ABSTRACT
Background: The fractionated picosecond laser produces microscopic lesions in the epidermis and dermis, which are known as laser-induced optical breakdown (LIOB) and intra-dermal laser-induced cavitation (LIC). There have been multiple histological reports on these phenomena, although some have been challenged on the grounds of similarity to artifacts. Asian skins, with a higher melanin content, may react differently to this treatment, and present literature is also lacking in this area.
Purpose: To observe and report the histological effect of different energy levels and parameters of the fractional 532 nm/1064 nm picosecond laser on Asian skin ex vivo.
Methods: Six skin samples were taken from clinically normal-looking perilesional areas and treated with different energy levels and parameters of the fractional 532 nm/1064 nm picosecond laser. The specimens were then sent to the lab for H&E staining, and the slides were reviewed by a dermatopathologist.
Results: Superficial, intra-epidermal LIOBs were seen in skin treating at higher laser energies; deep, intra-dermal LICs were seen in skin treated at lower energies. Lesion sizes and depths were consistent with previously reported values on Caucasian skins, and lesions were spaced in 600-μm intervals or its multiple.
Conclusions: The histological findings are consistent with results from other ethnicities, and the spacing of lesions is a strong indication of their validity as LIOBs or LICs.
Introduction
The picosecond laser has achieved satisfactory results in the treatment of tattoos, pigmentary disorders, enlarged pores, aging skin, and acne scars (Citation1–Citation4). Histologic studies of skin treated with the picosecond 755 nm laser fractional microlens optic revealed the formation of intra-epidermal cavities caused by a process known as laser-induced optical breakdown (LIOB), an important mechanism behind the rejuvenating effect of laser treatments (Citation5–Citation8). Since then, additional studies have shown similar reactions and histological changes in skins treated with the 532- and 1064-nm picosecond fractional holographic optic lasers (Citation7,Citation9–Citation11). However, previous results have been challenged on the grounds of the LICs’ similarity to artifacts, and literature involving Asian patients remain scarce. This study therefore aims to observe the histologic effects of different energy levels and parameters of the fractional 532- and 1064-nm picosecond lasers on Asian skin ex vivo.
Materials and methods
Skin samples were biopsied from clinically normal-looking perilesional areas in patients who underwent surgical excision for benign skin tumors. Patients with infectious or inflammatory dermatosis in lesional or perilesional areas were excluded, and six skin samples from two subjects were ultimately taken from the right arm of an East-Asian female (Patient A, age 36 years, Fitzpatrick skin type III) and the right thigh of an East-Asian male (Patient B, age 46 years, Fitzpatrick skin type IV). Both subjects were in good health and free of severe systemic diseases at the time of biopsy. Informed consent was obtained from both subjects before biopsy. Sample information is listed in .
Table 1. Specimens by subject demographics and biopsy sites.
After biopsy, skin samples were treated with a Nd:YAG laser system fitted with a holographic beam-splitting optic (PicoWay Resolve, Syneron-Candela Corporation, Wayland, MA) emitting 532- and 1064-nm picosecond-domain pulses. Each sample was treated by four consecutive shots at a repetition rate of 1 Hz. 1.9 mJ/microbeam and 2.3 mJ/microbeam energy settings were used for the 1064-nm wavelength, and 0.2 mJ/microbeam and 0.3 mJ/microbeam energy settings were used for the 532-nm wavelength.
Skin samples underwent laser treatment immediately after excision, under identical temperature and humidity levels, and were immersed in formalin solution before being sent to a pathology center for sectioning and staining with hematoxylin and eosin. The resulting slides were then examined by a well trained and qualified dermatopathologist looking specifically for intra-epidermal LIOBs and intra-dermal LICs. LIOB and LIC depths and sizes were recorded and photographed.
Results
Specimens 1 and 2 were treated using the fundamental 1064 nm Nd:YAG PicoWay Resolve applicator at 2.3 mJ/microbeam. Specimen 3 was treated with the same, except at 1.9 mJ/microbeam. Specimen 4 was treated using the frequency-doubled 532 nm Nd:YAG PicoWay Resolve applicator at 0.3 mJ/microbeam. Specimens 5 and 6 were treated using the same, except at 0.2 mJ/microbeam.
Intra-epidermal LIOBs were found in both specimens 1 and 2 ( and , respectively; both treated with 1064 nm, 2.3mJ) post treatment, ranging between 15–100 μm in depth and 30–55 μm in width. The outer skin barrier was unaffected and remained intact. Neither specimen showed intra-dermal LIC formation.
Figure 1. Histology from specimen No.1 demonstrating intra-epidermal LIOB (Hematoxylin and eosin (H&E), 100X).
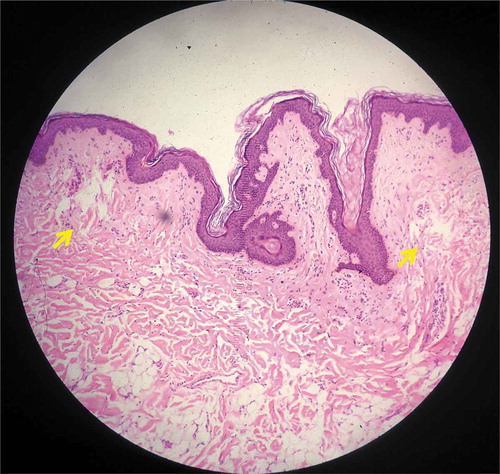
Figure 2. Histology from specimen No.2 demonstrating intra-epidermal LIOB (Hematoxylin and eosin (H&E), 100X).
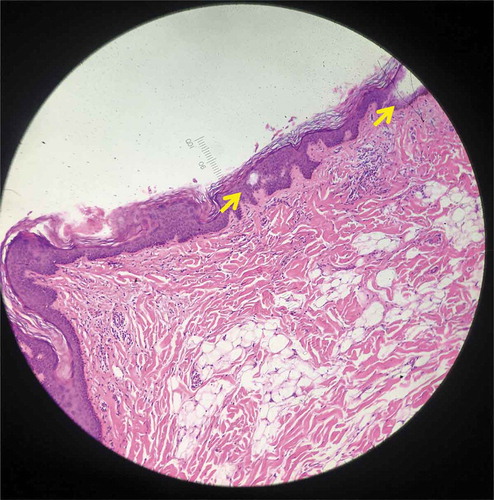
In contrast, no intra-epidermal LIOBs were found in specimen 3 (; treated with 1064 nm, 1.9 mJ). Instead, intra-dermal LICs were found, ranging from 155 to 540 μm in depth, 295–340 μm in length, and 430–540 μm in width. There was no significant damage to the stratum corneum, epidermis, or overlying dermis.
Figure 3. Histology from specimen No.3 demonstrating intra-dermal LIC (Hematoxylin and eosin (H&E), 100X).
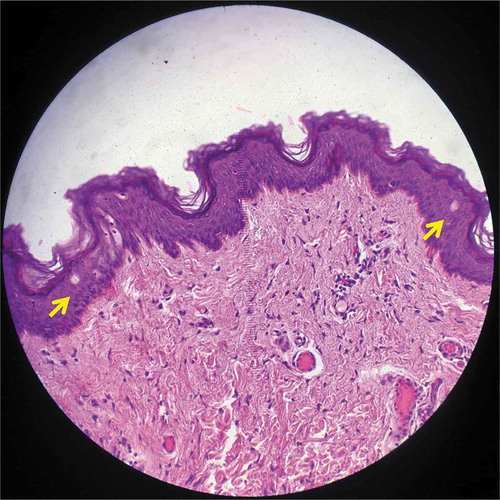
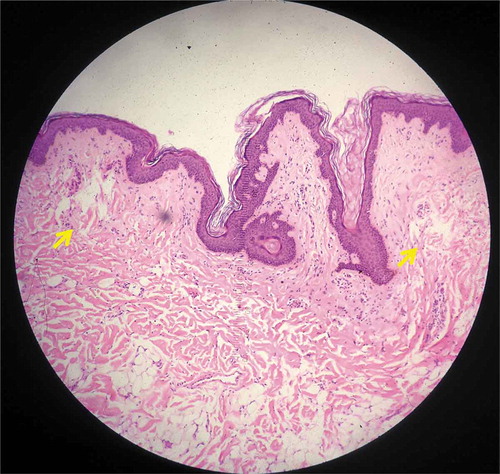
Both intra-epidermal LIOBs and vacuolated keratinocytes were found in specimen 4 (; treated with 532 nm, 0.3 mJ). LIOB depth ranged from 25 to 60 μm, with a size of 25 μm. No intra-dermal LICs were noted, and the outer skin layer remained intact.
Figure 4. Histology from specimen No.4 demonstrating intra-epidermal LIOB (Hematoxylin and eosin (H&E), 200X).
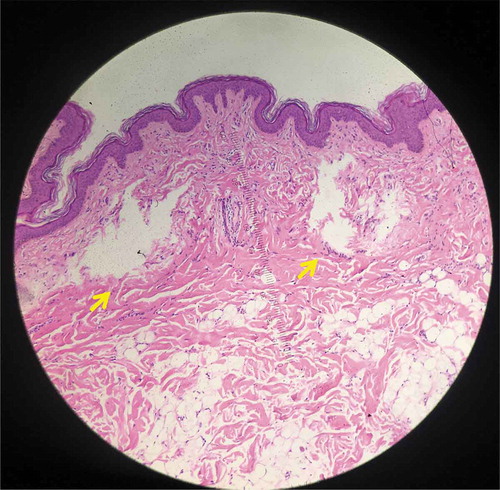
No intra-epidermal LIOBs were found in specimens 5 and 6 ( and , respectively; treated with 532 nm, 0.2 mJ). Intra-dermal LICs were noted, ranging from 215 to 970 μm in depth, 120–520 μm in length, and 180–260 μm in width. There was no significant damage to the stratum corneum, epidermis, or overlying dermis.
Figure 5. Histology from specimen No.5 demonstrating intra-dermal LIC (Hematoxylin and eosin (H&E), 100X).
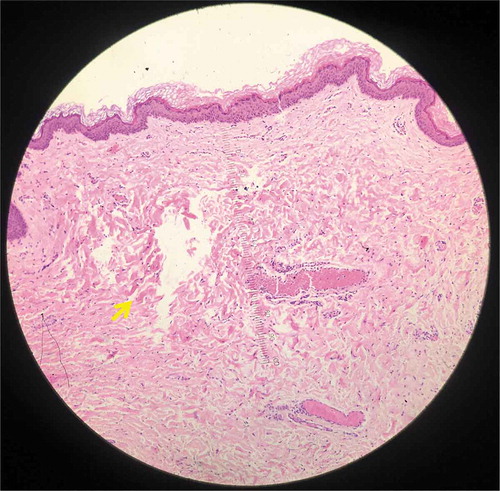
Figure 6. Histology from specimen No.6 demonstrating intra-dermal LIC (Hematoxylin and eosin (H&E), 100X).
Treatment parameters (including wavelength, duration, fluence, frequency, and repetition) and results (including lesion site and observed depth, length, and width) are summarized in .
Table 2. Specimen treatment parameters and results.
Discussion
Epidermal cell vacuolization was first reported following q-switched laser treatments on pigmented skin by Anderson et al. (Citation12), who reported vacuole formation in the cytoplasm of predominantly basal cells, caused by the explosive vaporization of melanosomes. Picosecond and nanosecond pulses are able to overwhelm the thermal relaxation time of melanosomes and rapidly heat them to supra-boiling temperatures.
Several studies have discussed the skin’s reaction to fractional picosecond laser treatment. Tanghetti et al. reported in 2016 that skin treated using a 755-nm picosecond Alexandrite laser with a fractional microlens optic showed intra-epidermal cavities (named LIOBs) in the treated tissue (Citation7,Citation8). LIOBs are hypothesized to form from a single free electron “seed”, released from laser-induced thermoionization of melanin, which creates a cascade that ultimately results in a very localized ball of hot plasma; this plasma ball then destroys local tissue with little or no collateral damage (Citation7,Citation8). Not surprisingly, LIOBs are found more often in skin types 3 or above due to increased melanin content. This is clinically significant when taking into account the melanin difference between Asian and Caucasian skins – histologic studies (Citation13–Citation15) have shown that Asian skins express larger sized melanosomes and higher percentages of individual melanosomes when compared to Caucasian skins. These key differences increase the rates of laser-induced complications such as blistering, post-inflammatory hyperpigmentation, or hypopigmentation in Asian patients, but may also influence the amount, depth, and size of LIOBs generated by treatment. LIOBs at the dermal-epidermal junction were sometimes noted to be filled with blood, and adjacent dermal hemorrhage has been noted via histology (Citation7,Citation8).
A fundamental reason for the interest in LIOBs lies in their link to local skin rejuvenation. For example, Brauer JA et al. reported an increase of dermal collagen and elastin in patients who underwent picosecond laser treatment (Citation16),and Habbema et al. reported new collagen formation around areas of optical breakdown (Citation17). These studies suggest microlesional histological changes such as LIOBs to be the basis for the rejuvenation and skin-remodeling effects of the picosecond laser.
Balu et al. reported LIOBs in skin after treatment with the fractional picosecond Nd:YAG laser holographic beamsplitter at 532 nm and 1064 nm, as measured by in vivo multiphoton-microscopy.Citation11 In 2017, Jennings et al. (Citation7) studied skin treated with the same laser, and reported focal areas of intra-epidermal vacuoles with superficial dermal hemorrhage. Focal areas of epidermal necrosis consistent with surface ablation were also seen in specimens treated at the highest energy settings of the 532-nm wavelength.
Results in this study were similar to the results reported by Jennings(Citation7), in that intra-epidermal LIOBs were found in treated skin at both the 532- and 1064-nm wavelengths. Lesion size was not often reported, but one in vivo study measured the size of intra-epidermal vacuoles to be 35–65 μm in diameter(Citation6), which is comparable to our result of 25–55 μm. In contrast, dermal lesions were much larger, with a diameter of at least 120 μm, and some reaching a length or width of over 500 μm.
Lesion depth was more commonly reported. We found intra-dermal LICs at a depth of 215–970 μm and 155–540 μm when using the 532- and 1064-nm wavelengths, respectively. Similarly, Schomacker and Bhawalkar reported a focus depth of 500μm when using the Nd:YAG picosecond laser system with a holographic beam-splitting optic(Citation10). Habbema et al. reported a focus depth of between 100 and 750 μm when using the flash lamp pumped SLM TEM00 1064nm Nd:YAG laser – the much larger numerical aperture allows for a sharper focus, and subsequently a better penetration(Citation17).
Interestingly, we have found a correlation between energy settings and lesion depth: higher energy settings produced more superficial intra-epidermal LIOBs, while the opposite produced deeper intra-dermal LICs. Though seemingly counterintuitive at first glance, the underlying mechanism (Citation10) is quite simple. When a high-energy laser beam passes the threshold for intra-epidermal LIOBs formation, the remaining laser energy is highly absorbed by the plasma surrounding the LIOB, and very little energy reaches the dermis. Conversely, when a low-energy laser beam bypasses the epidermis without triggering the threshold, this energy instead focuses into intra-dermal LICs through chromophore-assisted subsurface ablation. Although this phenomenon was reported by Ribé in 2016 (Citation9), to the best of our knowledge, it has not been demonstrated in Asian skins before.
Also, while previous histological studies have sometimes been challenged on the grounds of LIOBs or LICs appearing like histological artifacts, our results demonstrate lesion formation at regular intervals, with clear margins and more or less intact shapes. Furthermore, the distances between lesions were measured to be around 600 μm, or a multiple of 600 μm – this corresponds nicely with the official foci distance of the PicoWay applicator, and is a strong indicator that our observed lesions are indeed LIOBs or LICs, instead of artifacts.
One major limitation of our study lies in its ex vivo conditions, which differs from in vivo conditions in terms of temperature, water content, dermal capillary distribution, and local erythrocyte (hemoglobin) concentration. Accordingly, we noted less post-treatment dermal hemorrhage in comparison with in vivo studies, where dermal blood flow may have damaged capillaries and caused focal dermal hemorrhage when laser energy was absorbed by hemoglobin. Lack of circulation is also evident through the collapsed capillaries in the papillary dermis.
Conclusion
To the best of our knowledge, this is the first histological report of the Asian skin treated by the fractionated picosecond 532/1064 nm Nd:YAG laser. Our results agree with current literature concerning Caucasian skin and affirms the effective mechanism of the Nd:YAG picosecond laser across various skin types. We also found an inverse relationship between lesion depth and energy level, which remains a seldom-reported phenomenon of unknown esthetic treatment ramifications – since lesion formation lies at the center of the picosecond laser’s clinical efficacy, it is theoretically possible for the deeper and much larger intra-dermal LICs to have a greater rejuvenating effect than the more superficial intra-epidermal LIOBs. Further study on the mechanism of such lesions and its link to clinical efficacy will be invaluable to physicians who aim to use this novel technology for esthetic treatment.
References
- Bernstein EF, Schomacker KT, Basilavecchio LD, Plugis JM, Bhawalkar JD. Treatment of acne scarring with a novel fractionated, dual-wavelength, picosecond-domain laser incorporating a novel holographic beam-splitter. Lasers Surg Med. 2017;49(9):796–802.doi:10.1002/lsm.v49.9.
- Bernstein EF, Schomacker KT, Basilavecchio LD, Plugis JM, Bhawalkar JD. A novel dual-wavelength, Nd: YAG,picosecond-domain laser safely and effectively removes multicolor tattoos. Lasers Surg Med. 2015 Sep;47(7):542–48. doi:10.1002/lsm.v47.7.
- Guss L, Goldman MP, Wu DC. Picosecond 532 nm neodymium-doped yttrium aluminium garnet laser for the treatment of solar lentigines in darker skin types: safety and efficacy. Dermatol Surg. 2017 Mar;43(3):456–59.
- Bernstein EF, Schomacker KT, Paranjape AS, Bhawalkar JD. Treatment of photoaging with a dual- wavelength, 532 nm and 1,064 nm picosecond- domain laser producing a fractionated treatment beam using a holographic optic. J Drugs Dermatol. 2017 Nov;16(11):1077–82.
- Tanghetti EA. The histology of skin treated with a picosecond alexandrite laser and a fractional lens array. Lasers Surg Med. 2016 Sep;48(7):646–52. doi:10.1002/lsm.v48.7.
- Tanghetti EA, Tartar DM.Comparison of the cutaneous thermal signatures over twenty-four hours with a picosecond alexandrite laser using a flat or fractional optic. J Drugs Dermatol. 2016;15(11):1347–52.
- Tanghetti E, Jennings JA. Comparative study with a 755 nm picosecond alexandrite laser with a diffractive lens array and a 532 nm/1,064 nm Nd: YAG with a holographic optic. Lasers Surg Med. 2018 Jan;50(1):37–44. doi:10.1002/lsm.22752.
- Mirkov M, Sierra R, Tanghetti E.Theoretical analysis of the mechanism producing the histologically observed epidermal changes with a picosecond alexandrite laser with diffractive lens array. Lasers Surg Med. 2016;48(S27):1.
- Ribé A Histological study to evaluate the mechanism of action of the new fractional handpieces of a picosecond laser (532 and 1064nm) in human skin. 7th 5-Continent-Congress Meeting. 2016.
- Schomacker K, Bhawalkar JD. PicoWay® resolveTM histologies, epidermal (LIOBs) and dermal (LICs) lesions, mechanisms of action. Syneron Candela Clinical Bulletin. 2016
- Balu M, Lentsch G, Korta D, König K, Kelly KM, Tromberg BJ, Zachary CB. In vivo multiphoton- microscopy of picosecond-laser induced optical breakdown in human skin. Lasers Surg Med. 2017 Aug;49(6):555–62. doi:10.1002/lsm.v49.6.
- Anderson AA, Margolis RJ, Watanabe S, Flotte TF, Hruza GJ, Dover JS. Selective photothermolysis of cutaneous pigmentation by q-switched Nd: YAG laser pulses at 1064, 532, and 355 nm. J Invest Dermatol. 1989 Jul;93(1):28–32. doi:10.1111/1523-1747.ep12277339.
- Chan HH, Alam M, Kono T, Dover JS. Clinical application of lasers in Asians. Dermatol Surg. 2002 Jul;28(7):556–63. doi:10.1046/j.1524-4725.2002.01307.x.
- Thong HY, Jee SH, Sun CC, Boissy RE. The patterns of melanosome distribution in keratinocytes of human skin as one determining factor of skin colour. Br J Dermatol. 2003 Sep;149(3):498–505. doi:10.1046/j.1365-2133.2003.05473.x.
- Zaidi KU, Ali AS, Ali SA, Naaz I. Microbial tyrosinases: promising enzymes for pharmaceutical, food bioprocessing, and environmental industry. Biochem Res Int. 2014;2014:854687. doi:10.1155/2014/854687.
- Brauer JA, Kazlouskaya V, Alabdulrazzaq H, Bae YS, Bernstein LJ, Anolik R, Heller PA, Geronemus RG. Use of a picosecond pulse duration laser with specialized optic for treatment of facial acne scarring. JAMA Dermatol. 2015 Mar;151(3):278–84. doi:10.1001/jamadermatol.2014.3045.
- Habbema L, Verhagen R, Van Hal R, Liu Y, Varghese B. Minimally invasive non-thermal laser technology using laser- induced optical breakdown for skin rejuvenation. J Biophotonics. 2012 Feb;5(2):194–99. doi:10.1002/jbio.201100083.
