 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background
Next-generation, higher-valency pneumococcal conjugate vaccines (PCVs), 15-valent PCV V114 and 20-valent PCV (PCV20), have been assessed by comparing their immune responses across serotypes shared with the 13-valent PCV (PCV13). Without efficacy or real-world vaccine effectiveness (VE) it becomes important to relate IgG titers to VE to aid in the interpretation of the immune response elicited by V114 and PCV20.
Methods
We estimated the protective antibody concentrations for each serotype in 7-valent PCV (PCV7) and PCV13 which were then used to predict the serotype-specific VE for each PCV7 and PCV13 non PCV7 serotype present in V114 and PCV20.
Results
The predicted effectiveness of V114 was comparable to PCV7 and PCV13 for 11 of the 13 shared serotypes (1, 4, 5, 6B, 7F, 9 V, 14, 18C, 19A, 19F, and 23F), with improved effectiveness against serotype 3 and decreased effectiveness against serotype 6A. PCV20 had predicted effectiveness comparable to PCV7 and PCV13 for 7 of the 13 shared serotypes (5, 6A, 7F, 9 V, 18C, 19F, and 23F), with decreased effectiveness against the remaining serotypes (1, 3, 4, 6B, 14, and 19A).
Conclusions
Prediction of serotype-specific VE values suggests that V114 retains greater effectiveness than PCV20 toward most serotypes present in PCV7 and PCV13.
Plain Language Summary
Pediatric pneumococcal conjugate vaccines (PCVs) first became available in 2000, when the seven-valent PCV (PCV7) was approved. Since then, PCV7 has been replaced by higher-valency vaccines, including the ten-valent (PCV10) and thirteen-valent (PCV13) vaccines and, more recently, fifteen- and twenty-valent vaccines (V114 and PCV20, respectively). The increase in valency provides broader serotype coverage against invasive pneumococcal disease (IPD) in children. However, IPD due to serotypes contained in PCV7 and PCV13 continue to be observed. In the current study, we used a previously published method to estimate the vaccine effectiveness of V114 and PCV20 in a US and Puerto Rican pediatric population that is recommended to receive a 3 + 1 dosing schedule.
1. Introduction
Pediatric pneumococcal conjugate vaccines (PCVs) became available in 2000, when the seven-valent PCV (PCV7) first received regulatory approval [Citation1,Citation2]. Since then, PCV7 has been replaced by higher-valency vaccines, including PCV10 and PCV13. More recently, V114, a 15-valent PCV (VAXNEUVANCE, Merck & Co., Inc., Rahway, NJ, USA), was developed to prevent disease from serotypes 22F and 33F as well as the original 13 serotypes contained in PCV13 (see Supplementary Table S1) [Citation3–5]. Concurrently, a 20-valent vaccine (PCV20; PREVNAR 20, Pfizer, Inc.) was developed: it targets serotypes 8, 10A, 11A, 12F, and 15B in addition to all the serotypes in V114 [Citation6]. The increase in valency provides broader serotype coverage against invasive pneumococcal disease (IPD) in children. However, IPD due to serotypes contained in the original PCV7 (6B and 19F) and also PCV13 (3 and 19A) continues to be observed [Citation7–12].
It has also been observed that, as valency increases in PCVs, the elicited immune response has decreased for the shared serotypes [Citation13]. One study reported an average decrease of ~ 23% from PCV7 to PCV13 in the serotype-specific geometric mean concentrations (GMCs) of anticapsular immunoglobulin G (IgG) against PCV7 serotypes in infants [Citation14]. IgG concentrations that fail to reach the protective concentration threshold for a particular serotype may allow breakthrough disease due to infection by that serotype. Conversely, an immune response that meets and/or exceeds the protective threshold concentration ensures that progress achieved to date with currently licensed vaccines is maintained.
Because of the effectiveness of current PCVs against IPD, it is no longer feasible or ethical to perform placebo-controlled clinical trials to assess efficacy, hence new PCVs are licensed primarily based on their immunogenicity (as assessed by IgG serum titers) and opsonophagocytic activity [Citation15,Citation16]. Effectiveness may be measured after several years of use in a population, measured by surveillance of disease and carriage. Without efficacy or real-world vaccine effectiveness (VE), which can take years to estimate for IPD, it becomes important to relate IgG titers to VE to contextualize and aid in interpretation of the immune response elicited by each successive generation of PCVs.
We previously described and qualified a method for relating IgG concentrations to the serotype-specific VE of a PCV via determination of the serotype-specific protective concentration for each serotype [Citation17]. In the current study, we used this same method to estimate the VE of V114 and PCV20 against the serotypes they have in common with PCV7/PCV13 in a pediatric population (from US and Puerto Rico) that is recommended to receive a 3 + 1 dosing regimen.
2. Methods
2.1. Summary of the method
The method for estimating VE is adapted from prior work by Siber et al [Citation18]. We extended that method to allow for estimation of serotype-specific PCV effectiveness. Our extended method was shown to provide predictions consistent with observations when applied to serotype-specific VE against IPD caused by PCV7 serotypes in pediatric populations (vaccinated with PCV13) [Citation17]. The method is presented schematically in , and the details are given in two prior publications [Citation17,Citation19].
Figure 1. Schematic depiction of the modeling method.
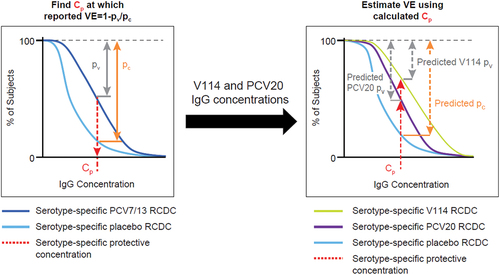
Here we used this method to determine the serotype-specific VE for V114 and PCV20 across serotypes in common with PCV7 and with (nonPCV7 serotypes in) PCV13. The target population was pediatric subjects (ages 6 months to 5 years) from the United States and Puerto Rico (hereafter referred to as ‘US’) vaccinated with a 3 + 1 dosing regimen (3 primary doses at 2, 4, 6 months and a booster at 12–15 months). The first step in the method used serotype-specific real-world VEs of PCV7 and PCV13 and simulated reverse cumulative distribution curves (RCDCs) from the known serotype-specific IgG concentrations after vaccination with placebo, PCV7, and PCV13 to derive the protective antibody concentration (Cp) for each PCV7 and PCV13 (nonPCV7) serotype. The second step in the method used available serotype-specific IgG concentrations assessed in V114 and PCV20 clinical trials and simulated RCDCs for each shared (PCV13) serotype, and this information was combined with the Cp values (for the respective serotypes) to predict the corresponding VE for each PCV7 and PCV13 (nonPCV7) serotype in V114 and PCV20.
2.2. Data sources
2.2.1. Vaccine effectiveness
Real-world serotype-specific VE values for PCV7 and PCV13 (nonPCV7 serotypes) were from two US-based pediatric (6-months to 5 years of age) matched case-control studies (, with a summary of the study details provided in the Appendix) [Citation20,Citation21]. VE estimates for PCV13 against the PCV7 serotypes were not available in a US pediatric population due to a lack of IPD cases. Thus, we used the Whitney et al. analysis as input for the PCV7 serotypes [Citation20].
Table 1. Summary-level input data and sources.
Further, there is no VE data available exclusively for pediatric subjects with only 3 primary doses (at 2, 4, 6 months) in the United States. Although we used post-primary series (PPS) titers, in the present analysis we are not predicting VE in infants with only 3 primary doses, we are predicting VE in the entire pediatric population where a 3 + 1 dosing schedule is recommended. Therefore, the VE inputs from Whitney [Citation20] and Moore [Citation21], with variation in vaccination completion, ensures that our predictions reflect real-world circumstances, including individuals who become infected with IPD prior to completing the recommended series, or who do not complete the series. The observation period for IPD cases for PCV7 was from 2001 to 2003, while those for PCV13 were observed from 2010 to 2014.
2.2.2. Immunogenicity data
Immunogenicity data for serotypes in PCV7 [Citation18], PCV13 [Citation6,Citation22–24], V114 [Citation22–24], and PCV20 [Citation6] were obtained from US-based clinical trials (). Summary-level or individual-level serotype-specific IgG concentration data for the indicated serotypes were extracted at the PPS time point (defined as 1 month after the third dose) in healthy infants given a 3 + 1 dosing regimen. The PPS timepoint was chosen as it reflects the immune response elicited within the first year of life (when children are at the highest risk of IPD), as well as to remain consistent with previous analyses [Citation18,Citation25] and with the qualification of the method used [Citation19]. The PPS timepoint is used for determining the Cp. The rationale for this is that infants <1 year old have immature immune systems as compared to toddlers and older children, and thus are at higher risk for IPD and its complications. As such the bar is highest for this age group in terms of sufficient vaccine-induced antibodies that would prevent disease. PPS placebo IgG concentrations were not available in the US pediatric population for the PCV13 serotypes. Thus, pre-vaccination IgG concentrations elicited in 2-month-old pediatric participants were assumed to reflect PPS timepoint placebo concentrations. The pre-vaccination IgG concentrations used in this analysis are assumed to reflect a lack maternal antibodies and are consistent with PPS timepoint placebo concentrations observed in a previous study with a placebo arm [Citation18,Citation26]. Summary-level, serotype-specific placebo IgG concentrations were extracted from data reported at the pre-vaccination time point in a US-based phase 3 trial in healthy infants concomitantly administered Vaxelis (hexavalent combination vaccine, Merck & Co., Inc., Rahway, NJ, USA) and PCV13 (antibodies against pneumococcal serotypes in PCV13 were a secondary endpoint) [Citation27].
IgG concentrations measured by different assays are not directly comparable, so IgG concentrations measured by enhanced chemiluminescence (ECL) [Citation28] and Luminex-based 13-plex direct immunoassay (dLIA) [Citation29] were normalized to the World Health Organization’s standard ELISA (in accordance with WHO recommendations) using a published concordance equation.
In this equation, αi and βi denote the intercept and slope estimates relating the ECL (or dLIA) assay to the ELISA for the ith pneumococcal serotype (i = 1, 2, 3, … , 13). Supplementary Table S2 lists the slope and intercept values used to normalize the IgG concentrations measured using the ECL assay and the IgG concentrations measured using the dLIA assay. The IgG concentrations presented in were normalized using these parameter estimates in the equation above.
2.3. Data analysis
For each serotype/vaccine combination, the published GMC, the published VEs and their geometric standard deviations (GSDs; calculated from the published 95% confidence intervals [CIs]), and/or the subject-level IgG concentrations were used as inputs for Monte Carlo simulations (5,000 iterations). In cases where summary-level data were used (placebo, PCV7, PCV13 from trial NCT04382326 [Citation6], and PCV20 IgG concentrations, and all VE input), each simulation sampled a new GMC and VE from a lognormal distribution; uncertainty in the GSD was accounted for by generating GSD estimates around the reported GSD assuming a chi-square distribution. When subject-level IgG concentration data were available (for PCV13 from trials NCT02987972 [Citation22], NCT03620162 [Citation23], and NCT03893448 [Citation24] for V114 IgG concentrations), the subject-level data were bootstrapped (i.e. sampled with replacement) for each serotype from individual IgG concentrations post-vaccination. The number of subjects simulated or bootstrapped was the same as the number of subjects in the respective vaccine trial arm (i.e. if there were 125 subjects in the PCV13 vaccine arm, 125 subjects were simulated or bootstrapped with replacement, depending on the data type). For PCV13-nonPCV7 serotypes, summary-level IgG concentrations [Citation6] were pooled with bootstrapped individual-level IgG concentrations [Citation22–24] to capture the GMC and distribution of PPS IgG concentrations for the US infant population.
Each simulation solved for a serotype-specific Cp based on the RCDCs generated (using clinical trial data) for placebo, PCV7, or PCV13 and the published real-world VE for PCV7 or PCV13 (nonPCV7 serotypes) (, left panel). Then, the serotype-specific VEs of V114 and PCV20 were predicted based on the aforementioned Cp value and the RCDCs generated (using clinical trial data) for placebo and next-generation PCV (V114 or PCV20) (, right panel). Within each simulation, the absolute difference in predicted VE of V114 and PCV20 was calculated by taking the difference in predicted VE (V114 VE minus PCV20 VE). Analysis was performed in R version 4.2.1. Simulated protective IgG threshold concentrations (μg/mL), VE values (%), and absolute percentage difference between V114 and PCV20 were summarized (details below) as medians with 95% CIs.
3. Results
3.1. Estimated protective antibody concentrations
Protective antibody concentrations derived from VE source data for PCV7 and PCV13 (nonPCV7 serotypes) are shown in . Estimated protective antibody concentrations, 30 days after PPS, ranged from 0.11 (serotype 23F) to 0.95 µg/mL (serotype 14) for PCV7 serotypes and from 0.23 (serotype 3) to 0.90 µg/mL (serotype 6A) for PCV13-nonPCV7 serotypes.
Table 2. Estimated protective antibody concentrations.
3.2. Predicted vaccine effectiveness
shows the predicted serotype-specific VE values for V114 and PCV20 against IPD and the observed VE for PCV7 and PCV13 (nonPCV7 serotypes) in a US pediatric population that is recommended to receive a 3 + 1 dosing schedule. The predicted serotype-specific effectiveness of V114 ranged from 87% to 99% for PCV7 serotypes and from 69% to 98% for PCV13-nonPCV7 serotypes. V114 serotype-specific effectiveness was comparable (defined as being within 15% points of the observed VE) to PCV7 for each of the shared serotypes and was comparable to all but two of the serotypes shared with PCV13 (nonPCV7 serotypes); V114 was predicted to be less effective against serotype 6A and more effective against serotype 3 compared to PCV13 (). The predicted serotype-specific effectiveness of PCV20 ranged from 74% to 99% for PCV7 serotypes and from 64% to 92% for PCV13-nonPCV7 serotypes. PCV20 serotype-specific effectiveness was comparable to PCV7 for four of the shared serotypes and was comparable to three of the serotypes shared with PCV13 (nonPCV7 serotypes). PCV20 was predicted to be less effective against serotypes 4, 6A, and 14 compared to PCV7 and less effective against serotypes 1, 3, and 19A compared to PCV13 (nonPCV7 serotypes) ().
Table 3. Observed and predicted vaccine effectiveness.
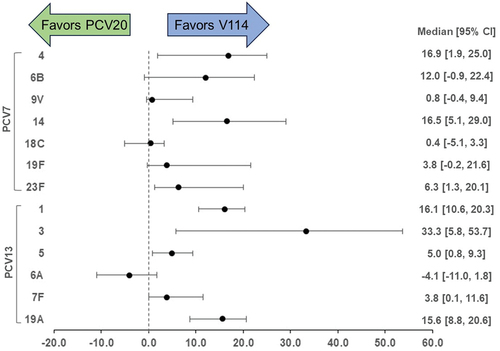
shows the difference between V114 and PCV20 serotype-specific predicted VE values. V114 had a higher predicted VE for 12 of the 13 shared serotypes. The results are further summarized in the Discussion section of the manuscript.
Figure 2. Absolute difference in predicted vaccine effectiveness for V114 and PCV20.
4. Discussion
Assessing the likely public health impact of new PCVs requires understanding the effectiveness of PCVs against IPD in the absence of real-world effectiveness data. Effectiveness estimates can inform vaccination policy decisions by predicting the impact of a new vaccine on the burden of disease for vaccine serotypes. In this study, a previously qualified method [Citation17] was applied to predict, using the PPS timepoint, the serotype-specific VE of V114 (a new 15-valent vaccine) and of PCV20 (a new 20-valent vaccine) in a US pediatric population recommended to receive a 3 + 1 dosing schedule.
For V114, the predicted VE was comparable to previously observed values for all the serotypes shared with PCV7 (4, 6B, 9 V, 14, 18C, 19F, 23F) and for most of the serotypes shared with PCV13 (nonPCV7 serotypes 1, 5, 7F, 19A). Two serotypes (3 and 6A) had a substantially different predicted VE in V114 as compared to PCV13. For serotype 3, V114 was predicted to have greater effectiveness against IPD than PCV13 (98% vs. 80%), and for serotype 6A, V114 was predicted to have lower effectiveness against IPD than PCV13 (69% vs. 86%; ). As this is a post-hoc analysis in a model-based meta-analysis context, no formal, prespecified statistical hypothesis testing was conducted. The public health impact of differences in VE can be estimated when combined with epidemiological data on the relative incidence rates of disease caused by these serotypes, as discussed further below.
For PCV20, the predicted VE was generally comparable to the previously-observed values for serotypes 9 V, 18C, 19F, and 23F in PCV7 and serotypes 5, 6A, and 7F in PCV13 (nonPCV7 serotypes). A decrease in VE was predicted for the other serotypes compared to PCV7 (4, 6B, and 14) and PCV13 (nonPCV7 serotypes 1, 3, and 19A). Again, no formal, prespecified statistical hypothesis testing was conducted. The decreases in PCV20 VE values as compared to PCV7 and PCV13 (nonPCV7 serotypes) were driven by the lower IgG concentrations elicited by PCV20 as compared to PCV7 and PCV13, with an average decrease of 38% overall (Supplementary Table S3).
V114 maintains or increases VE relative to PCV7 and PCV13 (nonPCV7 serotypes) and PCV20 despite eliciting lower concentrations in some serotypes when compared to PCV7 and PCV13 (nonPCV7 serotypes). IgG concentrations in V114 compared to PCV7 and PCV13 (nonPCV7 serotypes) were lower for nearly all of the shared serotypes, with a mean (across serotypes) decrease of 17% (with some serotypes decreasing as much as 54%; Supplementary Table S3). These results are consistent with the observation that the higher-valency PCVs induce lower immunogenicity (to shared serotypes) than a lower valency PCV. Nevertheless, the predicted serotype-specific VE values for V114 remained comparable to those for PCV7 and PCV13 (nonPCV7 serotypes), while, in PCV20, several serotype-specific VE values had notable decreases compared to PCV7 and PCV13 (nonPCV7 serotypes). This difference in results when comparing V114 and PCV20’s predicted VE can be explained by V114’s IgG concentration distributions: V114 induced concentrations were consistently greater than the estimated protective threshold concentrations (Cp), whereas PCV20’s decrease in immunogenicity for several serotypes was enough to drop the IgG concentration distribution below the estimated Cp.
The recent iteration of next-generation PCVs lacks head-to-head clinical trials and real-world effectiveness data that would help contextualize the differences in immunogenicity and the impact that may have on VE. To summarize the difference between V114 and PCV20 serotype-specific predicted VE values, the absolute difference in predicted VE of V114 and PCV20 (V114 VE minus PCV20 VE) was calculated for each of the 5,000 simulations and summarized as a median and 95% CI. As shown in , V114 had a higher predicted VE for 12 of the 13 shared serotypes after accounting for uncertainties in the inputs (including uncertainty in VE for real-world PCV7 and PCV13 VE, and in IgG concentrations for placebo, PCV7, PCV13, V114, and PCV20); these uncertainties were captured by the 5,000 simulations conducted for each serotype. The overall higher predicted VEs for V114 compared to PCV20 were due to the higher IgG concentrations elicited by V114 (). The effect of the IgG concentrations on the predicted VE differences is exemplified by serotype 3. The largest difference in immunogenicity between V114 and PCV20 was for this serotype, where V114 showed a 71% increase in GMC (relative to PCV13) while PCV20 showed a 31% decrease in GMC (Supplementary Table S3). This translated into a large difference in predicted VE, 33.3% points (). The predicted VE favored PCV20 for only one serotype (6A) out of 13, with a predicted VE difference of only 4%.
These predicted differences may have public health implications. The introduction of PCV13 was followed by a decreasing burden of disease due to serotype 6A, whereas the burden of disease caused by serotype 3 has remained high [Citation12,Citation30–33]. In the United States, IPD cases due to serotype 3 increased by 34% between 2015 and 2017 [Citation12]. Furthermore, serotype 3 has been found to be associated with severe infections and high mortality [Citation34,Citation35]. These disease burden and epidemiology statistics are evidence of the importance of the VE difference and why it matters for public health, supporting the necessity of keeping the strong protection against the serotypes that are covered by PCV13 with added protection against IPD caused by serotype 3.
The PCV13 VE used as input for serotype 3 (80%) [Citation21] is potentially higher than expected given the epidemiology of serotype 3 in the United States. Around 2011, just after the introduction of PCV13, there was a drop in IPD cases among children less than 5 years of age, consistent with a high VE [Citation36]. Since 2015, however, the reported number of cases returned to levels close to what was seen before the introduction of PCV13 [Citation36], suggesting the VE could be lower than the initial estimate. To address this possibility, a sensitivity analysis was performed again, this time using the serotype 3 VE from Andrews et al. (26%) [Citation25] as an input to the model: this showed (again) that V114 is likely to have improved serotype 3 VE relative to PCV20 (as well as PCV13). Using a lower VE for serotype 3 led to a larger predicted Cp of 0.77 μg/mL (data not shown). This reduced the predicted serotype 3 VE substantially for both V114 (56% vs. 98% originally) and PCV20 (11% vs. 64% originally; ). Based on the serotype 3 VE input used above from Moore et al. [Citation21], the difference in predicted VE between V114 and PCV20 was 33% points in favor of V114 over PCV20 (). Using the lower and potentially more epidemiologically realistic VE of 26% from Andrews et al. [Citation25], the difference in predicted VE between V114 and PCV20 was even greater (45% points higher for V114; ).
Table 4. Predicted effectiveness for serotype 3 using Andrews et al. (2014) PCV13 effectiveness estimates as input.
The VE values from Andrews et al. [Citation25] reflect PCV effectiveness in a United Kingdom pediatric population given a 2 + 1 regimen, not a US pediatric population given a 3 + 1 regimen. Hence, the Andrews et al. VE was used only for the sensitivity analysis and not for the original analysis above (which focused on the US/Puerto Rico pediatric population that is recommended to receive a 3 + 1 regimen).
4.1. Limitations
The modeling method is subject to several limitations, which are described in detail in previous publications [Citation17,Citation19]. In addition to the method and data limitations described before, there are also limitations on the application of VE estimates outside of the population that informed the prediction. In particular, the predictions using this method are specific to the population and vaccination regimen that were used for the VE and IgG inputs. As such, results presented herein cannot necessarily be extrapolated to other populations, other dosing regimens, or other disease outcomes ( applies only to IPD in US children under the age of 5 administered a 3 + 1 regimen). Specifically, other pneumococcal disease outcomes such as carriage and mucosal diseases are understood to have generally higher Cp values, and thus the lower antibody levels elicited by new vaccines for shared serotypes compared to PCV13 and PCV7 could impact protection against these more common disease outcomes somewhat differently. In addition, differences in vaccination completion (i.e. number of doses received) can impact Cp values at the subject and population levels. Any difference in completion thus influences the VE, which is used to estimate the Cp (and subsequently V114/PCV20 VE). Although some completion differences exist between the populations in the source datasets (subjects received ‘one or more doses’ in some cases [Citation20,Citation21]) and our modeled population, we have assumed that those differences are small enough that the correct relationship between immunogenicity and VE is being used for model prediction. Another limitation of our method is that it cannot predict the effectiveness of novel serotypes for which neither efficacy nor effectiveness data are available, including novel serotypes 22F and 33F (included in V114 and PCV20).
5. Conclusions
In this work, we applied a previously described immunogenicity-based VE prediction method that enables prediction (in the absence of real-world VE observations) of serotype-specific VE for V114 and PCV20 in a US pediatric population that is recommended to receive a 3 + 1 regimen). The results indicate that V114 has effectiveness comparable to that of PCV7 and PCV13 (nonPCV7 serotypes) for most shared serotypes and that PCV20 has comparable effectiveness for half of the serotypes it shares with PCV7 and PCV13 (nonPCV7 serotypes). The predicted VEs further suggest that V114 has the potential to have an even higher public health impact than previously approved pediatric vaccines, in part due to having a VE comparable to PCV7 and PCV13 (nonPCV7 serotypes) as well as evoking stronger immunogenicity against a prevalent and impactful pathogen like serotype 3. Although PCV20 May reduce the public health burden from the 7 additional serotypes included in the vaccine, its low immunogenicity against serotype 3 suggests that it is unlikely to reduce IPD cases caused by serotype 3, and these cases account for a significant proportion of residual pediatric IPD burden. The modeling facilitates interpretation of differences in immunogenicity between vaccines in terms of expected effectiveness, helping decision-makers to ensure that future PCVs will maintain and, hopefully, enhance control of IPD.
Declaration of interest
All authors were employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA at the time of the study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have received honoraria for their review work. In addition, one reviewer discloses that they are an investigator on industry-sponsored vaccine trials. A second reviewer acknowledges receiving fees for participating in advisory boards and funds for an unrestricted research grant, all paid directly to home institution, from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Author contributions
Conception and design: JR, JRS, NB, JW, TW. Analysis and interpretation of the data: JR, JRS, KLY, NB, JW, TW. Drafting the manuscript: JR, KLY, NB, JW, TW. Revising the manuscript for intellectual content: JR, JRS, KLY, NB, JW, TW. All authors approve the final version of the manuscript and agree to be accountable for all aspects of the work.
Supplemental Material
Download (15.6 KB)Acknowledgments
The authors thank Melissa Stauffer, PhD, in collaboration with ScribCo, for medical writing assistance.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article or its supplementary materials.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2023.2292773.
Additional information
Funding
References
- Masomian M, Ahmad Z, Gew LT, et al. Development of next generation streptococcus pneumoniae vaccines conferring broad protection. Vaccines (Basel). 2020 Mar 17;8(1):132. doi: 10.3390/vaccines8010132
- Scelfo C, Menzella F, Fontana M, et al. Pneumonia and invasive pneumococcal diseases: The role of pneumococcal conjugate vaccine in the era of multi-drug resistance. Vaccines (Basel). 2021 Apr 22;9(5):420. doi: 10.3390/vaccines9050420
- Greenberg D, Hoover PA, Vesikari T, et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (pcv15) in healthy infants. Vaccine. 2018 Oct 29;36(45):6883–6891. eng. doi: 10.1016/j.vaccine.2018.02.113.
- Platt HL, Greenberg D, Tapiero B, et al. A phase ii trial of safety, tolerability and immunogenicity of v114, a 15-valent pneumococcal conjugate vaccine, compared with 13-valent pneumococcal conjugate vaccine in healthy infants. Pediatr Infect Dis J. 2020 Aug;39(8):763–770. eng. doi: 10.1097/INF.0000000000002765
- Sobanjo-Ter Meulen A, Vesikari T, Malacaman EA, et al. Safety, tolerability and immunogenicity of 15-valent pneumococcal conjugate vaccine in toddlers previously vaccinated with 7-valent pneumococcal conjugate vaccine. Pediatric Infectious Disease Journal Query. 2015 Feb;34(2):186–94. eng. doi: 10.1097/INF.0000000000000516
- A phase 3, randomized, double-blind trial to evaluate the safety and immunogenicity of a 20-valent pneumococcal conjugate vaccine in healthy infant. Eudract no. 2019-003305-10 [cited 2023 Jun 26]. Available from: https://www.clinicaltrialsregister.eu/ctr-search/trial/2019-003305-10/results
- Balsells E, Guillot L, Nair H, et al. Serotype distribution of streptococcus pneumoniae causing invasive disease in children in the post-pcv era: a systematic review and meta-analysis. PLoS One. 2017;12(5):e0177113. doi: 10.1371/journal.pone.0177113
- Kandasamy R, Voysey M, Collins S, et al. Persistent circulation of vaccine serotypes and serotype replacement after 5 years of infant immunization with 13-valent pneumococcal conjugate vaccine in the United Kingdom. J Infect Dis. 2020 Mar 28;221(8):1361–1370. doi: 10.1093/infdis/jiz178
- Rockett RJ, Oftadeh S, Bachmann NL, et al. Genome-wide analysis of streptococcus pneumoniae serogroup 19 in the decade after the introduction of pneumococcal conjugate vaccines in australia. Sci Rep. 2018 Nov 16;8(1):16969. doi: 10.1038/s41598-018-35270-1
- Adebanjo TA, Pondo T, Yankey D, et al. Pneumococcal conjugate vaccine breakthrough infections: 2001-2016. Pediatrics. 2020 Mar;145(3). doi: 10.1542/peds.2019-0836
- Mungall BA, Hoet B, Nieto Guevara J, et al. A systematic review of invasive pneumococcal disease vaccine failures and breakthrough with higher-valency pneumococcal conjugate vaccines in children. Expert Rev Vaccines. 2022 Feb;21(2):201–214. eng. doi: 10.1080/14760584.2022.2012455
- Varghese J, Chochua S, Tran T, et al. Multistate population and whole genome sequence-based strain surveillance of invasive pneumococci recovered in the USA during 2017. Clin Microbiol Infect. 2020 Apr;26(4):512 e1–512 e10. doi: 10.1016/j.cmi.2019.09.008
- Hu T, Weiss T, Bencina G, et al. Comprehensive value assessments for new pediatric pneumococcal conjugate vaccines. J Med Econ. 2021 Jan;24(1):1083–1086. doi: 10.1080/13696998.2021.1970974
- Yeh SH, Gurtman A, Hurley DC, et al. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine in infants and toddlers. Pediatrics. 2010 Sep;126(3):e493–505. doi: 10.1542/peds.2009-3027
- Rid A, Saxena A, Baqui AH, et al. Placebo use in vaccine trials: recommendations of a who expert panel. Vaccine. 2014 Aug 20;32(37):4708–4712. doi: 10.1016/j.vaccine.2014.04.022
- World Health Organization. Recommendations for the production and control of pneumococcal conjugate vaccines. WHO Technical Report Series 9272005.
- Ryman J, Weaver J, Hu T, et al. Predicting vaccine effectiveness against invasive pneumococcal disease in children using immunogenicity data. NPJ Vaccines. 2022 Nov 7;7(1):140. doi: 10.1038/s41541-022-00538-1
- Siber GR, Chang I, Baker S, et al. Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine. 2007 May 10;25(19):3816–3826. doi: 10.1016/j.vaccine.2007.01.119
- Ryman J, Weaver J, Yee KL, et al. Predicting effectiveness of the v114 vaccine against invasive pneumococcal disease in children. Expert Rev Vaccines. 2022 Oct;21(10):1515–1521. doi: 10.1080/14760584.2022.2112179
- Whitney CG, Pilishvili T, Farley MM, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: A matched case-control study. Lancet. 2006 Oct 28;368(9546):1495–1502. doi: 10.1016/S0140-6736(06)69637-2
- Moore MR, Link-Gelles R, Schaffner W, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: a matched case-control study. Lancet Respir Med. 2016 May;4(5):399–406. doi: 10.1016/S2213-2600(16)00052-7
- A study to evaluate the safety, tolerability, and immunogenicity of two lots of v114 in healthy infants (v114-008). Clinicaltrials.Gov id: Nct02987972]. [cited 2022 Feb 8]. Available from: https://clinicaltrials.gov/ct2/show/NCT02987972
- A study to evaluate the interchangeability of v114 and Prevnar 13™ in healthy infants (v114-027/pneu-direction). [Clinicaltrials.Gov id: Nct03620162]. [cited 2022 Feb 8]. Available from: https://clinicaltrials.gov/ct2/show/NCT03620162
- Safety, tolerability, and immunogenicity of v114 in healthy infants (v114-029) ( pneu-ped). Clinicaltrials.Gov id: Nct03893448. [cited 2022 Feb 8]. Available from: https://clinicaltrials.gov/ct2/show/NCT03893448
- Andrews NJ, Waight PA, Burbidge P, et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis. 2014 Sep;14(9):839–846. doi: 10.1016/S1473-3099(14)70822-9
- Mussi-Pinhata MM, Ward S, Laimon L, et al. Effect of maternal immunization with 10-valent pneumococcus conjugate vaccine (pcv-10), 23-valent pneumococcus polysaccharide vaccine, or placebo on the immunogenicity of pcv-10 in human immunodeficiency virus-exposed uninfected infants: a randomized clinical trial. Clin Infect Dis. 2022 Sep 29;75(6):996–1005. doi: 10.1093/cid/ciac026
- A study of v419 given concomitantly with Prevnar 13™ and rotateq™ (p. v419–006). Clinicaltrials.Gov id: Nct01340937. [cited 2022 Feb 8]. Available from: https://clinicaltrials.gov/ct2/show/NCT01340937
- Nolan KM, Zhang Y, Antonello JM, et al. Enhanced antipneumococcal antibody electrochemiluminescence assay: validation and bridging to the who reference elisa. Bioanalysis. 2020 Oct;12(19):1363–1375. doi: 10.4155/bio-2020-0023
- Tan CY, Immermann FW, Sebastian S, et al. Evaluation of a validated luminex-based multiplex immunoassay for measuring immunoglobulin g antibodies in serum to pneumococcal capsular polysaccharides. mSphere. 2018 Aug 8;3(4):e00127–18. eng. doi: 10.1128/mSphere.00127-18
- de Miguel S, Domenech M, Gonzalez-Camacho F, et al. Nationwide trends of invasive pneumococcal disease in Spain from 2009 through 2019 in children and adults during the pneumococcal conjugate vaccine era. Clin Infect Dis. 2021 Dec 6;73(11):e3778–e3787. doi: 10.1093/cid/ciaa1483
- Ladhani SN, Collins S, Djennad A, et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and wales, 2000-17: a prospective national observational cohort study. Lancet Infect Dis. 2018 Apr;18(4):441–451. doi: 10.1016/S1473-3099(18)30052-5
- Zintgraff J, Gagetti P, Napoli D, et al. Invasive streptococcus pneumoniae isolates from pediatric population in Argentina for the period 2006-2019. Temporal progression of serotypes distribution and antibiotic resistance. Vaccine. 2022 Jan 24;40(3):459–470. doi: 10.1016/j.vaccine.2021.12.008
- Nanduri SA, Petit S, Smelser C, et al. Epidemiology of invasive early-onset and late-onset group b streptococcal disease in the United States, 2006 to 2015: multistate laboratory and population-based surveillance. JAMA Pediatr. 2019 Mar 1;173(3):224–233. doi: 10.1001/jamapediatrics.2018.4826
- De Miguel S, Latasa P, Yuste J, et al. Age-dependent serotype-associated case-fatality rate in invasive pneumococcal disease in the autonomous community of Madrid between 2007 and 2020. Microorganisms. 2021 Nov 3;9(11):2286. doi: 10.3390/microorganisms9112286
- Lapidot R, Shea KM, Yildirim I, et al. Characteristics of serotype 3 invasive pneumococcal disease before and after universal childhood immunization with pcv13 in massachusetts. Pathogens. 2020 May 21;9(5):396. doi: 10.3390/pathogens9050396
- Pilishvili T, Gierke R, Farley M, et al. Epidemiology of invasive pneumococcal disease (ipd) following 18 years of pneumococcal conjugate vaccine (pcv) use in the United States. Open Forum Infect Dis. 2020;7(Supplement 1):S736–S737. doi: 10.1093/ofid/ofaa439.1651
