ABSTRACT
Introduction
Routine infant primary series and toddler booster vaccination are associated with waning of antibody levels over time, which can lead to an increased incidence of vaccine-preventable diseases. A diphtheria-tetanus-pertussis (DTP) booster vaccination at school-entry (aged 4–7 years) allows continued protection against these diseases and is included in many national immunization programs.
Areas covered
The available immunogenicity and safety data from 6 clinical studies of a diphtheria-tetanus-acellular pertussis-inactivated poliovirus vaccine (DTaP-IPV [Tetraxim®]) used as a school-entry booster vaccination were identified using a PubMed search or on file at Sanofi. The studies spanned a 15-year period (1995–2010) and were performed in different populations using different study designs, so all data were reviewed descriptively (no meta-analyses were conducted). Additionally, post-marketing experience was reviewed.
Expert opinion
Each vaccine antigen is highly immunogenic, and the safety profile of the vaccine is satisfactory. Post-marketing evaluations have shown the effectiveness of a school-age booster, particularly against increased pertussis disease incidence around the time of school entry and the associated risk of spreading the disease through contact with younger vulnerable infants. School-entry provides an ideal opportunity to implement DTaP-IPV vaccination to close the gap between waning immunity from the previous infant/toddler vaccination and future adolescent vaccination.
1. Introduction
1.1. Burden of diphtheria, tetanus, pertussis, and poliomyelitis disease and the medical rationale of a school-entry booster
Since the introduction of broad vaccination programs targeting diphtheria (D), tetanus (T), pertussis (P), and poliomyelitis in 1974 as part of the WHO Expanded Programme on Immunization [Citation1], most children are now immunologically primed against these diseases [Citation2,Citation3] during infancy, and DTP-polio booster vaccinations are usually recommended at older ages to ensure the persistence of high protection. Such routine vaccination programs have dramatically reduced the burden of these diseases. However, in areas with suboptimal vaccine coverage rates (VCR) or a suboptimal vaccination schedule (i.e. the absence of recommended booster doses), these diseases can still cause vaccine-preventable morbidity and mortality in children. Recently, with the coronavirus disease 2019 (COVID-19) pandemic, there have been significant reductions in VCR in certain regions, leading to an increase in the pool of individuals who are susceptible to these vaccine preventable diseases [Citation4–7].
Strains of Corynebacterium diphtheriae or Corynebacterium ulcerans can cause diphtheria disease. In countries with robust diphtheria vaccination programs, the incidence of diphtheria is extremely low [Citation8–10]. However, diphtheria remains endemic in some areas of the world and regular small diphtheria outbreaks/resurgence are reported, mainly from Southeast Asia, the Indian subcontinent, South America, Africa, and Eastern Europe [Citation11–15]. This reflects inadequate VCR and demonstrates the importance of sustaining high levels of immunity through the highest possible coverage in childhood, adolescence, and adulthood [Citation16]. Individuals who are unvaccinated or incompletely vaccinated can also contract diphtheria during travel to endemic areas, as the bacterium spreads mainly through respiratory droplets. The World Health Organization (WHO) recommends a three-dose primary series as the foundation for building lifelong immunity to diphtheria. But in the absence of natural boosting, the humoral immunity conferred by primary vaccination wanes over time [Citation10,Citation16,Citation17] and booster doses are hence needed for continued protection. WHO’s recommendations include a minimum of three booster doses: one during the second year of life (at 12–23 months of age), one at primary school entry (4–7 years of age), and one during adolescence (9–15 years of age) [Citation16].
Tetanus is a non-communicable disease with no natural immunity, caused by Clostridium tetani spores, and cannot be eliminated or eradicated [Citation17,Citation18]. Prevention through vaccination is the best option against this non-eradicable disease [Citation18]. After the third primary immunization dose, each additional dose increases antitoxin antibody levels and prolongs the duration of immunity [Citation18]. A school-entry booster is needed to compensate for waning of immunity induced by primary vaccination, and provides protection throughout adolescence [Citation19].
The primary aim of pertussis vaccination is to reduce the risk of severe pertussis in infants and young children who suffer the highest morbidity and mortality when infected [Citation20]. Pertussis vaccination coverage is variable across countries, and pertussis disease continues to be of public health concern as a re-emerging infectious disease, with outbreaks or trends toward increased incidence being reported in several parts of the world [Citation21–27]. Moreover, school-age children, in whom immunity acquired from primary series vaccination has waned, constitute a reservoir of susceptible individuals to pertussis disease, and are recognized as a source of infection of younger infants as vaccination will not protect them from colonization and therefore transmission [Citation28–31]. Bordetella pertussis in the event of pertussis disease is highly transmissible, with a secondary attack rate up to 90% among non-immune household contacts [Citation20]. The impact of school-entry boosting of immunity against the disease is both to protect school-age children directly and also to confer indirect protection to other populations via herd immunity, with reduced coughing due to pertussis leading to less transmission to household contacts [Citation32–34].
Since 1988, the WHO Global Polio Eradication Initiative has made tremendous progress toward the elimination of wild poliovirus transmission globally, and mass vaccination programs have contributed to the global eradication of wild poliovirus (WPV) serotype 2 and serotype 3 [Citation35]. Currently, polio cases due to circulating WPV serotype 1 remain in Afghanistan and Pakistan, but since 2018 there has been an increasing number of acute flaccid paralysis cases due to circulating vaccine-derived polioviruses (cVDPV), almost exclusively poliovirus type 2 and mainly in Pakistan, Afghanistan, Ethiopia, and Western Africa countries [Citation36,Citation37]. Hence, the goal of global eradication remains unmet (February 2022) and may be jeopardized by this continuous circulation of WPV1 and cVDPV2 associated with the suboptimal routine use of the bivalent oral poliovirus vaccine type 1 and 3 (bOPV1&3). Additionally, the COVID-19 pandemic has disrupted vaccination programs and the incidence of polio was higher in 2020 than the previous 5 years. Polio vaccination recommendations vary according to the epidemiologic background of a given country, and should target sustained high VCR in all populations in support of the global commitment to eradicate polio [Citation38].
Many countries have implemented DTP-based booster vaccination recommendations at school-entry. In Europe, various recommendations for primary series with or without first toddler booster schedules can be found (the so-called 2 + 1, 3 + 1, or 3 + 0 regimens), and a large majority of European countries also recommend a school-entry booster for DTP and polio [Citation39]. This is also the case in the United States [Citation40], Canada [Citation41], Australia [Citation42], a handful of countries in Asia [Citation43], and Latin America [Citation44,Citation45].
1.2. History of the development of the DTaP-IPV vaccine (Tetraxim)
A DTP vaccine that included a two-component acellular pertussis vaccine valence (containing Pertussis Toxoid [PT] and filamentous hemagglutinin [FHA] antigens) (i.e. DTaP) was developed by Sanofi and its ancestral companies (Institut Mérieux, Pasteur Mérieux, Sérums & Vaccins, Pasteur Mérieux Connaught, Aventis Pasteur). This DTaP vaccine is manufactured in France and constitutes the core of various combinations with other pediatric vaccine antigens, including a tetravalent DTaP-IPV vaccine (Tetravac®/Tetraxim®), a pentavalent DTaP-IPV//PRP~T vaccine (Pentavac®/Pentaxim®), and a hexavalent DTaP-IPV-HB-PRP~T vaccine (Hexyon®/Hexaxim®/ Hexacima®), which have been studied extensively [Citation46–49].
The clinical development of this DTaP backbone began in 1986 with a Phase I safety study in adults, and continued with several Phase II dose–response studies to establish safety and immunogenicity [Citation50,Citation51]. These clinical studies constituted the Phase I and II parts of the Clinical Development Program (CDP) of the DTaP-IPV product that was subsequently developed. In three Phase II studies, conducted in the United Kingdom (UK), the United States (US), and Senegal, the DTaP vaccine was reported to have a better safety profile than a whole-cell pertussis (wP)-containing vaccine [Citation52–55]. As there is no established immunological correlate of protection for pertussis, evidence of efficacy against pertussis disease for Sanofi’s 2-component aP vaccine valence was demonstrated in a large-scale Phase III efficacy study conducted in rural Senegal between 1990 and 1994 [Citation56]. In this randomized, double-blind, cluster-controlled trial, the relative efficacy for DTaP versus DTwP was assessed in >4,000 children vaccinated at 2-4-6 months of age and followed up weekly to 4 years. Results showed that in children aged up to 18 months, the protective efficacy induced by the DTaP vaccine over the 12 month-period after the third dose was comparable to the DTwP vaccine (RR aP/wP = 1.13, 95% CI: 0.66–1.95) when using the stringent and specific WHO pertussis case definition (i.e. ≥21 consecutive days of paroxysmal cough) associated with Bordetella pertussis detection by polymerase chain reaction (PCR). In children aged over 18 months, this ratio increased, indicating a shorter duration of protection for the DTaP vaccine beyond this age and suggesting the need for booster vaccination during the second year of life [Citation56]. In addition, the absolute efficacy of the DTaP and DTwP vaccines was estimated through a prospective nested household-case-contact study in the same area and same follow-up period. When considering cases involving 21 or more days of paroxysmal cough that had PCR-validated detection of B. pertussis, efficacy of 85% (95% CI: 58–89%) for DTaP and 96% (95% CI: 86–99%) for the DTwP vaccine were estimated. For cases of 21 or more days of paroxysmal cough but without laboratory validation, efficacy of 74% (95% CI: 51–86%) was reported for the DTaP vaccine.
Based on this DTaP backbone, Sanofi developed the DTaP-IPV pediatric combination vaccine (trade name Tetraxim® or Tetravac®, depending on the country), hereafter denominated Tetraxim. The vaccine contains purified diphtheria toxoid (≥20 IU), purified tetanus toxoid (≥40 IU), purified pertussis toxoid (25 µg), purified filamentous hemagglutinin (25 µg), inactivated type 1 poliovirus (Mahoney) (D-antigen, 40 units), inactivated type 2 poliovirus (MEF1) (D-antigen, 8 units), inactivated type 3 poliovirus (Saukett) (D-antigen, 32 units), aluminum hydroxide (0.3 mg), formaldehyde (10 µg), 2-phenoxyethanol (50% solution: 2.5 µl phenoxyethanol, 2.5 µL ethanol anhydrous), Medium 199 (phenol red free) (0.05 mL), and water for injection (≤0.5 mL).
Tetraxim is licensed for active immunization against D, T, P, and poliomyelitis for primary vaccination in infants and for booster vaccination in children who have previously received a primary vaccination with this vaccine or any wP- or aP-based vaccine.
The use of pediatric combination vaccines provides the advantages of reducing the number of injections and the number of vaccination visits, which can help improve acceptance and compliance and thus optimize VCR and timeliness of vaccination at the population level [Citation57–62]. The combination of DTaP and IPV in Tetraxim reduces the number of injections, simplifying the administration procedure and reducing the risk of a missed dose [Citation63]. From a logistical point of view, it also simplifies vaccine purchase, storage, and record-keeping.
With the later deployment of penta- and then hexavalent vaccines as primary series and toddler booster vaccines around the globe, nowadays Tetraxim is almost exclusively used as a school-entry booster vaccine. This review presents the clinical experience with Tetraxim as a school-entry booster vaccine, and its epidemiological impact and effectiveness in the real-world setting.
2. Body
2.1. Clinical trial experience – Tetraxim as a school-entry booster
The clinical development of Tetraxim was conducted in various geographical regions, including Europe, Asia, and Latin America. More than 3600 subjects received at least one dose of Tetraxim during its clinical development. PubMed was searched up to February 2022 for studies reporting the safety and/or immunogenicity of Tetraxim; in addition, unpublished data (on file at Sanofi) were included where appropriate. As the present review focuses on Tetraxim used as a school-entry booster, only the clinical trials performed with the objective of assessing the immunogenicity and safety of Tetraxim as a school-entry booster, regardless of the type of primary series vaccines (i.e. wP- or aP-based vaccines), were included (). All these clinical studies were sponsored by Sanofi (or its ancestral companies) (Study 1 [data on file], Study 2 [Citation64], and Study 6 [Citation65]) or SP-MSD (Study 3 [Citation66] and Study 5 [Citation67,Citation68]), except Study 4 [Citation69] which was sponsored by GlaxoSmithKline (GSK). All were conducted according to global and local Good Clinical Practices and the applicable regulations in force at the time. As the studies were conducted over a long period of time (from 1995 to 2010) in different populations and using different study designs, meta-analyses were not performed and all data were reviewed descriptively on a trial-by-trial basis without head-to-head comparisons.
Table 1. Summary of DTaP-IPV or Tetraxim immunogenicity and safety clinical studies for the school-entry booster vaccination.
2.2. Serological evaluation
The serological assays were managed by Sanofi’s clinical immunology laboratories, either in France or in the US (except for the study sponsored by GSK [Citation69], for which serological assays were performed at GSK laboratories). For tests conducted by Sanofi’s laboratories, the individual assays used to assess the immune responses were comprehensively validated and antibodies titers/concentration were determined as follows:
Diphtheria: anti-diphtheria toxoid antibodies were evaluated using either toxin or seroneutralization on Vero cells using a micrometabolic inhibition or a pH development, which measured the ability of antibodies to neutralize the biological effects of the diphtheria toxin, or immunoglobulin G enzyme-linked immunoassay (ELISA), with results expressed in IU/mL [Citation70,Citation71].
Tetanus: anti-tetanus toxoid antibodies were evaluated using an immunoglobulin G ELISA, with results expressed in IU/mL [Citation17].
Pertussis: anti-PT and anti-FHA antibodies were evaluated using an immunoglobulin G ELISA, with results expressed in EU/mL calibrated against the US Reference Pertussis Antisera (human) CBER lot 3 [Citation72]. (In some studies, additional titration of the anti-PT was performed by seroneutralization on Chinese Hamster Ovary cells [data not presented]) [Citation73,Citation74].
Polio: anti-polio type 1, 2, and 3 antibodies were evaluated using a wild-type poliovirus neutralization assay on Vero cells [Citation75–78].
2.3. Immunogenicity of tetraxim
2.3.1. Analysis of immunogenicity
Antibody concentrations against D and T ≥ 0.01 IU/mL and ≥0.1 IU/mL and antibody titers for the three poliovirus serotypes ≥1:5 or ≥1:8 (1/dil [dilution]) were assessed. For anti-polio antibodies, in older studies ≥1:5 was used and replaced by ≥1:8 according to later recommendations, although the two thresholds have been shown to correlate well and so no re-analysis was done for the studies that used the ≥1:5 threshold. For pertussis, since a correlation between the serological response to antigens and protection against pertussis is not well established, studies reported anti-pertussis antigens seroconversion rates (defined as a ≥ fourfold increase in antibody titer from pre- to post-vaccination) for both primary and booster vaccination [Citation73].
Serum samples were usually obtained before primary or booster vaccination and approximately 1 month after the last primary series dose or booster dose. Seroprotection (i.e. the percentages of infants with antibody levels above pre-defined protective levels for anti-D, -T, -polio 1, 2, 3) and seroconversion rates (i.e. the percentages of infants showing a ≥ fourfold increase in antibodies to the pertussis antigens [PT and FHA]) were calculated with their 95% confidence interval (95% CI). Antibody concentrations or titers were summarized using geometric mean concentrations (GMCs: anti-D, anti-T, anti-polio 1, 2, 3) and geometric mean titers (GMTs: anti-PT, anti-FHA), calculated by taking the antilog of the mean of the log-transformed concentrations/titers, with their 95% CI.
2.3.2. Antibodies decline after primary series vaccination during infancy, regardless of the type of vaccine or immunization schedule
Antibody levels and the percentage of subjects who were seroprotected were evaluated before and after the administration of the Tetraxim school-entry booster in Studies 2, 3 and 6 () [Citation64–66,Citation79]. In these studies, seroprotection rates 1 month post-toddler booster were also available from previous trials. In Studies 1 and 5, as infant vaccination was performed per routine practice, immunogenicity after the toddler booster dose was not assessed. Regardless of the type of vaccine used for the primary series and toddler booster dose (i.e. either wP-based or aP-based vaccines), and after a 3 + 1 schedule, there was a decline in antibodies against D, T, and poliovirus, translating into a reduced proportion of subjects still considered as seroprotected against these diseases using the full/long term protection threshold of 0.1 IU/mL (anti-D and anti-T) and ≥1:5 or ≥1:8 (1/dil) (anti-polio).
Table 2. Geometric mean antibody concentrations and titers for diphtheria, tetanus, pertussis (PT, FHA) and polio type 1, 2 and 3 before and one month after school-entry booster dose.
Table 3. Percentage of subjects reaching seroprotection rate (anti-D, anti-T, anti-polio 1, 2, 3) or seroconversion rate (anti-PT, anti-FHA) before and 1 month after school-entry booster dose.
In Study 2, children (N = 161) were previously primed with a pentavalent wP combined vaccine (DTwP-IPV-Hib, Pentacoq®) at 2–3-4 months of age and received a toddler booster of Pentaxim® at 12–16 months (sequential wP/aP 3 + 1 schedule) [Citation64]. Just before administration of the school-entry booster at 5–6 years of age, seroprotection rates for diphtheria had declined to 49% (vs. 100% after the toddler booster), and rates for tetanus had declined to 76% (vs. 100% after the toddler booster). The proportion of subjects still seroprotected against the three types of poliovirus ranged from 94% to 99%. Antibody concentrations had dramatically decreased for pertussis antigens, from 196 EU/mL to 2.9 EU/mL for anti-PT, and from 206 EU/mL to 21.5 EU/mL for anti-FHA ().
In Study 3, children (N = 232) were previously primed by a 3-dose primary series at either 2-3-4 or 2-4-6 months and a toddler booster of Pentaxim® at 12–16 months of age [Citation66]. Just before administration of the school-entry booster at 5–6 years of age, seroprotection rates for diphtheria had declined to 28% (vs. 97% after the toddler booster), and rates for tetanus had declined to 47% (vs. 100% after the toddler booster). The proportion of subjects still seroprotected against the three types of poliovirus ranged from 94% to 96%. The GMCs against each antigen had declined in the interval between the toddler booster and school-entry age. Antibody concentrations to pertussis antigens declined from 136.85 EU/mL to 2.71 EU/mL for anti-PT, and from 282 EU/mL to 43.32 EU/mL for anti-FHA ().
In Study 6, children (N = 123) were previously primed with Pentaxim® at 2-4-6 months and received a booster of the same vaccine at 18–19 months of age [Citation65,Citation79] (pure aP 3 + 1 schedule). Just before administration of the school-entry booster at 4–6 years of age, seroprotection rates for diphtheria had declined to 60.2% (vs. 95% after toddler booster) but were maintained for tetanus (99.2%). Also, 100% of subjects were still seroprotected against polio type 1, 2 and 3. However, antibody concentrations to pertussis antigens declined from 307.4 EU/mL to 10.9 EU/mL for anti-PT, and from 271.9 EU/mL to 24.3 EU/mL for anti-FHA ().
These results indicate that antibody concentrations/titers would be expected to continue to decline in the absence of booster, highlighting the need for a booster injection at school-entry age to reinforce humoral immunity and guarantee longer persistence of protection.
2.3.3. Immunogenicity of Tetraxim when given as a school-entry booster
Tetraxim used as a school-entry booster is associated with strong anamnestic responses to all antigens [Citation64–69], regardless of the time between the last DTP-polio vaccine and the school-entry booster being variable depending on the study (). For diphtheria and tetanus, 100% of children achieved seroprotection (≥0.1 IU/mL) 1 month after the Tetraxim school-entry booster in each study except for diphtheria in Study 3 (99.6%) (). For the three types of polioviruses, 100% also achieved seroprotection 1 month after the school-entry booster of Tetraxim. For pertussis, between 89.1% and 99% of children seroconverted for anti-PT, and between 79% and 92.6% seroconverted for anti-FHA (, Study 4 excluded as the study sponsor [GSK] used a different definition). In terms of geometric mean concentrations and geometric mean ratio (GMR: post-vaccination/pre-vaccination), for the Tetraxim booster, anti-PT increased from 2.2 to 128.8 EU/mL (GMR: 59.1) (Study 1), 2.90 to 246 EU/mL (GMR: 84) (Study 2), 2.71 to 129.31 (GMR: 49.4) (Study 3), 4.3 to 98.2 EU/mL (GMR not available) (Study 5) and 10.9 to 190 (GMR: 17.4) (Study 6), and anti-FHA increased from 11.8 to 257.2 EU/mL (GMR: 22.6) (Study 1), 21.5 to 426 EU/mL (GMR: 19.9) (Study 2), 43.32 to 467.04 (GMR: 11.0) (Study 3), 5.4 to 191.4 EU/mL (GMR not available) (Study 5), and 24.3 to 356 EU/mL (GMR: 14.6) (Study 6).
Tetraxim as a school-entry booster was highly immunogenic regardless of the previous vaccination history, i.e. primed with aP-based vaccines (either Pentaxim® [Studies 3 [Citation66] and 6 [Citation65]], or a DTaP-based combination vaccine [Study 4] [Citation69]), or wP-based vaccines (Studies 1 [data on file], 2 [Citation64] and 5 [Citation67,Citation68]). In Study 1, when compared to a school-entry booster with a DTwP-IPV vaccine (Tetracoq®, Sanofi), the post-vaccination geometric mean antibody concentrations and GMR was significantly higher for Tetraxim than for DTwP-IPV for most antigens (D, T, polio 1 and 3, PT, and FHA) (data not shown).
Tetraxim was also highly immunogenic regardless of the previous vaccination schedule, i.e. after a 2 + 1 schedule (Study 4 [Citation69]), a 3 + 0 schedule (Study 5 [Citation67,Citation68]) or a 3 + 1 schedule (Studies 1 [data on file], 2 [Citation64], 3 [Citation66], and 6 [Citation65]).
2.3.4. Long-term persistence of antibodies (5-year follow-up)
In Study 5 [Citation67,Citation68], children (N = 200) were followed for up to 5 years to investigate the kinetics of antibodies at 1, 3 and 5 years after the pre-school booster dose of either Tetraxim (DTaP-IPV) or a reduced – diphtheria and pertussis antigens-content vaccine (Td5ap-IPV, Repevax®/Adacel Polio® [product name depends on the country]) given at 3.5–5 years of age following a 3 + 0 regimen [Citation68]. After a rapid decline for all antibodies in the first year after pre-school booster vaccination, a high proportion of children maintained seroprotective antibody levels against D, T, and poliovirus for at least 5 years (). At this time, all participants had anti-D antibody concentrations ≥0.01 IU/mL, and 75.00% (Tdap-IPV) and 78.95% (DTaP-IPV) of children had titers ≥0.1 IU/mL. Similarly, all participants had anti-tetanus antibody concentrations ≥0.01 IU/mL at the 5-year follow-up visit, and 100% (Tdap-IPV) and 89.47% (DTaP-IPV) of children had titers ≥0.1 IU/mL. Seroprotection rates for all polio antigens were high: all participants had anti-polio 2 titer ≥1:8 for polio type 2, seroprotection rates for anti-polio 1 were 97.87% (Tdap-IPV) and 100% (DTaP-IPV) and for polio type 3 were 95.74% (Tdap-IPV) and 97.37% (DTaP-IPV). Persisting antibody levels against pertussis antigens were also observed for PT and FHA after 5 years.
Table 4. Long-term persistence of antibodies at 1, 3, and 5 years in study 5.
These data demonstrate that the Tetraxim pre-school booster dose helps to maintain high immunity from early childhood up until early adolescent age.
2.4. Safety of Tetraxim
The safety of a school-entry booster dose of Tetraxim was assessed in each of the clinical studies presented in . In two of these studies, Tetraxim was co-administered with either a measles-mumps-rubella-varicella vaccine (Priorix Tetra™) (Study 4 [Citation69]) or a measles-mumps-rubella vaccine (M-M-M™II) (Study 5 [Citation67,Citation68]). The safety profile was established using standard methods and by soliciting the occurrence of specific symptoms after vaccination (solicited events). Parents were asked to use diary cards to record any pre-listed local and systemic reactions occurring within several days following vaccination (within 4 days in the Study 4 [i.e. GSK study] [Citation69], or within 7 days in Study 1 [data on file], Study 2 [Citation64], Study 3 [Citation66], Study 5 [Citation67,Citation68], and Study 6 [Citation65] [i.e. SP or SP-MSD studies]). As these clinical trials were conducted at different periods of time, with different study designs and objectives, the pre-defined list of solicited events varied between studies. All other adverse events occurring within the 4- or 7-day period following vaccination and any unsolicited adverse events occurring up to 30 days after vaccination were also reported and analyzed (unsolicited adverse events). All serious adverse events were reported throughout each trial.
This section aims to review: 1) the safety profile of a Tetraxim school-entry booster in a population of children primed and boosted during infancy with a vaccine from the same family (i.e. DTaP-IPV//PRP~T [Pentaxim]), or with a wP vaccine; 2) the safety profile of Tetraxim vs. a wP-based vaccine at school-entry in a population of children primed and boosted with a wP-based vaccine during infancy; 3) the safety profile of standard pediatric dose DTaP-IPV (Tetraxim) vs. reduced-content diphtheria and pertussis vaccines (Tdap-IPV) when given at school-entry age.
2.4.1. Safety of Tetraxim in subjects primed with Pentaxim (same antigens) (Studies 3 and 6) or with a DTwP-based vaccine (Pentacoq®) (Study 2)
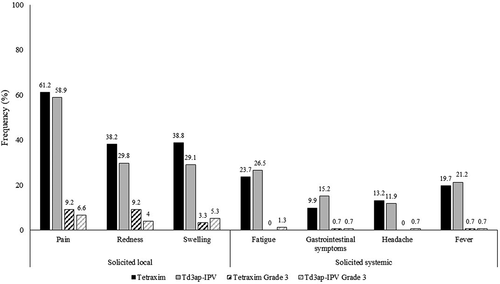
Study 2 [Citation64], Study 3 [Citation66], and Study 6 [Citation65] included a description of the safety of a Tetraxim booster dose at school-entry age after previous vaccination with a wP vaccine (Study 2) or an aP vaccine (Study 2, Study 3, and Study 4). Most children experienced at least one solicited reaction (). Most adverse events were solicited reactions that were transient and mild to moderate in severity. Local pain at the injection site was consistently the most frequently injection site reaction, and around 50% or more subjects experienced a solicited systemic reaction. No safety signal was raised during any of these clinical trials, and no serious adverse event was assessed as related to vaccination. There was no influence of the type of vaccine (ie, wP or aP) used in the primary vaccination series.
Table 5. Incidence of solicited local and solicited systemic reactions occurring up to 7 days after a school-entry booster dose of Tetraxim.
2.4.2. Comparative safety of DTaP-IPV (Tetraxim®) and a DTwP-IPV vaccine (Tetracoq®) given as second booster to 4 to 7 years old children
Study 1 (data on file) had a primary objective of assessing the safety of one dose of DTaP-IPV (Tetraxim®) given as a second booster to children aged 4 to 7 years in comparison to DTwP-IPV (Tetracoq®) () in children primed with DTwP-IPV (Tetracoq®) in a 3 + 1 schedule.
Figure 1. Comparative reactogenicity between DTaP-IPV (Tetraxim®) and DTwP-IPV (Tetracoq®) (Study 1).
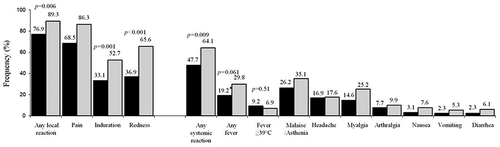
Statistical comparisons of the frequency of local and systemic reactions showed that Tetraxim was better tolerated overall than the wP-containing vaccine, with statistically fewer children presenting with any local reaction, induration, redness, and any systemic reaction. The severity of local reactions in terms of duration, size, and intensity was also lower with Tetraxim than the wP vaccine, suggesting that the aP vaccine is associated with generally milder local reactions. Overall, systemic reactions were observed more frequently in the DTwP-IPV group than in the Tetraxim group (except for fever ≥39°C which was unexpectedly high in both groups, although it should be noted that the study was carried out during the winter season when upper respiratory infections were common). Headache, myalgia, arthralgia, nausea, vomiting and diarrhea were also more frequent in the wP-containing vaccine group, although the statistical significance was not assessed. Two serious adverse events were reported (one case of paroxysmal supraventricular tachycardia [Bouveret’s syndrome] in a child with a history of tachycardia, and one case of planned adenoidectomy); both were assessed by the investigators to have no relationship with the vaccination.
2.4.3. Description of the safety of standard pediatric dose DTaP-IPV (Tetraxim®) vs. reduced antigen content vaccine (Tdap-IPV [Repevax®/Adacel®-Polio or Boostrix® Polio]) given as a school-entry booster
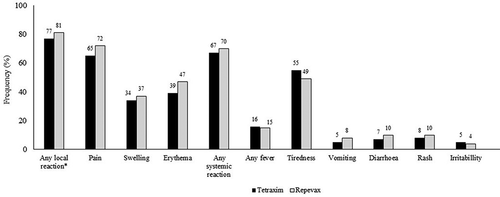
In Study 5 [Citation67,Citation68], the reactogenicity of Tetraxim given as a school-entry booster at 3.5 to 5 years of age was compared to a reduced diphtheria and pertussis antigen content vaccine (Tdap-IPV, Repevax®/Adacel®-Polio [depending on the country]), in children primed with DTwP-Hib tetravalent vaccine plus OPV vaccine in a 3 + 0 schedule (MMR vaccine [M-M-R™ II, MSD] was concomitantly administered at school-entry age) (). Overall, the incidence of both local and systemic reactions was similar across groups, indicating no evidence of higher reactogenicity of Tetraxim. The incidence of most solicited reactions actually tended to be higher in the reduced antigen vaccine group. Both the standard and the reduced antigen vaccines were thus well tolerated. It should be noted, however, that in the UK, routine immunization currently includes only 3 DTP-based vaccine doses at 2-3-4 months of age with no booster during the second year of life (3 + 0 regimen). It remains possible that the full-dose diphtheria vaccine may be more reactogenic in countries other than the UK where the school-entry booster usually corresponds to the fifth dose of diphtheria toxoid that children receive (i.e. the 3 + 1 + 1 regimen). No serious adverse events were reported in the study.
Figure 2. Description of the frequency of local and systemic adverse events of DTaP-IPV (Tetraxim ®) and Tdap-IPV (Repevax®) up to 7 days following vaccination (Study 5).
In Study 4 [Citation69], the safety profile of DTaP-IPV (Tetraxim®) or Tdap-IPV (Boostrix™ Polio) given as a school-entry booster at 5–6 years of age were described in children who had previously received 3 doses of a DTaP-based vaccine at 3, 5, and 12 months of age (2 + 1 schedule). All children received a live attenuated measles-mumps-rubella-varicella vaccine (Priorix Tetra™, GSK) concomitantly with the school-entry booster (). Overall, the incidence of both solicited local and systemic reactions were similar in both groups, although local injection site reactions were lower in the reduced antigen vaccine group compared to Tetraxim. However, the study was not powered to detect statistically significant differences between the two vaccines. Reaction rates were of the same order of magnitude as in other studies with Tetraxim used as a school-entry booster, with no increased reactogenicity associated with the concomitant administration of measles-mumps-rubella-varicella vaccine. No serious adverse events were reported in the study.
2.5. Co-administration of Tetraxim with other vaccines
In Study 4 [Citation69] and Study 5 [Citation67,Citation68], Tetraxim was co-administered with either measles-mumps-rubella-varicella vaccine or measles-mumps-rubella vaccine, respectively.
In Study 4, the immune responses to Tetraxim and measles-mumps-rubella-varicella vaccine antigens when administered simultaneously were high and showed no interference, and both vaccines showed an acceptable safety profile. In Study 5, the immune responses to Tetraxim antigens were not impaired by the co-administration of the measles-mumps-rubella vaccine, and Tetraxim was well tolerated (the immune response to measles-mumps-rubella antigens was not part of the objectives of Study 5 and hence was not evaluated).
Having the opportunity to co-administer several school-age-recommended pediatric vaccines at the same time increases the chance of timely vaccination and is associated with improved VCR [Citation80,Citation81].
Similarly, two other DTaP-IPV vaccines (Quadracel™ and Infanrix polio™/Kinrix™, GSK) have shown no interference following co-administration with measles-mumps-rubella and varicella vaccines [Citation82–84]. In a previous study (Study E2I23 [Data on File]), Tetraxim showed no interference when coadministered with a bivalent HepB-Hib vaccine in infant vaccination. Data also support the co-administration with a Haemophilus influenzae type b polysaccharide conjugated to tetanus protein vaccine [Citation85,Citation86]. No studies have specifically evaluated the co-administration of a DTaP-IPV booster vaccine with flu vaccines, and although no data are available for co-administration of Tetraxim with meningococcal vaccines, data support the co-administration with Hexaxim (which contains the same DTaP-IPV antigens) with meningococcal vaccines [Citation87,Citation88].
2.6. Real world experience
Tetraxim was first licensed in 1998 and has since been approved in more than 90 countries worldwide including 25 countries in the European Economic Area. As of February 2022, it is estimated that more than 72 million doses have been distributed worldwide (data on file). The post-marketing assessments of vaccine safety through continuous safety monitoring and signal detection activities have not raised any new significant safety concerns, confirming the satisfactory safety profile of Tetraxim reported from the clinical studies and the positive benefit-risk profile when Tetraxim is used in the approved indications.
Real-world evidence of the effectiveness of Tetraxim against pertussis disease comes from the epidemiological surveillance of pertussis disease in countries where Tetraxim has been widely implemented as school-entry booster vaccine for a long period of time. Norway introduced Tetraxim as a booster vaccine in children 7 to 8 years of age in 2006 following 8 years of routine use of aP vaccines as a 2 + 1 regimen in infants and toddlers [Citation89], and the incidence of pertussis disease decreased 65%, from a median of 204 per 100,000 cases between 1997 and 2005 to 71 per 100,000 cases between 2008 and 2016 in 5–11 year old children [Citation89,Citation90]. A similar effect was seen in Finland. After infants priming with a wP-based vaccine in a 3 + 1 schedule since the 1970s, a Tdap booster was introduced in the Finnish National Immunization Program at 6 years of age in 2003, and changed in 2005 to DTaP-IPV booster at 4 years of age. Tetraxim was the only preschool booster vaccine used at 4 years of age between 2005 and 2008 in Finland, and has been the only one used from 2011 until the time of this review. Additionally, an adolescent booster Tdap vaccine was introduced in Finland for 14 year olds in 2005 [Citation91] and in the same year the primary infant vaccination recommendation switched from wP- to aP-based vaccines in a 2 + 1 schedule [Citation92]. Median incidence of pertussis disease in Finnish children 4 to 8 years of age decreased 70%, from 43 per 100000 between 1994 and 2004 to 13 per 100000 between 2006 and 2016 [Citation93,Citation94]. Similar data have been reported from Sweden, where both the two-component aP-containing vaccine from Sanofi (Tetraxim) and the three-component aP-containing vaccine from GSK (Infanrix-Polio) have been used as school-entry booster vaccines. Sweden has a long record of pertussis surveillance [Citation95]: an enhanced surveillance program was implemented in 1997, which includes mandatory reporting of pertussis and laboratory case confirmation, along with case investigation. In 1996 a primary series immunization schedule at 3, 5 and 12 months of age (2 + 1) with aP-containing vaccines was introduced, and the vaccine coverage rate has been high and stable at 97%–98% since that time, leading to significant decline in pertussis incidence [Citation32,Citation96]. The data generated in this surveillance program have also provided further information regarding the duration of protection afforded by the 2 + 1 dose pertussis immunization program. As has been seen in all countries where universal infant pertussis vaccination has been introduced, Sweden observed a shift in pertussis occurrence to older age groups. The increased incidence of pertussis to 5–6 years of age in Sweden added to the body of data signaling the waning of the protection afforded by the first three doses. This led to a catch-up program for 10 year old children born in 1997 which was subsequently replaced by the introduction of a second booster dose at 5 to 6 years of age from 2007 onwards [Citation32]. Subsequent to the introduction of this pre-school booster dose, a reduction in the age-specific incidence of pertussis was noted in the age groups targeted by the school-entry booster, as well as among infants below 3 months of age who are too young to be vaccinated. This latter observation is suggestive of a herd effect [Citation32].
For the D, T, and IPV antigens, correlates of protection are clearly established, therefore, immunogenicity results are considered to be predictive of vaccine efficacy and efficacy trials are usually not required [Citation73]. Additionally, the effectiveness of D, T and IPV vaccine components similar to those included in Tetraxim is long-established and illustrated with sharp decreases in the incidence of diphtheria, maternal and neonatal tetanus, injury-associated tetanus, and poliomyelitis in all countries that have introduced these vaccines with high coverage in routine pediatric immunization programs [Citation16,Citation18,Citation38].
3. Discussion
Tetraxim given as a school-entry booster is highly immunogenic and induces strong anamnestic responses to all antigens, confirming that the primary vaccinations induced good immune memory, regardless of primary vaccine type (wP-containing vaccine or aP-containing vaccine), and regardless of the primary immunization schedule (2 + 1, 3 + 0 or 3 + 1 regimen). The pre-school booster dose contributes to the maintenance of high immunity from early childhood up until adolescence, as has been evidenced by data from the long-term antibody persistence follow-up study of Tetraxim in the UK (Study 5) [Citation68]. In this study, the kinetics of antibody persistence were assessed for the standard pediatric vaccine formulation of DTaP-IPV (Tetraxim) and for reduced antigen content vaccines (Tdap and Tdap-IPV). In all groups, a sharp decline in the level of all antibodies was observed in the first-year post-booster, followed by continued but slower decline up to the 5-year follow-up timepoint. High proportions of subjects maintained seroprotective antibody levels (against D, T, and poliovirus) and still had persistent anti-PT and anti-FHA antibodies 5 years after the school-entry booster dose. Similar findings were also reported for the anti-pertussis antibody kinetics following DTaP-IPV booster vaccination in Norwegian children at 7–8 years of age [Citation89]. Additionally, using these 5-year persistence data, mathematical modeling of the antibody kinetics up to 9 years after this pre-school booster dose showed that children were predicted to maintain good protection against most antigens until 13–14 years of age, regardless of the type of vaccine received at pre-school age [Citation97]. The declining trends and the fact that the majority were expected to have undetectable levels of anti-PT antibody after 9 years supported the need for a further booster dose in adolescence [Citation97]. Similarly, another study, which assessed antibody persistence approximately 5 years after a school-entry booster with either a DTaP or Tdap vaccine at 4–6 years of age showed that more than 94% of subjects remained seroprotected (≥0.1 IU/mL) against both T and D, while the rate of subjects with anti-PT antibodies ≥5 EU/mL was lower (>44%) [Citation98].
Some countries use vaccines with reduced diphtheria and pertussis antigen content (Tdap, or Tdap-IPV) to boost immunity at school-age entry. There is no indisputable evidence based on clinical protection data to preferentially support the standard pediatric formulation (i.e. full dose of diphtheria and pertussis antigens) over the reduced dose formulation for this school-age booster [Citation93,Citation99,Citation100]. It is, however, the authors’ opinion that the inclusion of a school-age booster dose for diphtheria, tetanus, pertussis, and poliomyelitis in the national immunization program in a given country (either standard pediatric formulation or reduced-dose formulation) with a target of high and sustainable VCR is more important than the type of vaccine used. The need for a school-entry booster is supported by data showing the decline of antibodies and the risk of disease by school age after a complete primary series and toddler booster. The risk of resurgence of vaccine-preventable diseases such as pertussis or diphtheria is not negligible in a situation where a population is under-immunized.
Vaccine efficacy data against pertussis disease support the use of Tetraxim. It should be noted that efficacy estimates have relied on several factors, such as pertussis case definition, study population, epidemiological setting, or circulating strain predominance at the time of the study. No head-to-head efficacy trial has been conducted with currently licensed aP-containing vaccines, which prevents any direct comparison of vaccine efficacy across studies. However, on the other hand, data from countries that use Tetraxim as a school-entry booster over a long period of time (Norway, Finland) have demonstrated good control of pertussis disease by this vaccine. Additionally, the three other diseases (diphtheria, tetanus, and poliomyelitis) have also virtually disappeared from these countries.
Pre-school or school-entry usually represents a good opportunity for booster or catch-up vaccinations [Citation101], allowing the level of antibodies to be reset at the maximum possible levels for the next few years. Evidence from different countries that have implemented school-entry boosters [Citation32,Citation33] also support that some degree of herd immunity against pertussis can be conferred to other age groups, notably infants who are too young to be vaccinated or who are incompletely vaccinated. The duration of protection conferred by this pertussis school-entry booster vaccination has been studied in different settings. All show a decline in pertussis antibody levels in the years following vaccination and an associated increase in the risk of infection and disease [Citation34,Citation89,Citation102,Citation103], and no single vaccine type (aP- or wP-based vaccines, standard pediatric formulation or reduced-dose formulation) would produce lifelong immunity. While the relative duration of protection against pertussis conferred by wP and aP vaccines is still debated, this has to be balanced with the higher reactogenicity of wP vaccines and the fact that immunity conferred by either vaccine after a school entry booster wanes by adolescence anyway. This again illustrates the need for regular subsequent booster vaccinations to maintain lifelong immunity against pertussis disease [Citation104]. Therefore, the development of new generation of pertussis vaccines that could aim to provide broad and sustainable protection against infection and transmission, along with a satisfactory safety profile, is being further investigated [Citation105,Citation106]. It has been suggested that the nature of the immune priming is an important factor, and while current aP vaccines have demonstrated effectiveness in preventing pertussis disease, they induce a T-helper (Th)2 dominated immune response, whereas a Th1/Th17 dominated immune response targeting the bacterial cell at the mucosal surface and inducing bacterial clearance from the airway would prevent asymptomatic carriage of B. pertussis [Citation107]. A series of systematic reviews focusing on Bordetella pertussis epidemiology, disease burden, and mortality in different geographical regions (Europe, Asia, Middle East and Africa) have highlighted the circulation of Bordetella pertussis in young children, with continued circulation in adolescents and adults [Citation108–111]. The true burden of pertussis disease in these populations is likely to be underestimated, due to mild clinical presentation, under-diagnosis, and a lack of robust epidemiological surveillance systems.
School-entry booster vaccination with Tetraxim in children for whom it is the fourth or fifth dose of a diphtheria-containing vaccine shows an acceptable safety profile. As expected, it is better tolerated than a DTwP-containing vaccine (Study 1 [data on file]), and as well tolerated as the reduced diphtheria and pertussis antigen vaccine (booster formulation) [Citation67]. This constitutes an important factor for vaccination acceptance. Tetraxim was well tolerated in various situations (i.e. irrespective of the immunization schedule used during infancy, and regardless of the type of vaccine used previously) and can therefore fit into any national immunization program. The inclusion of the IPV valence is important in the context of a shift toward the anticipated cessation of oral poliovirus vaccine use in the future, due to concerns regarding the risk of VDPVs and vaccine-associated paralytic poliomyelitis. The extra IPV dose at school-entry age in both high and low-/middle-income countries, compared to only giving a DTP vaccine only, allows humoral immunity against poliovirus to be sustained, as well as providing a catch-up opportunity for any previous missed doses of polio vaccine without impairing the immune responses to the other antigens (D, T, PT, FHA).
Generally speaking, the safety profile of the same class of DTaP-IPV vaccines (pediatric formulation) when used as a school-entry booster has been reported to be acceptable in post-marketing evaluations, confirming the good safety profile described in the clinical studies. Post-marketing surveillance studies have noted the occurrence of large injection site reactions (>50 mm) including extensive limb swelling, which tends to occur more frequently after a fourth or fifth dose. These events usually resolve within a few days without sequelae [Citation112–114]. There has been no evidence of increased risk for events such as meningitis/encephalitis, seizures, stroke, Guillain-Barre syndrome, Steven-Johnson syndrome, anaphylaxis, or serious local reactions [Citation115,Citation116]. However, data from one clinical trial have reported that the use of an adolescent-adult formulation Tdap vaccine (Adacel®, Sanofi) in children aged 4–6 years led to less frequent injection site reactions and fever than a pediatric DTaP-IPV vaccine formulation (Quadracel®, Sanofi), although these adverse reactions remain common no matter the type of vaccine used [Citation117]. The WHO position paper on diphtheria vaccine recommends low-dose diphtheria toxoid for immunization of individuals aged ≥7 years [Citation16]. Other factors such as prior vaccination history, pre-vaccination diphtheria antitoxin level, type of adjuvant, or type of preservative may also be linked to the safety profile of a vaccine and should be explored. An exploratory study from Rowe et al [Citation118] assessed various DTaP and DTaP-IPV vaccines administered to children aged 4–6 years and suggested a possible association between injection site reactions and a recall of the Th2-polarized immune memory resulting from the DTaP priming, which includes stimulation of vaccine-specific IgE production leading to a greater risk of large local reactions in the 24–72 hours following booster vaccination. Interestingly, findings of Rowe et al. also suggested that concomitant DTaP and IPV boosting at the same site significantly reduced Th2-associated local reactions. This hypothesis requires further confirmation in larger studies, and the mechanism by which aP-containing boosters predispose to extensive limb swelling is not yet fully elucidated [Citation113].
The introduction and high uptake of a school-entry DTaP-IPV booster vaccine in a national vaccination program is a public health measure that would ensure continuous protection against these vaccine-preventable diseases during childhood. Particularly, a DTaP-IPV vaccine would be highly beneficial not only to boost those who are fully vaccinated up to that point, but also to those who may have missed at least one dose. This is particularly important in the current context of the COVID-19 pandemic, which has adversely affected childhood vaccination uptake overall, with estimated global coverage of the third DTP dose decreasing from 86% in 2019 to 83% in 2020 and a 30% increase in children who have received no vaccines (zero dose children) compared to 2019 [Citation119]. Such immunity gaps in the population could increase the likelihood of disease resurgence in the future, and catch-up vaccinations, even 3–4 years later than planned, could have an important role [Citation4,Citation119].
4. Conclusion
The use of Tetraxim as school-entry booster has been extensively evaluated, and high immune responses to all Tetraxim antigens have been reported. The safety profile was acceptable and as expected for this class of vaccine. School-entry children act as a reservoir for pathogen transmission in the entire population, and so vaccinating this population indirectly protects other populations including vulnerable infants via herd immunity. When vaccination schedules and VCRs are not optimal, there is a significant risk of the resurgence of diseases. Therefore, school-entry booster vaccination allows the gap between infant vaccination and adolescent vaccination to be closed.
5. Expert opinion
Routine infant primary series and toddler booster vaccination with DTP-based vaccines are characterized by waning of antibody levels over time. Associated with the waning of antibody levels is a reduction of protection, evidenced by lower seroprotection rates against diphtheria and tetanus, and increased real-world incidence of pertussis disease (in the absence of correlates of protection for pertussis) in school-age children in the absence of a school-entry booster. The widespread implementation of a DTaP-IPV vaccination at school-entry age (4–7 years of age) can close the gap in immunity between the toddler booster vaccination and adolescent vaccination. In addition to the direct benefits for the vaccinated population, indirect benefits have also been described, notably for young infants who are too young to have received their first pertussis vaccination or have not yet completed their infant vaccination series. Unvaccinated school-age children are reservoirs of pertussis disease and can act as vectors for transmission of disease to younger siblings. Household transmission studies have shown that this link is weakened by school-age vaccination, which helps achieve herd immunity and protect unvaccinated vulnerable younger infants. The inclusion of an acellular pertussis rather than whole-cell pertussis antigen is associated with fewer injection site and systemic reactions, which is likely to improve vaccine acceptance and decrease vaccine hesitancy, thereby leading to real improvements in vaccine coverage rates. Furthermore, the inclusion of the IPV antigen is crucial in the context of global polio eradication and the move away from oral poliovirus vaccines that are associated with the risk of emergence of circulating vaccine-derived polioviruses and the persistence of vaccine-associated paralytic poliomyelitis cases in certain regions and in certain conditions of OPV use. In countries that already routinely use a school-age DTaP-IPV booster vaccination, ongoing surveillance for effectiveness and also long-term studies of antibody persistence up to the time of adolescent booster vaccination are important to fully define its impact in a range of settings. In countries that implement a school-entry booster, such surveillance should be set up so that the benefits can be clearly evaluated over a 5–10 year timescale. There have been no safety concerns related to the use of the DTaP-IPV booster vaccination at school-entry in extensive clinical development and post-marketing evaluation. The risk of resurgence of vaccine-preventable diseases in an under-immunized population is too great to be ignored, and the implementation of routine school-age vaccination of DTaP-IPV provides an ideal opportunity to allow antibody levels to be reset and protection assured for the following years. Such an opportunity is particularly important in the current context of the COVID-19 pandemic, during which childhood vaccination programs have been disrupted in most regions globally.
Article highlights
Antibody levels against diphtheria, tetanus, pertussis, and polio wane over time following routine infant primary series and toddler booster vaccination, regardless of the schedule, supporting the need for further booster vaccinations to sustain protection against these vaccine-preventable diseases.
School-entry age children act as a reservoir for pathogen transmission in the entire population; vaccinating school-entry children indirectly protects other populations including vulnerable infants via herd immunity.
If the immunization schedule and/or coverage rates are not optimal, there is a significant risk of resurgence of diseases (e.g. pertussis).
The implementation of a school-entry age booster vaccination against diphtheria, tetanus, and pertussis can help to close the gap between the waned immunity following primary series and toddler booster vaccination and the time of adolescent booster vaccination, and ensure protection in the intervening years.
The inclusion of an inactivated poliovirus vaccine is important in the context of global polio eradication to guarantee long-term protection against paralytic disease due to any form of poliovirus, and to move away from oral poliovirus vaccines due to their association with continued vaccine-derived poliovirus circulation where poorly deployed.
The combination of the diphtheria, tetanus, pertussis, and inactivated poliovirus antigens in a single vaccine leads to improved vaccine coverage. Improved compliance to vaccination recommendations is also linked to the inclusion of less reactogenic acellular pertussis antigens rather than whole-cell pertussis antigens.
School-entry provides an ideal opportunity to sustain continued protection through the implementation of routine vaccination against diphtheria, tetanus, pertussis, and polio, and to catch up on any previously missed doses.
Vaccination coverage decreases with age, and school-entry can be the last opportunity to provide routine widespread vaccination to children.
Author contributions
All authors made substantial contributions to the development of the work, the analysis and interpretation of the data, either drafted the work (CH) or reviewed it critically (JCV-Z, DM, EV), approved the final version to be published, and are fully accountable for all aspects of the work.
Declaration of interest
All authors are employees of Sanofi and own Sanofi shares. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer has disclosed that they have participiated in vaccine trials and Advisory Boards for SanofiPasteur on other vaccines than DTaP-IPV.
Acknowledgments
The authors thank and acknowledge the contribution and participation of the children and parents/legal guardians in the clinical studies that are reviewed, as well as the participating hospitals and investigational staff.
The authors would also like to acknowledge Cesar Mascarenas and Fabrice Guitton, both employees of Sanofi, for their valuable input into the development of this article.
Dr Andrew Lane (Lane Medical Writing) provided medical writing assistance, funded by Sanofi, in the preparation and development of the manuscript in accordance with the European Medical Writers Association guidelines and Good Publication Practice.
Additional information
Funding
References
- Henderson RH. The expanded programme on immunization of the World Health Organization. Rev Infect Dis. 1984 May-Jun;6(Suppl 2):S475–9.
- WHO. Immunization and vaccine-preventable diseases. [cited 2022 May 4]. Available from: https://www.who.int/data/gho/data/themes/immunization
- WHO. Polio (Pol3) immunization coverage amonth 1-year-olds (%). [cited 2022 May 4]. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/polio-(pol3)-immunization-coverage-among-1-year-olds-(-)
- Cohen R, Ashman M, Taha MK, et al. Pediatric Infectious Disease Group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect Dis Now. 2021 Aug;51(5):418–423.
- Moura C, Truche P, Sousa Salgado L, et al. The impact of COVID-19 on routine pediatric vaccination delivery in Brazil. Vaccine. 2022 Apr 1 40(15):2292–2298.
- Patel Murthy B, Zell E, Kirtland K, et al. Impact of the COVID-19 pandemic on administration of selected routine childhood and adolescent vaccinations - 10 U.S. Jurisdictions, March-September 2020. Morbidity Mortality Weekly Rep. 2021 Jun 11 70(23):840–845.
- SeyedAlinaghi S, Karimi A, Mojdeganlou H, et al. Impact of COVID-19 pandemic on routine vaccination coverage of children and adolescents: a systematic review. Health Sci Rep. 2022 Mar;5(2):e00516.
- WHO. Diphtheria reported cases. Available from: https://apps.who.int/immunization_monitoring/globalsummary/timeseries/tsincidencediphtheria.html. Accessed 4 May 2022.
- WHO. Review of the epidemiology of diphtheria - 2000-2016. Available from: https://www.who.int/immunization/sage/meetings/2017/april/1_Final_report_Clarke_april3.pdf. Accessed 4 May 2022.
- Clarke KEN, MacNeil A, Hadler S, et al. Global epidemiology of Diphtheria, 2000-2017. Emerg Infect Dis. 2019 Oct;25(10):1834–1842.
- Finger F, Funk S, White K, et al. Real-time analysis of the diphtheria outbreak in forcibly displaced Myanmar nationals in Bangladesh. BMC Med. 2019 Mar 12 17(1):58.
- Karyanti MR, Nelwan EJ, Assyidiqie IZ, et al. Diphtheria epidemiology in Indonesia during 2010-2017. Acta Med Indones. 2019 Jul;51(3):205–213.
- Polonsky JA, Ivey M, Mazhar MKA, et al. Epidemiological, clinical, and public health response characteristics of a large outbreak of diphtheria among the Rohingya population in Cox’s Bazar, Bangladesh, 2017 to 2019: a retrospective study. PLoS Med. 2021 Apr;18(4):e1003587.
- Sangal L, Joshi S, Anandan S, et al. Resurgence of diphtheria in North Kerala, India, 2016: laboratory supported case-based surveillance outcomes. Front Public Health. 2017;5:218.
- Paniz-Mondolfi AE, Tami A, Grillet ME, et al. Resurgence of vaccine-preventable diseases in Venezuela as a regional public health threat in the Americas. Emerg Infect Dis. 2019 Apr;25(4):625–632.
- WHO.Diphtheria vaccine: WHO position paper.August 2017;Weekly Epidemiological Rec. 2017 Aug 4 9231 417–435.
- Roper M, Wassilak S, Scobie H. Tetanus toxoid. Plotkin SA, Orenstein WA, Offit PA, et al., editors. Vaccines. 7th ed. PA:Elsevier; 2018. p.1052–1079.
- WHO. Tetanus vaccines: WHO position paper - February 2017. Weekly Epidemiological Rec. 2017 Feb 10;92(6):53–76.
- Borrow R, Balmer P, Roper M The Immunological Basis for Immunization Series—Module 3: tetanus Update 2018. Immunization, Vaccines and Biologicals, WHO Edition World Health Organization, Geneva. 2018. Available from: https://apps.who.int/iris/bitstream/handle/10665/275340/9789241513616-eng.pdf?sequence=1&isAllowed=y. Accessed 4 May 2022. Accessed 4 May 2022.
- WHO. Pertussis vaccines: WHO position paper - September 2015. Weekly Epidemiological Rec. 2015 Aug 28;90(35):433–458.
- Falleiros Arlant LH, de Colsa A, Flores D, et al. Pertussis in Latin America: epidemiology and control strategies. Expert Rev Anti Infect Ther. 2014 Oct;12(10):1265–1275.
- Gambhir M, Clark TA, Cauchemez S, et al. A change in vaccine efficacy and duration of protection explains recent rises in pertussis incidence in the United States. PLoS Comput Biol. 2015 Apr;11(4):e1004138.
- Gentile A, Bricks L, Avila-Aguero ML, et al. Pertussis in Latin America and the Hispanic Caribbean: a systematic review. Expert Rev Vaccines. 2019 Aug;18(8):829–845.
- Huang H, Gao P, Gao Z, et al. A big pertussis outbreak in a primary school with high vaccination coverage in northern China: an evidence of the emerging of the disease in China. Vaccine. 2018 Dec 18 36(52):7950–7955.
- van der Maas Na, Mooi FR, de Greeff Sc, et al. Pertussis in the Netherlands, is the current vaccination strategy sufficient to reduce disease burden in young infants? Vaccine. 2013 Sep 23 31(41):4541–4547.
- Worby CJ, Kenyon C, Lynfield R, et al. Examining the role of different age groups, and of vaccination during the 2012 Minnesota pertussis outbreak. Sci Rep. 2015 Aug 17 5(1):13182.
- Pillsbury A, Quinn HE, McIntyre PB. Australian vaccine preventable disease epidemiological review series: pertussis, 2006-2012. Commun Dis Intell Q Rep. 2014 Sep 30;38(3):E179–94.
- de Greeff Sc, Mooi FR, Westerhof A, et al. Pertussis disease burden in the household: how to protect young infants. Clinl Infect Dis. 2010 May 15 50(10):1339–1345.
- de Celles M D, Magpantay FMG, King AA, et al. The impact of past vaccination coverage and immunity on pertussis resurgence. Sci Transl Med. 2018 Mar 28;10(434):eaaj1748. doi:10.1126/scitranslmed.aaj1748.
- Jardine A, Conaty SJ, Lowbridge C, et al., Who gives pertussis to infants? Source of infection for laboratory confirmed cases less than 12 months of age during an epidemic. Commun Dis Intell Q Rep. 2009;34(2): 116–121. 2010 Jun. Sydney
- Skoff TH, Kenyon C, Cocoros N, et al. Sources of infant pertussis infection in the United States. Pediatrics. 2015 Oct;136(4):635–641.
- The Public Health Agency of Sweden. Pertussis surveillance in Sweden: twenty-year report. Available from: https://www.folkhalsomyndigheten.se/contentassets/14d7068f02604bb496a319895fd9ad47/pertussis-sweden-twenty-year-report-18068.pdf. Accessed 4 May 2022.
- de Greeff Sc, Mooi FR, Schellekens JFP, et al. Impact of acellular pertussis preschool booster vaccination on disease burden of pertussis in The Netherlands. Pediatr Infect Dis J. 2008 Mar;27(3):218–223.
- Domenech de Celles M, Rohani P, King AA. Duration of immunity and effectiveness of diphtheria-tetanus-acellular pertussis vaccines in children. JAMA Pediatr. 2019 Jun 1; 173(6):588–594.
- Global Polio Eradication Initiative. Available from: https://polioeradication.org/. Accessed 4 May 2022.
- Global Polio Eradication Initiative. Global wild poliovirus 2016-2021. Available from: https://polioeradication.org/wp-content/uploads/2021/09/Weekly-GPEI-Polio-Analyses-WPV-20210831.pdf. Accessed 4 May 2022.
- Global Polio Eradication Initiative. Circulating vaccine-derived poliovirus. Available from: https://polioeradication.org/polio-today/polio-now/this-week/circulating-vaccine-derived-poliovirus/. Accessed 4 May 2022.
- WHO. Polio vaccines: WHO position paper. Weekly Epidemiological Rec. 2016 March;91(12):145–168.
- European Centre for Disease Prevention and Control. Vaccine schedules in all countries in the EU/EEA. Available from: https://vaccine-schedule.ecdc.europa.eu/. Accessed 4 May 2022.
- Centers for Disease Control. Recommended child and adolescent immunization schedule for ages 18 years or younger, United States. Available from: https://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf. Accessed 4 May 2022.
- Government of Canada. Recommended immunization schedules: canadian Immunization Guide. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-1-key-immunization-information/page-13-recommended-immunization-schedules.html. Accessed 4 May 2022.
- Australian Government Department of Health. National Immunisation Program Schedule – from 1 July 2020. Available from: https://www.health.gov.au/health-topics/immunisation/immunisation-throughout-life/national-immunisation-program-schedule#national-immunisation-program-schedule-from-1-july-2020. Accessed 4 May 2022.
- Nicholson N, Adkins E, Karyanti MR, et al. What is the true burden of diphtheria, tetanus, pertussis and poliovirus in children aged 3–18 in Asia? A systematic literature review. Inter J Infect Dis. 2022;117:116–129.
- Argentina Ministry of Health. National vaccination calendar: information about vaccines and the vaccination schedule. Available from: https://www.argentina.gob.ar/salud/vacunas. Accessed 4 May 2022.
- Ministerio de Salud de Costa Rica. Esquema de vacunaciόn oficial en Costa Rica. Available from: https://www.ministeriodesalud.go.cr/index.php/centro-de-informacion/material-comunicacion/vacunas-3/5351-esquema-de-vacunacion-oficial-en-costa-rica-menores-de-edad/file. Accessed 4 May 2022.
- McCormack PL. DTaP-IPV-Hep B-Hib vaccine (Hexaxim®): a review of its use in primary and booster vaccination. Paediatr Drugs. 2013 Feb;15(1):59–70.
- Plotkin SA, Liese J, Madhi SA, et al. A DTaP-IPV//PRP~T vaccine (Pentaxim): a review of 16 years’ clinical experience. Expert Rev Vaccines. 2011 Jul;10(7):981–1005.
- Syed YY. DTaP-IPV-HepB-Hib vaccine (Hexyon®): an updated review of its use in primary and booster vaccination. Paediatr Drugs. 2019 Oct;21(5):397–408.
- Vidor E, Plotkin SA. Immunogenicity of a two-component (PT & FHA) acellular pertussis vaccine in various combinations. Hum Vaccines. 2008 Sep-Oct;4(5):328–340.
- Auerbach BS, Lake AM, Wilson ME, et al. Dose-response to a two-component acellular pertussis vaccine in infants and comparison with whole-cell vaccine. Biologicals. 1998 Jun;26(2):145–153.
- Edwards KM, Decker MD, Halsey NA, et al. Differences in antibody response to whole-cell pertussis vaccines. Pediatrics. 1991 Nov;88(5):1019–1023.
- Decker MD, Edwards KM. The multicenter acellular pertussis trial: an overview. J Infect Dis. 1996 Nov;174(Suppl 3):S270–5.
- Decker MD, Edwards KM, Steinhoff MC, et al. Comparison of 13 acellular pertussis vaccines: adverse reactions. Pediatrics. 1995 Sep;96(3 Pt 2):557–566.
- Miller E, Ashworth LA, Redhead K, et al. Effect of schedule on reactogenicity and antibody persistence of acellular and whole-cell pertussis vaccines: value of laboratory tests as predictors of clinical performance. Vaccine. 1997 Jan;15(1):51–60.
- Simondon F, Yam A, Gagnepain JY, et al. Comparative safety and immunogenicity of an acellular versus whole-cell pertussis component of diphtheria-tetanus-pertussis vaccines in Senegalese infants. Eur J Clin Microbiol Infect Dis. 1996 Dec;15(12):927–932.
- Simondon F, Preziosi M-P, Yam A, et al. A randomized double-blind trial comparing a two-component acellular to a whole-cell pertussis vaccine in Senegal. Vaccine. 1997 Oct;15(15):1606–1612.
- Decker M, Edwards K, Bogaerts H. Combination vaccines. In: Plotkin SA, Orenstein WA, Offit PA, et al., editors. Vaccines. 7th ed. PA: Elsevier; 2018. p. 198–227.
- Maman K, Zollner Y, Greco D, et al. The value of childhood combination vaccines: from beliefs to evidence. Hum Vaccin Immunother. 2015;11(9):2132–2141.
- Marshall GS, Happe LE, Lunacsek OE, et al. Use of combination vaccines is associated with improved coverage rates. Pediatr Infect Dis J. 2007 Jun;26(6):496–500.
- Decker MD. Principles of pediatric combination vaccines and practical issues related to use in clinical practice. Pediatr Infect Dis J. 2001 Nov;20(11 Suppl):S10–8.
- Kalies H, Grote V, Verstraeten T, et al. The use of combination vaccines has improved timeliness of vaccination in children. Pediatr Infect Dis J. 2006 Jun;25(6):507–512.
- Dodd D. Benefits of combination vaccines: effective vaccination on a simplified schedule. Am J Manag Care. 2003 Jan;9(1 Suppl):S6–12.
- Institute for Safe Medication Practices (ISMP). Recommendations for practitioners to prevent vaccine errors part 2: analysis of ISMP vaccine errors reporting program (ISMP VERP) 2015. Available from: https://www.ismp.org/resources/recommendations-practitioners-prevent-vaccine-errors-part-2-analysis-ismp-vaccine-errors. Accessed 4 May 2022.
- Langue J, Matisse N, Pacoret P, et al. Persistence of antibodies at 5-6 years of age for children who had received a primary series vaccination with a pentavalent whole-cell pertussis vaccine and a first booster with a pentavalent acellular pertussis vaccine: immunogenicity and tolerance of second booster with a tetravalent acellular vaccine at 5-6 years of age. Vaccine. 2004 Mar 29 22(11–12):1406–1414.
- Pancharoen C, Chotpitayasunondh T, Chuenkitmongkol S, et al. Long-term immunogenicity assessment of a DTaP-IPV//PRP-T vaccine given at 2, 4, 6 and 18-19 months of age, and immunogenicity and safety of a DTaP-IPV vaccine given as a booster dose at 4 to 6 years of age in Thai children. Southeast Asian J Trop Med Public Health. 2012 May;43(3):687–698.
- Mallet E, Matisse N, Mathieu N, et al. Antibody persistence against diphtheria, tetanus, pertussis, poliomyelitis and Haemophilus influenzae type b (Hib) in 5-6-year-old children after primary vaccination and first booster with a pentavalent combined acellular pertussis vaccine: immunogenicity and tolerance of a tetravalent combined acellular pertussis vaccine given as a second booster. Vaccine. 2004 Mar 29 22(11–12):1415–1422.
- Collins CL, Salt P, McCarthy N, et al. Immunogenicity and safety of a low-dose diphtheria, tetanus and acellular pertussis combination vaccine with either inactivated or oral polio vaccine as a pre-school booster in UK children. Vaccine. 2004 Oct 22 22(31–32):4262–4269.
- John T, Voysey M, Yu LM, et al. Immunogenicity of a low-dose diphtheria, tetanus and acellular pertussis combination vaccine with either inactivated or oral polio vaccine compared to standard-dose diphtheria, tetanus, acellular pertussis when used as a pre-school booster in UK children: a 5-year follow-up of a randomised controlled study. Vaccine. 2015 Aug 26 33(36):4579–4585.
- Ferrera G, Cuccia M, Mereu G, et al. Booster vaccination of pre-school children with reduced-antigen-content diphtheria-tetanus-acellular pertussis-inactivated poliovirus vaccine co-administered with measles-mumps-rubella-varicella vaccine: a randomized, controlled trial in children primed according to a 2 + 1 schedule in infancy. Hum Vaccin Immunother. 2012 Mar;8(3):355–362.
- Scheifele D, Ochnio J The immunological basis for immunization series—Module 2: diphtheria Update 2009: immunization, Vaccines and Biologicals, WHO Edition World Health Organization, Geneva. 2009. Available from: https://apps.who.int/iris/bitstream/handle/10665/44094/9789241597869_eng.pdf?sequence=1&isAllowed=y. Accessed 4 May 2022.
- Tiwari T, Wharton M. Diphtheria toxoid. In: Plotkin SA, Orenstein WA, Offit PA, et al., editors. Vaccines. 7th ed. PA: Elsevier; 2018. p. 261–275.
- Lynn F, Reed GF, Meade BD. Collaborative study for the evaluation of enzyme-linked immunosorbent assays used to measure human antibodies to Bordetella pertussis antigens. Clin Diagn Lab Immunol. 1996 Nov;3(6):689–700.
- Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccin Immunol. 2010 Jul;17(7):1055–1065.
- Wirsing von Konig C The immunological basis for immunization series—Module 4: pertussis. immunization, vaccines and biologicals, WHO Edition World Health Organization, Geneva. 2009. Available from: http://apps.who.int/iris/bitstream/handle/10665/44311/9789241599337_eng.pdf?sequence=1. Accessed 4 May 2022.
- Vidor E, Orenstein WA. Poliovirus vaccine. In: Plotkin SA, Orenstein WA, Offit PA, et al., editors. Vaccines. 7th ed. PA: Elsevier; 2018. p. 841–865.
- Robertson S The immunological basis for immunization series—Module 6: poliomyelitis. immunization, vaccines and biologicals, WHO Edition World Health Organization, Geneva 1993. WHO/EPI/GEN/93.16. Available from: https://www.who.int/ihr/polio1993en.pdf. Accessed 4 May 2022.
- Albrecht P, Enterline JC, Boone EJ, et al. Poliovirus and polio antibody assay in HEp-2 and Vero cell cultures. J Biol Stand. 1983 Apr;11(2):91–97.
- WHO. Manual of laboratory methods for testing of vaccines used in the WHO Expanded Programme on Immunization: titration of human sera for neutralizing antibodies to poliovirus. WHO/VSQ/97.04 Geneva1997, p65–71. Available from: http://apps.who.int/iris/bitstream/handle/10665/63576/WHO_VSQ_97.04_(parts1-2).pdf?sequence=1. Accessed 4 May 2022.
- Chotpitayasunondh T, Thisyakorn U, Pancharoen C, et al. Antibody persistence after primary and booster doses of a pentavalent vaccine against diphtheria, tetanus, acellular pertussis, inactivated poliovirus, haemophilus influenzae type B vaccine among Thai children at 18-19 months of age. Southeast Asian J Trop Med Public Health. 2012 Mar;43(2):442–454.
- King GE, Hadler SC. Simultaneous administration of childhood vaccines: an important public health policy that is safe and efficacious. Pediatr Infect Dis J. 1994 May;13(5):394–407.
- Zhao Z, Smith PJ, Hill HA. Evaluation of potentially achievable vaccination coverage with simultaneous administration of vaccines among children in the United States. Vaccine. 2016 Jun 8; 34(27):3030–3036.
- Mosley JF 2nd, Smith LL, Parke CK, et al. Quadracel: vaccination against diphtheria, tetanus, pertussis, and poliomyelitis in children. P & T. 2016Apr; 414: 238–253.
- Study Group MMR. A second dose of a measles-mumps-rubella vaccine administered to healthy four-to-six-year-old children: a phase III, observer-blind, randomized, safety and immunogenicity study comparing GSK MMR and MMR II with and without DTaP-IPV and varicella vaccines co-administration. Hum Vaccin Immunother. 2019;15(4):786–799.
- Klein NP, Weston WM, Kuriyakose S, et al. An open-label, randomized, multi-center study of the immunogenicity and safety of DTaP-IPV (Kinrix) co-administered with MMR vaccine with or without varicella vaccine in healthy pre-school age children. Vaccine. 2012 Jan 11 30(3):668–674.
- Kang JH, Lee HJ, Kim KH, et al. The immunogenicity and safety of a combined DTaP-IPV//Hib vaccine compared with individual DTaP-IPV and Hib (PRP~T) vaccines: a randomized clinical trial in South Korean infants. J Korean Med Sci. 2016 Sep;31(9):1383–1391.
- Lagos R, Kotloff K, Hoffenbach A, et al. Clinical acceptability and immunogenicity of a pentavalent parenteral combination vaccine containing diphtheria, tetanus, acellular pertussis, inactivated poliomyelitis and Haemophilus influenzae type b conjugate antigens in two-, four- and six-month-old Chilean infants. Pediatr Infect Dis J. 1998 Apr;17(4):294–304.
- Vesikari T, Borrow R, Da Costa X, et al. Concomitant administration of a fully liquid, ready-to-use DTaP-IPV-HB-PRP-T hexavalent vaccine with a meningococcal serogroup C conjugate vaccine in infants. Vaccine. 2017 Jan 11 35(3):452–458.
- Vesikari T, Borrow R, Da Costa X, et al. Concomitant administration of a fully liquid ready-to-use DTaP-IPV-HB-PRP-T hexavalent vaccine with a meningococcal ACWY conjugate vaccine in toddlers. Vaccine. 2018 Dec 18 36(52):8019–8027.
- Aase A, Herstad TK, Jorgensen SB, et al. Anti-pertussis antibody kinetics following DTaP-IPV booster vaccination in Norwegian children 7-8 years of age. Vaccine. 2014 Oct 14 32(45):5931–5936.
- Norwegian Surveillance System for Communicable Diseases (MSIS). Available from: http://www.msis.no/. Accessed 4 May 2022.
- Finnish Institute for Health and Welfare. Infectious diseases and vaccinations: dTaP-IPV vaccine for children (4-in-1). Available from: https://thl.fi/en/web/infectious-diseases-and-vaccinations/vaccines-a-to-z/diphtheria-tetanus-whooping-cough-polio-and-hib-combination-vaccines/dtap-ipv-vaccine-for-children-4-in-1-. Accessed 4 May 2022.
- Rapola S. National immunization program in Finland. Int J Circumpolar Health. 2007 Dec;66(5):382–389.
- The Public Health Agency of Sweden. Evaluation of immunogenicity and effectiveness of low dose dTap-IPV vaccine used as booster in 4-8 year old children: a rapid literature review. Available from: https://www.folkhalsomyndigheten.se/contentassets/61c155f8099646ccb2d9b80dfde820c6/evaluation-tap-ipv-vaccine-01192-2017.pdf. Accessed 4 May 2022.
- Elomaa A, He Q, Minh NN, et al. Pertussis before and after the introduction of acellular pertussis vaccines in Finland. Vaccine. 2009 Sep 4 27(40):5443–5449.
- The Public Health Agency of Sweden. Pertussis surveillance in Sweden: 22nd annual report. Available from: https://www.folkhalsomyndigheten.se/contentassets/3ddd2fc3d4e94b56a31c2372777e2552/pertussis-surveillance-sweden-twenty-second-annual-report.pdf. Accessed 4 May 2022.
- Olin P, Hallander HO. Marked decline in pertussis followed reintroduction of pertussis vaccination in Sweden. Eurosurveillance. 1999 Dec;4(12):128–129.
- Voysey M, Kandasamy R, Yu LM, et al. The predicted persistence and kinetics of antibody decline 9 years after pre-school booster vaccination in UK children. Vaccine. 2016 Jul 29 34(35):4221–4228.
- Zepp F, Habermehl P, Knuf M, et al. Immunogenicity of reduced antigen content tetanus-diphtheria-acellular pertussis vaccine in adolescents as a sixth consecutive dose of acellular pertussis-containing vaccine. Vaccine. 2007 Jul 20 25(29):5248–5252.
- Public Health Agency of Canada. Statement on the booster for 4-6 year-olds for protection against pertussis. Available from: https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/healthy-living/statement-booster-4-6-year-olds-protection-against-pertussis-advisory-committee-statement-national-advisory-committee-immunization-naci/pertussis-booster-2014-coqueluche-rappel-eng.pdf. Accessed 4 May 2022.
- Gabutti G, Trucchi C, Conversano M, et al. Booster vaccination: the role of reduced antigen content vaccines as a preschool booster. Biomed Res Int. 2014;2014:541319.
- Marchal C, Belhassen M, Guiso N, et al. Vaccination coverage rates for Diphtheria, Tetanus. Poliomyelitis and Pertussis booster vaccination in France between 2013 and 2017: Learnings from an analysis of National Health System Real-World Data. Vaccine. 2021 Jan 15;39(3):505–511.
- Klein NP, Bartlett J, Rowhani-Rahbar A, et al. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med. 2012 Sep 13 367(11):1012–1019.
- McGirr A, Fisman DN. Duration of pertussis immunity after DTaP immunization: a meta-analysis. Pediatrics. 2015 Feb;135(2):331–343.
- Martinon-Torres F, Heininger U, Thomson A, et al. Controlling pertussis: how can we do it? A focus on immunization. Expert Rev Vaccines. 2018 Apr;17(4):289–297.
- Hozbor D. New pertussis vaccines: a need and a challenge. Adv Exp Med Biol. 2019;1183:115–126.
- Poolman JT. Shortcomings of pertussis vaccines: why we need a third generation vaccine. Expert Rev Vaccines. 2014 Oct;13(10):1159–1162.
- Kapil P, Merkel TJ. Pertussis vaccines and protective immunity. Curr Opin Immunol. 2019 Aug;59:72–78.
- Macina D, Evans KE Bordetella pertussis in School-Age Children, Adolescents and Adults: a Systematic Review of Epidemiology and Mortality in Europe. Infect Dis Ther. 2021 Dec;10(4):2071–2118. doi:10.1007/s40121-021-00520-9.
- Macina D, Evans KE. Bordetella pertussis in school-age children, adolescents, and adults: a systematic review of epidemiology, burden, and mortality in Asia. Infect Dis Ther. 2021 Sep;10(3):1115–1140.
- Macina D, Evans KE. Bordetella pertussis in School-age children, adolescents, and adults: a systematic review of epidemiology, burden, and mortality in the Middle East. Infect Dis Ther. 2021 Jun;10(2):719–738.
- Macina D, Evans KE. Bordetella pertussis in school-age children, adolescents, and adults: a systematic review of epidemiology, burden, and mortality in Africa. Infect Dis Ther. 2021 Sep;10(3):1097–1113.
- Rennels MB, Deloria MA, Pichichero ME, et al. Extensive swelling after booster doses of acellular pertussis-tetanus-diphtheria vaccines. Pediatrics. 2000 Jan;105(1):e12.
- Southern J, Waight PA, Andrews N, et al. Extensive swelling of the limb and systemic symptoms after a fourth dose of acellular pertussis containing vaccines in England in children aged 3-6years. Vaccine. 2017 Jan 23 35(4):619–625.
- Woo EJ, Burwen DR, Gatumu SN, et al. Extensive limb swelling after immunization: reports to the vaccine adverse event reporting system. Clinl Infect Dis. 2003 Aug 1 37(3):351–358.
- Daley MF, Yih WK, Glanz JM, et al. Safety of diphtheria, tetanus, acellular pertussis and inactivated poliovirus (DTaP-IPV) vaccine. Vaccine. 2014 May 23 32(25):3019–3024.
- Lee SM, Kim SJ, Chen J, et al. Post-marketing surveillance to assess the safety and tolerability of a combined diphtheria, tetanus, acellular pertussis and inactivated poliovirus vaccine (DTaP-IPV) in Korean children. Hum Vaccin Immunother. 2019;15(5):1145–1153.
- Langley JM, Predy G, Guasparini R, et al. An adolescent-adult formulation tetanus and diptheria toxoids adsorbed combined with acellular pertussis vaccine has comparable immunogenicity but less reactogenicity in children 4-6 years of age than a pediatric formulation acellular pertussis vaccine and diphtheria and tetanus toxoids adsorbed combined with inactivated poliomyelitis vaccine. Vaccine. 2007 Jan 22 25(6):1121–1125.
- Rowe J, Yerkovich ST, Richmond P, et al. Th2-associated local reactions to the acellular diphtheria-tetanus-pertussis vaccine in 4- to 6-year-old children. Infect Immun. 2005 Dec;73(12):8130–8135.
- Muhoza P, Danovaro-Holliday MC, Diallo MS, et al. Routine vaccination coverage - worldwide. Morbidity Mortality Weekly Rep. 2020 [2021 Oct 29];70(43):1495–1500.