ABSTRACT
Background
Updating vaccines is essential for combatting emerging coronavirus disease 2019 (COVID-19) variants. This study assessed the public health and economic impact of a booster dose of an adapted vaccine in the United Kingdom (UK).
Methods
A Markov-decision tree model estimated the outcomes of vaccination strategies targeting various age and risk groups in the UK. Age-specific data derived from published sources were used. The model estimated case numbers, deaths, hospitalizations, medical costs, and societal costs. Scenario analyses were conducted to explore uncertainty.
Results
Vaccination targeting individuals aged ≥65 years and the high-risk population aged 12-64 years was estimated to avert 701,549 symptomatic cases, 5,599 deaths 18,086 hospitalizations 56,326 post-COVID condition cases, and 38,263 lost quality-adjusted life years (QALYs), translating into direct and societal cost savings of £112,174,054 and £542,758,682, respectively. The estimated economically justifiable price at willingness-to-pay thresholds of £20,000 and £30,000 per QALY was £43 and £61, respectively, from the payer perspective and £64 and £82, respectively, from the societal perspective. Expanding to additional age groups improved the public health impact.
Conclusions
Targeting individuals aged ≥65 years and those aged 12-64 years at high risk yields public health gains, but expansion to additional age groups provides additional gains.
Disclaimer
As a service to authors and researchers we are providing this version of an accepted manuscript (AM). Copyediting, typesetting, and review of the resulting proofs will be undertaken on this manuscript before final publication of the Version of Record (VoR). During production and pre-press, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal relate to these versions also.1 Introduction
As of 26 May 2024, nearly 25 million cases and approximately 232,100 deaths attributable to COVID-19 have been reported in the UK [1]. The UK government has prioritized vaccination against COVID-19 as a primary mechanism to reduce its spread [2]. The UK was the first country worldwide to approve the emergency use of a COVID-19 vaccine and initiate a mass vaccination campaign in December 2020 [2]. By the end of 2023, 79% of the UK population had been vaccinated with at least one dose of a COVID-19 vaccine, and 75% of the population had been vaccinated with a complete primary series of a COVID-19 vaccine [1]. Vaccination against COVID-19 is particularly important for those considered at a higher risk of developing severe COVID-19, including older adults and individuals with underlying conditions [3]. Older individuals aged ≥65 years constitute 18% of the UK population [4], and 24.4% of the UK population (18% of those aged <70 years and 66% of those aged ≥70 years) has an underlying condition [5].
COVID-19 has continuously evolved throughout the pandemic, resulting in new variants. The Omicron variant emerged in November 2021 and rapidly became the dominant variant in circulation in the UK [9]. The effectiveness of the initial COVID-19 vaccines was reduced against the Omicron variant [10,11,12,13]. Therefore, bivalent vaccines were developed to offer a higher level of protection against Omicron variants [14,15]. In May 2023, the World Health Organization (WHO) evaluated the impact of COVID-19 vaccines on the then most current variants of concern and recommended new formulations to control the spread of emerging Omicron variants [16]. In response, the Joint Committee on Vaccination and Immunisation (JCVI) recommended that COVID-19 vaccines should be updated to incorporate a formulation targeting XBB lineages [17].
For older adults and high-risk individuals, the WHO vaccine recommendations published in 2023 recommend receiving a booster dose either six or 12 months following the previous dose depending on the age and risk level [18]. However, the uptake of booster vaccines may be limited by vaccine hesitancy. In the UK, vaccine hesitancy has been associated with a younger age, the female gender, ethnicity, low income, low educational levels, high reliance on information from social media, etc. [6,7,8]. Emphasizing the benefits of vaccination and addressing vaccination barriers are crucial for increasing the uptake of vaccines [8].
The objective of this modeling study was to quantify the introduction and expanded coverage of an adapted COVID-19 booster vaccine in the United Kingdom (UK) to determine its public health and economic impact in different age groups. To test our hypothesis that expanding vaccination to lower age groups would have a substantial public health impact, a simulation model was used to project the effect of vaccination with an adapted COVID-19 vaccine in the UK over a hypothetical year based on empirical epidemiological data collected during a period dominated by XBB lineages of the Omicron variant (XBB 1.5-adapted vaccine was used as a proxy for adapted vaccines). The results of this study could be used as a reference for the development and implementation of future adapted vaccines that offer levels of vaccine effectiveness (VE) [19,20,21,22,23,24] and duration of protection (DoP) [25,26,27] similar to those observed in adapted vaccines in recent years.
2 Methods
2.1 Model Structure and Overview
The impact of administering a single dose of a variant-adapted booster vaccine was estimated by modifying a previously reported combined decision tree-Markov model () [28] constructed based on the International Society for Pharmacoeconomics and Outcomes Research and the Society for Medical Decision Making (ISPOR-SMDM) guidelines [29]. The model was programmed in Microsoft Excel. The model started at the beginning of the COVID-19 vaccination season; immunity from vaccination or infection was assumed to have fully waned, and all individuals began the model in the susceptible health state following the approach used in studies modeling seasonal flu vaccination [34]. At model entry, a single booster dose of the adapted vaccine was administered. Then, the Markov component of the model monitored transitions across the health states over a hypothetical year ().
Figure 1. Model Structure. (a) Markov cohort model. (b) Decision tree component. Green circles represent decision points, and red triangles represent decision tree endpoints.
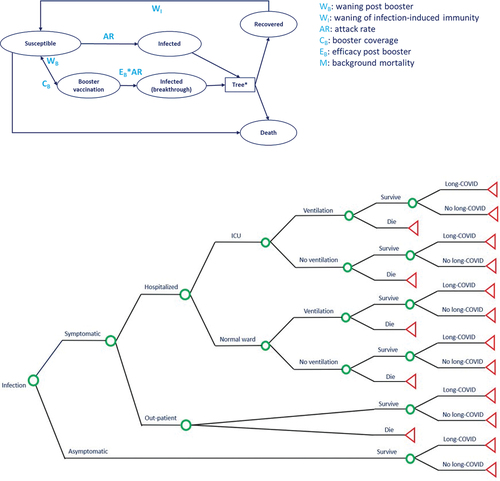
Because immunity conferred by COVID-19 vaccines has a limited duration and COVID-19 evolves rapidly, resulting in new variants and uncertainty in long-term parameter estimation, a one-year time horizon was selected. Health state transitions occurred in weekly cycles based on age-specific parameters derived from the literature. Individuals transitioned from the susceptible to the infected health state based on age-specific yearly attack rates that were adjusted by VE inputs to simulate the reduced transition from the vaccinated to the infected health state. Infection could be asymptomatic or symptomatic. Only patients with symptomatic infection were hospitalized or received outpatient care. Hospitalized patients could be admitted to a general ward or intensive care unit (ICU), and patients in the ICU could receive mechanical ventilation. All symptomatic patients could develop post-COVID condition (PCC) regardless of whether they were hospitalized based on research demonstrating the PCC burden in hospitalized and non-hospitalized populations [35,36,37,38,39]. The outcome probabilities were governed by the decision-tree component of the model (). Individuals in the vaccinated and recovered health states transitioned to the susceptible health state once vaccine-induced immunity and infection-induced immunity were considered to have waned, which occurred at a rate of the reciprocal of the DoP.
2.2 Model Outcomes
This analysis adopted the UK National Health Service (NHS) perspective and the societal perspective. The model estimated the following health outcomes: COVID-19 infections, hospitalizations, PCC, and deaths. The model estimated the following economic outcomes: number of vaccine doses administered, COVID-19-related costs (i.e., testing, treatment, and PCC management costs), and productivity losses. Additionally, the model estimated the economically justifiable price (EJP) at willingness-to-pay (WTP) thresholds of £20,000 and £30,000. The EJP was computed as the vaccine acquisition price that would ensure cost-effectiveness at a specified WTP threshold. A 3.5% discount rate was applied to all outcomes according to the National Institute for Health and Care Excellent (NICE) guidelines [40].
2.3 Model Inputs
2.3.1 Population Inputs
The UK population size stratified by age was sourced from the Office of National Statistics (ONS) [41]. The population was stratified into a high-risk population (individuals with ≥1 underlying health conditions conferring risk of severe COVID-19 and those aged ≥65 years) and a standard-risk population (individuals aged <65 years without underlying health conditions). The prevalence of underlying health conditions, including chronic respiratory disease, heart disease, kidney disease, neurological conditions, diabetes mellitus, asthma, and severe obesity, was informed by UK data derived from electronic health records [5].
2.3.2 Infection Inputs
The proportion of the susceptible population that acquired a COVID-19 infection was defined by age-specific attack rates derived from the UK Health Security Agency (Supplementary ) [42,43].
Table 1. Base-case results in the UK population aged ≥65 years and high-risk population aged 12 to 64 years
Table 2: Incremental impact of alternative age/risk-based vaccination
2.3.3 Vaccine Inputs
The model assumed that booster vaccination was available to all adults aged ≥18 years in the UK. Vaccination uptake by age was informed by the Department of Health and Social Care (DHSC) (Supplementary ) [44]. The model was not specific to a specific vaccine product. Based on real-world evidence from studies investigating an XBB 1.5-adapted vaccine, VE was assumed to be 50% against infection [20,21], 60% against symptomatic infection [20,21,22], and 70% against hospitalization [22,23,24], with a DoP of 6 months [25,26,27]. Immunity conferred by prior infection was assumed to persist for 3 months.
Table 3: Scenario Analysis
2.3.4 Health Inputs
The probability of symptomatic infection by age was informed by Moore et al. [46]. The probability of hospitalization by age was informed by Nyberg et al. [47]. The probability of hospitalization in either a general ward or ICU by age was informed by Nyberg et al. [47]. The probability of ICU admission by age was informed by Yang et al. [48]. The probability of mechanical ventilation in the ICU was informed by Yang et al. [48] (Supplementary Table 4).
The probability of COVID-19-related death by age was informed by Nyberg et al. [47] (Supplementary Table 5). The same probability of death was assumed for all patients admitted to the ICU regardless of the receipt of mechanical ventilation due to data availability limitations.
The following risk ratios derived from published odds/hazard ratios were applied to account for the higher risk of severe COVID-19 infection in the high-risk population: hospitalization risk: 1.7; risk of severe infection requiring ICU admission: 1.12; and mortality risk: 2.15 [49]. No asymptomatic patients were assumed to develop PCC; the probability of PCC among symptomatic outpatients was informed by Hanson et al. [50]; and the probability of PCC among symptomatic inpatients was assumed to be 100% based on the DHSC (Supplementary Table 6) [44].
2.3.5 Cost Inputs
All costs were inflated to 2023 and are reported in pound sterling (£). Vaccine price was excluded from the analysis, but a deployment cost of £10.06 per vaccination line, including payment to general practitioners (GPs), was included [44]. The deployment cost used in past studies (£25) [51] was assumed to include pandemic response costs, such as IT setup, based on the National Audit Office [52]. Including only the GP cost for vaccine administration is appropriate given the transition to an endemic era. Asymptomatic patients were assumed to have neither taken a COVID-19 test nor received treatment in any setting. Symptomatic patients treated as outpatients were assumed to have taken one COVID-19 test (to confirm diagnosis), while symptomatic patients admitted to either a general ward or ICU were assumed to have taken two COVID-19 tests (to confirm diagnosis and recovery). The COVID-19 test price (£31) was informed by the Scottish Government [53]. Patients were assumed to have two GP visits at £39 per visit [54]. The over-the-counter pain medication cost (£0.48) was derived from Menni et al. [55].
The total cost of treatment in a general ward or ICU with or without ventilation was informed by the NHS [56] (Supplementary Table 7; see Supplemental Material for further details). Patients suffering from PCC accrued annual medical costs of £2,267 based on NICE estimates [60]. The model further considered indirect costs, including patients’ and caregivers’ productivity losses due to COVID-19 infection, PCC, and COVID-19-related mortality. Productivity losses were computed using the workforce participation rate and weekly labor cost derived from the Office of National Statistics [61] (Supplementary Table 8).
2.3.6 Health Utilities
To calculate patients’ health-related quality of life (HRQoL), utility decrements attributed to COVID-19 infection, acute treatment, and PCC treatment were applied to relevant outcomes and subtracted from age-specific utility norms of the general population [62]. Due to the limited availability of COVID-19-specific utility values, utility values for outpatient cases and ICU cases were obtained from other infectious diseases (e.g., influenza) [63,64,65,66]. Utility values specific for COVID-19 inpatients and PCC patients were available in the literature [67,68]. It was assumed that the NHS was operating at full capacity and that elective care procedures would need to be postponed due to the significant burden on the healthcare system during the respiratory season. To consider the impact of COVID-19 hospitalizations on the increase in the wait time for elective care, a bed cost multiplier approach was adopted instead because this approach considers the cost impact without requiring a bed capacity model. The decrement in QALYs due to outpatient treatment was determined by multiplying the utility decrement associated with COVID-19 symptoms by the duration of those symptoms [55]. The QALY decrement due to hospitalization was determined by multiplying the respective utility decrement associated with hospital outcomes by the duration of stay [69,70]. A short-term QALY decrement was considered for all patients receiving mechanical ventilation to account for the extended recovery period and impact on QoL [71]. QALYs lost due to premature death were considered over a lifetime horizon (Supplementary Table 9).
2.4 Analysis
In the base-case analysis, the outcomes of vaccination were explored in the UK population aged ≥65 years and the high-risk population aged 12-64 years. We explored four additional vaccination strategies targeting a) individuals aged ≥50 years and the high-risk population aged 12-49 years, b) individuals aged ≥30 years and the high-risk population aged 12-29 years, c) individuals aged ≥65 years, and d) individuals aged ≥75 years. To explore parameter uncertainty, a series of scenario analyses was conducted by varying the prevalence of PCC, PCC treatment costs, inpatient treatment costs, vaccine deployment costs, DoP, and discount rate. A one-way deterministic sensitivity analysis was conducted to evaluate the influence of parameter uncertainty on the results by varying the base-case values by ±20%.
3 RESULTS
3.1 Base-case Results
The public health and economic impact of COVID-19 booster vaccination in the UK population aged ≥65 years and the high-risk population aged <65 years was assessed. In terms of the public health impact, compared to no additional annual vaccination, booster vaccination was estimated to avert 701,549 symptomatic cases, 5,599 deaths, 683,463 outpatient cases, 18,086 hospitalizations, 56,326 PCC cases, and 38,263 lost QALYs. In terms of the economic impact, compared to no additional annual vaccination, booster vaccination resulted in cost savings of £112,174,054 in total direct costs and £542,758,682 in societal costs. From the payer perspective, the EJP at WTP thresholds of £20,000 and £30,000 was £43 and £61, while from the societal perspective, the EJP was £64 and £82, respectively ().
Next, the incremental impact of booster vaccination in alternative age and risk groups was explored, revealing that the impact of vaccination varied across the age groups. Extending the vaccination strategy to adults aged ≥50 years and the high-risk population 12-49 years resulted in greater benefits compared to the base-case strategy, with 916,157 symptomatic cases, 5,862 deaths, 895,653 outpatient cases, 20,504 hospitalizations, and 70,839 PCC cases prevented and 43,535 QALYs gained; this vaccination strategy results in cost savings of £121,745,646 in total direct costs and £704,787,114 in total societal costs, with an EJP at WTP thresholds of £20,000 and £30,000 of £37 and £53 from the payer perspective and £59 and £75 from the societal perspective, respectively. These health and economic benefits increased as the vaccination strategy extended to include additional age groups. The greatest benefits were observed with the vaccination strategy targeting the population aged ≥30 years and the high-risk population aged 12-29 years, with the highest numbers of symptomatic cases (1,451,178), deaths (5,913), outpatient cases (1,427,083), hospitalizations (24,096), and PCC cases (104,722) prevented and the greatest QALY gain (48,929); consequently, this vaccination strategy presented the highest cost savings in total direct costs (£174,992,231) and total societal costs (£1,212,143,704), with an EJP at WTP thresholds of £20,000 and £30,000 of £31 and £4 from the payer perspective and £59 and £72 from the societal perspective, respectively ().
We further explored the impact of vaccination strategies limiting vaccination to only those aged ≥65 years and those aged ≥75 years. Although the estimated gains in the strategy targeting the population aged ≥65 years were higher than those in the strategy targeting the population aged ≥75 years, both strategies resulted in lower benefits than those observed in the base-case analysis, which additionally targeted the high-risk population aged 12-64 years ().
3.2 Scenario Analysis
A set of scenario analyses was conducted to explore parameter uncertainty. In Scenarios 1 and 2, the prevalence of PCC in both outpatient and inpatient settings was varied, resulting in higher savings in incremental direct medical costs, productivity costs, and discounted QALYs and a higher EJP from both the payer and societal perspectives compared to the base case, while the cost savings, QALYs and EJP in Scenario 1 were higher than those in Scenario 2. In Scenarios 3 and 4, the costs associated with the treatment of PCC were varied by ±20%, resulting in higher incremental direct medical costs and EJP from both perspectives in Scenario 3 and lower incremental direct medical costs and EJP in Scenario 4 compared to the base case. However, the productivity costs and discounted QALYs were similar to those in the base case. In Scenarios 5 and 6, the inpatient cost parameters were multiplied by 1.1 and 2, respectively (compared to 1.55 used in the base case), resulting in lower cost savings in direct medical costs and EJP in Scenario 5 and higher direct medical cost savings and EJP in Scenario 6, with no change observed in incremental productivity costs or discounted QALYs. In Scenario 7, the inclusion of a £25.06 vaccine deployment cost resulted in an incremental cost increase and an EJP decrease, with similar incremental productivity costs and discounted QALYs compared to the base case. In Scenario 8, increasing the DoP of infection-induced immunity from three to four months slightly decreased the incremental direct and societal cost savings, incremental discounted QALYs, and EJP. Finally, in Scenario 9, decreasing the discount rate to 1.5% did not impact the incremental direct medical costs but increased the incremental productivity costs, incremental discounted QALYs, and EJP ().
3.3 Deterministic Sensitivity Analysis
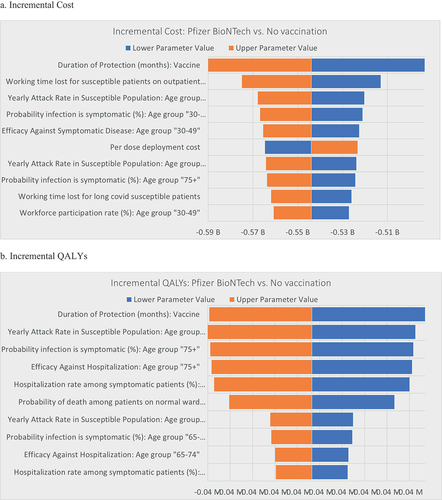
A one-way deterministic sensitivity analysis was conducted to assess the potential influence of parameter uncertainty on the results of the base-case analysis. displays a tornado diagram illustrating the top 10 parameters with the greatest influence. The parameters with the greatest impact on the incremental cost were the VE DoP, working time lost for susceptible patients receiving outpatient care, and the yearly attack rate in the susceptible population aged 30-49 years. The parameters with the greatest impact on the incremental QALYs were the VE DoP, yearly attack rate in the susceptible populated aged ≥75 years, and probability of a symptomatic infection in those aged ≥75 years.
4 Discussion
This study assessed the public health and economic impact of COVID-19 booster vaccination in the UK population. The base-case analysis results suggest that booster vaccination in the older adult and high-risk population offers significant benefits by averting symptomatic cases (701,549), deaths (5,599), outpatient cases (683,462), hospitalizations (18,086), and PCC cases (56,326), resulting in substantial direct medical cost (£112,174,054) and societal cost savings (£542,758,682). The analysis revealed variations in the impact of vaccination across different age groups. The benefits observed in the base-case analysis increased as the vaccination strategies incorporated additional age groups, with the greatest benefits observed in the vaccination strategy targeting all individuals aged ≥30 years and the high-risk population aged 12-29 years, with 1,451,178 symptomatic cases, 5,913 deaths, 1,427,082 outpatient cases, 24,096 hospitalizations, and 104,722 PCC cases prevented, 48,929 QALYs gained, and cost savings of £174,992,231 in medical costs and £1,212,143,704 in societal costs. In the scenario analyses, variations in the model parameters resulted in different levels of cost savings and QALYs gained. Varying the PCC prevalence resulted in higher cost savings, productivity costs, discounted QALYs, and EJP. Varying the PCC treatment cost and inpatient cost parameters impacted the cost savings and EJP. Changing the vaccine deployment cost resulted in increased costs and a decreased EJP. Increasing the DoP slightly impacted the cost savings, discounted QALYs, and EJP at the £20,000 WTP. Decreasing the discount rate increased the societal costs, discounted QALYs, and EJP. Overall, these findings highlight the importance and benefits of booster vaccination in mitigating the health and economic burdens of COVID-19.
Previous studies have shown that older adults are more susceptible to severe COVID-19 outcomes, with a higher-risk of severe COVID-19 infections, hospitalizations, and mortality [72,73]. Therefore, older adults have been prioritized in vaccination campaigns worldwide [73]. Although older adults have typically been defined as those aged ≥65 years [74], different definitions have been proposed [75]. For this reason, alternative vaccination strategies targeting different age groups were assessed. The vaccination strategy yielding the greatest benefits (i.e., targeting individuals aged ≥30 years and the high-risk population aged 12-29 years) also covers older adults, who are at the greatest risk of severe COVID-19 [72]. Additionally, vaccination in younger and low-risk populations reduces transmission to older adults and the high-risk population through indirect effects [76].
Notably, this study is subject to certain limitations. This study utilized a static model instead of a dynamic model due to the limited availability of data related to the transmission of COVID-19 and adapted vaccines; thus, certain aspects of disease dynamics, such as indirect effects, were not considered. Despite this limitation, many studies have used static models to study infectious diseases [28,77,78], and static models are considered favorable for studies investigating economic outcomes [79]. The model primarily relied on UK-specific inputs, thereby limiting the generalizability of the findings to other countries or regions that differ in circulating variants, levels of disease burden, and vaccine accessibility. The same VE inputs were applied to the standard- and high-risk populations due to the lack of granular data. Due to limitations in data availability, newer variants were not considered. The modeling study was informed by data collected during a period dominated by XBB lineages of the Omicron variant; additionally, the VE inputs were derived from studies investigating an XBB 1.5-adapted vaccine [20,21,22,23,24]; therefore, the XBB 1.5-adapted vaccine was used as a proxy for adapted vaccines. However, importantly, future variants may differ in their severity, infectiousness, and response to vaccines, necessitating future studies to continually assess and adapt vaccination strategies. Notably, it remains difficult to capture the current state of the COVID-19 situation accurately using available data because COVID-19 is constantly evolving. Finally, vaccine hesitancy, an important consideration in efforts to increase the uptake of COVID-19 vaccination, was not considered in this study.
Despite these limitations, our results highlight the significant benefits that could be realized by increasing vaccination coverage in younger individuals in the UK. Booster vaccination programs with expanded coverage have the potential to alleviate the burden on the UK’s healthcare system and off substantial medical and societal cost savings. Based on these data, policymakers making decisions regarding the inclusion of booster vaccines in national vaccination programs in the UK should consider increasing vaccination coverage among standard-risk and younger populations to reduce cases, hospitalizations, medical costs, and productivity losses.
5 Conclusions
Altogether, the results of this study illustrate that COVID-19 booster vaccination in the UK would result in significant public health and economic benefits. The vaccination strategy targeting individuals aged ≥30 years and individuals at a high risk aged 12-29 years would result in the greatest benefits. This study offers valuable information for policymakers and healthcare practitioners seeking to develop and implement vaccination strategies in the UK. Future research should focus on monitoring the efficacy of adapted vaccines against emerging variants of SARS-CoV-2 and optimizing vaccination campaigns as the virus continues to evolve.
Declarations of interest
C. Harrison, J. Yang, and R. Butfield, are employees of Pfizer and may own stocks. B. Yarnoff, is an employee of Evidera Inc., which received funding from Pfizer in connection to the study and with the development of this manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors were involved in the conception and design of the study and contributed to the analysis and interpretation. All authors critically revised manuscript drafts, approved the final version of the manuscript, and agree to be accountable for all aspects of the work.
Ethics statement
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
UK_COVID_SupplementaryMaterialV2_12June2024.docx
Download MS Word (60.5 KB)Acknowledgements
The authors wish to thank Lucie Bouin, Charles Reynard and Solene De Boisvilliers for their analytical assistance throughout model development. Medical writing was provided by Ruth Sharf, PhD of Evidera Inc and was funded by Pfizer Inc.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14760584.2024.2383343
Additional information
Funding
REFERENCES
- World health Organization. WHO COVID-19 dashboard. (Ed.^(Eds) (2024)
- Baraniuk C Covid-19: How the UK vaccine rollout delivered success, so far. BMJ, 372 (2021). n421 10.1136/bmj.n421
- World Health Organization. Coronavirus disease (COVID-19). (Ed.^(Eds) (2023)
- Centre for Ageing Better. Our Ageing Population. In: State of Ageing 2023-24. (Ed.^(Eds) (2023)
- Walker JL, Grint DJ, Strongman H, et al. UK prevalence of underlying conditions which increase the risk of severe COVID-19 disease: a point prevalence study using electronic health records. BMC Public Health. 2021;21(1):1–14. doi: 10.1186/s12889-021-10427-2
- Freeman D, Loe BS, Chadwick A, et al. COVID-19 vaccine hesitancy in the UK: the Oxford coronavirus explanations, attitudes, and narratives survey (oceans) II. Psychol Med. 2022;52(14):3127–3141. doi: 10.1017/S0033291720005188
- Allington D, McAndrew S, Moxham-Hall V, et al. Coronavirus conspiracy suspicions, general vaccine attitudes, trust and coronavirus information source as predictors of vaccine hesitancy among UK residents during the COVID-19 pandemic. Psychol Med. 2023;53(1):236–247. doi:10.1017/S0033291721001434
- Husted M, Gibbons A, Cheung W-Y, Keating S. COVID-19 vaccination hesitancy in adults in the United Kingdom: Barriers and facilitators to uptake. Health Psychology. 2023;42(8):584–592. doi: 10.1037/hea0001256
- Elliott P, Bodinier B, Eales O, et al. Rapid increase in omicron infections in England during December 2021: REACT-1 study. Science. 2022;375(6587):1406–1411. doi: 10.1126/science.abn8347
- Lau JJ, Cheng SMS, Leung K, et al. Real-world COVID-19 vaccine effectiveness against the omicron BA.2 variant in a SARS-CoV-2 infection-naive population. Nat Med. 2023;29(2):348–357. doi: 10.1038/s41591-023-02219-5
- Rosenblum HG, Wallace M, Godfrey M et al. Interim recommendations from the Advisory Committee on Immunization Practices for the use of bivalent booster doses of COVID-19 vaccines—United States, October 2022. Morbidity and Mortality Weekly Report, 71(45), 1436–1441 (2022).
- Andrews N, Stowe J, Kirsebom F et al. Covid-19 vaccine effectiveness against the Omicron (B. 1.1. 529) variant. New England Journal of Medicine, 386(16), 1532–1546 (2022).
- Powell AA, Kirsebom F, Stowe J, et al. Effectiveness of BNT162b2 against COVID-19 in adolescents. The Lancet Infectious Diseases. 2022;22(5):581–583. doi: 10.1016/S1473-3099(22)00177-3
- Pather S, Muik A, Rizzi R, et al. Clinical development of variant-adapted BNT162b2 COVID-19 vaccines: the early omicron era. Expert Rev Vaccines. 2023;22(1):650–661. doi:10.1080/14760584.2023.2232851
- Barda N, Lustig Y, Indenbaum V, et al. Immunogenicity of Omicron BA.1-adapted BNT162b2 vaccines: randomized trial, 3-month follow-up. Clinical Microbiology and Infection. 2023;29(7):918–923. doi: 10.1016/j.cmi.2023.03.007
- World Health Organization. Statement on the antigen composition of COVID-19 vaccines. (Ed.^(Eds) (2023)
- Department of Health and Social Care. JCVI statement on COVID-19 vaccination in spring 2024 and considerations on future COVID-19 vaccination, 4 December 2023. (Ed.^(Eds) (2023)
- World Health Organization.WHO SAGE roadmap on uses of COVID-19 vaccines in the context of OMICRON and substantial population immunity: an approach to optimize the global impact of COVID-19 vaccines at a time when Omicron and its sub-lineages are the dominant circulating variants of concern, based on public health goals, evolving epidemiology, and increasing population-level immunity, first issued 20 October 2020, updated: 13November 2020, updated: 16 July 2021, update: 21 January 2022, latest update: 30 March 2023. (Ed.^(Eds) (World Health Organization, 2023)
- Krammer F, Ellebedy AH. Variant-adapted COVID-19 booster vaccines. Science. 2023;382(6667):157–159. doi:10.1126/science.adh2712
- Huiberts AJ, Hoeve CE, de Gier B et al. Effectiveness of Omicron XBB. 1.5 vaccine against infection with SARS-CoV-2 Omicron XBB and JN. 1 variants, prospective cohort study, the Netherlands, October 2023 January 2024. Eurosurveillance, 29(10), 2400109 (2024).
- Skowronski DM, Zhan Y, Kaweski SE, et al. 2023/24 mid-season influenza and Omicron XBB. 1.5 vaccine effectiveness estimates from the Canadian Sentinel Practitioner Surveillance Network (SPSN). Eurosurveillance. 2024;29(7):2400076. doi: 10.2807/1560-7917.ES.2024.29.7.2400076
- Tartof SY, Slezak JM, Frankland TB et al. BNT162b2 XBB1. 5-adapted Vaccine and COVID-19 Hospital Admissions and Ambulatory Visits in US Adults. medRxiv, 2023.2012. 2024.23300512 (2023).
- Hansen CH, Moustsen-Helms IR, Rasmussen M, Søborg B, Ullum H, Valentiner-Branth P. Short-term effectiveness of the XBB. 1.5 updated COVID-19 vaccine against hospitalisation in Denmark: a national cohort study. The Lancet Infectious Diseases. 2024;24(2):e73–e74. doi:10.1016/S1473-3099(23)00746-6
- van Werkhoven CH, Valk A-W, Smagge B et al. Early COVID-19 vaccine effectiveness of XBB. 1.5 vaccine against hospitalisation and admission to intensive care, the Netherlands, 9 October to 5 December 2023. Eurosurveillance, 29(1), 2300703 (2024).
- Nguyen NN, Houhamdi L, Delorme L, et al. Reinfections with different SARS-CoV-2 omicron subvariants, France. Emerg Infect Dis. 2022;28(11):2341–2343. doi:10.3201/eid2811.221109
- Eythorsson E, Runolfsdottir HL, Ingvarsson RF, Sigurdsson MI, Palsson R. Rate of SARS-CoV-2 Reinfection During an Omicron Wave in Iceland. JAMA Netw Open. 2022;5(8):e2225320. doi:10.1001/jamanetworkopen.2022.25320
- Centers for Disease Control and Prevention (CDC). Reinfections and COVID-19. (Ed.^(Eds) (2022)
- Di Fusco M, Marczell K, Deger KA et al. Public health impact of the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) in the first year of rollout in the United States. Journal of Medical Economics, 25(1), 605–617 (2022).
- Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–7. Medical Decision Making, 32(5), 733–743 (2012).
- Cooper I, Mondal A, Antonopoulos CG. A SIR model assumption for the spread of COVID-19 in different communities. Chaos, Solitons & Fractals. 2020;139:110057. doi:10.1016/j.chaos.2020.110057
- Amaro JE, Dudouet J, Orce JN. Global analysis of the COVID-19 pandemic using simple epidemiological models. Applied Mathematical Modelling. 2021;90:995–1008. doi:10.1016/j.apm.2020.10.019
- Calafiore GC, Novara C, Possieri C. A time-varying SIRD model for the COVID-19 contagion in Italy. Annual reviews in control. 2020;50:361–372. doi:10.1016/j.arcontrol.2020.10.005
- Ndaïrou F, Area I, Nieto JJ, Torres DF. Mathematical modeling of COVID-19 transmission dynamics with a case study of Wuhan. Chaos, Solitons & Fractals. 2020;135:109846. doi:10.1016/j.chaos.2020.109846
- Peasah SK, Azziz-Baumgartner E, Breese J, et al. Influenza cost and cost-effectiveness studies globally–a review. Vaccine. 2013;31(46):5339–5348. doi:10.1016/j.vaccine.2013.09.013
- Li J, Zhou Y, Ma J, et al. The long-term health outcomes, pathophysiological mechanisms and multidisciplinary management of long COVID. Signal Transduct Target Ther. 2023;8(1):416. doi: 10.1038/s41392-023-01640-z
- O’Mahoney LL, Routen A, Gillies C et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: A systematic review and meta-analysis. EClinicalMedicine, 55 (2023). 101762 10.1016/j.eclinm.2022.101762
- Tufts J, Guan N, Zemedikun DT et al. The cost of primary care consultations associated with long COVID in non-hospitalised adults: a retrospective cohort study using UK primary care data. BMC Primary Care, 24(1), 245 (2023).
- Sandmann FG, Tessier E, Lacy J et al. Long-term health-related quality of life in non-hospitalized coronavirus disease 2019 (COVID-19) cases with confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in England: longitudinal analysis and cross-sectional comparison with controls. Clinical Infectious Diseases, 75(1), e962–e973 (2022).
- Soare I-A, Ansari W, Nguyen JL, et al. Health-related quality of life in mild-to-moderate COVID-19 in the UK: a cross-sectional study from pre-to post-infection. Health Qual Life Outcomes. 2024;22(1):12. doi: 10.1186/s12955-024-02230-5
- National Institute for Health and Care Excellence (NICE). Developing NICE guidelines: the manual. (Ed.^(Eds) (2014)
- Office for National Statistics. Population estimates for the UK, England, Wales, Scotland and Northern Ireland: mid-2021. (Ed.^(Eds) (2022)
- UK Health Security Agency. Weekly national Influenza and COVID-19 surveillance report Week 27 report (up to week 26 data) 7 July 2022. (2022).
- UK Health Security Agency. Weekly National Influenza and COVID-19 surveillance report Week 19 report (up to week 18 data) 11 May 2023. (Ed.^(Eds) (2023)
- Department of Health and Social Care. COVID-19 autumn 2023 vaccination programme: cost effectiveness impact assessment. (Ed.^(Eds) (2023)
- UK Health Security Agency. National flu and COVID-19 surveillance reports: 2022 to 2023 season. (Ed.^(Eds) (2023)
- Moore S, Hill EM, Tildesley MJ, Dyson L, Keeling MJ. Vaccination and non-pharmaceutical interventions for COVID-19: a mathematical modelling study. The lancet infectious diseases. 2021;21(6):793–802. doi:10.1016/S1473-3099(21)00143-2
- Nyberg T, Ferguson NM, Nash SG et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B. 1.1. 529) and delta (B. 1.617. 2) variants in England: a cohort study. The Lancet, 399(10332), 1303–1312 (2022).
- Yang J, Andersen KM, Rai KK, et al. Healthcare resource utilisation and costs of hospitalisation and primary care among adults with COVID-19 in England: a population-based cohort study. BMJ Open. 2023;13(12):e075495. doi: 10.1136/bmjopen-2023-075495
- Mason KE, Maudsley G, McHale P, et al. Age-adjusted associations between comorbidity and outcomes of COVID-19: a review of the evidence from the early stages of the pandemic. Front Public Health, 1124 (2021). 9 10.3389/fpubh.2021.584182
- Hanson SW, Abbafati C, Aerts JG et al. A global systematic analysis of the occurrence, severity, and recovery pattern of long COVID in 2020 and 2021. MedRxiv, (2022).
- Department of Health and Social Care. JCVI statement on COVID-19 vaccination in spring 2024 and considerations on future COVID-19 vaccination, 4 December 2023. (Ed.^(Eds) (2024)
- Department of Health and Social Care. The rollout of the COVID-19 vaccination programme in England. (Ed.^(Eds)
- Scottish Government. Cost of processing a PCR test for Covid-19: FOI release. (Ed.^(Eds) (2021)
- Personal Social Services Research Unit. Unit Costs of Health and Social Care 2022. (Ed.^(Eds) (2023)
- Menni C, Valdes AM, Polidori L, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. The Lancet. 2022;399(10335):1618–1624. doi: 10.1016/S0140-6736(22)00327-0
- NHS. NHS Reference Costs - National Schedule of NHS Costs 2020-21. (Ed.^(Eds) (2022)
- NHS England. 2020/21 National Cost Collection Data Publication. (Ed.^(Eds) (2022)
- NHS England Digital. Hospital Episode Statistics 2020-2021. (Ed.^(Eds)
- Neri M, Brassel S, Schirrmacher H, et al. Vaccine-preventable hospitalisations from seasonal respiratory diseases: what is their true value? Vaccines (Basel). 2023;11(5):945. doi: 10.3390/vaccines11050945
- National Institute for Health and Care Excellence. NICE Final Draft Guidance - Therapeutics for People with COVID-19. (Ed.^(Eds) (2023)
- Office for National Statistics. Earnings and hours worked, age group: ASHE Table 6. (Ed.^(Eds) (2023)
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value in Health. 2010;13(5):509–518. doi:10.1111/j.1524-4733.2010.00700.x
- van Hoek AJ, Underwood A, Jit M, Miller E, Edmunds WJ. The impact of pandemic influenza H1N1 on health-related quality of life: a prospective population-based study. PloS one, 6(3), e17030 (2011).
- Cuthbertson BH, Roughton S, Jenkinson D, MacLennan G, Vale L. Quality of life in the five years after intensive care: a cohort study. Critical care, 14, 1–12 (2010).
- Griffiths J, Hatch RA, Bishop J et al. An exploration of social and economic outcome and associated health-related quality of life after critical illness in general intensive care unit survivors: a 12-month follow-up study. Critical care, 17(3), 1–12 (2013).
- Marti J, Hall P, Hamilton P, et al. One-year resource utilisation, costs and quality of life in patients with acute respiratory distress syndrome (ARDS): secondary analysis of a randomised controlled trial. J Intensive Care. 2016;4(1):1–11. doi: 10.1186/s40560-016-0178-8
- Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID‐19 infection: a cross‐sectional evaluation. J Med Virol. 2021;93(2):1013–1022. doi: 10.1002/jmv.26368
- Evans RA, Leavy OC, Richardson M, et al. Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study. The Lancet Respiratory Medicine. 2022;10(8):761–775. doi: 10.1016/S2213-2600(22)00127-8
- Vekaria B, Overton C, Wiśniowski A, et al. Hospital length of stay for COVID-19 patients: data-driven methods for forward planning. BMC Infect Dis. 2021;21(1):1–15. doi: 10.1186/s12879-021-06371-6
- Hazard D, Kaier K, von Cube M et al. Joint analysis of duration of ventilation, length of intensive care, and mortality of COVID-19 patients: a multistate approach. BMC medical research methodology, 20, 1–9 (2020).
- Sandmann FG, Davies NG, Vassall A, et al. The potential health and economic value of SARS-CoV-2 vaccination alongside physical distancing in the UK: a transmission model-based future scenario analysis and economic evaluation. The Lancet Infectious Diseases. 2021;21(7):962–974. doi: 10.1016/S1473-3099(21)00079-7
- World Health Organization. Interim statement on booster doses for COVID-19 vaccination. (Ed.^(Eds) (2021)
- Thye AY-K, Tan LT-H, Law JWF, Letchumanan V. COVID-19 Booster Vaccines Administration in Different Countries. Progress In Microbes & Molecular Biology, 4(1) (2021).
- Orimo H, Ito H, Suzuki T, Araki A, Hosoi T, Sawabe M. Reviewing the definition of “elderly”. Geriatrics & gerontology international, 6(3), 149–158 (2006).
- United Nations Economic and Social Commission for Asia and the Pacific. Social development and ageing societies. (Ed.^(Eds)
- Park HJ, Gonsalves GS, Tan ST, et al. Comparing frequency of booster vaccination to prevent severe COVID-19 by risk group in the United States. Nat Commun. 2024;15(1):1883. doi: 10.1038/s41467-024-45549-9
- Xu Z, Zhang H, Huang Z. A continuous markov-chain model for the simulation of COVID-19 epidemic dynamics. Biology (Basel). 2022;11(2):190. doi:10.3390/biology11020190
- Dehghan Shabani Z, Shahnazi R. Spatial distribution dynamics and prediction of COVID‐19 in Asian countries: Spatial Markov chain approach. Regional Science Policy & Practice. 2020;12(6):1005–1025. doi:10.1111/rsp3.12372
- Lochen A, Anderson RM. Dynamic transmission models and economic evaluations of pneumococcal conjugate vaccines: a quality appraisal and limitations. Clin Microbiol Infec, 27(10), 1546–1557 (2021).