ABSTRACT
Introduction
Human papillomavirus (HPV) infection, which poses significant disease burden, is decreasing following implementation of vaccination programs. Synthesized evidence on HPV vaccine real-world benefit was published in 2016. However, long-term impact of vaccination, and how vaccination programs influence infection rates and disease outcomes, requires further examination.
Areas covered
We systematically reviewed observational studies on HPV vaccination within MEDLINE, EMBASE, and Google Scholar from 2016 to 2020, involving 14 years of follow-up data. We identified 138 peer-reviewed publications reporting HPV vaccine impact or effectiveness. Outcomes of interest included rates of infection at different anatomical sites and incidence of several HPV-related disease endpoints.
Expert opinion
The expansion of HPV vaccination programs worldwide has led to a reduction in genital infection and significant decreases in incidence of HPV-related disease outcomes. Therefore, the WHO has set goals for the elimination of cervical cancer as a public health concern. To track progress toward this requires an understanding of the effectiveness of different vaccination initiatives. However, the impact on males, and potential benefit of gender-neutral vaccination programs have not been fully explored. To present an accurate commentary on the current outlook of vaccination and to help shape policy therefore requires a systematic review of available data.
1. Introduction
The causative role of human papillomavirus (HPV) in a range of diseases, including cervical cancer, anogenital warts (AGWs), a proportion of head and neck cancers (including oropharynx, oral cavity, and larynx), and anogenital precancerous and cancerous lesions, and recurrent respiratory papillomatosis (RRP), presents a significant global health concern [Citation1–3]. Global targets have therefore been set to reduce the prevalence of HPV, and the World Health Organization (WHO) has launched a significant initiative for the elimination of cervical cancer as a global public health problem [Citation4].
To achieve the WHO goal, robust and resilient HPV vaccination programs, as well as cervical screening and precancerous and cancer treatment programs, are needed. Vaccination programs have been progressively introduced since 2006, and four vaccines which protect against different HPV genotypes have been developed and licensed to date [Citation5,Citation6]. These include: two bivalent vaccines which target high-risk types HPV-16 and HPV-18; a quadrivalent vaccine which additionally targets the low-risk genotypes HPV-6 and HPV-11, and a nonavalent vaccine which further targets high-risk types HPV-31/33/45/52/58 [Citation5,Citation7]. The high-risk genotypes 16 and 18, which are targeted by all vaccines, cause 70% of cervical cancers and 64–85% of HPV-related neoplasms found at other sites; the seven high-risk types targeted by the nonavalent vaccine cause 90% of cervical cancers and 84–95% of HPV-related neoplasms found at other sites [Citation8]. The quadrivalent and nonavalent vaccines additionally protect against the low-risk genotypes 6 and 11, which account for 85–90% of AGWs and RRP cases [Citation9,Citation10].
As per the WHO’s definition, as of June 2020 national or partial vaccination programs have been introduced in 107 countries since initial licensing in 2006, with this number expected to further increase by the end of 2021 [Citation11]. Significant variation in the design of national programs exists, as the vaccination recommendations note use of different dosing schedules to target populations of different age groups and gender [Citation12]. Gender-neutral vaccine (GNV) schedules have become more common in recent years, with 33 WHO Member States having started vaccinating boys by the end of 2019, and six countries implementing GNV in 2020/21 (Merck & Co., Inc., Rahway, NJ, USA, data on file) [Citation13]. The US was the first country to recommend GNV in 2011, followed by Australia in 2013 [Citation12]. GNV has been proposed to provide gender equity, greater individual and herd protection against infection and disease, and increased program resilience should vaccination coverage change [Citation14]. Randomized controlled trials have shown that GNV increased protection against HPV, with high coverage in males conveying significant herd effects to unvaccinated females [Citation15]. Examination of these herd effects has therefore become important, along with the investigation of real-world impact and effectiveness of GNV on HPV-related outcomes in men, as well as women. Further new developments include the emergence of off-label one-dose vaccination schedules, which have now been recommended by the WHO, and on which substantial research is ongoing [Citation16,Citation17].
To enable tracking and evaluation of the performance of vaccination programs, and gauge progress toward meeting the WHO elimination goals, it is important to assess the impact and effectiveness of HPV vaccines in real-world settings [Citation18–20]. Several studies have revealed the impact and/or effectiveness of HPV vaccination in different settings with heterogeneous study design, although inconsistencies in the opinions reported by reviews of long-term outcomes related to the HPV vaccine vary among the scientific community [Citation12,Citation19,Citation21–24]. Due to the decades-long lag-time between HPV infection and the development of cancer, investigating invasive cancer incidence as an outcome has been a major challenge in both randomized clinical trial settings and the real-world setting [Citation25]. However, in countries where HPV vaccination programs have been established for longer periods and comprehensive surveillance exists, data on these endpoints have recently started to become available. Indeed, in 2020, a nationwide Swedish demographic and health register study demonstrated a significant reduction in cervical cancer incidence, following introduction of HPV vaccination to women in the nation in 2006 and more recently similar data from Denmark confirmed this reduction [Citation26,Citation27].
In this study, we will provide an update to the Garland et al. 2016 review of real-world data, which previously captured both the effectiveness and impact data of the 4 vHPV vaccine since 2007–2016 [Citation28]. Garland et al. 2016 reported that incidence of vaccine-type (VT) HPV-6/11/16/18 infection and AGWs had decreased by up to 90% after the introduction of the quadrivalent vaccine [Citation28]. Total low-grade cervical cytological abnormalities were also reduced by up to 45%, and high-grade histologically confirmed cervical lesions by 64% (cervical intraepithelial neoplasia [CIN] 2+) and 72% (CIN3+) [Citation28]. However, data on malignancies, including true invasive cancer, have only recently become available. As time since vaccine implementation increases and GNV initiatives are introduced in more countries, new studies with longer follow-up periods have since been published and will be summarized in this review. We have also expanded the scope of review to a wider range of non-cervical outcomes. Evidence on new endpoints, such as RRP, and oral and anal infection and disease, will be synthesized. Additionally, a comprehensive analysis has been conducted to examine the role of GNV program in reducing the burden of HPV-related disease, and to help inform stakeholders and vaccination strategies worldwide.
2. Methods
2.1. Search strategy and selection criteria
A systematic literature review (SLR) update was conducted to evaluate the real-world impact and effectiveness of the HPV vaccines. MEDLINE, EMBASE, and Google Scholar were last searched on April 1st, 2020 for peer-reviewed observational studies on the HPV vaccines, according to predefined search terms (Supplementary Table 1). This update aimed to capture vaccine impact and effectiveness data on previously reported HPV-related endpoints of genital HPV infections, AGWs, and cervical lesions from March 1st, 2016–March 31st, 2020. The search strategy was further expanded to include new endpoints from January 2007–March 31st, 2020, including RRP, oral and anal HPV infections, and oropharyngeal and anal lesions. These searches were performed separately. Searching for outcomes of interest was conducted within select full-text articles, with additional abstracts retrieved where necessary. Observational studies on the impact and effectiveness of the 4 vHPV and/or the 9 vHPV vaccine on HPV infection were eligible for inclusion (however, of note, available published data on the 9 vHPV vaccine were very sparse at the time of this review). We excluded studies which only reported on the 2 v vaccine Cervarix® to follow the same methods as the previous SLR [Citation28]. Of note, reviews of population impact studies including the 2 v vaccine Cervarix® have been summarized elsewhere [Citation29].
The selection criteria for this SLR were based on the PICOTS framework (Supplementary Table 2). All titles and abstracts were assessed independently by two reviewers according to this predefined inclusion/exclusion criteria, with disputes resolved through discussion. Full-text selection was then performed by one reviewer, with 10% of non-selected papers checked by a second reviewer as a quality control measure.
The manuscript uses certain terms which have been defined and explained here: 1) vaccine impact, defined as the population-prevented fraction of infection or disease, assessed by comparing prevalence or incidence in the vaccine era to a comparable population from the pre-vaccine era or by measuring population-level trends over time, and; 2) vaccine effectiveness (VE) defined as the proportion of infection or disease prevented among vaccinated individuals, estimated by comparing the incidence in vaccinated vs. unvaccinated individuals within similar populations [Citation30,Citation31]. Throughout this manuscript, we refer to herd protection effects. This occurs when sufficient vaccination coverage of the population results in a reduction of circulating transmissible infection and disease in the population in general, extending the protective effects to those who have not been vaccinated. It is noteworthy, however, that these individuals are not protected when moving out of the herd and into another unvaccinated group [Citation32]. The elimination of HPV and HPV-related diseases are discussed in this manuscript. Here, the WHO’s elimination of cervical cancer as a public health problem refers to a target incidence of below the proposed threshold of four per 100,000 women-years [Citation4]. Where noted, eradication of HPV/HPV-related disease refers to permanent reduction to zero.
2.2. Synthesis of evidence
Data extracted from each study included: country, data source, impact vs. VE, study population, gender, population size, age group, dosing schedule, study period, primary outcome, incident/prevalent, outcome data, outcome data source, exposure data source, main statistical analyses, effect measure, covariates/confounders considered, and limitations listed. For the outcome ‘HPV infection,’ we distinguished between vaccine-type HPV infections (VT) rates and non-vaccine type infections (non-VT) rates, respectively. Reductions/declines are reported here as they were in the papers identified. If not reported, the reduction/decline was calculated based on the measure reported (the original authors were not contacted for clarification); for example:
reduction = (prevalence pre-vaccination − prevalence post-vaccination) ÷ prevalence pre-vaccination × 100.
3. Results
3.1. Search results
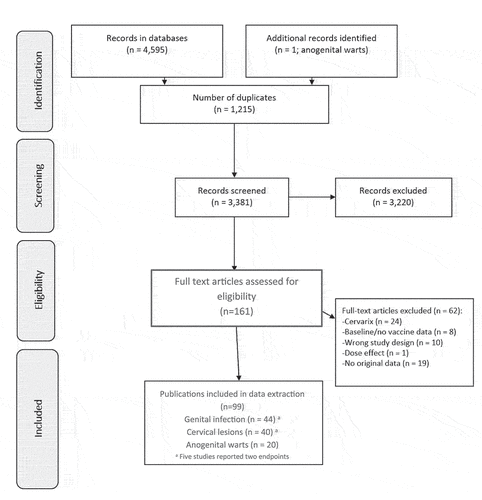
In the search of the HPV endpoints of genital HPV infection, cervical lesions, and genital warts, a total of 3,381 publications were screened as part of the five-year update, leading to 161 full texts being reviewed. Of these, 99 met the prespecified PICOTS inclusion criteria and reported on the impact and effectiveness of the HPV vaccines (). Some publications reported on multiple studies, i.e. data on different endpoints or from different countries, with impact and effectiveness analyses counted separately, resulting in a total of 122 studies being extracted from these publications. Full citations, along with a detailed summary of data extraction from each publication, are given in Supplementary Tables 3A, 3B, and 3C.
Of the 122 studies extracted, 62 detailed the impact of HPV vaccines, and 60 discussed vaccine effectiveness. Of these, 57 reported changes in genital HPV infection (44 publications), while 44 reported changes on the cervical lesion endpoint (40 publications), and 21 on the AGWs endpoint (20 publications). Studies from 22 countries were included, a majority of which used 4 vHPV (). Some countries utilized 2 vHPV in addition to 4 vHPV in their national immunization program, and the ‘HPV vaccines’ referred to throughout are therefore inclusive of this. Despite being included in the search terms, no studies reported on the impact or effectiveness of the more recently licensed 9 vHPV vaccine. This five-year update additionally reported on Asia and Africa, and included 12 new countries: Bhutan, India, Israel, Italy, Japan, Luxembourg, Mongolia, Norway, Rwanda, Spain, South Korea, Switzerland, and the UK (Supplementary Figure 1).
Table 1. SLR endpoints: Summary of studies reporting the 4vHPV impact (I) and vaccine effectiveness (VE) in 23 countries
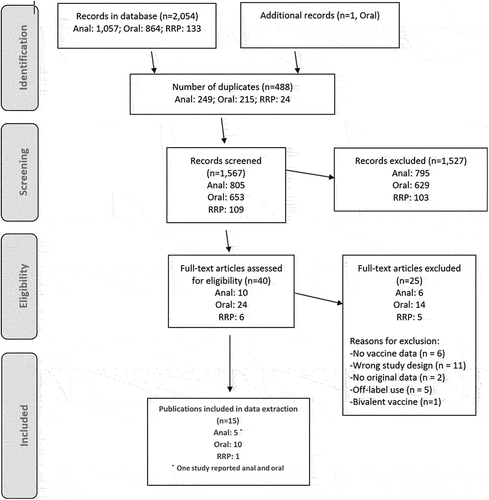
In the search for additional HPV endpoints, 1,567 publications were screened with 40 articles selected for full-text 230 review (; full citations listed in Supplementary Table 4). Sixteen studies were included from 15 publications reporting on the impact or effectiveness of 4vHPV on oral infection, anal endpoints, or RRP (). Of these, five studies reported on anal endpoints; four on anal infection, and one on high-grade anal intraepithelial neoplasia (HGAIN). ten studies discussed oral infection, and one study related to RRP, which was of juvenile-onset type (JoRRP). These studies reported data from five countries, including Australia, Norway, Sweden, Colombia, and the US. Studies on the original cervical infection, AGWs, and cervical lesion endpoints were undertaken in Australia, Norway, Sweden, and the US. In total, data from 138 studies from 23 countries were represented within this review
3.2. Reductions in HPV infection across populations
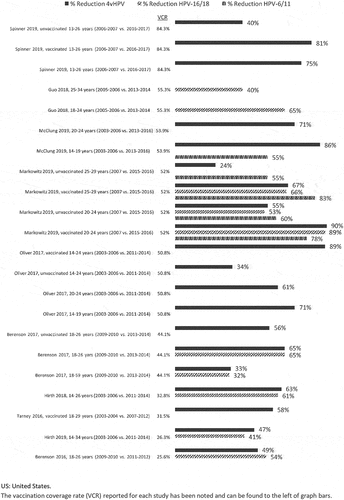
We observed consistent decreases in VT-HPV positivity rates in females among age groups targeted by national immunization programs, of up to 96% [Citation33–35]. As time since program implementation increased, vaccination coverage rates (VCR, typically given as a proportion) increased and HPV infection rates declined. The greatest reductions in HPV infection rates were observed in younger age groups (that is, in regions where vaccination is initiated at relatively young ages according to policy, and in individuals who are likely sexually naïve; and Supplementary Figure 2).
In addition to HPV reductions in vaccinated females, two US studies reported decreases in HPV genital infections among unvaccinated females [Citation36,Citation37]. Berenson et al. 2017 reported a significant decline among unvaccinated females aged 18–26 over time (when VCR was 44.1%), from 19.5% in 2009–2010 to 9.7% in 2013–2014 (prevalence ratio 0.44, 95% CI 0.22–0.91) [Citation36]. Oliver et al. 2017 also observed a 34% decrease in 4vHPV infection rates among unvaccinated females when VCR was 50.8% [Citation37]. In contrast, Tarney et al. 2016 saw no reduction in infection in unvaccinated US females, potentially due to a lower VCR of 31.8% [Citation38]. A general trend was observed across the US, Canada, Scandinavia, and Australia, of decreases in 4vHPV infection associated with increases in VCR, particularly among younger age cohorts. However, these data should be interpreted qualitatively and with some caution due to variability in reported VCR across years, and potential variations in vaccination policies () [Citation37–46]. Several studies also noted herd protection effects in female populations and evidence of cross-protection for phylogenetically related HPV-16/18 was also reported [Citation28,Citation42,Citation43,Citation47–52].
HPV infection rates among predominantly unvaccinated men was assessed for the first time by three studies () [Citation40,Citation53,Citation54]. The first of these demonstrated a 73% reduction in HPV among Australian men following high coverage of the 4vHPV vaccine in a female-only program [Citation53]. As the first US study to compare VT and non-VT-HPV rates, Gargano et al. 2017 found the lowest 4vHPV infection rate in younger men (1.8% in 14–19 year olds and 4% in 20–24 year olds). In the absence of vaccine impact, it would be expected that prevalence be considerably higher in the 20–24 year age group: however, this pattern was only observed for non-VT-HPV types suggesting indirect herd protection effects from female HPV vaccination [Citation54]. Further, Machalek et al. 2017 compared HPV rates in younger and older unvaccinated Australian heterosexual males 16–35 years of age in a national survey (≤25 years vs. >25 years) and reported a 78% decline in those ≤25 years, likely due to herd protection effects from the female program [Citation47].
Limited data has been published examining the direct vaccine impact or effectiveness on males. One US study One US study reported a statistically significant reduction in oral VT-HPV rates among vaccinated vs. unvaccinated men from 2011 to 2014 (0.0% vs. 2.13%; model-adjusted p = 0.007) [Citation55]. However, other studies did not find a significant impact related to vaccination. Han et al. 2017 conducted the first population-based study to date in the US examining the proportion of 9vHPV VT, showing similar infection rates between vaccine-eligible and non-eligible populations [Citation56,Citation57,Citation58]. However, the overall rate of HPV vaccination among eligible men was low, at 10.7%. In an additional Australian study, the prevalence of penile 4vHPV VT rates was low at 2.6% in young heterosexual males (17–19 years), prior to the introduction of male vaccination implementation (2014–2015). The study further observed a non-statistically significant reduction of 0.7% (p = 0.371) in the male vaccination period (2016–2017), the recruited population of whom 55% had received >1 vaccine dose, suggesting but not confirming possible herd protection effects from the female program.
3.3. Anogenital warts
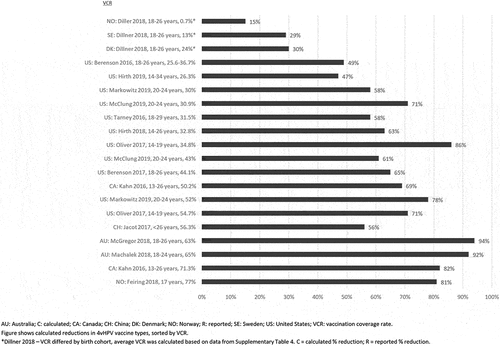
Compared to our previous review, additional data from Italy, Israel, Norway, Spain, and the UK are reported in this review. Declines in the incidence of AGWs continue to be observed within vaccine-targeted female age groups, with larger decreases (up to 88%) observed in younger age groups (Supplementary Figure 3) [Citation59].
Declines in AGW among unvaccinated males (likely attributable to herd protection from female vaccination) have been described in several studies (Supplementary Figure 4) [Citation60–64]. Cocchio et al. 2017 observed reductions in hospitalization rates due to penile warts for 12–20-year-old males, and Flagg et al. 2018 reported a reduction in AGW rates among men aged 15–24, further implying herd protection effects of female HPV vaccination in men of a corresponding age () [Citation60,Citation61]. Orumaa et al., 2020, highlighted the benefit of high VCR and the effect of single cohort vs. multicohort vaccination at program initiation. In Denmark, where multi-cohort vaccination of older age groups was introduced with 70% coverage, rapid decreases among women and men were observed in genital warts (by 18% and 10.7%, respectively, each year immediately after vaccine introduction). While Norway, which introduced single-cohort vaccination with 24% coverage at the same time, showed only modest decreases (4.8% among women and 1.9% in men, each year immediately after vaccine introduction) [Citation65]. Additional studies discussed the effectiveness in settings of low vaccine coverage among females, and in opportunistic (non-organized) programs [Citation62,Citation66,Citation67]. Notably, Herweijer et al. 2018 found that, when opportunistic vaccination was offered in Sweden and led to ~30% coverage, AGW prevalence decreased similarly in both males and females (albeit with delays of >1 year in males), despite vaccination only being offered to females [Citation66].
Table 2. Vaccine impact on female and male AGWs in the US
3.4. Cervical lesions
Recent studies demonstrated reduced rates of 4vHPV VT high-grade and low-grade cervical lesions in vaccine-targeted females. The greatest reductions were observed in younger age groups (14–17 years), with up to 73% reduction in CIN3+ among vaccinated females (Supplementary Figure 5) [Citation68]. In addition to our previous review, HPV vaccine impact and effectiveness on cervical lesions was additionally reported in Belgium, India, Luxembourg, New Zealand, South Korea, and Japan.
The outcomes of HPV vaccination has now also been reported in Japan despite the short window of vaccination, with studies observing vaccine-related benefits within four years of program implementation. This supports the reinstatement of Japan’s mandatory program, which was suspended in 2013 after media coverage of adverse effects [Citation69–75]. Karube et al. 2019 showed the rates of HPV-16/18 decreased from 36.7% to 5.8% (p = 0.00013) among women aged 18–24 years in a setting of a high VCR of 68.2% [Citation75]. Matsumoto et al. 2019 additionally showed a decrease in high-grade cervical lesions attributable to HPV-16/18 (from 50% to 0% in CIN1 and from 83.3% to 45.0% in CIN2/3, respectively, in women <25-years old) [Citation74].
In addition to the inclusion of real-world data from more countries compared to our previous review, sufficient time has now passed for longer-term effects of HPV vaccination to be observed, including cervical high-grade lesions. In Australia, where HPV vaccination programs were implemented in 2007, histologically confirmed cervical high-grade lesions have begun to show a statistically significant decline [Citation76]. The observed impact of vaccination extends to 25–29-year-old females (who experienced a 17% decline from 2012 to 2014), and 30–34-year-old females, whose trend in rates of high-grade lesions has begun to decline [Citation77]. Data from the US have also shown significant reductions in adenocarcinoma in situ in the 21–24-year age group, by 22.1% (95% CI −33.9–-8.2) [Citation78]. In both Australia and the US, reductions were also reported across all grades of cervical lesions [Citation79,Citation80]. Benard et al. 2017 described reductions in all grades of cervical intraepithelial neoplasia by an annual percentage decrease of 9% (95% CI, −12.0 to −5.8; p < 0.001) in New Mexico [Citation80].
Some inconsistencies were observed in relation to the number of doses and associated reductions in different grades of disease observed (for example, numerous publications reported on only ≥1 vaccine dose, with the associated vaccine effectiveness ranging in those papers from 18% to 64%; dependent on whether the outcome assessed was abnormal cytology, CIN2+, or CIN 3+; see Supplementary Figure 5) [Citation76,Citation81–83]. Vaccine effectiveness was, however, well-supported in those receiving a complete dosing series on schedule [Citation21,Citation83–87].
3.5. Non-cervical disease endpoints
A total of 16 studies were identified which reported on non-cervical endpoints (, ,, Supplementary Table 4).
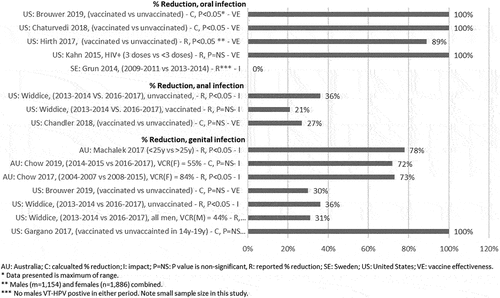
A consistent overall decrease in anal infection prevalence has been reported (). Variability, however, was observed by HPV type [Citation86,Citation88]. A significant reduction in HGAIN was observed among vaccinated men who have sex with men, compared to unvaccinated men who have sex with men in one study [Citation89]. In years one and two after vaccination, statistical significance in the estimate was observed, with hazard ratios of 0.42 (95%CI: 0.22–0.82, p = 0.01) and 0.50 (95%CI: 0.26–0.98, p = 0.05), respectively. This was not the case in year 3 (HR = 0.52; 95%CI: 0.27–1.02, p = 0.06), potentially due to loss of follow-up of participants in the longer-term study [Citation89].
Several studies demonstrated a substantial decrease in HPV-related oral infection, with some establishing significance. One study by Hirth et al. 2017 reported that vaccinated adults had lower rates of oral VT-HPV (VE=89%, p<0.001) and oral HPV-16/18 (VE=85%, p=0.006) compared to unvaccinated adults [Citation95]. In addition, Chaturvedi et al. 2018 showed a statistically significant reduction in oral HPV prevalence among men in a cross-sectional study (0.0% vs 2.13%; model-adjusted p=0.007) [Citation55]. However, other studies demonstrated a non-statistically significant trend of lower oral HPV prevalence in vaccinated vs. unvaccinated populations [Citation90,Citation91,Citation92]. As oral HPV infection is relatively rare compared to anal and genital HPV infections, many published studies likely included too small a population to definitively show an effect of vaccination on this endpoint [Citation33,Citation86,Citation91–94]. statistically significant trend of lower.
The impact of 4vHPV on RRP was reported by one study. Novakovic et al. 2018 observed an annual decrease in JoRRP incidence, as caused by HPV-6 and HPV-11, by a factor of 0.614 per calendar year following Australia’s implementation of their extensive 4vHPV female-only program with catch-up [Citation96].
4. Discussion
This literature review has shown the impact and effectiveness of HPV vaccination across multiple endpoints and expanded upon the previous Garland et al. review [Citation28]. Since the prior SLR, vaccine programs have matured, allowing for an evaluation of real-world effectiveness and impact in multiple countries, across multiple HPV-related endpoints [Citation28]. Also, the role of GNV has been described, and male outcomes have been discussed in further detail.
Reductions in infection, AGWs and cervical lesions were identified in Australia, Europe, North America, and New Zealand. This updated SLR identified novel data from additional countries related to the impact and effectiveness of 4vHPV, including low-income (Bhutan and Rwanda) and lower-middle-income countries (India and Mongolia), which is particularly relevant given that these setting face the largest disease burdens [Citation97]. Data provided by the identified studies suggest the largest benefit of HPV vaccination is obtained when females are vaccinated with a complete vaccination schedule at sustained high coverage, and program commencement is before sexual debut. Albeit slightly lower, there is also a benefit to vaccinating males and females above the adolescent age. Additionally, evidence from this review has shown the benefit of implementing multi-cohort vaccination strategies in seeing outcomes faster, as opposed to single cohort programs. We found that HPV vaccination is associated with substantial and consistent declines in VT-HPV infection and HPV-related disease endpoints. However, effectiveness was generally found to decrease with increasing age at vaccination of the vaccinated cohort. Data were primarily derived from studies among women. The observed inverse correlation between age at vaccination and vaccine effectiveness correlates with the likelihood of HPV exposure prior to vaccination and is expected on the grounds of the vaccines not being therapeutic but prophylactic [Citation82].
The number of countries with GNV programs is growing, with countries such as the US, Australia, Switzerland, Austria, Norway, Sweden, and Canada choosing to vaccinate boys as well as girls. Studies in countries offering gender-neutral HPV vaccination show reductions in VT-HPV infection, and declining cases of AGWs in non-vaccinated females and males. These effects are seen in countries with both high and low vaccine coverage, indicating strong herd protection effects. For example, data from a 2021 US report showed significant declines in 4vHPV infection in both vaccinated and unvaccinated females from the pre-vaccine era to 2015–2018 [Citation98]. Data regarding the real-world direct effect of male HPV vaccination are, thus far, more inconsistent and complicated to measure due to the potential for herd effects conferred by concurrent female vaccination. There is an emergence of benefit, for example demonstration of reduced anal, penile, and oral VT-HPV prevalence in men who have sex with men pre and post-male vaccination program establishment in Australia. However, further studies in the post-GNV era are needed [Citation99]. In contrast, the indirect effects of vaccination for males have been well reported, with declines in AGW prevalence among heterosexual men attributed to females receiving HPV vaccination [Citation60,Citation62,Citation64,Citation82,Citation100–102]. As vaccination rates among men remained low in the US from 2011 to 2014, observed declines in AGW rates among young men were credited to herd protection effects from women within similar age groups (), who were most likely to be sexual partners to men of the same age [Citation47,Citation61]. Data from Sweden also strongly support the presence of herd effects in males deriving from vaccinated females [Citation66].
Despite this, data suggest that HPV elimination goals will be supported by GNV when sufficient vaccine is available. GNV provides particular resiliency against disruptions in vaccine dissemination, such as have occurred recently due to the COVID-19 pandemic [Citation103]. Current female-only vaccination programs do not have high enough VCR to deliver necessary herd effects to males to achieve HPV-16/18 elimination. Vänskä et al. 2020 compared female-only and GNV when 2vHPV was administered, finding that GNV produced approximately 150% higher herd protection effects than girls-only vaccination [Citation14]. The authors predicted elimination of HPV-18/31/33 in young adults in 20 years with 75% coverage of GNV. Eventual HPV-16 elimination was also predicted under these circumstances.
A recent review by de Sanjosé and Bruni, 2020, called for planning of global gender-neutral HPV vaccination [Citation104]. The authors recognize the merits of GNV, including the role such programs could play in increasing and maintaining high HPV vaccination coverage, and reducing the disease burden associated with infection in both males and females. They noted that herd protection effects associated with GNV may be the most cost-effective longer-term HPV prevention strategy, but this does not outweigh the direct benefit of reducing the cervical cancer burden at this time with female-only programs. Further, current WHO Strategic Advisory Group of Experts on Immunization recommends that girls aged 9–14 should be prioritized for vaccination [Citation105]. Given the evidence of benefits of HPV vaccine in males and females, there has been a large increase in the number of vaccination programs, including boys and men and multi-age cohorts since 2017 [Citation106]. As a result, a global effort has been established and HPV vaccine manufacturing capacity has been significantly expanded. Particularly, the availability of vaccine has been enhanced significantly with both increases in manufacturing reliability and decreases in short-term demand due to the COVID pandemic. Therefore, country readiness and implementation becomes an important issue for program start-up and expansion, especially including GNV and multi-age cohort programs.
4.1. Caveats and limitations
Given the wide heterogeneity associated with studies included in this review, including variety of outcomes, diversity of populations, and countries with different programs, the results could only focus on general trends [Citation28]. Additionally, the benefit of vaccination on HPV-related cancers cannot be fully determined in randomized clinical trials due to long latency periods following exposure, which require long follow-up [Citation107]. There are limited data on the impact and effectiveness of HPV vaccination on other outcomes, such as oropharyngeal, vulvar, vaginal, penile lesions, and RRP; future updates will be needed when these data become available. Nevertheless, expected reductions in HPV infection and lesions are becoming increasingly evident, which suggests that HPV vaccination can provide benefit regarding all aspects of HPV-related disease. Finally, the male vaccination program is still limited and with short follow-up data currently available. Therefore, more evidence on the direct vaccine impact and effectiveness in males is needed to fully understand the benefit of GNV strategies. Due to the relatively recent licensing of the 9vHPV vaccine in 2014, no reports were identified within this review on the impact and effectiveness of 9vHPV. However, several studies provide a good baseline to monitor its impact by including prevalence of the additional five genotypes. Further studies can be expected as uptake of 9vHPV becomes more widespread.
In addition, to fully evaluate the benefit and risk of vaccine, vaccine safety data should be considered beyond impact and effectiveness evidence. However, it is not in the scope of this study.
4.2. Future challenges
Considering the high number of reported studies in the past four years, an increasing number of HPV impact and effectiveness studies can be expected from countries, which have implemented programs in recent years. Studies such as Lehtinen et al., 2018, have begun to report on the effects of GNV in Scandinavia, including herd protection and cross-protection (against non-VT HPVs). As the number of GNV initiatives increase, additional data on the anticipated direct effect in males and indirect effects in unvaccinated females will become available from a wider geographical area [Citation15].
As vaccine programs mature and a larger number of cohorts are vaccinated each year, it is expected that the impact of HPV vaccination on cancer outcomes will become apparent. Studies from 2017 to 2018 showed early effects of HPV vaccination on cervical cancer, highlighting a decreasing trend in cervical cancer incidence in young women in the US, but noting the need to confirm with further data [Citation108,Citation109]. Lei et al., 2020, recently reported a substantially reduced risk of invasive cervical cancer among HPV-vaccinated individuals in a large cohort of 1,672,983 Swedish girls and women aged 10–30 years old. The incidence rate ratio of cervical cancer for vaccinated vs. unvaccinated women was 0.51 (95% CI: 0.32–0.82) after adjustment for age, with estimated cumulative incidence of 47 cases per 100,000 persons with vaccination, and 94 cases per 100,000 persons without [Citation26]. Additional data on the invasive cancer endpoint has just come through from Denmark and further data are expected to become available in future years, assuming the trend of a small number of papers being published each year continues [Citation27]. When these are available, a further update can be conducted to include this endpoint, and better gauge the long-term effects of HPV vaccination.
Unlike cervical cancer, routine screening for oral and anal cancers does not currently exist; thus, the opportunities to examine these endpoints, as well as infection at these anatomic sites, are limited. Despite this limitation, the published literature demonstrates reductions in oral HPV infection and anal disease among vaccinated cohorts. It is therefore possible that effectiveness studies for these endpoints will become more common and, as vaccine dissemination increases among males, impact studies may be feasible. One recent study from Spain, for example, showed the effectiveness of 4vHPV vaccination in preventing anal HSIL in men who have sex with men, with a 48-month follow-up [Citation110]. Further, a recent Danish study reported reduced rates of high-grade vulvaginal lesions for women vaccinated at age 16 years or younger compared to unvaccinated women [Citation111]. As GNV programs continue to be implemented, further studies with follow-up of similar or longer length will become increasingly available. Even for very rare outcomes, such as RRP, impact can be established retrospectively, as demonstrated by the first such study from Australia detailing the impact of HPV vaccination RRP incidence [Citation96].
4.3. Conclusions
HPV vaccination programs continue to support a marked reduction in new HPV infections and corresponding disease outcomes. The data included in this review can inform vaccination initiatives and evaluations around the world. As infections with high-risk HPV types can now be protected against by immunization, near-complete disease elimination may become an achievable target, coupled with cervical screening and treatment for those with disease. Neoplastic disease from HPV poses a significant public health burden; its elimination would therefore result in vast benefits. Further research on the impact and effectiveness of HPV vaccines will aid realization of this goal, not the least studies on HPV vaccines with an extended spectrum of genotypes.
5. Expert opinion
The impact and effectiveness of HPV vaccination continues to be demonstrated through increasingly extensive real-world data, data that include longer follow-up time and more detailed assessments of patient endpoints. Due to the long period from persistent oncogenic HPV infection to neoplasia, early studies demonstrated effectiveness against more proximal endpoints such as reductions in vaccine-type HPV infections and precancers. It is so pleasing to see that now three countries have demonstrated dramatic vaccine induced reductions in cervical cancer incidence. In countries with gender-neutral vaccination (GNV) programs, we expect to see more rapid declines in cervical cancer incidence and in time significant reductions in other HPV-related cancers occurring among men such as cancers of anus, penis, and head and neck.
Despite clear evidence of the benefit of HPV vaccines, a notable proportion of the population remain vaccine hesitant, with public mistrust detailed as an underlying cause [Citation112,Citation113]. This is an important consideration for combatting the anti-vaccine movement and requires deeper understanding and reflection, however the long-term hope is to instill an epistemic trust, i.e. the willingness to consider new knowledge as trustworthy and relevant. In addition to issues surrounding hesitancy, vaccine access in countries in greatest need remains low. A concerted global effort to improve worldwide vaccination rates is needed as recent WHO data indicate that only 13% of young girls eligible for vaccination have completed the vaccine series [Citation114]. It is expected that this number will rise with the continuing expansion of knowledge of the benefits of HPV vaccination and as additional countries roll out national HPV vaccination programs. Studies in countries with GNV programs show the benefit of reaching all age eligible adolescents with vaccine as there are marked and more rapid declines in HPV infection and HPV-related diseases than observed with female only vaccine programs. Currently, there are over 50 countries and regions instituting GNV programs. As GNV programs mature, long-term follow-up data will be available to evaluate the direct benefits to HPV-related diseases among males as well as females.
New data are emerging on HPV vaccine doses and timing of doses, including a single-dose vaccine schedule, and the permissive off-label use of single-dose schedules is de facto accepted in some regions [Citation16,Citation17,Citation115]. This is an active area of research with several ongoing efficacy randomized clinical trials as well as immunogenicity trials. Efficacy trials are important since the minimum level of antibody needed for protection is unknown. Nonetheless, considering the lower immunogenicity and incomplete seroconversion observed after a single dose of 9vHPV vaccine compared to two- and three-dose regimens (56–98% after one dose depending on the HPV type and 99–100% after two or three doses, in a recently reported trial in 9–14-year-olds) [Citation116], it will be important to thoroughly assess the long-term effectiveness of single-dose HPV vaccination against HPV-related disease in these trials and other studies. Of note, the ongoing trials do not include assessment of efficacy of single-dose HPV vaccination in males nor efficacy against HPV-related diseases.
Since the WHO Call to Action, many countries have considered or initiated implementation of new HPV vaccination programs or have expanded existing programs. This led to a doubling in the demand for HPV vaccines in 2018, compared to 2017. A concerted effort has been made, including improvements in the production process by key manufacturers, as well as new vaccine producers in the market such that within the next year or two an abundant and sufficient vaccine supply to meet demands will be available [Citation105]. Therefore, country readiness and implementation becomes an important issue for program start-up and expansion, especially including GNV and multi-age cohort programs.
Vaccination and screening are closely interrelated and complementary cervical cancer prevention measures. Empirical studies imply considerable shift in accuracy of existing screening technology after HPV-vaccinated cohorts enter in the screening [Citation117], and modeling suggests that with high coverage vaccination of young girls and as they reach screening age, the risk for cervical cancer may be so low it may not be cost-effective to continue screening [Citation118]. Although adapting screening inevitably will be context specific, furthering the screening programs currently in place will allow identification of women who are HPV-positive, prior to the emergence of HPV-related diseases. This is particularly important in high-risk groups, such as patients living with HIV [Citation119,Citation120].
One future development of note could be the extension of self-sampling: the collection of cervicovaginal HPV DNA samples or first-void urine (as an even less invasive option) by women at screen eligible ages. Research has highlighted the increased acceptance of these methods by end users as well as validation in the laboratory, and thus increases in participation rates should result [Citation121–125]. Additionally, the switch from cytology- to HPV-based screening will confer an added benefit by providing data needed to monitor the vaccination program [Citation126]. With HPV-based screening, it is essential to optimize the triage approach for those that are HPV-positive, to discriminate between transient and persistent infections. Research is on-going evaluating different post-HPV positive triage methods, such as genome-wide HPV methylation assessments and extended genotyping and other biomarkers of progression [Citation127–132], to improve specificity of HPV testing as a primary screen, among both high-risk and low-risk populations. Of note, findings by Smith et al. suggest that colposcopy should be considered for women whose first primary test is HPV16/18 positive, regardless of cytology result, owing to a high risk of detecting cancer in this group (1%), even in a previously well-screened population [Citation133].
The SARS-CoV-2 pandemic has impacted both vaccination and screening programs. Interruption of some routine HPV vaccine programs led to a decreased coverage, and we will likely observe increases in genital wart, cervical pre-cancer, and cervical cancer incidences in the near future unless action is taken to recover vaccine coverage [Citation134]. The demand for SARS-CoV-2 testing is also competing with HPV testing, compounded by a shortage of staff [Citation135]. As a silver lining, once the demand for COVID-19 testing decreases, this should release expert workforce and platform capacity for high-throughput HPV testing. On the bright side, innovation around molecular COVID-19 testing systems may also result in solutions that address the shortage of rapid low-cost HPV testing systems for low-resource settings.
The pandemic has taught the community about the public health benefit of vaccination and the need for testing and screening. We hope that after the pandemic, we will have increased confidence and a better understanding of the importance of virus testing and screening worldwide.
Article highlights
This review captures recent 5 years’ publication of real-world impact and effectiveness of human papillomavirus (HPV) vaccines in females and males, which represent at least a 14-year follow up real word impact and effectiveness since the launch of quadrivalent vaccine in 2006.
Data identified demonstrate statistically significant decreases in vaccine-type HPV genital, oral and anal infections, and prevention of recurrent respiratory papillomatosis, anal, and cervical lesions.
There is now robust and expansive data available to support HPV vaccination direct and indirect benefit from a variety of settings and populations including different countries, different outcome assessments, and different vaccination program strategies.
Understanding the impact on both male and female outcomes, that are due to herd effects and direct benefits of female-only and gender-neutral vaccination initiatives, can inform policy and ensure tracking of progress toward the elimination of HPV-related disease outcomes as a public health issue.
Declaration of interest
W(V) Wang, S Kothari, C Koro, A Luxembourg and AJ Saah are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and potentially own stock and/or hold stock options in Merck & Co., Inc., Rahway, NJ, USA. . P95 (a healthcare research company specializing in epidemiology and pharmacovigilance) were contracted to perform the systematic literature review by Merck & Co., Inc.; J Skufca, M Baay and T Verstraetenare employees of P95. M Nygård has received research grants though her affiliating institute from MSD Norway. SM Garland and AR Giuliano have received research funding to their institutions, payment for board membership and speaker fees from MSD. K Sundström has received research funding and consultancy fees from MSD to her affiliating institution. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (2.5 MB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2022.2129615
Additional information
Funding
References
- Forman D, CJ L, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30:F12–F23.
- Bruni L, Albero G, Serrano B, et al. Human papillomavirus and related diseases report HPV center 2019 Accessed 12 January 2021. Available from: https://www.hpvcentre.net/statistics/reports/XWX.pdf
- Castellsagué X, Alemany L, Quer M, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016;108(6):6.
- World Health Organization. Global strategy to accelerate the elimination of cervical cancer as a public health problem. 2020.
- de Sanjose S, Brotons M, LaMontagne DS, et al. Human papillomavirus vaccine disease impact beyond expectations. Curr Opin Virol. 2019;39:16–22.
- Wu T, Hu Y-M, Li J, et al. Immunogenicity and safety of an E. coli-produced bivalent human papillomavirus (type 16 and 18) vaccine: a randomized controlled phase 2 clinical trial. Vaccine. 2015;33(32):3940–3946.
- de Martel C, Plummer M, Vignat J, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–670.
- Serrano B, Brotons M, Bosch FX, et al. Epidemiology and burden of HPV-related disease. Best Pract Res Clin Obstetrics Gynaecol. 2018;47:14–26.
- Garland SM, Steben M, Sings HL, et al. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199(6):805–814.
- Pou AM, Rimell FL, Jordan JA, et al. Adult respiratory papillomatosis: human papillomavirus type and viral coinfections as predictors of prognosis. SAGE Publications Sage Los Angeles; 1995.
- Bruni L, Saura-Lazaro A, Montoliu A, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010-2019. Prev Med. 2021;144:106399.
- Drolet M, Benard E, Perez N, et al. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet. 2019;394(10197):497–509.
- World Health Organization. Immunization coverage 2020 Accessed 12 January 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/immunization-coverage
- Vanska S, Luostarinen T, Baussano I, et al. Vaccination with moderate coverage eradicates oncogenic human papillomaviruses if a gender-neutral strategy is applied. J Infect Dis. 2020 Aug 17;222(6):948–956.
- Lehtinen M, Soderlund-Strand A, Vanska S, et al. Impact of gender-neutral or girls-only vaccination against human papillomavirus-Results of a community-randomized clinical trial (I). Int J Cancer. 2018;142(5):949–958.
- World Health Organization. One-dose human papillomavirus (HPV) vaccine offers solid protection against cervical cancer 2022 Accessed 30 April 2022. Available from: https://www.who.int/news/item/11-04-2022-one-dose-human-papillomavirus-(hpv)-vaccine-offers-solid-protection-against-cervical-cancer
- Center for Vaccine Innovation and Access. Single-Dose HPV Vaccine Evaluation Consortium 2022 Accessed 17 April 2022 [updated April 2022]. Available from: https://www.path.org/programs/center-for-vaccine-innovation-and-access/single-dose-hpv-vaccine-evaluation-consortium/
- Schiller JT, Lowy DR. Explanations for the high potency of HPV prophylactic vaccines. Vaccine. 2018;36(32):4768–4773.
- Drolet M, Benard E, Boily MC, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2015 May;15(5):565–580.
- World Health Organization. Human papillomavirus vaccines: WHO position paper. Vaccine. 2017;35(43):5753–5755.
- Johnson Jones ML, Gargano JW, Powell M, et al. Effectiveness of 1, 2, and 3 doses of human papillomavirus vaccine against high-grade cervical lesions positive for human papillomavirus 16 or 18. Am J Epidemiol. 2020 Apr 2;189(4):265–276.
- Tabrizi SN, Brotherton JM, Kaldor JM, et al. Fall in human papillomavirus prevalence following a national vaccination program. J Infect Dis. 2012 Dec 1;206(11):1645–1651.
- Harper DM, DeMars LR. HPV vaccines – a review of the first decade. Gynecol Oncol. 2017;146(1):196–204.
- Ramondetta L. Response to Harper and De Mars, HPV vaccines: a review of the first decade. Gynecol Oncol Rep. 2017 Nov;22:113–114.
- Hariri S, Schuler MS, Naleway AL, et al. Human papillomavirus vaccine effectiveness against incident genital warts among female health-plan enrollees, United States. American Journal of Epidemiology. 2018;187(2):298–305.
- Lei J, Ploner A, Elfstrom KM, et al. HPV Vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020 Oct 1;383(14):1340–1348.
- Kjaer SK, Dehlendorff C, Belmonte F, et al. Real-world effectiveness of human papillomavirus vaccination against cervical cancer. Journal of the National Cancer Institute: JNCI; 2021.
- Garland SM, Kjaer SK, Munoz N, et al. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of real-world experience. Clin Infect Dis. 2016 Aug 15;63(4):519–527.
- Brown DR, Joura EA, Yen GP, et al. Systematic literature review of cross-protective effect of HPV vaccines based on data from randomized clinical trials and real-world evidence. Vaccine. 2021;39(16):2224–2236.
- World Health Organization. Vaccine efficacy, effectiveness and protection 2021 Accessed 19 July 2022. Available from: https://www.who.int/news-room/feature-stories/detail/vaccine-efficacy-effectiveness-and-protection
- Hanquet G, Valenciano M, Simondon F, et al. Vaccine effects and impact of vaccination programmes in post-licensure studies. Vaccine. 2013 Nov 19;31(48):5634–5642.
- Oxford Vaccine Group. Herd immunity (Herd protection): University of Oxford. Accessed 21 March 2022]. Available from: https://vk.ovg.ox.ac.uk/vk/herd-immunity
- Enerly E, Flingtorp R, Christiansen IK, et al. An observational study comparing HPV prevalence and type distribution between HPV-vaccinated and -unvaccinated girls after introduction of school-based HPV vaccination in Norway. PLoS One. 2019;14(10):e0223612.
- Goggin P, Sauvageau C, Gilca V, et al. Low prevalence of vaccine-type HPV infections in young women following the implementation of a school-based and catch-up vaccination in Quebec, Canada. Hum Vaccin Immunother. 2018;14(1):118–123.
- Heard I, Tondeur L, Arowas L, et al. Effectiveness of human papillomavirus vaccination on prevalence of vaccine genotypes in young sexually active women in France. J Infect Dis. 2017;215(5):757–763.
- Berenson AB, Hirth JM, Chang M. Change in human papillomavirus prevalence among U.S. Women Aged 18-59 Years, 2009-2014. Obstet Gynecol. 2017 2017-1-1;130(4):693–701.
- Oliver SE, Unger ER, Lewis R, et al. Prevalence of human papillomavirus among females after vaccine introduction-national health and nutrition examination survey, United States, 2003-2014. J Infect Dis. 2017;216(5):594–603.
- Tarney CM, Klaric J, Beltran T, et al. Prevalence of Human Papillomavirus in Self-Collected Cervicovaginal Swabs in Young Women in the United States Between 2003 and 2012. Obstet Gynecol. 2016;128(6):1241–1247.
- Dillner J, Nygård M, Munk C, et al. Decline of HPV infections in Scandinavian cervical screening populations after introduction of HPV vaccination programs. Vaccine. 2018 Jun 18;36(26):3820–3829.
- Machalek DA, Garland SM, Brotherton JML, et al. Very low prevalence of vaccine human papillomavirus (HPV) types among 18 to 35 year old Australian women, nine years following implementation of vaccination. J Infect Dis. 2018;217(10):1590–1600.
- Feiring B, Laake I, Christiansen IK, et al. Substantial decline in prevalence of vaccine-type and nonvaccine-type human papillomavirus (HPV) in vaccinated and unvaccinated girls 5 years after implementing HPV Vaccine in Norway. J Infect Dis. 2018 Nov 5;218(12):1900–1910.
- Kahn JA, Widdice LE, Ding L, et al. Substantial Decline in Vaccine-Type Human Papillomavirus (HPV) Among Vaccinated Young Women During the First 8 Years After HPV Vaccine Introduction in a Community. Clin Infect Dis. 2016;63(10):1281–1287.
- McGregor S, Saulo D, Brotherton JML, et al. Decline in prevalence of human papillomavirus infection following vaccination among Australian Indigenous women, a population at higher risk of cervical cancer: the VIP-I study. Vaccine. 2018 Jul 5;36(29):4311–4316.
- Jacot-Guillarmod M, Pasquier J, Greub G, et al. Impact of HPV vaccination with Gardasil(R) in Switzerland. BMC Infect Dis. 2017;17(1):790.
- Hirth J, McGrath CJ, Kuo YF, et al. Impact of human papillomavirus vaccination on racial/ethnic disparities in vaccine-type human papillomavirus prevalence among 14-26 year old females in the U.S. Vaccine. 2018 Nov 29;36(50):7682–7688.
- Hirth JM, Kuo YF, Starkey JM, et al. Regional variations in human papillomavirus prevalence across time in NHANES (2003-2014). Vaccine. 2019 Jul 9;37(30):4040–4046.
- Machalek DA, Chow EP, Garland SM, et al. Human Papillomavirus Prevalence in Unvaccinated Heterosexual Men After a National Female Vaccination Program. J Infect Dis. 2017;215(2):202–208.
- Markowitz LE, Naleway AL, Lewis RM, et al. Declines in HPV vaccine type prevalence in women screened for cervical cancer in the United States: evidence of direct and herd effects of vaccination. Vaccine. 2019 Jun 27;37(29):3918–3924.
- Sarr EHM, Mayrand MH, Coutlee F, et al. Exploration of the effect of human papillomavirus (HPV) vaccination in a cohort of pregnant women in Montreal, 2010-2016. Heliyon. 2019 Aug;5(8):e02150.
- Saccucci M, Ding L, et al. Non-vaccine-type human papillomavirus prevalence after vaccine introduction: no evidence for type replacement but evidence for cross-protection. Sex Transm Dis. 2018;45(4):260–265.
- Covert C, Ding L, Brown D, et al. Evidence for cross-protection but not type-replacement over the 11 years after human papillomavirus vaccine introduction. Hum Vaccin Immunother. 2019 Jan;11:1–8.
- Garland SM, Cornall AM, Brotherton JML, et al. Final analysis of a study assessing genital human papillomavirus genoprevalence in young Australian women, following eight years of a national vaccination program. Vaccine. 2018 May 31;36(23):3221–3230.
- Chow EPF, Machalek DA, Tabrizi SN, et al. Quadrivalent vaccine-targeted human papillomavirus genotypes in heterosexual men after the Australian female human papillomavirus vaccination programme: a retrospective observational study. Lancet Infect Dis. 20172017-1-1;17(1):68–77.
- Gargano JW, Unger ER, Liu G, et al. Prevalence of genital human papillomavirus in males, United States, 2013-2014. J Infect Dis. 2017;215(7):1070–1079.
- Chaturvedi AK, Graubard BI, Broutian T, et al. Effect of Prophylactic Human Papillomavirus (HPV) Vaccination on Oral HPV Infections Among Young Adults in the United States. J Clin oncol. 2018;36(3):262–267.
- Han JJ, Beltran TH, Song JW, et al. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among us adult men: national health and nutrition examination survey (NHANES) 2013-2014. JAMA Oncol. 2017;3(6):810–816.
- Chow EPF, Tabrizi SN, Fairley CK, et al. Prevalence of human papillomavirus in teenage heterosexual males following the implementation of female and male school-based vaccination in Australia: 2014-2017. Vaccine. 2019 Oct 31;37(46):6907–6914.
- Widdice LE, Bernstein DI, Franco EL, et al. Decline in vaccine-type human papillomavirus prevalence in young men from a Midwest metropolitan area of the United States over the six years after vaccine introduction. Vaccine. 2019 Oct 23;37(45):6832–6841.
- Ali H, McManus H, O’Connor CC, et al. Human papillomavirus vaccination and genital warts in young Indigenous Australians: national sentinel surveillance data. Med J Aust. 2017;206(5):204–209.
- Cocchio S, Baldovin T, Bertoncello C, et al. Decline in hospitalization for genital warts in the Veneto region after an HPV vaccination program: an observational study. BMC Infect Dis. 2017;17(1):249.
- Flagg EW, Torrone EA. Declines in Anogenital Warts Among Age Groups Most Likely to Be Impacted by Human Papillomavirus Vaccination, United States, 2006-2014. Am J Public Health. 2018;108(1):112–119.
- Lurie S, Mizrachi Y, Chodick G, et al. Impact of quadrivalent human papillomavirus vaccine on genital warts in an opportunistic vaccination structure. Gynecol Oncol. 2017;146(2):299–304.
- Steben M, Tan Thompson M, Rodier C, et al. A review of the impact and effectiveness of the quadrivalent human papillomavirus vaccine: 10 years of clinical experience in Canada. J Obstet Gynaecol Can. 2018 Dec;40(12):1635–1645.
- Checchi M, Mesher D, Mohammed H, et al. Declines in anogenital warts diagnoses since the change in 2012 to use the quadrivalent HPV vaccine in England: data to end 2017. Sex Transm Infect. 2019 Aug;95(5):368–373.
- Orumaa M, Kjaer SK, Dehlendorff C, et al. The impact of HPV multi-cohort vaccination: real-world evidence of faster control of HPV-related morbidity. Vaccine. 2020 Feb 5;38(6):1345–1351.
- Herweijer E, Ploner A, Sparen P. Substantially reduced incidence of genital warts in women and men six years after HPV vaccine availability in Sweden. Vaccine. 2018;36(15):1917–1920.
- Thone K, Horn J, Mikolajczyk R. Evaluation of vaccination herd immunity effects for anogenital warts in a low coverage setting with human papillomavirus vaccine-an interrupted time series analysis from 2005 to 2010 using health insurance data. BMC Infect Dis. 2017;17(1):564.
- Silverberg MJ, Leyden WA, Lam JO, et al. Effectiveness of catch-up human papillomavirus vaccination on incident cervical neoplasia in a US health-care setting: a population-based case-control study. Lancet Child Adolesc Health. 2018 Oct;2(10):707–714.
- Ozawa N, Ito K, Tase T, et al. Beneficial effects of human papillomavirus vaccine for prevention of cervical abnormalities in Miyagi, Japan. Tohoku J Exp Med. 2016;240(2):147–151.
- Ozawa N, Ito K, Tase T, et al. Lower incidence of cervical intraepithelial neoplasia among young women with human papillomavirus vaccination in Miyagi, Japan. Tohoku J Exp Med. 2017;243(4):329–334.
- Tanaka H, Shirasawa H, Shimizu D, et al. Preventive effect of human papillomavirus vaccination on the development of uterine cervical lesions in young Japanese women. J Obstet Gynaecol Res. 2017;43(10):1597–1601.
- Matsumoto K, Yaegashi N, Iwata T, et al. Early impact of the Japanese immunization program implemented before the HPV vaccination crisis. Int J Cancer. 2017;141(8):1704–1706.
- Ueda Y, Yagi A, Nakayama T, et al. Dynamic changes in Japan’s prevalence of abnormal findings in cervical cervical cytology depending on birth year. Sci Rep. 2018 Apr 4;8(1):5612.
- Matsumoto K, Yaegashi N, Iwata T, et al. Reduction in HPV16/18 prevalence among young women with high-grade cervical lesions following the Japanese HPV vaccination program. Cancer Sci. 2019 Dec;110(12):3811–3820.
- Karube A, Saito F, Nakamura E, et al. Reduction in HPV 16/18 prevalence among young women following HPV vaccine introduction in a highly vaccinated district, Japan, 2008-2017. J Rural Med. 2019 May;14(1):48–57.
- Brotherton JM, Budd A, Rompotis C, et al. Is one dose of human papillomavirus vaccine as effective as three?: a national cohort analysis. Papillomavirus Res. 2019 Dec;8:100177.
- Brotherton JM, Gertig DM, May C, et al. HPV vaccine impact in Australian women: ready for an HPV-based screening program. Med J Aust. 2016 Mar 21;204(5):184–184e1.
- Cleveland AA, Gargano JW, Park IU, et al. Cervical adenocarcinoma in situ: human papillomavirus types and incidence trends in five states, 2008-2015. Int J Cancer. 2020 Feb 1;146(3):810–818.
- Robertson G, Robson SJ. Excisional Treatment of Cervical Dysplasia in Australia 2004-2013: a Population-Based Study. J Oncol. 2016;2016:3056407.
- Benard VB, Castle PE, Jenison SA, et al. Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol. 2017;3(6):833–837.
- Kim J, Bell C, Sun M, et al. Effect of human papillomavirus vaccination on cervical cancer screening in Alberta. Cmaj. 2016;188(12):E281–8.
- Verdoodt F, Dehlendorff C, Kjaer SK. Dose-related effectiveness of quadrivalent human papillomavirus vaccine against cervical intraepithelial neoplasia: a Danish nationwide cohort study. Clin Infect Dis. 2020 Feb 3;70(4):608–614.
- Dehlendorff C, Sparén P, Baldur-Felskov B, et al. Effectiveness of varying number of doses and timing between doses of quadrivalent HPV vaccine against severe cervical lesions. Vaccine. 2018 Oct 15;36(43):6373–6378.
- Racey CS, Albert A, Donken R, et al. cervical intraepithelial neoplasia rates in british columbia women: a population-level data linkage evaluation of the school-based HPV immunization program. J Infect Dis. 2020 Jan 1;221(1):81–90.
- Hofstetter AM, Ompad DC, Stockwell MS, et al. Human papillomavirus vaccination and cervical cytology outcomes among urban low-income minority females. JAMA Pediatr. 2016;170(5):445–452.
- Schlecht NF, Diaz A, Shankar V, et al. Risk of delayed human papillomavirus vaccination in inner-city adolescent women. J Infect Dis. 2016;214(12):1952–1960.
- Rodriguez AM, Zeybek B, Vaughn M, et al. Comparison of the long-term impact and clinical outcomes of fewer doses and standard doses of human papillomavirus vaccine in the United States: a database study. Cancer. 2020 Apr 15;126(8):1656–1667.
- Chandler E, Ding L, Gorbach P, et al. Epidemiology of any and vaccine-type anogenital human papillomavirus among 13-26-year-old young men after HPV vaccine introduction. J Adolesc Health. 2018 Jul;63(1):43–49
- Swedish KA, Factor SH, Goldstone SE. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort study. Clin Infect Dis. 2012 Apr;54(7):891–898.
- Schlecht NF, Burk RD, Nucci-Sack A, et al. Cervical, anal and oral HPV in an adolescent inner-city health clinic providing free vaccinations. PLoS One. 2012;7(5):e37419.
- Schlecht NF, Masika M, Diaz A, et al. Risk of oral human papillomavirus infection among sexually active female adolescents receiving the quadrivalent vaccine. JAMA Network Open. 2019 Oct 2;2(10):e1914031.
- Kahn JA, Rudy BJ, Xu J, et al. Behavioral, immunologic, and virologic correlates of oral human papillomavirus infection in HIV-infected youth. Sex Transm Dis. 2015;42(5):246–252.
- Grün N, Ährlund-Richter A, Franzén J, et al. Oral human papillomavirus (HPV) prevalence in youth and cervical HPV prevalence in women attending a youth clinic in Sweden, a follow up-study 2013-2014 after gradual introduction of public HPV vaccination. Infect Dis (Lond). 2015 Jan;47(1):57–61.
- Brouwer AF, Eisenberg MC, Carey TE, et al. Multisite HPV infections in the United States (NHANES 2003-2014): an overview and synthesis. Prev Med. 2019 Jun;123:288–298.
- Hirth JM, Chang M, Resto VA. Prevalence of oral human papillomavirus by vaccination status among young adults (18-30years old). Vaccine. 2017 Jun 14;35(27):3446–3451.
- Novakovic D, Cheng ATL, Zurynski Y, et al. A prospective study of the incidence of juvenile-onset recurrent respiratory papillomatosis after implementation of a national HPV vaccination program. J Infect Dis. 2018 Jan 4;217(2):208–212.
- Gallagher KE, Erio T, Baisley K, et al. The impact of a human papillomavirus (HPV) vaccination campaign on routine primary health service provision and health workers in Tanzania: a controlled before and after study. BMC Health Serv Res. 2018 Mar 12;18(1):173.
- Rosenblum HG, Lewis RM, Gargano JM, et al. Declines in prevalence of human papillomavirus vaccine-type infection among females after introduction of vaccine — United States, 2003–2018. MMWR Morb Mortal Wkly Rep. 2021;70:415–420.
- Chow EP, Tabrizi SN, Fairley CK. Prevalence of human papillomavirus in young men who have sex with men after the implementation of gender-neutral HPV vaccination: a repeated cross-sectional study. Lancet Infect Dis. 2021;21;10:1448–1457.
- Steben M, Thompson MT, Rodier C, et al. A review of the impact and effectiveness of the quadrivalent human papillomavirus vaccine: 10 years of clinical experience in Canada. J Obstet Gynaecology Canada. 2018;40(12):1635–1645.
- Flagg EW, Torrone EA. Declines in anogenital warts among age groups most likely to be impacted by human papillomavirus vaccination, United States, 2006–2014. Am J Public Health. 2018;108(1):112–119.
- Orumaa M, Kjaer SK, Dehlendorff C, et al. The impact of HPV multi-cohort vaccination: real-world evidence of faster control of HPV-related morbidity. Vaccine. 2020;38(6):1345–1351.
- Organization WH. Closing immunization gaps caused by COVID-19 2020 Accessed 21 March 2022. Available from: https://cdn.who.int/media/docs/default-source/immunization/catch-up/closing-immunization-gaps-caused-by-covid-19-v11.pdf?sfvrsn=8a91a2bd_2
- de Sanjose S, Is BL. It now the time to plan for global gender-neutral HPV vaccination? J Infect Dis. 2020 Aug 17;222(6):888–889.
- Garland SM, Stanley MA, Giuliano AR, et al. IPVS statement on ”Temporary HPV vaccine shortage: implications globally to achieve equity”. Papillomavirus Res. 2020 Jun;9:100195.
- Bruni L, Saura-Lázaro A, Montoliu A, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev Med. 2021;144:106399.
- Enerly E, Berger S, Kjær SK, et al. Use of real-world data for HPV vaccine trial follow-up in the Nordic region. Contemp Clin Trials. 2020 May;92:105996.
- Guo F, Cofie LE, Berenson AB. Decreasing trends in cervical cancer incidence among young women (15-34 years) in the United States during the human papillomavirus (HPV) Vaccine Era. Cancer Epidemiol Biomarkers Prev. 2017;26(3):3.
- Guo F, Cofie LE, Berenson AB. Cervical Cancer Incidence in Young U.S. Females After Human Papillomavirus Vaccine Introduction. Am J Prev Med. 2018 Aug;55(2):197–204.
- Hidalgo-Tenorio C, Pasquau J, Omar-Mohamed M, et al. Effectiveness of the Quadrivalent HPV Vaccine in Preventing Anal ≥ HSILs in a Spanish Population of HIV+ MSM Aged > 26 Years. Viruses. 2021 Jan 20;13(2):2.
- Dehlendorff C, Baandrup L, Kjaer SK. Real-world effectiveness of Human Papillomavirus vaccination against vulvovaginal high-grade precancerous lesions and cancers. JNCI. 2021;113(7):869–874.
- Goldenberg M.Public trust in vaccines. 34th International Papillomavirus Conference; 2021 November
- London School of Hygiene and Tropical Medicine. Vaccine Confidence Project Accessed 5 January 2021 . Available from: https://www.vaccineconfidence.org/the-confidence-project
- WHO/UNICEF. Estimates of national immunization coverage analytics 2021 Accessed 21 March 2022. Available from: https://data.unicef.org/resources/immunization-coverage-estimates-data-visualization
- gov.uk. Off-label vaccines - An introductory guide for healthcare professionals 2019 Accessed 21 March 2022. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/832917/Off_label_cold-chain_healthcare_professionals.pdf
- Bornstein J, Roux S, Kjeld Petersen L, et al. Three-Year Follow-up of 2-Dose Versus 3-Dose HPV Vaccine. Pediatrics. 2021;147(1):1.
- El-Zein M, Richardson L, Franco EL. Cervical cancer screening of HPV vaccinated populations: cytology, molecular testing, both or none. J Clin Virol. 2016;76:S62–S68.
- Hall MT, Simms KT, Lew J-B, et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health. 2019;4(1):e19–e27.
- Stelzle D, Tanaka LF, Lee KK, et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob Health. 2021 Feb;9(2):e161–e169.
- Rohner E, Bütikofer L, Schmidlin K, et al. Cervical cancer risk in women living with HIV across four continents: a multicohort study. Int J Cancer. 2020 Feb 1;146(3):601–609.
- Nishimura H, Yeh PT, Oguntade H, et al. HPV self-sampling for cervical cancer screening: a systematic review of values and preferences. BMJ Glob Health. 2021;6(5):e003743.
- Nelson EJ, Maynard BR, Loux T, et al. The acceptability of self-sampled screening for HPV DNA: a systematic review and meta-analysis. Sex Transm Infect. 2017;93(1):56–61.
- Aarnio R, Isacson I, Sanner K, et al. Comparison of vaginal self‐sampling and cervical sampling by medical professionals for the detection of HPV and CIN2+: a randomized study. Int J Cancer. 2021;148(12):3051–3059.
- De Pauw H, Donders G, Weyers S, et al. Cervical cancer screening using HPV tests on self-samples: attitudes and preferences of women participating in the VALHUDES study. Arch Public Health. 2021;79(1):1–9.
- Hashim D, Engesæter B, Baadstrand Skare G, et al. Real-world data on cervical cancer risk stratification by cytology and HPV genotype to inform the management of HPV-positive women in routine cervical screening. Br J Cancer. 2020;122(11):1715–1723.
- Machalek DA, Roberts JM, Garland SM, et al. Routine cervical screening by primary HPV testing: early findings in the renewed National Cervical Screening Program. Med j Aust. 2019;211(3):113–119.
- Cook DA, Krajden M, Brentnall AR, et al. Evaluation of a validated methylation triage signature for human papillomavirus positive women in the HPV FOCAL cervical cancer screening trial. Int J Cancer. 2019;144(10):2587–2595.
- Dick S, Kremer WW, De Strooper LM, et al. Long-term CIN3+ risk of HPV positive women after triage with FAM19A4/miR124-2 methylation analysis. Gynecol Oncol. 2019;154(2):368–373.
- Vink F, Lissenberg-Witte BI, Meijer C, et al. FAM19A4/miR124-2 methylation analysis as a triage test for HPV-positive women: cross-sectional and longitudinal data from a Dutch screening cohort. Clin Microbiol Infect. 2021;27(1):125.e1–125.
- Torres-Ibarra L, Cuzick J, Lorincz AT, et al. Comparison of HPV-16 and HPV-18 genotyping and cytological testing as triage testing within human papillomavirus–based screening in Mexico. JAMA network open. 2019;2(11):e1915781–e1915781.
- Arbyn M, Rezhake R, Yuill S, et al. Triage of HPV-positive women in Norway using cytology, HPV16/18 genotyping and HPV persistence. Br J Cancer. 2020;122:1577–1579.
- Stoler MH, Baker E, Boyle S, et al. Approaches to triage optimization in HPV primary screening: extended genotyping and p16/Ki‐67 dual‐stained cytology—Retrospective insights from ATHENA. Int J Cancer. 2020;146(9):2599–2607.
- Smith MA, Sherrah M, Sultana F, et al. National experience in the first two years of primary human papillomavirus (HPV) cervical screening in an HPV vaccinated population in Australia: observational study .BMJ. 2022;376:e068582.
- Daniels V, Saxena K, Roberts C, et al. Impact of reduced human papillomavirus vaccination coverage rates due to COVID-19 in the United States: a model based analysis. Vaccine. 2021;39(20):2731–2735.
- Poljak M, Cuschieri K, Waheed DEN, et al. Impact of the COVID-19 pandemic on human papillomavirus-based testing services to support cervical cancer screening. Acta Dermatovenerol Alp Pannonica Adriat. 2021;30(1):21–26.