ABSTRACT
Introduction
Vaccines are powerful tools for controlling microbial infections and preventing epidemics. To enhance the immune response to antigens, effective subunit vaccines or mRNA vaccines often require the combination of adjuvants or delivery carriers. In recent years, with the rapid development of immune mechanism research and nanotechnology, various studies based on the optimization of traditional adjuvants or various novel carriers have been intensified, and the construction of vaccine adjuvant delivery systems (VADS) with both adjuvant activity and antigen delivery has become more and more important in vaccine research.
Areas covered
This paper reviews the common types of vaccine adjuvant delivery carriers, classifies the VADS according to their basic carrier types, introduces the current research status and future development trend, and emphasizes the important role of VADS in novel vaccine research.
Expert opinion
As the number of vaccine types increases, conventional aluminum adjuvants show limitations in effectively stimulating cellular immune responses, limiting their use in therapeutic vaccines for intracellular infections or tumors. In contrast, the use of conventional adjuvants as VADS to carry immunostimulatory molecules or deliver antigens can greatly enhance the immune boosting effect of classical adjuvants. A comprehensive understanding of the various delivery vehicles will further facilitate the development of vaccine adjuvant research.
1. Introduction and background
Vaccines are preventive biological products used for human immunization to prevent and control the occurrence and prevalence of diseases. In recent years, vaccination has emerged as the most cost-effective means to protect against various pathogens and prevent the spread of infectious diseases. Vaccines have successfully eradicated or effectively prevented and controlled once-fatal diseases such as smallpox virus, poliovirus, human papillomavirus (HPV), and hepatitis B virus (HBV) [Citation1–4]. However, with the emergence of new or highly mutated pathogens such as human immunodeficiency virus (HIV), Ebola virus, influenza virus, coronavirus, monkeypox virus, etc., there is still an urgent need for more safe and effective vaccines with universal applicability to combat the threat of emerging infectious diseases [Citation5–9].
Conventional vaccines are typically made from inactivated or attenuated whole microorganisms that induce antigen-specific immune responses in the host, but there is a potential threat of strain transformation into virulent mutants. Recent rapid advances in immunology, cell biology, and bioengineering technologies have greatly advanced vaccine research, leading to the development of structurally designed subunit vaccines or mRNA vaccines with known sequences that can trigger an immune response while maintaining a good safety profile [Citation10,Citation11]. However, subunit vaccines tend to exhibit poor immunogenicity compared to conventional vaccines and require personalized adjuvants to achieve effective antigen delivery and enhance the level of immune response [Citation12]. Current mRNA vaccines, on the other hand, need to be delivered and expressed in vivo through the encapsulation of lipid nanoparticles (LNPs), which have been shown to have some adjuvant activity [Citation13].
Therefore, adjuvants have become an integral part of vaccine research. The concept of ‘Adjuvant’ was first introduced by Gaston Ramon in 1925 and is defined as ‘a mixture of one or more components used in combination with a specific vaccine antigen to accelerate, enhance, broaden, prolong, or modify the type of antigen-specific immune response’ [Citation14]. It was not until 1932 that Alum adjuvant was approved as the first human vaccine adjuvant for clinical use [Citation15]. However, it was not until 1994 that Virosome was used as the second human vaccine adjuvant for the hepatitis A vaccine (Epaxal®), and the development of adjuvants has been described as one of the slowest processes in medical history [Citation16]. Spanning 62 years from 1932 to 1994, no new adjuvant was used in human vaccines. However, with the increasing number of vaccine types and the discovery of small-molecule immunostimulants, the use and mechanism of action of vaccine adjuvants have gradually been investigated, leading to the broadening of the types of adjuvants () [Citation17]. In 1997, Cox and Coulter further defined adjuvants as ‘a series of substances or procedures that can enhance the immune response to vaccine antigens’ and considered that adjuvants are no longer limited to a certain component, but a series of rational designs that can effectively enhance the immune response to antigens [Citation18]. Accordingly, based on the functional properties of adjuvants, Guy proposed that they can be divided into three types: delivery vehicles or adjuvants with reservoir effects that enhance immune response by delivering antigen more efficiently to antigen-presenting cells (APCs) or lymph nodes; molecules with immune boosting or immunomodulatory properties; and adjuvants that combine both of these functions, i.e. delivery vehicles with one or more immunomodulatory molecules to form a specific vaccine adjuvant delivery system (VADS) [Citation19,Citation20].
Figure 1. Overview of the main adjuvant types used in licensed human vaccines. Note: The figure illustrates various adjuvants, including ssRNA (Single-Stranded Ribonucleic Acid), LPS (Lipopolysaccharide), O/W (oil-in-water) emulsions, AS (Adjuvant System; examples are AS01B, AS03, AS04), PRR (Pattern Recognition Receptor), TLR (Toll-like Receptor), and LNP (Lipid Nanoparticle).
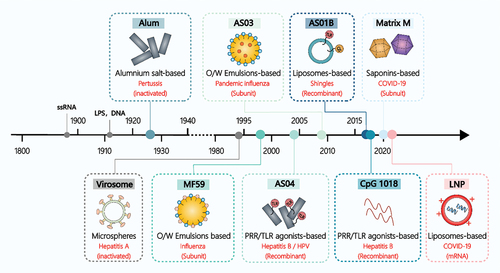
The concept of VADS originates from the Vaccine Delivery System (VDS), which plays a pivotal role in optimizing vaccine design strategies [Citation21]. VDS encompasses a variety of substances or delivery methods that transport attenuated, inactivated, or genetically modified pathogens, as well as their associated antigens, into the immune system. There, they persist for extended periods and maintain their antigenic effects. This is achieved through rational design methods, innovative delivery approaches, and the implementation of adjuvant strategies, aiming to bridge the efficacy gap between VDS and conventional vaccines. The application of delivery systems in vaccine design not only enhances the immune response by modifying antigen delivery and recruiting APCs but also prolongs immune stimulation by protecting antigens, influencing antigen localization, storage, and delayed release. Consequently, it reduces the number of immunizations, streamlines vaccination procedures, and enhances the overall effectiveness of vaccination [Citation22]. In other words, VADS possesses two fundamental functions – adjuvant activity and antigen delivery – which distinguish it from both Adjuvant Systems (AS) and Delivery Systems (DS). Consequently, VADS is regarded as a more encompassing concept in adjuvant design, amalgamating elements of adjuvant and delivery systems, while remaining an integral part of the vaccine delivery system, and playing a pivotal role in vaccine design. Therefore, the development of carriers with both delivery and adjuvant activities, along with the construction of VADS, emerges as a significant research strategy in the field of adjuvant research.
In summary, this review paper offers an overview of VADS composition, describes the application of different delivery carriers in vaccine adjuvants, and underscores the importance of constructing VADS. This includes considerations of immune response mechanisms, design features, safety, and stability. Furthermore, the paper discusses the development prospects of delivery vectors in vaccine adjuvant research and identifies key challenges that need to be addressed. The aim of this comprehensive reference is to contribute to the advancement of VADS research.
2. Composition and application of VADS
Vaccine formulations necessitate three critical components: an effective antigen, a potent adjuvant, and an efficient delivery system or vector capable of transporting these components to APCs, intracellular organelles, or appropriate sites such as lymph nodes (LNs) and mucosa [Citation23]. Consequently, during vaccine development, researchers investigate various delivery techniques to optimize antigen expression efficiency and enhance vaccine efficacy by designing novel adjuvants with optimized immune activation activity, such as immune responses elicited by subunit vaccines [Citation24].
For adjuvant design, there are three commonly employed strategies: The first and most conventional approach involves synthesizing the adjuvant and immunogen independently, either by adsorbing the immunogen and adjuvant molecules for direct administration or by encapsulating them in the same polymer as an encapsulating material, ensuring that both essential components are delivered together into the same APCs [Citation25]. Another approach utilizes the encapsulating material itself as an adjuvant, as seen in mRNA vaccines where ionizable lipids possess innate immunostimulatory activity as the encapsulating component of the lipid nanoparticle (LNP). This strategy, however, requires careful optimization to balance delivery efficiency and adjuvant activity, and extensive screening is needed to identify suitable encapsulation materials. Alternatively, a chimeric adjuvant can be designed by combining the adjuvant with the immunogen itself to maximize the immunostimulatory effect of both components. This approach can be directly applied to existing delivery systems or designed with unique adjuvant components based on the immunogen structure to enhance delivery efficiency.
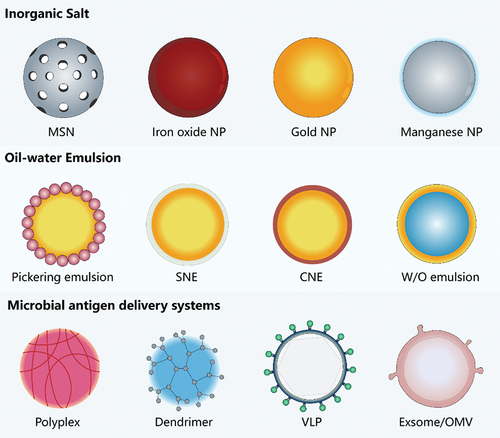
The importance of delivery carriers in adjuvant research is undeniable, as they can amplify the immune benefits of adjuvants, enable antigen-adjuvant co-delivery, or prolong antigen activity utilizing carrier properties. In summary, the construction and development of effective VADS with novel carriers possessing both immune stimulation and delivery functions form the foundation and future of successful vaccines [Citation26]. Several common adjuvant delivery carriers can be classified into three types: inorganic salts, oil-water emulsions, and microparticle antigen delivery systems ().
Table 1. Types of adjuvant carrier in clinical development.
2.1. Inorganic salt-based delivery carriers
2.1.1. Aluminum salt-based adjuvants
The term ‘Alum’ is often used to refer to all aluminum-containing adjuvants, and it is believed that the structural differences between various Alums allow them to exhibit distinct adjuvant activities. Consequently, by modifying their physicochemical properties, adding other immune enhancers, or combining them with different ions or compounds to form new aluminum-containing adjuvant systems, it is possible to stimulate the induction of stronger, faster, or more balanced Th1-type and Th2-type immune responses. Developing aluminum adjuvant-based delivery systems is a crucial strategy for advancing new vaccine adjuvants.
Conventional aluminum adjuvants consist of nanometer-sized particles as their basic unit, but in aqueous solutions, they tend to aggregate into micron-sized particles ranging from 1–20 μm [Citation32]. Li et al. compared the level of immunostimulation of Ovalbumin (OVA) antigen combined with micron- and nano-sized aluminum hydroxide adjuvants and discovered that nano-sized aluminum adjuvants induced stronger antigen-specific antibody responses and caused milder inflammatory responses, indicating a correlation between adjuvant activity and particle size [Citation33]. To induce a more robust Th1-like immune response, nanoscale aluminum adjuvants were further functionalized by combining them with immune enhancers or through surface modification. For example, the phosphophilicity of aluminum adjuvants, which attracts phosphate or phosphate group-containing molecules, was used to adsorb small molecule immune enhancers, such as monophosphorylated lipid A (MPL), cytosine-phosphate-guanine oligodeoxynucleotide (CpG ODN), and imidazoquinoline (IMDG) [Citation34–36]. Among them, the AS04 adjuvant system, consisting of aluminum hydroxide and Toll-like receptor 4 (TLR4) agonist MPL, has been successfully applied in HPV16/18 bivalent cervical cancer vaccine (Cervarix®) and HBV vaccine (Fendrix®), with higher antibody levels induced by HBV and HPV vaccines containing AS04 compared to those containing aluminum adjuvant alone [Citation37]. The combination of aluminum adjuvant with CpG1018 demonstrated better protection than either component alone in the SCB-2019 COVID-19 vaccine, which is now approved for emergency use in China [Citation38]. When carrying the agonist 3 M–052, a small molecule immunostimulant such as TLR7/8 agonist, a water-soluble nanosuspension (3 M–052-AF) formed with phospholipid DSPG as a co-lipid can be adsorbed by aluminum hydroxide through ligand exchange and is now being used in vaccine studies for HIV and novel coronaviruses [Citation39]. Additionally, molecular modification of the aluminum adjuvant surface is an effective strategy to enhance its activation level. For example, PEI-modified aluminum hydroxide nanoparticles can enhance antigen transport and promote cross-presentation of dendritic cells (DCs), and are used in tumor vaccine adjuvant studies [Citation40].
2.1.2. Other inorganic adjuvants and hybrid particles
In addition to traditional inorganic salt adjuvants based on aluminum, inorganic ions such as manganese, zinc, and calcium also exhibit adjuvant activity and can be used alone or in combination to enhance adjuvant efficiency [Citation41]. One approach involves partially replacing the anion of aluminum adjuvants to form a complex or heterogeneous aluminum adjuvant, where the newly added component directly modifies the aluminum adjuvant and mineralizes to form a precipitate. For example, Imject®, consisting of a combination of crystalline magnesium hydroxide (40 mg/mL) and amorphous aluminum hydroxide (40 mg/mL), is commonly employed as an aluminum-containing adjuvant in experimental or basic immunological studies [Citation42]. Layered double hydroxide (LDH) nanoparticles with a 1:1 Mg:Al ratio and an interlayer of nitrate (MgAl-NO3) have also been utilized as drug delivery vehicles, efficiently promoting dendritic cell (DC) activation and maturation as a delivery system for DNA vaccines [Citation43]. Another example is aluminum risediphosphonate adjuvant (FH002C), prepared using risedronate, which has been demonstrated to induce more effective humoral and cellular immune responses than traditional aluminum adjuvants, particularly in terms of cellular immune responses [Citation44]. In other words, using the ‘phosphophilic’ property of the aluminum adjuvant, organophosphonic acid molecules are mineralized to form stable organic-inorganic hybrid micro-nanoparticles. These are hybrid particles containing both organic and inorganic groups, and they enhance, accelerate, and regulate the strength, speed, and type of immune response.
Moreover, other inorganic salts like calcium phosphate, known for their good biocompatibility, have been considered as potential replacements for aluminum adjuvants and are used as adjuvants in Tdap vaccines in Europe. Manganese ion-based adjuvants have been found to exhibit nanosize and activate the cGAS-STING pathway, Which detects viral infections and triggers immune responses in vivo, and thus manganese ions, as a typical STING agonist leading to a more robust antitumor immune response compared to aluminum adjuvants [Citation45]. Additionally, a novel mRNA delivery system with high immunogenicity, termed IC8/Mn lipid nanoparticles (IC8/Mn LNPs), has been constructed by introducing manganese ions into the LNP delivery system. Manganese ions not only promote the maturation of APCs by activating the STING pathway but also enhance mRNA expression by facilitating lysosomal escape [Citation46]. Iron oxide nanoparticles (IONPs) are another class of adjuvants approved by the Food and Drug Administration (FDA) for clinical use [Citation47]. Certain IONPs, such as supramagnetic iron oxides ranging from 30–120 nm in size formed by lipid bilayers encapsulating magnetic iron oxide crystals (Fe3O4), and manganese ferrite nanoparticles encapsulated by citrate, have been shown to be effectively internalized by APCs and induce antigen-specific Th1 immune responses in mice, triggering pro-inflammatory cytokines against tuberculosis and malaria in preclinical settings [Citation48]. Notably, 100% of mice were protected from 4T1 triple-negative breast cancer metastasis 80 days after receiving two doses of a magnetosome vaccine (containing Fe3O4 core and CpG adjuvant) supplemented with PD-1 immune checkpoint blockade [Citation49]. Mesoporous silica nanomaterials (MSNs), characterized by high porosity, biocompatibility, and surface modifiability, have also been utilized as carrier systems for constructing adjuvant delivery systems, representing an ideal strategy for enhancing the effectiveness of tumor immunotherapy [Citation50].
2.2. Oil-water emulsion as delivery vehicles
Emulsions have been employed as vaccine adjuvants since the 1930s to enhance immune responses. In simple emulsions, hydrophilic active pharmaceutical ingredients (APIs) are encapsulated in a water-in-oil (W/O) emulsion, while hydrophobic APIs are encapsulated in an oil-in-water (O/W) emulsion. Emulsion carriers play a crucial role in protecting the encapsulated API and improving the solubility and bioavailability of the drug, which can potentially function as vaccine adjuvants [Citation51]. Complete Freund’s adjuvant (CFA) is considered the gold standard for immune enhancement, but its use in human vaccines is restricted due to the non-metabolizable mineral oil component that causes significant side effects [Citation52]. It was not until 1997 that the first O/W emulsion-based adjuvant, MF59, was approved as a human vaccine adjuvant for the Fluad® influenza vaccine [Citation53].
Currently, oil phases in O/W emulsions such as MF59, AS03, and AF03, all contain squalene. Squalene-containing emulsions, being fully metabolizable lipids in the human cholesterol synthesis pathway, have been found to facilitate solubilization, modified release, and cellular uptake of drugs, adjuvants, and vaccines, making them suitable for producing stable and nontoxic nano-emulsions (NE) [Citation54,Citation55]. Emulsions, as thermodynamically unstable systems, are typically formulated with stabilizers such as Tween 80, Span 80, etc [Citation56]. The immunostimulant D-L-α-tocopherol (vitamin-E) has been added to AS03 for GSK’s influenza A/H1N1 vaccine Pandemrix® [Citation57]. Notably, the mineral oil and mannitol monooleate family of surfactants present in the W/O emulsion MontanideTM have demonstrated good tolerability in humans and have been tested with varying success in AIDS, malaria, cancer, and influenza vaccines [Citation58]. Montanide ISA-51 combined with oligodeoxynucleotide CpG ODN has shown synergistic enhancement of the Th1 immune response and is widely used in many vaccines [Citation59].
From a formulation perspective, W/O emulsions require emulsification of the aqueous antigen solution with the oil phase, while O/W emulsions enable simple mixing of the aqueous antigen solution with a preformed emulsion. This allows for separate storage of the antigen and adjuvant, which is particularly relevant in pandemic influenza preparedness programs. The adjuvant can be stockpiled separately in large quantities and quickly applied to any emerging pandemic strain for the preparation of the corresponding vaccine. Although some studies have suggested that MF59 is less effective in physical interaction with antigens and may not possess antigen delivery capability [Citation60], it can still play an important role as an emulsion-based adjuvant. It has been widely used in human vaccines as a vital component of the vaccine adjuvant delivery system. Such as, a cationic nanoemulsion (CNE) delivery system, based on the introduction of MF59 adjuvant consisting of cationic lipid DOTAP, has been used to deliver mRNA for the development of influenza mRNA vaccines [Citation61]. Studies have shown that mRNA delivered using CNE is well tolerated and immunogenic in various animal models, making CNEs a promising alternative for relatively heat-resistant mRNA vaccine carriers. Xun Sun’s team modified MF59 adjuvant and successfully prepared a nano-aluminum emulsion adjuvant in combination with aluminum adjuvant to construct a new VADS (AlNEs) that adsorbs the model antigen ovalbumin (OVA) and immune enhancer CpG by a simple mixing step on the surface of AlNEs (referred to as AlNEs-OVA-CpG) [Citation62]. After subcutaneous injection, AlNEs-OVA-CpG effectively drains to lymph nodes, delivers both cargoes to lymph node-resident APCs, and escapes from lysosomes to the cytoplasm, thus enhancing antigen cross-presentation with promising prospective applications. Collectively, these studies suggest that emulsion-based adjuvant delivery systems, composed of emulsions with their own biocompatible properties, hold excellent potential for development.
2.3. Microparticle antigen delivery system
2.3.1. Liposome-based adjuvant delivery systems
Bangham et al. first observed phospholipid bilayers in water through electron microscopy in the early 1960s, which spontaneously assembled due to hydrophobic interactions [Citation63]. These spheres were later named ‘liposomes’ by Sessa and Weissmann in 1968 [Citation64]. In 1974, Gregoriadis et al. demonstrated the inherent adjuvant activity of liposomes, as they elicited an effective antibody immune response to encapsulated antigens, such as diphtheria toxoid, in mice following immunization [Citation65]. The advent of nanotechnology in recent years has allowed for the modification of immune enhancers and targeting molecules, enabling liposomes to more precisely target immune cells and even organelles. This results in lysosomal escape and enhanced antigen cross-presentation, significantly improving vaccine efficacy [Citation66]. For instance, liposomes can bind to functional molecules containing toll-like receptor agonists/ligands (TLRas), C-type lectin receptor agonists/ligands (CLRas), NOD-like receptor agonists/ligands (NLRas), saponin and squalene derivatives, or cationic lipids. In October 2017, the FDA approved the liposome-based adjuvant system AS01B for use in the herpes zoster vaccine Shingrix® for individuals aged 50 years and older [Citation67]. The AS01B adjuvant system consists of 50–100 nm DOPC/Cholesterol/MPLA liposomes in an aqueous suspension containing QS21 [Citation68,Citation69]. Immunostimulatory complexes (ISCOMs) are a unique liposome structure characterized by nano-sized, rigid cage-like particles with anionic properties [Citation70]. Antigen-free ISCOM particles are referred to as ISCOMATRIX [Citation71]. The composition of ISCOMs may help reduce saponin hemolysis toxicity while retaining Th1-type immune adjuvant activity, such as Matrix M adjuvant (a mixture of Matrix-A and Matrix-C) [Citation72].
In the case of AS01B, for example, the adjuvant is administered in a ready-to-use formulation, bypassing the process of antigen-adjuvant adsorption. Consequently, it may exhibit similarities to emulsions, which do not demonstrate antigen-presenting effects. However, this type of adjuvant has structural characteristics typical of liposomes, and optimization based on its formulation, such as the introduction of an aluminum adjuvant, enables it to retain its potent adjuvant properties while facilitating antigen adsorption [Citation73]. Liposomes also play a pivotal role in mRNA vaccines [Citation74]. The rapid spread of the COVID-19 pandemic significantly accelerated the real-world application of mRNA technology, leading to the development of various delivery vehicles based on liposomes, including lipid nanoparticles (LNPs). LNPs consist of four main components: cationic lipids, auxiliary phospholipids, PEGylated lipids, and cholesterol [Citation75]. Among these, ionizable lipids are a critical component of LNPs, as they give a positive charge to liposomes, allowing for spontaneous assembly with negatively charged mRNA to form LNPs under acidic conditions. Once inside cells, as the physiological pH increases, LNPs can electrostatically bind to endosomal membranes and facilitate cytoplasmic release [Citation76]. Currently, LNPs are pivotal to COVID-19 mRNA vaccines, safeguarding and transporting mRNA effectively into cells. Their successful application is evident in the two COVID-19 mRNA vaccines authorized by Pfizer/BioNTech and Moderna, which have shown significant disease prevention efficacy [Citation77]. These vaccines deliver mRNA encoding the SARS-CoV-2 spike protein into the host cell’s cytoplasm. The mRNA then translates into the spike protein, which acts as an antigen to mediate an immune response to the virus. The LNP composition of the two mRNA vaccines is remarkably similar, both containing an ionizable lipid – ALC-0315 (Pfizer) and SM-102 (Moderna) – which are positively charged at low pH (to complex the RNA) and neutral at physiological pH (to minimize potential toxic effects and facilitate payload release) [Citation74]. The lipids carry hydrocarbon chains linked by biodegradable ester groups, ensuring safe mRNA clearance post-delivery. The vaccines also include polyglycolylated lipids to minimize antibody binding of serum proteins and clearance by phagocytes, thus extending somatic circulation. Furthermore, distearoyl phosphatidylcholine (DSPC) and cholesterol contribute to the packaging of mRNA into the carrier and enhance liposome stability [Citation78]. Therefore, LNPs can encapsulate and deliver therapeutic drugs to specific body sites and release their contents at desired intervals, offering a valuable platform for treating various diseases. In addition to mRNA, LNPs can also deliver other small molecule immunostimulants, such as STING agonists, resulting in STING-LNP complexes that exhibit enhanced adjuvant effects. STING-LNPs can effectively prolong STING degradation and sustainably activate the tumor immune response [Citation79].
2.3.2. Multimeric particle-based adjuvant delivery systems
Polymeric nanoparticles, which exhibit desirable characteristics such as good bioavailability, high drug loading, controlled release, and stability, have found widespread applications in carrying vaccine antigens, immunomodulators, or both, either by conjugating them to the surface or encapsulating them within the core. Polymers can be classified as solid, non-gelatinous particles or hydrogel-based nanoparticles, and they significantly enhance antigen uptake by APCs through passive phagocytosis or receptor-mediated active endocytosis. In addition to the nanocarrier delivery systems mentioned earlier, polymer-based delivery systems have also been employed for mRNA delivery [Citation80]. For instance, compounds with higher molecular weights, such as chitosan, polyethyleneimine, and polyurethane, which consist of simple structural units repeatedly linked by covalent bonds, can serve as effective carriers to facilitate in vivo mRNA delivery through the formation of polymeric particles or polymeric micelles, despite their low inherent adjuvant activity [Citation81,Citation82].
In recent years, Pickering emulsions have emerged as a promising approach, offering superior anti-aggregation and anti-flocculation properties compared to traditional emulsions, and have been widely explored in biopharmaceuticals, food, and cosmetics [Citation83]. These particle-stabilized emulsions involve solid particles irreversibly adsorbed at the two-phase interface to alter the spatial site resistance for stabilization. They retain the force-dependent deformation ability and lateral mobility of the antigens, while exhibiting higher biosafety and antigen loading capacity [Citation84]. Various types of particles, such as silica particles, metal particles, natural clays, polymer particles, proteins, cellulose, and starch, have been utilized to prepare Pickering emulsions. These emulsions combine the advantages of conventional emulsions, such as multiple encapsulation, protection, and controlled release, while also offering a relatively simple preparation process (which can be prepared in a single step) and long-term stability that is challenging to achieve with surfactants. For example, a study introduced poly(lactic-co-glycolic acid) (PLGA) nanoparticles and squalene to prepare an antigen-carrying Pickering emulsion, which exhibited flexibility and lateral mobility as an adjuvant, providing an effective, safe, and widely applicable strategy to enhance adaptive immunity against infections and diseases. Furthermore, studies based on mesoporous PDA particles (MPDA) assembled at the water/1,3,5-trimethylbenzene (TMB) interface with primary polydopamine PDA particles and Pluronic F127 stable emulsions revealed that MPDA emulsions carrying the TLR8 agonist imidazoquinoline exhibited adjuvant activity and effectively activated the tumor immune response, prolonging the survival of tumor-bearing mice [Citation85]. Moreover, a Pickering emulsion (PAPE) composed of aluminum adjuvant as solid particles stabilized with squalene has been suggested as a safe and effective adjuvant platform for enhanced COVID-19 vaccination [Citation86].
2.3.3. Biological vector-based adjuvant delivery systems
Vaccine delivery systems often include biological vectors, such as viruses and bacteria, which are widely studied for their ability to mimic the host immune system and enhance antigens in a manner similar to natural infection [Citation87]. Common viral vectors, including adenoviruses, adeno-associated viruses, poxviruses, and retroviruses, often possess self-adjuvant properties, simplifying vaccine formulation while activating innate immunity and promoting antigen expression, thus enhancing vaccine efficacy [Citation88]. The ChAdOx1 vector, an adeno-associated viral vector based on a monkey adenovirus expressing the SARS-CoV-2 spike protein, has been approved for human use in the UK due to its low immunogenicity and high safety [Citation89]. However, limitations such as limited loading capacity, intrinsic immunogenicity of the virus, and preexisting immunity to adenovirus types used as vaccine vectors still need to be addressed.
Virus-like particles (VLPs) are another delivery system option, consisting of multimeric nanostructures composed of one or more structural proteins of the virus in the absence of genetic material. VLPs can bind to different functional molecules while maintaining a virus-like structure, but without any pathogenicity or infectivity [Citation90]. VLPs serve as nanocarriers for efficient display of antigenic epitopes or delivery of small molecules, and virosomes, a type of VLP, can be used as both vaccine carriers and adjuvants. However, VLPs often require additional adjuvants and multiple doses to achieve an optimal immune response, despite their versatility in delivering proteins, peptides, nucleic acids, and drugs. Therefore, the development of an efficient antigen delivery adjuvant system that stimulates both humoral and cellular immunity is crucial for VLP vaccines to elicit adequate immune protection [Citation91,Citation92].
Using VLPs as a biological template, researchers have constructed a silica-based vaccine delivery system known as VLP@Silica, in which VLP forms a raspberry-like structure through self-assembly with silica while maintaining intact antigenic structure and immunogenicity [Citation93]. In bone marrow-derived DC cells, VLP@Silica induces secretion of Th1 and Th2 type cytokines and promotes VLP escape and intracellular delivery through lysosomal swelling triggered by silica lysis. Preclinical studies have demonstrated that coronavirus-neutralizing antibody levels are significantly enhanced by using Alum and Matrix-M1 adjuvant-loaded VLPs of SARS-CoV and MERS-CoV full-length S proteins. Furthermore, virosomes, which are synthetic, biodegradable VLPs ranging in size from 80–150 nm and composed of an outer covering of viral phospholipids and glycoproteins with an empty core for drug/antigen delivery, have been utilized in licensed vaccines such as Epaxal® for hepatitis B and Inflex V® for influenza [Citation94]. The ongoing development of virosomal vaccines and immunotherapies highlights the tremendous potential of this platform in designing vaccines against emerging infections and underscores the need for large-scale clinical evaluation.
Bacteria are common biological vectors that utilize lipopolysaccharides in Gram-negative bacteria, phospholipid wall acids in Gram-positive bacteria, and other pathogen-associated pattern molecules (PAMPs), such as flagellin that are inherently characteristic of bacteria and recognized by pattern recognition receptors (PRRs) to produce inflammatory cytokines or express anti-pathogen genes [Citation95]. Thus, in addition to antigen production and delivery, the intrinsic properties of bacterial vectors give them potential immunostimulatory adjuvant activity [Citation96].
Among these bacterial vectors, outer membrane vesicles (OMVs) are secreted by Gram-negative bacteria and play a role not only in pathogenesis, intercellular communication, and stress response, but also in immune regulation and in the establishment and homeostasis of the intestinal flora [Citation97]. OMVs are non-replicating entities that mimic bacterial immunogenicity, making them an attractive vaccine platform that can significantly reduce infection and morbidity by mimicking pathogens that induce humoral and/or cellular immunity without causing associated disease. In addition, OMVs possess nanoscale size, are able to enter lymphatic vessels, and are taken up by APCs, which gives them natural adjuvant properties that drive antigen-specific antibody and cellular immune responses. OMVs have been found to act as an effective adjuvant, significantly enhancing humoral and cellular immune responses. Biomineralized OMVs (OMV@CaP) can effectively promote reprogramming of the tumor environment, making them a potential candidate for adjuvant delivery research platforms for tumor vaccines [Citation98].
Moreover, exosomes, a specific type of extracellular vesicles (EVs) derived from multivesicular vesicles (MVBs), are also utilized as delivery vehicles in tumor therapy, vaccine, and adjuvant development [Citation99]. Generally 30–150 nm in size, exosomes are secreted by late endosomes and contain cell-specific proteins, lipids, and nucleic acids that can act as signaling molecules to other cells, thus altering the function of other cells. They play important roles in pathophysiology, including antigen presentation in immunity, tumor growth and migration, and repair of tissue damage. Exosomes secreted by different cells have distinct compositions and functions, and can be used as biomarkers for disease diagnosis [Citation100]. With a lipid bilayer membrane structure, exosomes provide protection for the substances they encapsulate and can target specific cells or tissues, making them a potential targeted drug delivery system. Lung stem cell-derived exosomes (LSC-Exo) in combination with the SARS-CoV-2 RBD antigen have been found to exhibit more advantageous targeted delivery and immune enhancement effects than liposomes () [Citation101].
Figure 2. Illustration of innovative carriers employed as VADS. Note: MSN: mesoporous silica nanoparticles; NP: nanoparticle; Manganese NP (‘MnJ’); SNE: stable nano-emulsion containing phospholipids such as DSPC (‘ANE, adjuvant nano-emulsion’); CNE: cationic nano-emulsion (DOTAP); OMV: Outer membrane vesicles.
3. The importance of carriers in VADS
3.1. Basic component features
Adjuvant components have transitioned from naturally sourced to synthetic compounds. The realm of novel adjuvants now includes inorganic salts, emulsions, liposomes, and synthetic small molecules (). In the development of novel adjuvants, adjuvant components with well-defined molecular structures, such as PAMPs and their chemically synthesized derivatives (CpG, MPL), or synthetic small molecules (R848, IMQ) [Citation110], have been extensively employed, along with significant advancements in nanotechnology and immunological research. Adjuvant development has progressively shifted from an empirical to a rational design phase [Citation111]. Currently, only seven adjuvants, apart from aluminum adjuvants – MF59, AS03, AS04, CpG ODN, AS01B, and Matrix-M – have been approved by the FDA for human vaccines [Citation112]. Although these human vaccine adjuvants have received approval and widespread use, their molecular mechanisms in humans remain unclear. Among these human vaccine adjuvants, except for CpG ODN, which is a single small molecule, all others are adjuvant complexes with carrier activity or co-loaded with small molecules. It has been demonstrated that using aluminum adjuvant loaded with CpG ODN in the SARS-CoV-2 recombinant vaccine resulted in a stronger immune advantage, further suggesting that the delivery carrier may play a crucial role in understanding the adjuvant immune enhancement mechanism [Citation113,Citation114]. In fact, several CpG carrier-based tumor nanovaccines, constructed using different loading modes of CpG, such as electrostatic adsorption, covalent bonding, hydrophilic interactions, and DNA self-assembly, are currently undergoing clinical investigation [Citation115].
Table 2. Types of immunomodulatory molecules.
The characteristics of delivery carriers, as a vital component of the VADS, impact the activity of the adjuvant complexes they compose. The size of the delivery carriers is generally manifested in distinct nanostructures [Citation116]. According to the FDA, nanotechnology is defined as the utilization of unique properties of objects that function as a whole in the range of 1–1000 nm, and these structures are capable of more efficiently manipulating or delivering the immune active ingredient to the target site [Citation117]. Consequently, nanoparticles with unique immune properties, formed by differences in physicochemical properties such as size, shape, charge, porosity, and hydrophobicity, can be employed to enhance delivery targeting, carry large amounts of antigen, and modulate its release over extended periods to amplify antigen supply to the body’s immune system and protect it from degradation while maintaining stability [Citation118]. Moreover, the broad application of nanotechnology in vaccine adjuvants enables carriers with nanostructures to interact with biological barriers and transport bioactive molecules without altering antigenicity, providing adjuvant activity by enhancing antigen delivery to the immune system or by amplifying the innate immune response, and exhibiting outstanding biocompatibility [Citation119].
3.2. Mechanisms of action for common VADS
As the first approved adjuvant for human vaccines, aluminum adjuvant is extensively used with various antigens; however, its immune response behavior varies across immunogens [Citation120]. It demonstrates weaker performance against peptides, recombinant subunit proteins in monomeric form (e.g. varicella zoster virus glycoprotein E), nucleic acids, and other antigens compared to recombinant multimerized antigen VLPs. Furthermore, the dominant Th2 response ‘deactivates’ aluminum adjuvants against intracellular viruses (e.g. HIV), bacterial infections, and tumor therapy. The prevailing hypothesis involves the reservoir effect, which includes activation of NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasomes and complement activation [Citation121]. Thus, it has been suggested that the adjuvant action mechanism may result from multiple pathways acting together. In the body’s immune response, the adaptive immune response primarily relies on the level and specificity of the initial ‘danger’ signal perceived by the innate immune cells after infection or vaccination. Guy posits that adjuvant initiation of an effective immune response necessitates different signals, which can be classified into signal 0 (antigen recognition and APC activation), signal 1 (antigen presentation), and signal 2 (co-stimulation) according to the type of Th activated, activating Th0, Th1, and Th2 cells () [Citation19,Citation122,Citation123].
Figure 3. Mechanisms of action for various VADS.
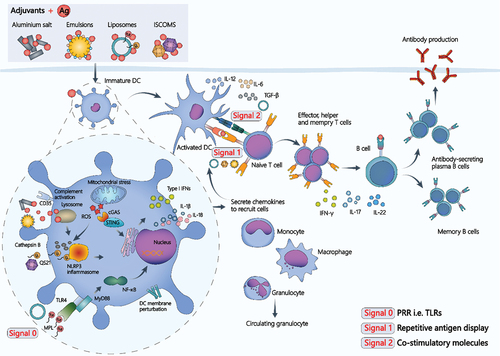
Liposomes have been extensively employed in VADS construction due to their excellent biocompatibility, various drug loading methods, high drug loading capacity, simple preparation, ease of surface modification to produce desired functions, and self-adjuvant effect, among other factors. It has been proposed that liposomes can effectively function as adjuvants regardless of whether the loading antigen is encapsulated within the lipid membrane, attached to the lipid membrane, or merely mixed with it. This property simplifies the production and immunization processes [Citation124]. Liposomes, with their unique membrane structure, have also been investigated for binding various functional molecules such as PRRs. Intrinsic immune cells can recognize PAMPs through PRRs, and PRR activation is essential for triggering inflammation and subsequent development of adaptive immune responses [Citation125]. Currently, immunostimulatory complexes composed of liposomes and TLRs have been successfully utilized as VADS in vaccines to combat different infections, such as with AS01B [Citation126]. This system employs liposomes (DOPC and cholesterol) as carriers and binds two immunostimulatory active molecules, MPL and QS21 [Citation127]. AS01B, carrying the antigen, is simultaneously injected into the muscle, and after being taken up by APCs, MPL activates APCs via TLR4, while QS-21 activates NLRP3 inflammasomes, leading to the release of IL-1β and IL-18 (signal 0) [Citation128]. The synergistic effect of MPL and QS-21 increases chemokine release, circulating granulocytes, and enhances monocyte and dendritic cell recruitment. In draining lymph nodes, highly activated dendritic cells efficiently induce differentiation of CD4+ T cells into memory CD4+ T cells and effector CD4+ T cells. Cytokines such as IL-2, TNF-α, CD40L, and IFN-γ secreted by effector CD4+ T cells stimulate the division of naive B cells into plasma cells and memory B cells (signal 2).
The immune enhancement mechanism of emulsion-based vaccine adjuvants, such as MF59 and AS03, exhibits a reservoir effect similar to that of aluminum adjuvants. These adjuvants can generate a transient local immune environment at the injection site, promoting cytokine and chemokine production, and recruitment of cells to the injection site. This allows migration of activated antigen-loaded APCs to the draining lymph nodes, where APCs can initiate naïve CD4+ T cells (signal 0 and signal 1). Chemokine-driven immune cell recruitment is a key feature of MF59 and AS03 immune activation mechanisms compared to liposomal structures. Furthermore, the inclusion of α-tocopherol as an immunostimulant sets AS03 apart from other oil-in-water emulsion-based adjuvants used in human vaccines. The adjuvant system comprises the surfactant polysorbate 80 and two biodegradable oils, squalene and dl-α-tocopherol, in a phosphate-buffered saline (PBS). AS03 employs synthetic α-tocopherol, a widely distributed tautomer of vitamin E in nature and the form preferentially absorbed by the body, found in various tissues. Vitamin E is chiefly recognized for its antioxidant activity, which serves to maintain cell membrane integrity by preventing lipid oxidation [Citation129]. It also influences gene expression and intracellular signaling, and dietary supplementation of vitamin E has been suggested to possess immunomodulatory properties, such as mitigating aging-related impairment of naive T-cell activation and macrophage prostaglandin E2 overproduction [Citation57]. Studies have demonstrated that AS03’s mode of action relies strictly on the temporal and spatial co-localization of the antigen, and that staggered or separate administration of antigen and adjuvant results in a decline in specific antibody titers or even a complete lack of immune response. This indicates that AS03 primarily functions as an adjuvant system and lacks certain antigen delivery capabilities. However, modifying nanoemulsions, such as by introducing aluminum adjuvant, phospholipid groups, or oil-water distribution, can facilitate targeted antigen delivery while enhancing emulsion immune potentiation.
In therapeutic tumor vaccines, Jing Jie et al. discovered that MF59 adjuvant, when used as a carrier in combination with CpG 1826 to construct a complex adjuvant system, could significantly prolong CpG retention time, induce a Th1-prone cellular immune response, and overcome CpG molecule instability to reduce individual differences in tumor growth and extend the survival of prophylactic and therapeutic mice [Citation130]. Therefore, different delivery vectors may exhibit distinct immune-enhancing mechanisms depending on the vector’s nature or the combined functional molecules. Constructing VADS may produce a cascade effect that further enhances adjuvant activity while reducing side effects and avoiding concentrated transient stimulation (e.g. the combination of liposomes with QS21 reduces the hemolytic activity of QS21) and induces the generation of effective Th1 and Th2 immune responses [Citation128,Citation131].
4. Conclusion
Addressing the challenges of weak immunogenicity, instability of antigens, and the necessity for T-cell response in vaccine research necessitates the progressive development of novel adjuvant research. This aims to nurture desirable vaccine adjuvants, essential for combating both emerging and existing infectious diseases.Vaccine adjuvant research has recently shifted from empirical methods toward rational design, focusing on potent immune targets. This involves harnessing natural or synthetic materials with adjuvant properties and developing VADS that synergize adjuvant activity with antigen delivery or protection.
VADS symbolizes a comprehensive adjuvant design approach, fusing adjuvant and delivery system concepts, and is critical to vaccine design. For instance, the inclusion of various metal ions or immunostimulatory molecules into traditional aluminum adjuvants leads to inorganic salt-based VADS, which enhance antigen delivery and provoke specific immune responses. Utilizing LNPs to encapsulate naked mRNA forms an mRNA vaccine, protecting the mRNA from degradation and enhancing the immune response. Additionally, modifying nanoemulsions to carry effector molecules with immunostimulatory effects, like nanoaluminum, can improve the in vivo stability of these molecules and enhance biocompatibility.
Despite these advancements, VADS research still faces hurdles, such as lack of long-term safety and efficacy data in human populations and difficulties with system stability and large-scale production. Developing new VADS with simpler components and cost-effective production materials is therefore vital. As the field continues to evolve with new vaccines based on VADS and innovative delivery systems, the scope of vaccine research expands, offering promising therapeutic prospects for various diseases and fostering multidisciplinary collaborations.
5. Expert opinion
The modern concept of vaccines emerged in the late 18th century when Edward Jenner successfully prevented smallpox infection by using pus from cowpox-infected milkmaids for vaccination. Early vaccines primarily consisted of whole organisms, developed to protect against pathogenic infections. However, as human society advanced, the concept of therapeutic vaccines surfaced. The inclusion of adjuvants, such as aluminum adjuvants, emulsions, liposomes, virosomes, and polymers, in vaccines can significantly enhance their efficacy and provide protection for immunocompromised populations. Adjuvants aim to improve immune responses by recruiting immune cells to the inoculation site, promoting antigen uptake by APCs, and activating innate immune cells. While conventional adjuvants can achieve this to some extent, the transient or weak adaptive immune response may not suffice against pathogenic microorganisms, such as viruses [Citation132,Citation133].
VADS have thus been developed, combining carriers like aluminum adjuvants, emulsions, liposomes, virosomes, and polymers with functional molecules. These carriers serve not only as adjuvants for enhanced immune stimulation but also as delivery vehicles targeting immune cells, enabling sustained or signal-triggered release and resulting in a multifunctional VADS. The establishment of the VADS concept may facilitate FDA approval of new adjuvant products, such as nano-emulsion/squalene-based MF59, liposome/TLR4/QS21-based AS01B, and alum/TLR4-based AS04, which have been utilized in various vaccines as adjuvants and carriers for delivering bioactive ingredients.
Nonetheless, during vaccine formulation studies, understanding the interaction mechanisms between adjuvants, antigens, and antigen delivery systems is crucial for initiating vaccine effects. Adjuvants’ physicochemical properties can vary, and their effects on antigen interaction mechanisms are not fully understood due to differences in adjuvants’ and antigens’ physicochemical properties. Thus, continuous investigation of the key properties of different carriers, adjuvant activity, delivery efficiency, and small molecules’ mechanisms of action is essential for developing novel vaccine adjuvants, optimizing VADS, and improving vaccine formulations.
Article highlights
In order to tackle challenges in vaccine research such as weak immunogenicity of antigens, instability, or the necessity for a T-cell response for disease prevention, it is crucial to focus on the development of novel adjuvants.
Vaccine adjuvant development strategies consist of two principal types: utilizing natural or synthetic materials with adjuvant properties, and creating VADS that couple adjuvant activity with antigen delivery or protection.
The carriers used in VADS can be grouped into three main categories based on their characteristics: inorganic salt-based, oil-water emulsions, and microparticle antigen delivery systems.
VADS constructed from varying base vectors often display distinct immune-enhancing mechanisms. Further analysis of their overall immune enhancement principles can provide direction for the rational design of innovative adjuvants.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or mending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
S.L. and N.S. designed and funded the study. R.Z wrote the original draft, reviewed and edited the manuscript, N.X., and S.L. participated in discussion and interpretation of the manuscript.
Data availability statement
The data that support the findings of this study are openly available in PubMed at https://pubmed.ncbi.nlm.nih.gov/.
Additional information
Funding
References
- Spinney L. Smallpox and other viruses plagued humans much earlier than suspected. Nature. 2020;584(7819):30–32. doi: 10.1038/d41586-020-02083-0
- Minor PD. An introduction to poliovirus: Pathogenesis, vaccination, and the endgame for global eradication. Methods Mol Biol. 2016;1387:1–10.
- Rosalik K, Tarney C, Han J. Human Papilloma virus vaccination. Viruses. 2021;13(6):1091. doi: 10.3390/v13061091
- Pattyn J, Hendrickx G, Vorsters A, et al. Hepatitis B vaccines. J Infect Dis. 2021;224(12 Suppl 2):S343–S351. doi: 10.1093/infdis/jiaa668
- Wijesundara DK, Jackson RJ, Ramshaw IA, et al. Human immunodeficiency virus-1 vaccine design: where do we go now? Immunol Cell Biol. 2011;89(3):367–374. doi: 10.1038/icb.2010.118
- Nicastri E, Kobinger G, Vairo F, et al. Ebola virus disease: Epidemiology, clinical features, management, and prevention. Infect Dis Clin North Am. 2019;33(4):953–976. doi: 10.1016/j.idc.2019.08.005
- Javanian M, Barary M, Ghebrehewet S, et al. A brief review of influenza virus infection. J Med Virol. 2021;93(8):4638–4646. doi: 10.1002/jmv.26990
- Sadeghalvad M, Mansourabadi AH, Noori M, et al. Recent developments in SARS-CoV-2 vaccines: A systematic review of the current studies. Rev Med Virol. 2023;33(1):e2359. doi: 10.1002/rmv.2359
- Gong Q, Wang C, Chuai X, et al. Monkeypox virus: a re-emergent threat to humans. Virol Sin. 2022;37(4):477–482. doi: 10.1016/j.virs.2022.07.006
- Moyle PM, Toth I. Modern subunit vaccines: development, components, and research opportunities. ChemMedchem. 2013;8(3):360–376. doi: 10.1002/cmdc.201200487
- Pardi N, Hogan MJ, Porter FW, et al. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–279. doi: 10.1038/nrd.2017.243
- Mao L, Chen Z, Wang Y, et al. Design and application of nanoparticles as vaccine adjuvants against human corona virus infection. J Inorg Biochem. 2021;219:111454. doi: 10.1016/j.jinorgbio.2021.111454
- Mendes BB, Conniot J, Avital A, et al. Nanodelivery of nucleic acids. Nat Rev Methods Primers. 2022;2(1). doi: 10.1038/s43586-022-00104-y
- Rappuoli R. Rational design of vaccines. Nat Med. 1997;3(4):374–376. doi: 10.1038/nm0497-374
- Park WH, Schroder MC. Diphtheria toxin-antitoxin and toxoid: A comparison. Am J Public Health Nations Health. 1932;22(1):7–16. doi: 10.2105/AJPH.22.1.7
- Bovier PA. Epaxal®: a virosomal vaccine to prevent hepatitis a infection. Expert Rev Vaccines. 2008;7(8):1141–1150. doi: 10.1586/14760584.7.8.1141
- Del Giudice G, Rappuoli R, Didierlaurent AM. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin Immunol. 2018;39:14–21. doi: 10.1016/j.smim.2018.05.001
- Cox JC, Coulter AR. Adjuvants–a classification and review of their modes of action. Vaccine. 1997;15(3):248–256. doi: 10.1016/S0264-410X(96)00183-1
- Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5(7):505–517. doi: 10.1038/nrmicro1681
- Wang N, Qian R, Liu T, et al. Nanoparticulate carriers Used as vaccine adjuvant delivery systems. Crit Rev Ther Drug Carrier Syst. 2019;36(5):449–484. doi: 10.1615/CritRevTherDrugCarrierSyst.2019027047
- Garcia A, De Sanctis JB. An overview of adjuvant formulations and delivery systems. APMIS. 2014;122(4):257–267. doi: 10.1111/apm.12143
- Petkar KC, Patil SM, Chavhan SS, et al. An overview of nanocarrier-based adjuvants for vaccine delivery. Pharmaceutics. 2021;13(4):455. doi: 10.3390/pharmaceutics13040455
- Moyer TJ, Zmolek AC, Irvine DJ. Beyond antigens and adjuvants: formulating future vaccines. J Clin Invest. 2016;126(3):799–808. doi: 10.1172/JCI81083
- Anderluzzi G, Schmidt ST, Cunliffe R, et al. Rational design of adjuvants for subunit vaccines: The format of cationic adjuvants affects the induction of antigen-specific antibody responses. J Control Release. 2021;330:933–944. doi: 10.1016/j.jconrel.2020.10.066
- Garcon N, Chomez P, Van Mechelen M. GlaxoSmithKline adjuvant systems in vaccines: concepts, achievements and perspectives. Expert Rev Vaccines. 2007;6(5):723–739. doi: 10.1586/14760584.6.5.723
- Paston SJ, Brentville VA, Symonds P, et al. Cancer vaccines, adjuvants, and delivery systems. Front Immunol. 2021;12:627932. doi: 10.3389/fimmu.2021.627932
- Bian L, Zheng Y, Guo X, et al. Intramuscular inoculation of AS02-adjuvanted Respiratory Syncytial Virus (RSV) F subunit vaccine shows better efficiency and safety than subcutaneous inoculation in BALB/c mice. Front Immunol. 2022;13:938598. doi: 10.3389/fimmu.2022.938598
- Behzad H, Huckriede ALW, Haynes L, et al. GLA-SE, a synthetic toll-like receptor 4 agonist, enhances T-cell responses to influenza vaccine in older adults. J Infect Dis. 2012;205(3):466–473. doi: 10.1093/infdis/jir769
- Marques RF, de Melo FM, Novais JT, et al. Immune system modulation by the adjuvants poly (I: C) and montanide ISA 720. Front Immunol. 2022;13:910022. doi: 10.3389/fimmu.2022.910022
- van Dissel JT, Joosten SA, Hoff ST, et al. A novel liposomal adjuvant system, CAF01, promotes long-lived mycobacterium tuberculosis-specific T-cell responses in human. Vaccine. 2014;32(52):7098–7107. doi: 10.1016/j.vaccine.2014.10.036
- Clegg CH, Roque R, Perrone LA, et al. GLA-AF, an emulsion-free vaccine adjuvant for pandemic influenza. Plos One. 2014;9(2):e88979. doi: 10.1371/journal.pone.0088979
- Hem SL, Hogenesch H. Relationship between physical and chemical properties of aluminum-containing adjuvants and immunopotentiation. Expert Rev Vaccines. 2007;6(5):685–698. doi: 10.1586/14760584.6.5.685
- Li X, Aldayel AM, Cui Z. Aluminum hydroxide nanoparticles show a stronger vaccine adjuvant activity than traditional aluminum hydroxide microparticles. J Control Release. 2014;173:148–157. doi:10.1016/j.jconrel.2013.10.032
- Zhao Q, Sitrin R. Surface phosphophilicity of aluminum-containing adjuvants probed by their efficiency for catalyzing the P–O bond cleavage with chromogenic and fluorogenic substrates. Anal Biochem. 2001;295(1):76–81. doi: 10.1006/abio.2001.5175
- Didierlaurent AM, Morel S, Lockman L, et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol. 2009;183(10):6186–6197. doi: 10.4049/jimmunol.0901474
- Fox CB, Orr MT, Van Hoeven N, et al. Adsorption of a synthetic TLR7/8 ligand to aluminum oxyhydroxide for enhanced vaccine adjuvant activity: A formulation approach. J Control Release. 2016;244(Pt A):98–107. doi: 10.1016/j.jconrel.2016.11.011
- Garcon N, Di Pasquale A. From discovery to licensure, the adjuvant system story. Hum Vaccin Immunother. 2017;13(1):19–33. doi: 10.1080/21645515.2016.1225635
- Richmond P, Hatchuel L, Dong M, et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397(10275):682–694. doi: 10.1016/S0140-6736(21)00241-5
- Kasturi SP, Rasheed MAU, Havenar-Daughton C, et al. 3M-052, a synthetic TLR-7/8 agonist, induces durable HIV-1 envelope–specific plasma cells and humoral immunity in nonhuman primates. Sci Immunol. 2020;5(48). doi: 10.1126/sciimmunol.abb1025
- Dong H, Wen Z-F, Chen L, et al. Polyethyleneimine modification of aluminum hydroxide nanoparticle enhances antigen transportation and cross-presentation of dendritic cells. Int J Nanomedicine. 2018;13:3353–3365. doi: 10.2147/IJN.S164097
- Li X, Wang X, Ito A. Tailoring inorganic nanoadjuvants towards next-generation vaccines. Chem Soc Rev. 2018;47(13):4954–4980. doi: 10.1039/C8CS00028J
- Hem SL, Johnston CT, HogenEsch H. Imject alum is not aluminum hydroxide adjuvant or aluminum phosphate adjuvant. Vaccine. 2007;25(27):4985–4986. doi: 10.1016/j.vaccine.2007.04.078
- Li A, Qin L, Zhu D, et al. Signalling pathways involved in the activation of dendritic cells by layered double hydroxide nanoparticles. Biomaterials. 2010;31(4):748–756. doi: 10.1016/j.biomaterials.2009.09.095
- Wu Y, Huang X, Yuan L, et al. A recombinant spike protein subunit vaccine confers protective immunity against SARS-CoV-2 infection and transmission in hamster. Sci Transl Med. 2021;13(606):eabg1143. doi: 10.1126/scitranslmed.abg1143
- Wang C, Guan Y, Lv M, et al. Manganese increases the sensitivity of the Cgas-STING pathway for double-stranded DNA and is required for the host defense against DNA viruses. Immunity. 2018;48(4):675–687 e7. doi: 10.1016/j.immuni.2018.03.017
- Fan N, Chen K, Zhu R, et al. Manganese-coordinated mRNA vaccines with enhanced mRNA expression and immunogenicity induce robust immune responses against SARS-CoV-2 variants. Sci Adv. 2022;8(51):eabq3500. doi: 10.1126/sciadv.abq3500
- Mulens-Arias V, Rojas JM, Barber DF. The use of iron oxide nanoparticles to reprogram macrophage responses and the immunological tumor microenvironment. Front Immunol. 2021;12:693709. doi: 10.3389/fimmu.2021.693709
- Li F, Nie W, Zhang F, et al. Engineering magnetosomes for high-performance cancer vaccination. ACS Cent Sci. 2019;5(5):796–807. doi: 10.1021/acscentsci.9b00060
- Guo Y, Tang L. A magnetic nanovaccine enhances cancer immunotherapy. ACS Cent Sci. 2019;5(5):747–749. doi: 10.1021/acscentsci.9b00325
- Mahony D, Cavallaro AS, Stahr F, et al. Mesoporous silica nanoparticles act as a self-adjuvant for ovalbumin model antigen in mice. Small. 2013;9(18):3138–3146. doi: 10.1002/smll.201300012
- Tayeb HH, Sainsbury F. Nanoemulsions in drug delivery: formulation to medical application. Nanomedicine (Lond). 2018;13(19):2507–2525. doi: 10.2217/nnm-2018-0088
- Dube JY, McIntosh F, Zarruk JG, et al. Synthetic mycobacterial molecular patterns partially complete Freund’s adjuvant. Sci Rep. 2020;10(1):5874. doi: 10.1038/s41598-020-62543-5
- O’Hagan DT, Ott GS, Nest GV, et al. The history of MF59 ® adjuvant: a phoenix that arose from the ashes. Expert Rev Vaccines. 2013;12(1):13–30. doi: 10.1586/erv.12.140
- Klucker MF, Dalençon F, Probeck P, et al. AF03, an alternative squalene emulsion-based vaccine adjuvant prepared by a phase inversion temperature method. J Pharm Sci. 2012;101(12):4490–4500. doi: 10.1002/jps.23311
- Mendes A, Azevedo-Silva J, Fernandes JC. From sharks to yeasts: squalene in the development of vaccine adjuvants. Pharmaceuticals (Basel). 2022;15(3):265. doi: 10.3390/ph15030265
- Ravera F, Dziza K, Santini E, et al. Emulsification and emulsion stability: The role of the interfacial properties. Adv Colloid Interface Sci. 2021;288:102344. doi: 10.1016/j.cis.2020.102344
- Garcon N, Vaughn DW, Didierlaurent AM. Development and evaluation of AS03, an adjuvant system containing alpha-tocopherol and squalene in an oil-in-water emulsion. Expert Rev Vaccines. 2012;11(3):349–366. doi: 10.1586/erv.11.192
- Aucouturier J, Dupuis L, Deville S, et al. Montanide ISA 720 and 51: a new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev Vaccines. 2002;1(1):111–118. doi: 10.1586/14760584.1.1.111
- van Doorn E, Liu H, Huckriede A, et al. Safety and tolerability evaluation of the use of montanide ISA™51 as vaccine adjuvant: A systematic review. Hum Vaccin Immunother. 2016;12(1):159–169. doi: 10.1080/21645515.2015.1071455
- O’Hagan DT, Ott GS, De Gregorio E, et al. The mechanism of action of MF59 – an innately attractive adjuvant formulation. Vaccine. 2012;30(29):4341–4348. doi: 10.1016/j.vaccine.2011.09.061
- Brito LA, Chan M, Shaw CA, et al. A cationic nanoemulsion for the delivery of next-generation RNA vaccines. Mol Ther. 2014;22(12):2118–2129. doi: 10.1038/mt.2014.133
- Chen Z, Hao X, Wang H, et al. Smart combination of aluminum hydroxide and MF59 to induce strong cellular immune responses. J Control Release. 2022;349:699–711. doi: 10.1016/j.jconrel.2022.07.032
- Budai M, Szogyi M. Liposomes as drug carrier systems. Preparation, classification and therapeutic advantages of liposomes. Acta Pharm Hung. 2001;71(1):114–118.
- Akbarzadeh A, Rezaei-Sadabady R, Davaran S, et al. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8(1):102. doi: 10.1186/1556-276X-8-102
- Gregoriadis G. Immunological adjuvants: a role for liposomes. Immunol Today. 1990;11(3):89–97. doi: 10.1016/0167-5699(90)90034-7
- Wang N, Chen M, Wang T. Liposomes used as a vaccine adjuvant-delivery system: From basics to clinical immunization. J Control Release. 2019;303:130–150. doi: 10.1016/j.jconrel.2019.04.025
- Syed YY. Recombinant Zoster vaccine (Shingrix((r))): A review in Herpes Zoster. Drugs Aging. 2018;35(12):1031–1040. doi: 10.1007/s40266-018-0603-x
- Didierlaurent AM, Laupèze B, Di Pasquale A, et al. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev Vaccines. 2017;16(1):55–63. doi: 10.1080/14760584.2016.1213632
- Alving CR, Rao M, Steers NJ, et al. Liposomes containing lipid A: an effective, safe, generic adjuvant system for synthetic vaccines. Expert Rev Vaccines. 2012;11(6):733–744. doi: 10.1586/erv.12.35
- Morein B, Lövgren K, Höglund S, et al. The ISCOM: an immunostimulating complex. Immunol Today. 1987;8(11):333–338. doi: 10.1016/0167-5699(87)90008-9
- Baz Morelli A, Becher D, Koernig S, et al. ISCOMATRIX: a novel adjuvant for use in prophylactic and therapeutic vaccines against infectious diseases. J Med Microbiol. 2012;61(7):935–943. doi: 10.1099/jmm.0.040857-0
- Magnusson SE, Altenburg AF, Bengtsson KL, et al. Matrix-M™ adjuvant enhances immunogenicity of both protein- and modified vaccinia virus Ankara-based influenza vaccines in mice. Immunol Res. 2018;66(2):224–233. doi: 10.1007/s12026-018-8991-x
- Alving CR, Matyas GR, Torres O, et al. Adjuvants for vaccines to drugs of abuse and addiction. Vaccine. 2014;32(42):5382–5389. doi: 10.1016/j.vaccine.2014.07.085
- Tenchov R, Bird R, Curtze AE, et al. Lipid nanoparticles─From liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15(11):16982–17015. doi: 10.1021/acsnano.1c04996
- Chaudhary N, Weissman D, Whitehead KA. mRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat Rev Drug Discov. 2021;20(11):817–838. doi: 10.1038/s41573-021-00283-5
- Ramachandran S, Satapathy SR, Dutta T. Delivery Strategies for mRNA Vaccines. Pharmaceut Med. 2022;36(1):11–20. doi: 10.1007/s40290-021-00417-5
- Li Y, Tenchov R, Smoot J, et al. A comprehensive review of the global efforts on COVID-19 vaccine development. ACS Cent Sci. 2021;7(4):512–533. doi: 10.1021/acscentsci.1c00120
- DeFrancesco L. Whither COVID-19 vaccines? Nat Biotechnol. 2020;38(10):1132–1145. doi: 10.1038/s41587-020-0697-7
- Nakamura T, Sato T, Endo R, et al. STING agonist loaded lipid nanoparticles overcome anti-PD-1 resistance in melanoma lung metastasis via NK cell activation. J Immunother Cancer. 2021;9(7):e002852. doi: 10.1136/jitc-2021-002852
- Li M, Li Y, Li S, et al. The nano delivery systems and applications of mRNA. Eur J Med Chem. 2022;227:113910. doi: 10.1016/j.ejmech.2021.113910
- Zhang Y, Wang Z, Gemeinhart RA. Progress in microRNA delivery. J Control Release. 2013;172(3):962–974. doi: 10.1016/j.jconrel.2013.09.015
- Carroll EC, Jin L, Mori A, et al. The vaccine adjuvant Chitosan promotes cellular immunity via DNA sensor Cgas-STING-Dependent induction of type I interferons. Immunity. 2016;44(3):597–608. doi: 10.1016/j.immuni.2016.02.004
- Albert C, Beladjine M, Tsapis N, et al. Pickering emulsions: Preparation processes, key parameters governing their properties and potential for pharmaceutical applications. J Control Release. 2019;309:302–332. doi: 10.1016/j.jconrel.2019.07.003
- Xia Y, Wu J, Wei W, et al. Exploiting the pliability and lateral mobility of pickering emulsion for enhanced vaccination. Nat Mater. 2018;17(2):187–194. doi: 10.1038/nmat5057
- Zhu M, Shi Y, Shan Y, et al. Recent developments in mesoporous polydopamine-derived nanoplatforms for cancer theranostics. J Nanobiotechnology. 2021;19(1):387. doi: 10.1186/s12951-021-01131-9
- Peng S, Cao F, Xia Y, et al. Particulate alum via pickering emulsion for an enhanced COVID-19 vaccine adjuvant. Adv Mater. 2020;32(40):e2004210. doi: 10.1002/adma.202004210
- Curley SM, Putnam D. Biological nanoparticles in vaccine development. Front Bioeng Biotechnol. 2022;10:867119. doi: 10.3389/fbioe.2022.867119
- Lai CM, Lai YK, Rakoczy PE. Adenovirus and adeno-associated virus vectors. DNA Cell Biol. 2002;21(12):895–913. doi: 10.1089/104454902762053855
- van Doremalen N, Lambe T, Spencer A, et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586(7830):578–582. doi: 10.1038/s41586-020-2608-y
- Qian C, Liu X, Xu Q, et al. Recent progress on the versatility of virus-like particles. Vaccines (Basel). 2020;8(1):139. doi: 10.3390/vaccines8010139
- Yilmaz IC, Ipekoglu EM, Bulbul A, et al. Development and preclinical evaluation of virus-like particle vaccine against COVID-19 infection. Allergy. 2022;77(1):258–270. doi: 10.1111/all.15091
- Hills RA, Howarth M. Virus-like particles against infectious disease and cancer: guidance for the nano-architect. Curr Opin Biotechnol. 2022;73:346–354. doi: 10.1016/j.copbio.2021.09.012
- Li M, Liang Z, Chen C, et al. Virus-like particle-templated silica-adjuvanted nanovaccines with enhanced humoral and cellular immunity. ACS Nano. 2022;16(7):10482–10495. doi: 10.1021/acsnano.2c01283
- Mischler R, Metcalfe IC. Inflexal V a trivalent virosome subunit influenza vaccine: production. Vaccine. 2002;20(Suppl_5):B17–23. doi: 10.1016/S0264-410X(02)00512-1
- Cui B, Liu X, Fang Y, et al. Flagellin as a vaccine adjuvant. Expert Rev Vaccines. 2018;17(4):335–349. doi: 10.1080/14760584.2018.1457443
- Vasou A, Sultanoglu N, Goodbourn S, et al. Targeting pattern recognition receptors (prr) for vaccine adjuvantation: From synthetic PRR agonists to the potential of defective interfering particles of viruses. Viruses. 2017;9(7):186. doi: 10.3390/v9070186
- Sartorio MG, Pardue EJ, Feldman MF, et al. Bacterial outer membrane vesicles: From discovery to applications. Annu Rev Microbiol. 2021;75(1):609–630. doi: 10.1146/annurev-micro-052821-031444
- Qing S, Lyu C, Zhu L, et al. Biomineralized bacterial outer membrane vesicles potentiate safe and efficient tumor microenvironment reprogramming for anticancer therapy. Adv Mater. 2020;32(47):e2002085. doi: 10.1002/adma.202002085
- Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30(1):255–289. doi: 10.1146/annurev-cellbio-101512-122326
- Vlassov AV, Magdaleno S, Setterquist R, et al. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820(7):940–948. doi: 10.1016/j.bbagen.2012.03.017
- Wang Z, Popowski KD, Zhu D, et al. Exosomes decorated with a recombinant SARS-CoV-2 receptor-binding domain as an inhalable COVID-19 vaccine. Nat Biomed Eng. 2022;6(7):791–805. doi: 10.1038/s41551-022-00902-5
- Friedman-Klabanoff DJ, Berry AA, Travassos MA, et al. Low dose recombinant full-length circumsporozoite protein-based Plasmodium falciparum vaccine is well-tolerated and highly immunogenic in phase 1 first-in-human clinical testing. Vaccine. 2021;39(8):1195–1200. doi: 10.1016/j.vaccine.2020.12.023
- Liang H, Poncet D, Seydoux E, et al. The TLR4 agonist adjuvant SLA-SE promotes functional mucosal antibodies against a parenterally delivered ETEC vaccine. NPJ Vaccines. 2019;4(1):19. doi: 10.1038/s41541-019-0116-6
- Olafsdottir TA, Lingnau K, Nagy E, et al. IC31 ® , a two-component novel adjuvant mixed with a conjugate vaccine enhances protective immunity against pneumococcal disease in neonatal mice. Scand J Immunol. 2009;69(3):194–202. doi: 10.1111/j.1365-3083.2008.02225.x
- Li L, Honda-Okubo Y, Baldwin J, et al. Covax-19/Spikogen® vaccine based on recombinant spike protein extracellular domain with Advax-CpG55.2 adjuvant provides single dose protection against SARS-CoV-2 infection in hamsters. Vaccine. 2022;40(23):3182–3192. doi: 10.1016/j.vaccine.2022.04.041
- Sultan H, Salazar AM, Celis E. Poly-ICLC, a multi-functional immune modulator for treating cancer. Semin Immunol. 2020;49:101414. doi: 10.1016/j.smim.2020.101414
- Gai WW, Zhang Y, Zhou D-H, et al. PIKA provides an adjuvant effect to induce strong mucosal and systemic humoral immunity against SARS-CoV. Virol Sin. 2011;26(2):81–94. doi: 10.1007/s12250-011-3183-z
- Portielje JE, Gratama J, van Ojik HH, et al. IL-12: a promising adjuvant for cancer vaccination. Cancer Immunol Immunother. 2003;52(3):133–144. doi: 10.1007/s00262-002-0356-5
- Yoon HA, Aleyas AG, George JA, et al. Cytokine GM-CSF genetic adjuvant facilitates prophylactic DNA vaccine against pseudorabies virus through enhanced immune responses. Microbiol Immunol. 2006;50(2):83–92. doi: 10.1111/j.1348-0421.2006.tb03773.x
- Wang L, He Y, He T, et al. Lymph node-targeted immune-activation mediated by imiquimod-loaded mesoporous polydopamine based-nanocarriers. Biomaterials. 2020;255:120208. doi: 10.1016/j.biomaterials.2020.120208
- Pulendran B, P SA, O’Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov. 2021;20(6):454–475. doi: 10.1038/s41573-021-00163-y
- Iwasaki A, Omer SB. Why and how vaccines work. Cell. 2020;183(2):290–295. doi: 10.1016/j.cell.2020.09.040
- Nanishi E, Borriello F, O’Meara TR, et al. An aluminum hydroxide: CpG adjuvant enhances protection elicited by a SARS-CoV-2 receptor binding domain vaccine in aged mice. Sci Transl Med. 2022;14(629):eabj5305. doi: 10.1126/scitranslmed.abj5305
- Bajoria S, Kaur K, Kumru OS, et al. Antigen-adjuvant interactions, stability, and immunogenicity profiles of a SARS-CoV-2 receptor-binding domain (RBD) antigen formulated with aluminum salt and CpG adjuvants. Hum Vaccin Immunother. 2022;18(5):2079346. doi: 10.1080/21645515.2022.2079346
- Chen W, Jiang M, Yu W, et al. CpG-Based nanovaccines for cancer immunotherapy. Int J Nanomedicine. 2021;16:5281–5299. doi: 10.2147/IJN.S317626
- Das A, Ali N. Nanovaccine: an emerging strategy. Expert Rev Vaccines. 2021;20(10):1273–1290. doi: 10.1080/14760584.2021.1984890
- Smith DM, Simon JK, Baker JR. Applications of nanotechnology for immunology. Nat Rev Immunol. 2013;13(8):592–605. doi: 10.1038/nri3488
- Di J, Du Z, Wu K, et al. Biodistribution and non-linear gene expression of mRNA LNPs affected by delivery route and particle size. Pharm Res. 2022;39(1):105–114. doi: 10.1007/s11095-022-03166-5
- Keikha R, Daliri K, Jebali A. The use of nanobiotechnology in immunology and vaccination. Vaccines (Basel). 2021;9(2):74. doi: 10.3390/vaccines9020074
- Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19(12):1597–1608. doi: 10.1038/nm.3409
- Sun B, Wang X, Ji Z, et al. NLRP3 inflammasome activation induced by engineered nanomaterials. Small. 2013;9(9–10):1595–1607. doi: 10.1002/smll.201201962
- Schijns VE. Induction and direction of immune responses by vaccine adjuvants. Crit Rev Immunol. 2001;21(1–3):75–85. doi: 10.1615/CritRevImmunol.v21.i1-3.50
- Storni T, KUNDIG T, SENTI G, et al. Immunity in response to particulate antigen-delivery systems. Adv Drug Deliv Rev. 2005;57(3):333–355. doi: 10.1016/j.addr.2004.09.008
- Andrade S, Ramalho MJ, Loureiro JA, et al. Liposomes as biomembrane models: Biophysical techniques for drug-membrane interaction studies. J Mol Liq. 2021;334:116141. doi: 10.1016/j.molliq.2021.116141
- Fitzgerald KA, Kagan JC. Toll-like receptors and the control of immunity. Cell. 2020;180(6):1044–1066. doi: 10.1016/j.cell.2020.02.041
- Coccia M, Collignon C, Hervé C, et al. Cellular and molecular synergy in AS01-adjuvanted vaccines results in an early IFNγ response promoting vaccine immunogenicity. NPJ Vaccines. 2017;2(1):25. doi: 10.1038/s41541-017-0027-3
- Pifferi C, Fuentes R, Fernandez-Tejada A. Natural and synthetic carbohydrate-based vaccine adjuvants and their mechanisms of action. Nat Rev Chem. 2021;5(3):197–216. doi: 10.1038/s41570-020-00244-3
- Shi S, Zhu H, Xia X, et al. Vaccine adjuvants: Understanding the structure and mechanism of adjuvanticity. Vaccine. 2019;37(24):3167–3178. doi: 10.1016/j.vaccine.2019.04.055
- Wu D, Meydani SN. Age-associated changes in immune function: impact of vitamin E intervention and the underlying mechanisms. Endocr Metab Immune Disord Drug Targets. 2014;14(4):283–289. doi: 10.2174/1871530314666140922143950
- Jie J, Liu G, Feng J, et al. MF59 promoted the combination of CpG ODN1826 and MUC1-MBP vaccine-induced antitumor activity involved in the enhancement of DC maturation by prolonging the local retention time of antigen and down-regulating of IL-6/STAT3. Int J Mol Sci. 2022;23(18):10887. doi: 10.3390/ijms231810887
- Kumari P, Ghosh B, Biswas S. Nanocarriers for cancer-targeted drug delivery. J Drug Target. 2016;24(3):179–191. doi: 10.3109/1061186X.2015.1051049
- Aoshi T, Koyama S, Kobiyama K, et al. Innate and adaptive immune responses to viral infection and vaccination. Curr Opin Virol. 2011;1(4):226–232. doi: 10.1016/j.coviro.2011.07.002
- Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022;23(2):186–193. doi: 10.1038/s41590-021-01122-w
