ABSTRACT
Introduction
Healthcare workers (HCWs) susceptible to hepatitis B represent an important public health concern. National and international guidelines recommend assessing the hepatitis B immune status of all HCWs and possibly vaccinating those found to be seronegative (non-responders). We conducted a meta-analysis to estimate the rate of hepatitis B sero-susceptibility among HCWs in Italy and to explore possible options for the management of non-responders.
Areas Covered
Nineteen studies, selected from scientific articles available in the Scopus, MEDLINE/PubMed and ISI Web of Knowledge databases between 1 January 2016 and 22 April 2022, were included. The prevalence of HBV-susceptible HCWs was 27.1% (95%CI = 23.2–31.7%). In a comparison by sex (males vs. females) the RR was 1.16 (95%CI = 1.03–1.31), and by full-cycle vaccination period (adolescence vs. infancy) the RR was 0.30 (95%CI = 0.25–0.37). Occupational health screenings for hepatitis B, with subsequent vaccination of non-responders, and exclusion of susceptible HCWs from high-risk settings have been common management strategies.
Expert opinion
It is highly probable that a proportion of the next generation of medical students and HCWs will not show circulating IgG on serologic evaluation. Therefore, more targeted efforts are needed to identify these individuals and actively immunize them.
1. Introduction
According to the U.S. Centers for Disease Control and Prevention (CDC) recommendations, healthcare workers (HCWs) should have presumptive evidence of immunity due to hepatitis B vaccination [Citation1]. In addition, an anti-HBsAg (anti-HBs) antibody titer ≥10 mIU/mL assessed by serologic testing is required to be considered protected. HCWs who are not seroprotected should receive another complete cycle of hepatitis B vaccine; if the anti-HBsAg titer remains <10 mIU/mL after two complete series, the subject is considered a ‘non-responder’ [Citation2].
These recommendations are essential for all HCWs, especially those working in contact with patient body fluids or in infectious disease wards. Nevertheless, there is good evidence of significant susceptibility to hepatitis B among HCWs. A 2021 study [Citation3] described a significant percentage of fully vaccinated Dutch HCWs susceptible to HBV (2%), linked to a decline in IgG levels after immunization.
Susceptible and/or non-responders HCWs pose a risk to both themselves and patients in hospitals and clinics, and therefore are a major public health concern. Many studies have reported the Incidence of sharps and needle-stick injuries and mucocutaneous exposure to blood among healthcare workers [Citation4–6], and thus the risk of infection is substantial.
Mass vaccination programs against hepatitis B have been incorporated into national immunization programs in more than 150 countries. Italy was the first industrialized country to adopt a universal hepatitis B vaccination strategy. The first hepatitis B vaccination strategy was introduced in 1981 for the immunization of hemodialysis patients and healthcare personnel; in 1983 the active offer of the anti-HBV vaccine was extended through targeted campaigns to vulnerable population groups [Citation7]. In 1991, vaccination was made mandatory and extended to the entire population through a universal ‘two-cohort’ vaccination strategy that included:
Routine vaccination of all newborns (three doses at 3, 5, 11 months of age);
Vaccination of 12-years-old children (three doses at 0,1,6 months);
HBsAg testing in all pregnant women to prevent perinatal infection;
vaccination of adults in high-risk groups [Citation7].
Vaccination provision for 12-year-olds continued for 12 years, only to be discontinued in 2003; it allowed, in 12 years, to get 24 cohorts of individuals immunologically protected from the risk of infection. This strategy was able to reduce the number of acute hepatitis cases already documented by data from the SEIEVA (Integrated Epidemiological System of Acute Viral Hepatitis) surveillance system [Citation8], particularly in the age groups targeted by the vaccine campaign; however, hepatitis B has not yet been eliminated.
The Italian Ministry of Health, in accordance with international guidelines [Citation2], recommends, in addition to universal vaccination, screening of HCWs by measuring anti-HBs in order to verify seroprotection. In fact, in subjects with a negative anti-HBs result (<10 mIU/mL), a booster dose of the vaccine is recommended followed, after 1 month, by an additional blood test to understand whether an immunological memory exists [Citation9]; moreover, since 2012, Medical School students are equated with healthcare professionals as they are exposed to a similar biological risk during training activities and therefore the same recommendations for HBV prophylaxis are valid [Citation10]. Nevertheless, there are no Italian national data on hepatitis B vaccination coverage and immunization status of HCWs.
To estimate the prevalence in Italy of HCWs fully vaccinated against HBV without circulating antibodies, we conducted a systematic review of relevant literature and a meta-analysis. Options suggested by these studies for the management of non-responders were also analyzed. We also included students and residents in the School of Medicine in the HCWs category, considering that the 2017–2019 Italian Plan for Vaccination Prevention equates these categories by biological risk [Citation10].
2. Body
2.1. Methods
The protocol of the systematic review was set up following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist [Citation11]. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under reference acknowledgment number anonymized. The population, intervention, comparison, and outcome (PICO) framework was used to formulate the review question. The resulting question was ‘prevalence and management of HCWs vaccinated against HBV in Italy without circulating anti-HBsAg .’
2.1.1. Search strategy, selection criteria and data extraction
Systematic searches were conducted in the Scopus, MEDLINE/PubMed, and ISI Web of Knowledge databases; records were ordered by best match. Research articles, clinical trials, and letters to the editor published between 1 January 2016 and 22 April 2022 were included in the search. The following terms were used for the search strategy: (healthcare worker* OR physician OR nurse OR resident OR student) AND (hepatitis B OR HBV) AND (Ital*). Studies in English or Italian were included. Abstracts without full-text, reviews and meta-analyses, original studies that did not report epidemiologic data (editorials, commentaries, etc.), studies in which susceptibility was evaluated by surveys or those in which only vaccination coverage was reported, all studies that focused on issues unrelated to the purpose of this review (vaccine hesitancy, vaccine knowledge, attitudes, etc.), and all studies not set in Italy were excluded. When necessary, authors of the eligible studies were contacted to obtain additional information. The references of all articles were reviewed to identify further studies. The list of papers was screened by title and/or abstract independently by two reviewers who applied the predefined inclusion/exclusion criteria. Discrepancies were recorded and resolved by consensus.
Data extracted included year, sample size, number of susceptible HCWs, number of non-responders, number of seroconversion after booster dose, professional category, Italian region, and options for management of susceptible HCWs.
2.1.2. Quality assessment
The methodological quality of the selected studies was assessed using the Newcastle–Ottawa Scale (NOS), adapted for the assessment of cross-sectional studies [Citation12]. It is divided into nine categories controlling for three aspects of quality (selection, comparability and outcome/exposure), and scores range from 0 to 10. The quality of a study was considered high if the NOS score was between 7 and 10, intermediate if the NOS score was between 4 and 6, and low if it was between 0 and 3.
The risk of bias for each study was independently assessed by two researchers. Discrepancies were recorded and resolved by consensus.
2.1.3. Pooled analysis
Five different meta-analysis groups were performed: the first included all HCWs, the second compared HCWs and Medical School students/residents, the third estimated the rate of seroconversion after booster dose, the fourth compared susceptibility by sex (males vs. females), and the fifth compared susceptibility by age at the time of full-cycle vaccination (adolescence/adulthood vs. infancy). For comparisons by sex and age, Risk Ratio (RR) and 95% confidence interval (95%CI) were calculated. In addition, a separate analysis was carried out using only high-quality papers, when possible.
The pooled proportion in the meta-analysis was calculated using the double Freeman-Tukey arcsine transformation to stabilize variances and DerSimonian-Laird weights for random effects-models, with the estimated heterogeneity obtained from the inverse-variance fixed-effects model. The pooled prevalence and associated 95% Wald confidence interval were plotted, and a forest plot was drawn. The I2 statistic was calculated as a measure of the proportion of the overall variance attributable to heterogeneity between studies rather than chance. Heterogeneity between studies from different groups was also assessed. A value of p < 0.05 was considered an index of statistical significance of heterogeneity.
Funnel plots were used to assess publication bias. A distribution of studies with a symmetrical funnel shape indicated no significant bias, while an asymmetrical funnel indicated publication bias. Egger’s test for small-study effects was also performed.
To evaluate stability, a sensitivity analysis was conducted, in which among the studies included in this systematic review, one study was excluded at a time and conclusions based on the others were reevaluated to avoid severe bias.
Statistical analysis was conducted using STATA MP17 and Review Manager 5.4.1 software.
Strategies to promote vaccination among susceptible HCWs and characteristics of serosusceptible HCWs were collected from all available studies and the respective findings were compared, with special attention to the evidence presented in several included papers.
3. Results
3.1. Identification of relevant studies
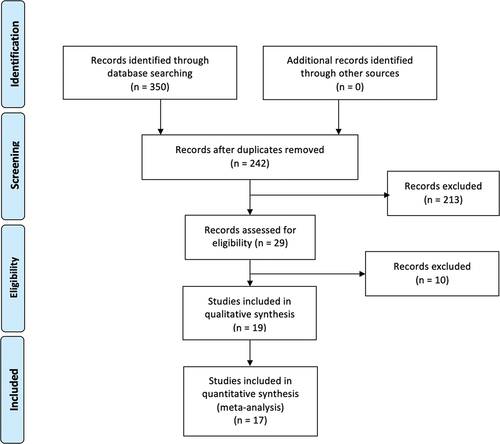
The flow-chart, constructed following the PRISMA guide [Citation12] (), shows the process of article selection.
According to the aforementioned inclusion criteria, 24 articles were identified in ISI Web of Knowledge, 18 in Scopus, and 29 in MEDLINE/PubMed. After exclusion of duplicate articles in the two databases, 29 eligible studies were identified [Citation13–41]. Of these, 6 were excluded because they evaluated the same phenomenon in more recent and comprehensive articles already included in the review [Citation32–37], and 4 because they did not fulfill the inclusion criteria [Citation38–41]. Thus, a total of 19 studies were found to be eligible [Citation13–31] (). The remaining 213 studies did not meet the inclusion criteria.
Table 1. Characteristics of the selected studies included in meta-analysis and systematic review.
3.2. Quality assessment
The NOS was applied appropriately to the included studies, and 89.5% were determined to be of high quality (). The impact of study quality was assessed in a sub-analysis.
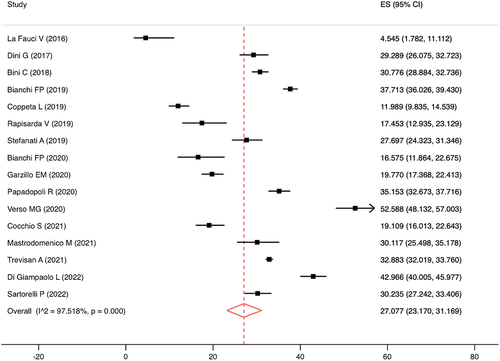
3.3. Pooled analysis. Meta-analysis showed that the pooled prevalence of fully vaccinated subjects without circulating anti-HBsAg, estimated from 24.653 Italian HCWs, was 27.1% (95%CI = 23.2–31.7%), in accordance with an I2 of 97.5% and a p-value for the heterogeneity test of <0.0001 (). Based only on high-quality articles, the pooled prevalence was 29.4% (95%CI = 25.4–33.6%; I2 = 97.6; p < 0.0001).
A sub analysis by professional category was performed, which showed that the pooled prevalence was higher among Medical School students/residents (33.7%; 95%CI = 30.8–37.3%; I2 = 95.2; p < 0.0001), compared with HCWs (15.5%; 95%CI = 10.9–20.7%; I2 = 83.7; p < 0.0001), in agreement with a p-value for heterogeneity between sub-groups of <0.0001. Sensitivity analysis by quality showed no severe distortion.
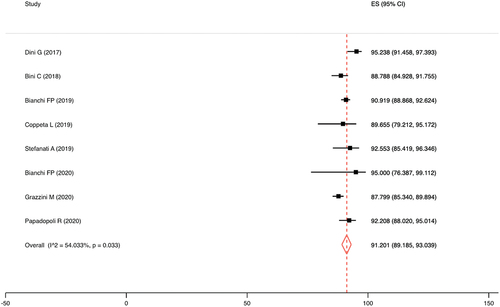
The seroconversion rate after the HBV booster dose was 91.2% (95%CI = 89.2–93.0%), in concordance with an I2 of 54.0% and a p-value for the heterogeneity test of 0.033 ().
Comparing hepatitis B serosusceptibility between male and female HCWs, the RR was 1.09 (95%CI = 0.95–1.25; I2 = 88.0%; p < 0.001). Considering only high-quality articles, the RR was 1.16 (95%CI = 1.03–1.31; I2 = 83.0%; p < 0.001).
Comparing hepatitis B serosusceptibility between the time of full cycle vaccination (adolescence/adulthood vs. infancy), the RR was 0.30 (95%CI = 0.25–0.37; I2 = 86.0%; p < 0.001). Sub-analysis by quality was not performed, as all included studies were rated as high-quality.
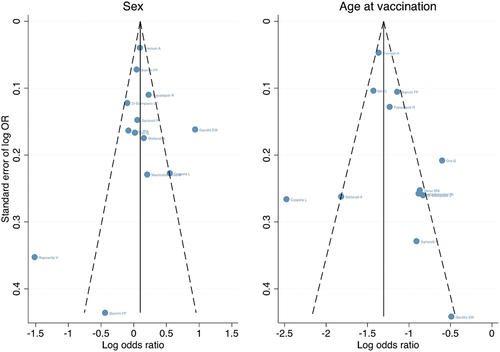
Sensitivity analysis did not reveal severe distortion by any specific study. In the publication bias analysis, there was no obvious asymmetry in the funnel plots and no strong evidence of publication bias (). The p-value of Egger’s test was 0.825 for the sex-based sub-analysis and 0.330 for the age-based sub-analysis.
3.4. Suggestions and procedures for the management of non-responders
Most of the included studies [Citation13,Citation14,Citation16,Citation17,Citation20,Citation22–24,Citation26–28] reported a higher proportion of serosusceptible HCWs among those vaccinated in infancy compared with those vaccinated during adolescence or adulthood; explanations for this evidence were the different maturity of the immune system between the two groups and/or the type of vaccine used in adolescents and adults. Nevertheless, those vaccinated in infancy were more frequently negative for anti-HBs at first follow-up, but they more often showed a booster effect after the ‘challenge’ dose [Citation27]. Otherwise, no significant differences were found between subjects vaccinated >20 years and those vaccinated <20 years [Citation14,Citation16].
Regarding sex, several studies reported no difference [Citation13–16,Citation23,Citation26,Citation28,Citation29], while four papers [Citation17,Citation19,Citation20,Citation24] observed a significant difference in serosusceptibility in males compared with females. Nurses appear to be more seroprotected, probably because of more direct and frequent contact with patients and their body fluids than other health professionals [Citation19].
Several studies have reported that immune memory remains intact for at least 15–20 years after the primary vaccination series [Citation16,Citation20,Citation23,Citation24,Citation26,Citation27]. The response to the booster dose appears to be related to baseline levels of anti-HBs; a booster dose of HBV vaccine may be insufficient to induce an immunologic response in a substantial proportion of subjects who have received a primary HBV vaccination but have undetectable anti-HBs titers [Citation21,Citation22,Citation26]. Indeed, a suggested precautionary measure might be to introduce a booster dose before the anti-HBs titer vanishes and becomes undetectable [Citation21]. Cocchio S et al. [Citation15] reported good persistence of protective anti-HBs titers in HCWs at occupational risk of HBV for up to 30 years if their initial titer after the primary vaccination cycle was greater than 100 mIU/mL. Anti-HBs titers appear to have different kinetics in boosted and non-boosted subjects, with a rapid decay among boosted subjects; however, a subject who seroconverts after the booster dose but loses circulating antibodies at a subsequent serologic test should be considered immune to hepatitis B, because the persistence of cellular memory has already been demonstrated [Citation22].
Ten studies described the management of non-responders with a booster dose of the vaccine [Citation18,Citation20–28], seroconversion with values >90% was reported in nine of them [Citation18,Citation20,Citation21,Citation24–28]. Four studies described the management of HCWs still seronegative after the fourth dose [Citation18,Citation20,Citation21,Citation23]; Grazzini M et al. [Citation20] described the use of a fifth dose followed by serologic evaluation; if still negative, a sixth dose was proposed. This management showed a high rate of seroconversion but low adherence by health personnel. To face this issue, the authors planned, in addition to counseling activities, to promote HBV re-vaccination in non-seroprotected HCWs and students by distributing pamphlets and publishing posters in order to improve awareness among HCWs [Citation20]. In three studies [Citation18,Citation21,Citation23], the vaccination protocol consisted of two doses of hepatitis B vaccine administered 1 and 6 months after the booster dose, followed 28 days later by a second blood test to recheck IgG titers. If the value determined in the reevaluation exceeded the cutoff, the HCW was classified as seroconverted; if the titer was still negative, the subject was considered a ‘non-responder,’ with reassessment for HBV infection advocated in all exposure events, with immunoglobulin administration when needed. Two papers reported that the screening described above was on voluntary basis and immunization was not compulsory, with no consequences in terms of suitability for work in case of refusal [Citation21,Citation23]; at the end of screening, the Occupational Health physician of the Bari Policlinico University-General Hospital planned job alternatives for each worker based on susceptibility status. For nonimmune employees who declined the vaccine(s), a ban on working in occupational sites with patients at high infectious risk was endorsed [Citation18]. This immunization procedure [Citation18,Citation23] reported great vaccination aptitude among susceptible health personnel and a seroconversion rate >80% after booster doses. No severe adverse events were was recorded after vaccination.
All authors determined that screening of health personnel is indispensable to avoid nosocomial infections and that promotion of an appropriate vaccination protocol should be a priority of Occupational Medicine Departments. Convincing communication strategies should be planned to educate all seronegative health workers to immunize [Citation20,Citation30]; in fact, interventions to overcome misconceptions and mistrust of vaccine prophylaxis are indicated as indispensable factors for health personnel compliance with vaccination [Citation20]. Lastly, two studies advocated compulsory immunization for both health personnel and Medical School students/residents [Citation18,Citation31].
4. Discussion
Our meta-analysis estimated a susceptibility rate for hepatitis B among fully vaccinated HCWs in Italy of 27% (95%CI = 23–32%); considering other vaccine-preventable diseases, this value is higher than that reported for Italian (9%; 95%CI = 6–13%) and European (13%; 95%CI = 10–17%) HCWs for measles [Citation42,Citation43]. However, more than 91% (95%CI = 89–93%) of subjects responded to the booster dose, demonstrating the persistence of cellular immune memory. This memory lasts at least 15–20 years, as confirmed for other vaccine-preventable diseases, such as measles [Citation42,Citation44], rubella [Citation45], mumps [Citation46], varicella [Citation47,Citation48] and pertussis [Citation49,Citation50]. Less than 10% of HCWs who did not respond to the booster dose should represent individuals who did not develop immunity after the full course of vaccination; in these cases, it seems to be appropriate to complete a second full course of vaccination, which appears to be able to seroconvert 80–90% of the non-responders to the primary full cycle [Citation18,Citation21,Citation23].
To our knowledge, this is the first study to find that male HCWs were less likely than female HCWs to have circulating anti-HBs IgG, through estimating a Risk Ratio (RR = 1.16; 95%CI = 1.03–1.31). Sex differences in response to vaccination or infection have been examined in several studies [Citation43,Citation51–54], but our analysis is the first to demonstrate these differences for hepatitis B vaccination. Females generally have more effective immune responses after immunization and against infection, with immunologic, hormonal, genetic, microbiotic, and environmental factors possibly contributing to the difference between males and females.
Considering the age of vaccination, our systematic review showed that subjects vaccinated during adolescence and adulthood reported a higher prevalence of circulating antibodies than those vaccinated in infancy (regardless of the time elapsed from the last vaccine dose to antibody evaluation); this evidence has been confirmed by our meta-analysis, which estimated a RR of 0.30 (95%CI = 0.25–0.37). In fact, the infant immune system is considered immature, with a restricted immunoglobulin repertoire that exhibits low-affinity antibody responses and impaired T-cell function with poor B-cell and T-cell interaction. The Th2/regulatory T cell-type response and reduced somatic hypermutation of B cells, which predominate in early infancy, result in reduced immune tolerance and humoral response, which transitions to a Th1-type response and progressive maturation of immunoglobulin class switching and related responses during the first year of life [Citation16]. In addition, the administration of more immunogenic vaccines in adolescence/adulthood than in childhood may be another explanation [Citation17]. Moreover, our sub-group analysis per professional category revealed more than twice the prevalence of subjects without circulating antibodies among students/residents compared with HCWs; actually, professional category is a proxy for the real risk factor, that is, the age of vaccination. Indeed, most HCWs have been vaccinated during adolescence/adulthood, while most students/residents during infancy.
Few studies have described the management of susceptible HCWs, but the protocol developed by Bianchi FP et al. [Citation18,Citation23] and Papadopoli R et al. [Citation21] has demonstrated high efficacy and safety. However, the cost-effectiveness of this protocol needs to be evaluated. As reported in the literature, several cases of sharps and needle-stick injuries have been reported among HCWs and medical students [Citation4,Citation5], and a 2020 study [Citation55] asserted that anti-HBs testing possibly followed by vaccination might be more cost-effective than post-exposure management for Hepatitis B. Consider also that new technologies are being developed to evaluate cellular immunity memory, but their use in clinical practice is still far off [Citation56]. Further studies are needed to clarify this point.
The main limitation of this study was the large heterogeneity between papers, as suggested by the I2 values; yet, the use of random-effects analysis mitigated the bias. However, a strength of our paper was the considerable sample size as a result of the assortment of selected studies, which enhanced the statistical analysis by providing a clearer interpretation of HBV immunity status among Italian health personnel. Furthermore, considering that numerous papers have examined a recent cohort of HCWs, this analysis is up-to-date and consistent. Lastly, sub-analyses by sex and age at vaccination provided data, including RR value, not previously described in the scientific literature. In the future, similar meta-analyses should embrace more papers to conduct sub-analyses by age, occupation, and geographic area.
5. Conclusion
The various evidence emerging from this review, confirmed by recent literature, underlines that unimmunized and unresponsive health workers pose a serious public health concern . Therefore, national and international public health organizations should encourage the drafting of advanced policies to address HBV risk, particularly in high-risk nosocomial sites. Efforts to educate health personnel and medical students need to be fortified [Citation57], as they have so far proven to be insufficient to bridge the immunization gap. The most recent proposal in the literature is compulsory vaccination of health personnel [Citation18,Citation31], in order to decrease the risk of nosocomial transmission to patients and the workers themselves. Three Italian regions have ratified a detailed law making immunization semi-compulsory for health personnel; this law is centered on fitness for work evaluated by occupational health physicians [Citation43], similar to the procedure described by Bianchi FP et al. [Citation23].
A fundamental issue is the management of HCWs vaccinated during infancy; in fact, currently and increasingly in the future, students and health workers born after 1992 are beginning to attend wards and caring for patients. Given the increased likelihood of finding negative serology, the question that public health institutions should ask themselves is to understand and decide whether it is appropriate to periodically administer a booster dose of the vaccine (along the lines of the tetanus vaccine booster) or to promote the screening described above by setting the seroprotection cutoff at 10 mIU/ml as recommended by the CDC [Citation58]. Indeed, this strategy should allow a high level of antibody titers to be maintained over time and thus keep a nosocomial setting safe. Cost-effectiveness analyses and evaluations of health aspects are needed to answer this question.
Article highlights
Good evidence of significant susceptibility to hepatitis B among HCWs is reported in the scientific literature
Italy was the first industrialized country to adopt a universal vaccination strategy against hepatitis B
Our meta-analysis estimated a hepatitis B susceptibility rate among fully vaccinated HCWs in Italy of 27%
more than 91% (95%CI=89-93%) of subjects responded to the booster dose
Unimmunized and unresponsive HCWs are a real public health concern
Future HCWs vaccinated at a young age will probably not show circulating antibodies
A booster dose administered periodically or promotion of the screening described above seems necessary
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or mending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
FPB and ST conceived the study. FPB and AM did the literature research. FPB did the metanalysis. GM and PS participated in the design of the metanalysis. CAG and LV supervisioned the metanalysis. FPB and ST codrafted the first version of the article.
Additional information
Funding
References
- CDC. Hepatitis B vaccination: information for healthcare providers. Available on: https://www.cdc.gov/vaccines/vpd/hepb/hcp/index.html. Last accessed on 2022 Apr 10.
- Advisory Committee on Immunization Practices; Centers for Disease Control and Prevention (CDC). Immunization of health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011 Nov 25;60(RR–7):1–45.
- van Leeuwen LPM, Doornekamp L, Goeijenbier S, et al. Evaluation of the hepatitis b vaccination programme in medical students in a Dutch University hospital. Vaccines (Basel). 2021 Jan 20; 9(2):69.
- Rapisarda V, Loreto C, Vitale E, et al. Incidence of sharp and needle-stick injuries and mucocutaneous blood exposure among healthcare workers. Future Microbiol. 2019 Jun;14:27–31.
- Veronesi L, Giudice L, Agodi A, et al. A multicentre study on epidemiology and prevention of needle stick injuries among students of nursing schools. Ann Ig. 2018 Sep-Oct;30(5 Supple 2):99–110.
- Mengistu DA, Tolera ST, Demmu YM. Worldwide prevalence of occupational exposure to needle stick injury among healthcare workers: a systematic review and meta-Analysis. Can J Infect Dis Med Microbiol. 2021;2021:9019534. Jan 29.
- Romanò L, Mele A, Pariani E, et al. Update in the universal vaccination against hepatitis B in Italy: 12 years after its implementation. Eur J Public Health. 2004;14(Suppl):S19.
- Istituto Superiore di Sanità. Epidemiology - SEIEVA data. Last accessed on 2022 Apr 21. Available on: https://www.epicentro.iss.it/en/hepatitis/data-seieva.
- Italian Ministry of Health. National Vaccine Prevention Plan 2017-2019.Last accessed on 2022 Apr 18 Available on: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf.
- Italian Ministry of Health. National Vaccine Prevention Plan 2012-2014. Last accessed on 2022 Apr 18. Available on: https://www.salute.gov.it/imgs/C_17_pubblicazioni_1721_allegato.pdf.
- von Elm E, Altman DG, Egger M, et al. STROBE iniziative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 Mar 29; 372:n71
- Di Giampaolo L, Costantini E, Di Nicola M, et al. Titer of anti-HBs in health professions trainees: prevalence of antibody coverage in a University of Central Italy. Hum Vaccin Immunother. 2022 Dec 31; 18(1):1886805.
- Sartorelli P, Occhialini F, Miceli R, et al. The seroprevalence of the hepatitis B virus in Italian medical students after 3 decades since the introduction of universal vaccination. Int J Occup Med Environ Health. 2022 Feb 15; 35(1):75–80.
- Cocchio S, Baldo V, Volpin A, et al. Persistence of anti-Hbs after up to 30 years in health care workers vaccinated against hepatitis B virus. Vaccines (Basel). 2021 Apr 1;9(4):323.
- Mastrodomenico M, Muselli M, Provvidenti L, et al. Long-term immune protection against HBV: associated factors and determinants. Hum Vaccin Immunother. 2021 Jul 3; 17(7):2268–2272.
- Trevisan A, Mason P, Nicolli A, et al. Future healthcare workers and hepatitis B vaccination: a new generation. Int J Environ Res Public Health. 2021 Jul 22;18(15):7783.
- Bianchi FP, Vimercati L, Mansi F, et al. Compliance with immunization and a biological risk assessment of health care workers as part of an occupational health surveillance program: the experience of a university hospital in southern Italy. Am J Infect Control. 2020 Apr;48(4):368–374.
- Garzillo EM, Arnese A, Coppola N, et al. HBV vaccination status among healthcare workers: a cross-sectional study. J Infect Prev. 2020 Jan;21(1):23–27.
- Grazzini M, Arcangeli G, Mucci N, et al. High chance to overcome the non-responder status to hepatitis B vaccine after a further full vaccination course: results from the extended study on healthcare students and workers in Florence, Italy. Hum Vaccin Immunother. 2020 Apr 2;16(4):949–954.
- Papadopoli R, De Sarro C, Torti C, et al. Is there any opportunity to provide an HBV vaccine booster dose before anti-HBS titer vanishes? Vaccines (Basel). 2020 May 16; 8(2):227.
- Verso MG, Costantino C, Marrella A, et al. Kinetics of anti-Hepatitis B surface antigen titers in nurse students after a two-year follow-up. Vaccines (Basel). 2020 Aug 21; 8(3):467.
- Bianchi FP, Gallone MS, Gallone MF, et al. HBV seroprevalence after 25 years of universal mass vaccination and management of non-responders to the anti-Hepatitis B vaccine: an Italian study among medical students. J Viral Hepat. 2019 Jan;26(1):136–144.
- Coppeta L, Pompei A, Balbi O, et al. Persistence of immunity for hepatitis B virus among heathcare workers and Italian medical students 20 years after vaccination. Int J Environ Res Public Health. 2019 Apr 29; 16(9):1515.
- Rapisarda V, Nunnari G, Senia P, et al. Hepatitis B vaccination coverage among medical residents from Catania University hospital, Italy. Future Microbiol. 2019 Jun;14:41–44.
- Stefanati A, Bolognesi N, Sandri F, et al. Long-term persistency of hepatitis B immunity: an observational cross-sectional study on medical students and resident doctors. J Prev Med Hyg. 2019 Sep 30; 60(3):E184–E190.
- Bini C, Grazzini M, Chellini M, et al. Is hepatitis B vaccination performed at infant and adolescent age able to provide long-term immunological memory? An observational study on healthcare students and workers in Florence, Italy. Hum Vaccin Immunother. 2018 Feb 1; 14(2):450–455.
- Dini G, Toletone A, Barberis I, et al. Persistence of protective anti-HBs antibody levels and anamnestic response to HBV booster vaccination: a cross-sectional study among healthcare students 20 years following the universal immunization campaign in Italy. Hum Vaccin Immunother. 2017 Feb;13(2):440–444.
- La Fauci V, Riso R, Facciolà A, et al. Response to anti-HBV vaccine and 10-year follow-up of antibody levels in healthcare workers. Public Health. 2016 Oct;139:198–202.
- Bechini A, Bonanni P, Grazzini M, et al. Need to take special care of non-responders to hepatitis B vaccination among health-care workers, students and chronic patients. Hum Vaccin Immunother. 2021 Feb 1; 17(2):580–582.
- Trevisan A. Long-term persistence of immunity after hepatitis B vaccination: a fact, not a fancy. Hum Vaccin Immunother. 2017 Apr 3; 13(4):916–917.
- Trevisan A, Giuliani A, Scapellato ML, et al. Sex disparity in response to hepatitis B vaccine related to the age of vaccination. Int J Environ Res Public Health. 2020 Jan 2; 17(1):327.
- Verso MG, Lo Cascio N, Noto Laddeca E, et al. Predictors of Hepatitis B Surface Antigen Titers two decades after vaccination in a cohort of students and post-graduates of the Medical School at the University of Palermo, Italy. Ann Agric Environ Med. 2017 Jun 12; 24(2):303–306.
- Pileggi C, Papadopoli R, Bianco A, et al. Hepatitis B vaccine and the need for a booster dose after primary vaccination. Vaccine. 2017 Nov 1; 35(46):6302–6307.
- Trevisan A, Frasson C, De Nuzzo D, et al. Significance of anti-HB levels below 10 IU/L after vaccination against hepatitis B in infancy or adolescence: an update in relation to sex. Hum Vaccin Immunother. 2020;16(2):460–464.
- Verso MG, Costantino C, Vitale F, et al. Immunization against hepatitis B surface antigen (HBsAg) in a cohort of nursing students two decades after vaccination: surprising feedback. Vaccines (Basel). 2019 Dec 19; 8(1):1.
- Stefanati A, Brosio F, Kuhdari P, et al. Studio di incidenza sugli infortuni biologici nei medici in formazione specialistica dell’Azienda Ospedaliero - Universitaria di Ferrara e stato immunitario nei confronti delle principali infezioni prevenibili [Incidence of biological accidents at work and immune status for vaccine-preventable diseases among resident physicians in specialist training at Ferrara University Hospital]. Ig Sanita Pubbl. 2017 Nov-Dec;73(6):633–648. Italian.
- Ledda C, Rapisarda V, Maltezou HC, et al. Coverage rates against vaccine-preventable diseases among healthcare workers in Sicily (Italy). Eur J Public Health. 2021 Feb 1; 31(1):56.
- Lamberti M, De Rosa A, Garzillo EM, et al. Erratum to: vaccination against hepatitis b virus: are Italian medical students sufficiently protected after the public vaccination programme? J Occup Med Toxicol. 2016Feb;15;11:3.
- Campagna M, Argiolas F, Soggiu B, et al. Current preventive policies and practices against vaccine-Preventable diseases and tuberculosis targeted for workers from hospitals of the Sardinia Region, Italy. J Prev Med Hyg. 2016;57(2):E69–74.
- Coppeta L, D’Alessandro I, Pietroiusti A, et al. Seroprevalence for vaccine-preventable diseases among Italian healthcare workers. Hum Vaccin Immunother. 2021 May 4; 17(5):1342–1346.
- Bianchi FP, Mascipinto S, Stefanizzi P, et al. Prevalence and management of measles susceptibility in healthcare workers in Italy: a systematic review and meta-analysis. Expert Rev Vaccines. 2020 Jul;19(7):611–620.
- Bianchi FP, Stefanizzi P, Trerotoli P, et al. Sex and age as determinants of the seroprevalence of anti-measles IgG among European healthcare workers: a systematic review and meta-analysis. Vaccine. 2022 May 20; 40(23):3127–3141.
- Bianchi FP, Mascipinto S, Stefanizzi P, et al. Long-term immunogenicity after measles vaccine vs. wild infection: an Italian retrospective cohort study. Hum Vaccin Immunother. 2021 Jul 3; 17(7):2078–2084.
- Bianchi FP, De Nitto S, Stefanizzi P, et al. Immunity to rubella: an Italian retrospective cohort study. BMC Public Health. 2019 Nov 8; 19(1):1490.
- Bianchi FP, De Nitto S, Stefanizzi P, et al. Long time persistence of antibodies against Mumps in fully MMR immunized young adults: an Italian retrospective cohort study. Hum Vaccin Immunother. 2020 Nov 1; 16(11):2649–2655.
- Bianchi FP, Tafuri S, Larocca AMV, et al. Long -term persistence of antibodies against varicella in fully immunized healthcare workers: an Italian retrospective cohort study. BMC Infect Dis. 2021 May 25; 21(1):475.
- Trevisan A, Nicolli A, De Nuzzo D, et al. Varicella seroepidemiology and immunization in a cohort of future healthcare workers in the pre-vaccination era. Int J Infect Dis. 2020 Jul;96:228–232.
- Versteegen P, Valente Pinto M, Barkoff AM, et al. Responses to an acellular pertussis booster vaccination in children, adolescents, and young and older adults: a collaborative study in Finland, the Netherlands, and the United Kingdom. EBioMedicine. 2021 Mar;[Epub 2021 Feb 26];65:103247.
- Ruggieri A, Anticoli S, D’Ambrosio A, et al. The influence of sex and gender on immunity, infection and vaccination. Ann Ist Super Sanita. 2016 Apr-Jun;52(2):198–204.
- Flanagan KL, Fink AL, Plebanski M, et al. Sex and Gender Differences in the Outcomes of Vaccination over the Life Course. Annu Rev Cell Dev Biol. 2017 Oct 6; 33:577–599
- Morris GP. Understanding sex-related differences in immune responses. Sci Transl Med. 2020 Jul;12(554):eabd3631.
- Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016 Oct;16(10):626–638.
- Ortona E, Pierdominici M, Rider VE. Sex Hormones and Gender Differences in Immune Responses. Front Immunol. 2019;10:1076.
- Souza CL, Salgado TA, Sardeiro TL, et al. Post-vaccination anti-HBs testing among healthcare workers: more economical than post-exposure management for Hepatitis B. Rev Lat Am Enfermagem. 2020 Jun 19; 28:e3278
- Cassaniti I, Calarota SA, Adzasehoun KM, et al. Memory T cells specific for HBV enumerated by a peptide-based cultured enzyme-linked immunospot assay in healthy HBV-vaccinated subjects. Hum Vaccin Immunother. 2016 Nov;12(11):2927–2933.
- Barbato D, De Lillo F, La Torre G, et al. Validation of a questionnaire about knowledge and perception of biological risk among biomedical students of Sapienza University of Rome. Clin Ter. 2019 Nov-Dec;170(6):e430–e434.
- Schillie S, Murphy TV, Sawyer M, et al. Centers for Disease Control and Prevention (CDC). CDC guidance for evaluating health-care personnel for hepatitis B virus protection and for administering postexposure management. MMWR Recomm Rep. 2013 Dec 20;62(RR–10):1–19.