ABSTRACT
Introduction
Influenza is a vaccine-preventable disease. Due to the evolving nature of influenza viruses, the composition of vaccines has to be updated annually. Most of the current influenza vaccines are still produced in embryonated chicken eggs, a well-established process with some limitations.
Area covered
This review focuses on the recombinant DNA technology using baculovirus expression vector system a modern method of manufacturing licensed influenza vaccines. The speed, scalability, biosafety and flexibility of the process, together with the reliability of the hemagglutinin in the vaccine, represent a significant advance toward new platforms for vaccine production.
Expert opinion
The scenario of vaccine production in the next years seems to be particularly interesting, involving a transition from the current egg-based production to new technologies, such as the cell culture platform, the RNA technology, the plant-based system, and the DNA vaccine. This latter offers great advantages over egg- and cell-based influenza vaccine production. The universal vaccine remains the goal of researchers and ideally would avoid the need for annual reformulation and re-administration of seasonal vaccines. The lesson learned from the COVID-19 pandemic highlights the importance of having different technologies available and able to promptly respond to a great demand of vaccines worldwide.
1. Introduction
Influenza is a global public health problem; vaccination is the most effective way of controlling seasonal influenza infections and preventing possible pandemics [Citation1,Citation2]. The World Health Organization (WHO) estimates that annual influenza epidemics affect approximately 5–15% of the population in the Northern hemisphere and cause about 290,000 to 650,000 respiratory deaths, worldwide [Citation3,Citation4]. The disease can affect all age-groups, but some groups are more at risk than others. Vaccination recommendations differ among countries; however, the WHO recommends annual vaccination for pregnant women at any stage of pregnancy, children from 6 months to 5 years old, the elderly, individuals with chronic medical conditions and health workers [Citation4].
Due to the evolving nature of influenza viruses, the composition of vaccines has to be updated annually in order to include the seasonal viruses predicted to circulate during the next influenza season. Updating is a complex process that involves intense collaboration among the WHO Global Influenza Surveillance and Response System, regulatory authorities, vaccine manufacturers and public health laboratories [Citation5]. Since 1998, the WHO has issued recommendations for the composition of seasonal influenza vaccines twice per year: once in February for the Northern hemisphere and once in September for the Southern hemisphere, which means that the vaccine composition is decided upon almost a year in advance of the seasonal influenza peak [Citation6]. When there is a good match between vaccine strains and the circulating viruses, vaccination provides 70–90% protection in healthy adults younger than 65 years [Citation7]. Sometimes, mutations in circulating influenza viruses, including the unpredictable late appearance of an antigenic variant virus, and the mutations that the viruses may undergo during the manufacturing process, have resulted in vaccine mismatch and reduced vaccine effectiveness (VE) [Citation4,Citation6]. This occurred in the 2014–2015 Northern hemisphere influenza season, when an H3N2 variant virus emerged and little or no VE was observed. However, with only few exceptions, retrospective studies have shown good correspondence between the viruses recommended by the WHO for inclusion in the vaccine and the viruses circulating in the next influenza season [Citation6,Citation8,Citation9].
The degree of protection elicited by influenza vaccines depends on a complex interplay among vaccine composition and circulating viruses, the subjects vaccinated (i.e. age and health status), previous exposure to influenza, product-specific factors, such as formulation and the use of adjuvants, manufacturing, and timeliness [Citation2,Citation4].
Current seasonal vaccines contain three or four different influenza viruses recommended by the WHO. The trivalent vaccine is composed of A(H1N1) virus, A(H3N2) virus, and one lineage of B virus (Yamagata or Victoria). Owing to frequent mismatch and the co-circulation of both lineages of B virus, since the 2013–2014 Northern hemisphere season, the WHO has recommended the inclusion of both lineages, resulting in a quadrivalent vaccine that provides wider protection against influenza B viruses [Citation10].
Three different classes of influenza vaccines have been licensed so far: inactivated, live attenuated and recombinant influenza vaccines (RIVs). The inactivated influenza vaccines (IIV) consist of whole virus, split virus or subunits, and are administered intramuscularly or subcutaneously. The whole virus vaccines have been extensively used in humans but are no longer in use in most parts of the world, owing to their relatively high reactogenicity. Conventionally, subunit or split virus vaccines are the most widespread [Citation2,Citation11]. Traditional vaccines are produced in 9 to 11-day-old pathogen-free embryonated chicken eggs, harvested from the allantoic cavity and then treated, according to the type of vaccine. After harvesting, the whole virus is chemically inactivated with formaldehyde or β-propiolactone, concentrated, and purified in order to remove non-viral protein contaminants. Split virus and subunit vaccines are produced in the same way but undergo a further purification process; this exposes all the viral proteins, but results in the partial loss of the original viral organization and the viral RNA (split virus vaccine), or hemagglutinin (HA) and neuraminidase (NA) only (subunit vaccines). Vaccination with split virus or subunit vaccines usually induces an immune response against HA, and these vaccines contain a standard dose of 15 µg of HA of each influenza virus. In 2009, the Food and Drug Administration (FDA) licensed a high-dose influenza vaccine containing 60 µg of HA per each virus strain, four times more than the standard dose, for use in older adults (≥65 years old) [Citation12]. In 2020, the high-dose vaccine received marketing authorization in 25 European countries for use in adults 60 years of age and older [Citation13]. Despite the higher frequency of local and systemic reactions, the high-dose vaccine is well tolerated, induces a better and significantly higher immune response, and provides better protection against laboratory-confirmed influenza infection than the standard dose [Citation14–19].
RIVs have recently been licensed and contain HA only.
The live attenuated influenza vaccines (LAIVs) are administered intranasally to healthy subjects from 24 months to 49 years of age, depending on the country-specific regulations (United States, Europe, Russia, and India). Intranasal administration mimics the natural pathway of infection and induces a broader humoral and cellular response than IIVs, in addition to mucosal IgA responses in the upper respiratory tract. There is evidence that LAIVs provide protection against both well matched and antigenically drifted influenza strains [Citation5,Citation11,Citation20–22]. However, some safety concerns are persisting on using LAIVs in addition to the tumultuous recent history due to the lesser efficacy against the H1N1 circulating virus [Citation21,Citation23,Citation24].
The majority of influenza vaccines (roughly 85–90%) are currently produced in embryonated chicken eggs [Citation25] (hereafter referred to as eggs), an old-fashioned but well-established production system. Although the egg-based production confers several benefits, some limitations have emerged, and other platforms and technologies have been developed or are under development [Citation5].
This review focuses on a modern recombinant DNA technology based on baculovirus expression vector system for manufacturing licensed influenza vaccines, compared to the egg- and cell-culture platforms.
2. Egg- and cell-culture platforms
The egg-based production of influenza vaccines is a well-established process that was developed over 70 years, and extensive safety data are available [Citation5]. The process is cost-effective, allowing global access to the vaccine, and high titers of virus can be obtained, with a production capacity of 413 million doses of trivalent influenza vaccine every year worldwide [Citation7,Citation26].
Despite being the most widely used system for vaccine manufacture, egg-based production has some limitations. First, a large number of eggs are needed in a short-time period, one or two eggs are required for each dose of vaccine, the process is labor-intensive and cumbersome, and the eggs need to be from specific pathogen-free flocks [Citation5,Citation26,Citation27]. Moreover, the purification process is difficult, yielding a partially purified vaccine that could contain small amounts of egg protein. In addition, as allergy to eggs is one of the most common allergies in the pediatric population, and given the theoretical risk of an anaphylactic reaction, these vaccines have been not recommended to subjects with egg allergy [Citation5,Citation28–31]. Specifically, influenza vaccines have been contraindicated in subjects with severe egg allergic reaction. On the contrary, studies have shown that subjects with egg allergy can receive influenza vaccines since the amount of egg protein present in the vaccine is insufficient to trigger an allergic reaction [Citation28,Citation32–35]. Finally, antibiotics and preservatives are often used in the process, in order to maintain adequate sterility [Citation36].
In the event of an avian influenza outbreak, the virus could be lethal for eggs, resulting in a shortage of eggs and low titers [Citation5,Citation26]. Moreover, egg-based production takes a long time; as highlighted during the 2009 H1N1 pandemic, the process was unable to produce and distribute enough doses of vaccines against a new influenza virus in a short time [Citation37]. Indeed, the complete process, from the selection of candidate vaccine viruses (CVVs) to vaccine availability, typically takes 6–8 months. Furthermore, unexpected events, such as low virus yield or the late appearance of an antigenic variant virus, can affect the process, resulting in delays in vaccine production [Citation5,Citation7].
It is also noteworthy that some viruses, such as H3N2, grow poorly in eggs and can acquire some adaptive changes, leading to specific amino acid substitutions at the tip of the HA and close to the receptor-binding site. These adaptations have been seen to alter antigenicity and reduce VE [Citation5,Citation38]. A study conducted by Skowronski et al. [Citation39] showed that, during the 2012–2013 season, the low VE was due to a mutation acquired by the egg-adapted vaccine strain rather than to antigenic drift in circulating viruses. This was in contrast with the traditional concept that low VE was related to the mismatch between the circulating viruses and the vaccine viruses recommended by the WHO. This study highlights the importance of monitoring the annual vaccine constituents, which could impair VE even if the antigenic integrity of circulating viruses is maintained during the influenza season. Furthermore, a retrospective analysis of influenza seasons from 2011–2012 to 2017–2018 showed that there was little to no antigenic similarity between circulating H3N2 viruses and the seed virus of the egg-based vaccines in half of the seasons evaluated [Citation40]. However, the scenario is more complicated and seems that the low VE in some influenza seasons (i.e. 2012–2013, 2017–2018) is likely multifactorial and related to age-specific vaccine response, underlying medical conditions, NA drift, heterogeneous response to influenza vaccine and immune history rather than egg adaptations alone [Citation41–45].
The many shortcomings of egg-based vaccine production have prompted the development of alternative technologies. In 2017, Seqirus announced the successful production of the first H3N2 cell-based seasonal influenza vaccine using a CVV isolated and grown in cells [Citation46]. This innovative technology avoids the egg-adapted changes associated with traditional manufacturing methods and constitutes a great achievement, given that egg-adapted mutations are very likely to be maintained if egg-derived virus seeds are used for the production of influenza vaccines in other substrates [Citation5]. A cell-based quadrivalent inactivated influenza vaccine (Flucelvax Tetra in Europe; Flucelvax Quadrivalent in the United States) was approved for the 2019–2020 season, completing the transition to a product completely isolated and grown in cells [Citation47,Citation48].
Since 1995, the WHO has supported the development of an alternative influenza virus cultivation system [Citation49], and the cell culture platform has become one attractive alternative for vaccine production. The system is independent of the supply of eggs, the final product is free from egg protein, and cells can be cryopreserved and reconstituted as needed [Citation5,Citation50]. The process has greater flexibility, scalability and a shorter production cycle, does not require additives in the production process, and allows pathogenic viruses to be produced safely. Viruses adapted for growth in cells seem not to undergo egg-adaptation and to be representative of the circulating strains, remaining unchanged in cell culture [Citation5,Citation51,Citation52]. However, concerns have been raised regarding mutations that influenza viruses can develop after serial passaging in conventional cell culture [Citation53,Citation54]. Three cell lines are widely studied and applied to influenza vaccine production: Madin-Darby Canine Kidney (MDCK), Vero and PER.C6 [Citation5]. Trivalent and quadrivalent cell-derived vaccines have proved to be well tolerated and display a good safety profile [Citation27]. Furthermore, cell-derived vaccines have demonstrated to be non-inferior to egg-grown vaccines and in some circumstances even more immunogenic against influenza B viruses [Citation55,Citation56]. However, data to assess the effectiveness of these vaccine compared with their egg-based counterparts are still limited [Citation57]. The cell-derived method also has some disadvantages. As yet, experience of vaccine production in cells is somewhat limited. Moreover, the process needs new and qualified production facilities, is more expensive than egg-based production (costs are approximately 40% higher [Citation7]), shows variation among batches, and carries a risk of mycoplasma contamination [Citation5,Citation58]. Regulatory authorities require proof that vaccine is pathogen-free, without oncogenic agents, and a characterization of the cell substrate after manipulations (i.e. immortalization of primary cells) [Citation1,Citation5,Citation59]. In addition, the volumetric yield of influenza virus is lower than in the egg-based process [Citation58] and adapting the new virus to the cell substrate may be a major challenge [Citation36].
3. Recombinant DNA technology
The first influenza vaccine produced by means of modern recombinant DNA technology was Flublok, manufactured by Protein Sciences, Meriden, CT, acquired by Sanofi Pasteur in 2017. Flublok was first approved by the FDA in 2013 for use in persons aged 18–49 years in the United States; in 2017, it was replaced by Flublok Quadrivalent for use in subjects 18 years of age and older [Citation60,Citation61]. The quadrivalent vaccine, named Supemtek, has been authorized by the European Medicines Agency (EMA) for use in adults in the European Union in November 2020 [Citation62].
The novelty of this vaccine lies in the technology used, which enables RIV to be created synthetically without the need for CVV, seed viruses grown in eggs, and eggs for the manufacturing process [Citation63].
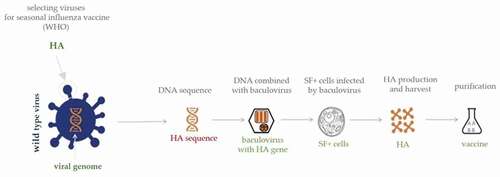
The starting point is the viral DNA sequence, in order to make the HA of the influenza virus; this DNA is then combined with a baculovirus, resulting in a ‘recombinant’ virus [Citation63,Citation64]. The host range of baculoviruses is restricted to invertebrates, the baculovirus most commonly used for manufacturing purposes being Autographa californica multiple-capsid nuclear polyhedrosis virus [Citation65]. Baculoviruses are able to infect insect cells, which do not support the replication of most types of viruses that cause human diseases, the only exceptions being arboviruses, which are sporadically reported to replicate in insect cells [Citation66]. However, the baculovirus-insect cell system is limited by the nature of insect cell glycosylation. As a result, N-glycans are different from those produces by mammalian cells, may lack terminal sialic acid residues, and consists of simple paucimannose structures [Citation67–70]. The vaccine is produced in a non-transformed, non-tumorigenic continuous cell line (expresSF+ insect cells) derived from Sf9 cells of the fall armyworm, Spodoptera frugiperda. This cell line is grown in suspension and serum-free medium, two primary requirements for large-scale manufacturing and safety [Citation71,Citation72] (). RIV contains three times as much HA as IIV [Citation73]; the antigens are highly purified full-length proteins containing the HA1-HA2 regions and the transmembrane domain and are not cleaved. Since it is not known whether the cleavage site is involved in the immune response, no significant differences should be found between the immune response to cleaved or uncleaved HA, at least in adults and the elderly, and the mechanism of action of this type of vaccine should be similar to that of IIV, i.e. antibody response against the HA [Citation74–76].
This novel technology presents some features that make it more appealing and interesting than vaccine production in eggs and/or cells. First of all, the baculovirus expression vector system employing recombinant technology allows a level of control over the whole process that is unreachable with the other current platforms. Indeed, the recombinant baculovirus codes for the required antigen and, since growth in other substrates (such as eggs and/or cells) is not required, the final product can exactly match the influenza virus sequence without the risk of adaptive changes that reduce VE [Citation65,Citation74]. Moreover, it has been proved that the HA produced in insect cells retains its native structure after purification and is correctly translated and biologically active [Citation77]. As this method of vaccine production is relatively rapid (requiring as little as 45 days after receipt of the virus) [Citation72] and the process has been successfully scaled to allow the rapid delivery of a large number of doses, this technology enables the annual vaccine preparation to be promptly modified in response to the unpredictable late appearance of an antigenic variant virus or to a pandemic strain. In addition, since the starting point is the DNA sequence, the WHO’s selection of viruses for inclusion in the vaccine composition can be delayed until more surveillance and epidemiological data are available [Citation64,Citation66,Citation76,Citation78]. Finally, since no live influenza viruses are produced, this production system does not require high-level biosafety facilities, which means that manufacturing personnel are not exposed to live viruses and that the production of seasonal and/or pandemic influenza vaccines is more rapid and economical [Citation75]. The final product is highly purified, without having undergone inactivation and/or extraction processes, and is free from pathogens, preservatives (such as thimerosal), antibiotics, adjuvants and, significantly from a clinical perspective, any residual egg proteins [Citation65,Citation74,Citation75,Citation77].
Several studies have evaluated the safety, efficacy, and immunogenicity of RIVs in preventing seasonal influenza in adults ≥18 years old [Citation73,Citation76,Citation78–83]. highlights the studies supported licensure of quadrivalent RIV (RIV4) for adults 50 years of age and older, adults 18–49 years and children aged 6 to 17 years compared to IIV produced in eggs [Citation73,Citation84,Citation85]. Overall, the data show that the vaccine is well tolerated and immunogenic in adults and the elderly. The high purity of the antigen allows the administration of higher doses (45 µg of HA per strain) without a significant increase in side-effects. Previous studies on high-dose vaccines have proved that these types of vaccine are able to produce an enhanced immunologic response in adults and the elderly, while maintaining a favorable safety profile [Citation86–88]. Notably, Dunkle et al. [Citation79] performed a randomized, double-blind, multicenter clinical trial in the US in 2014–2015 influenza season aimed at comparing RIV4 to a standard-dose quadrivalent IIV produced in eggs in persons 50 years of age and older. The trial assessed the relative vaccine efficacy against reverse-transcriptase-chain-reaction (RT-PCR)-confirmed influenza-like illness occurred from 14 days or more after vaccination. The results showed a 30% lower probability of influenza-like illness with RIV than egg-derived vaccine. This trial showed that RIV, as compared with standard vaccine, improved protection against laboratory confirmed influenza like-illness in adults 50 years of age or older. An exploratory study performed in pediatric subjects showed that the safety and immunogenicity of the RIV4 were comparable to those of IIV with most convincing data in 9–17 years old cohort than those aged 6–8 years [Citation84].
Table 1. Overview of clinical studies supporting RIV4 licensure. rHA: recombinant hemagglutinin.
RIV elicits strong immunogenicity, a long-lasting immune response and, notably, provides cross-protection against drifted influenza viruses, a feature that could be of particular importance during mismatched influenza seasons. The mechanism behind the cross-protection seems to be related to a difference in the glycosylation of the HA produced in the cells of lepidopteran insects, which allows greater accessibility by B-cells to the stalk domain, the most highly conserved region of the HA [Citation89–91]. Notably, it has been reported that against H3N2 virus, the vaccine was more immunogenic than IIV in the elderly [Citation82].
As reported by a systematic review released by the European Center for Disease Prevention and Control (ECDC) [Citation57], the body of knowledge of newer and enhanced influenza vaccines is still limited. Data related to efficacy or effectiveness for recombinant HA influenza vaccines were limited to two efficacy randomized controlled trials (RCTs) [Citation1,Citation2,Citation79,Citation81]. Recombinant HA was found to provide a greater protective effect against overall influenza viruses compared with placebo/no vaccination in 2007–2008 influenza season [Citation81], and when compared to traditional standard-dose influenza vaccination during the 2014–2015 season by Dunkle and colleagues (moderate-certainty evidence) [Citation79]. In this RCT, the relative VE was shown to be 30% more protective in prevention of influenza disease over quadrivalent standard-dose comparator. It is speculated that improved vaccine performance may be attributable to either the restriction of mutations seen with egg-based vaccines, or the higher dose of antigen seen in this type of influenza vaccine (based on recombinant technology). It should be recognized that during both seasons in which the clinical trials were conducted, there was significant mismatch between the vaccine strains and the circulating wild-type viruses. The findings [Citation57] suggest that the safety profile of recombinant HA influenza vaccines is largely similar to that of traditional standard-dose influenza vaccines in terms of local and systemic effects (low-moderate certainty evidence). Collectively, the results of the RIV3/RIV4 studies suggest that recombinant HA vaccines may offer better protection than no vaccination or standard-dose influenza vaccines with some possible cross protection to drift variants. Other studies are needed to better characterize these newer and enhanced vaccines and the increased use will provide larger data [Citation57].
4. Conclusions
Recombinant DNA technology represents a significant advance toward new platforms for the production of influenza vaccines. The speed, scalability, biosafety, and flexibility of the manufacturing process, together with the reliability of the HA in the vaccine, a protein genetically identical to the one selected by the WHO for inclusion in the annual seasonal influenza vaccine, are valuable advantages over the egg- and cell-based platforms [Citation65,Citation92].
This new technology would allow a prompt response to the emergence of a new pandemic strain and seems to confer cross-protection even in the event of mismatched influenza seasons. Overall, recombinant DNA technology is an important milestone in the field of influenza vaccine production and would appear to be the future of production.
5. Expert opinion
Influenza is a vaccine-preventable disease. However, the evolving nature of influenza viruses and their ability to escape the immune system make them a public health challenge. Vaccination is the most effective way of controlling seasonal influenza infections and the most important strategy for preventing possible pandemic events. Most of the current influenza vaccines are still produced and/or isolated in eggs, an old-fashioned production system established in the 1940s. However, two other technologies have been developed: the cell culture platform and DNA-based technology. This latter offers great advantages over egg- and cell-based influenza vaccine production and could be defined as an ‘anticipated modernization of influenza vaccine production’ [Citation92]. The ideal method of production should possess some indispensable characteristics: great flexibility and scalability, especially in the event of a pandemic; the virus included in the vaccine should be representative of the circulating strains; production should be economical and safe, and also yield large quantities; the process should be standardized and controlled and allow production to be started as soon as the sequence of the new influenza strain is available [Citation5]. Recombinant DNA technology displays many of these characteristics, and, with further improvement of the technology, it may be possible in the future to postpone vaccine strain selection to a time much closer to the beginning of the influenza season. In addition, from a public health point of view, these vaccines would be particularly useful during seasons characterized by the predominance of influenza strains antigenically mismatched to the seasonal vaccine. Moreover, the inclusion of a known amount of purified recombinant NA in this vaccine could constitute a further useful advantage of the current technology, since there is evidence of protective efficacy of the NA protein [Citation92].
The scenario of vaccine production in the next years seems to be particularly interesting and promising, involving a transition from the current egg-based production to new technologies, such as the RNA technology, the plant-based system and the DNA vaccine in addition to the one mentioned before.
The emergency use authorization of COVID-19 vaccines, one of which (the Pfizer-BioNTech COVID-19 Vaccine) was officially approved by FDA on 23 August 2021 [Citation93] and marketed as Comirnaty, makes the expectation of RNA vaccines against influenza disease realistic in the next years. In July 2021, Moderna, Inc. announced that mRNA-1010, Moderna’s first quadrivalent influenza vaccine candidate, has entered the clinic aimed at evaluating its safety, reactogenicity, and immunogenicity in healthy adults 18 years and older in the US [Citation94]. Other companies such as Seqirus, Sanofi/Translate Bio have announced plans to accelerate the development of influenza RNA vaccines [Citation95]. This technology is not entirely new, and scientists have been studying RNA vaccines for decades [Citation96]. The first study dates back to 1993 highlighting the usefulness of mRNA technology for immunization against influenza viruses [Citation97], however only in the early 2000s this technology has been taken into consideration again. Animal studies have provided promising data on cellular and antibody response, underlining a broadly protective immune response [Citation98–100]. Results from the first human trials of mRNA vaccines against H10N8 and H7N9 influenza viruses in healthy adults showed a robust humoral immune response with acceptable tolerability profile [Citation97,Citation101]. Overall, this technology has some beneficial features. First of all, the process is rapid, highly scalable and ideally the production could start within 6–8 weeks after the publication of the antigen sequence. This may allow a timely and effective response in the case of pandemic caused by a new influenza virus, the possibility to postpone the WHO selection to accurately target the dominant circulating strains or to promptly response to viral antigenic drift. The mRNA is noninfectious, non-integrating platform with no potential risk of infection or integrating into the host cell DNA and the absence of anti-vector immunity allows repeated administrations. Basically, RNA vaccines work using the host cell machinery for translating the mRNA into the corresponding antigen, mimicking a viral infection and producing a strong humoral and immune response [Citation96,Citation102–104].
In recent decades, the use of plants as bio-factories for vaccines production has aroused the attention of manufacturers. The plant-based vaccine production offers some advantages over the egg-based production such as the scalability of the process, plants require the same growth conditions as just one and only glass-house space and/or land and the risk of contamination by human pathogens is minimized. In addition, once the genetic sequence from a pathogen has been isolated, the protein production can usually be started within just a few weeks [Citation5,Citation105]. A phase 1–2 clinical trial in healthy adults receiving one intramuscular dose of a seasonal influenza plant-based quadrivalent virus-like particle (QVLP) reported promising results. The QVLP vaccine was well tolerated and induced strong and cross-reactive humoral and cellular response. Notably, the long-lasting response persisted for at least 6 months and could be partially attributed to the parallel induction of CD4 T cell help by this kind of vaccine [Citation106]. Recent phase 3 efficacy studies of QVLP in adults (18–64 years) and older subjects (≥65 years) confirmed the protection provided by the vaccine against both A (especially H3N2) and B strains in adults and showed to be non-inferior to a commercial quadrivalent IIV in the elderly even in a season characterized by mismatched strains [Citation107]. Altogether, these results showed that the plant platform is very promising in the world of vaccines.
Even DNA vaccine seems to be a promising technology in development since the 1990’. These vaccines do not require the growth of live virus, are temperature stable, noninfectious, non-replicating and the production process is rapid and cheap. The great advantage is that the target sequences of clinical isolates can be used as soon as available and are able to induce both humoral and cellular immune responses. The route of administration is critical and different devices have been evaluated, such as patches, gene-gun, and electroporation. The main concerns are with regard to safety and the potential integration of the plasmid DNA into host genome, the development of anti-DNA antibodies resulting in auto-immune disease and antibiotic resistance [Citation108–113]. However, a phase 1 randomized clinical trial in children and adolescents priming with trivalent DNA vaccine and boosting with trivalent IIV provided evidence that the strategy is safe and well tolerated [Citation114]. So far, no DNA vaccines have been approved for use in humans.
Beyond the technologies described in this review that are only some of those that have been developed so far, the universal vaccine remains the goal of researchers representing the ‘game changer’ in the fight against influenza [Citation115]. Ideally, a universal vaccine would avoid the need for annual reformulation and re-administration of seasonal vaccines and would confer a long-lived antibody response [Citation11]. However, owing to the significant differences between influenza A and B types, the prospect of having a ‘universal vaccine’ able to provide universal protection against all influenza A and B viruses seems unrealistic [Citation5]. In this regard, a WHO document released in 2017 reported that a universal influenza vaccine able to provide protection against influenza A viruses (group 1 or 2) could be expected to be in advanced clinical development within the next ten years, but not within the next five years [Citation116]. Various approaches have been studied, based either on stimulating the cellular immune response or on identifying potential conserved epitopes, such as the HA stalk domain, that may be a potential target in the attempt to elicit broadly cross-reactive neutralizing antibodies. In addition to HA, other proteins, such as M1, nucleoprotein, NA, and Matrix-2, have also been seen to have conserved epitopes and need to be investigated further [Citation5,Citation117–121]. By contrast, an influenza vaccine able to provide universal protection against influenza B viruses is unlikely to be in advanced clinical development within the next ten years [Citation116].
Overall, several new technologies and platforms have been or are being developed. However, many more data on the safety and immunogenicity of next-generation influenza vaccines in humans are needed.
The lesson learned from the COVID-19 pandemic highlights the importance of having different technologies available and able to promptly respond to a great demand and supply of vaccines worldwide. Based on these assumptions, the next decades may represent a milestone in the field of next-generation vaccines against influenza viruses.
Article highlights
Influenza is a global public health problem; vaccination is the most effective way of controlling seasonal influenza infections and preventing possible pandemics.
Due to the evolving nature of influenza viruses, the composition of vaccines has to be updated annually in order to include the seasonal viruses predicted to circulate during the next influenza season.
The majority of influenza vaccines are currently produced in embryonated chicken eggs, a well-established production system with some limitations that impact vaccine manufacturing.
Recombinant DNA technology using baculovirus-based expression system represents a modern method with a significant advance toward new platforms for the production of influenza vaccines.
The main advantages of the baculovirus-based expression system are the fully control over the whole process, the final product can exactly match the influenza virus sequence, the speed, and scalability. This technology enables the annual vaccine preparation to be promptly modified in response to the unpredictable late appearance of an antigenic variant virus or to a pandemic strain. No live viruses are required, and the final product is highly purified without any residual egg proteins.
Quadrivalent recombinant influenza vaccine, based on baculovirus expression vector system to express recombinant haemagglutinin in insect cells, is the only one authorized for use in adults in US and Europe.
Other platforms are under development, some of which are RNA technology, the plant-based system, and the DNA vaccine.
Declaration of interest
E Montomoli is the Chief Scientific Officer of VisMederi srl and VisMederi Research srl. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Funding
This paper was not funded.
References
- Onions D, Egan W, Jarrett R, et al. Validation of the safety of MDCK cells as a substrate for the production of a cell-derived influenza vaccine. Biologicals. 2010;38(5):544–551.
- Trombetta CM, Montomoli E. Influenza immunology evaluation and correlates of protection: a focus on vaccines. Expert Rev Vaccines. 2016;15(8):967–976.
- World Health Organization. Influenza, Seasonal Influenza, Data and statistics. 2016 2021 November 18]; Available from: https://www.euro.who.int/en/health-topics/communicable-diseases/influenza/data-and-statistics.
- World Health Organization. Influenza (Seasonal). 2018 2020 Nov 18]; Available from: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal).
- Trombetta CM, Marchi S, Manini I, et al. Challenges in the development of egg-independent vaccines for influenza. Expert Rev Vaccines. 2019;18(7):737–750.
- Hampson A, Barr I, Cox N, et al. Improving the selection and development of influenza vaccine viruses - Report of a WHO informal consultation on improving influenza vaccine virus selection, Hong Kong SAR, China.Vaccine. 2015 [18-20 Nov];35(8):1104–1109.
- Chen JR, Liu YM, Tseng YC, et al. Better influenza vaccines: an industry perspective. J Biomed Sci. 2020;27(1):33.
- World Health Organization. Improving influenza vaccine virus selection: Report of the 4th WHO informal consultation, Hong Kong SAR, China, 2015 Nov 18-20. 2015 18/11/2021]; Available from: https://apps.who.int/iris/handle/10665/249599.
- eCDC. WHO issues recommendation on the composition of influenza virus vaccines for the northern hemisphere 2016-2017. 2016 2021 Nov 18]; https://www.ecdc.europa.eu/en/news-events/who-issues-recommendation-composition-influenza-virus-vaccines-northern-hemisphere-2016.
- Pitrelli A. Introduction of a quadrivalent influenza vaccine in Italy: a budget impact analysis. J Prev Med Hyg. 2016;57(1):E34–40.
- Krammer F. The human antibody response to influenza A virus infection and vaccination. Nat Rev Immunol. 2019;19(6):383–397.
- CDC. Licensure of a high-dose inactivated influenza vaccine for persons aged ≥65 years (fluzone high-dose) and guidance for use — United States, 2010. MMWR Morb Mortal Wkly Rep. 2010 Apr 30;59(16):485–486.
- Sanofi. Sanofi to build new facility in Canada to increase global availability of high-dose influenza vaccine. 2021.
- Gravenstein S, Davidson HE, Taljaard M, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccination on numbers of US nursing home residents admitted to hospital: a cluster-randomised trial. Lancet Respir Med. 2017;5(9):738–746.
- Falsey AR, Treanor JJ, Tornieporth N, et al. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis. 2009;200(2):172–180.
- Kaka AS, Filice GA, Myllenbeck S, et al. Comparison of side effects of the 2015-2016 high-dose, inactivated, trivalent influenza vaccine and standard dose, inactivated, trivalent influenza vaccine in adults >/=65 years. Open Forum Infect Dis. 2017;4(1):ofx001.
- DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371(7):635–645.
- Wilkinson K, Wei Y, Szwajcer A, et al. Efficacy and safety of high-dose influenza vaccine in elderly adults: a systematic review and meta-analysis. Vaccine. 2017;35(21):2775–2780.
- CDC. Fluzone High-Dose Seasonal Influenza Vaccine. 2021 18/Nov/2021]; Available from: https://www.cdc.gov/flu/prevent/qa_fluzone.htm.
- Sridhar S, Brokstad KA, Cox RJ. Influenza vaccination strategies: comparing inactivated and live attenuated influenza vaccines. Vaccines (Basel). 2015;3(2):373–389.
- Jang YH, Seong BL. Immune Responses elicited by live attenuated influenza vaccines as correlates of universal protection against influenza viruses. Vaccines (Basel). 2021;9(4):353. DOI:10.3390/vaccines9040353.
- Wong SS, Webby RJ. Traditional and new influenza vaccines. Clin Microbiol Rev. 2013;26(3):476–492.
- Jhaveri R. Live attenuated influenza vaccine: is past performance a guarantee of future results? Clin Ther. 2018;40(8):1246–1254.
- American Academy of Pediatrics. AAP: No flu vaccine preference for 2020-’21 season 2020 [2021 Nov 18; https://publications.aap.org/aapnews/news/7233?autologincheck=redirected.
- Sharon DM, Nesdoly S, Yang HJ, et al. A pooled genome-wide screening strategy to identify and rank influenza host restriction factors in cell-based vaccine production platforms. Sci Rep. 2020;10(1):12166.
- Perez Rubio A, Eiros JM. Cell culture-derived flu vaccine: present and future. Hum Vaccin Immunother. 2018;14(8):1874–1882.
- Perez-Rubio A, Ancochea J, Eiros Bouza JM. Quadrivalent cell culture influenza virus vaccine. Comparison to egg-derived vaccine. Hum Vaccin Immunother. 2020;16(8):1746–1752.
- James JM, Zeiger RS, Lester MR, et al. Safe administration of influenza vaccine to patients with egg allergy. J Pediatr. 1998;133(5):624–628.
- Croegaert KA, Ithman MM, Spurgin AL, et al. Influenza vaccine safety in patients with egg allergy. J Am Pharm Assoc. 2013;53(2):214–216.
- Greenhawt M. Live attenuated influenza vaccine for children with egg allergy. Vol. 351. h6656. London: British Medical Association; 2015.
- Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127(3):594–602.
- CDC. Flu Vaccine and People with Egg Allergies. 2020 11/11/2021]; Available from: https://www.cdc.gov/flu/prevent/egg-allergies.htm.
- Des Roches A, Paradis L, Gagnon R, et al. Egg-allergic patients can be safely vaccinated against influenza. J Allergy Clin Immunol. 2012;130(5):1213–1216 e1.
- Kelso JM. Administering influenza vaccine to egg-allergic persons. Expert Rev Vaccines. 2014;13(8):1049–1057.
- Yang HJ. Safety of influenza vaccination in children with allergic diseases. Clin Exp Vaccine Res. 2015;4(2):137–144.
- Buckland BC. The development and manufacture of influenza vaccines. Hum Vaccin Immunother. 2015;11(6):1357–1360.
- Houser K, Subbarao K. Influenza vaccines: challenges and solutions. Cell Host Microbe. 2015;17(3):295–300.
- Paules CI, Sullivan SG, Subbarao K, et al. Chasing seasonal influenza - the need for a universal influenza vaccine. N Engl J Med. 2018;378(1):7–9.
- Skowronski DM, Janjua NZ, De Serres G, et al. Low 2012-13 influenza vaccine effectiveness associated with mutation in the egg-adapted H3N2 vaccine strain not antigenic drift in circulating viruses. PLoS One. 2014;9(3):e92153.
- Rajaram S, Van Boxmeer J, Leav B, et al. Retrospective evaluation of mismatch from egg-based isolation of influenza strains compared with cell-based isolation and the possible implications for vaccine effectiveness . OFID, OFID. Editor. 2018; 5(Supp_1):S69.
- Klein NP, Fireman B, Goddard K, et al. Vaccine effectiveness of cell-culture relative to egg-based inactivated influenza vaccine during the 2017-18 influenza season. PLoS One. 2020;15(2):e0229279.
- Liu F, Gross FL, Jefferson SN, et al. Age-specific effects of vaccine egg adaptation and immune priming on A(H3N2) antibody responses following influenza vaccination. J Clin Invest. 2021;131(8). DOI:10.1172/JCI146138
- Krietsch Boerner L. The Flu Shot and the Egg. ACS Cent Sci. 2020;6(2):89–92.
- Izurieta HS, Chillarige Y, Kelman J, et al. Relative effectiveness of influenza vaccines among the United States elderly, 2018-2019. J Infect Dis. 2020;222(2):278–287.
- Cobey S, Gouma S, Parkhouse K, et al. Poor immunogenicity, not vaccine strain egg adaptation, may explain the low h3n2 influenza vaccine effectiveness in 2012-2013. Clin Infect Dis. 2018;67(3):327–333.
- Seqirus. Seqirus announces next major advancement in cell-based influenza vaccine technology. 2017.
- Buhler S, Ramharter M. Flucelvax Tetra: a surface antigen, inactivated, influenza vaccine prepared in cell cultures. ESMO Open. 2019;4(1):e000481.
- Lamb YN. Cell-based quadrivalent inactivated influenza virus vaccine (flucelvax((R)) tetra/flucelvax quadrivalent((R))): a review in the prevention of influenza. Drugs. 2019;79(12):1337–1348.
- World Health Organization. Cell culture as a substrate for the production of influenza vaccines: memorandum from a WHO meeting. Bull World Health Organ. 1995;73(4):431–435.
- DeMarcus L, Shoubaki L, Federinko S. Comparing influenza vaccine effectiveness between cell-derived and egg-derived vaccines, 2017-2018 influenza season. Vaccine. 2019;37(30):4015–4021.
- Pöri P. Development of vaccines. Metropolia University of Applied Sciences; 2018. p. 40.
- Park YW, Kim YH, Jung HU, et al. Comparison of antigenic mutation during egg and cell passage cultivation of H3N2 influenza virus. Clin Exp Vaccine Res. 2020;9(1):56–63.
- Harding AT, Heaton NS. Efforts to improve the seasonal influenza vaccine. Vaccines (Basel). 2018;6(2):1–12.
- Skowronski DM, Sabaiduc S, Chambers C, et al. Mutations acquired during cell culture isolation may affect antigenic characterisation of influenza A(H3N2) clade 3C.2a viruses. Euro Surveill. 2016;21(3):30112.
- Song JY, Cheong HJ, Lee J, et al. Immunogenicity and safety of a cell culture-derived inactivated trivalent influenza vaccine (NBP607): a randomized, double-blind, multi-center, phase 3 clinical trial. Vaccine. 2015;33(41):5437–5444.
- Divino V, Krishnarajah G, Pelton SI, et al. A real-world study evaluating the relative vaccine effectiveness of a cell-based quadrivalent influenza vaccine compared to egg-based quadrivalent influenza vaccine in the US during the 2017-18 influenza season. Vaccine. 2020;38(40):6334–6343.
- eCDC. Systematic review of the efficacy, effectiveness and safety of newer and enhanced seasonal influenza vaccines. 2020.
- Kim EH, Kwon HI, Park SJ, et al. Generation of a high-growth influenza vaccine strain in MDCK cells for vaccine preparedness. J Microbiol Biotechnol. 2018;28(6):997–1006.
- FDA. Characterization and qualification of cell substrates and other biological materials used in the production of viral vaccines for infectious disease indications. 2010.
- Protein Sciences Corporation. Superior Protection by Flublok® Influenza Vaccine in Seniors Documented in New England Journal of Medicine. 2017 2021 Nov 22]; Available from: https://www.prnewswire.com/news-releases/superior-protection-by-flublok-influenza-vaccine-in-seniors-documented-in-new-england-journal-of-medicine-300478298.html.
- SanofiPasteur. Flublok (influenza vaccine) for intramuscular injection. 2018.
- EMA. Supemtek 2020 2021 Nov 22]; Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/supemtek.
- CDC. How Influenza (Flu) Vaccines Are Made. 2021 2021 Nov 22]; Available from: https://www.cdc.gov/flu/prevent/how-fluvaccine-made.htm.
- Buckland B, Boulanger R, Fino M, et al. Technology transfer and scale-up of the Flublok recombinant hemagglutinin (HA) influenza vaccine manufacturing process. Vaccine. 2014;32(42):5496–5502.
- Felberbaum RS. The baculovirus expression vector system: a commercial manufacturing platform for viral vaccines and gene therapy vectors. Biotechnol J. 2015;10(5):702–714.
- Cox MM, Karl Anderson D. Production of a novel influenza vaccine using insect cells: protection against drifted strains. Influenza Other Respir Viruses. 2007;1(1):35–40.
- Margine I, Martinez-Gil L, Chou YY, et al. Residual baculovirus in insect cell-derived influenza virus-like particle preparations enhances immunogenicity. PLoS One. 2012;7(12):e51559.
- Shi X, Jarvis DL. Protein N-glycosylation in the baculovirus-insect cell system. Curr Drug Targets. 2007;8(10):1116–1125.
- Harrison RL, Jarvis DL. Protein N-glycosylation in the baculovirus-insect cell expression system and engineering of insect cells to produce “mammalianized” recombinant glycoproteins. Adv Virus Res. 2006;68:159–191.
- Kost TA, Condreay JP, Jarvis DL. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol. 2005;23(5):567–575.
- Aucoin MG, Mena JA, Kamen AA. Bioprocessing of baculovirus vectors: a review. Curr Gene Ther. 2010;10(3):174–186.
- Cox MM, Hashimoto Y. A fast track influenza virus vaccine produced in insect cells. J Invertebr Pathol. 2011;107:S31–41. Suppl.
- Treanor JJ, Schiff GM, Hayden FG, et al. Safety and immunogenicity of a baculovirus-expressed hemagglutinin influenza vaccine: a randomized controlled trial. JAMA. 2007;297(14):1577–1582.
- Cox MM, Hollister JR. FluBlok, a next generation influenza vaccine manufactured in insect cells. Biologicals. 2009;37(3):182–189.
- Cox MM, Patriarca PA, Treanor J. FluBlok, a recombinant hemagglutinin influenza vaccine. Influenza Other Respir Viruses. 2008;2(6):211–219.
- King JC Jr., Cox MM, Reisinger K, et al. Evaluation of the safety, reactogenicity and immunogenicity of FluBlok trivalent recombinant baculovirus-expressed hemagglutinin influenza vaccine administered intramuscularly to healthy children aged 6-59 months. Vaccine. 2009;27(47):6589–6594.
- Wang K, Holtz KM, Anderson K, et al. Expression and purification of an influenza hemagglutinin–one step closer to a recombinant protein-based influenza vaccine. Vaccine. 2006;24(12):2176–2185.
- Cox MM, Izikson R, Post P, et al. Safety, efficacy, and immunogenicity of Flublok in the prevention of seasonal influenza in adults. Ther Adv Vaccines. 2015;3(4):97–108.
- Dunkle LM, Izikson R, Patriarca P, et al. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med. 2017;376(25):2427–2436.
- Baxter R, Patriarca PA, Ensor K, et al. Evaluation of the safety, reactogenicity and immunogenicity of FluBlok(R) trivalent recombinant baculovirus-expressed hemagglutinin influenza vaccine administered intramuscularly to healthy adults 50-64 years of age. Vaccine. 2011;29(12):2272–2278.
- Treanor JJ, El Sahly H, King J, et al. Protective efficacy of a trivalent recombinant hemagglutinin protein vaccine (FluBlok(R)) against influenza in healthy adults: a randomized, placebo-controlled trial. Vaccine. 2011;29(44):7733–7739.
- Treanor JJ, Schiff GM, Couch RB, et al. Dose-related safety and immunogenicity of a trivalent baculovirus-expressed influenza-virus hemagglutinin vaccine in elderly adults. J Infect Dis. 2006;193(9):1223–1228.
- Safdar A, Rodriguez MA, Fayad LE, et al. Dose-related safety and immunogenicity of baculovirus-expressed trivalent influenza vaccine: a double-blind, controlled trial in adult patients with non-Hodgkin B cell lymphoma. J Infect Dis. 2006;194(10):1394–1397. R.B.Couch.
- Dunkle LM, Izikson R, Patriarca PA, et al. Safety and Immunogenicity of a recombinant influenza vaccine: a randomized trial. Pediatrics. 2018;141(5). DOI:10.1542/peds.2017-3021
- Dunkle LM, Izikson R, Patriarca PA, et al. Randomized comparison of immunogenicity and safety of quadrivalent recombinant versus inactivated influenza vaccine in healthy adults 18-49 years of age. J Infect Dis. 2017;216(10):1219–1226.
- Sullivan SJ, Jacobson R, Poland GA. Advances in the vaccination of the elderly against influenza: role of a high-dose vaccine. Expert Rev Vaccines. 2010;9(10):1127–1133.
- Keitel WA, Couch RB, Cate TR, et al. High doses of purified influenza A virus hemagglutinin significantly augment serum and nasal secretion antibody responses in healthy young adults. J Clin Microbiol. 1994;32(10):2468–2473.
- Keitel WA, Cate TR, Atmar RL, et al. Increasing doses of purified influenza virus hemagglutinin and subvirion vaccines enhance antibody responses in the elderly. Clin Diagn Lab Immunol. 1996;3(5):507–510.
- Wang CC, Chen JR, Tseng YC, et al. Glycans on influenza hemagglutinin affect receptor binding and immune response. Proc Natl Acad Sci U S A. 2009;106(43):18137–18142.
- Chen JR, Yu YH, Tseng YC, et al. Vaccination of monoglycosylated hemagglutinin induces cross-strain protection against influenza virus infections. Proc Natl Acad Sci U S A. 2014;111(7):2476–2481.
- Tseng YC, Wu CY, Liu ML, et al. Egg-based influenza split virus vaccine with monoglycosylation induces cross-strain protection against influenza virus infections. Proc Natl Acad Sci U S A. 2019;116(10):4200–4205.
- Dunkle LM, Izikson R. Recombinant hemagglutinin influenza vaccine provides broader spectrum protection. Expert Rev Vaccines. 2016;15(8):957–966.
- FDA. FDA Approves First COVID-19 Vaccine. 2021 2021 Nov 22]; Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine.
- Moderna. Moderna Announces First Participant Dosed in Phase 1/2 Study of Its Quadrivalent Seasonal Flu mRNA Vaccine. 2021 2021 Nov 22]; Available from: https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-first-participant-dosed-phase-12-study-its/.
- Seqirus. Seqirus Announces Investment in Next-Generation Influenza Vaccine Technology, Self-Amplifying Messenger RNA (sa-mRNA). 2021 2021 Nov 22]; https://www.seqirus.com/news/seqirus-invests-in-self-amplifying-messenger-rna-technology.
- Maruggi G, Zhang C, Li J, et al. mRNA as a transformative technology for vaccine development to control infectious diseases. Mol Ther. 2019;27(4):757–772.
- Bahl K, Senn JJ, Yuzhakov O, et al. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against h10n8 and h7n9 influenza viruses. Mol Ther. 2017;25(6):1316–1327.
- Pardi N, Parkhouse K, Kirkpatrick E, et al. Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies. Nat Commun. 2018;9(1):3361.
- Petsch B, Schnee M, Vogel AB, et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat Biotechnol. 2012;30(12):1210–1216.
- Kowalczyk A, Doener F, Zanzinger K, et al. Self-adjuvanted mRNA vaccines induce local innate immune responses that lead to a potent and boostable adaptive immunity. Vaccine. 2016;34(33):3882–3893.
- Feldman RA, Fuhr R, Smolenov I, et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine. 2019;37(25):3326–3334.
- Zhang C, Maruggi G, Shan H, et al. Advances in mRNA vaccines for infectious diseases. Front Immunol. 2019;10:594.
- Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–279.
- Scorza FB, Pardi N. New kids on the block: RNA-based influenza virus vaccines. Vaccines (Basel). 2018;6(2):1–15.
- Medicago. Plant-derived vaccines. Biopharmadealmakers. 2018.
- Pillet S, Aubin E, Trepanier S, et al. A plant-derived quadrivalent virus like particle influenza vaccine induces cross-reactive antibody and T cell response in healthy adults. Clin Immunol. 2016;168:72–87.
- Ward BJ, Makarkov A, Seguin A, et al. Efficacy, immunogenicity, and safety of a plant-derived, quadrivalent, virus-like particle influenza vaccine in adults (18-64 years) and older adults (>/=65 years): two multicentre, randomised phase 3 trials. Lancet. 2020;396(10261):1491–1503.
- Medjitna TD, Stadler C, Bruckner L, et al. DNA vaccines: safety aspect assessment and regulation. Basel. 2006;126:261–270. discussion 327.
- Guilfoyle K, Major D, Skeldon S, et al. Protective efficacy of a polyvalent influenza A DNA vaccine against both homologous (H1N1pdm09) and heterologous (H5N1) challenge in the ferret model. Vaccine. 2021;39(34):4903–4913.
- Lee LYY, Izzard L, Hurt AC. A review of DNA vaccines against influenza. Front Immunol. 2018;9:1568.
- Kalenik BM, Gora-Sochacka A, Stachyra A, et al. Response to a DNA vaccine against the H5N1 virus depending on the chicken line and number of doses. Virol J. 2020;17(1):66.
- Andersen TK, Bodin J, Oftung F, et al. Pandemic PREPAREDNESS AGAINST INFLUENZA: DNA VACCINE FOR RAPID RELIEf. Front Immunol. 2021;12:747032.
- Rockman S, Laurie KL, Parkes S, et al. New Technologies for Influenza Vaccines. Microorganisms. 2020;8(11):1745.
- Houser KV, Yamshchikov GV, Bellamy AR, et al., V.R.C.s. team. DNA vaccine priming for seasonal influenza vaccine in children and adolescents 6 to 17 years of age: a phase 1 randomized clinical trial. PLoS One. 2018;13(11):e0206837.
- Beans C. Researchers getting closer to a “universal” flu vaccine. Proc Natl Acad Sci U S A. 2022;119(5). 10.1073/pnas.2123477119
- WHO. WHO preferred product characteristics for next-generation influenza vaccines. 2017. p. 1–42.
- Lee YT, Kim KH, Ko EJ, et al. New vaccines against influenza virus. Clin Exp Vaccine Res. 2014;3(1):12–28.
- Henry C, Palm AE, Krammer F, et al. From original antigenic sin to the universal influenza virus vaccine. Trends Immunol. 2018;39(1):70–79.
- Subbarao K, Matsuoka Y. The prospects and challenges of universal vaccines for influenza. Trends Microbiol. 2013;21(7):350–358.
- Pica N, Palese P. Toward a universal influenza virus vaccine: prospects and challenges. Annu Rev Med. 2013;64(1):189–202.
- Egorov AY. The challenges of creating a universal influenza vaccine. Microbiol Independent Res J. 2016;3(1):31–41