During this devastating period of COVID-19, a new threat has been detected in many parts of the world known as monkeypox. A multi-country outbreak of monkeypox has now surpassed over 30,000 suspected cases, 16,016 laboratory-confirmed cases and 5 reported deaths in accordance with the WHO published report on 25 July 2022 [Citation1,Citation2]. In addition to the United States (US), incidents are on the rise in Europe, Brazil, Canada, Australia, UK and 70 other nations () [Citation3]. Monkeypox epidemics occur mostly in western and central Africa and very rarely spread abroad [Citation4]. Recently, the US declared monkeypox a public health emergency with more than 8934 cases as of 11 August 2022 [Citation5]. This is the most unusual spread of contagious monkeypox virus in the US, with 99% of cases in males. It is a devastating situation around globe due to emergence of viral variants and new viral outbreaks [Citation6–10].
Figure 1. Cases of monkeypox in nonendemic countries as reported by the World Health Organization (WHO) and by Ruters; accessed 11 August 2022 [Citation3,Citation5,Citation11]. Created with biorender.com.
![Figure 1. Cases of monkeypox in nonendemic countries as reported by the World Health Organization (WHO) and by Ruters; accessed 11 August 2022 [Citation3,Citation5,Citation11]. Created with biorender.com.](/cms/asset/37d04b9f-55ef-46e8-92c4-039f63a8d48b/ierv_a_2113515_f0001_oc.jpg)
The name monkeypox derives from the initial discovery of the virus in monkeys in a Danish laboratory in 1958. The first human case was reported in a child in the Democratic Republic of the Congo in 1970 [Citation12]. The monkeypox virus is an orthopoxvirus, which is a virus genus that contains the smallpox-causing variola virus. Monkeypox is a zoonosis, a disease transferred to individuals from sick animals [Citation13]. Poxviruses are a type of DNA virus with numerous genes that replicate in the host cell cytoplasm and not the nucleus and are super stable in the environment. Although large DNA viruses have a low mutation rate, this is not truly a hindrance for poxviruses when they encounter a new environment, as they already harbor genetic diversity and can recombine and adapt by amplifying gene numbers. The most well-known poxvirus outbreak was the devastatingly lethal, unbearable, and history-changing smallpox disease in humans. After killing over 500 million people since 1880, the variola virus came under control using an original ‘Jennerian’ vaccine based on inoculation with a related virus (vaccinia) found in animals, which provided cross protection against the variola virus, but was less likely to cause significant disease [Citation14]. In 1980, the variola virus was eradicated, leaving humans with only a single endemic poxvirus, the relatively benign molluscum contagiosum virus, which is distantly related to the variola-like ‘orthopox’ viruses. Given their significant diversity and generalist predisposition, poxviruses frequently jump from one host species to another (particularly from rodents). One of the best-known examples is monkeypox or cowpox, both of which are rodent-borne [Citation15]. Vaccinia viruses have caused bovine vaccinia (BV) in Brazil and India, following the end of vaccination [Citation16]. The lack of investment in research, the unreadiness of health professionals and agencies to cope with the illness, and the propensity of dairy products as alternate routes of infection pose the issue of how BV should be addressed going forward. The highest limit R0 of 1.0 for the Central African clade shows that the viruses can continue human-to-human transmission and remain in human populations. Further studies are required to determine whether the unusual spread of the virus has a genetic foundation [Citation17]. The genome of the monkeypox virus is much larger than the genomes of the SARS-CoV-2 and hepatitis B viruses, which are six and sixty times larger, respectively [Citation17]. As databases include limited nucleotide sequences, numerous ambiguities persist. People with monkeypox may develop symptoms including fever, headache, muscle aches, exhaustion, or swollen lymph nodes.
According to the World Health Organization (WHO), instances occur near tropical rainforests populated by virus-carrying animals. A monkeypox viral infection has been discovered in squirrels, poached rats from the Gambia, dormice, and some monkey species. Nevertheless, human-to-human propagation of the monkeypox virus is restricted. This may occur via blood and other body fluids, sores on the skin, the respiratory system, or the eyes, nose, or mouth, as well as through virus-contaminated things such as bedding and clothes [Citation18]. Monkeypox is not like COVID-19. R0 is approximately 1, and the transmission mechanisms are entirely different [Citation19]. According to the latest UK Health Security Agency technical briefing, the pandemic continues to expand, and case data clearly show that the virus is transmitted predominantly via intimate or sexual contact. Propagation of monkeypox persists predominantly in the linked sexual networks of homosexual, bisexual, and males who have sex with other men [Citation20]; the virus is predominantly being identified in these communities, but anyone can contract and pass on the virus. The finding of monkeypox DNA in urine and feces samples has highlighted the need for wastewater management and monitoring. The UK and many other nonendemic nations have experienced unparalleled community transmission of monkeypox virus among homosexual, bisexual, and other males who have sex with men. Rectal discomfort and penile edema were discovered to be new clinical manifestations of monkeypox infection [Citation21]. During the present monkeypox epidemic, patients with vesicular and pustular ocular lesions should be considered for monkeypox, especially if there are epidemiological ties or risk factors [Citation22]. As per a multicenter, prospective, observational cohort study [Citation23], monkeypox causes genital, perianal, and oral lesions and complications including proctitis and tonsillitis. Because of the variability of presentations, clinicians should have a low threshold for suspicion of monkeypox. Lesion swabs showed the highest viral loads, which, combined with the history of sexual exposure and the distribution of lesions, suggests close contact is probably the dominant transmission route in the current outbreak.”
Although it was initially detected in 1970 in the Democratic Republic of the Congo, the epidemiology has changed. Deadly monkeypox has already been detected in 10 African countries and 9 other nations [Citation3,Citation24]. The number of cases has grown by at least a factor of ten, and the median age of onset has shifted from young children in the 1970s to young adults in recent decades. This may be a result of the discontinuation of smallpox vaccines, which offered some coverage against monkeypox [Citation25]. The case fatality rate for the West African clade was 3.6%, whereas it was 10.6% for the Central African clade. Consequently, monkeypox is progressively becoming a worldwide concern. Surveillance and detection strategies are crucial for comprehending the dynamic epidemiology of this disease’s resurgence [Citation20]. According to Ishidro et al. [Citation26], ‘An in-depth mutational analysis suggests the action of host APOBEC3 in viral evolution as well as signs of potential monkeypox virus human adaptation in ongoing microevolution.’
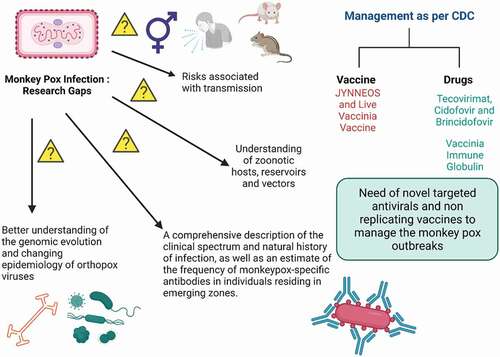
It is feared that the monkeypox virus might evolve into a more potent human infection. Perhaps this is due to the genetic composition of the virus, anthropogenic factors, variations in host behavior, and the fact that smallpox vaccination is no longer administered due to the elimination of the variola virus [Citation27]. In this regard, anyone born post 1980 are not vaccinated and are at risk of disease. Moreover, real-time polymerase chain reaction (PCR) experiments have been utilized to identify two genes targeted by the monkeypox virus [Citation28]. A test for immunoglobulin M (IgM) antibodies was conducted since anti-vaccine IgM antibodies from earlier smallpox immunizations should not be detected [Citation29]. Diagnostic tests may also concentrate on cellular immune responses to certain infections. Counting orthopoxvirus-specific T cells is one such approach that is now under investigation [Citation30]. While primates, including monkeys, are susceptible to infection, it is believed that squirrels and other rodents serve as reservoir hosts for the virus. Further spread of this orthopox virus must be carefully contained and not allow novel (variant) viruses to adapt further to the human population (). Tecorivimat is a recently approved antiviral medicine for orthopoxvirus-associated illnesses, including monkeypox, based on animal models and human evidence for safety, bioavailability, and therapeutic efficacy [Citation31,Citation32]. Tecovirimat did not cause side effects and appeared to lead to a decrease in viral levels and a speedier recovery. Based on comparable results, the FDA authorized a second smallpox medication, brincidofovir, in 2021.
Expert Opinion: The Health Security Agency of the United Kingdom has issued revised recommendations for administering the JYNNEOS vaccination to males deemed to be at a greater risk of exposure. Although the virus is not officially classified as a sexually transmitted illness, it may be transferred via close and personal contact during sex. Smallpox vaccination is also effective against monkeypox. It will be administered mostly after exposure to the pathogen to avoid severe sickness and symptoms [Citation33]. Recent interim guidance, created with the assistance and advice of the Strategic Advisory Group of Experts Ad hoc Working Group on smallpox and monkeypox vaccinations, offers the first WHO guidelines on vaccines and immunization for monkeypox [Citation34]. Global mass vaccination efforts are needed, especially in endemic areas, through surveillance and contact tracing. Postexposure prophylaxis with an adequate second- or third generation ‘safer’ vaccination is advised for contacts of patients, preferably within four days of first exposure, to avoid illness development. Frontline healthcare workers need to be vaccinated first as a part of a preventive strategy to avoid further transmission. According to the USFDA, individuals with occupational exposure to orthopoxviruses (e.g. researchers who work with monkeypox virus samples) receive the ACAM2000 or JYNNEOS vaccine as preexposure prophylaxis. There is currently no vaccination against monkeypox that is safe to use during pregnancy [Citation35]. Because MVA-BN is a nonreplicating vaccine, there is no need to be concerned about its use during pregnancy. Monkeypox is distinguished by continued upper respiratory tract viral DNA shedding notwithstanding the healing of skin lesions, offering unique problems for preventing transmission and gaining control of the disease’s progress. Needless to add, it is critical to investigate the safety and efficacy of suitable antivirals for use in humans afflicted with the monkeypox virus, particularly immunocompromised individuals.
Control of future pox threats would involve limiting global zoonosis, population-level surveillance, and shutting down transmission chains. Safeguarding the world population against this new virus threat by adequate vaccines and antivirals should be paramount. To manage current and future outbreaks, a globally focused one-health strategy for disease prevention and treatment is needed.
Declaration of interest
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants, or patents received or mending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
V.P. Chavda would like to dedicate this work to L M College of Pharmacy as a part of the 75th year celebration of the college. V. Apostolopoulos would like to thank the Immunology and Translational Research Group and the Mechanisms and Interventions in Health and Disease Program within the Institute for Health and Sport, Victoria University Australia for their support. V. Apostolopoulos was supported by Victoria University, VIC Australia.
Additional information
Funding
References
- WHO. Multi-country monkeypox outbreak: situation update. 2022 [cited 2022 Jul 10]. Available from: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON396
- WHO. Multi-country outbreak of monkeypox, external situation report #2 - 25 July 2022. [cited 2022 Aug 11]. Available from: https://www.who.int/publications/m/item/multi-country-outbreak-of-monkeypox–external-situation-report–2—25-july-2022
- World Health Organization (WHO). Multi-country monkeypox outbreak in non-endemic countries. Dis Outbreak News. 2022 [cited 2022 Aug 10]. Available from: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON385
- Chavda VP, Apostolopoulos V. Rare monkeypox: is it really a threat to the elderly? Maturitas. 2022;163:90–91. ** This work discuss the outbreak of monkeypox
- CDC. U.S. Map & Case Count. 2022 [cited 2022 Aug 11]. Available from: https://www.cdc.gov/poxvirus/monkeypox/response/2022/us-map.html
- Chavda VP, Apostolopoulos V. Global impact of delta plus variant and vaccination. Expert Rev Vaccines. 2022;null–null. DOI:10.1080/14760584.2022.2044800
- Chavda VP. Omicron variant (B.1.1.529) of SARS-CoV-2: threat for the elderly? Maturitas. 2020;158:78–81.
- Chavda VP, Patel AB, Vaghasiya DD. SARS-CoV-2 variants and vulnerability at the global level. J Med Virol. 2022;94(7):2986–3005. * Summarizes the impact of diffrent variants of SARS-CoV-2.
- Chavda VP, Hanuma Kumar Ghali EN, Yallapu MM, et al. Therapeutics to tackle Omicron outbreak. Immunotherapy. 2022;14(11):833–838.
- Chavda VP, Apostolopoulos V. Tomato flu outbreak in India. Lancet Respir Med. n.d.;2022:100021. *Discusses the tomato flu outbreak in india
- Factbox: Monkeypox cases around the world, Healthc. Pharm. Compil. by Andrey Sychev Louise Rasm. Gdansk Ed. by Milla Nissi Mark Potter. 2022 [cited 2022 Jul 10]. Available from: https://www.reuters.com/business/healthcare-pharmaceuticals/monkeypox-cases-around-world-2022-05-23/
- Brown K, Leggat PA. Human Monkeypox: current state of knowledge and implications for the future, trop. Med Infect Dis. 2016;1:8.
- Beer EM, Rao VB, Holbrook MR. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS NeglTrop Dis. 2019;13(10):e0007791.
- Baxby D. Edward Jenner’s role in the introduction of smallpox vaccine BT - history of vaccine development. In: Plotkin SA, editor. History of vaccine development. New York NY: Springer; 2011. p. 13–19. DOI:10.1007/978-1-4419-1339-5_3.
- Moore M, Monkeypox ZF. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; n.d. 2022 Jan [Updated 2022 May 22]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK574519/
- José da Silva Domingos I, Silva de Oliveira J, Lorene Soares Rocha K, et al. Twenty years after bovine vaccinia in Brazil: where we are and where are we going? Pathogens. 2021;10(4):406. (Basel, Switzerland).
- Quarleri J, Delpino MV, Galvan V. Monkeypox: considerations for the understanding and containment of the current outbreak in non-endemic countries. GeroScience. 2022. DOI:10.1007/s11357-022-00611-6
- Ligon BL. Monkeypox: a review of the history and emergence in the Western hemisphere, Semin. Pediatr Infect Dis. 2004;15:280–287.
- Lancet T. Monkeypox: a global wake-up call. Lancet. 2022;400(10349):337. * Discuss the current monkeypox scenario
- Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox—A potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16(2):e0010141.
- Patel A, Bilinska J, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410.
- Benatti SV, Venturelli S, Comi N, et al. Ophthalmic manifestation of monkeypox infection. Lancet Infect Dis. 2022. DOI:10.1016/S1473-3099(22)00504-7
- Tarín-Vicente EJ, Alemany A, Agud-Dios M, et al. Mitjà, clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet. 2022. DOI:10.1016/S0140-6736(22)01436-2
- Wilson ME. Re-emerging diseases: overview. In: Quah SE, editor. S.R.B.T.-I.E. of P.H. Academic Press: Oxford. 2017. p. 269–277. DOI:10.1016/B978-0-12-803678-5.00374-X.
- Grosenbach DW, Hruby DE. Preliminary screening and in vitro confirmation of orthopoxvirus antivirals. Methods Mol Biol. 2019;2023:143–155.
- Isidro J, Borges V, Pinto M, et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat Med. 2022. DOI:10.1038/s41591-022-01907-y
- Weaver JR, Isaacs SN. Monkeypox virus and insights into its immunomodulatory proteins. Immunol Rev. 2008;225:96–113.
- Li Y, Olson VA, Laue T, et al. Detection of monkeypox virus with real-time PCR assays. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2006;36:194–203.
- Karem KL, Reynolds M, Braden Z, et al. Damon, characterization of acute-phase humoral immunity to monkeypox: use of immunoglobulin M enzyme-linked immunosorbent assay for detection of monkeypox infection during the 2003 North American outbreak. Clin Diagn Lab Immunol. 2005;12:867–872.
- Hammarlund E, Lewis MW, Carter SV, et al. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat Med. 2005;11(9):1005–1011.
- Russo AT, Grosenbach DW, Brasel TL, et al. Effects of treatment delay on efficacy of tecovirimat following lethal aerosol monkeypox virus challenge in cynomolgus macaques. J Infect Dis. 2018;218(9):1490–1499.
- Adler H, Gould S, Hine P, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022. DOI:10.1016/S1473-3099(22)00228-6
- Townsend MB, Keckler MS, Patel N, et al. Humoral immunity to smallpox vaccines and monkeypox virus challenge. Proteomic Assess Clin Correl. 2013;87:900–911.
- WHO. Vaccines and immunization for monkeypox: interim guidance. WHO/MPX/Immunization/2022.1. 2022 [cited 2022 Jul 10]. Available from: https://www.who.int/publications/i/item/who-mpx-immunization-2022.1
- Khalil A, Samara A, O’Brien P, et al. Monkeypox vaccines in pregnancy: lessons must be learned from COVID-19. Lancet Glob Heal. 2022;10(9):e1230–e1231. * Discuss the impact of monkeypox during pregnancy