ABSTRACT
Background
This study evaluates the immunogenicity and reactogenicity of BNT162b2 and mRNA-1273 booster doses after the primary two-dose BNT162b2 series in Japan and is the first report from Western Pacific region.
Research design and Methods
Healthcare workers receiving the two-dose BNT162b2 series and eligible for booster vaccination were enrolled. Self-reported adverse reactions were recorded for 8 days. Antibody titer was measured at baseline and on day 28.
Results
A total of 2,931 and 890 subjects received BNT162b2 (homologous) and mRNA-1273 (heterologous) booster vaccinations, respectively. The anti-SARS-CoV-2 spike protein IgG titer increased by 50.9- and 64.3-fold in the homologous and heterologous groups, respectively. Immunogenicity was greater with increasing age, regardless of sex. Adverse reactions were mild to moderate and decreased with age. The most common adverse reactions were injection-site pain (92.2%), fatigue (71.8%), headache (58.3%), and fever ≥37.5°C (46.5%). Two cases of non-severe myocarditis occurred in the heterologous group and resolved without clinical sequelae.
Conclusion
Homologous booster schedules had fewer reported adverse reactions; heterologous boosters elicited greater immunogenicity. Among different age groups, subjects aged 60 or over had the lowest immunogenicity before the booster, and both homologous and heterologous boosters restored vaccine immunogenicity level comparable to those of younger age groups.
1. Introduction
Coronavirus disease 2019 (COVID-19) has been declared a global health crisis by the World Health Organization in May 2020 and claimed the lives of millions of people worldwide. In Japan, there have been more than 4 million confirmed cases and almost 22,000 deaths due to COVID-19 as of February 2022 [Citation1]. Early on during the pandemic, researchers found that age and certain comorbidities were associated with an increased risk of severe illness and death, and the world quickly began developing novel preventions and treatments. The first vaccine against COVID-19 approved in Japan was the mRNA-based BNT162b2 vaccine, with mass vaccination prioritizing medical staff and other high-risk populations commencing in February 2020 [Citation2,Citation3]. The mRNA-1273 vaccine, also mRNA-based, received approval by the Ministry of Health, Labor and Welfare of Japan later in the same year. The vaccination rate in Japan was initially low at 4% in April 2021, but soon picked up due to governmental efforts and increased public awareness of the pandemic crisis, eventually reaching at least 61.8% of the targeted population by October 2021 [Citation4].
Japanese society initially showed reluctance to the mass COVID-19 vaccination program because of the perceived effectiveness and adverse events associated with the vaccines [Citation5,Citation6], possibly stemming from an earlier human papillomavirus vaccine controversy. Japan’s real-world results of COVID-19 vaccine efficacy, immunogenicity, and reactogenicity, mainly of the BNT162b2, were published soon after the commencement of the COVID-19 vaccination program [Citation3,Citation7–14]. While BNT162b2 was found to elicit a robust anti-severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) spike protein (anti-Spike) antibody response and was found to be effective in reducing SARS-CoV-2 infection [Citation3,Citation7–13], age, comorbidity, sex, and the time between the first and second doses were reported to be associated with the antibody titers in vaccine recipients [Citation8,Citation9,Citation11,Citation13]. BNT162b2 and mRNA-1273 induced similar types of systemic and injection-site reactions, but the incidence of adverse reactions was higher in females, younger individuals, and those receiving the mRNA-1273 vaccine [Citation14].
With the emergence of new, more transmissible SARS-CoV-2 variants as well as reports of waning immunity and breakthrough infections occurring after the primary two-dose vaccine series in Japan [Citation3,Citation12], and around the world, a third or even fourth booster dose has been proposed for maintaining sufficient protection. Retrospective studies demonstrated that three doses were more effective than two doses of the BNT162b2 vaccine in protecting against both SARS-CoV-2 infection and severe outcomes in the general population [Citation15–17]. Safety and efficacy of a third dose of BNT162b2 Covid-19 Vaccine has been demonstrated [Citation18]. Furthermore, prospective studies in high-risk populations found that the third homologous dose of mRNA-1273 or BNT162b2 vaccines was able to induce neutralizing humoral and cellular immunity [Citation19,Citation20]. Various heterologous vaccination booster strategies have been tested to overcome the difficulties in vaccine distribution and allocation, and almost all combinations were reported to show good immunogenicity and neutralization activity [Citation21–25]. In the COV-BOOST trial conducted in the UK, age was associated with reactogenicity, but not immunogenicity, of the third immunization [Citation23]. However, neither the frequency of adverse events nor the level of immune response were found to be associated with age in the phase 1/2 trial in the US [Citation24].
In this prospective study, we aim to assess the immunogenicity and reactogenicity of two booster immunization protocols (i.e. BNT162b2/BNT162b2/BNT162b2 [homologous] and BNT162b2/BNT162b2/mRNA-1273 [heterologous]) among healthcare workers in Japan. Participants received the two-dose primary BNT162b2 series early on in the national rollout. Factors theoretically affecting the antibody titer and adverse reaction rate were also tested.
2. Methods
2.1. Study design
The current prospective study was an extension of the national COVID-19 vaccine rollout program in healthcare workers of seven hospitals from the National Hospital Organization and four hospitals from the Japan Community Health Care Organizations in Japan. The priming dose of the BNT162b2 vaccine was initiated on 17 February 2021, the second BNT162b2 vaccination was administered in March 2021, and the third immunization of anti-SARS-CoV-2 vaccine (either BNT162b2 or mRNA-1273) was administered starting from 1 December 2021 and 17 December 2021, respectively (8 months after the second vaccination). Questionnaires recording reactogenicity (adverse reaction) were distributed and filled out by study participants at each medical institution starting from the day of the third vaccination (Day 0) for 8 days (Day 7), and any adverse reaction after Day 7 were recorded on the day of occurrence. Blood was withdrawn from participants who gave additional consent at baseline and 28 days after the third vaccination for antibody titer measurement. Information regarding adverse reaction, serious adverse events, and confirmed SARS-CoV-2 infection were recorded electronically throughout the study period.
2.2. Participants
Healthcare workers who had completed two BNT162b2 vaccinations and were eligible for the third immunization as a booster dose were enrolled for study participation. The type of booster vaccine administered was chosen by the participants. All participants were offered the option of immunogenicity test with no additional selection criteria, except that the participants in the immunological cohort were asked to sign additional consent for the blood sampling protocol. Signed informed consent was obtained from all individual participants included in the study.
2.3. Outcomes and data collection
The reactogenicity parameters recorded daily in the questionnaire included injection-site reactions (pain, burning sensation, redness, swelling, itching, induration), systemic reactions (fatigue, headache, fever ≥37.5°C, nasal discharge), and sick-leaves. Serum anti-Spike and anti-nucleocapsid protein (anti-N) antibody titers were measured in all blood samples; subjects who tested positive for anti-N IgG, indicating a history of SARS-CoV-2 infection were excluded from the analysis for antibody titers. Adverse reaction and serious adverse events including anaphylaxis, thrombosis, myocarditis/pericarditis, convulsions, Guillain-Barré syndrome, acute disseminated encephalomyelitis, thrombocytopenic purpura, vasculitis, aseptic meningitis, encephalitis or encephalopathy, meningitis, arthritis, facial nerve paralysis, and vasovagal reflex were noted and reported to the COVID-19 Vaccine Secretariat. All personal identifiable information was removed prior to the data analysis of the present study.
2.4. Laboratory methods
Serum was tested for IgG antibodies against the receptor binding domain of the SARS-CoV-2 spike protein (anti-Spike) and the SARS-CoV-2 nucleocapsid protein (anti-N). Qualitative detection was outsourced to a certified laboratory and performed using commercial automatic electrochemiluminescence immunoassays. Elecsys Anti-SARS-CoV-2 S RUO for anti-spike IgG and Elecsys Anti-SARS-CoV-2 RUO for anti-N IgG were purchased from Roche Diagnostics K.K., Tokyo, Japan. Samples were prepared according to the manufacturer’s instructions and analyzed on the Roche cobas platform (Roche Diagnostics K.K.).
2.5. Data and statistical analysis
Data were analyzed using IBM SPSS 28.2. The statistical results of continuous variables are presented as the mean and standard deviation (SD), while those of categorical variables are presented as percentages. Differences in categorical variables between subgroups were compared using the Pearson’s Chi-squared test or Fisher’s exact test and those of continuous variables using the Wilcoxon rank sum test, Mann–Whitney U test, or Kruskal–Wallis test. Antibody titers of anti-Spike IgG are further presented as geometric mean (GM) with 95% confidence interval (CI), and the ratio of GM between groups was also calculated. For association analysis, data were stratified by age and sex. A p-value of <0.05 was considered statistically significant in all tests.
2.6. Ethics
The study was approved by The Tokushukai Group Ethics Committee (TGE01856701) prior to initiation and was conducted in accordance with the principles of the Declaration of Helsinki.
2.7. Role of the funding source
The funder has no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
3. Results
3.1. Patient demographics
A total of 3,821 subjects received either the BNT162b2 (n = 2,931, henceforth the homologous group) and the mRNA-1273 (n = 890, henceforth the heterologous group) as the third dose after primary two-dose BNT162b2 immunization. Among these subjects, 500 (17.1%) and 497 (55.8%) subjects in the BNT162b2 and mRNA-1273 group, respectively, gave consent and underwent blood sampling for anti-Spike and anti-N IgG titer quantification (henceforth the immunology cohort).
summarizes the baseline characteristics of the enrolled study subjects. In the overall and immunology cohorts, 66.6% and 58.8% of the subjects were females, respectively. The mean age of the two cohorts was 41.4 ± 12.1 and 42.0 ± 12.0 years, and the most common comorbidities were hypertension (8.0% and 8.8%), hyperlipidemia (4.6% and 7.1%), and atopic dermatitis (3.0% and 3.3%). Prior to the third immunization against SARS-CoV-2, 0.7% of the total cohort and 0.8% of the immunology cohort tested positive for SARS-CoV-2. The mean time interval between the second and third COVID-19 vaccinations was 266.5 ± 15.8 days in the overall cohort and 275.5 ± 17.0 days in the immunology cohort (). The three most common occupations of the study participants in the analyzed cohorts and subgroups were nurses, doctors, and administrators (Supplementary Table S1).
Table 1. Demographics and characteristics of subjects receiving BNT162b2 and mRNA-1273.
3.2. Immunogenicity of BNT162b2 and mRNA-1273 vaccines as the third immunization
In the BNT162b2 homologous group, the geometric mean anti-Spike IgG titer was 382.5 (352.3 − 415.4) U/mL at baseline and increased to 19,762.6 (18,615.6 − 20,980.2) on Day 28, with at a ratio of 50.9 (47.2 − 54.8) in the homologous group ( and ). The anti-Spike IgG titer ratio increased with age (p < 0.001) and was highest in subjects aged ≥60 years (100.6 [76.0 − 133.1]) () and ). The geometric mean anti-Spike IgG titer on Day 28 was similar between males and females in the homologous group (21,229.0 vs 18,693.9, p < 0.057) () and ).
Figure 1. Comparison of anti-Spike IgG titer level at baseline (before 3rd vaccination) and Day 28 after 3rd vaccination. BNT162b2: cohort with homologous BNT162b2 prim and BNT162b2 boost vaccine. mRNA-1273: cohort with heterologous BNT162b2 prime + mRNA-1273 booster.
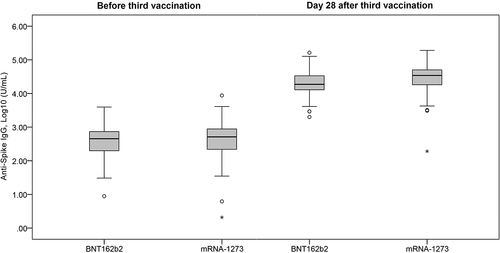
Figure 2. Distribution of anti-Spike IgG titer level at baseline (before 3rd vaccination) and Day 28 after 3rd vaccination by age groups (a) and sex (b). BNT162b2: cohort with homologous BNT162b2 prim and BNT162b2 boost vaccine. mRNA-1273: cohort with heterologous BNT162b2 prime + mRNA-1273 booster.
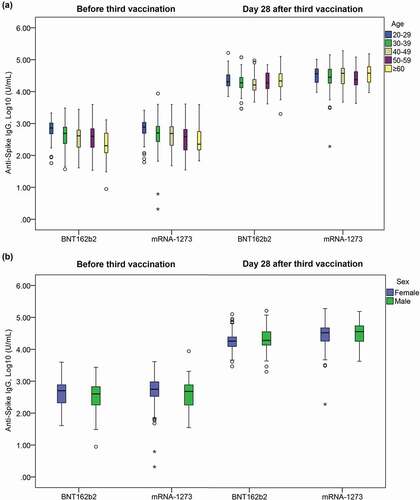
Table 2. Anti-Spike IgG at baseline and day 28 after 3rd vaccination by vaccine type, age, and sex.
In the mRNA-1273 heterologous group, the geometric mean anti-Spike IgG titer was 454.4 (417.8 − 494.2) at baseline and increased to 29,500.0 (27,566.9 − 31,568.7) on Day 28, with at ratio of 64.3 (59.8 − 69.1) ( and ). The anti-Spike IgG titer ratio also significantly increased with age (p < 0.001) and was highest in the subjects aged ≥60 years (112.5 [81.3 − 155.7]) () and ). Like the homologous group, the geometric mean anti-Spike IgG titer on Day 28 was similar between males and females in the heterologous group (31,335.0 vs 26,067.5, p < 0.189) () and ).
The heterologous group achieved a significantly higher overall anti-Spike IgG titer ratio than the homologous group (p < 0.001, ). This trend was observed when the subjects were stratified by sex (p ≤ 0.001) and age groups (p < 0.001 − 0.012), except in those ≥60 years-old, where the anti-Spike IgG titer ratios were comparable (p = 0.333) ().
3.3. Reactogenicity of BNT162b2 and mRNA-1273 vaccines as the third immunization
summarizes the distribution of self-reported adverse reactions in the homologous and heterologous groups of the overall cohort. The incidence of adverse reactions after the third vaccination was generally comparable to the adverse reactions after the second vaccination [Citation12]. The most common adverse reactions after the third immunization were pain at injection site (92.2%), systemic fatigue (71.8%), headache (58.3%), and fever ≥37.5°C (46.5%) ().
Table 3. Common adverse reactions during the first 8 days after 2nd and 3rd vaccinations.
The incidence of injection-site adverse reactions and systemic adverse reactions were significantly higher in heterologous vaccination compared to homologous vaccination ().
summarizes the distribution of self-reported adverse reactions in the homologous and heterologous groups during the first 8 days after immunization. Overall, 39.9% of subjects in the homologous group experienced a fever of ≥37.5°C, and of those 21.3% experienced a fever of ≥38.0°C. In the heterologous group, 66.7% experienced a fever of ≥37.5°C and of those 47.2% were ≥38.0°C (). The proportions of subjects reporting a fever of ≥37.5°C were significantly higher in the heterologous group than the homologous group and this was most commonly reported on Days 0−2 (p < 0.001−0.003, Supplementary Table S2). The incidence of fever did not differ notably between males and females, but a decreasing trend could be observed when stratified by age groups in both the homologous and heterologous groups (all p < 0.001) (Supplementary Table S3).
Figure 3. Distribution of self-reported adverse reactions during the first 8 days after vaccination (Day 0–7) by severity of the adverse reactions. The adverse reactions were categorized into (a) Injection-site reactions, or (b) Systemic reactions. Severity of adverse reaction were stratified into mild, moderate, or severe. Fever was stratified as ≥37.5°C and ≥38.0°C. Date of injection was defined as Day 0.
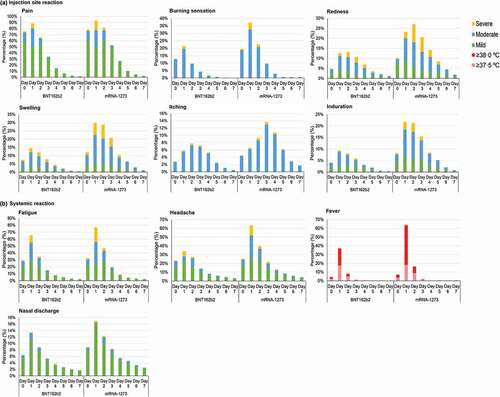
Most of the adverse reactions were mild to moderate () without sequalae and resolved without medical attention or after taking antipyretics and/or analgesics. Supplementary Table S4 presents the use of antipyretic and/or analgesics after third vaccination. The proportions of subjects reporting taking antipyretics and/or analgesics were significantly higher in the heterologous group (p < 0.001). Overall, the days of sick leave during the first 8 days after booster vaccination were similar to those after the second vaccination. Less than 10% of subjects were absent from work after the third vaccination, most sick leave was taken on day 1 after immunization (Supplementary Figure S1). Differences in days of sick leave were observed (p < 0.017), the heterologous group had higher proportion of subjects taking sick leave one day after booster immunization (8.0%), compared to homologous group (6.4%) (Supplementary Table S5). Apart from injection-site itching, all other common injection-site adverse reactions and systemic adverse reactions showed a similar pattern over the first 8 days after the third immunization, with the incidence of adverse reaction being highest on day 1 post-immunization in both the homologous and heterologous groups ().
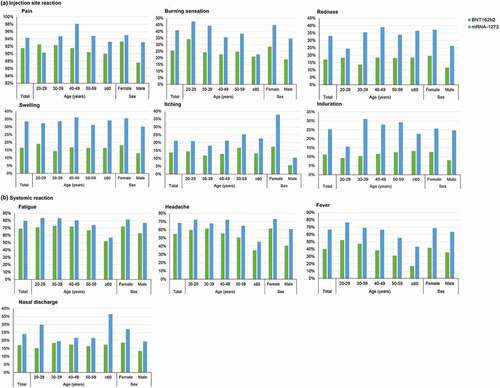
The incidence of injection-site adverse reaction and systemic adverse reaction was significantly higher in the heterologous than the homologous group (all p < 0.001) and generally followed a decreasing trend with age (Supplementary Table S3). As illustrated in , the incidence of systemic adverse reaction including fatigue, headache and fever ≥37.5°C showed a decreasing trend in those ≥60 years (, Supplementary Table S3).
Figure 4. Self-reported adverse reactions during the first 8 days after 3rd vaccination, by age groups and sex. The adverse reactions were categorized into (a) Injection-site reactions, or (b) Systemic reactions.
Analysis on the difference in anti-Spike IgG response by comorbidity were attempted, however, subjects with different comorbidities had very different anti-Spike IgG titer level at baseline in the mRNA-1273 cohort, thus no meaningful result was obtained.
Two cases of suspected myocarditis and one serious adverse event (prostate cancer) were reported in the homologous group, but these were not related to vaccination. Neither case of myocarditis was serious, and both resolved without clinical sequelae. No suspected adverse reactions requiring reporting to the regulatory agency, or serious adverse event, were noted in the heterologous subgroup.
4. Discussion
This study presents the immunogenicity and reactogenicity of two mRNA vaccines (BNT162b2 and mRNA-1273) as the third dose after primary BNT162b2 vaccination in a prospective extension study of the vaccination roll-out program in healthcare workers initiated in Japan in 2021. Both mRNA vaccines were immunogenic and successfully elicited the anti-Spike antibody response. In particular, the elicited response from the third immunization of either mRNA vaccine was greatest among older subjects. The incidence of adverse reaction after the third immunization was generally comparable to that after the second dose and comparable in all ages. Among our study cohort with two BNT162b2 vaccinations, the heterologous mRNA-1273 protocol induced a higher anti-Spike IgG response, as measured by the geometric mean at day 28, as well as a higher incidence of adverse reaction than the homologous BNT162b2 protocol, but the two protocols were comparable in immunogenicity and reactogenicity in those ≥60 years.
Differences in the immune response to SARS-CoV-2 infection by age and sex have been noted in literatures [Citation26]. A study by Hirotsu et al. found that the anti-Spike antibody titer increased by almost 40-fold at one week after the second dose of BNT162b2 [Citation8], and age and sex were not associated with the anti-Spike antibody titer level at early phase after the second dose. However, an age of >60 years has been associated with a reduced antibody response after two doses of the BNT162b2 vaccine [Citation9]. In a study evaluating the immunogenicity of two doses of BNT162b2 reported that while the anti-receptor binding domain antibody titer was 761.6 U/ml at least 13 weeks after the second vaccination in 464 hospital employees, the antibody titer was higher in employees who were younger and were female [Citation13]. Similarly, younger age and female sex were positively associated with antibody titer levels after two doses of BNT162b2 in Kageyama et al. [Citation11]. In our present study, we also found that the antibody titer at baseline was inversely correlated with increasing age at 9 months after primary doses of BNT162b2, and that higher anti-Spike antibody titer level was found in females. However, this did not appear to influence the immunogenicity of the third dose, which elicited comparable anti-Spike IgG titer levels across the age groups at 28 days post immunization regardless of sex in the current study. At present, we do not have clear mechanistic explanations for the observed differences between age groups and sex. Factors including racial differences, the period between booster doses, and the time of blood sampling may affect anti-Spike IgG response [Citation27]. It is also possible that additional unknown factors play a role in the regulation of IgG titer. The mechanism and factors influencing antibody response remain to be investigated.
In the few studies that reported the results of a third homologous dose of BNT162b, a homologous third dose of BNT162b2 given around 100 days after the second dose increased the anti-Spike IgG titer from 763 to 27,242 ELU/ml at 28 days. Moreover, the anti-Spike antibody response elicited was 8.11-fold greater than the control quadrivalent meningococcal conjugate vaccine [Citation23]. In an observational study conducted in Israel, three doses of BNT162b2 with a 5-month period between the second and third dose was estimated to be 93% effective for preventing related-hospital admissions, 92% effective for severe disease, and 88% effective for death [Citation17]. Another study found that the crude estimated effectiveness of anti-SARS-CoV-2 infection after the third homologous dose of the BNT162b2 vaccine was 92.9% and 89.1% after statistical adjustment for patient demographics [Citation15], and the effectiveness in preventing SARS-CoV-2 infection and related severe illnesses and hospitalization could be observed in individuals older than 60 years of age [Citation16]. In special populations regularly taking immunosuppressants or immune modulators, shorter homologous booster regimens (i.e. given 2–3 months after the second dose) of mRNA-based anti-SARS-CoV-2 vaccines were also successfully immunogenic and effective [Citation19,Citation20]. Despite the period between the second and third homologous dose of BNT162b2 being greater in our study, the anti-Spike IgG titers observed were comparable to that reported by Munro et al. [Citation23] The effectiveness of our third dose immunization protocol in preventing SARS-CoV-2 infection, related severe illnesses and hospitalization is not yet available due to the short follow-up period after the third immunization. Previous reports indicate that neutralizing antibody titers are highly predictive of protection and that omicron-neutralizing antibodies could be successfully induced by BNT162b2 immunization [Citation28,Citation29]. Based on the present observations of anti-Spike IgG titers, we expect that three doses of BNT162b2 could be expected to effectively protect individuals, including people ≥60 years, from COVID-19. The effectiveness analysis of our third-dose immunization protocol in preventing SARS-CoV-2 related severe disease will be carried out with longer follow-up data.
Heterologous booster vaccination has been shown to elicit a stronger neutralizing response against compared with homologous BNT123b2. A large-scale randomized trial conducted in the UK found that mRNA-1273 given as a third dose around 3 months after the base priming-boost regimen of the BNT162b2 vaccine induced an anti-Spike antibody response that was 11.49-fold higher than controls 28 days later, while a third homologous dose of BNT162b2 was only 8.11-fold greater [Citation23]. Another study evaluating heterologous and homologous combinations of third dose booster regimens conducted in the US found that the homologous regimen of BNT162b2/BNT162b2/BNT162b2 increased the anti-Spike antibody titer from 224 U/ml before the third dose to 3,345 U/ml 14 days later, and the heterologous regimen of BNT162b2/BNT162b2/mRNA-1273 increased anti-Spike antibodies from 534 to 5,256 U/ml [Citation24]. In these two studies, there were no differences between older and younger participants in the amount of induced antibodies [Citation23,Citation24]. A recent study also reported that heterologous mRNA-1273 booster vaccination induced a stronger neutralizing response against the Omicron variant compared with homologous BNT123b2 [Citation25], however the differences in mean spike antibody titer were significant only in older individuals. Our present results are in line with that these studies that the heterologous mRNA-1273 protocol elicited a stronger anti-Spike IgG response than the homologous BNT162b2 protocol.
The frequency of anaphylaxis after one or more doses of BNT162b2 was around 201.2 cases per million doses administered among healthcare workers enrolled in the mass vaccination program in Japan [Citation7]. Injection-site pain and fatigue were the most reported adverse reaction after the second homologous dose of either BNT162b2 or mRNA-1273, but the rate of adverse reactions was higher in individuals who received mRNA-1273 [Citation14]. In study participants who received either two doses of BNT162b2 or mRNA-1273, adverse reactions were observed more frequently after the second dose, in females, and in younger individuals [Citation12,Citation14]. The rates of self-reported systemic adverse reactions, such as fever, fatigue, headache, or pain, were significantly higher after the second dose than the first dose of these two mRNA-based vaccines, but this was not observed with injection-site reactions [Citation12,Citation14]. mRNA-1273 as the third immunization after the primary BNT162b2 regimen was found to induce more local reactions and systemic adverse reactions, particularly chills, headache, fatigue, and muscle ache, than BNT162b2 at either half or the full dose [Citation23]. Individuals older than 70 years were found to experience less reactogenicity than younger individuals in the combination regimen study [Citation23]. However, the US study did not find remarkable differences between these two regimens or between age groups in adverse reaction [Citation24]. Our results demonstrated that the third dose was similar in reactogenicity to the second dose of immunization and that mRNA-1273 induced more adverse reactions than BNT162b2 after the two-dose BNT162b2 series [Citation12]. Nevertheless, no serious adverse events or severe myocarditis were noted in either protocol in our study. In addition, those ≥60 years were less likely to experience systemic adverse reactions. This is potentially advantageous in this age group, who can benefit from either the homologous BNT162b2 or heterologous mRNA-1273 booster dose.
This study has several limitations. First, the study subjects received vaccinations in accordance with the vaccine roll-out program without randomization; therefore, it should be noted that the BNT162b2 and mRNA-1273 subgroups were not comparable in all baseline demographics. Our study cohort consisted of healthcare workers and therefore in terms of age distribution, the majority of participants were of working-age, this may potentially constrain the generalizability of our results, especially for the elder age groups. Finally, the correlation between the anti-Spike IgG response and effectiveness against COVID-19, and breakthrough antibody responses remain to be confirmed with long-term follow-up. This study focused on mRNA vaccines, future studies may include examining the efficacy and adverse reaction in subjects who received recombinant viral vectors, and on the most effective regimen combination with less vaccine injections.
5. Conclusion
In conclusion, this prospective study is the first report from Western Pacific region demonstrating that both BNT162b2 and mRNA-1273 vaccines are immunogenic third-dose boosters after two-dose BNT162b2 immunization. The adverse reactions induced by either vaccine were mostly mild to moderate, which were ameliorated and manageable within one week after immunization. Overall, homologous booster using BNT162b2 had less adverse reactions, heterologous booster using mRNA-1273 elicited greater immunogenicity. Compared to younger age groups, the immunogenicity before the booster was lowest among individuals ≥60 years, both homologous and heterologous boosters restored vaccine immunogenicity level comparable to that of younger age groups. The finding should be emphasized to both policymakers and the general public in Japan.
Declaration of interests
All authors had received grants from Welfare and Labor Administration Promotion Survey Project (Emerging/re-emerging infectious diseases and vaccination policy promotion research project) [Grant number: 20HA2013]. Toshio Naito had received a consulting fee from Nobelpharma Co., Ltd, All of which has no role in the study design, data collection and analysis, or decision to publish this study.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Authors contributions
Conception and design: TN, NT, SK, YK, MT, SH, and SI; Data acquisition and collection: MT and SI; Provision of study materials and methodology: NT, SK, YK; Analysis and interpretation of data: TN and SI; Drafting of the manuscript: TN; Validation/Visualization: TN; Critical revision of the manuscript: TN, NT, SK, YK, MT, SH, and SI; Funding acquisition/Software/Supervision: SI. All authors reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage. All authors agreed to take responsibility and be accountable for the contents of the article and to share responsibility to resolve any questions raised about the accuracy or integrity of the published work.
Ethical approval
The study was approved by The Tokushukai Group Ethics Committee (TGE01856701) prior to initiation and was conducted in accordance with the principles of the Declaration of Helsinki.
Supplemental Material
Download MS Word (353 KB)Acknowledgments
The authors would like to thank 4DIN Ltd, Japan for data analysis and assisting with preparing the manuscript and other materials for journal submission.
Data availability statement
The datasets generated during and/or analysed during the current study are not publicly available due to patient confidentiality, but the derived data supporting the findings of this current study are available from the corresponding author Prof. Ito and Prof. Tobita on reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2022.2093722
Additional information
Funding
References
- World Health Organization. WHO coronavirus (COVID-19) dashboard. [cited 2022 Mar 24]. Available from: https://covid19.who.int/region/wpro/country/jp
- Yasuhiro F. Pharmaceuticals and medical devices agency. special approval for emergency on first COVID-19 vaccine in Japan 2021. [ cited 2022 March 15]. Available from: https://www.pmda.go.jp/english/about-pmda/0003.pdf
- Naito T, Yan Y, and Tabe Y, et al. Real-world evidence for the effectiveness and breakthrough of BNT162b2 mRNA COVID-19 vaccine at a medical center in Japan. Hum Vaccin Immunother. 2022;18(1):1–2.
- Mori H, Naito T. A rapid increase in the COVID-19 vaccination rate during the Olympic and Paralympic games 2021 in Japan. Hum Vaccin Immunother. 2022;18(1):2010440.
- Okubo R, Yoshioka T, Ohfuji S, et al. COVID-19 vaccine hesitancy and its associated factors in Japan. Vaccines. 2021;9(6):662.
- Machida M, Nakamura I, Kojima T, et al. Acceptance of a COVID-19 vaccine in Japan during the COVID-19 pandemic. Vaccines. 2021;9(3):210.
- Hashimoto T, Ozaki A, Bhandari D, et al. High anaphylaxis rates following vaccination with the Pfizer BNT162b2 mRNA vaccine against COVID-19 in Japanese healthcare workers: a secondary analysis of initial post-approval safety data. J Travel Med. 2021;28(7):taab090.
- Hirotsu Y, Amemiya K, Sugiura H, et al. Robust antibody responses to the BNT162b2 mRNA vaccine occur within a week after the first dose in previously infected individuals and after the second dose in uninfected individuals. Front Immunol. 2021;12:722766.
- Mitsunaga T, Ohtaki Y, Seki Y, et al. The evaluation of factors affecting antibody response after administration of the BNT162b2 vaccine: a prospective study in Japan. PeerJ. 2021;9:e12316.
- Yoshimura Y, Sasaki H, Miyata N, et al. Antibody response after COVID-19 vaccine BNT162b2 on health care workers in Japan. J Infect Chemother. 2021;27(12):1713–1715.
- Kageyama T, Ikeda K, Tanaka S, et al. Antibody responses to BNT162b2 mRNA COVID-19 vaccine and their predictors among healthcare workers in a tertiary referral hospital in Japan. Clin Microbiol Infect. 2021;27(12):1861.e1–1861.e5.
- Saita M, Yan Y, Ito K, et al. Reactogenicity following two doses of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers in Japan. J Infect Chemother. 2022;28(1):116–119.
- Otsuka S, Hiraoka K, Suzuoki M, et al. Antibody responses induced by the BNT162b2 mRNA COVID-19 vaccine in healthcare workers in a single community hospital in Japan. J Infect Chemother. 2022;28(4):539–542.
- Kitagawa H, Kaiki Y, Sugiyama A, et al. Adverse reactions to the BNT162b2 and mRNA-1273 mRNA COVID-19 vaccines in Japan. J Infect Chemother. 2022;28(4):576–581.
- Saciuk Y, Kertes J, Shamir Stein N, et al. Effectiveness of a third dose of BNT162b2 mRNA vaccine. J Infect Dis. 2022;225(1):30–33.
- Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against COVID-19 in Israel. N Engl J Med. 2021;385(15):1393–1400.
- Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398(10316):2093–2100.
- Moreira ED, Kitchin N, and Xu X, et al. Safety and efficacy of a third dose of BNT162b2 Covid-19 vaccine. N Engl J Med. 2022;386(20):1910–1921.
- Hall VG, Ferreira VH, and Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385(13):1244–1246.
- Speer C, Tollner M, Benning L, et al. Third COVID-19 vaccine dose with BNT162b2 in patients with ANCA-associated vasculitis. Ann Rheum Dis. 2022;81(4):593–595.
- Estrada JA, Cheng CY, and Ku SY, et al. Immunobridging Study to Evaluate the Neutralizing Antibody Titer in Adults Immunized with Two Doses of Either ChAdOx1-nCov-19 (AstraZeneca) or MVC-COV1901. Vaccines. 2022;10(5):655.
- Intapiboon P, Seepathomnarong P, Ongarj J, et al. Immunogenicity and safety of an intradermal BNT162b2 mRNA vaccine booster after two doses of inactivated SARS-CoV-2 vaccine in healthy population. Vaccines. 2021;9(12):1375.
- Munro APS, Janani L, and Cornelius V, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398(10318):2258–2276.
- Atmar RL, Lyke KE, and Deming ME, et al. Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386(11):1046–1057.
- Poh XY, Tan CW, Lee IR, et al. Antibody response of heterologous vs homologous mRNA vaccine boosters against the SARS-CoV-2 Omicron variant: interim results from the PRIBIVAC study, A randomized clinical trial. Clin Infect Dis. 2022. ciac345. DOI:10.1093/cid/ciac345
- Ciarambino T, Para O, Giordano M. Immune system and COVID-19 by sex differences and age. Womens Health. 2021;17:17455065211022262.
- Shaw RH, Liu X, Stuart ASV, et al. Effect of priming interval on reactogenicity, peak immunological response, and waning after homologous and heterologous COVID-19 vaccine schedules: exploratory analyses of Com-COV, a randomised control trial. Lancet Respir Med. 2022;S2213-2600(22):00163.
- Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211.
- Kotaki R, Adachi Y, Moriyama S, et al. SARS-CoV-2 Omicron-neutralizing memory B-cells are elicited by two doses of BNT162b2 mRNA vaccine. Sci Immunol. 2022;7:eabn8590.
