ABSTRACT
Introduction
Technological innovations have been instrumental in advancing vaccine design and protective benefit. Improvements in the safety, tolerability, and efficacy/effectiveness profiles have profoundly reduced vaccine-preventable global disease morbidity and mortality. Here we present an original vaccine platform, the Multiple Antigen Presenting System (MAPS), that relies on high-affinity interactions between a biotinylated polysaccharide (PS) and rhizavidin-fused pathogen-specific proteins. MAPS allows for flexible combinations of various PS and protein components.
Areas covered
This narrative review summarizes the underlying principles of MAPS and describes its applications for vaccine design against bacterial and viral pathogens in non-clinical and clinical settings.
Expert opinion
The utilization of high-affinity non-covalent biotin–rhizavidin interactions in MAPS allows for combining multiple PS and disease-specific protein antigens in a single vaccine. The modular design enables a simplified exchange of vaccine components. Published studies indicate that MAPS technology may support enhanced immunogenic breadth (covering more serotypes, inducing B- and T-cell responses) beyond that which may be elicited via PS- or protein-based conjugate vaccines. Importantly, a more detailed characterization of MAPS-based candidate vaccines is warranted, especially in clinical studies. It is anticipated that MAPS-based vaccines could be adapted and leveraged across numerous diseases of global public health importance.
Plain Language Summary
Existing conjugate vaccines, consisting of pathogen-derived polysaccharides (PSs) and carrier proteins unrelated to the target pathogen, have helped to significantly reduce morbidity and mortality of several bacterial diseases. However, the worldwide burden of infectious diseases targeted by conjugate vaccines is still high. This is mainly due to high pathogen diversity and ongoing evolution, and innovative approaches are needed to respond to these challenges. Multiple Antigen Presenting System (MAPS) is an original vaccine technology that relies on strong molecular interactions between biotin and rhizavidin. MAPS is highly adaptable, as different PS and protein components can be precisely combined and easily exchanged, with limited damage to immunogenic epitopes (PS and protein features recognized by the immune system). Unlike existing conjugate vaccines, MAPS complexes contain pathogen-specific proteins, able to elicit broad immune responses directed against the pathogen. To date, investigational MAPS-based vaccines have been evaluated in several non-clinical studies; one candidate pneumococcal vaccine has been evaluated in early phase clinical studies in healthy children and adults (including older adults). In these clinical studies, the MAPS-based vaccine candidate was well tolerated and induced robust immune responses. If the favorable profile of MAPS-based vaccines is confirmed in further studies, these vaccines could be used against infectious diseases associated with significant morbidity and mortality.
1. Introduction
Estimated 3.5–5.0 million lives are saved worldwide every year as a consequence of vaccination. The success of vaccines at improving public health is a direct result of the seminal technological advances that have shaped vaccine development [Citation1,Citation2].
Most recently, mRNA and viral vector technologies have allowed for swift development and deployment of effective vaccines against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [Citation3]; it is likely that similarly urgent innovations in vaccination may be needed in the future to combat emerging and new pathogens. Furthermore, there remains an opportunity for improvement across a wide range of long-established vaccines, regarding responses to vaccines (e.g. tolerability profiles, efficacy/effectiveness, extent, and duration of protection) as well as practical considerations (e.g. manufacturing, cold chain, stability, and distribution) [Citation4–6].
The Multiple Antigen Presenting System (MAPS) is an innovative technology, with potential benefits across many vaccine-preventable diseases. MAPS combines pathogen-specific polysaccharide (PS) and protein components via a high-affinity non-covalent biotin–rhizavidin interaction [Citation7]. This narrative review provides an overview of MAPS technology development, non-clinical and clinical evaluations to date, and discusses future applications and differentiated potential of MAPS.
2. Vaccines history and technology
The field of vaccinology has benefited from several key enhancements, including changes in the choice of immunogenic targets (e.g. from the whole pathogen to specific subunits, toxoids, polysaccharides, DNA, or mRNA), improved technologies for target identification (e.g. reverse vaccinology), strategies to improve vaccine immunogenicity (e.g. use of adjuvants) and enhance vaccination compliance and coverage (e.g. combination and multivalent vaccines) [Citation1,Citation6,Citation8].
Vaccines against Streptococcus pneumoniae (pneumococcal vaccines) represent one clear example of technological innovations driving vaccine development [Citation9–11]. Following initial development as inactivated whole-cell preparations, multivalent formulations containing pneumococcal capsular polysaccharides (CPSs) from up to 23 different serotypes were licensed. One 23-valent formulation (pneumococcal polysaccharide vaccine [PPSV23]) is still recommended in older adults aged ≥65 years and in certain populations at high risk for pneumococcal infection [Citation12,Citation13]. Nevertheless, the efficacy of the pure CPS vaccines against invasive pneumococcal disease is a matter of some controversy and, importantly, these vaccines are not reliably immunogenic in children under 2 years, in whom the burden of pneumococcal disease is particularly high [Citation10,Citation11].
Introduction of pneumococcal conjugate vaccines (PCVs), in which the CPS is covalently linked to a carrier protein that is not related to the targeted disease (i.e. disease-unrelated carrier protein), enabled the production of memory B cells via activation of T helper 2 (Th2) cells in immunized individuals [Citation10,Citation11,Citation14,Citation15] and significantly reduced the worldwide burden of invasive pneumococcal disease [Citation16]. Unlike the pure CPS formulations, PCVs were found to induce T-cell-dependent and persistent PS-specific antibody responses also in young children and mediate extensive protection through herd immunity [Citation11]. Currently licensed PCV formulations contain CPS from up to 20 serotypes [Citation17–22]. Similar conjugation technology has been applied to vaccines (licensed and/or investigational) against a number of other pathogens, such as Neisseria meningitidis (meningococcus), Salmonella enterica Typhi and Paratyphi, Haemophilus influenzae type B, group B Streptococcus, and Shigella [Citation15]. For example, for PCVs, a common feature of conjugate vaccines against the listed bacteria is induction of T-cell-mediated anti-PS antibody responses [Citation15,Citation23,Citation24].
While conjugation technology can improve vaccine immunogenicity, few limitations remain. In the case of pneumococcal vaccines, the number of serotypes that can be included without deleterious impact on the immunogenicity profile is limited. One of the mechanisms hypothesized to underlie this limitation is carrier-induced epitopic suppression, leading to a decreased immune response with every increase in valency [Citation11,Citation25]. Furthermore, the inclusion of serotype-specific CPS in conjugate vaccines does not always confer protection; in current PCV formulations, the serotype 3 PS component has not had a demonstrable impact on rates of invasive disease caused by this serotype [Citation17,Citation26]. Conjugate vaccines have also been associated with serotype replacement, whereby the prevalence of serotypes that are not included in the vaccine formulation has increased [Citation25]. After the introduction of PCV13 (a 13-valent PCV), a significant rise in invasive pneumococcal disease caused by non-vaccine serotype strains was observed in children in North America and Europe [Citation10]. Serotype replacement is also an issue among (older) adults, who have been found to be colonized by a wider variety of disease-causing pneumococcal serotypes than children [Citation10]. One hypothesis to explain the observed serotype replacement is that removing vaccine-targeted serotypes from the population opens a niche for non-vaccine-targeted serotypes [Citation25]. With over 100 currently known pneumococcal serotypes [Citation10], of which any can increase in prevalence, it is difficult to predict how vaccines may influence serotype replacement and which epidemiological shifts may occur. The effectiveness of the recently licensed 15- and 20-valent PCV formulations at reducing the pneumococcal disease burden remains to be determined. Finally, manufacturing of conjugate vaccines can be complex, especially for multivalent vaccines, where the multi-step conjugation reactions may affect a vaccine’s immunogenic potential [Citation11,Citation27].
Beyond pneumococcal vaccines, other potential limitations of current vaccination approaches include sub-optimal efficacy in specific populations (e.g. infants, older adults, immunocompromized individuals, and persons with underlying medical conditions), lack of universal protection against pathogens with inherent diversity, and limited duration of protection. Furthermore, key considerations such as ensuring health equity (i.e. availability across ethnicities and geographical regions) must also be tackled [Citation5,Citation28,Citation29].
Vaccines for established and new pathogens continue to be evaluated to bring new or improved solutions. Strategies include but are not limited to novel viral vectors [Citation30–33], updated mRNA technologies [Citation34,Citation35], novel adjuvants/adjuvant systems [Citation36], bioconjugation [Citation37–39] and Generalized Modules for Membrane Antigens (GMMA) [Citation40–42]. The development of new vaccines is supported by ongoing advances in the fields of structural biology, reverse vaccinology, synthetic biology, and more recently, artificial intelligence and machine learning. For the purposes of this review, we will focus on MAPS.
MAPS is an innovative vaccine platform and represents an original approach for antigen presentation and delivery, with the potential to ensure robust and broad immune responses against different pathogens [Citation7,Citation43]. The ability of MAPS-based complexes to induce both humoral and cellular responses coupled with broader serotype coverage may be a promising advance in modern vaccine development [Citation7,Citation43].
3. MAPS technology
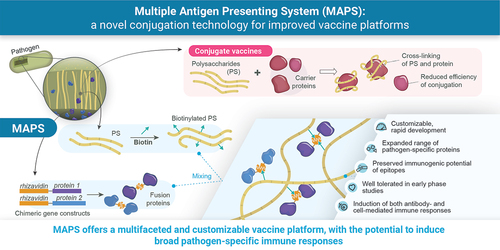
The MAPS approach relies on the formation of a macromolecular complex consisting of two components, joined by non-covalent affinity-based coupling: biotinylated PS and pathogen-specific proteins genetically fused to rhizavidin (from Rhizobium etli), an avidin-like biotin-binding moiety (see also Graphical Abstract) [Citation7]. After mixing these two main components together, the MAPS complexes are isolated using standard purification techniques. Depending on the individual PS target, the diameter of MAPS complexes can range between 20 nm and several hundred nm [Citation7].
The interaction between biotin and avidin moieties is mediated by the esterophilic heterocycle of biotin and biotin-binding sites on the avidin polymer. The small size of biotin, its ability to bind different molecules through its hydrophilic carboxylic acid chain, and the strength of its interaction with avidin have enabled varied use of the biotin-avidin coupling [Citation44]. In MAPS-based vaccines, rhizavidin is used as biotin-binding moiety [Citation7,Citation45–52]. In contrast to tetrameric avidin and streptavidin, rhizavidin is a dimer containing a proline residue after a flexible GGSG stretch in its loop between β-strands 7 and 8, leading to its unique and high-affinity interaction with biotin [Citation53]. The dissociation rate constant between D-biotin and rhizavidin was found to be significantly higher than those of avidin and streptavidin [Citation53].
Some advantages of this system include ease of manufacturing, versatility, and flexibility to easily adapt to different pathogens or in response to changes in disease epidemiology, while theoretically also minimizing possible epitope damage by avoiding covalent bindings [Citation7]. The MAPS platform lends itself to vaccine optimization, as the PS:protein ratio can be precisely controlled, and the protein components can be easily exchanged. Furthermore, by combining PS with pathogen-specific, conserved proteins instead of disease-unrelated carrier proteins, the breadth of the immune response induced by MAPS vaccines is increased, comprising multipronged antibody- and cell-mediated immune responses to both PS and protein antigens [Citation7,Citation43].
4. Non-clinical and clinical evaluation of MAPS-based vaccines
Investigational MAPS-based rhizavidin-containing vaccines have been evaluated in several non-clinical studies [Citation7,Citation43,Citation45–47,Citation49–52,Citation54] and clinical trials [Citation48], with a large body of results not yet described in peer reviewed publications [Citation45,Citation47,Citation54]. Two clinical studies regarding the safety and immunogenicity of a pneumococcal investigational vaccine (one in adults, NCT04265911; one in toddlers from the United States, NCT04525599) are completed and results are being prepared for publication.
Immunization with MAPS-based vaccines was shown to induce robust anti-PS immunoglobulin G (IgG) production [Citation7,Citation46,Citation47,Citation51], high levels of opsonophagocytic activity (OPA) [Citation7,Citation48,Citation51] and Th1/Th17 cellular immune responses in non-clinical and clinical study settings [Citation7,Citation48–50,Citation52].
4.1. Evidence from non-clinical studies
An important aspect of MAPS is its ability to induce not only anti-PS antibodies but also anti-protein humoral (usually IgG) and cell-mediated (e.g. cytotoxic and Th1 and Th17) immune responses (). The elicited immune responses are thus broader than those generated using classical conjugation technology. It has also been recently demonstrated that the MAPS-induced anti-PS and anti-protein antibodies can provide synergistic immunogenic and protective effects [Citation43,Citation45].
Table 1. Overview of immune responses induced by MAPS-based vaccines in non-clinical studies, by disease area.
4.1.1. MAPS-induced humoral immunity: anti-PS and anti-protein antibodies
PS-specific IgG titers and OPA levels were several fold higher in MAPS-immunized versus PCV13-immunized mice [Citation7] in the initial non-clinical study of candidate pneumococcal MAPS-based vaccine formulations. The described anti-PS responses were shown to be dependent on cluster-of-differentiation-4-expressing (CD4+) T cells, as depletion of these cells nearly abolished production of PS-specific antibodies.
The protein components may aid the anti-CPS responses induced by MAPS vaccines and the likely mechanisms have recently been described [Citation43] (). Inclusion of a pathogen-specific protein in a MAPS vaccine results in greater natural boosting of the antibody response to the PS components of the vaccine when the host is subsequently exposed to the organism [Citation43].
Figure 1. Schematic representation of MAPS-induced immune responses to polysaccharide and protein antigens (largely based on [Citation43]).
![Figure 1. Schematic representation of MAPS-induced immune responses to polysaccharide and protein antigens (largely based on [Citation43]).](/cms/asset/b2bdf332-158e-42bc-b275-a9b2f5e6882e/ierv_a_2299384_f0001_oc.jpg)
In addition to anti-PS responses, MAPS complexes evaluated in non-clinical studies were shown to induce IgG responses specific for protein antigens from Mycobacterium tuberculosis (Mtb) [Citation7,Citation50], pneumococcus [Citation7], Staphylococcus aureus [Citation52], S. enterica serovars Typhimurium, Enteritidis, Typhi, and Paratyphi A [Citation46,Citation51], and SARS-CoV-2 spike protein [Citation49]. Furthermore, when given at the equivalent amount of individual components, MAPS-based vaccines induced higher protein-specific responses than the uncoupled protein antigens [Citation7,Citation52]. Additionally, a study in non-human primates indicate that MAPS-induced antigen-specific antibodies may have distinct effector functionalities, since antibodies against the SARS-CoV-2 spike protein bound with high affinity to FCγRIIa and FCγRIIb in the presence of the spike protein or its receptor-binding domain [Citation49]. The MAPS-induced antibodies promote phagocytic function in monocytes and neutrophils and antibody-dependent complement deposition [Citation49].
4.1.2. MAPS-induced cellular immunity
Induction of CD4+ T cells producing interferon-gamma (IFN-γ) and interleukin-17A (IL-17A) was detected after immunization of mice with MAPS complexes containing Mtb proteins coupled to the pneumococcal CPS from serotype 3, but not with uncoupled Mtb proteins [Citation7]. With the same PS backbone, the size of the MAPS complex was found to likely impact the level of induced cellular responses [Citation7]. Induction of Th1 and Th17 responses, characterized by IFN-γ and IL-17A secretion upon stimulation of peripheral blood cells of immunized animals with the target protein antigens, was also detected in other studies, with different PS and protein antigen combinations used in the MAPS vaccines [Citation49,Citation50,Citation52]. O’Hara et al. [Citation50] found that mice immunized with an Mtb-targeting MAPS vaccine had increased total numbers of CD4+ and CD8+ T cells in peripheral blood and nasal tissues, but not in the lung or spleen [Citation50]. Immunization with MAPS vaccines induced distinct CD4+ and CD8+ T-cell subpopulations. Specifically, in MAPS-immunized animals, CD4+ and/or CD8+ effector memory T cell levels were significantly increased in the systemic compartments (blood and spleen) and tissue-resident memory T cells in local tissues (nasal passages and lungs) [Citation50]. MAPS-induced memory CD4+ splenic and lung T cells were specific to the target protein antigens. Upon ex vivo stimulation, the cells were found to produce IFN-γ (Th1 response), tumor necrosis factor-alpha (TNF-α) (Th1 response), IL-17A (Th17 response), or granzyme B (cytotoxic CD4+ T cells). A similar trend was detected for cytotoxic CD8+ responses, with IFN-γ or granzyme B production in the spleen, but only granzyme B in lung [Citation50]. In another study investigating a MAPS-based vaccine targeting SARS-CoV-2 [Citation49], antigen-specific T-cell responses, in terms of IFN-γ-producing T cells (CD4+ Th1, CD8+), TNF-α-producing CD8+ T cells, Th1 and Th17 cell frequencies, and IL-4-producing CD4+ T cells (Th2), were significantly higher in MAPS – than placebo-immunized macaques [Citation49].
4.1.3. Protective capacity of MAPS-based vaccines
MAPS-immunized animals were protected against invasive disease and/or death compared to placebo-immunized animals in infection challenges in several studies [Citation45,Citation49,Citation51,Citation52]. Cieslewicz et al. [Citation49] showed that the SARS-CoV-2 mRNA is more quickly and efficiently cleared from nasal and broncho-alveolar tissues in macaques immunized with a spike-protein-based MAPS vaccine compared to placebo [Citation49]. Two of the most detailed characterizations of MAPS-induced protective responses were reported in studies focusing on S. aureus and Mtb [Citation50,Citation52]. Interestingly, in a S. aureus study, MAPS-induced protection was mediated by various immune mechanisms depending on the infection route (i.e. intravenous or subcutaneous injection, intranasal inoculation) [Citation52]. Humoral responses were found to mediate protection against bacteremia and, together with cellular responses, to protect the animals against dermonecrosis, while cellular responses were both essential and sufficient for protection against skin abscesses and gastrointestinal (GI) colonization [Citation52]. Either IFN-γ or IL-17A secretion (induced by MAPS vaccination) is sufficient for protection against skin abscesses, while both cytokines are required for optimal protection against GI colonization. Similar importance of joint IFN-γ- and IL-17A-driven responses was noted in an Mtb study, where a MAPS-based vaccine (containing several Mtb proteins [full-length or fragments]: ESAT6, CFP10, TB9.8, TB10.4, MPT64, MPT83, and MPT51) effectively lowered bacterial load in the lung and spleen in an Mtb infection challenge [Citation50]. The mechanism of MAPS-mediated protection observed in the same study was likely multifaceted: broad cellular responses (including Th1, Th17, cytotoxic CD4+ and CD8+ cells, gamma delta T [γδT] and natural killer T [NKT] cells), tissue-resident memory T cells in nasal passages and lung tissues, and balanced secretion of IFN-γ and IL-17A. Furthermore, the co-administration of the MAPS-based Mtb vaccine with Bacille Calmette-Guérin boosted cellular immunity to Mtb antigens and improved bacterial clearance (especially from the lungs) beyond the levels of either vaccine alone [Citation50].
Another study investigated the protective capacity of pneumococcal MAPS-based vaccines, specifically the synergistic effect of antibodies against CPS and pathogen-specific proteins, in a lethal sepsis model in mice [Citation45]. Mice were challenged with a pneumococcal strain of serotype 3, against which PCV13 vaccines in humans are not effective [Citation45]. If mice were immunized with sera from rabbits immunized with PCV13- or MAPS-based vaccine (containing CPS3, CP1, and a detoxified pneumolysin mutant SPP2), the latter group had a significantly higher median survival rate after a highly lethal infection challenge with a strain of serotype 3. These data strongly suggest a synergistic effect of pneumolysin- and CPS3-specific antibodies in mediating protection against serotype 3 pneumococcal infection [Citation45].
Taken together, the above non-clinical studies illustrate the importance of the multifaceted MAPS-induced immune responses in conferring optimal protection against disease-associated morbidity and/or mortality in animal models.
4.2. Clinical evaluation of MAPS-based vaccines
4.2.1. MAPS-induced humoral immunity: anti-PS and anti-protein antibodies
The first clinical study of a pneumococcal MAPS-based vaccine indicated a similar profile of humoral responses in adults (aged 18–64 and 65–85 years) as observed in non-clinical studies [Citation48]. At 30 days post-immunization with the investigational MAPS-based (AFX3772) and PCV13 control vaccine, anti-PS IgG geometric mean concentrations (GMCs) were comparable for shared serotypes and significantly higher for AFX3772-unique serotypes after AFX3772 versus PCV13 immunization [Citation48]. Antibody OPA levels follow a similar trend as anti-PS IgG GMCs for most serotypes [Citation48]. Moreover, the GMCs of IgG titer against sp1500 + sp0785 (the two pneumococcal proteins included in the MAPS vaccine) increased several folds over baseline at 1-month post-immunization with the AFX3772, but not with the control PCV13 vaccine [Citation48].
4.2.2. MAPS-induced cellular immunity
The findings of a clinical study of the candidate pneumococcal AFX3772 vaccine are consistent with the described non-clinical T-cell data [Citation48]. Upon ex vivo stimulation of peripheral blood mononuclear cells from the participants of this study with the purified antigenic fusion proteins, the Th17 responses (i.e. Th17 cell frequencies, IL-17 and IL-22 production) were significantly greater for individuals who received AFX3772 (at the highest dose group) versus PCV13 recipients at Day 30 [Citation48]. Based on its composition and described immunogenicity findings, the investigational AFX3772 vaccine has the potential to induce multipronged immune responses to both pneumococcal PS and proteins, thereby providing better protection against different vaccine and possibly non-vaccine pneumococcal serotypes.
4.2.3. Safety profiles of MAPS-based vaccines
Both the published [Citation48] and unpublished [Citation48] phase 1–2 clinical data indicate an acceptable safety profile of the 24-valent investigational AFX3772 vaccine. In a phase 2 study in older adults (aged 65–85 years), the overall frequencies of administration-site and systemic AEs were comparable after AFX3772 or PCV13 administration in PCV13-naive participants [Citation48]. Tenderness and pain were the most frequent administration-site AEs, while fatigue, headache, and myalgia were the most frequently reported systemic AEs. Frequencies of all AEs tended to be highest for the highest-dose AFX3772 formulation (5 μg of each PS), but the difference versus PCV13 was not statistically significant. There was no clear correlation between the vaccine dosage and the frequencies of treatment-emergent AEs, and no vaccine-related serious AEs were reported [Citation48].
5. Conclusions
Despite major advances in vaccine technology, further developments are needed to enhance vaccine immunogenicity and effectiveness and to design vaccines that convey protection against evolving and emerging pathogens. The non-clinical and clinical data on MAPS-based vaccines suggest the broad applicability of MAPS and distinct functionalities of induced immune responses.
To date, the MAPS technology has been successfully evaluated in non-clinical studies across several bacterial targets, including S. pneumoniae, S. aureus, M. tuberculosis, Group B Streptococcus, Shigella, Salmonella, and a viral target, SARS-CoV-2. The initial phase 1/2 clinical trial of an investigational pneumococcal vaccine AFX3772 confirmed the non-clinical immunogenicity findings. Induction of specific T-cell responses, especially Th1 and Th17, together with antibody-mediated immunity is an important feature of MAPS-based vaccines, as these multifaceted responses may provide protection against a wider variety of pathogens compared to traditional conjugate vaccines. Further clinical studies are warranted to better characterize the AFX3772 and evaluate other MAPS-based vaccine candidates in human participants. Favorable outcomes of these clinical studies will be key to confirm the utility of MAPS for necessary innovations in modern vaccinology.
6. Expert opinion
MAPS technology is unique in that it leverages a well-known biotin–rhizavidin interaction to create novel, versatile, and adaptable antigen-presenting systems able to induce multifaceted immune responses. The process of generating MAPS complexes is now well established and can be tailored to various existing and emerging pathogens.
Several studies have indicated that MAPS-induced immunity is broad, characterized both by humoral (e.g. PS- and protein-specific IgG) and cellular (e.g. Th1, Th17, cytotoxic CD4+ and CD8+ T cells, γδT, and NKT cells) responses, owing largely to the inclusion of pathogen-specific proteins in MAPS complexes. In the sphere of pneumococcal vaccines, MAPS technology may help circumvent the issue of serotype replacement, by inducing serotype-specific and serotype-independent immune responses. Furthermore, MAPS may be a valuable approach when broad immune responses are required to enhance protection against the target disease. The modular and flexible natures of MAPS may be utilized to dissect mechanisms of protective immunity and help determine correlates of protection for diseases with complex pathogenesis.
Further research should focus on the following aspects: to evaluate and improve the use of MAPS system on the smaller PS and/or synthetic oligosaccharides; to study the underlying molecular mechanism as how MAPS-based vaccines may elicit different types of cellular responses; to explore the possibility of engineering a stable, monomeric rhizavidin derivative that could adapt fusion with protein antigens that oligomerize. Furthermore, not all protein antigens may tolerate a fusion with rhizavidin (e.g. steric hindrances, suboptimal epitope presentation, and loss of physiological activity). Thus, it is important to assess in which cases and how MAPS-based formulations may be limited concerning the choice of antigenic targets. In addition, the feasibility of the MAPS technology for other pathogens and the optimal approach for each (e.g. number and type of PS and protein components, valency, choice and addition of adjuvants for more challenging pathogens, and administration route) need to be evaluated. Existing and future vaccine candidates will also need to be evaluated in at-risk populations, such as infants, older adults and frail populations, individuals who may be immunocompromized due to disease and/or therapy, and people with underlying comorbidities and/or medical conditions. Furthermore, it is important to determine both vaccine efficacy and the real-world safety and effectiveness of MAPS-based vaccines.
The true impact of MAPS-generated multifaceted immune profiles on clinical outcomes will have to be evaluated for different pathogens and in specific populations. This will be particularly important to determine the long-term safety profile and persistence of MAPS-induced immune responses and the corresponding needs for revaccination.
Despite the currently open questions regarding MAPS-based vaccines, we believe this is a promising technology for modern vaccine development. MAPS technology may offer immunological and practical solutions to many of the challenges in vaccinology. Should MAPS be successfully used for immunization against common pathogens, the relatively simple production and customizable design may lead to improved vaccine coverage in previously underserved or vulnerable populations. Applications of MAPS technology to other noninfectious disease areas may also be possible in the future. Finally, its adaptable design may support more rapid vaccine development in response to changes in disease epidemiology by age, ethnicity, and geographic location, and allow for tailored approaches to combat diseases (e.g. for targeting specific pneumococcal serotypes contributing to invasive pneumococcal disease) of public health consequences.
Article highlights
Several aspects of infectious diseases, such as diverse mechanisms of pathogenesis, high diversity and evolution of etiological agents, serotype replacement, and emergence of new pathogens, call for development of novel and more effective vaccines.
Multiple Antigen Presenting System (MAPS) is a unique vaccine platform that uses high-affinity biotin–rhizavidin interaction to combine polysaccharides and target-pathogen-specific proteins into macromolecular complexes.
MAPS complexes are stable, precisely defined (controlled ratio of components), modular (components easily exchanged and combined), flexible (combination of components of different origin/property), and epitope-preserving (single-point attachment between polysaccharide and protein).
In non-clinical studies, MAPS-based candidate vaccines against six bacterial and one viral target were able to induce broader (antibody- and cell-mediated) immune responses compared to licensed vaccines targeting the same pathogens or purified protein controls.
In clinical studies, a 24-valent MAPS-based pneumococcal candidate vaccine (AFX3772) was shown to be well tolerated and induced robust immune responses in healthy adults (including older adults).
Inclusion of conserved pathogen-specific proteins in MAPS complexes may potentiate the protection mediated by polysaccharide-specific immunity via eliciting serotype-independent and multifaceted humoral and cellular responses (e.g. helper T cells [Th]1 and Th17 and cytotoxic T cells).
MAPS technology may provide a platform to support advanced vaccine development across various disease targets and overcome some inherent limitations of current technologies.
Abbreviations
AE, adverse event; CD4+ and CD8+, cluster-of-differentiation-4- and −8-expressing; CPS, capsular polysaccharide; γδT, gamma delta T cells; GI, gastrointestinal; GMC, geometric mean concentration; GMMA, Generalized Modules for Membrane Antigens; IFN-γ, interferon-gamma; IgG, immunoglobulin G; IL-17A, interleukin-17A; MAPS, Multiple Antigen Presenting System; Mtb, Mycobacterium tuberculosis; NKT, natural killer T cells; OPA, opsonophagocytic activity; PCV, pneumococcal conjugate vaccine; PPSV23, pneumococcal polysaccharide vaccine; PS, polysaccharide; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; Th1, Th2, and Th17, T helper cells 1, 2, and 17; TNF-α, tumor necrosis factor-alpha.
Declaration of interests
R Malley is a former employee of GSK and was an Affinivax board member and scientific founder at the time this manuscript was initiated. R Malley receives consulting fees from GSK as a consultant in the field of vaccines and discloses GSK support for attending meetings and/or travel and participation to the GSK Advisory board. F Zhang and YJ Lu received consulting fees from Affinivax and GSK as consultants. R Malley, F Zhang, and YJ Lu declare potential future payments and royalties as equity holders of Affinivax and named co-inventors of MAPS technology. R Malley, F Zhang, and YJ Lu have issued or pending patents on MAPS technology. S Sebastian has issued and pending patents on Multivalent Pneumococcal Vaccine. DO Willer has restricted share stock ownership in GSK. S Sebastian and DO Willer are employees of the GSK group of companies. None of the authors have any other financial or non-financial interests to declare. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors contributed substantially to the conception and design of the review article and interpreting the relevant literature and were involved in writing the review article and revised it for intellectual content. All authors contributed equally to manuscript development and have approved the final version of the manuscript.
Acknowledgments
The authors thank Akkodis Belgium for providing manuscript writing (Irena Zurnic Bönisch) and coordination support on behalf of GSK.
Additional information
Funding
References
- Bonanni P, Santos JI. Vaccine evolution. Perspect Vaccin. 2011;1(1):1–24. doi: 10.1016/j.pervac.2011.05.001
- World Health Organization. Vaccines and immunization. [cited 2023 Jun 20]. Available from: https://www.who.int/health-topics/vaccines-and-immunization#tab=tab_1
- Brice Y, Morgan L, Kirmani M, et al. COVID-19 vaccine evolution and beyond. Neurosci Insights. 2023;18:26331055231180543. doi: 10.1177/26331055231180543
- Lofano G, Mallett CP, Bertholet S, et al. Technological approaches to streamline vaccination schedules, progressing towards single-dose vaccines. NPJ Vaccines. 2020;5(1):88. doi: 10.1038/s41541-020-00238-8
- Piot P, Larson HJ, O’Brien KL, et al. Immunization: vital progress, unfinished agenda. Nature. 2019;575(7781):119–129. doi: 10.1038/s41586-019-1656-7
- Soni D, Bobbala S, Li S, et al. The sixth revolution in pediatric vaccinology: immunoengineering and delivery systems. Pediatr Res. 2021;89(6):1364–1372. doi: 10.1038/s41390-020-01112-y
- Zhang F, Lu YJ, Malley R. Multiple antigen-presenting system (MAPS) to induce comprehensive B- and T-cell immunity. Proc Natl Acad Sci U S A. 2013;110(33):13564–13569.
- Vesikari T, Sadzot-Delvaux C, Rentier B, et al. Increasing coverage and efficiency of measles, mumps, and rubella vaccine and introducing universal varicella vaccination in Europe: a role for the combined vaccine. Pediatr Infect Dis J. 2007;26(7):632–638. doi: 10.1097/INF.0b013e3180616c8f
- Austrian R. A brief history of pneumococcal vaccines. Drugs Aging. 1999;15(Supplement 1):1–10. doi: 10.2165/00002512-199915001-00001
- Briles DE, Paton JC, Mukerji R, et al. Pneumococcal vaccines. Microbiol Spectr. 2019;7(6). doi: 10.1128/microbiolspec.GPP3-0028-2018
- Oliveira GS, Oliveira MLS, Miyaji EN, et al. Pneumococcal vaccines: past findings, present work, and future strategies. Vaccines. 2021;9(11):1338. doi: 10.3390/vaccines9111338
- Centers for Disease Control and Prevention. Pneumococcal Polysaccharide VIS. [cited 2023 Nov 23]. Available from: https://www.cdc.gov/vaccines/hcp/vis/vis-statements/ppv.html
- Daniels CC, Rogers PD, Shelton CM. A Review of pneumococcal vaccines: current polysaccharide vaccine recommendations and future protein antigens. J Pediatr Pharmacol Ther. 2016;21(1):27–35. doi: 10.5863/1551-6776-21.1.27
- Rappuoli R. Glycoconjugate vaccines: Principles and mechanisms. Sci Transl Med. 2018;10(456):eaat4615. doi: 10.1126/scitranslmed.aat4615
- Rappuoli R, De Gregorio E, Costantino P. On the mechanisms of conjugate vaccines. Proc Natl Acad Sci U S A. 2019;116(1):14–16. doi: 10.1073/pnas.1819612116
- Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein–polysaccharide conjugate vaccines. Nat Rev Immunol. 2009;9(3):213–220. doi: 10.1038/nri2494
- Andrews NJ, Waight PA, Burbidge P, et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis. 2014;14(9):839–846. doi: 10.1016/S1473-3099(14)70822-9
- Cannon K, Cardona JF, Yacisin K, et al. Safety and immunogenicity of a 20-valent pneumococcal conjugate vaccine coadministered with quadrivalent influenza vaccine: a phase 3 randomized trial. Vaccine. 2023;41(13):2137–2146. doi: 10.1016/j.vaccine.2022.11.046
- Food and Drug Administration. Vaxneuvance. [cited 2023 Jul 28]. Available from: https://www.fda.gov/vaccines-blood-biologics/vaccines/vaxneuvance
- Food and Drug Administration. Prevnar 20. [cited 2023 Jul 28]. Available from: https://www.fda.gov/vaccines-blood-biologics/vaccines/prevnar-20
- Rupp R, Hurley D, Grayson S, et al. A dose ranging study of 2 different formulations of 15-valent pneumococcal conjugate vaccine (PCV15) in healthy infants. Hum Vaccin Immunother. 2019;15(3):549–559. doi: 10.1080/21645515.2019.1568159
- Stacey HL, Rosen J, Peterson JT, et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (PCV-15) compared to PCV-13 in healthy older adults. Hum Vaccin Immunother. 2019;15(3):530–539. doi: 10.1080/21645515.2018.1532249
- Bazhenova A, Gao F, Bolgiano B, et al. Glycoconjugate vaccines against Salmonella enterica serovars and shigella species: existing and emerging methods for their analysis. Biophys Rev. 2021;13(2):221–246. doi: 10.1007/s12551-021-00791-z
- Crum-Cianflone N, Sullivan E. Meningococcal vaccinations. Infect Dis Ther. 2016;5(2):89–112. doi: 10.1007/s40121-016-0107-0
- Løchen A, Croucher NJ, Anderson RM. Divergent serotype replacement trends and increasing diversity in pneumococcal disease in high income settings reduce the benefit of expanding vaccine valency. Sci Rep. 2020;10(1):18977. doi: 10.1038/s41598-020-75691-5
- Kwun MJ, Ion AV, Cheng H-C, et al. Post-vaccine epidemiology of serotype 3 pneumococci identifies transformation inhibition through prophage-driven alteration of a non-coding RNA. Genome Med. 2022;14(1):144. doi: 10.1186/s13073-022-01147-2
- Moffitt KL, Malley R. Next generation pneumococcal vaccines. Curr Opin Immunol. 2011;23(3):407–413. doi: 10.1016/j.coi.2011.04.002
- UNICEF. Immunization. [cited 2023 Jun 20]. Available from: https://data.unicef.org/topic/child-health/immunization/#status
- World Health Organization. Immunization coverage. [cited 2023 Jun 20]. Available from: https://www.who.int/news-room/fact-sheets/detail/immunization-coverage
- Colloca S, Barnes E, Folgori A, et al. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci Transl Med. 2012;4(115):115ra112. doi: 10.1126/scitranslmed.3002925
- Gilbert SC, Warimwe GM. Rapid development of vaccines against emerging pathogens: the replication-deficient simian adenovirus platform technology. Vaccine. 2017;35(35):4461–4464. doi: 10.1016/j.vaccine.2017.04.085
- Pinschewer DD. Virally vectored vaccine delivery: medical needs, mechanisms, advantages and challenges. Swiss Med Wkly. 2017;147:w14465.
- Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther. 2004;10(4):616–629. doi: 10.1016/j.ymthe.2004.07.013
- Park JW, Lagniton PNP, Liu Y, et al. mRNA vaccines for COVID-19: what, why and how. Int J Biol Sci. 2021;17(6):1446–1460. doi: 10.7150/ijbs.59233
- Ulmer JB, Mansoura MK, Geall AJ. Vaccines ‘on demand’: science fiction or a future reality. Expert Opin Drug Discov. 2015;10(2):101–106. doi: 10.1517/17460441.2015.996128
- Pulendran B, Arunachalam P, O’Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov. 2021;20(6):454–475. doi: 10.1038/s41573-021-00163-y
- Harding CM, Feldman MF. Glycoengineering bioconjugate vaccines, therapeutics, and diagnostics in E. coli. Glycobiology. 2019;29(7):519–529. doi: 10.1093/glycob/cwz031
- Kämpf MM, Braun M, Sirena D, et al. In vivo production of a novel glycoconjugate vaccine against Shigella flexneri 2a in recombinant Escherichia coli: identification of stimulating factors for in vivo glycosylation. Microb Cell Fact. 2015;14(1):12. doi: 10.1186/s12934-015-0195-7
- Romano MR, Berti F, Rappuoli R. Classical- and bioconjugate vaccines: comparison of the structural properties and immunological response. Curr Opin Immunol. 2022;78:102235. doi: 10.1016/j.coi.2022.102235
- Micoli F, MacLennan CA. Outer membrane vesicle vaccines. Semin Immunol. 2020;50:101433. doi: 10.1016/j.smim.2020.101433
- Piccioli D, Bartolini E, Micoli F. GMMA as a ‘plug and play’ technology to tackle infectious disease to improve global health: context and perspectives for the future. Expert Rev Vaccines. 2022;21(2):163–172. doi: 10.1080/14760584.2022.2009803
- Tennant SM, MacLennan CA, Simon R, et al. Nontyphoidal salmonella disease: Current status of vaccine research and development. Vaccine. 2016;34(26):2907–2910. doi: 10.1016/j.vaccine.2016.03.072
- Zhang F, Thompson C, Ma N, et al. Carrier proteins facilitate the generation of antipolysaccharide immunity via multiple mechanisms. MBio. 2022;13(3):e0379021. doi: 10.1128/mbio.03790-21
- Lesch HP, Kaikkonen MU, Pikkarainen JT, et al. Avidin-biotin technology in targeted therapy. Expert Opin Drug Deliv. 2010;7(5):551–564. doi: 10.1517/17425241003677749
- Besin G, Stevenson T, Malley R, et al. Synergistic protective effect of antibodies against polysaccharide type 3 and pneumococcal proteins in a highly virulent type 3 invasive disease model in mice (abstract 360/#606). Toronto (Canada): ISPPD; 2022. Available from: https://info.kenes.com/flip/isppd22/
- Boerth EM, Gong J, Roffler B, et al. Induction of broad immunity against invasive salmonella disease by a quadrivalent combination salmonella MAPS vaccine targeting Salmonella Enterica serovars typhimurium, enteritidis, typhi, and paratyphi a. Vaccines. 2023;11(11):1671. doi: 10.3390/vaccines11111671
- Boerth EM, Zhang F, Gong J, et al. A MAPS Vaccine Against Shigella Flexneri 2a, 3a, And 6 And Shigella Sonnei (Abstract ADJ08). Vaccines Against Shigella and ETEC (VASE) Conference; Washington (DC); 2022. Available from: https://custom.cvent.com/6DCDF4E0CF23495C882F4A5114961CE5/files/event/7f7e0f2602e8495e86b26300e082e0c5/3eea70d0ecf3455b9c29d204ad5c9d3a.pdf
- Chichili GR, Smulders R, Santos V, et al. Phase 1/2 study of a novel 24-valent pneumococcal vaccine in healthy adults aged 18 to 64 years and in older adults aged 65 to 85 years. Vaccine. 2022;40(31):4190–4198. doi: 10.1016/j.vaccine.2022.05.079
- Cieslewicz B, Makrinos D, Burke H, et al. Preclinical immunogenicity and efficacy of a Multiple Antigen-Presenting System (MAPSTM) SARS-CoV-2 vaccine. Vaccines. 2022;10(7):1069. doi: 10.3390/vaccines10071069
- O’Hara JM, Wakabayashi S, Siddiqi N, et al. A MAPS vaccine induces multipronged systemic and tissue-resident cellular responses and protects mice against mycobacterium tuberculosis. MBio. 2023;14(1):e0361122. doi: 10.1128/mbio.03611-22
- Zhang F, Boerth EM, Gong J, et al. A bivalent MAPS vaccine induces protective antibody responses against salmonella typhi and paratyphi A. Vaccines. 2022;11(1):91. doi: 10.3390/vaccines11010091
- Zhang F, Ledue O, Jun M, et al. Protection against staphylococcus aureus colonization and infection by B- and T-Cell-mediated mechanisms. MBio. 2018;9(5):e01949–01918. doi: 10.1128/mBio.01949-18
- Helppolainen SH, Nurminen KP, Määttä JAE, et al. Rhizavidin from Rhizobium etli: the first natural dimer in the avidin protein family. Biochem J. 2007;405(3):397–405. doi: 10.1042/BJ20070076
- Malley R. Glycoconjugates and MAPS. World vaccine congress; Washington (DC), USA; 2023.