ABSTRACT
Objectives
Despite national recommendations for use of pneumococcal vaccines, rates of community-acquired pneumonia (CAP) and invasive pneumococcal disease (IPD) remain high in Germany. New pneumococcal conjugate vaccines (PCVs) with expanded coverage have the potential to reduce the pneumococcal disease burden among adults.
Methods
Using a Markov model, we evaluated the lifetime outcomes/costs comparing 20-valent PCV (PCV20) with standard of care (SC) vaccinations for prevention of CAP and IPD among adults aged ≥60 years and at-risk adults aged 18–59 years in Germany. PCV20 also was compared with sequential vaccination with 15-valent PCV (PCV15) followed by PPSV23 in a scenario analysis.
Results
Over the course of a lifetime (82 years), use of PCV20vs. SC would prevent 54,333 hospitalizations, 26368 outpatient CAP cases, 10946 disease-related deaths yield 74,694 additional life-years (LYs), while lowering total medical costs by 363.2 M €. PCV20 remained cost saving (i.e. dominant) versus SC even in numerous sensitivity analyses, including a sensitivity analysis assuming moderate effectiveness of the SC pneumococcal polysaccharide vaccine against noninvasive pneumococcal CAP. In several scenario analyses and a probabilistic sensitivity analysis, PCV20 was also cost-saving compared toPCV15 PPSV23 vaccination.
Conclusions
One dose of PCV20 among adults aged ≥60 years and adults aged 18–59 years with moderate- and high-risk conditions wouldsubstantially reduce pneumococcal disease, save lives, and be cost saving compared with SC.
1. Introduction
Streptococcus pneumoniae is a major cause of invasive (IPD) and noninvasive diseases and associated with high rates of hospitalizations and premature death, especially among older adults [Citation1]. Community-acquired pneumonia (CAP) is the most common manifestation with more than 250,000 hospitalizations and 200,000 outpatient visits annually among adults in Germany [Citation1–3]. Interpretation of IPD trends in adults recorded by Germany’s national reference center are difficult due to improvements in disease reporting over time [Citation4]. However, several European countries with robust IPD surveillance systems have reported an increase in IPD incidence among older adults in the 4 years prior to the COVID-19 pandemic [Citation5–7].
Three vaccines have been available in Germany until recently to prevent pneumococcal disease: a 23-valent pneumococcal polysaccharide vaccine (PPSV23), a 13-valent pneumococcal conjugate vaccine (PCV13), and 10-valent PCV (PCV10) [Citation8]. The national Standing Committee on Vaccination (STIKO) recommends PCVs for children aged ≤24 months (2 + 1 schedule for term infants), and sequentially administered PCV13 and PPSV23 (PCV13PPSV23) for children and adolescents aged 2–15 years with underlying medical conditions [Citation8]. A single dose of PPSV23 is recommended for immunocompetent individuals aged ≥16 years with chronic medical conditions and for all immunocompetent adults aged ≥60 years. A single dose of PCV13 followed by PPSV23 (with at least 6-month interval) is recommended for all adults aged ≥16 years with immunocompromising conditions [Citation8]. According to STIKO recommendations, revaccination with PPSV23 after at least 6 years may be considered for individuals aged ≥16 years who have previously received PPSV23 [Citation8]. Currently, STIKO is assessing the risk benefit of higher-valent PCVs and respective recommendation is not published yet.
As a result of the indirect effects of pediatric PCV vaccination, the burden of IPD due to serotypes included in 10- and 13-valent PCVs has decreased among adults reaching a steady state in the last years [Citation7]. Nevertheless, the overall burden of pneumococcal disease among adults has increased, highlighting the need for vaccines with expanded serotype coverage [Citation7]. Next-generation PCVs have the potential to further reduce the burden of pneumococcal disease in Germany [Citation9].
Meanwhile, two higher-valent PCVs – a 20-valent PCV (PCV20) and a 15-valent PCV (PCV15) – that include the serotypes contained in PCV13 as well as additional epidemiologically important serotypes have been approved for use in adults by the European Medicines Agency (EMA) [Citation10–12]. PCV20 contains the serotypes in PCV15 (1, 3, 4, 5, 6A, 6B, 7F, 9 V, 14, 18C, 19A, 19F, 23F, 22F, 33F) plus five additional serotypes (8, 10A, 11A, 12F, 15B) [Citation13,Citation14]. Although 19 of the 20 serotypes in PCV20 are also present in PPSV23, PCV20 is expected to provide more robust protection due to its T-cell dependent immune response, generation of immunologic memory and induction of an antigenically broader and more durable antibody response [Citation15,Citation16]. Here, we evaluated the cost-effectiveness of PCV20 compared with the current standard of care (SC) and, alternatively, compared with PCV15 PPSV23, among adults aged 18 years and older in Germany. We take the payer perspective and evaluate the current German population over a lifetime.
2. Methods
2.1. Model description
The model utilizes a probabilistic framework and a Markov-type process to depict the lifetime risk of clinical outcomes and economic costs of pneumococcal diseases (i.e. IPD, all-cause CAP) in a hypothetical population of adults in Germany (). The model population is initially defined based on age (i.e. in one-year increments) and risk profile (i.e. low-risk (no comorbidities considered as risk factors for pneumococcal disease), moderate-risk (at least one chronic medical condition, no immunodeficiency), or high-risk (any primary or secondary immunodeficiency) [Citation17] for pneumococcal disease. Persons may transition to a higher risk group (i.e. from low-risk to moderate-risk, from moderate-risk to high-risk), but not to a lower risk group, during the modeling horizon. Transition probabilities between risk groups are described in Supplement Section 2.1. Persons may receive PPSV23 alone, PCV13 PPSV23, PCV15 PPSV23, PCV20 alone, or no vaccine at model entry.
Figure 1. Cost-effectiveness model schematic.
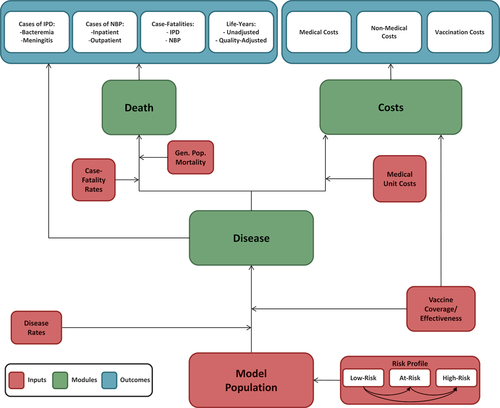
The model analyses a closed cohort that is vaccinated in year 1 with revaccination according to the vaccination strategy. Expected clinical outcomes and economic costs are projected for the model population on an annual basis, based on age, risk profile, disease/fatality risk, vaccination status, vaccine type, and time since vaccination. IPD is assumed to only include bacteremia and meningitis, and all-cause CAP is stratified by setting of care (inpatient vs. outpatient). Bacteremic CAP were classified as IPD. Persons vaccinated at model entry were at lower risk of future IPD and all-cause CAP. The magnitude of vaccine-associated risk reduction depended on the individual’s age and risk profile, clinical presentation (i.e. IPD or all-cause CAP), the vaccine(s) administered, time since vaccination, and the proportion of IPD and all-cause CAP assumed to be vaccine-preventable. Only disease caused by vaccine serotypes was reduced by vaccination.
Expected costs of medical treatment for IPD and all-cause CAP are generated based on event rates and unit costs in relation to the setting of care (i.e. inpatient vs. outpatient), age, and risk profile [Citation18]. Costs of vaccination – including the vaccine and its administration – are tallied in the year(s) in which vaccination occurs. The value of morbidity- and mortality-related work productivity loss also is tallied in the model. Clinical outcomes and economic costs are projected over the specified period of interest (e.g. remaining years of life from model entry [max. 82 years]) for the alternative vaccination strategies considered, and include expected: numbers of cases of IPD and all-cause CAP (inpatient and outpatient); number of deaths due to IPD and all-cause CAP; number of life-years (unadjusted and quality-adjusted); costs of medical treatment for IPD and all-cause CAP; value of morbidity- and mortality-related work loss; and costs of vaccination. Future life-years and costs were discounted annually, and a healthcare system perspective was employed for the base case, while the societal perspective was assessed in sensitivity analyses. The model was programmed in Microsoft Excel 2016 as a macro-enabled workbook (.xlsm).
2.2. Model estimation
Model parameter values were estimated primarily based on published literature; unpublished sources were employed as needed. Most model parameter values can be found in . Methods of estimation of model inputs are summarized below; more detailed descriptions are available in Supplementary Material.
Table 1. Base case input values×.
Population. The model population included all adults in Germany aged ≥18 years (N = 69.4 M), stratified across five age groups (18–49, 50–59, 60–64, 65–74, and 75–99 years) based on German census projections for 2022 [Citation19]. The population was grouped into low-risk (immunocompetent without chronic medical conditions predisposing to pneumococcal disease), moderate-risk (immunocompetent with ≥1 chronic medical condition predisposing to pneumococcal disease), and high-risk (immunocompromised) groups based on the medical conditions specified in STIKO pneumococcal vaccination recommendations [Citation8,Citation17]. The prevalence of low-risk, moderate-risk and high-risk status in the German population was determined based on Pelton et al. [Citation17] (Supplementary Material Table S1).
Disease Incidence. Values employed for disease incidence are summarized in Supplementary Material (Tables 4–5). Annual incidence of IPD was estimated by age and risk profile using age-specific disease rates, age-specific population distributions by risk profile, and risk-specific disease rates (for details see Supplement Section 2.3) [Citation17,Citation20,Citation31,Citation34]. IPD rates were apportioned between bacteremia and meningitis based on hospitalization data published by the Federal Statistical Office [Citation21]. Age- and risk-specific annual incidence of inpatient and outpatient all-cause CAP, respectively, were based on a recent study that employed 2016–2019 data and age-specific population distributions [Citation3,Citation19,Citation20].
Serotype Distribution and Vaccine Coverage. Values employed for serotype coverage are summarized in Supplementary Material (Tables 9–10). The proportion of IPD due to vaccine serotypes in year 1 of the modeling horizon was based on unpublished German National Reference Laboratory data provided to Pfizer [Pfizer GmbH, data on file] (Supplemental Table S9). The proportion of all-cause CAP due to pneumococcal serotypes contained in the respective vaccines [Citation35] in year 1 of the modeling horizon was based on the prevalence of vaccine serotypes in adults admitted to hospital with all-cause CAP in Germany between 2017 and 2018; vaccine serotypes in pneumonia patients were detected using urine antigen detection assays (UAD) (Supplemental Table S10) [Citation35].
Indirect Effects. Longstanding use of PCV13 in Germany has led to reductions in PCV13-type disease among unvaccinated adults through indirect effects (‘herd effects’). We assumed PCV13-type disease has reached a steady state but incorporated potential herd effects from pediatric use of PCV15 and PCV20. The model assumes that PCV15 and PCV20 will be recommended for children in Germany by model years 2 and 3, respectively. Therefore, herd effects for serotypes unique to PCV15 were assumed to begin in model year 3, and in model year 4 for serotypes unique to PCV20 (due to later approval of PCV20 compared to PCV15 for infants). Lacking German data, reductions in the proportion of IPD and CAP due to vaccine-serotypes in adults due to indirect effects from pediatric PCV15/PCV20 vaccination were extrapolated from the reductions in IPD caused by PCV13 non-PCV7 serotypes (excluding serotype 3) observed among adults following the introduction of pediatric PCV13 in the United States (US) [Pfizer GmbH, data on file]. In unvaccinated adults, we assume pediatric herd effects will reduce vaccine-type disease due to serotypes newly introduced into pediatrics by approximately 80% within the 5 years after introduction and approximately 90% within 10 years of introduction, as detailed in Table S11 in the supplement. The relative reductions in the prevalence of PCV20 serotypes among CAP patients assumed in the model are presented in Supplemental Table S10. Serotype replacement was assumed not to occur in the base case, thus the proportion of disease due to vaccine serotypes was re-based annually (model years 3–10) to account for the reduction in overall disease [Pfizer GmbH, data on file]. No indirect effects from adult vaccination were included in the model.
Number of deaths and Case fatality risks (CFR). Annual deaths were estimated by age and risk profile using age-specific disease rates and CFRs, and age-specific population distributions (Supplementary Material Tables 6–8) [Citation17,Citation20]. CRFs of IPD, hospitalized CAP, and outpatient CAP was based on published literature and reports [Citation17,Citation20,Citation22] (Supplementary Material Table S7). Age-specific all-cause German mortality was apportioned across risk groups based on assumed relative risk for mortality and age-specific risk distributions (Supplementary Material Table S8) [Citation17,Citation19].
Effectiveness of PCVs. Effectiveness data of PCVs are shown in Supplementary Material Tables 12–14. In year 1, effectiveness of PCVs against VT-IPD (VT-IPD) and vaccine-type CAP (VT-CAP) for low- and moderate-risk persons aged ≥18 years was based on data from the Community-Acquired Pneumonia Immunization Trial in Adults (CAPiTA) [Citation25,Citation26]. When discussing VT-IPD or VT-CAP cases, we mean IPD or CAP cases that are infected by serotypes covered by the vaccine a patient could have been vaccinated with in a given scenario. For high-risk persons aged 18–99 years, vaccine effectiveness (VE) against VT-IPD and VT-CAP in year 1 was assumed to be 80% of values for low/moderate-risk persons of the same age [Citation27]. In line with findings from CAPiTA and post-hoc analyses from the trial, initial VE-PCV against VT disease was assumed to be durable for 5 years [Citation25,Citation29], then to decline 5% annually during years 6–10, 10% annually during years 11–15, and to be zero from year 16 through the end of the modeling horizon [Citation26].
Scenario analyses were conducted using the VE assumptions used by the US Centers for Disease Control and Prevention (CDC) and Hoshi and colleagues in their evaluations of higher-valent PCVs [Citation36,Citation37]. For these scenarios, a VE lower than base-case PCV VE was assumed, as well as steeper waning of effectiveness, lower VE against ST3 disease, lower VE in immunocompromised individuals, and higher VE against VT-CAP not caused by ST3 in low-risk adults. Details are available in Supplemental Table S24.
Effectiveness of PPSV23. Initial age- and risk-specific VE-PPSV23 against VT-IPD was derived from a recent real-world effectiveness study conducted in the United Kingdom aged ≥18 years [Citation32] (Supplemental Table S13). VE-PPSV23 against VT-IPD was assumed to decline linearly beyond the first year of the modeling horizon, to 76.2% of initial VE by year 5 and to no effectiveness by year 10 [Citation33]. Consistent with evidence from recent publications, VE-PPSV23 against VT-CAP was assumed to be 0% [Citation38–40]. No boosting or blunting of immune response for the serotypes shared between both vaccines was assumed from vaccination with both PCV and PPSV23; the individual was assumed to benefit from both vaccines with the effects of each vaccine waning independently. Scenario analyses used CDC assumptions on PPSV23 VE (Supplemental Table S24). The initial VE of PPSV23 assumed by CDC was higher than our base case estimate, and the waning of VE was longer (15-year waning period). CDC also assumed some effectiveness against VT-CAP in contrast to the base case assumption used in previous assessments, while serotype 3 VE was assumed to be the same as the overall VE of PPSV23. Details are available in Supplemental Table S23.
Costs. Age-specific payer costs of IPD hospitalization (bacteremia and meningitis, respectively) were from a previously published cost-effectiveness analysis of pneumococcal vaccination in Germany which employed estimates based on national tariffs/diagnosis-related groups [Citation23,Citation41]. The costs were updated to the calendar year 2022 and expressed in Euro (2022 Euro) using the healthcare component of the consumer price index [Citation23,Citation41]. Age- and risk-specific costs of all-cause CAP requiring hospitalization and outpatient care only, respectively, were derived healthcare claims data and included insurer paid amounts for all healthcare encounters that occurred within 30 days of the start of the CAP episode (i.e. date of hospital admission or date of outpatient encounter for CAP) [Citation18].
The prices of PPSV23 (33.88 €), PCV13 (72.50 €), PCV15 (76.74 €), and PCV20 (76.74 €) were based on published list prices [Citation42]. The cost of vaccine administration was assumed to be 8.19 € [Citation23] (Supplementary Material Tables 16–17).
Utilities. General population health state utilities were the same as those employed in the aforementioned published cost-effectiveness analysis (Supplementary Material Table S15) [Citation23]. An annual utility decrement for persons who experienced IPD or CAP was applied during the year in which the event occurred. The one-year excess QALY loss attributed to all-cause CAP or IPD was 0.13 [Citation43] and to outpatient all-cause CAP was 0.004 [Citation26,Citation44].
2.3. Analyses
Base Case. Clinical outcomes and economic costs were projected over a lifetime (82 years) for the model population under PCV20 and SC (i.e. age/risk-dependent use of PPSV23 or PCV13 PPSV23 with PPSV23 revaccination). Vaccine coverage for PCV20 and PCV15- PPSV23 was based on published estimates of vaccine coverage for adults aged 18–59 with underlying conditions and all adults aged ≥60 years in 2020 and was assumed to be the same regardless of vaccine strategy (Supplementary Material Tables 18–19) [Citation24,Citation45]. In SC, all persons who received PCV13 at model entry were assumed to receive PPSV23 if alive in year 2; all persons who received a dose of PPSV23 (i.e. at model entry or in year 2) as part of SC were also assumed to receive a second dose of PPSV23 if alive 6 years after administration of the first dose. To convert values (costs and life years) received in the future to current time equivalents, we discounted future costs, life-years (LYs), and quality-adjusted LYs (QALYs) at 3.0% annually; analyses were conducted from the payer perspective (i.e. excluding patient paid amounts) [Citation46,Citation47].
Sensitivity. Sensitivity analyses were conducted to evaluate the robustness of base case results to changes in key model parameter values, including: changes to incidence parameters, case fatality rates, costs, proportion of all-cause CAP requiring hospitalization, proportion of PPSV23 revaccination (SC only), vaccine effectiveness, utilities, and herd effects [Citation33,Citation48]. To test extreme values and the impact of other sources, we changed the single parameters. The values used for the one-way sensitivity analyses are described in section 4 of the appendix. To test the joint uncertainty of the underlying parameter estimates, we performed a second-order stochastic probabilistic sensitivity analysis. For the typical probability distributions, we followed the guidance in Briggs et al. [Citation49,Citation50,Citation51]. For inputs such as proportions and utility values, we used the beta distribution which is confined by the interval 0–1. For costs, we used the gamma distribution (interval 0-infinity). Whenever possible, 95% confidence intervals (CIs), and standard errors (SEs) were used. Dependencies between parameters were accounted for. We performed the PSA with 1,000 iterations. The values used in the PSA are shown in Supplementary Material (Table S28).
Scenario. Several scenario analyses were conducted in which more than one parameter was varied and the impact on the results was tested (Supplementary Material Section 5). First, PCV20 and PCV15 PPSV23 (without PPSV23 revaccination) were compared; second, the societal perspective was adopted; third, the set of vaccine effectiveness parameters (including waning) were changed using sources from independent bodies such as CDC; forth, several different sources for case fatality rates and costs were changed; and finally, a scenario was analyzed where the lowest PCV and high PPV efficacy assumptions and low CAP cost assumptions were combined.
3. Results
Throughout a lifetime, use of PCV20 in lieu of SC in the German adult population would prevent an additional 2,801 (1.3%) IPD cases, 51532 (0.2%) hospitalized CAP cases, 26368 (0.2%) outpatient CAP cases, and 10,946 (0.2%) disease-related deaths (). This translates to 107 prevented IPD cases, 2,038 hospitalized CAP cases, and 1,048 outpatient CAP cases per year. Throughout a lifetime cohort of German adults, PCV20 vaccination would save 363.2 million € of the medical care costs and vaccination costs would increase by 6.5 million €, resulting in a 356.7 million € reduction in total costs. Also, PCV20 vaccination would save 74,694 additional total LYs, and 49,655 additional QALYs. The PCV20 strategy is dominant compared to SC (i.e. it resulted in more life years and fewer costs). In one-way sensitivity analyses assuming lower pneumococcal disease incidence, lower case fatality risks, treatment costs, lower PCV20 VE, or higher VE of PPSV23, PCV20 remained cost-saving (). In a probabilistic sensitivity analysis (), PCV20 remained cost saving over any combination of parameter variation within the ranges shown in Supplementary Material (Table S28).
Figure 2. Probabilistic sensitivity analysis PCV20 vs PPSV23/PCV13->PPSV23.
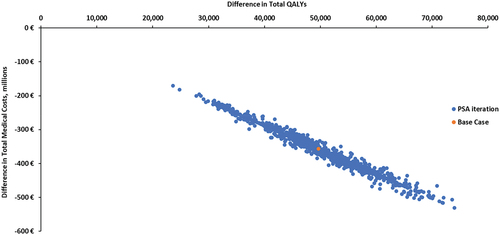
Table 2. Clinical and economic outcomes of PCV20 vs PPSV23/PCV13+PPSV23 among adults ages 18-99 in Germany (N=69,466,087).
The impact of model assumption was further explored in several scenarios varying more than one model parameter (). In one scenario PCV20 was compared to PCV15 PPSV23 instead of SC (, scenario 1). In this scenario PCV20 continued to result in better outcomes and would prevent 928 cases of IPD, 12485 cases of CAP requiring hospitalization, 5,978 cases of CAP in the outpatient setting, and 2,776 disease-related deaths more than PCV15 PPSV23. In scenario 1, medical care and vaccination costs would be 97.0 million € and 357.0 million € lower, respectively, and total costs would be lower by 454.0 million €. PCV20 would add 20,277 total LYs and 13,298 QALYs and therefore would be also cost-saving compared to PCV15 PPSV23.
Table 3. Clinical and economic outcomes – scenario analyses.
Other scenario analyses evaluated the effects of model inputs assuming a lower initial VE and faster wanting pattern for PCV13 and PCV20, reduced PCV13/PCV20 VE against serotype 3, combined with full effectiveness of PPSV23 against serotype 3, and some effectiveness of PPSV23 against CAP (, scenario 3, for model inputs see Supplemental Table S24). This scenario modeled smaller clinical benefits and smaller cost savings of PCV20 compared to SC and PCV20 would only prevent 947 cases of IPD, 644 cases of hospitalized CAP, and 1,460 cases of CAP in the outpatient setting more than the SC. Medical care would be lower by 17.1 million € and vaccination costs would increase by 6.5 million €. Thus, total costs would be lower by 10.6 million €. PCV20 would add 3,664 total LYs and 3,169 QALYs and was therefore dominant compared with SC. Scenario analyses assuming a societal perspective instead of a payer’s perspective, alternative CFRs, and alternative costs did not substantially change the total LY and QALY saved, or the cost saved compared to the base case and PCV20 remained cost-saving (, scenarios 2, 4, 5, and 6).
Only in the most extreme scenario that assumed reduced VE of PCV20 against serotype 3, combined with full effectiveness of PPSV23 against serotype 3, some effectiveness of PPSV23 against CAP as well as lower treatment costs for pneumococcal disease PCV20 was not cost saving, but showed a cost-effectiveness ratio of 214 € per life year and 247 € per QALY saved (, scenario 7).
4. Discussion
To the best of our knowledge, this is the first evaluation of the potential public health and economic impacts of next-generation pneumococcal vaccination strategies for adults in Germany. The results of our analyses suggest that replacing SC with a single dose of PCV20 for adults aged ≥60 years without underlying diseases and adults aged 18–59 years with moderate- and high-risk conditions would reduce the burden of pneumococcal disease and pneumococcal-related deaths, thereby reducing healthcare costs and resource usage and would be cost saving overall [Citation20]. Findings also suggest PCV20 would prevent more cases of disease and associated deaths and would reduce total costs compared with PCV15 followed by PPSV23. PCV20 remained cost-saving in one-way and probabilistic sensitivity analyses as well as scenario analyses that varied inputs for cost, disease burden, and vaccine effectiveness. Specifically, PCV20 remained cost-saving if moderate effectiveness of PPSV23 against noninvasive pneumococcal CAP was assumed (Suzuki 2019, Lawrence 2021), although costs averted and live years gained were 18% to 29% and 19% to 30% lower compared to our base case that assumed PPSV23 was not protective against noninvasive pneumococcal CAP, respectively [Citation33,Citation48].
Our findings are consistent with several recently published analyses considering use of PCV20 in other settings, including England, Denmark, the US, and Canada [Citation52–54]. Mendes and colleagues and Olsen and colleagues found that PCV20 was cost saving compared with current recommendations (i.e. PPSV23) in a cohort of all adults aged ≥65 years and moderate- and high-risk adults aged 18–64 years in England and Denmark, respectively [Citation52,Citation53]. In an analysis conducted by the US CDC Advisory Committee on Immunization Practices (ACIP) considering all adults aged ≥65 years and adults aged 19–64 years with chronic medical or immunocompromising conditions, a single dose of PCV20 was found to be cost saving compared with 2019 ACIP recommendations, which included age/risk-dependent use of [Citation37] PPSV23 alone or PCV13 PPSV23 [Citation37]. Likewise, recent analyses by the Public Health Agency of Canada found PCV20 was likely to be cost-effective in Canadian adults aged ≥65 years [Citation55]. We note that in a recent analysis by Smith et al., PCV20 use (vs. 2019 ACIP recommendations) among elderly US adults was found to cost $172,491 per QALY, however, differences in findings (compared with aforementioned US CDC analysis) are believed to be due, at least in part, to variation in model populations (e.g. Smith et al. excluded immunocompromised patients) as well as key parameter values (e.g. rates of pneumonia were assumed by Smith et al. to be lower) [Citation54]. Comparisons between the present study and the Smith et al. study are complicated by the differences between the German and US health systems, the standard of care comparator vaccine, as well as difference in model structure and populations. However, a key difference appears to be the incidence of vaccine-preventable disease in comparable population segments; with higher incidence PCV20-type CAP among unvaccinated low-risk adults 65 years of age assumed in our model than in the model by Smith et al. A similar pattern is seen across age and risk segments included in the models, and the present study also including high-risk adults amongst whom the vaccine is more likely to be cost-effective given their elevated rates of disease, a group excluded from the US analysis.
Our analyses have several limitations. First, we aimed at minimizing a potential conflict of interest arising from the author affiliation by strictly adhering to published practice guidelines [Citation46,Citation47,Citation56–60], using the most recent and highest quality data, providing detailed description of the methodology, and by addressing uncertainties with numerous sensitivity analyses. Specifically, we addressed uncertainties around vaccine effectiveness and duration of protection by performing sensitivity analyses that use model inputs for PCV20 and PPSV23 effectiveness based on assumptions by the US CDC [Citation36] and cost data used for the RKI model that were higher for meningitis, similar for bacteremia, and lower for CAP when compared to the respective medical costs in the base case [Citation22]. Second, we aimed at using model parameters based on German data. However, this was not always possible. When German data were not available, data from other high-income European countries with similar healthcare systems were applied. It was assumed that reductions in disease attributable to the seven new serotypes included in PCV20 (i.e. PCV20 non-PCV13 serotypes) among adults via pediatric use of next-generation vaccines higher-valent PCVs would be similar to reductions observed in PCV13 non-PCV7 type disease among adults following the introduction of pediatric PCV13 and that serotype replacement would not occur; however, the extent to which the magnitude and patterns of vaccine-type disease reduction may vary and whether replacement will occur is unknown.
In the base case, we did not model serotype replacement, explicitly. It is unknown to what extent replacement of serotypes newly covered by PCV15 and PCV20 will take place. Model simulations suggest that replacement may be less for high-valency PCVs [Citation61,Citation62]. However, we tested the impact of our base case assumptions on the overall results. The analyses were not very sensitive to assumptions on serotype replacement.
Also, the model did not consider adverse events originating from vaccination by PCV20, PCV15, PPSV23, or PCV13. As adverse events after pneumococcal vaccination resulting in healthcare seeking are rare and event rates are similar for the different pneumococcal vaccines, it is unlikely that the omission of adverse events from our model has introduced bias that would have affected our main conclusions. On the contrary, PCV20 is only administered once. Hence, the risk of adverse events is lower in the strategy where PCV20 is administered once compared to other strategies with vaccination with comparable safety profile administered more than once.
Another area of uncertainty surrounds vaccine effectiveness. Next-generation PCVs have been licensed based on studies demonstrating non-inferior immunogenicity compared the PCV13 or PPV13 [Citation14,Citation63–65]. Effectiveness of PCV20 and PCV15, respectively, against VT-IPD and VT-CAP was therefore assumed to be the same as VE-PCV13 against VT disease from CAPiTA [Citation25]. While it is anticipated that the additional serotypes in higher-valent PCVs will perform similarly to PCV13 in terms of disease prevention, it is possible that the effectiveness of PCV20 and/or PCV15 may [Citation32] differ from PCV13. Masala and colleagues estimated that increased valency may decrease vaccine effects [Citation62]. We tested this by simulating lower effectiveness of PCVs. Changes to the assumptions resulted in fewer cases avoided and smaller life expectancy increase. However, PCV20 kept being the dominant strategy avoiding more cases, increasing life-expectancy while costing less than the standard of care. The effectiveness of PPSV23 against VT-IPD was assumed to decline linearly as observed in a test-negative design study from Japan [Citation33]. We also note that the findings presented herein likely underestimate the impact of PCV20 use among adults in Germany because multiple studies evaluating the impact of PCV13 against all-cause CAP have reported larger reductions than what would be expected if accounting only for etiologically confirmed vaccine-type pneumonia [Citation66–68].
Some recently published real-world data analyses suggest that PPSV23 may provide limited protection against VT-CAP, but because there is considerable inconsistency across studies, we do not believe these data are sufficiently robust to employ in base case analyses [Citation33,Citation38,Citation40,Citation48,Citation69]. Nonetheless, we conducted scenario analyses that assumed moderate PPSV23 effectiveness against VT-CAP based on findings from Lawrence et al. and Suzuki et al., respectively [Citation33,Citation48]. Under these assumptions, SC and PCV15→PPSV23 prevented more cases of all-cause CAP and associated deaths (compared with base case analyses), but PCV20 remained dominant against both strategies in all analyses conducted, with cost savings ranging from 248 to 292 million € (vs. SC) and from 432 to 442 million € (vs. PCV15→PPSV23).
Current STIKO recommendations include revaccination with PPSV23 on a case-by-case basis only, however, estimates of the proportion of adults who are revaccinated are not available. We therefore assumed in base case analyses that all persons in the SC strategy who received PPSV23 alone or PCV13→PPSV23 at model entry received a second dose of PPSV23 (i.e. revaccination) 6 years later. Because this likely overestimates the cost – and associated benefits – of vaccination in the SC strategy, we conducted a sensitivity analysis in which only 10% of persons were revaccinated. With lower rates of revaccination, the costs of vaccination in SC dropped dramatically (nearly 50% compared with base case). Nonetheless, total costs with PCV20 were still slightly lower and PCV20 remained dominant against SC.
We used case fatality rates from a recently published article on the burden of pneumococcal disease [Citation20]. The mortality rates of CAP estimated in that paper were higher than previous estimates (2015) [Citation22], so we tested the impact of more recent higher CFR in sensitivity analyses using lower CFR, which were applied by a previous model of PCV13 cost-effectiveness [Citation22]. PCV20 strategy was still the dominating strategy, meaning it saved more life-years while costing less than the standard of care strategy.
The analyses presented herein were conducted from the payer perspective which does not reflect the true costs of medical care, such as patient paid amounts. Finally, our model, like all such health economic models, simplifies reality. For example, long-term consequences of pneumococcal disease – including exacerbations of underlying conditions such as COPD or asthma – also were not considered, despite evidence that such sequelae are common and costly [Citation70–74].
5. Conclusions
Use of a single dose of PCV20 among adults aged ≥60 years and adults aged 18–59 years with moderate- and high-risk conditions would reduce the burden of pneumococcal diseases, save more lives, and would be cost saving compared with SC. PCV20 alone would also be cost saving compared with use of PCV15→PPSV23 [Citation75–79]. The availability of a single-dose PCV20 strategy will likely improve vaccine uptake compared to sequential regimens which are often not completed, leading to greater benefit in terms of pneumococcal disease prevention. The simplicity of a single-dose strategy for pneumococcal disease prevention among adults may also reduce burden on providers, payers, and the healthcare system overall.
Future research examining the real-world impact of PCV20 use on pneumococcal disease burden in Germany should aim to capture other potential benefits of a single-dose vaccination strategy for pneumococcal disease prevention among adults.
Article highlights
Pneumococcal disease causes significant morbidity and mortality among adults in Germany
New, higher valent vaccines have the potential to reduce disease burden and associated costs in vulnerable populations
Over a lifetime, 20-valent pneumococcal conjugate vaccine was found to be cost-saving compared with current standard of care for pneumococcal disease prevention among adults in Germany
Declaration of interest
F Kühne, J Friedrich, R Sprenger, C Theilacker, C von Eiff, and J Vietri are employees of Pfizer. Ernestine Mahar was an employee of Pfizer Deutschland GmbH at the time of analysis, but not at the time of publication. All of which may hold stock or stock options. Mark Atwood is an employee of Policy Analysis Inc. (PAI), which received financial support from Pfizer for this study. K Achtert, F Püschner, D Urbanski-Rini, and J Schiller are employees of the Private Institute for Applied Health Services Research (inav), which received financial support from Pfizer for this study. Pfizer is the manufacturer one of the vaccines investigated in this paper. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium for their review work. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (299.5 KB)Acknowledgments
The authors would like to thank Reiko Sato and Julia Schiffner-Rohe for many valuable discussions and Mark van der Linden for providing IPD data. Further, we would like to thank Ahuva Hanau for medical writing.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2023.2262575
Additional information
Funding
References
- Pletz MW, Bahrs C. [Pneumococcal vaccination]. Internist. 2021;62(8):807–815. doi: 10.1007/s00108-021-01100-2
- Ambulant erworbene Pneumonie: Bundesauswertung zum Erfassungsjahr 2019. IQTIG–Institut für Qualitätssicherung und Transparenz im Gesundheitswesen B; 2020.
- Theilacker C, Sprenger R, Leverkus F, et al. Population-based incidence and mortality of community-acquired pneumonia in Germanys. PLoS One. 2021;16(6):e0253118. doi: 10.1371/journal.pone.0253118
- Robert Koch Institut. SurvStat@RKI 2.0 Web-basierte Abfrage der Meldedaten gemäß Infektionsschutzgesetz (IfSG) 2021. Rober Koch Institut; 2021.
- Hanquet G, Krizova P, Dalby T, et al. Serotype replacement after introduction of 10-valent and 13-valent pneumococcal conjugate vaccines in 10 countries, Europe. Emerg Infect Dis. 2022;28(1):137–138. doi: 10.3201/eid2801.210734
- Ladhani SN, Collins S, Djennad A, et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000-17: a prospective national observational cohort study. Lancet Infect Dis. 2018;18(4):441–451. doi: 10.1016/S1473-3099(18)30052-5
- van der Linden M, Imöhl M, Perniciaro S, et al. Limited indirect effects of an infant pneumococcal vaccination program in an aging population. PLoS One. 2019;14(8):e0220453. doi: 10.1371/journal.pone.0220453
- Ständige Impfkommission. Empfehlungen der Ständigen Impfkommission (STIKO) beim Robert Koch-Institut 2021. Epidemiologisches Bull. 2021;34:3–63.
- Hurley D, Griffin C, Young M, et al. Safety, tolerability, and immunogenicity of a 20-valent pneumococcal conjugate vaccine (PCV20) in adults 60 to 64 years of age. Clin Infect Dis. 2021;73(7):e1489–e1497. doi: 10.1093/cid/ciaa1045
- European Medicines Agency [Press Release]. Vaxneuvance: pneumococcal polysaccharide conjugate vaccine (15-valent, adsorbed). [cited 2021 Oct 14]. Available from: https://www.ema.europa.eu/en/documents/smop-initial/chmp-summary-positive-opinion-vaxneuvance_en.pdf.
- European Medicines Agency. Apexxnar product infomation. 2022
- European Medicines Agency [Press Release]. Apexxnar (pneumococcal polysaccharide conjugate vaccine, 20-valent, adsorbed) an overview of apexxnar and why it is authorised in the EU. [cited 2020 Mar 2]. Available from: https://www.ema.europa.eu/en/documents/overview/apexxnar-epar-medicine-overview_en.pdf.
- Pfizer Inc. [Press Release]. U.S. FDA approves PREVNAR 20™, Pfizer’s pneumococcal 20-valent conjugate vaccine for adults ages 18 years or older. [cited 2021 Jun 8]. Available from: https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-prevnar-20tm-pfizers-pneumococcal-20-valent
- Merck & Co. Inc. [Press Release]. Merck announces U.S. FDA approval of VAXNEUVANCE™ (pneumococcal 15-valent conjugate vaccine) for the prevention of invasive pneumococcal disease in adults 18 years and older caused by 15 serotypes. 2021 [cited 2021 Dec 16]. Available from: https://www.merck.com/news/merck-announces-u-s-fda-approval-of-vaxneuvance-pneumococcal-15-valent-conjugate-vaccine-for-the-prevention-of-invasive-pneumococcal-disease-in-adults-18-years-and-older-caused-by-15-serot/
- Clutterbuck EA, Lazarus R, Yu L-M, et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis. 2012;205(9):1408–1416. doi: 10.1093/infdis/jis212
- Davies LRL, Cizmeci D, Guo W, et al. Polysaccharide and conjugate vaccines to streptococcus pneumoniae generate distinct humoral responses. Sci Transl Med. 2022;14(656):eabm4065. doi: 10.1126/scitranslmed.abm4065
- Pelton SI, Shea KM, Farkouh RA, et al. Rates of pneumonia among children and adults with chronic medical conditions in Germany. BMC Infect Dis. 2015;15(1):470. doi: 10.1186/s12879-015-1162-y
- Mahar E, Sprenger R, Diesing J, et al. Case Fatality risk and attributable costs in patients treated for community-acquired pneumonia in Germany: a population-based healthcare claims data cohort study. 2022. PREPRINT. doi: 10.2139/ssrn.4118607
- Statistisches Bundesamt (Destatis). Population: Germany reporting date, age years [Table 12411-0005]. 2022 [cited 2022 Feb]. Available from: https://www.destatis.de/DE/Home/_inhalt.html.
- Deb A, Podmore B, Barnett R, et al. Clinical and economic burden of pneumococcal disease among individuals aged 16 years and older in Germany. Epidemiol Infect. 2022;150:e204. doi: 10.1017/S0950268822001182
- Statistisches Bundesamt. Gesundheitsberichterstattung des Bundes. 2021. Available from: https://www.gbe-bund.de/gbe/pkg_isgbe5.prc_menu_olap?p_uid=gast&p_aid=47092962&p_sprache=D&p_help=2&p_indnr=550&p_version=10&p_ansnr=38796254.
- Kuhlmann A, Treskova M, Graf von der Schulenburg J-M. Pneumokokkenerkrankungen bei Erwachsenen: Gesundheitsökonomische Evaluation unterschiedlicher Impfszenarien in Deutschland. Rober Koch Institut; 2016.
- Kuchenbecker U, Chase D, Reichert A, et al. Estimating the cost-effectiveness of a sequential pneumococcal vaccination program for adults in Germany. PLoS One. 2018;13(5):e0197905. doi: 10.1371/journal.pone.0197905
- Rieck T, Steffen A, Feig M, et al. Impfquoten bei Erwachsenen in Deutschland – Aktuelles aus der KV-Impfsurveillance. Epid Bull. 2022;49:3–23.
- Bonten MJM, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372(12):1114–1125. doi: 10.1056/NEJMoa1408544
- Mangen M-J, Rozenbaum MH, Huijts SM, et al. Cost-effectiveness of adult pneumococcal conjugate vaccination in the Netherlands. Eur Respir J. 2015;46(5):1407–1416. doi: 10.1183/13993003.00325-2015
- Klugman KP, Madhi SA, Huebner RE, et al. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349(14):1341–1348. doi: 10.1056/NEJMoa035060
- French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-Infected adults. N Engl J Med. 2010;362(9):812–822. doi: 10.1056/NEJMoa0903029
- Patterson S, Webber C, Patton M, et al. A post hoc assessment of duration of protection in CAPiTA (community acquired pneumonia immunization trial in adults). Trials Vaccinol. 2016;5:92–96. doi: 10.1016/j.trivac.2016.04.004
- McLaughlin JM, Jiang Q, Isturiz RE, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine against hospitalization for community-acquired pneumonia in older US adults: a test-negative design. Clinl Infect Dis. 2018;67(10):1498–1506. doi: 10.1093/cid/ciy312
- van Hoek AJ, Andrews N, Waight PA, et al. The effect of underlying clinical conditions on the risk of developing invasive pneumococcal disease in England. J Infect. 2012;65(1):17–24. doi: 10.1016/j.jinf.2012.02.017
- Djennad A, Ramsay ME, Pebody R, et al. Effectiveness of 23-valent polysaccharide pneumococcal vaccine and changes in invasive pneumococcal disease incidence from 2000 to 2017 in those aged 65 and over in England and Wales. EClinicalMedicine. 2018;6:42–50. doi: 10.1016/j.eclinm.2018.12.007
- Suzuki M, Dhoubhadel BG, Ishifuji T, et al. Serotype-specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia in adults aged 65 years or older: a multicentre, prospective, test-negative design study. Lancet Infect Dis. 2017;17(3):313–321. doi: 10.1016/S1473-3099(17)30049-X
- Reinert RR, Haupts S, van der Linden M, et al. Invasive pneumococcal disease in adults in North-Rhine Westphalia, Germany, 2001-2003. Clin Microbiol Infect. 2005;11(12):985–991. doi: 10.1111/j.1469-0691.2005.01282.x
- Bahrs C, Kesselmeier M, Kolditz M, et al. Pneumococcal serotype distribution in adults with community-acquired pneumonia in Germany and coverage of an investigational 20-valent conjugate vaccine: a longitudinal analysis from the CAPNETZ cohort 2013-2018. 31st European Congress of Clinical Biology and Infectious Diseases; July 9–12, 2021. 2021.
- Hoshi SL, Shono A, Seposo X, et al. Cost-effectiveness analyses of 15- and 20-valent pneumococcal conjugate vaccines for Japanese elderly. Vaccine. 2022;40(49):7057–7064. doi: 10.1016/j.vaccine.2022.10.010
- Leidner AJ Summary of three economic models assessing pneumococcal vaccines in US adults. In: ACIP Meeting. Atanta (GA): National Center for, I, Respiratory Diseases. Immunization Services, D; 2021.
- Chandler T, Furmanek S, Carrico R, et al. 23-valent pneumococcal polysaccharide vaccination does not prevent community-acquired pneumonia hospitalizations due to vaccine-type streptococcus pneumoniae. Microorganisms. 2022;10(3):560. doi: 10.3390/microorganisms10030560
- Dunne EM, Cilloniz C, von Mollendorf C, et al. Pneumococcal vaccination in adults: what can we learn from observational studies that evaluated PCV13 and PPV23 effectiveness in the same population? Arch Bronconeumol. 2023;59(3):157–164. doi: 10.1016/j.arbres.2022.12.015
- Heo JY, Seo YB, Choi WS, et al. Effectiveness of pneumococcal vaccination against pneumococcal pneumonia hospitalization in older adults: a prospective, test-negative study. J Infect Dis. 2021;225(5):836–845. doi: 10.1093/infdis/jiab474
- Statistisches Bundesamt (Destatis). Consumer price index: Germany reporting date, application [Table 61111]. 2022 [cited 2022 Dec]. Available from: https://www.destatis.de/DE/Home/_inhalt1.html.
- LAUER-FISCHER GmbH. WEBAPO® InfoSystem. LAUER-TAXE® Kompetenz online. 2023.
- Mangen M-J, Huijts SM, Bonten MJM, et al. The impact of community-acquired pneumonia on the health-related quality-of-life in elderly. BMC Infect Dis. 2017;17(1):208. doi: 10.1186/s12879-017-2302-3
- Melegaro A, Edmunds WJ. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine. 2004;22(31–32):4203–4214. doi: 10.1016/j.vaccine.2004.05.003
- Robert Koch-Institut. Impfquoten bei Erwachsenen in Deutschland, STIKO: Bestätigung der Pneumokokken-Impfempfehlung. Epidemiologisches Bull. 2020;47:27–30.
- IQWiG. Allgemeine Methoden Version 6.1 vom 24.01.2022. 2022. Available from: https://www.iqwig.de/methoden/allgemeine-methoden-v6-1.pdf.
- Ständige Impfkommission. STIKO. Modelling methods for predicting epidemiological and health economic effects of vaccinations – guidance for analyses to be presented to the German Standing Committee on vaccination (STIKO). 2016 [last updated 2016 Mar 16]. Berlin.
- Lawrence H, Pick H, Baskaran V, et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against vaccine serotype pneumococcal pneumonia in adults: a case-control test-negative design study. PLOS Med. 2020;17(10):e1003326. doi: 10.1371/journal.pmed.1003326
- Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford, UK: Oxford University Press; 2006.
- Hoshi S-L, Kondo M, Okubo I. Economic evaluation of immunisation programme of 23-valent pneumococcal polysaccharide vaccine and the inclusion of 13-valent pneumococcal conjugate vaccine in the list for single-dose subsidy to the elderly in Japan. PLoS One. 2015;10(10):e0139140.
- Kolditz M, Tesch F, Mocke L, et al. Burden and risk factors of ambulatory or hospitalized CAP: a population based cohort study. Respir med. 2016;121:32–38. doi: 10.1016/j.rmed.2016.10.015
- Mendes D, Averin A, Atwood M, et al. Cost-effectiveness of using a 20-valent pneumococcal conjugate vaccine to directly protect adults in England at elevated risk of pneumococcal disease. Expert Rev Pharmacoecon Outcomes Res. 2022;22(8):1285–1295. doi: 10.1080/14737167.2022.2134120
- Olsen J, Schnack H, Skovdal M, et al. Cost-effectiveness of 20-valent pneumococcal conjugate vaccine in Denmark compared with PPV23. J Med Econ. 2022;25(1):1240–1254. doi: 10.1080/13696998.2022.2152235
- Smith KJ, Wateska AR, Nowalk MP, et al. Higher-valency pneumococcal conjugate vaccines: an exploratory cost-effectiveness analysis in U.S. Seniors. Am J Preventive Med. 2021;61(1):28–36. doi: 10.1016/j.amepre.2021.01.023
- Wierzbowski A, Pless R, Hildebrand K. On behalf of the National Advisory Committee on Immunization (NACI). Summary of the NACI Statement on Public Health Level recommendations on the use of pneumococcal vaccines in adults, including the use of 15-valent and 20-valent conjugate vaccines. Can Commun Dis Rep. 2023;49(2/3):81–86. doi: 10.14745/ccdr.v49i23a08
- Briggs AH, Weinstein MC, Fenwick EA, et al. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM modeling good research practices task force working group-6. Med Decis Making. 2012;32(5):722–732. doi: 10.1177/0272989X12458348
- Caro JJ, Briggs AH, Siebert U, et al. Modeling good research practices–overview: a report of the ISPOR-SMDM modeling good research practices task force-1. Med Decis Making. 2012;32(5):667–677. doi: 10.1177/0272989X12454577
- Gold M, Siegel J, Russell L, et al. Cost-effectiveness in health and medicine. (NY): Oxford University Press; 1996.
- Siebert U, Alagoz O, Bayoumi AM, et al. State-transition modeling: a report of the ISPOR-SMDM modeling good research practices task force-3. Med Decis Making. 2012;32(5):690–700. doi: 10.1177/0272989X12455463
- Ultsch B, Damm O, Beutels P, et al. Methods for health economic evaluation of vaccines and Immunization decision frameworks: a consensus framework from a European vaccine economics community. PharmacoEconomics. 2016;34(3):227–244. doi: 10.1007/s40273-015-0335-2
- Croucher NJ, Løchen A, Bentley SD. Pneumococcal vaccines: host Interactions, population dynamics, and design principles. Annu Rev Microbiol. 2018;72(1):521–549. doi: 10.1146/annurev-micro-090817-062338
- Masala GL, Lipsitch M, Bottomley C, et al. Exploring the role of competition induced by non-vaccine serotypes for herd protection following pneumococcal vaccination. J R Soc Interface. 2017;14(136):20170620. doi: 10.1098/rsif.2017.0620
- Essink B, Sabharwal C, Cannon K, et al. Pivotal phase 3 randomized Clinical trial of the Safety, tolerability, and immunogenicity of 20-valent pneumococcal conjugate vaccine in adults aged ≥18 years. Clinl Infect Dis. 2022;75(3):390–398. doi: 10.1093/cid/ciab990
- Mohapi L, Pinedo Y, Osiyemi O, et al. Safety and immunogenicity of V114, a 15-valent pneumococcal conjugate vaccine, in adults living with HIV. AIDS. 2022;36(3):373–382. doi: 10.1097/QAD.0000000000003126
- Song J-Y, Chang C-J, Andrews C, et al. Safety, tolerability, and immunogenicity of V114, a 15-valent pneumococcal conjugate vaccine, followed by sequential PPSV23 vaccination in healthy adults aged ≥50 years: a randomized phase III trial (PNEU-PATH). Vaccine. 2021;39(43):6422–6436. doi: 10.1016/j.vaccine.2021.08.038
- Gessner BD, Isturiz R, Snow V, et al. The rationale for use of clinically defined outcomes in assessing the impact of pneumococcal conjugate vaccines against pneumonia. Expert Rev Vaccines. 2021;20(3):269–280. doi: 10.1080/14760584.2021.1889376
- Hsiao A, Hansen J, Timbol J, et al. Incidence and estimated vaccine effectiveness against hospitalizations for all-cause pneumonia among older US adults who were vaccinated and not vaccinated with 13-valent pneumococcal conjugate vaccine. JAMA Netw Open. 2022;5(3):e221111–e221111. doi: 10.1001/jamanetworkopen.2022.1111
- Lewnard JA, Bruxvoort KJ, Fischer H, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine against medically attended lower respiratory tract infection and pneumonia among older adults. Clin Infect Dis. 2022;75(5):832–841. doi: 10.1093/cid/ciab1051
- Kim JH, Chun BC, Song JY, et al. Direct effectiveness of pneumococcal polysaccharide vaccine against invasive pneumococcal disease and non-bacteremic pneumococcal pneumonia in elderly population in the era of pneumococcal conjugate vaccine: a case-control study. Vaccine. 2019;37(21):2797–2804. doi: 10.1016/j.vaccine.2019.04.017
- Bornheimer R, Shea KM, Sato R, et al. Risk of exacerbation following pneumonia in adults with heart failure or chronic obstructive pulmonary disease. PLoS One. 2017;12(10):e0184877. doi: 10.1371/journal.pone.0184877
- Campling J, Jones D, Chalmers J, et al. Clinical and financial burden of hospitalised community-acquired pneumonia in patients with selected underlying comorbidities in England. BMJ Open Respir Res. 2020;7(1):e000703. doi: 10.1136/bmjresp-2020-000703
- Corrales-Medina VF, Alvarez KN, Weissfeld LA, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313(3):264–274. doi: 10.1001/jama.2014.18229
- Hughes GJ, Wright LB, Chapman KE, et al. Serotype-specific differences in short- and longer-term mortality following invasive pneumococcal disease. Epidemiol Infect. 2016;144(12):2654–2669. doi: 10.1017/S0950268816000856
- Myint PK, Hawkins KR, Clark AB, et al. Long-term mortality of hospitalized pneumonia in the EPIC-Norfolk cohort. Epidemiol Infect. 2016;144(4):803–809. doi: 10.1017/S0950268815001971
- Black CL, Williams WW, Warnock R, et al. Pneumococcal vaccination among medicare beneficiaries occurring after the advisory committee on immunization practices recommendation for routine use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults aged ≥65 years. MMWR Morb Mortal Wkly Rep. 2017;66(27):728–733. doi: 10.15585/mmwr.mm6627a4
- Morga A, Kimura T, Feng Q, et al. Compliance to advisory committee on immunization practices recommendations for pneumococcal vaccination. Vaccine. 2022;40(15):2274–2281. doi: 10.1016/j.vaccine.2022.03.005
- Perniciaro S, van der Linden M. Pneumococcal vaccine uptake and vaccine effectiveness in older adults with invasive pneumococcal disease in Germany: a retrospective cohort study. Lancet Reg Health- Eur. 2021;7:100126. doi: 10.1016/j.lanepe.2021.100126
- Sprenger R, Häckl D, Kossack N, et al. Pneumococcal vaccination rates in immunocompromised patients in Germany: a retrospective cohort study to assess sequential vaccination rates and changes over time. PLoS One. 2022;17(3):e0265433. doi: 10.1371/journal.pone.0265433
- Yang X, Zhang D, Ou W. Pneumococcal vaccination patterns among persons aged 65 years or older in the United States: a retrospective database analysis. Vaccine. 2018;36(49):7574–7579. doi: 10.1016/j.vaccine.2018.10.015
