV vaccination on AGE and RVGE burden in adults aged ≥60 years
In a 2020 national surveillance study that included laboratory-confirmed outpatient RVGE cases, the authors reported that outpatient RVGE incidence was reduced from 32.1 to 20.2 per 100,000 persons per year in Germany from the pre- to post-vaccination period [Citation46]. This finding contrasts with modeled results in 2014 by Weidemann et al., using data from the communicable disease reporting system for Germany and an infectious disease model, that predicted that mass vaccination would lead to an increase in RVGE incidence among older adults due to decreased natural immunity in this age-group [Citation60].
In the UK, two studies (Thomas et al. [2017] and Atchison et al. [2016]) reported that the incidence rate ratio (RR) for RVGE hospitalizations after vaccine introduction was 0.94 and 0.86 respectively, when compared to the pre-vaccination period, indicating a reduction in RVGE hospitalizations after vaccine introduction when adjusting for yearly care seeking trends [Citation34,Citation35].
In Canada and the US, the estimated incidence RR suggested a decrease in the rate of RVGE hospitalizations among those 65 and above following vaccine introduction, when accounting for secular trends in the increase of rate of AGE hospital admissions [Citation19,Citation23,Citation24,Citation47,Citation67]. Additionally, the two studies conducted in Canada suggested a slight reduction in the monthly rate of AGE-related ED and outpatient visits in the post-vaccination period [Citation47,Citation67]. However, the hospitalization rate increased among older adults from the pre- to the post-vaccination period in Australia (n = 3) [Citation27,Citation29,Citation30]. Authors noted that this increase could be due to increased testing after RV vaccine introduction.
In Ireland, Yandle et al. reported a significant decrease in the prevalence among adults aged ≥65 years between 2015 (1.86%) and 2019 (0.56%; P < 0.001) [Citation78].
One study reported on the costs of RVGE hospitalization averted post-vaccination in the US [Citation23]. Lopman et al. analyzed hospital inpatient data associated with AGE and RVGE for patients aged ≥65 years from 2000 to 2008 and reported a median cost of RVGE hospitalization averted following vaccination of $10,260 per patient [Citation23].
3.4. Quality assessment
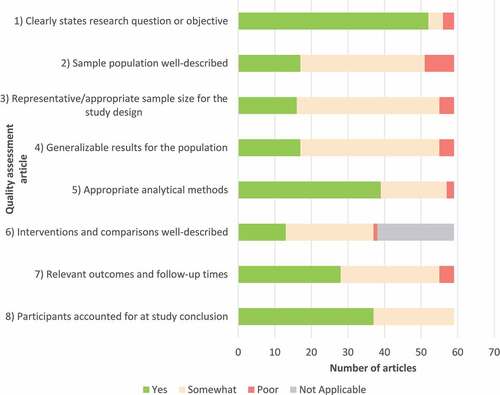
All studies identified in this review described the data sources in sufficient detail to determine the study rationale, define the study sample, intervention and outcomes, and steps taken to analyze the outcomes ().
Of the 59 included studies, the majority (n = 52) clearly described the research question or objective. Most included studies (n = 39) clearly described the analytical methods used to evaluate RV outcomes among the sample population.
Although many studies defined the outcomes of the research, these were not consistently defined. Many of the studies identified only reported one outcome of interest, usually as secondary or exploratory outcomes, rather than the primary outcome of the research. Outcomes reporting on older adults (≥60 years) were often less well defined than the primary sample or were missing complete demographic data and overall, there was a lack of well-designed studies specifically assessing the burden of RV among older adults (≥60 years).
4. Discussion
In this systematic literature review, we reviewed 59 publications that provided evidence on RV burden, seroprevalence, and possible vaccination impact in older adults ≥60 years of age. The incidence rate of RVGE was generally higher in older age groups (≥85 years of age) and also higher in inpatients versus outpatients [Citation21,Citation45]. Our results suggest that the burden of RV is not insignificant in older adults. In this review, up to 33% of all AGE in adults ≥60 years old was caused by RV, with prevalence increasing to 62% in one study for adults 65 and above [Citation31]. Results varied greatly between countries. Additionally, community-acquired RVGE was associated with hospitalizations lasting 4–5 days [Citation23,Citation76], a similar duration to that reported among children with RVGE [Citation79,Citation80]. However, one study reported length of stay associated with nosocomial transmission to be 19 days in older adults with a case-fatality rate of 8.6% compared to 0.8% for community-acquired RVGE [Citation44]. RV mortality increases among those with hospital-acquired infection, indicating the role of other co-morbidities among older adults with RVGE [Citation44]. Our results also suggest that pediatric RV vaccination can confer beneficial effect to older adults through herd immunity.
Our results are in line with evidence found by a recent systematic literature review of RV prevalence among older children and adults [Citation81]. Our review captured the majority (N = 9/14) of the same papers included for older adults for the same time horizon, with only those that had broad age ranges, without age disaggregated data being excluded in this review. However, our review was broader and also captured publications reporting on clinical and economic outcomes among older adults. Arakaki et. al. also found large variations in the prevalence of RV in older adults, which they defined as individuals older than 50 years of age [Citation81]. The authors reported an RV positivity in AGE patients between 1% and 15% [Citation81]. However, older individuals remain an understudied group, with >50% of the AGE in this age-group being due to unspecified causes after testing for multiple pathogens in multi-country studies [Citation40,Citation81,Citation82].
The majority of studies that analyzed hospitalization rates due to RVGE before and after pediatric RV vaccine introduction among those 60 and above suggested that pediatric vaccination reduced the burden in older age-groups, which was likely due to herd immunity [Citation19,Citation23,Citation24]. An Austrian surveillance study reported that following the introduction of universal RV vaccination for all infants, decreasing hospitalization rates were observed in children of all age groups, including those ineligible for vaccination [Citation83]. Similarly, surveillance data from Australia and the US has shown that infant vaccination resulted in reduced cases and hospitalizations, respectively [Citation24,Citation84,Citation85]. This suggests that herd protection may be conferred to non-vaccinated groups, including older adults [Citation24,Citation83–85]. Three studies from Australia reported an increase in hospitalization rate post-vaccination, which the authors noted could have been due to increased awareness and willingness to test for RV in the post-vaccination age. Variations among countries may further be driven by differences in contact patterns and household structure [Citation19,Citation23,Citation24]. A study by Dorleans et al. in Denmark revealed that contact with children was a risk factor to develop RV infection among adults [Citation86]. Several household studies reported RV transmission within households affecting older children and adults [Citation87–89].
There are several key strengths and limitations to this study. The search was conducted in a robust and comprehensive manner in accordance with standard Cochrane and PRISMA guidelines [Citation16,Citation17], and captured global data. The search was also conducted over a wide time frame to ensure that all key publications were captured across both the pre- and post-vaccination periods. Of note, we only included articles published in the English language in this study, potentially leading to the exclusion of relevant articles in other languages and thus introducing reporting bias.
A limitation of this study is that we did not search explicitly for outbreaks in long-term care facilities for the elderly. However, RVGE outbreaks are frequent in elderly care facilities, with RV being related to 11% of AGE outbreaks in elderly care facilities in England and 19% in France by Inns et al. and Barrett et al., respectively [Citation15,Citation90]. These outbreaks can be associated with significant morbidity and mortality but estimates of disease burden can be imprecise because RV testing is rarely performed [Citation15]. The absence of these search terms related to outbreaks within this review may thus lead to an underestimation of the burden of disease within this population.
Additionally, this literature review only evaluated the burden of RVGE associated with group A RV, as this is the predominant strain of RV that infects humans. Studies have investigated other species such as RV C which are most commonly isolated in animals, including pigs and dogs, with the aim of establishing the risks of this serotype to humans when transmitted from animals [Citation37,Citation64,Citation71]. Nilsson et al. and Iturriza-Gomara et al. reported seroprevalence of RV C in the general population of adults aged 61–70 years of 30% and 46% in Sweden and the UK, respectively [Citation37,Citation71]. These studies reveal that the burden of RV C should not be overlooked when considering the burden of RV. However, as RV C is usually only considered in the context of animal infections these studies fall outside the scope of this review.
A further potential limitation of the studies included in this review is the use of serology to determine the prevalence of RV among older adults. Lewnard et al. reported that acquired serum IgA and IgG titers are associated with partial protection against infection and therefore differences in primary-, secondary- and subsequent infections may confound serology data used to calculate incidence rates [Citation91].
The main challenge encountered, which is not a limitation of this study but a characteristic of the scientific landscape, relates to the current lack of studies in this specific area. There were very few included articles with the primary objective of exploring the burden of RV in older adults. The high level of heterogeneity between studies in terms of the outcomes measured and the definitions of age groups limited comparisons. Given the potential adverse effects of AGE among older adults, additional research on the burden of RV in this population is needed. Furthermore, despite the global scope of this study, this review predominantly identified publications from high-income countries in Asia-Pacific, Europe, and North America. This review did not apply limits based on country or region; therefore, this suggests a lack of observational evidence on RVGE among older adults in low- and middle-income countries. There is, however, evidence to suggest that the burden would not be negligible. A household study from Ecuador showed that once a child has RVGE, other family members are affected as well [Citation87]. While additional studies exploring the incidence and prevalence of RV in older adult age groups are needed, we found that RV imposes a clinical burden on older individuals, and that pediatric vaccination may have a beneficial impact on those ≥60 years of age through herd immunity.
5. Expert opinion
This review summarized evidence on RVGE burden and RV pediatric vaccination impact in older adults, which can assist in understanding the overall disease burden among older adults and demonstrating the potential value of pediatric vaccination programs. We found high variation in the epidemiological burden of RV in older adults and suggestive evidence on the benefits of pediatric vaccination in older adults through herd protection, given large database studies and studies in which the RV prevalence in stool samples of older adults from the pre-to-post vaccination period decreased. Notably, RV prevalence varies greatly with the setting, country, and patient age.
Quantifying the existing burden of RVGE among older adults across countries can assist in appropriately planning and managing healthcare use in this age group [Citation4]. Older adults are at increased risk of adverse outcomes connected to AGE [Citation4]. As a result, determining the etiology of diarrhea may impact treatment and outcomes [Citation4]. However, both the current review and a previous review by Arakaki et al. [Citation81] have noted that the percentage of diarrhea caused by RV varies regionally, which decreases the generalizability of country-specific findings. Both reviews found that there was substantial heterogeneity in the prevalence of RV among older adults [Citation81]. RV positivity ranged from 1% to 15%, however >50% of the AGE in this age-group was due to unspecified causes after testing for multiple pathogens in multi-country studies [Citation40,Citation81,Citation82]. That is, without testing, it may be difficult to evaluate regional and country-specific prevalence estimates of RV. Of note, an outbreak study in France reported that laboratory testing was only performed for 56% of outbreaks that occurred in long-term care facilities between 2010 and 2012 [Citation15].
Evidence suggests that the introduction of RV vaccination in children caused a decrease in RV incidence among older adults in the post-vaccination era. Furthermore, evidence of herd immunity has previously been observed in studies among infants and children, following the introduction of universal mass vaccination programs in several countries. For example, analysis of a national US medical claims database by Mast et al. indicated that there was a sustained and substantial decrease in the seasonal RV medical claims pattern after the introduction of RV vaccination in infants [Citation92]. Furthermore, Paulke-Korinek et al. reported decreasing hospitalization rates among children of all age groups following the introduction of universal mass vaccination in Austria, despite only infants being eligible for vaccination [Citation83]. However, herd immunity needs to be investigated further in lower-income countries, as most studies have been conducted in high-income countries.
This review suggested that pediatric vaccination may benefit older adults through herd protection. In Canada and the US, studies suggested a decrease in the rate of RVGE hospitalizations among those 65 and above following vaccine introduction, after accounting for secular trends in the increase of AGE hospital admissions [Citation19,Citation23,Citation24,Citation47,Citation67]. Similarly, studies in Europe reported a decreased RVGE incidence from the pre- to the post-vaccination period [Citation34,Citation35,Citation46,Citation78]. These results may be confounded by a lack of historical RV testing in some populations [Citation27,Citation29,Citation30,Citation47,Citation67]. Of note, several household studies have shown RV transmission within households [Citation87–89]. Therefore, and depending on the household structure, RV vaccination may contribute to a reduction in the SECincidence of RVGE among family contacts, reducing the burden of RV on healthcare services [Citation36]. SEC Our results both emphasize the importance of RV testing in the elderly, and the potential impact of pediatric vaccination in reducing the burden of RV among older adults.
Article highlights
RV prevalence in older adults with diarrhea varies with the setting, country, and patient age.
Seroprevalence of RVA varied greatly across age groups in the pre-vaccination period.
Evidence suggests that the introduction of RV vaccination in children caused a decrease in RV incidence among older adults in the post-vaccination era.
There is a lack of well-designed studies assessing the burden of RV among older adults (≥60 years), and few studies were designed with the primary objective of exploring the burden of RV in older adults.
Future research exploring the burden on RV in older adults may be relevant.
Author contributions
A Karakusevic, P Devaney, A Enstone, N Kanibir, S Hartwig, and C Carias contributed to study conception and study design. All authors contributed to the writing of the manuscript. All authors reviewed and approved the final version of the manuscript.
Declaration of interest
N Kanibir, S Hartwig, and C Carias are employees of MSD subsidiaries of Merck & Co., Inc., Kenilworth, NJ, USA, the manufacturer of the rotavirus vaccine RotaTeqTM (Rotavirus Vaccine, Live, Oral, Pentavalent), and may own stocks and/or stock options. A Karakusevic, P Devaney, and A Enstone are employees of Adelphi Values PROVE™. Adelphi Values PROVE™ was compensated by MSD for the conduct of the study and development of the manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium for their review work. Peer reviewers on this manuscript have no other relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (86.3 KB)Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Written requests can be sent to the corresponding author.
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Crawford SE, Ramani S, Tate JE, et al. Rotavirus infection. Nat Rev Dis Primers. 2017;3:17083.
- Estes MK, Greenberg HB. Rotaviruses. In: Knipe D, PM H, editors. Fields Virology. 6th Edition ed. Philadelphia: Wolter Kluwer Health/Lippincott Williams & Wilkins; 2013. p. 1347–1401.
- Atkinson W. Epidemiology and prevention of vaccine-preventable diseases. Washington, D.C.: Department of Health & Human Services, Centers for Disease Control and Prevention; 2006.
- Troeger C, Blacker BF, Khalil IA, et al., Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the global burden of disease study 2016. Lancet Infect Dis. 18(11): 1211–1228. 2018.
- Thirteenth International Rotavirus Symposium. Paper presented at: Rotavirus Symposium; 29th-31st August 2018; Minsk, Belarus.
- Roth GA, Abate, D., Abate, K. H, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1736–1788.
- Carias C, Hartwig S, Kanibir MN, et al. 1381. Rotavirus Gastroenteritis among older adults: discussion based on a systematic literature review. Open Forum Infect Dis. 2020;7(Suppl 1):S700–1.
- Desselberger U. Viral gastroenteritis. Med (Abingdon). 2017;45(11):690–694
- Burnett E, Parashar UD, Tate JE. Global impact of rotavirus vaccination on diarrhea hospitalizations and deaths among children <5 years old: 2006–2019. J Infect Dis. 2020;222(10):1731–1739.
- Hall AJ, Rosenthal M, Gregoricus N, et al. Incidence of acute gastroenteritis and role of norovirus, Georgia, USA, 2004-2005. Emerg Infect Dis. 2011;17(8):1381–1388.
- Anderson EJ, Shippee DB, Weinrobe MH, et al. Indirect protection of adults from rotavirus by pediatric rotavirus vaccination. Clin Infect Dis. 2013;56(6):755–760.
- Keddy KH. Old and new challenges related to global burden of diarrhoea. Lancet Infect Dis. 2018;18(11):1163–1164.
- G. B D. Mortality Causes of Death, C., Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–171.
- Markkula J, Hemming-Harlo M, Salminen MT, et al. Rotavirus epidemiology 5–6 years after universal rotavirus vaccination: persistent rotavirus activity in older children and elderly. Infect Dis (Auckl). 2017;49(5):388–395.
- Barret AS, Jourdan-da Silva N, Ambert-Balay K, et al. Surveillance for outbreaks of gastroenteritis in elderly long-term care facilities in France, November 2010 to May 2012. Euro Surveill. 2014;19(29). DOI:10.2807/1560-7917.ES2014.19.29.20859.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- Higgins JP, Thomas, J., Chandler, J, et al. Cochrane handbook for systematic reviews of interventions. Hoboken NJ: John Wiley & Sons; 2019.
- National Institute for Health and Care Excellence (NICE). Appendix H methodology checklist: qualitative studies. [ cited 2021 Feb]; Available from: https://www.nice.org.uk/process/pmg6/resources/the-guidelines-manual-appendices-bi-2549703709/chapter/appendix-h-methodology-checklist-qualitative-studies.
- Gastanaduy PA, Curns AT, Parashar UD, et al. Gastroenteritis hospitalizations in older children and adults in the United States before and after implementation of infant rotavirus vaccination. JAMA. J Am Med Assoc. 2013;310(8):851–853.
- Grytdal SP, DeBess E, Lee LE, et al. Incidence of norovirus and other viral pathogens that cause acute gastroenteritis (AGE) among kaiser permanente member populations in the United States, 2012-2013. PLoS ONE. 2016;11(4):e0148395.
- Cardemil CV, Balachandran, N., Kambhampati, A, et al. Incidence, etiology, and severity of acute gastroenteritis among prospectively enrolled patients in 4 Veterans affairs hospitals and outpatient centers, 2016-18. Clin Infect Dis. 2021;73(9):e2729–2738.
- Lopman BA, Hall AJ, Curns AT, et al. Increasing rates of gastroenteritis hospital discharges in US adults and the contribution of norovirus, 1996-2007. Clinl Infect Dis. 2011;52(4):466–474.
- Lopman BA, Curns AT, Yen C, et al. Infant rotavirus vaccination may provide indirect protection to older children and adults in the United States. J Infect Dis. 2011;204(7):980–986.
- Baker JM, Tate JE, Steiner CA, et al. Longer-term direct and indirect effects of infant rotavirus vaccination across all ages in the United States in 2000-2013: analysis of a large hospital discharge data set. Clinl Infect Dis. 2019;68(6):976–983.
- Burke RM, Mattison CP, Marsh Z, et al. Norovirus and other viral causes of medically attended acute gastroenteritis across the age spectrum: results from the MAAGE study in the United States. Clin Infect Dis. 2021;68(6):e913–e920.
- Wilhelm CM, Hanna SL, Welch CA, et al. Viral gastroenteritis in Charleston, West Virginia, in 2007: from birth to 99 years of age. Infect Control Hosp Epidemiol. 2010;31(8):816–821.
- Dey A, Wang H, Menzies R, et al. Changes in hospitalisations for acute gastroenteritis in Australia after the national rotavirus vaccination program. Med j Aust. 2012;197(8):453–457.
- Fletcher S, Sibbritt D, Stark D, et al. Descriptive epidemiology of infectious gastrointestinal illnesses in Sydney, Australia, 2007-2010. West Pac Surveill Response J: WPSAR. 2015;6(4):7–16.
- Fathima P, Jones MA, Moore HC, et al. Impact of rotavirus vaccines on gastroenteritis hospitalisations in Western Australia: a time-series analysis. J Epidemiol. 2021;31(8):480–486.
- Field EJ, Vally H, Grimwood K, et al. Pentavalent rotavirus vaccine and prevention of gastroenteritis hospitalizations in Australia. Pediatrics. 2010;126(3):e506–e512.
- Marshall J, Botes J, Gorrie G, et al. Rotavirus detection and characterisation in outbreaks of gastroenteritis in aged-care facilities. J Clin Virol. 2003;28(3):331–340.
- Donato CM, Roczo-Farkas, S., Kirkwood, C. D, et al. Rotavirus disease and genotype diversity in older children and adults in Australia. J Infect Dis. 2020;X(X):1–11.
- Phillips G, Lopman B, Rodrigues LC, et al. Asymptomatic rotavirus infections in England: prevalence, characteristics, and risk factors. Am J Epidemiol. 2010;171(9):1023–1030.
- Thomas SL, Walker JL, Fenty J, et al. Impact of the national rotavirus vaccination programme on acute gastroenteritis in England and associated costs averted. Vaccine. 2017;35(4):680–686.
- Atchison CJ, Stowe J, Andrews N, et al. Rapid declines in age group-specific rotavirus infection and acute gastroenteritis among vaccinated and unvaccinated individuals within 1 year of rotavirus vaccine introduction in England and Wales. J Infect Dis. 2016;213(2):243–249.
- Hungerford D, Vivancos R, Read JM, et al. Rotavirus vaccine impact and socioeconomic deprivation: an interrupted time-series analysis of gastrointestinal disease outcomes across primary and secondary care in the UK. BMC Med. 2018;16(1):10.
- Iturriza-Gomara M, Clarke, I., Desselberger, U., et al. Seroepidemiology of group C rotavirus infection in England and Wales. Eur J Epidemiol. 2004;19(6):589–595.
- Cox MJ, Medley GF. Serological survey of anti-group A rotavirus IgM in UK adults. Epidemiol Infect. 2003;131(1):719–726.
- Shen H, Zhang J, Li Y, et al. The 12 gastrointestinal pathogens spectrum of acute infectious diarrhea in a sentinel hospital, Shenzhen, China. Front Microbiol. 2016;7(NOV):1926.
- Zhang Z, Lai S, Yu J, et al. Etiology of acute diarrhea in the elderly in China: a six-year observational study. PLoS One. 2017;12(3):e0173881.
- Kuang X, Gong X, Zhang X, et al. Genetic diversity of group A rotavirus in acute gastroenteritis outpatients in Shanghai from 2017 to 2018. BMC Infect Dis. 2020;20(1):596. 10.1186/s12879-020-05279-x.
- Wang YH, Kobayashi N, Zhou D-J, et al. Molecular epidemiologic analysis of group A rotaviruses in adults and children with diarrhea in Wuhan city, China, 2000-2006. Arch Virol. 2007;152(4):669–685.
- Jia L, Lin, C., Gao, Z., et al. Prevalence and factors associated with different pathogens of acute diarrhea in adults in Beijing, China. The Journal of Infection in Developing Countries. 2016;10(11):1200–1207.
- Beck-Friis T, Andersson M, Gustavsson L, et al. Burden of rotavirus infection in hospitalized elderly individuals prior to the introduction of rotavirus vaccination in Sweden. J Clin Virol. 2019;119:1–5.
- Morton VK, Thomas MK, McEwen SA. Estimated hospitalizations attributed to norovirus and rotavirus infection in Canada, 2006-2010. Epidemiol Infect. 2015;143(16):3528–3537.
- Marquis A, Koch J. Impact of routine rotavirus vaccination in Germany: evaluation five years after its introduction. Pediatr Infect Dis J. 2020;39(7):E109–E116.
- Wilson SE, Rosella LC, Wang J, et al. Population-level impact of Ontario’s infant rotavirus immunization program: evidence of direct and indirect effects. PLoS ONE. 2016;11(5):e0154340.
- Leblanc D, Inglis, G. D., Boras, V. F, et al. The prevalence of enteric RNA viruses in stools from diarrheic and non-diarrheic people in southwestern Alberta, Canada. Arch Virol. 2017;162(1):117–128.
- McAuliffe GN, Anderson, T. P., Stevens, M., et al. Systematic application of multiplex PCR enhances the detection of bacteria, parasites, and viruses in stool samples. J Infect. 2013;67(2):122–129.
- Ferdous F, Ahmed S, Farzana FD, et al. Aetiologies of diarrhoea in adults from urban and rural treatment facilities in Bangladesh. Epidemiol Infect. 2015;143(7):1377–1387.
- Seo J-H, Park JJ, Lim J-Y, et al. Changes in anti-group A rotavirus antibody seroprevalence and levels in the western Gyeongnam Province of Korea over 16 years. J Korean Med Sci. 2013;28(1):55–61.
- Tatte VS, Gentsch JR, Chitambar SD. Characterization of group A rotavirus infections in adolescents and adults from Pune, India: 1993-1996 and 2004-2007. J Med Virol. 2010;82(3):519–527.
- Kheyami AM, Areeshi, M. Y., Dove, W., et al. Characterization of rotavirus strains detected among children and adults with acute gastroenteritis in Gizan, Saudi Arabia. Saudi Med J. 2008;29(1): 90–93
- Faruque ASG, Malek MA, Khan AI, et al. Diarrhoea in elderly people: aetiology, and clinical characteristics. Scand J Infect Dis. 2004;36(3):204–208.
- Wilking H, Höhle M, Velasco E, et al. Ecological analysis of social risk factors for rotavirus infections in Berlin, Germany, 2007-2009. Int J Health Geogr. 2012;11(1):37.
- Pang XL, Preiksaitis JK, Lee BE. Enhanced enteric virus detection in sporadic gastroenteritis using a multi-target real-time PCR panel: a one-year study. J Med Virol. 2014;86(9):1594–1601.
- Huhulescu S, Kiss R, Brettlecker M, et al. Etiology of acute gastroenteritis in three sentinel general practices, Austria 2007. Infection. 2009;37(2):103–108.
- de Wit MA, Koopmans M, Kortbeek L, et al. Etiology of gastroenteritis in sentinel general practices in the Netherlands. Clin Infect Dis. 2001;33(3):280–288.
- Tacharoenmuang R, Komoto S, Guntapong R, et al. High prevalence of equine-like G3P[8] rotavirus in children and adults with acute gastroenteritis in Thailand. J Med Virol. 2020;92(2):174–186.
- Weidemann F, Dehnert M, Koch J, et al. Modelling the epidemiological impact of rotavirus vaccination in Germany - A Bayesian approach. Vaccine. 2014;32(40):5250–5257.
- Satayarak J, Strauss ST, Duangdee C, et al. Prevalence and diversity of human rotavirus among Thai adults. J Med Virol. 2020;92(11):2582–2592.
- Kittigul L, Swangsri T, Pombubpa K, et al. Rotavirus infection in children and adults with acute gastroenteritis in Thailand. Southeast Asian J Trop Med Public Health. 2014;45(4):816–824.
- Baker JM, Alonso WJ. Rotavirus vaccination takes seasonal signature of childhood diarrhea back to pre-sanitation era in Brazil. J Infect. 2018;76(1):68–77.
- Kuzuya M, Fujii R, Hamano M, et al. Seroepidemiology of human group C rotavirus in Japan based on a blocking enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 2001;8(1):161–165.
- Friesema IHM, De Boer RF, Duizer E, et al. Aetiology of acute gastroenteritis in adults requiring hospitalization in the Netherlands. Epidemiol Infect. 2012;140(10):1780–1786.
- Ena J, Afonso-Carrillo RG, Bou-Collado M, et al. Epidemiology of severe acute diarrhea in patients requiring hospital admission. J Emergency Med. 2019;57(3):290–298.
- Wilson SE, Rosella LC, Wang J, et al. Equity and impact: ontario’s infant rotavirus immunization program five years following implementation. A population-based cohort study. Vaccine. 2019;37(17):2408–2414.
- Steenland MW, Joseph GA, Lucien MAB, et al. Laboratory-confirmed cholera and rotavirus among patients with acute diarrhea in four hospitals in Haiti, 2012-2013. Am J Trop Med Hyg. 2013;89(4):641–646.
- Selvarajan S, Reju S, Pushpanathan P, et al. Molecular characterisation and clinical correlates of rotavirus in children and adults in a tertiary care centre, Chennai, South India. Indian J Med Microbiol. 2017;35(2):221–227.
- Chia G, Ho HJ, Ng C-G, et al. An unusual outbreak of rotavirus G8P[8] gastroenteritis in adults in an urban community, Singapore, 2016. J Clin Virol. 2018;105:57–63.
- Nilsson M, Sigstam G, Svensson L. Antibody prevalence and specificity to group C rotavirus in Swedish sera. J Med Virol. 2000;60(2):210–215.
- Trop Skaza A, Beskovnik L, Zohar Cretnik T. Outbreak of rotavirus gastroenteritis in a nursing home, Slovenia, December 2010. Eurosurveillance. 2011;16(14):19837.
- Subelj M, Ucakar V. An outbreak of acute gastroenteritis associated with group A Rotavirus in long-term care facility in Slovenia. Wien Klin Wochenschr. 2015;127(11–12):415–420.
- Fernandez J, De Oña, M., Melón, S, et al. Noroviruses as cause of gastroenteritis in elderly patients. Aging Clin Exp Res. 2011;23(2):145–147.
- Andersson M, Lindh M. Rotavirus genotype shifts among Swedish children and adults-Application of a real-time PCR genotyping. J Clin Virol. 2017;96:1–6.
- Cardemil CV, Cortese, M. M., Medina-Marino, A, et al. Two rotavirus outbreaks caused by genotype G2P [4] at large retirement communities: cohort studies. Ann Intern Med. 2012;157(9):621–631.
- Piednoir E, Borderan GC, Borgey F, et al. Direct costs associated with a hospital-acquired outbreak of rotaviral gastroenteritis infection in a long term care institution. J Hosp Infect. 2010;75(4):295–298.
- Yandle Z, Coughlan S, Dean J, et al. Indirect impact of rotavirus vaccination on viral causes of acute gastroenteritis in the elderly. J Clin Virol. 2021;137:104780.
- Campbell D, Pockett RD, Adlard N, et al. Re-admissions to hospital within 30 days of hospital discharge with rotavirus gastroenteritis: a UK study on children under 5 years of age. Arch Dis Child. 2012;97(Suppl 1):A52–A53.
- Tajiri H, Takeuchi Y, Takano T, et al. The burden of rotavirus gastroenteritis and hospital-acquired rotavirus gastroenteritis among children aged less than 6 years in Japan: a retrospective, multicenter epidemiological survey. BMC Pediatr. 2013;13(1):83.
- Arakaki L, Tollefson D, Kharono B, et al., Prevalence of rotavirus among older children and adults with diarrhea: a systematic review and meta-analysis. Vaccine. 39(33): 4577–4590. 2021.
- Spina A, Kerr KG, Cormican M, et al. Spectrum of enteropathogens detected by the FilmArray GI Panel in a multicentre study of community-acquired gastroenteritis. Clin Microbiol Infect. 2015;21(8):719–728.
- Paulke-Korinek M, Kundi M, Rendi-Wagner P, et al., Herd immunity after two years of the universal mass vaccination program against rotavirus gastroenteritis in Austria. Vaccine. 29(15): 2791–2796. 2011.
- Baker JM, Dahl RM, Cubilo J, et al. Effects of the rotavirus vaccine program across age groups in the United States: analysis of national claims data, 2001-2016. BMC Infect Dis. 2019;19(1):186.
- Lambert SB, Faux, C. E., Hall, L, et al. Early evidence for direct and indirect effects of the infant rotavirus vaccine program in Queensland. Med j Aust. 2009;191(3):157–160.
- Dorleans F, Falkenhorst, G., Böttiger, B, et al. A case-control study of risk factors for rotavirus infections in adults, Denmark, 2005-2009. Epidemiol Infect. 2016;144(3):560–566.
- Lopman B, Vicuña Y, Salazar F, et al. Household transmission of rotavirus in a community with rotavirus vaccination in Quininde, Ecuador. PLOS ONE. 2013;8(7):e67763.
- Quee FA, de Hoog MLA, Schuurman R, et al. Community burden and transmission of acute gastroenteritis caused by norovirus and rotavirus in the Netherlands (RotaFam): a prospective household-based cohort study. Lancet Infect Dis. 2020;20(5):598–606.
- Cortese MM, Dahl RM, Curns AT, et al. Protection against gastroenteritis in US households with children who received rotavirus vaccine. J Infect Dis. 2015;211(4):558–562.
- Inns T, Wilson D, Manley P, et al. What proportion of care home outbreaks are caused by norovirus? An analysis of viral causes of gastroenteritis outbreaks in care homes, North East England, 2016–2018. BMC Infect Dis. 2020;20(1):1–8.
- Lewnard JA, Lopman BA, Parashar UD, et al. Naturally acquired immunity against rotavirus infection and gastroenteritis in children: paired reanalyses of birth cohort studies. J Infect Dis. 2017;216(3):317–326.
- Mast TC, Wang, F. T., Su, S, et al. Evidence of herd immunity and sustained impact of rotavirus vaccination on the reduction of rotavirus-related medical encounters among infants from 2006 through 2011 in the United States. Pediatr Infect Dis J. 34(6): 615–620. 2015.