ABSTRACT
Introduction
Rotavirus is one of the most common pathogens causing diarrhea in children <5 years and has a major impact on childhood morbidity and mortality. Since the implementation of rotavirus vaccines into childhood immunization programs across Europe, there has been a reduction in rotavirus burden, including hospitalizations, outpatient cases, costs, and deaths.
Areas covered
A systematic literature review identified publications describing the clinical and economic impact of rotavirus vaccinations across Europe, from their introduction in 2006 to the end of 2020. A total of 3,137 articles were identified, of which 46 were included in the review. Included articles reported the impact of rotavirus vaccination on disease in any age group.
Expert opinion
Rotavirus vaccination has resulted in substantial reductions in hospitalizations and rotavirus-associated costs across Europe, particularly in children <5 years. There is some evidence of herd protection afforded to older age groups where vaccine uptake is high among infants, highlighting the potential for vaccination to confer a greater societal benefit as programs become more established. Increasing vaccination coverage and continuing investment in widespread rotavirus vaccination programs across countries will likely increase the substantial public health benefits associated with vaccination and further reduce the clinical and economic burden of disease.
1. Introduction
Rotavirus (RV) is a highly infectious virus and the primary cause of severe gastroenteritis among infants and young children [Citation1]. RV is most often transmitted by the fecal-oral route, from person to person or via contaminated objects, surfaces or food [Citation2]. Early symptoms of the infection include fever, vomiting, and diarrhea [Citation3]. Additionally, RV can lead to death, most often in low-income countries, due to dehydration and lack of medical care [Citation1].
RV carries a large public health burden. Prior to vaccination, almost all children would be exposed to RV before they were 5 years old, and it was estimated that RV gastroenteritis (RVGE) resulted in approximately 450,000 deaths worldwide annually among children <5 years [Citation4–6]. In Europe, prior to the introduction of RV vaccines in 2006, RV was the leading cause of severe gastroenteritis among children <5 years [Citation7]. A report from 2006 estimated that 72,000 to 77,000 of RV cases were hospitalized in the European Union (EU) annually, with a median cost of €1,417 per case [Citation8]. Mortality estimates could only be made for some Northern and Western European countries (Germany, Finland, France, Ireland, and England and Wales), in which the mortality rate ranged from 0.5 to 3 deaths per million children <5 years per year pre-vaccine introduction [Citation8].
In 2006, two RV vaccines were licensed for use in Europe; Rotarix (RV1; GlaxoSmithKline Biologicals) and RotaTeq (RV5; Merck Sharp & Dohme Corp.) [Citation9,Citation10]. RV1 is administered in two doses from 6 to 24 weeks of age, and RV5 is administered in three doses from 6 to 32 weeks of age [Citation9,Citation10]. Both vaccines have been shown to be highly effective and safe in clinical trials and post-licensure observational studies [Citation9,Citation10]. In 2007 the World Health Organization (WHO) recommended that RV vaccines be introduced into national immunization programs (NIPs) of regions and countries where vaccine efficacy data suggested a significant public health impact, and where appropriate infrastructure and financing mechanisms were available to sustain vaccine utilization [Citation11]. In 2009, this recommendation was extended to all other regions globally [Citation1]. In 2016, RV vaccination use was estimated to have averted more than 28,000 deaths among children <5 years worldwide, and approximately 1.7 million hospitalizations (approximately 3.2 million hospitalizations were reported in 1990) [Citation1]. However, as of 2018, only 13 European countries provided a fully funded universal RV mass vaccination program () [Citation12].
Figure 1. Rotavirus recommendations in the EUa [Citation12–31].
![Figure 1. Rotavirus recommendations in the EUa [Citation12–31].](/cms/asset/eec28b70-be37-4f89-857d-fd7221d61920/ierv_a_2075851_f0001_oc.jpg)
Therefore, this systematic literature review sought to summarize and understand the burden of acute gastroenteritis (AGE) and RVGE before and after vaccination, and the impact of RV vaccination in the EU. While other reviews of RV vaccination impact have been published, with the progressive adoption of RV vaccination in the EU, new evidence regarding vaccination impact has become available. This review will also be relevant for modelers involved in quantifying the value of RV vaccination, and policy makers assessing the benefits and costs of RV vaccination.
2. Methods
2.1. Search strategy
A search was carried out in accordance with the Cochrane and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidance for systematic reviews [Citation32,Citation33]. The OVID platform was used to search Embase, EconLit, Evidence Based Medicine (EBM) Reviews, OVID MEDLINE® ALL, and EBM Reviews – Cochrane Database of Systematic Reviews for relevant evidence on the impact of RV vaccination in all age groups published from 1 January 2006 to 31 December 2020. The search terms used can be found in Table S1.
A gray literature search was conducted to capture any relevant literature that may not have been captured by the database search to supplement the findings. The sources for this search included the International Society for Pharmacoeconomics and Outcomes Research (IPSOR), the European Society for Pediatric Infectious Diseases (ESPID), the International Conference on Vaccines and Immunization, the Euro Global Summit and Expo on Vaccines, the International Rotavirus Symposium, the European Center for Disease Prevention and Control (ECDC), the WHO, and the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN). Additionally, the reference lists of all relevant papers were reviewed to identify further materials for inclusion, a stage referred to here as ‘hand searching.’
2.2. Study selection and data extraction
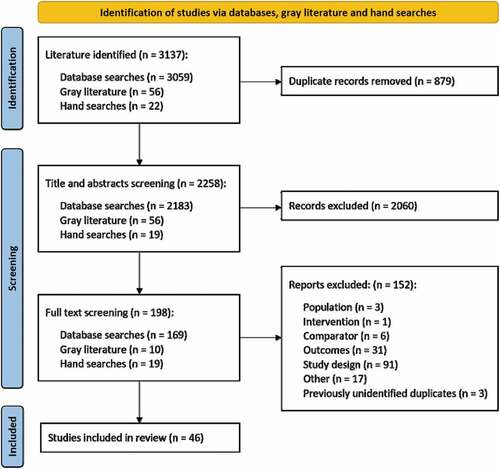
All identified references were reviewed for inclusion at the title and abstract screening stage by two reviewers using the population, comparators, outcomes, and study design (PICOS) framework (Table S2). Briefly, included articles were primary studies including populations of all ages, compared RV vaccination to no vaccination, and measured changes in the clinical or economic burden of RV in Europe. This review included all countries that were a member of the EU as of 1 January 2020 and a further three countries that were a part of the EU single market (Norway, Switzerland, and Iceland). There was no requirement for included studies to be conducted in countries where rotavirus vaccination was part of the NIP. Publications included at this stage underwent full-text screening by two reviewers using the same PICOS criteria. All discrepancies identified were resolved by consensus via a third researcher. Any articles that did not align with the PICOS criteria were excluded from this review.
Data were extracted on the clinical and economic burden of RVGE and AGE before and after vaccination. Data from articles included at the full-text screening stage were extracted into a data extraction form in Microsoft Excel by one reviewer and checked for accuracy by a second reviewer. Data were then checked by a senior reviewer.
2.3. Quality assessment for included studies
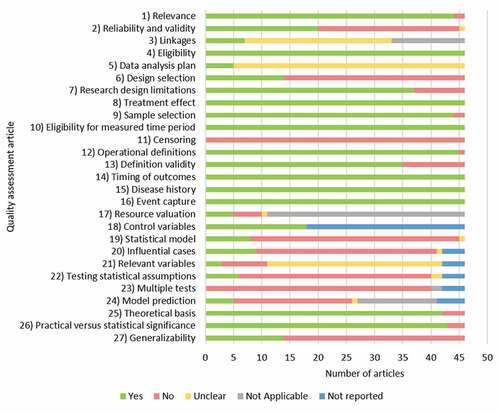
A full quality assessment of each of the included articles was completed using the ISPOR Task Force Checklist on Retrospective Database Studies (Table S3), to ensure the inclusion of quality data to inform reliable conclusions [Citation34]. Study quality was assessed by one reviewer and verified by a second reviewer, with discrepancies being resolved by consensus or involvement of a senior reviewer.
3. Results
3.1. Systematic literature search overview
A total of 3,137 articles were identified, of which 46 were included in the review: 37 from the OVID search, six from ‘hand searches’ and three from gray literature searches (, , Table S4, quality assessment in . Studies took place in Austria (n = 3), Belgium (n = 9), England and Wales (n = 1), England (n = 1), Estonia (n = 2), Finland (n = 6), Germany (n = 4), Ireland (n = 3), Italy (n = 2), Norway (n = 1), Scotland (n = 2), Spain (n = 6), Sweden (n = 1), and the United Kingdom (UK; n = 5).
Table 1. Overview of included studies (n = 46)
3.2. Clinical impact of RV vaccination
The clinical impact of RV vaccination was reported in 44 of the 46 identified studies (; further details on the findings can be found in the appendix in Tables S5, S6, S7, and S8. Outcomes of interest included the impact of RV vaccination on the burden of AGE or RVGE hospitalizations, outpatient visits, and mortality in children <5 years and on populations >5 years.
Table 2. Range of reductions in clinical outcomes following rotavirus vaccination (n = 44)
Some articles expressed the impact of vaccination on changes in the number of bed days or RV cases as whole numbers rather than percentages, with no reported pre-vaccination and post-vaccination results. For clinical outcomes, this manuscript presents only percentage changes where they were reported in the publication or were able to be calculated from reported pre-vaccination and post-vaccination data.
3.2.1. Incidence of hospitalizations for AGE <5 years
Twenty studies reported the incidence of AGE hospitalizations both before and after vaccine introduction, 14 of which reported results in children <5 years (Table S5) [Citation35–47]. After vaccine introduction, 13 of these studies reported a reduction in AGE hospitalizations among children <5 years, and the greatest reductions in AGE hospitalizations were reported for children ≤2 years [Citation35–47]. Reduction in number of hospital bed days for AGE ranged from 0.4% for AGE hospital admissions in Ireland for children aged 2 to 4 years (vaccination coverage rate [VCR] 85% to 89%) to 91.8% in Finland for children <5 years (with a higher VCR of ~96%) [Citation40,Citation44].
Seven of these studies reported both reductions and increases in AGE after vaccine introduction, depending on the age group. One additional study reported an overall increase in AGE admissions (overall increases ranged from 1.6% to 81.8%) [Citation36,Citation38–40,Citation42,Citation43,Citation45,Citation48]. Of note, for the majority of studies showing increases in disease, vaccination had been introduced for four years or less [Citation36,Citation38–40,Citation42,Citation43,Citation45,Citation48]. Some studies reporting increases in disease reported very low VCRs, although there was a high level of variation in VCRs (ranging from 17% to >95%) [Citation36,Citation38–40,Citation42,Citation43,Citation45,Citation48]. Additionally, the greatest increases in disease were generally observed in older pediatric age groups, specifically in children between 2 and 5 years [Citation36,Citation38–40,Citation42,Citation43,Citation45,Citation48].
3.2.2. Incidence of hospitalizations for RVGE <5 years
Forty studies reported the incidence of RVGE hospitalizations; 34 of which reported a decrease in hospitalizations for RVGE after vaccine introduction (Table S6) [Citation35,Citation36,Citation42–45,Citation47–74]. The impact of vaccination ranged from a 0.2% reduction in RVGE cases in children <5 years in Germany (with a VCR of 66%) to a 100.0% reduction in RVGE cases in children aged 6 months to 5 years in Belgium, Finland, and Spain (VCR ranged from 46% to ~96%) [Citation36,Citation44,Citation45,Citation65,Citation66]. The 0.2% reduction in Germany was a statistically significant finding (p value not reported), although the authors noted that the effect was marginal and likely due to low national vaccine uptake [Citation65]. Children <2 years often experienced greater reductions (up to 100%) in the incidence of RVGE [Citation44,Citation45].
Furthermore, some studies reported reductions in the number of RVGE hospital bed days in children <5 years in Belgium, Finland, and Spain (VCR ranged from 46% to ~96%) [Citation36,Citation44,Citation45,Citation66]. Twelve studies reported an increase in RVGE hospitalizations, seven of which had also reported reductions (increases ranged from 0.2% to 200.0%) [Citation36,Citation44,Citation49,Citation51,Citation53,Citation54,Citation56,Citation65–67,Citation70,Citation73]. Most of these increases were reported when vaccination had only been introduced for up to three years, and some studies reported very low VCRs (range from 18% to ~96%) [Citation36,Citation44,Citation49,Citation51,Citation53,Citation54,Citation56,Citation65–67,Citation70,Citation73].
3.2.3. Incidence of AGE among outpatients <5 years
Six studies reported on the incidence of AGE among outpatients, including primary care and emergency department visits (Table S7) [Citation39,Citation42,Citation43,Citation45,Citation46,Citation75]. All these studies reported reductions in the incidence of disease after the introduction of vaccination [Citation39,Citation42,Citation43,Citation45,Citation46,Citation75]. These reductions ranged from 1.0% (in children aged 3 years after two to three years of RV vaccine introduction) to 59.5% (in children <5 years after one to four years of vaccine introduction) in primary care cases [Citation39]. Both of these values were reported in the same study in Norway, which reported a high post-vaccination era VCR of 95% [Citation39].
While all six studies reported reductions in specific age groups, five of these also reported increases in the incidence of AGE in children <5 years (compared to the pre-vaccination period, ranging from 2.0% to 81.8%) [Citation39,Citation42,Citation43,Citation45,Citation75]. The increase of 81.8% was recorded among children aged 4 years in Finland despite a high VCR of >95% [Citation42]. However, this value was an outlier and the article highlighted that the overall total outpatient disease burden of AGE was reduced by 8.8% in the post-vaccination year when compared to the pre-vaccination year [Citation42]. Of note, while outpatient cases decreased after vaccine introduction, these reductions were not as substantial as the reductions in inpatient cases [Citation42].
3.2.4. Incidence of RVGE among outpatients <5 years
Seven studies reported the incidence of RVGE among outpatients, all of which reported reductions over time among children <5 years (Table S8) [Citation42,Citation43,Citation45,Citation52,Citation61,Citation67,Citation73]. These reductions ranged from 26.4% in RV-related general practice (GP) consultations in children <5 years in Scotland to 100.0% in outpatient RVGE cases in children aged 3 to 4 years in Finland [Citation43,Citation52]. These studies reported similarly high VCRs (93% and 91% to 93%, respectively), but the greatest reduction was observed five years after vaccine introduction, whereas the smallest reduction was observed from the year of vaccine introduction (2013) onwards [Citation43,Citation52].
3.2.5. Incidence of AGE among older age groups (≥5 years)
A total of 10 studies reported results of the impact of AGE hospitalizations in individuals ≥5 years [Citation35,Citation38,Citation40,Citation41,Citation44,Citation46–48,Citation51,Citation60]. All of these studies reported reductions in the number of hospitalizations recorded (Table S5) [Citation35,Citation38,Citation40,Citation41,Citation44,Citation46–48,Citation51,Citation60]. Reductions in AGE hospital admissions ranged from 0.9% in children aged 10 to 16 years at one year post-vaccine introduction to 37.6% in children aged 5 to 9 years at two years post-vaccine introduction in Ireland (with a VCR of 85% to 89%) [Citation40]. The greatest reduction in AGE hospital admissions for cohorts comprising only individuals ≥5 years was 37.6% for children aged 5 to 9 years in Ireland where there was a high VCR (85% to 89%; vaccination had been introduced for two years) [Citation40].
Two studies reported increases in AGE hospitalizations (by 1.0% to 97.1%). Of note, one study reported post-vaccination results only one to two years after vaccine introduction, and the other reported a low VCR of 34% [Citation40,Citation51]. Additionally, one of these studies recorded low hospitalization rates in both the pre-vaccination and post-vaccination periods, which may have influenced results [Citation40].
3.2.6. Incidence of RVGE among older age groups (≥5 years)
Eighteen studies explored RVGE hospitalizations in individuals aged 5 to >65 years, all of which reported reductions after the introduction of vaccination (Table S6) [Citation35,Citation41,Citation44,Citation47–51,Citation58,Citation60–62,Citation67–69,Citation71,Citation73,Citation76]. A study in Austria estimated a 100.0% reduction in the incidence of nosocomial RVGE among 6 to 10 and 11 to 18-year-olds, with a high VCR of 72% to 87% [Citation71]. The smallest decrease in nosocomial RVGE was reported in children aged 5 to 9 years in Germany (0.7%; VCR: 59% at one year after vaccine introduction to 80% at 4 to 5 years after vaccine introduction) [Citation67]. Conversely, three studies also reported increases in RVGE hospitalizations (by 0.8% to 57.1%), although the authors noted that some of the largest increases were recorded shortly after the introduction of RV vaccination (e.g. one year), and some were reported in areas with a low VCR (e.g. 34%) [Citation49,Citation51,Citation67].
3.2.7. Mortality due to RV-related disease
RV-related mortality was explored in three studies, from Austria (n = 1) and Belgium (n = 2; Table S9) [Citation60,Citation71,Citation74]. Two of these studies reported a 100.0% reduction; one in the number of deaths due to RVGE in individuals aged from 3 days to 16.5 years in Austria and the other in the number of deaths due to unspecified gastroenteritis in all age groups in Belgium [Citation60,Citation71,Citation74]. However, RV-related mortality was low in both pre-vaccination (three deaths and two deaths, respectively) and post-vaccination periods (zero deaths) [Citation60,Citation71]. Vaccination had been introduced for one to two years in the Austrian study and three to six years in the Belgian study [Citation60,Citation71]. VCRs were similar between the studies, reported at 72% to 87% in the Austrian study and 79% to 88% in the Belgian study [Citation60,Citation71]. The third study in Belgium reported a 36.4% decrease in crude RVGE mortality rate from less than a year (the transition period) to seven years post-vaccine introduction, with a much lower VCR of ~40% [Citation74].
3.3. Economic impact of RV vaccination
Eleven studies reported on the impact of RV vaccination on the economic burden of disease (, Table S10) [Citation37,Citation43,Citation44,Citation46,Citation51,Citation52,Citation54,Citation55,Citation70,Citation71,Citation77]. Ten studies reported on inpatient costs, four reported on outpatient costs, and two reported on indirect costs [Citation37,Citation43,Citation44,Citation46,Citation51,Citation52,Citation54,Citation55,Citation70,Citation71,Citation77].
Table 3. Range of reductions in economic outcomes following rotavirus vaccination (n = 11)
The 10 studies reporting inpatient costs recorded reductions from the pre-vaccination to the post-vaccination period [Citation37,Citation43,Citation44,Citation46,Citation51,Citation52,Citation54,Citation55,Citation70,Citation71]. A study in England recorded a reduction in costs of £12.505 million (2014 Great British Pounds [GBP]) due to AGE in GP, with a high VCR of 88% for course completion at one to two years post-vaccine introduction [Citation46]. When stratified by age group, this study showed the greatest reduction in costs in children <1 year [Citation46]. Two studies explored changes in bed occupancy due to RV and reported reductions across all timepoints measured for both community-acquired and nosocomial RVGE (up to a 94.9% decrease for which hospital bed occupancy was >25% in Finland) [Citation44,Citation70]. A study in Finland noted that the RV vaccination program annually pays for itself almost two times over [Citation43].
The four studies considering outpatient costs due to RVGE and AGE reported a reduction in costs after vaccine introduction in Finland (1999–2005 to 2014), England (2008–2013 to 2013–2015), Italy (2009–2012 to 2013–2016), and Scotland (before 2013 to 2013 onwards) [Citation43,Citation46,Citation52,Citation54]. One study reported averted costs of €23,724 for RVGE in outpatients aged 0 to 4 years in Finland [Citation43]. When stratified by age, the greatest reductions were observed in children aged 1 year (€7,195; VCR was 91% to 93% with a post-vaccination period five years after vaccine introduction) [Citation43]. A study in Italy reported total annual cost savings from reductions in emergency room and primary care admissions of €1,556,605 for children aged 0 to 59 months (VCR of 25% to 45% at a post-vaccination period zero to three years after vaccine introduction) [Citation54].
The two studies that reported indirect costs (including productivity losses and costs for caregivers) estimated that these costs reduced after vaccine introduction [Citation71,Citation77]. In Austria, estimated total costs for caregivers remained unchanged in older children (aged 6 to 18 years) but decreased substantially in younger age groups, by up to 86.2% for patients between 0 and 11 months [Citation71]. In this study, VCR was 72% to 87% and vaccination had been introduced one to two years prior to data collection [Citation71]. In Belgium, the overall reduction in work absences for first-time mothers ranged from 0.4% for average days absent from work per woman (at five years post-vaccine introduction) to 62.0% for short work absences per month (at one year post-vaccine introduction), both recorded in the RV season [Citation77]. Some increases in work absences were reported in this study, although the greatest of these were recorded in the non-RV season [Citation77]. VCR for this study was relatively high at >85% across time periods, providing an explanation for the high overall reductions observed [Citation77].
4. Discussion and conclusion
4.1. Discussion
The review collated evidence from a total of 46 publications. Of the studies captured, 24 were conducted in countries in which RV vaccines were fully funded via the NIP (Austria, Estonia, Finland, Germany, Ireland, Italy, Norway, and the UK) [Citation12,Citation35,Citation39–50,Citation52–55,Citation57–59,Citation61,Citation62,Citation65,Citation67,Citation68,Citation71–73,Citation75,Citation76]. Of the remaining 16 studies, one was conducted in Sweden where vaccination was only funded in some regions [Citation12,Citation78]. Nine studies were conducted in Belgium where RV vaccination is only partially publicly funded and a fixed co-payment from the parent is required [Citation12,Citation60,Citation63,Citation64,Citation66,Citation69,Citation70,Citation77,Citation79,Citation80]. Six studies were conducted in Spain where public funding for RV vaccination is only available for preterm infants, whereas for other children, parents must pay for RV vaccination out of pocket [Citation36–38,Citation51,Citation56,Citation74,Citation81]. Since this study, RV vaccination was added to the NIP for high-risk children in Sweden [Citation82]. While the search strategy included terms to capture publications investigating the humanistic impact of RV vaccination, no such publications met the PICOS criteria for inclusion in this systematic literature review. Overall, results show how the introduction of RV vaccines in Europe has decreased the clinical and economic burden associated with AGE and RVGE.
RV vaccination had a greater impact on reducing the clinical and economic burden of AGE and RVGE in children <5 years compared to older age groups. This is likely because these individuals had received RV vaccination, although several studies reported substantial reductions in AGE and RVGE in adults and older children who were not eligible for vaccination, suggesting the impact of indirect protection. However, it is important to note that only three studies considered AGE- or RVGE-related mortality and therefore the ability to draw conclusions regarding the impact of vaccination on AGE- and RVGE-related mortality for all age groups is limited. Furthermore, the recent introduction of vaccination in some countries lead to short post-vaccination data collection periods, and therefore the findings may not be representative of how reductions are sustained over longer periods of time. There was also a reduction in direct and indirect costs associated with AGE and RVGE after the introduction of RV vaccination. The authors of a study in Finland noted that the RV vaccination program annually pays for itself almost two times over [Citation43].
Our results are consistent with those reported in a literature review undertaken in 2015, which reported substantial reductions in AGE and RVGE disease following vaccine introduction in Europe [Citation83]. The current review captured additional data from European countries not included in the 2015 review [Citation35,Citation39,Citation48,Citation54,Citation62,Citation78,Citation83]. Our results are also consistent with reviews focusing on countries in Asia, Africa, and North and South America that have shown a substantial decrease in the incidence of AGE and RVGE after RV vaccination [Citation7,Citation84–86]. In children <5 years, there has been a significant reduction in the proportion of hospital admissions for AGE due to RV in all WHO regions that have introduced RV vaccines (p ≤ 0.05) [Citation7].
Of note, publications reviewed in this study also reported reductions in AGE and RVGE in the post-vaccination period in older age groups (individuals aged ≥5 years), particularly in areas with a high vaccination coverage, suggesting the existence of herd immunity effects resulting from the vaccination of younger populations [Citation40,Citation67]. This is consistent with results from the United States, where medical claims and hospital stay data analysis studies also suggested the existence of long-term herd protection provided by RV vaccination in the post-vaccination period [Citation87,Citation88].
While the majority of results were suggestive of the beneficial effects of RV vaccination, some papers reported both reductions and increases in AGE and RVGE incidence and costs after vaccine introduction [Citation36,Citation38–40,Citation42–45,Citation48,Citation49,Citation51,Citation53,Citation54,Citation56,Citation65–67,Citation70,Citation73]. These were most often reported in older pediatric age groups, likely due to low vaccination coverage or the recording of post-vaccination results less than a year after the vaccine was introduced (during the transition period) [Citation36,Citation38–40,Citation42–45,Citation48,Citation49,Citation51,Citation53,Citation54,Citation56,Citation65–67,Citation70,Citation73]. Furthermore, temporary increases in incidence of AGE and RVGE in older pediatric groups may be due to an age shift associated with the recent introduction of RV vaccination for younger children [Citation7].
One of the studies in Ireland, by Coveney et al., noted that low pre-vaccination rates may reflect the lack of access to pediatric hospital beds due to overcrowding, and therefore cases were not recorded [Citation40]. Furthermore, the authors highlighted that only two seasons post-vaccine introduction were observed in the study and suggested that the effect of vaccination would become more apparent over the following years [Citation40]. Furthermore, there may be a bias introduced by lack of routine testing for RV as the gastroenteritis etiology does not necessarily determine the treatment course [Citation35]. Therefore, such contextual factors may also be important in other studies.
The nine studies that took place in Belgium reported great variations in the impact of RV vaccination on incidence of AGE and RVGE (reaching increases in RVGE incidence of 200%), which likely reflects variation in the VCRs due to only partial funding through the NIP [Citation60,Citation63,Citation64,Citation66,Citation69,Citation70,Citation77,Citation79,Citation80]. VCRs in Belgium varied by region and time period and ranged from 15% in 2006 (region not reported) to >85% in Antwerp (time period not reported) [Citation69,Citation77]. Substantial variations in the impact of RV vaccination were also reported in the six studies conducted in Spain [Citation36–38,Citation51,Citation56,Citation74]. These variations may similarly be attributable to the low VCRs recorded in these studies (ranging from 12% to 51%), which may result from the lack of NIP recommendation and universal public funding for RV vaccination [Citation37,Citation81,Citation89]. Previous studies conducted in Spain and the United States (US) have demonstrated the dependence of RVGE burden on RV VCR, highlighting the importance of high VCRs in maximizing the impact of RV vaccines [Citation84,Citation85,Citation90]. By contrast, the six studies conducted in Finland, where RV vaccination is fully funded, reported mostly high VCRs (ranging from 30% to 97%) and consistent reductions in RVGE burden (ranging from 25% to 100%) [Citation41–45,Citation61]. These findings suggest that full recommendation and public funding of RV vaccines may improve VCRs and therefore further reduce the burden of RV.
Our study was not without limitations. There was no free text included in the search strategy for the population category of the OVID database search, potentially excluding any publications not linked to the key terms used and therefore limiting the number of relevant publications captured. The review included many studies that were retrospective, ecological, and descriptive in nature. Therefore, it is possible that effects measured were influenced by factors unrelated to RV vaccine introduction. For example, RV seasons vary per year. Therefore, extraneous factors such as natural fluctuation in the prevalence of RV could also impact results. Many studies did not report a sample size and vaccination coverage was not well defined, reducing the ability to contextualize results.
Furthermore, data were often based on hospital discharge records data and indirect costs were only estimated (through emergency department and pediatrician visits) in one study [Citation71]. In general, use of hospital discharge records could underestimate the burden of RVGE as not all cases reported received a laboratory confirmation of RV infection. Furthermore, although an RVGE-specific gastroenteritis ICD-10 code exists, few RV-positive hospitalizations are identified by this ICD-10 code [Citation91]. This is largely due to miscoding as other, non-RVGE-specific, gastroenteritis ICD-10 codes and under-testing for RV infection [Citation91]. Therefore, the true burden of RVGE is likely underestimated without the consideration of all ICD-10 codes for gastroenteritis [Citation91]. It is also important to note that AGE was not necessarily caused by RV infection and can be caused by other pathogens. The lack of data on indirect costs (including productivity losses) may underestimate the economic impact of vaccination. Finally, there were comparatively few data available on the impact in older age groups. Therefore, the ability to draw conclusions regarding herd immunity is limited.
4.2. Conclusion
This review found that RV vaccination reduced the burden of AGE and RVGE in countries within the EU 15 years after its introduction. While the greatest reductions were observed in children <5 years, the results also indicate a reduction in AGE and RVGE among older age groups. However, RV vaccination is not yet included in all NIPs in Europe. Expansion of RV vaccination programs to other countries could further reduce the burden of AGE and RVGE in Europe.
5. Expert opinion
This review summarizes real-world evidence on the impact of RV vaccination on hospitalizations and mortality due to AGE and RVGE across Europe. The review provides an update to previous findings as RV vaccination has been adopted more widely in the five years since the work by Karafillakis et al [Citation83]. The findings highlight the benefits of RV vaccination, demonstrating a substantial reduction in AGE- and RVGE-related hospitalizations and costs for children <5 years following vaccine introduction. Results also indicate a potential reduction in AGE- and RVGE-related mortality and a reduction in RV-related hospitalizations and costs in older age groups (≥5 years).
The reductions in AGE- and RVGE-related costs were observed relatively soon after vaccine introduction and the importance of these savings was summarized by a publication reporting that the vaccination program annually pays for itself almost two times over in Finland [Citation43]. Only two publications exploring the impact of RV vaccination on indirect costs were captured in this review (considering parent absenteeism from work and cost for caregivers), and future research may be required to further characterize the potential effects on these variables [Citation71,Citation77].
Research has suggested that pediatric vaccination rates have been negatively impacted due to the COVID-19 pandemic as a result of clinics closing or reduced opening hours, reluctance to receive a vaccine due to fear of transmission, lockdown measures, and transport disruptions [Citation92–98]. Timely RV vaccination is crucial as the vaccination cannot be administered as a catch-up dose after the indicated age range [Citation9,Citation10]. Thus, disruption to routine RV vaccinations may result in some children remaining unvaccinated and therefore more vulnerable to AGE and RVGE disease. In Spain, where routine RV vaccination was withheld for five months due to a temporary availability issue in 2010, AGE- and RVGE-related hospitalizations increased [Citation99,Citation100].
Since 2013, the WHO has recommended that RV vaccines be included in all NIPs and considered a priority, as part of a comprehensive strategy to control diarrheal diseases [Citation101]. As of 2021, several EU countries are yet to implement RV vaccination in their NIP (43% according to the European Center for Disease Prevention and Control [ECDC]) and several others that have implemented a national vaccination program have reported a low RV VCR [Citation102,Citation103]. This review demonstrates the real-world value of RV vaccination programs for countries who are yet to introduce universal pediatric RV vaccination or who report a low RV VCR. Therefore, increasing the VCR and introducing RV vaccination programs in additional countries may further reduce the burden of AGE and RVGE in Europe.
Article highlights
Rotavirus vaccination reduces the clinical and economic burden of diarrheal disease
Beneficial effects are most substantial in younger populations (<5 years)
Some studies indicate herd protection for older children after infant vaccination
Future research should continue to document vaccine impact as coverage increases
Author contributions
All authors substantially contributed to the conception and design of the study and interpretation of the relevant literature. All authors were involved in the writing of the manuscript or revision of the manuscript for intellectual content.
Declaration of interests
G Bencina, U Sabale, J Murtagh and C Carias are employees of MSD subsidiaries of Merck & Co., Inc., Kenilworth, NJ, USA, the manufacturer of the rotavirus oral vaccine and may own stocks and/or stock options. A Ahern, R Bhaila, and R Newman are employees of Adelphi Values PROVE™. Adelphi Values PROVE™ was compensated by MSD for the conduct of the study and development of the manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from Expert Review of Vaccines for their review work but have no other relevant financial relationships to disclose.
Supplemental Material
Download MS Word (506.4 KB)Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials. Written requests can be sent to the corresponding author.
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Troeger C, Khalil IA, Rao PC, et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018;172(10):958–965.
- Centers for Disease Control and Prevention (CDC). Rotavirus: Transmission 2021 [ Accessed 30/04/2021]. Available from: https://www.cdc.gov/rotavirus/about/transmission.html
- Centers for Disease Control and Prevention (CDC). Rotavirus: symptoms 2021 [ Accessed 05/May/2021]. Available from: https://www.cdc.gov/rotavirus/about/symptoms.html
- Dornbusch HJ, Vesikari T, Guarino A, et al. Rotavirus vaccination for all children or subgroups only? Comment of the European Academy of Paediatrics (EAP) and the European Society for Paediatric Infectious Diseases (ESPID) recommendation group for rotavirus vaccination. Eur J Pediatr. 2020;179(9):1489–1493.
- World Health Organization. Weekly Epidmiological Record 2009 [Cited 30/March/2021]. Available from: https://www.who.int/wer/2009/wer8450.pdf
- Steele AD, Madhi SA, Cunliffe NA, et al. Incidence of rotavirus gastroenteritis by age in African, Asian and European children: relevance for timing of rotavirus vaccination. Hum Vaccines Immunother. 2016;12(9):2406–2412.
- Aliabadi N, Antoni S, Mwenda JM, et al. Global impact of rotavirus vaccine introduction on rotavirus hospitalisations among children under 5 years of age, 2008-16: findings from the Global Rotavirus Surveillance Network. Lancet Glob Health. 2019;7(7):e893–e903.
- Pediatric ROTavirus European CommitTee (PROTECT) The paediatric burden of rotavirus disease in Europe. Epidemiol Infect. 2006. 0950–2688. Print
- GlaxoSmithKline Biologicals. Rotarix Prescribing Information 2019 [Cited 12/May/2021]. Available from: https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Rotarix/pdf/ROTARIX-PI-PIL.PDF
- Merck Sharp & Dohme Corp. RotaTeq prescribing Information 2020 [ Accessed 30/04/2021]. Available from: https://www.merck.com/product/usa/pi_circulars/r/rotateq/rotateq_pi.pdf
- World Health Organization. Rotavirus Vaccines: WHO position paper 2007 [Accessed 19/March/2022]. Available from: https://apps.who.int/iris/bitstream/handle/10665/240983/WER8232_285-295.PDF.
- Poelaert D, Pereira P, Gardner R, et al. A review of recommendations for rotavirus vaccination in Europe: arguments for change. Vaccine. 2018;36(17):2243–2253.
- ERR News. Algab väikelaste vaktsineerimine rotaviiruse vastu 2014 [Cited 19/March/2022]. Available from: https://www.err.ee/514868/algab-vaikelaste-vaktsineerimine-rotaviiruse-vastu
- LIKUMI. Vakcinācijas noteikumi 2022. [ Accessed 19/March/2022]. Available from: https://likumi.lv/ta/id/11215-vakcinacijas-noteikumi
- Terveyden ja Hyvinvoinnin Laitos. Infektiotaudit Ja Rokotukset Milloin Eri Rokotukset Ovat Alkaneet Suomessa? 2022 [Cited 19/March/2022]. Available from: https://thl.fi/fi/web/infektiotaudit-ja-rokotukset/tietoa-rokotuksista/kansallinen-rokotusohjelma/milloin-eri-rokotukset-ovat-alkaneet-suomessa-
- National Immunisation Office. Rotavirus: the Disease and The Vaccine 2022 [ Accessed 19/March/2022]. Aviailable from: https://www.hse.ie/eng/health/immunisation/hcpinfo/othervaccines/rotavirus/?msclkid=0a70297fba3f11ecb8cb8a316c7e8b50
- Gov UK Rotavirus immunisation programme for infants 2021 [ Accessed 19/March/2022]. Accessed https://www.gov.uk/government/collections/rotavirus-vaccination-progarmme-for-infants?msclkid=909d34a9ba4211ecb8d65e2dfe12f959
- World Health Organization. Vaccine introduction in Germany 2021 [ Accessed 19/March/2022]. Available from: https://immunizationdata.who.int/pages/vaccine-intro-by-country/deu.html?YEAR=
- Nacionalinis visuomenes sveikatos centras prie Sveikatos apsaugos ministerijos. Kitąmet vaikai bus skiepijami nuo rotavirusinės infekcijos 2017 [ Accessed 19/March/2022]. Available from: https://nvsc.lrv.lt/lt/naujienos/kitamet-vaikai-bus-skiepijami-nuo-rotavirusines-infekcijos
- Gov PL Komunikat w sprawie realizacji szczepień przeciwko rotawirusom w ramach obowiązkowego Programu Szczepień Ochronnych na rok 2021 [ Accessed 19/March/2022]. Accessed https://www.gov.pl/web/zdrowie/komunikat-w-sprawie-realizacji-szczepien-przeciwko-rotawirusom-w-ramach-obowiazkowego-programu-szczepien-ochronnych-na-rok-2021
- DerStandard. Neuer Impfplan mit Impfpflicht 2006 [ Accessed 19/March/2022]. Available from: https://www.derstandard.at/story/2356614/neuer-impfplan-mit-impfpflicht
- Bundesminiterium Soziales, Gesundheit, Pflege und Konsumentenschutz. Rotavirus (Brechdurchfall) 2020 [Cited 19/March/2022]. Available from: https://www.sozialministerium.at/Themen/Gesundheit/Uebertragbare-Krankheiten/Infektionskrankheiten-A-Z/Rotavirus-(Brechdurchfall).html
- Bundesminiterium Soziales, Gesundheit, Pflege und Konsumentenschutz., 2022 [Cited 19/March/2022]. Available from: sozialministerium.at/Themen/Gesundheit/Impfen/Kostenfreies-Kinderimpfprogramm.html
- Agenzia italiana del farmaco. Il calendario vaccinale del Piano Nazionale di Prevenzione Vaccinale 2017-2019 2019 [ Accessed 19/March/2022]. Available from: https://www.salute.gov.it/imgs/C_17_pagineAree_4829_listaFile_itemName_0_file.pdf
- Υγείας Υ. Εθνικό Πρόγραμμα Εμβολιασμών Παιδιών και Εφήβων 2022 [ Accessed 19/March/2022]. Available from: https://www.moh.gov.gr/articles/health/dieythynsh-dhmosias-ygieinhs/emboliasmoi/ethniko-programma-emboliasmwn-epe-paidiwn-kai-efhbwn/10289-ethniko-programma-emboliasmwn-paidiwn-kai-efhbwn-2022
- World Health Organization. Vaccine introduction in Albania 2021 [ Accessed 19/March/2022]. Available from: https://immunizationdata.who.int/pages/vaccine-intro-by-country/alb.html?YEAR=
- World Health Organization. Vaccine introduction in Luxembourg 2021 [ Accessed 19/March/2022]. Available from: https://immunizationdata.who.int/pages/vaccine-intro-by-country/lux.html?YEAR=
- Folkhälsomyndigheten. Barnvaccinationsprogrammet i Sverige 2019 – Årsrapport 2019 [ Accessed 19/March/2022]. Available from: https://www.folkhalsomyndigheten.se/publicerat-material/publikationsarkiv/b/barnvaccinationsprogrammet-i-sverige-2019/
- Folkhelseinstituttet. Rotavirusvaksine - veileder for helsepersonell 2019 [ Accessed 19/March/2022]. Available from: https://www.fhi.no/nettpub/vaksinasjonsveilederen-for-helsepersonell/vaksiner-mot-de-enkelte-sykdommene/rotavirusvaksinasjon—veileder-for/
- World Health Organization. Vaccine introduction in Bulgaria 2021 [ Accessed 13/March/2022]. Available from: https://immunizationdata.who.int/pages/vaccine-intro-by-country/bgr.html?YEAR=
- Mинистерство на здравеопазването. Национална програма за контрол и лечение на ротавирусните гастроентерити в Република България 2017-2021 г 2021 [ Accessed 13/March/2022]. Available from: https://www.strategy.bg/StrategicDocuments/View.aspx?lang=bg-BG&Id=1221
- Higgins JPT TJ, Chandler J, Cumpston M, et al. Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). Cochrane, 2021. [Cited 11/May/2021]. Available from: www.training.cochrane.org/handbook
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- Motheral B, Brooks J, Clark MA, et al. A checklist for retrospective database studies–report of the ISPOR task force on retrospective databases. Value Health. 2003;6(2):90–97.
- Hungerford DA-O, Vivancos R, Read JM, et al. Rotavirus vaccine impact and socioeconomic deprivation: an interrupted time-series analysis of gastrointestinal disease outcomes across primary and secondary care in the UK. BMC Med. 2018;16(10). DOI:10.1186/s12916-017-0989-z.
- Martinon-Torres F, Martinon-Torres N, Bouzon Alejandro M, et al. Acute gastroenteritis hospitalizations among children aged < 5 years before and after introduction of rotavirus vaccines: a hospital-based surveillance study in Galicia, Spain [Research Support, Non-U.S. Gov’t]. Hum Vaccines Immunother. 2012;8(7):946–952.
- Orrico-Sanchez A, Lopez-Lacort M, Perez-Vilar S, et al. Long-term impact of self-financed rotavirus vaccines on rotavirus-associated hospitalizations and costs in the Valencia Region, Spain. BMC Infect Dis. 2017;17(1). DOI: 10.1186/s12879-017-2380-2.
- Redondo O, Cano R, Simon L. Decline in rotavirus hospitalizations following the first three years of vaccination in Castile-La Mancha, Spain. Hum Vaccines Immunother. 2015;11(3):769–775.
- Bruun T, Salamanca BV, Bekkevold T, et al. Impact of the rotavirus vaccination program in Norway after four years with high coverage. Pediatr Infect Dis J. 2021;40(4):368–374.
- Coveney J, Barrett M, Fitzpatrick P, et al. National rotavirus vaccination programme implementation and gastroenteritis presentations: the paediatric emergency medicine perspective. Ir J Med Sci. 2020;189(1):327–332.
- Vesikari T, Uhari M, Renko M, et al. Impact and effectiveness of rotateq vaccine based on 3 years of surveillance following introduction of a rotavirus immunization program in Finland. Pediatr Infect Dis J. 2013;32(12):1365–1373.
- Leino T, Ollgren J, Salo H, et al. First year experience of rotavirus immunisation programme in Finland. Vaccine. 2012;31(1):176–182.
- Leino T, Baum U, Scott P, et al., Impact of five years of rotavirus vaccination in Finland - And the associated cost savings in secondary healthcare. Vaccine. 35(42): 5611–5617. 2017.
- Hartwig S, Uhari M, Renko M, et al. Hospital bed occupancy for rotavirus and all cause acute gastroenteritis in two Finnish hospitals before and after the implementation of the national rotavirus vaccination program with RotaTeq [Multicenter Study Observational Study Research Support, Non-U.S. Gov’t]. BMC Health Serv Res. 2014;14:632.
- Solastie A, Leino T, Ollgren J. Success of rotavirus vaccination in Finland, a register based study measuring impact beyond overall effectiveness. Vaccine. 2020;38(21):3766–3772.
- Thomas SL, Walker JL, Fenty J, et al. Impact of the national rotavirus vaccination programme on acute gastroenteritis in England and associated costs averted. Vaccine. 2017;35(4):680–686.
- Vaas HJ, Tamm E Impact of the national rotavirus immunisation programme on hospitalisation of children with all- cause and rotaviral gastroenteritis. ESPID 37th Annual Meeting, Ljubljana, Solvenia, May 6–11 2019.
- Koivumagi K, Toompere K, Soeorg H, et al. Acute gastroenteritis hospitalizations after implementation of universal mass vaccination against rotavirus. Vaccine. 2020;38(13):2879–2886.
- Paulke-Korinek M, Kollaritsch H, Aberle SW, et al. Sustained low hospitalization rates after four years of rotavirus mass vaccination in Austria. Vaccine. 2013;31(24):2686–2691.
- Forrest R, Jones L, Willocks L, et al. Impact of the introduction of rotavirus vaccination on paediatric hospital admissions, Lothian, Scotland: a retrospective observational study. Arch Dis Child. 2017;102(4):323–327.
- Redondo-Gonzalez O, Tenias-Burillo JM, Ruiz-Gonzalo J. Impact of self-financed rotavirus vaccines on hospital stays and costs in Spain after a 3-year introductory period. Epidemiol Infect. 2017;145(9):1773–1785.
- Heggie R, Murdoch H, Cameron C, et al. Cost-impact study of rotavirus vaccination programme in Scotland. Hum Vaccines Immunother. 2019;15(6):1265–1271.
- Restivo V, Caracci F, Sannasardo CE, et al. Rotavirus gastroenteritis hospitalization rates and correlation with rotavirus vaccination coverage in Sicily [Research Support, Non-U.S. Gov’t]. Acta Biomed Ateneo Parmense. 2018;89(3):437–442.
- Costantino C, Restivo V, Tramuto F, et al. Universal rotavirus vaccination program in Sicily: reduction in health burden and cost despite low vaccination coverage. Hum Vaccines Immunother. 2018;14(9):2297–2302.
- Crealey M, Hayden J, Delaney I, et al. Hospitalisation with rotavirus gastroenteritis before and after rotavirus vaccine introduction [Conference Abstract]. Arch Dis Child. 2019;104(Supplement 3):A107.
- Gil-Prieto R, Gonzalez-Escalada A, Alvaro-Meca A, et al. Impact of non-routine rotavirus vaccination on hospitalizations for diarrhoea and rotavirus infections in Spain. Vaccine. 2013;31(43):5000–5004.
- Pietsch C, Liebert UG. Rotavirus vaccine effectiveness in preventing hospitalizations due to gastroenteritis: a descriptive epidemiological study from Germany. Clin Microbiol Infect. 2019;25(1):102–106.
- Atchison CJ, Stowe J, Andrews N, et al. Rapid declines in age group-specific rotavirus infection and acute gastroenteritis among vaccinated and unvaccinated individuals within 1 year of rotavirus vaccine introduction in England and wales. J Infect Dis. 2016;213(2):243–249.
- Uhlig U, Kostev K, Schuster V, et al. Impact of rotavirus vaccination in Germany: rotavirus surveillance, hospitalization, side effects and comparison of vaccines. Pediatr Infect Dis J. 2014;33(11):e299–e304.
- Sabbe M, Berger N, Blommaert A, et al. Sustained low rotavirus activity and hospitalisation rates in the post-vaccination era in Belgium, 2007 to 2014. Eurosurveillance. 2016;21(27). DOI:10.2807/1560-7917.ES.2016.21.27.30273.
- Hemming M, Räsänen S, Huhti L, et al. Major reduction of rotavirus, but not norovirus, gastroenteritis in children seen in hospital after the introduction of RotaTeq vaccine into the National Immunization Programme in Finland. Eur J Pediatr. 2013;172(6):739–746.
- Armstrong G, Gallagher N, Cabrey P, et al. A population based study comparing changes in rotavirus burden on the Island of Ireland between a highly vaccinated population and an unvaccinated population. Vaccine. 2016;34(39):4718–4723.
- Raes M, Strens D, Vergison A, et al. Reduction in pediatric rotavirus-related hospitalizations after universal rotavirus vaccination in Belgium [Research Support, Non-U.S. Gov’t]. Pediatr Infect Dis J. 2011;30(7):e120–125.
- Standaert B, Alwan A, Strens D, et al. Improvement in hospital quality of care (Qoc) after the introduction of rotavirus vaccination: an evaluation study in Belgium. Hum Vaccines Immunother. 2015;11(9):2266–2273.
- Kittel PA. The impact of the recommendation of routine rotavirus vaccination in Germany: an interrupted time-series analysis [Observational Study]. Vaccine. 2018;36(2):243–247.
- Zeller M, Rahman M, Heylen E, et al. Rotavirus incidence and genotype distribution before and after national rotavirus vaccine introduction in Belgium. Vaccine. 2010;28(47):7507–7513.
- Marquis A, Koch J. Impact of Routine Rotavirus Vaccination in Germany: evaluation Five Years After Its Introduction. Pediatr Infect Dis J. 2020;39(7):e109–e116.
- Paulke-Korinek M, Rendi-Wagner P, Kundi M, et al. Universal mass vaccination against rotavirus gastroenteritis: impact on hospitalization rates in Austrian children. Pediatr Infect Dis J. 2010;29(4):319–323.
- Hanquet G, Ducoffre G, Vergison A, et al. Impact of rotavirus vaccination on laboratory confirmed cases in Belgium. Vaccine. 2011;29(29–30):4698–4703.
- Standaert B, Strens D, Li X, et al. The sustained rotavirus vaccination impact on nosocomial infection, duration of hospital stay, and age: the rotabis study (2005-2012). Infect Dis Ther. 2016;5(4):509–524.
- Zlamy M, Kofler S, Orth D, et al. The impact of Rotavirus mass vaccination on hospitalization rates, nosocomial Rotavirus gastroenteritis and secondary blood stream infections. BMC Infect Dis. 2013;13(1). DOI:10.1186/1471-2334-13-112.
- Inns T, Trindall A, Dunling-Hall S, et al. Introduction of a new Rotavirus vaccine: initial results of uptake and impact on laboratory confirmed cases in Anglia and Essex, United Kingdom. Hum Vaccines Immunother. 2015 [Jul];12(4):1040–1044.
- Marlow R, Muir P, Vipond B, et al. Assessing the impacts of the first year of rotavirus vaccination in the United Kingdom. Eurosurveillance. 2015;20(48). DOI: 10.2807/1560-7917.ES.2015.20.48.30077.
- Perez-Rubio A, Luquero FJ, Bachiller Luque MR, et al. Impact of the rotavirus vaccine in Valladolid, Spain: an interrupted time series analysis. Trials Vaccinol. 2016;5:84–87.
- Marlow RL M, Vipond B, Muir P, et al. Assessing the ongoing impact of rotavirus vaccination in the United Kingdom. ESPID 37th Annual Meeting, Ljubljana, Slovenia, May 6–11 2019.
- Akhtar T, Cargill J, Gerrard C, et al. Detection of rotavirus in paediatric oncology patients with diarrhoea: the impact of rotavirus vaccine. J Hosp Infect. 2018;99(2):185–187.
- Standaert B, Van De Mieroop E, Nelen V. Exploring the potential impact of rotavirus vaccination on work absenteeism among female administrative personnel of the City of Antwerp through a retrospective database analysis. BMJ Open. 2015;5(6):e007453.
- Ask LS, Liu C, Gauffin K, et al. The effect of rotavirus vaccine on socioeconomic differentials of paediatric care due to gastroenteritis in Swedish infants. Int J Environ Res Public Health. 2019;16(7):1095.
- Braeckman T, Van Herck K, Raes M, et al. Rotavirus vaccines in Belgium: policy and impact. Pediatr Infect Dis J. 2011;30(SUPPL. 1):S21–S24.
- Raes MS D, Benninghof B, Pereira P, et al. ESPID 38th Annual Meeting (Virtual Meeting). October 26-29 2020.
- Álvarez Aldeán J, Ares Segura S, Díaz González C, et al. Recommendations for vaccination against ROTAvirus in PREMature newborns (ROTAPREM) Recomendaciones para la vacunación frente al ROTAvirus de los recién nacidos PREMaturos (ROTAPREM). Anales de Pediatría. 2019;91(3):205.e1–205.e7.
- Zeevat F, Dvortsin E, Wondimu A, et al. Rotavirus vaccination of infants delayed and limited within the national immunization programme in the Netherlands: an opportunity lost. Vaccines (Basel). 2021;9(2):144.
- Karafillakis E, Hassounah S, Atchison C. Effectiveness and impact of rotavirus vaccines in Europe, 2006-2014. Vaccine. 2015;33(18):2097–2107.
- Burnett E, Parashar UD, Tate JE. Global impact of Rotavirus vaccination on diarrhea hospitalizations and deaths among children <5 years old: 2006-2019. J Infect Dis. 2020;222(10):1731–1739.
- Lopez-Lacort M, Orrico-Sanchez A, Martinez-Beneito MA, et al. Spatio-temporal impact of self-financed rotavirus vaccination on rotavirus and acute gastroenteritis hospitalisations in the Valencia region, Spain. BMC Infect Dis. 2020;20(1). DOI: 10.1186/s12879-020-05373-0.
- Mast TC, Heyman D, Dasbach E, et al. Planning for monitoring the introduction and effectiveness of new vaccines using real-word data and geospatial visualization: an example using rotavirus vaccines with potential application to SARS-CoV-2. Vaccine. 2021;7:100084.
- Baker JM, Dahl RM, Cubilo J, et al., Effects of the rotavirus vaccine program across age groups in the United States: analysis of national claims data, 2001–2016. BMC Infect Dis. 19(1): 186. 2019.
- Lopman BA, Curns A, Yen C, et al., Infant rotavirus vaccination may provide indirect protection to older children and adults in the United States. J Infect Dis. 204(7): 980–986. 2011.
- Díez-Domingo J, Garcés-Sánchez M, Giménez-Sánchez F, et al. What have we learnt about rotavirus in Spain in the last 10 years? Anales de Pediatría. 2019;91(3):166–179.
- Ruiz-Contreras J, Alfayate-Miguelez S, Carazo-Gallego B, et al. Rotavirus gastroenteritis hospitalizations in provinces with different vaccination coverage rates in Spain, 2013–2018. BMC Infect Dis. 2021;21(1). DOI:10.1186/s12879-021-06841-x.
- Jayasinghe S, Macartney K. Estimating rotavirus gastroenteritis hospitalisations by using hospital episode statistics before and after the introduction of rotavirus vaccine in Australia. Vaccine. 2013;31(6):967–972.
- World Health Organization. COVID-19 pandemic leads to major backsliding on childhood vaccinations, new WHO, UNICEF data shows 2021 [ Accessed November/26/2021]. Available from: https://www.who.int/news/item/15-07-2021-covid-19-pandemic-leads-to-major-backsliding-on-childhood-vaccinations-new-who-unicef-data-shows*Data indicate that the COVID19 pandemic had a*Data indicate that the COVID19 pandemic had a significant impact on routine vaccinations worldwide.
- DeSilva MB, Haapala J, Vazquez-Benitez G, et al. Association of the COVID-19 pandemic with routine childhood vaccination rates and proportion up to date with vaccinations across 8 US health systems in the vaccine safety datalink. JAMA Pediatr. 2022;176(1):68–77.
- Kujawski SA, Yao L, Wang HE, et al. Impact of the COVID-19 pandemic on pediatric and adolescent vaccinations and well child visits in the United States: a database analysis. Vaccine. 2022;40(5):706–713.
- Scarano SM, Lo Vecchio A. How to make up for Rotavirus vaccination missed during the COVID-19 pandemic? JAMA Pediatr. 2022;176(4):420.
- Bell S, Clarke R, Paterson P, et al. Parents’ and guardians’ views and experiences of accessing routine childhood vaccinations during the coronavirus (COVID-19) pandemic: a mixed methods study in England. PLOS ONE. 2021;15(12):e0244049.
- Causey K, Fullman N, Sorensen RJD, et al. Estimating global and regional disruptions to routine childhood vaccine coverage during the COVID-19 pandemic in 2020: a modelling study. Lancet. 2021;398(10299):522–534.
- McDonald HI, Tessier E, White JM, et al. Early impact of the coronavirus disease (COVID-19) pandemic and physical distancing measures on routine childhood vaccinations in England, January to April 2020. Euro Surveillance. 2020;25(19):2000848. bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin.
- Bouzón Alejandro M, Diez Domingo J, Martinón-Torres F, et al. Circovirus and impact of temporary withdrawal of rotavirus vaccines in Spain. Hum Vaccin. 2011;7(7):798–799.
- Martinón-Torres F, Aramburo A, Martinón-Torres N, et al. A reverse evidence of rotavirus vaccines impact. Hum Vaccines Immunother. 2013;9(6):1289–1291.
- World Health Organization. Rotavirus vaccines WHO position paper – January 2013 2013. [Accessed 2021 November 26]. Available from: https://apps.who.int/iris/bitstream/handle/10665/242024/WER8805_49-64.PDF?sequence=1&isAllowed=y
- European Centre for Disease Prevention and Control. Vaccine scheduler. Rotavirus infection: recommended vaccinations 2021 [ Accessed November/26/2021]. Available from: https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=32&SelectedCountryIdByDisease=−1
- Sheikh S, Biundo E, Courcier S, et al. A report on the status of vaccination in Europe. Vaccine. 2018;36(33):4979–4992.