ABSTRACT
Background
The Belgian Superior Health Council (SHC) preferentially recommended the 20-valent pneumococcal conjugate vaccine (PCV20) for adults aged ≥65 years, immunocompromised patients, and patients aged ≥50 years suffering from conditions that increase their risk for pneumococcal infections. The objective of this paper is to present the cost-utility of PCV20 compared to no vaccination and the alternative sequence of PCV15 followed by the 23-valent pneumococcal polysaccharide vaccine (PPV23) in this population.
Research Design and Methods
The analysis employed a static Markov model capturing lifetime risk of pneumococcal infections, associated disutility, mortality, and costs from different healthcare payer perspectives.
Results
Results indicated use of PCV20 among Belgian older and at-risk adults is highly cost-effective compared to no vaccination, with an incremental cost per quality-adjusted life-year (QALY) of €4,164. Compared to the sequential regimen (PCV15+PPV23), PCV20 vaccination is a cost-saving strategy. Subgroup analysis indicated PCV20 vaccination of at-risk adults aged 65–84 years would also be cost-saving from the national healthcare perspective.
Conclusion
Based on current knowledge, this analysis suggests that access to PCV20 should be proposed in all adults recommended for vaccination by the SHC as PCV20 prevents additional hospitalizations and deaths caused by pneumococcal infection at an affordable cost.
Plain Language Summary
Pneumococcal infections cause a high burden on infected patients and society. Vaccination of patients at risk of severe infection has been recommended for decades, but uptake of pneumococcal vaccines in adults has historically been low in Belgium, where patients have borne the vaccine costs and the recommended vaccination schedule required the sequential administration of two vaccines. A single PCV20 dose is recommended as the preferred vaccine for adults at risk due to age or other factors in Belgium as it is expected to provide lasting protection against more types of disease-causing pneumococcal bacteria as well as being simpler to administer than alternatives requiring multiple injections. Uptake is expected to improve with the recent reimbursement of the new PCV20 vaccine, though reimbursement covers only a portion of the recommended population. This paper presents a detailed analysis of the PCV20 cost-effectiveness in all adults at increased risk of severe pneumococcal disease, including immunocompromised adults younger than 65 years. Our analysis captures and compares the lifetime risk of pneumococcal disease and associated healthcare costs in an unvaccinated cohort, a cohort vaccinated with the alternative recommendation of PCV15 and PPV23 vaccines and a cohort vaccinated with PCV20. This cost-effectiveness analysis indicates that use of PCV20 will help decrease the number of pneumococcal disease cases, hospitalizations, and premature deaths at an affordable healthcare cost: PCV20 is a cost-effective option compared to no vaccination and a cost-saving option compared to the sequential regimen PCV15 followed by PPV23 in the Belgian adult population recommended for pneumococcal vaccination.
1. Introduction
Pneumococcal disease causes significant morbidity and mortality in the elderly and frail adults [Citation1–4]. This respiratory infectious disease caused by Streptococcus pneumoniae can be classified as invasive or non-invasive. More than 95 serotypes of Streptococcus pneumoniae have been identified but a limited number causes disease. Invasive pneumococcal disease (IPD), including bacteremia and meningitis, is rare but severe, requiring hospitalization and often leading to death or complications [Citation4,Citation5]. Non-invasive pneumococcal disease, typically non-bacteremic pneumonia (NBP) is more common but less severe; older adults and patients with underlying chronic condition(s) are, however, at higher risk to be admitted for long hospital stay [Citation6].
Published data on the incidence of pneumococcal infections acquired in the community are limited in Belgium. The epidemiology of invasive pneumococcal disease is closely tracked by a network of laboratories, but no accurate data are currently available for non-invasive pneumococcal diseases [Citation4]. Blommaert et al. have estimated that non-invasive pneumococcal infections were 4.78 times more prevalent in hospital than invasive cases [Citation1]. Based on this data source, the incidence of hospitalizations due to invasive pneumococcal disease was estimated at 44.55 per 100,000 adults aged 65 years and older in 2019 (pre-SARS-Cov-2 pandemic period). In this age group, the incidence of hospitalizations due to non-invasive pneumococcal disease is estimated at 212.95 per 100,000 older adults. Based on a general practitioners’ network database, it is assumed that twice as many non-invasive pneumococcal infection cases are treated in the ambulatory settings [Citation7].
With aging of the population, pneumococcal infections represent a growing threat [Citation8]. A targeted vaccination program will help to tackle this challenge, preventing this severe respiratory infection in a vulnerable population [Citation9].
There are two types of pneumococcal vaccines to protect adults against this respiratory infection: capsular polysaccharide and protein-polysaccharide conjugate vaccines. The 23-valent plain polysaccharide vaccine (PPV23) containing 23 serotypes of Streptococcus pneumoniae has been available for more than 30 years but effectiveness was assessed as poor in older adults and patients with underlying conditions [Citation10]. Vaccines with capsular polysaccharides conjugated to protein have been developed to address the shortcomings of plain polysaccharide pneumococcal vaccines but contain fewer disease-causing serotypes. Since the availability of the first conjugate vaccine PCV13 for adults (containing 13 serotypes) the Belgian Superior Health Council (SHC) has recommended sequential vaccination with PCV13 followed by PPV23 in all adults aged 65–84 years, immunocompromised adults, and patients 50 years of age and older with chronic underlying condition(s) [Citation2]. Vaccination of adults aged 85 years and older is determined on an individual basis. The adherence to the sequential schedule (PCV13+PPV23) was poor and adults’ protection low [Citation11]. The concurrent infant immunization program with PCV13 has led to a decline of the PCV13-serotype IPD cases in all age groups through herd protection, and at the same time an increase of non PCV13-serotype IPD was observed. Higher valency pneumococcal conjugate vaccines (PCV15 and PCV20) have consequently been developed to address the disease burden of non-PCV13 serotypes [Citation12]. In August 2022, the Belgian SHC updated its recommendation to include both new vaccines. Based on its higher serotype coverage and the simplified vaccination schedule, a single dose of PCV20 has been preferentially recommended for the adult pneumococcal vaccination [Citation2]. PCV15 followed by PPV23 (after a minimum of 8 weeks for patients with risk conditions or 1 year in healthy persons of 65 years and older) has been recommended as an alternative vaccination to the preferential single dose of PCV20.
Pneumococcal disease results in high economic burden in the older and at-risk adult population and has an impact on the patient’s quality of life [Citation13,Citation14].
In 2016, the KCE (Kenniscentrum-Centre d’Expertise) investigated the cost-effectiveness of the pneumococcal vaccines (PCV13 and PPV23) in the population aged 50 years and older [Citation1]. Even though the authors expressed difficulty in this analysis due to uncertainties in vaccine effectiveness, burden of disease influenced by the infant immunization program and willingness to pay for one additional QALY, they had a preference for a single dose vaccine with a large serotype coverage. Of note, the population selected for this analysis was not fully in line with the population recommended by the SHC as the patients’ risk profile was not taken into account. In another study that considered the patients’ risk profile as well as the updated infant immunization program, PCV13 compared to no vaccination was cost-effective [Citation15]. However, due to remaining uncertainties, the National Institute for Health and Disability Insurance (NIHDI) decided that PCV13 could not be reimbursed at the requested price.
Based on the preferred recommendation and the higher serotype coverage, a request for reimbursement of a single dose of PCV20 was again submitted to the NIHDI. This was granted for the subgroup of 65–80 year olds with underlying conditions [Citation16].
An overview of the scientific recommendations and funding of pneumococcal vaccines in the Belgian context have been provided in supplement 1 Figures S1 and S2.
The objective of the present analysis is to assess the cost-utility of PCV20 compared to no vaccination and, alternatively, PCV15 followed by PPV23 in the adult population recommended for pneumococcal vaccination by the SHC. Subgroup analysis was also conducted from different healthcare payer’s perspectives.
2. Methods
2.1. Overview
A cost-utility model was used to assess the health economic profile of PCV20. The choice for a cost-utility analysis is justified by the impact of pneumococcal vaccination on both mortality and quality of life of the vaccinated population. This analysis captures the benefits of pneumococcal vaccination over a lifetime horizon in a cohort vaccinated at model entry. Different cost perspectives have been considered for specific groups of adults: 1) the all health-care payers cost perspective for the adults recommended for vaccination by the SHC; 2) the NIHDI cost perspective for the adults who were granted reimbursement for PCV20; and 3) the patient perspective for the adults who are at increased risk of pneumococcal disease and have to fully finance access to the PCV20 vaccine.
2.2. Model structure and assumptions
The cost-utility analysis is based on a model originally developed to evaluate the cost-effectiveness of PCV13 [Citation15,Citation17]. The major changes in the structure of the model concern the addition of pneumococcal vaccination history and the inclusion of herd effect following general infant vaccination. This updated cost-utility model has already been published and customized in the United Kingdom, Italy, and Spain [Citation18–20].
The static Markov model describes the lifetime risk of clinical outcomes and economic costs of pneumococcal disease in the defined population. Detailed description of the model can be found in Mendes et al. [Citation18].
The model population is characterized by pre-specified age groups (18–49 years, 50–64 years, 65–74 years, 75–84 years, and 85–99 years) and by risk profile (low-, moderate- or high-risk of pneumococcal disease). The low-risk group is defined as healthy adults; the moderate risk group as immunocompetent adults with chronic underlying condition(s) associated with an increased risk of pneumococcal disease and the high-risk group as immunocompromised patients. Over time, the adults can only transition to a more severe risk profile. The present analysis targets all adults at high risk, adults aged 50 years and older at moderate risk, and adults aged 65–84 years at low risk, in accordance with the SHC’s recommendations. The NIHDI has recently granted reimbursement for adults aged 65–80 years with moderate risk. Due to the pre-specified age groups in the cost-effectiveness model, the analysis will refer to the adults aged 65–84 years with moderate risk to define this population.
Clinical outcomes include the number of cases of IPD and NBP as well as the number of deaths due to pneumococcal disease. IPD is defined by bacteremia and meningitis, and NBP is stratified by care setting (hospitalized vs. outpatient). The extent of vaccine-associated risk reduction depends on the pneumococcal disease type (IPD or NBP), as well as on time since vaccination, age, and risk profile. PCV20 has been approved for the prevention of IPD and pneumococcal pneumonia in adults, based on immune bridging with common serotypes in PCV13, which has demonstrated significant vaccine efficacy for vaccine type IPD and NBP in the CAPiTA study in adults [Citation21]. Risk of death from IPD, NBP, and other causes depends upon the patient’s age and risk profile.
The final clinical outcomes by intervention are expressed in number of life-years (quality of life-unadjusted and adjusted).
Costs related to the healthcare management of IPD and NBP are defined by age, risk profile, and care setting (hospitalized vs. outpatient). The cost of vaccination is applied in the year in which the vaccine is administered.
Clinical outcomes and related costs are estimated from model entry until death and discounted annually at 1.5% for benefits and 3.0% for costs. Because of the age of the population and in the absence of robust data on caregivers’ productivity loss, the societal perspective was not considered in the present analysis.
A model is necessarily a simplification of reality and some choices have been made. Firstly, meningitis-related sequelae, even if very severe and disabling, have not been included in the analysis as the risk is low (annual incidence <1 per 100,000). Due to lack of robust data, other long-term complications associated with pneumococcal disease were also disregarded. The inclusion of these complications would have resulted in a more accurate cost-effectiveness analysis of pneumococcal vaccine [Citation22]. Second, the maximum vaccine coverage (i.e. uptake) is assumed as of the first year of the analysis and not implemented progressively over the years. Finally, adverse events from vaccination have not been taken into consideration as these are limited and have a negligible impact on outcomes.
2.3. Inputs
2.3.1. Epidemiological, effectiveness, and (dis)utility data
Most of the data were retrieved from previous cost-effectiveness analyses of PCV13 and updated to consider the epidemiological evolution of pneumococcal disease (hospitalized and outpatient) as well as the enhanced protection due to the additional serotypes included in PCV20 [Citation1,Citation15]. Evidence of the epidemiological data has been improved by additional real-world data collection. Inputs included in the analysis have also been reviewed by a panel of Belgian experts (acknowledgments).
The model population included all adults aged 18–99 years with high risk, adults aged 50–99 years with moderate risk and adults aged 65–84 years with low risk, in accordance with the SHC’s recommendations.
The age groups-specific risk distribution of adults in the model population was estimated based on data from the INTEGO database. INTEGO is a Flemish morbidity registry that represents 102 general practice centers and contains medical records of more than 300,000 patients [Citation7,Citation23]. Patients included in this database were stratified according to their underlying conditions (defined as in the SHC’s recommendations) and immunocompromised status. The distribution of the risk profile (low, moderate, high) retrieved from this analysis was assumed to be representative of the Belgian population. The data have confirmed the increase of risk profile with age.
Age-specific incidence rates of IPD were obtained from the National Reference Laboratory and were reported for the year 2019 (pre-SARS-Cov-2 pandemic year, as advised by Belgian expert panel) [Citation24]. In line with KCE Report 274, these data were adjusted for underreporting of the laboratories network [Citation1]. As we have used a static model, an additional correction was applied to consider herd immunity due to the infant immunization program. The herd effect due to PCV13 pediatric vaccination was estimated over the 2019–2024 period based on the herd immunity observed during a previous PCV13 pediatric vaccination program (2011–2015) [Citation1]. The consulted Belgian expert panel does not expect additional herd effect on the 13 serotypes beyond year 2024. At that time, pediatric vaccination was assumed to switch to PCV15/PCV20. With the introduction of these new vaccines in the infant immunization program, similar herd immunity that observed with PCV13 has been assumed (namely a stabilization after a 50% decrease of the additional serotypes over 5 years). In the base case, we have assumed a decrease of pneumococcal disease incidence as no further serotype replacement is expected to occur. This assumption was tested in a scenario analysis in which herd effects were assumed to occur over a longer period of time; details on inputs employed in this alternative assumption can be found in Supplement 2.
Contrary to IPD, there is no Belgian surveillance system for pneumococcal NBP in place. The age-specific incidence of pneumococcal NBP has consequently been estimated based on the KCE Report 274: the incidence of pneumococcal NBP requiring hospitalization was assumed to be 4.78 times higher than the IPD hospitalization incidence [Citation1]. Based on this estimate, Streptococcus pneumoniae would be responsible for 22.5% of all-cause pneumonia hospitalizations in the adult population. The total number of hospitalizations due to community acquired all-cause pneumonia was obtained from the national hospital Technische Cel-Cellule Technique (TCT) database (ICD-10 codes J12-J18).
Age- and risk-specific incidence rates of outpatient NBP were derived from the INTEGO database, based on the ICPC-2 code for pneumonia (R81). Streptococcus pneumoniae was assumed to cause 10.5% of these pneumonia cases as reported in the KCE Report 274 and validated by the Belgian expert panel. The herd effect due to infant immunization is similarly applied as in IPD (Supplement 2).
Age- and risk-specific incidence rates of IPD and NBP were estimated based on age-specific incidence, underlying population risk distribution and the relative risk of all-cause pneumonia for moderate- and high-risk adults (vs. low-risk adults), retrieved from the INTEGO database [Citation23]. These estimates might be subject to future additional research.
In the absence of standard NBP surveillance, the serotype distribution reported in IPD was applied to the serotype distribution in NBP [Citation24]. While cost-effectiveness analyses of PCV20 in other European countries present a relative uniformity of the serotype distribution in IPD, a large range of serotype distributions is reported in NBP [Citation18–20].
Pneumococcal disease causes premature death in the older and at-risk adult populations. Age-specific mortality rates were derived from KCE Report 274 [Citation1,Citation15]. As mentioned by the authors of the KCE Report 274, the mortality rates might be considered conservative. The underlying conditions and age have an impact on the mortality rate [Citation6,Citation25]. A retrospective database analysis was conducted with a Belgian hospital network to estimate the relative risk of mortality between risk groups in hospital setting (Ecole de Santé Publique ULB database).
The Belgian age-specific utility values obtained from a national survey conducted in year 2018 were combined with risk-specific utilities from a study conducted on the impact of multimorbidity patterns on health‑related quality of life to estimate age- and risk-specific utility values [Citation26,Citation27].
The disutility due to pneumococcal disease (IPD and NBP) was derived from KCE Report 274 and assumed to be invariant by risk profile [Citation1].
The impact of vaccination on pneumococcal disease burden in the older and at-risk adult populations depended on vaccine uptake, vaccine effectiveness against S. pneumoniae and circulating serotypes. Conjugate vaccine (PCV20/PCV15) effectiveness was derived from the results of the CAPiTA study in low- to moderate-risk older adults and from other published data for high-risk adults [Citation17,Citation21,Citation28–30]. Based on the outcomes of these studies, effectiveness in high-risk adults was assumed to be 80% of the efficacy observed in the CAPiTA study. These effectiveness outcomes were assumed to be the same for all vaccine serotypes [Citation28,Citation29]. Conjugate vaccine effectiveness was assumed to remain constant over the first 5 years since vaccination and to progressively decrease over time by 5% annually from year 6 to year 10, by 10% annually from year 11 up to year 15 and to have no effect anymore as of year 16 since vaccination [Citation15,Citation17]. This waning of effectiveness has been applied in previous cost-effectiveness analyses [Citation15,Citation17–20,Citation31,Citation32].
Effectiveness of PPV23 in IPD was derived from real-world data as used in previously published cost-effectiveness analyses [Citation10,Citation18,Citation20,Citation31]. This effectiveness was assumed to decline linearly to end up in no effectiveness by year 10. It was assumed that PPV23 does not protect against pneumococcal NBP due to inconclusive results regarding its efficacy. The same assumption has been considered in other cost-effectiveness analyses [Citation18,Citation20,Citation31,Citation32].
Uptake of PCV20 was estimated from the Belgian expert panel’s advice, at a maximum of 15–18% in the adults recommended for vaccination. Similar uptake was assumed for the sequential regimen PCV15+PPV23. An uptake of 10% has been applied in the older adults who had been previously vaccinated. This uptake is lower than the current coverage with influenza vaccine, as observed in the INTEGO database (16–64%, depending on age and risk groups). These higher vaccine uptakes were considered in a scenario analysis.
The epidemiological, effectiveness, and (dis)utility inputs are reported in .
Table 1. Epidemiological, effectiveness, and (dis)utility inputs in the population recommended for vaccination by SHC.
2.3.2. Cost data
Cost inputs were derived from the best available data in Belgian settings. The age-specific cost data of pneumococcal disease requiring hospitalization were retrieved from the national hospital TCT-database [Citation33]. The ICD-10 codes representative of pneumococcal infections were classified as G001 (meningitis), A403 (sepsis due to Streptococcus pneumoniae) and J12-J18 (all causes pneumonia). No cost data after hospital discharge were identified. Risk-specific cost data were estimated by applying the relative ratio as derived from a retrospective study conducted in a network of 17 hospitals in Brussels and the south of Belgium (Ecole de Santé Publique ULB network).
The age-specific cost data of outpatient NBP were reported in two KCE Reports [Citation1,Citation34]. One source had restricted the cost of an outpatient NBP to the medication costs and one visit to a general practitioner [Citation1]. The other KCE report had a more comprehensive perspective of healthcare costs. Due to the high variability between both sources, the lowest value of both data sources has been applied in the base case analysis. This cost was subject to scenario analysis. The impact of the risk profile on the cost in ambulatory settings was estimated based on a retrospective study conducted on the INTEGO database.
All cost data were inflated to year 2023, as appropriate, based on health index, in accordance with the Belgian pharmaco-economic guidelines [Citation35,Citation36].
The cost of vaccination included the vaccine acquisition cost and the vaccine administration cost. As recommended by the SHC and the KCE experts, pneumococcal vaccination might occur during a routine visit to the general practitioner (GP) or together with the influenza vaccination [Citation1,Citation2]. As such, only half of the cost of a visit to the GP is consequently taken into consideration.
Cost inputs are reported in .
Table 2. Cost inputs in the population recommended for vaccination by the SHC, from all health-care payers’ perspective (NIHDI + patient).
2.3.3. Analysis
The base case presents 1) the results in the adults recommended for vaccination by the SHC (from NIHDI + patient perspective, in line with the Belgian pharmaco-economic guidelines), 2) the results in the subgroup of adults aged 65–84 years with one or more chronic underlying conditions, which are representative of the subgroup of 65 years and older that were granted reimbursement of PCV20 (from NIHDI perspective) and 3) the results of all adults recommended by the SHC that will fully pay PCV20 (from patient perspective), excluding the 65–84 years old with one or more underlying conditions.
The outcomes are expressed in terms of the number of avoided events (IPD, outpatient, and hospitalized NBP), healthcare costs/savings, and incremental cost per QALY. A one-way deterministic sensitivity analysis (OWSA), a probabilistic sensitivity analysis (PSA), and scenario analyses were conducted to evaluate the impact of value uncertainty or assumption on the cost-utility of PCV20.
In the OWSA, the values were varied, each in turn, by ±20%, keeping all other parameter values constant. In the PSA, values were sampled from the distribution of model parameters: beta distribution was applied to incidence rates, case-fatality rates, vaccine effectiveness, and (dis)utility values; normal distribution to herd effects and log-normal distribution to healthcare costs. The process was repeated 1,000 times. The results of the PSA are summarized in the cost-effectiveness curve. Scenario analyses were conducted considering alternative data sources and/or assumptions for selected model parameters, including cost of outpatient NBP and discount rates. Alternative source of information is applied for the cost of NBP in ambulatory care ([Citation1] vs [Citation34]). A scenario with different discount rates (0–5% on both costs and effects) has also been considered. The choice of these rates is based on the Belgian pharmaco-economic guidelines that have guided the present cost-effectiveness analysis [Citation36].
3. Results
3.1. Base case
3.1.1. In the adults recommended for vaccination by the Superior Health Council (NIHDI + patient perspective)
Among immunocompromised adults aged 18–99 years, immunocompetent adults with underlying conditions aged 50–99 years, and healthy adults aged 65–99 years (N = 3,274,957 in 2023), use of PCV20 (vs. no vaccination) would prevent 4,471 pneumococcal infections including 2,333 hospitalizations due to IPD or NBP and 2,138 NBP cases treated in outpatient settings as well as 227 premature deaths. The prevented pneumococcal diseases would generate €22,071,930 savings in the healthcare budget. The healthcare savings partially offset the cost of PCV20 vaccination, resulting in an incremental cost per QALY of €4,164 when compared to no vaccination, lower than the €35,000/QALY threshold considered in a previous publication for Belgium [Citation1].
When compared to the sequential regimen PCV15 + PPV23, the use of PCV20 would prevent 1,997 pneumococcal infections, including 987 hospitalizations. The choice for PCV20 over PCV15+PPV23 would also prevent 90 additional premature deaths and generate total healthcare savings of €26,907,807, vaccine cost included. Compared to the vaccination with PCV15+PPV23, PCV20 provides a small increase in QALYs as well as healthcare savings. PCV20 is cost-saving compared to PCV15+PPV23.
These results are reported in .
Table 3. Clinical and economic outcomes in the adult population recommended for pneumococcal vaccination by the SHC, lifetime horizon (NIHDI + patient perspective).
3.1.2. In the subgroup of adults aged 65–84 years, with one or more underlying conditions (NIHDI perspective)
Use of PCV20 among moderate-risk Belgian adults aged 65–84 years (N = 1,037,349 in 2023) would avert 2,332 pneumococcal infections, including 1,295 hospitalizations due to IPD or NBP, 1,038 cases of outpatient NBP, and 128 premature deaths. The avoided pneumococcal diseases will also be associated with €1,742,727 savings in healthcare budget. In this subgroup of older population, vaccination with PCV20 is a dominant intervention compared to no vaccination as it improves the clinical burden of pneumococcal disease and is responsible for healthcare savings relative to no pneumococcal vaccination.
These results are reported in .
Table 4. Clinical and economic outcomes in the 65–84 year olds with moderate risk, lifetime horizon (NIHDI perspective).
3.1.3. In the adults recommended for pneumococcal vaccination by the SHC but not reimbursed (patient perspective)
Among adults aged 50–64 years with one or more underlying conditions and healthy adults aged 65–84 years (N = 1,884,275 in 2023), use of PCV20 would prevent 1,771 pneumococcal infections, including 789 hospitalizations due to IPD or NBP and 982 NBP cases treated in outpatient settings. Reduction in disease would also result in 64 fewer premature deaths. Over this period, patients will also save a total of €188,307 on their healthcare budget due to the prevented cases of pneumococcal disease. Vaccination with PCV20 would therefore be a cost-effective option compared to no vaccination as it reduces the risk of pneumococcal disease and requires a short-term healthcare investment that will be partially off-set by future healthcare savings (cost/QALY = €21,378).
These results are reported in .
Table 5. Clinical and economic outcomes in the immunocompromised adults, the 50–64 year olds with underlying conditions and the healthy 65–84 year olds, lifetime horizon (patient perspective).
3.2. One-way deterministic sensitivity analysis (OWSA)
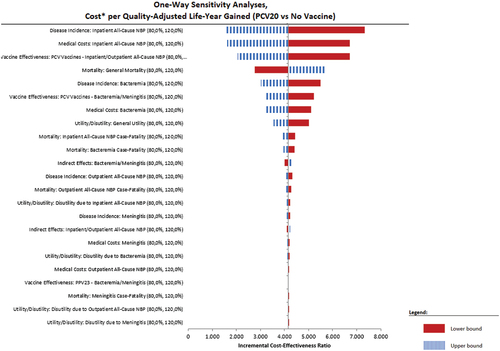
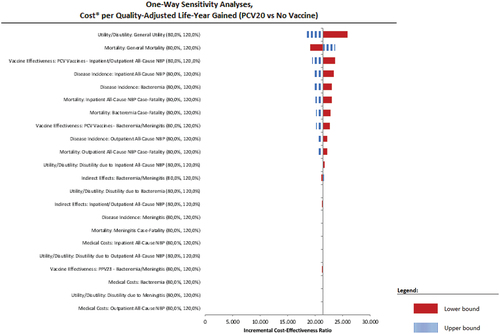
At a variation of ± 20% around the mean value of key parameters, PCV20 remains a very cost-effective intervention in the adults recommended for vaccination by the SHC (NIHDI + patient perspective), a cost-saving option in the adult subgroup of 65–84 year olds with one or more chronic condition(s) (NIHDI perspective) and a cost-effective intervention in the cohort of immunocompromised adults, the 50–64 year olds with chronic underlying conditions and the healthy 65–84 year olds.
We present in the tornado diagram of the two cost-effective cases.
3.3. Probabilistic sensitivity analysis (PSA)
The robustness of the cost-effectiveness outcomes has been further analyzed based on 1,000 probabilistic simulations. At each of the 1,000 Monte-Carlo iterations, a value has been chosen at random for each parameter. The potential values for each parameter have previously been defined based on a specified parametric distribution. These 1,000 simulations, in the comparison of PCV20 with no vaccination, are reported in a cost-effectiveness plane for the three analyzed cohorts (). An additional PSA has been conducted with the comparison to the sequential regimen PCV15+PPV23 in the total recommended adult population ().
Figure 3. Cost-effectiveness plane – All adults recommended by the SHC (NIHDI + patient perspective), PCV20 vs. no vaccination. X-axis represents difference in QALYs and y-axis represents the difference in costs; red line represents willingness to pay (€35,000).
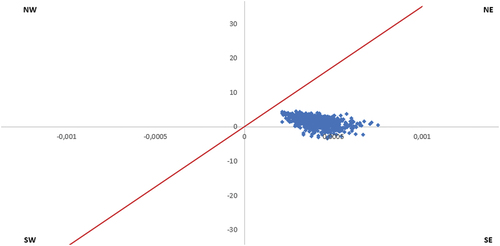
Figure 4. Cost-effectiveness plane – Adults recommended for vaccination and reimbursed (NIHDI perspective), PCV20 vs no vaccination. X-axis represents difference in QALYs; y-axis represents difference in costs; red line represents willingness to pay (€35,000).
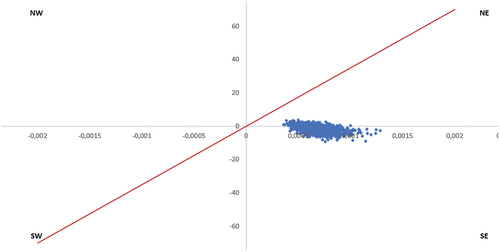
Figure 5. Cost-effectiveness plane – Adults recommended for vaccination but who have to fully finance access to vaccine (patient perspective), PCV20 vs. no vaccination. X-axis represents difference in QALYs; y-axis represents difference in costs; red line represents the willingness to pay (€35,000).
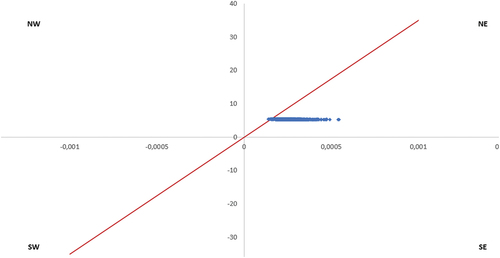
Figure 6. Cost-effectiveness plane – All adults recommended by the SHC (NIHDI + patient perspective), PCV20 vs. PCV15+PPV23. X-axis represents the difference in QALYs; y-axis represents difference in costs; red line represents willingness to pay (€35,000).
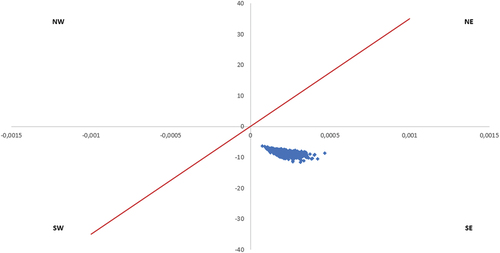
The PSA reports that in the adults recommended for vaccination by the SHC, 13% of the simulations are cost-saving (simulations in the Southeast quadrant of the cost-effectiveness plane) and 87% very cost-effective (in the bottom of Northeast quadrant of the CE plane) ().
The PSA also reports that in the adults recommended for vaccination by the SHC and reimbursed by NIHDI, 77% of the simulations are cost-saving (simulations in the Southeast quadrant of the cost-effectiveness plane) and 23% very cost-effective (in the bottom of Northeast quadrant of the CE plane) ().
The PSA reports that in the adults recommended for vaccination by the SHC but not reimbursed by NIHDI, 99% of the simulations are cost-effective at a threshold of €35,000 ().
The PSA also reports that in the adults recommended for vaccination by the SHC, 100% of the simulations are cost-saving (simulations in the Southeast quadrant of the cost-effectiveness plane) when PCV20 is compared to PCV15+PPV23 ().
Because the 1,000 simulations remain close to each other, we can conclude the robustness of the outcomes.
3.4. Scenario analysis
Scenario analyses have been conducted to assess some additional uncertainties and assumptions.
In accordance with the Belgian pharmaco-economic guidelines, the discount rate was varied, including 0% and 5% on both costs and effects [Citation36]. Due to the large cost variability according to the source considered, alternative medical cost for outpatient NBP has been applied (highest value).
There is uncertainty on the level of PCV20 uptake. In the base case, the uptake of PCV20 was assumed lower than the current coverage with influenza vaccine. In the scenario analysis, the same uptake of PCV20 as with influenza vaccine has been tested.
The cost-effectiveness analysis comparing PCV20 with the alternative vaccination strategy (PCV15 + PPV23) has also been considered in the two sub-analyses (NIHDI perspective and patient perspective), the NIHDI + patient perspective having been presented in detail in the base case.
The results of scenarios are presented in .
Table 6. Scenario analysis.
The specific group of immunocompromised patients has also been investigated in the scenario analysis. Vaccination with PCV20 was found to be cost-saving compared to no vaccination in this cohort, except in the youngest age group (adults aged 18–49 years) in whom PCV20 was found to be cost-effective with an ICER of €13,987/QALY, from NIHDI perspective.
PCV20 pneumococcal vaccination remains a cost-effective option in all scenarios.
4. Discussion
The older population and adults with underlying condition(s) are at increased risk of respiratory infections [Citation3]. In Belgium, a reimbursed vaccination program has long been limited to influenza protection in these vulnerable adults. With the SARS-Cov-2 pandemic, vaccination against this new respiratory infection has been encouraged in the risk population. Pneumococcal vaccination has been recommended for decades in the adults at increased risk of pneumococcal infections, but vaccination has only reached 18% to 32% of this population [Citation2,Citation11]. Reimbursement for PCV20 has recently been granted by the Belgian healthcare system for a subgroup of 65 year olds. A new step has been taken to better protect the vulnerable adults against a vaccine-preventable disease. Some other vulnerable patients would still benefit from PCV20, but they are required to pay for pneumococcal vaccination.
The present health economic analysis has concluded that PCV20 is cost-effective compared to no vaccination in all adults recommended for vaccination by the SHC, from the healthcare payer perspective. The reimbursement of vaccination with PCV20 in 65–84 years old adults with chronic underlying condition(s) is expected to be a cost-saving strategy from the NIHDI perspective. Vaccination in all other groups recommended by the SHC is also a cost-effective option, from a patient perspective.
The sensitivity analysis has confirmed the robustness of this conclusion with 100% probability of PCV20 to be cost-effective compared to no vaccination in the population recommended for vaccination by the SHC, from all healthcare payers’ perspective, NIHDI perspective, and patient perspective.
The scenario analysis confirms the cost-effectiveness of PCV20 in all target populations, from the different perspectives. PCV20 vaccination is also a cost-saving alternative compared to vaccination with PCV15 followed by PPV23, which supports the preferred recommendation for PCV20 by the SHC [Citation2].
Cost-effectiveness of PCV20 has been investigated in other countries, mainly compared to PPV23 vaccine, and in adults older than 65 years [Citation18–20,Citation31,Citation32,Citation37]. These analyses concluded that PCV20 was dominant in this population. The same outcome has been reported in the adult population with underlying conditions or with immunocompromised status when compared to PPV23 vaccine [Citation18,Citation31,Citation32].
The present health economic analysis has adopted a conservative, narrow approach of the benefits of vaccination. An analysis including broader benefits of vaccination might be relevant for future research [Citation22]. These additional benefits include the prevented long-term complications due to infection, the reduction of antibiotic use, and the associated positive impact on antibiotic resistance as well as the improvement on caregivers’ quality of life and productivity [Citation22,Citation38]. The long-term benefits of pneumococcal vaccination would include the prevented cardiovascular and thrombotic complications, the mortality risk and costs beyond hospital discharge which is particularly relevant in case of hospitalization with intensive care [Citation5,Citation34,Citation39–41].
The major limitation of our study is uncertainty with regard to parameter values. The greatest uncertainty concerns current and future rates of vaccine-preventable disease. Because of the impact that the COVID-19 pandemic has had on rates of infectious diseases, including pneumonia, pre-pandemic disease rates, and serotype coverage were employed, assuming that rates will soon return to pre-pandemic levels. Moreover, future reductions in IPD and NBP rates due to future infant immunization with PCV15 and PCV20 were modeled based on herd effects observed in IPD following widespread pediatric PCV13 use in the past (i.e. from 2011 to 2015), which may not be reflective of future experience with novel vaccines.
In addition, because data on serotype coverage for all-cause NBP are not commonly reported in Belgium, vaccine-type IPD serotype coverage was used to estimate the vaccine-type serotype distribution in NBP attributable to pneumococcus. This assumption represents a limitation and future research should provide more accuracy on this parameter. The INTEGO data source for outpatient NBP incidence also has its limitations as these regional (Flemish) data were assumed to be representative of the national population. Moreover, the reported pneumonia data did not distinguish between cases requiring general practitioner’s visits only and those that may ultimately require hospitalization. Therefore, double-counting of some cases of pneumonia may have occured; however, the extent of this double-counting is believed to be low.
Another area of parameter uncertainty concerns vaccine effectiveness. As PCV20 has been licensed based on immunobridging from PCV13, effectiveness was based principally on PCV13 data from the double-blind, randomized, placebo-controlled efficacy trial (CAPiTA) and post-hoc analyses [Citation21]. This trial, which followed 84,496 healthy and at-risk adults aged ≥65 years in the Netherlands for an average of 4 years, is one of the largest adult vaccine efficacy trials ever conducted. Inclusion of immunocompromised patients was, however, missing. During the CAPiTA study, PCV13 demonstrated protection against vaccine-type non-bacteremic pneumococcal community-acquired pneumonia (45.0% [95%CI: 14.2–65.3]), and vaccine-type IPD (75.0% [95%CI: 41.4–90.8]). The duration of protection extended over the 5-year study period. We acknowledge, however, that although the licensure of PCV20 is based on safety and establishing an immunobridge between PCV20 and PCV13 (for the PCV13 serotypes) and between PCV20 and PPV23 (for the seven additional serotypes in PCV20), the effectiveness of PCV20 may vary somewhat from PCV13.
Finally, our model, like all health economic models, simplifies reality to some extent. Our model does not, for example, account for sequelae of IPD, which can be severe.
Like previous cost-effectiveness analyses on pneumococcal vaccination, the outcomes are derived from a static model, but including a herd immunity parameter. The implementation of a dynamic model would have provided more accuracy in herd immunity and serotype replacement parameters over time, including post-vaccination dynamics, but would also have required more uncertain data with regard to the biology and serotypes’ natural history of pneumococcal infection [Citation42,Citation43].
5. Conclusions
Based on the best available data, this health economic analysis concludes that PCV20 is cost-effective in all adults recommended for vaccination by the SHC, from all healthcare payers’ perspective. PCV20 is cost-saving from the NIHDI perspective in the subgroup of 65 years old with underlying conditions who were granted reimbursement. For the risk adults recommended by the SHC for pneumococcal vaccination but for whom the reimbursement has not been granted, vaccination with PCV20 is still cost-effective from the patients’ perspective. Sensitivity analyses and scenario analyses have confirmed the robustness of these conclusions. Compared to a vaccination with PCV15 followed by PPV23, PCV20 is a cost-saving option.
Declaration of interests
A Mignon, A Taelman, and J Vietri are employees of Pfizer and own stock of Pfizer Inc. S Marbaix is an external consultant who has received consulting fees from Pfizer in connection with the development of this study and manuscript. A Averin and M Atwood are employees of PAI, which was a paid consultant to Pfizer in connection with the development of this study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium for their review work. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors have 1) substantially contributed to the conception, study design, execution, acquisition of data, analysis, and/or interpretation 2) have been involved in writing or substantially revised or critically reviewed the article 3) have agreed on the journal to which the article has been submitted 4) reviewed and agreed on all versions of the article before submission, during revision and the final version accepted for publication 5) agree to take responsibility and be accountable for the contents of the article and to share responsibility to resolve any questions raised about the accuracy or integrity of the published work.
Supplemental Material
Download Zip (74.9 KB)Acknowledgments
The following Belgian experts have provided recommendations on inputs of this analysis during a meeting on 30 March 2022 and/or 15 June 2022 : Dr. Pascal Van Bleyenbergh (Lung Disease, University Hospital Leuven), Prof. Dr. Emeritus Willy Peetermans (Internal Medicine, University Hospital Leuven), Prof. Dr. Bernard Vandercam (Infectious Diseases, University Hospital St Luc, Brussels), Prof. Dr. Bert Vaes (Primary Care, Catholic University Leuven) and Dr. Germaine Hanquet (Epidemiology, Consultant).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2023.2273892.
Additional information
Funding
References
- Blommaert A, Hanquet G, Willem L Use of pneumococcal vaccines in the elderly: an economic evaluation Health Technology Assessment (HTA). [Internet]. Brussels: Belgian Health Care Knowledge Centre (KCE), 2016. Report No.: 274. https://kce.fgov.be/en/publications/all-reports/use-of-pneumococcal-vaccines-in-the-elderly-an-economic-evaluation
- Conseil Superieur de la Santé. 2022. Vaccination antipneumococcique (adultes). [Internet]. Report No.: CSSN°9674. https://www.health.belgium.be/fr/avis-9674-vaccination-antipneumococcique-adultes
- Struyf T, Nuyts S, Tournoy J, et al. Burden of infections on older patients presenting to general practice: a registry-based study. Fam Pract. 2021;38(2):165–171. doi: 10.1093/fampra/cmaa105
- Verhaegen J, Flamaing J, De Backer W, et al. Epidemiology and outcome of invasive pneumococcal disease among adults in Belgium, 2009–2011. Eurosurveillance [Internet]. 2014;19(31): [[cited 2023 Mar 23]]. Available from: https://www.eurosurveillance.org/content/10.2807/1560-7917.ES2014.19.31.20869 doi: 10.2807/1560-7917.ES2014.19.31.20869
- Chen H, Matsumoto H, Horita N, et al. Prognostic factors for mortality in invasive pneumococcal disease in adult: a system review and meta-analysis. Sci Rep. 2021;11(1):11865. doi: 10.1038/s41598-021-91234-y
- De Foor J, Senterre C, Leclercq P, et al. Profile of hospitalised elderly patients in Belgium—analysis of factors affecting hospital costs. JEoA. 2020;15:100209. doi: 10.1016/j.jeoa.2019.100209
- Truyers C, Goderis G, Dewitte H, et al. The intego database: background, methods and basic results of a Flemish general practice-based continuous morbidity registration project. BMC Med Inform Decis Mak. 2014;14(1):48. doi: 10.1186/1472-6947-14-48
- Doherty TM, Connolly MP, Del Giudice G, et al. Vaccination programs for older adults in an era of demographic change. Eur Geriatr Med. 2018;9(3):289–300. doi: 10.1007/s41999-018-0040-8
- Mamouris P, Henrard S, Molenberghs G, et al. Pneumococcal vaccination prevented severe LRTIs in adults: a causal inference framework applied in registry data. J Clinical Epidemiol. 2022;143:118–127. doi: 10.1016/j.jclinepi.2021.12.008
- Djennad A, Ramsay ME, Pebody R, et al. Effectiveness of 23-valent polysaccharide pneumococcal vaccine and changes in invasive pneumococcal disease incidence from 2000 to 2017 in those aged 65 and over in England and Wales. EClinicalMedicine. 2018;6:42–50. doi: 10.1016/j.eclinm.2018.12.007
- Janssens A, Vaes B, Abels C, et al. Pneumococcal vaccination coverage and adherence to recommended dosing schedules in adults: a repeated cross-sectional study of the INTEGO morbidity registry. BMC Public Health. 2023;23(1):1104. doi: 10.1186/s12889-023-15939-7
- Méroc E, Fletcher MA, Hanquet G, et al. Systematic literature review of the epidemiological characteristics of pneumococcal disease caused by the additional serotypes covered by the 20-valent pneumococcal conjugate vaccine. Microorganisms. 2023;11(7):1816. doi: 10.3390/microorganisms11071816
- Shiri T, Khan K, Keaney K, et al. Pneumococcal disease: a systematic review of health utilities, resource use, costs, and economic evaluations of interventions. Value Health. 2019;22(11):1329–1344. doi: 10.1016/j.jval.2019.06.011
- Flamaing J, De Backer W, Van Laethem Y, et al. Pneumococcal lower respiratory tract infections in adults: an observational case-control study in primary care in Belgium. BMC Fam Pract. 2015;16(1):66. doi: 10.1186/s12875-015-0282-1
- Marbaix S, Peetermans WE, Verhaegen J, et al. Cost-effectiveness of PCV13 vaccination in Belgian adults aged 65-84 years at elevated risk of pneumococcal infection. PLoS ONE. 2018;13:e0199427.
- INAMI/RIZIV. Médicaments remboursables par l’assurance soins de santé [Internet]. 2022. [cited 2023 Sep 10]. Available from: https://webappsa.riziv-inami.fgov.be/SSPWebApplicationPublic/fr/Public/ProductSearch
- Mangen M-J, Rozenbaum MH, Huijts SM, et al. Cost-effectiveness of adult pneumococcal conjugate vaccination in the Netherlands. Eur Respir J. 2015;46(5):1407–1416. doi: 10.1183/13993003.00325-2015
- Mendes D, Averin A, Atwood M, et al. Cost-effectiveness of using a 20-valent pneumococcal conjugate vaccine to directly protect adults in England at elevated risk of pneumococcal disease. Expert Rev Pharmacoecon Outcomes Res. 2022;22(8):1285–1295. doi: 10.1080/14737167.2022.2134120
- Polistena B, Icardi G, Orsi A, et al. Cost-effectiveness of vaccination with the 20-valent pneumococcal conjugate vaccine in the Italian adult population. Vaccines. 2022;10(12):2032. doi: 10.3390/vaccines10122032
- Cantarero D, Ocaña D, Onieva-García MÁ, et al. Cost-utility analysis of the use of the 20-valent anti-pneumococcal vaccine (PCV20) in adults older than 60 years in Spain. Vaccine. 2023;41(36):S5342–5349. doi: 10.1016/j.vaccine.2023.07.016
- Bonten MJM, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372(12):1114–1125. doi: 10.1056/NEJMoa1408544
- Annemans L, Beutels P, Bloom DE, et al. Economic evaluation of vaccines: Belgian reflections on the need for a broader perspective. Value Health. 2021;24(1):105–111. doi: 10.1016/j.jval.2020.09.005
- INTEGO. Intego database; unpublished data. [Internet]. www.intego.be
- Sciensano. Report National reference Centre Streptococcus pneumoniae [Internet]. https://sciensano.be/sites/default/files/streptoccuspneumoniae_2019_2019_nrc_rapport.pdf_
- van Hoek AJ, Andrews N, Waight PA, et al. The effect of underlying clinical conditions on the risk of developing invasive pneumococcal disease in England. J Infect. 2012;65(1):17–24. doi: 10.1016/j.jinf.2012.02.017
- Van Wilder L, Charafeddine R, Beutels P, et al. Belgian population norms for the EQ-5D-5L, 2018. Qual Life Res. 2022;31(2):527–537. doi: 10.1007/s11136-021-02971-6
- Van Wilder L, Devleesschauwer B, Clays E, et al. The impact of multimorbidity patterns on health-related quality of life in the general population: results of the Belgian health interview survey. Qual Life Res. 2022;31(2):551–565. doi: 10.1007/s11136-021-02951-w
- French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-Infected adults. N Engl J Med. 2010;362(9):812–822. doi: 10.1056/NEJMoa0903029
- Klugman KP, Madhi SA, Huebner RE, et al. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349(14):1341–1348. doi: 10.1056/NEJMoa035060
- McLaughlin JM, Jiang Q, Isturiz RE, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine against hospitalization for community-acquired pneumonia in older US adults: a test-negative design. Clinical Infectious Diseases [Internet]. 2018 [cited 2023 Mar 23]. Available from: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciy312/5000157
- Olsen J, Schnack H, Skovdal M, et al. Cost-effectiveness of 20-valent pneumococcal conjugate vaccine in Denmark compared with PPV23. J Med Econ. 2022;25(1):1240–1254. doi: 10.1080/13696998.2022.2152235
- Malene MB, Oyvind H, Tor M, et al. Cost-effectiveness of 20-valent pneumococcal conjugate vaccine compared with 23-valent pneumococcal polysaccharide vaccine among adults in a Norwegian setting. Cost Eff Resour Alloc. 2023;21(1):52. doi: 10.1186/s12962-023-00458-4
- Cellule Technique (INAMI/SPF Santé Publique). Cellule Technique de traitement de données relatives aux hôpitaux [Internet]. 2020. [cited 2022 Mar 20]. Available from: https://tct.fgov.be/webetct/etct-web/national_data?lang=fr&menuSelected=apr_drg&selectedSideMenu=default&reset=true
- Beutels P, Blommaert A, Hanquet G, et al. Cost-effectiveness of 10- and 13-valent pneumococcal conjugate vaccines in childhood. [Internet]. 1st. BE: Belgian Health Care Knowledge Centre (KCE); 2011. [cited 2023 Mar 23]. Available from: doi: 10.57598/R155C
- Statbel. Consumer health index [Internet]. Brussels; 2023 [cited 2023 Jul 12]. Available from: https://statbel.fgov.be/en/themes/consumer-prices/health-index
- Cleemput I. Belgian guidelines for economic evaluations and budget impact analyses [Internet]. 2nd ed. BE: Belgian Health Care Knowledge Centre (KCE); 2012. [cited 2023 Mar 23].doi: 10.57598/R183C
- Hoshi S, Shono A, Seposo X, et al. Cost-effectiveness analyses of 15- and 20-valent pneumococcal conjugate vaccines for Japanese elderly. Vaccine. 2022;40(49):7057–7064. doi: 10.1016/j.vaccine.2022.10.010
- Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67(1):71–79. doi: 10.1136/thx.2009.129502
- Wang C-C, Peng C-L, Wang G-J, et al. Pneumococcal pneumonia and the risk of acute coronary syndrome: a population-based cohort study. Int J Cardiol. 2013;168(4):4480–4481. doi: 10.1016/j.ijcard.2013.06.134
- Hughes GJ, Wright LB, Chapman KE, et al. Serotype-specific differences in short- and longer-term mortality following invasive pneumococcal disease. Epidemiol Infect. 2016;144(12):2654–2669. doi: 10.1017/S0950268816000856
- Averin A, Shaff M, Weycker D, et al. Mortality and readmission in the year following hospitalization for pneumonia among US adults. Respir med. 2021;185:106476. doi: 10.1016/j.rmed.2021.106476
- Ultsch B, Damm O, Beutels P, et al. Methods for health economic evaluation of vaccines and immunization decision frameworks: a consensus framework from a European vaccine economics community. PharmacoEconomics. 2016;34(3):227–244. doi: 10.1007/s40273-015-0335-2
- Løchen A, Anderson RM. Dynamic transmission models and economic evaluations of pneumococcal conjugate vaccines: a quality appraisal and limitations. Clin Microbiol Infect. 2020;26(1):60–70. doi: 10.1016/j.cmi.2019.04.026