Abstract
Objective. Pulmonary surfactant is a complex molecule of lipids and proteins synthesized and secreted by type II alveolar cells into the alveolar epithelial lining. Both lipid and protein components are essential for lung function in postnatal life. Infection is a well-established cause of preterm delivery, and several inflammatory cytokines play a role in the mechanisms of preterm parturition. An increased concentration of inflammatory cytokines in amniotic fluid or fetal plasma has been linked to the onset of preterm parturition and fetal/neonatal injury, including cerebral palsy and chronic lung disease. Experimental evidence indicates that inflammatory mediators also regulate surfactant protein synthesis, and histologic chorioamnionitis is associated with a decreased incidence of hyaline membrane disease in neonates. This study was conducted to determine if amniotic fluid concentrations of surfactant protein (SP)-A, SP-B, and SP-D change in patients with and without intra-amniotic infection (IAI).
Materials and methods. A case–control study was conducted to determine amniotic fluid concentrations of SP-A, SP-B, SP-D, and total protein in patients who had an amniocentesis performed between 18 and 34 weeks of gestation for the detection of IAI in patients with spontaneous preterm labor with intact membranes (n = 42) and cervical insufficiency prior to the application of cerclage (n = 6). Amniotic fluid samples were selected from a bank of biological specimens and included patients with (n = 16) and without (n = 32) IAI matched for gestational age at amniocentesis. Intra-amniotic infection was defined as a positive amniotic fluid culture for microorganisms. Each group was further subdivided according to a history of corticosteroid administration within 7 days prior to amniocentesis into the following subgroups: (1) patients without IAI who had received antenatal corticosteroids (n = 21), (2) patients with IAI who had received antenatal corticosteroids (n = 9), (3) patients without IAI who had not received antenatal corticosteroids (n = 11), and (4) patients with IAI who had not received antenatal corticosteroids (n = 7). Amniotic fluid was obtained by transabdominal amniocentesis. SP-A, SP-B, and SP-D concentrations in amniotic fluid were determined by enzyme-linked immunosorbent assay (ELISA). Non-parametric statistics were used for analysis.
Results. Women with IAI had a higher median amniotic fluid concentration of SP-B and of SP-B/total protein, but not other SPs, than those without IAI (both p = 0.03). Among patients who had received antenatal corticosteroids, the median amniotic fluid concentration of SP-B and of SP-B/total protein was significantly higher in patients with IAI than in those without IAI (SP-B, IAI: median 148 ng/mL, range 37.3–809 ng/mL vs. without IAI: median 7.2 ng/mL, range 0–1035 ng/mL; p = 0.005 and SP-B/total protein, IAI: median 14.1 ng/mg, range 4.3–237.5 ng/mg vs. without IAI: median 1.45 ng/mg, range 0–79.5 ng/mg; p = 0.003). Among women who had not received antenatal corticosteroids, the median amniotic fluid concentrations of SP-B and of SP-B/total protein were not significantly different between patients with and without IAI (SP-B, IAI: median 4 ng/mL, range 0–31.4 ng/mL vs. without IAI: median 3.4 ng/mL, range 0–37 ng/mL; p = 0.8 and SP-B/total protein, IAI: median 0.55 ng/mg, range 0–6.96 ng/mg vs. without IAI: median 0.59 ng/mg, range 0–3.28 ng/mg; p = 0.9). The median amniotic fluid concentrations of SP-A, SP-A/total protein, SP-D, and SP-D/total protein were not significantly different between patients with and without IAI whether they received antenatal corticosteroids or not (all p > 0.05).
Conclusions. IAI was associated with an increased amniotic fluid concentration of SP-B in patients who received antenatal corticosteroids within 7 days prior to amniocentesis.
Introduction
Intrauterine infection has been implicated in the etiology of preterm birth and is present in approximately 25% of all preterm deliveries Citation[1],Citation[2]. Increased concentrations of pro-inflammatory cytokines in amniotic fluid and fetal plasma have been linked to the onset of preterm parturition and fetal or neonatal injury, including cerebral palsy Citation[3-5] and chronic lung disease Citation[6-8]. A recent study has suggested that neonates with histologic chorioamnionitis have a decreased incidence of hyaline membrane disease Citation[9], possibly through the increased production of surfactant protein (SP) Citation[9].
Pulmonary surfactants are composed of a lipid–protein complex. Currently, four types of SPs have been characterized: SP-A, SP-B, SP-C, and SP-D. SP-A and SP-D are hydrophilic and involved in host defense against infection, regulation of surfactant structure or homeostasis, and immunomodulation Citation[10-12]. In contrast, SP-B and SP-C are hydrophobic and essential for lung function after birth Citation[10-12]. Alveolar type II cells in the lung alveoli synthesize all four SPs and surfactant lipids. That all SPs are present in amniotic fluid is thought to be the result of lung liquid production and fetal respiration.
Several studies have reported a complex association between infection/inflammatory mediators and SP mRNA expression in lung tissues or protein concentration in bronchoalveolar lavage fluid Citation[13-15]. Animal experiments in pregnant ewes Citation[16-18], rabbits Citation[19], and mice Citation[20] have suggested that endotoxin and inflammatory cytokines regulate SP synthesis, the effects of which vary depending on the stage of pulmonary airway maturation (or gestational age) Citation[14],Citation[21], duration of exposure Citation[16],Citation[21], and routes of administration (i.e., intratracheal or intra-amniotic) Citation[17],Citation[22],Citation[23]. Moreover, the administration of glucocorticoids to animals Citation[24-27] or lung tissue explants Citation[28-31] modulates SP expression. However, there is a paucity of information on SP concentrations in human amniotic fluid in the context of intrauterine infection. The purpose of this study was to determine whether amniotic fluid concentrations of SP-A, SP-B, and SP-D change in patients with and without intra-amniotic infection (IAI).
Material and methods
Study design
A case–control study was conducted to determine the amniotic fluid concentrations of SP-A, SP-B, SP-D, and total protein in patients who had had an amniocentesis performed at between 18 and 34 weeks of gestation at Hutzel Women's Hospital, from July 1998 to December 2000. Amniotic fluid samples were selected from a bank of biological specimens and included samples from patients with (n = 16) and without (n = 32) IAI. Intra-amniotic infection was defined as a positive amniotic fluid culture for microorganisms. The groups were matched (1:2) for gestational age (within 2 weeks) at amniocentesis. The two groups were further subdivided according to history of corticosteroid administration (to induce fetal lung maturity) within 7 days prior to amniocentesis into the following subgroups: (1) patients without IAI who had received antenatal corticosteroids (n = 21), (2) patients with IAI who had received antenatal corticosteroids (n = 9), (3) patients without IAI who had not received antenatal corticosteroids (n = 11), and (4) patients with IAI who had not received antenatal corticosteroids (n = 7). Amniotic fluid was obtained by transabdominal amniocentesis. A sample of amniotic fluid was transported to the laboratory for aerobic, anaerobic, and genital Mycoplasma cultures. Amniotic fluid not required for clinical purposes was centrifuged and stored. The indication for amniocentesis was for the detection of IAI in patients with a diagnosis of: (1) spontaneous preterm labor and intact membranes (n = 42) and (2) cervical insufficiency prior to the application of cerclage (n = 6).
All women provided informed consent prior to the collection of amniotic fluid. The collection and utilization of the samples was approved by the Human Investigation Committee of the Wayne State University, Detroit, MI, USA, and approved for research purposes by the Institutional Review Board of the Eunice Kennedy Shriver National Institutes of Child Health and Human Development (NICHD). Many of these samples have been used in previous studies of inflammatory mediators, antibacterial peptides, and chemokines Citation[32-34].
Enzyme-linked immunosorbent assay for determining the SP-A, SP-B, and SP-D concentrations in amniotic fluid
The SP-A, SP-B, and SP-D concentrations in amniotic fluid were determined by a sandwich enzyme-linked immunosorbent assay (ELISA) system, which has been validated for amniotic fluid as previously described Citation[35-37]. For SP-A, the inter- and intra-assay coefficients of variation (CV) were 33.5% and 7.7%, respectively, and the sensitivity was 10 ng/mL. For SP-B, the inter- and intra-assay CV were 34.2% and 18.7%, respectively, and the sensitivity was 2 ng/mL. For SP-D, the inter- and intra-assay CV were 19.6% and 4.6%, respectively, and the sensitivity was 10 ng/mL.
Statistical analysis
The Mann–Whitney U-test was utilized to determine differences in the medians between groups. Contingency tables and Chi-square tests were employed for comparisons of proportions. A p value of <0.05 was considered significant. The statistical package used was SPSS 12.0 (SPSS Inc., Chicago, IL, USA).
Results
describes the clinical and obstetrical characteristics of the study population. There were no significant differences in the median maternal age and gestational age at amniocentesis between patients with and without IAI. Patients with IAI had a median gestational age at delivery and neonatal birth weight lower than those without IAI.
Table I. Clinical and obstetrical characteristics of patients with and without intra-amniotic infection (IAI).
SP-A was detected in all (46/46) samples, while SP-B was detected in 68.8% (33/48) of samples. SP-D was above the limit of detection in 40% (12/30) of cases.
Patients with IAI had a significantly higher median amniotic fluid concentration of SP-B than those without IAI (IAI: median 40.2 ng/mL, range 0–809 ng/mL vs. without IAI: median 6.2 ng/mL, range 0–1035 ng/mL; p = 0.03; see ). In contrast, there were no significant differences in the median amniotic fluid concentrations of SP-A and SP-D (see ). Similar results were observed after adjusting the amniotic fluid SP concentration according to the total protein concentration (SP/total protein ratio; see and ). Using analysis of covariance adjusting for the duration of sample storage and gestational age at amniocentesis, similar results were obtained.
Figure 1. Median amniotic fluid concentration of SP-B was significantly higher in patients with intra-amniotic infection (IAI) than those without IAI (IAI: median 40.2 ng/mL, range 0–809 ng/mL vs. without IAI: median 6.2 ng/mL, range 0–1035 ng/mL; p = 0.03). Similar result was observed after adjusting the amniotic fluid SP-B concentration according to the total protein concentration (SP-B/ total protein, IAI: median 7.19 ng/mg, range 0–237.5 ng/mg vs. without IAI: median 0.94 ng/mg, range 0–79.5 ng/mg; p = 0.03). *p < 0.05.
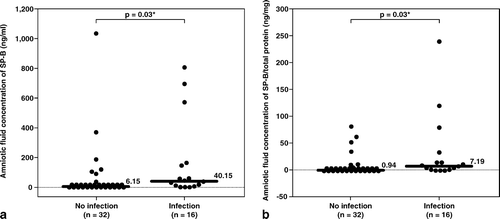
Table II. Amniotic fluid concentrations of SP-A and SP-D in patients with and without intra-amniotic infection (IAI).
Among patients who had received corticosteroid administration within 7 days before amniocentesis, there was no significant difference in the median gestational age at amniocentesis between patients with and without IAI (IAI: median 28.2 weeks, range 24.0–33.3 weeks vs. without IAI: median 30.0 weeks, range 23.0–33.3 weeks; p = 0.9; see ).
Table III. Clinical and obstetrical characteristics of patients who had and who had not received antenatal corticosteroids within 7 days prior to amniocentesis, classified by the presence or absence of intra-amniotic infection (IAI).
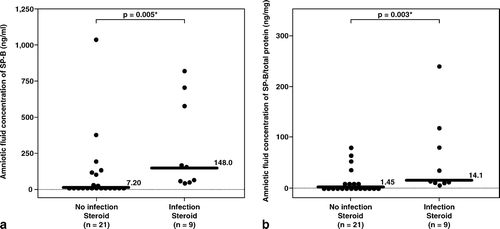
The median amniotic fluid concentrations of SP-B and of SP-B/total amniotic fluid protein were significantly higher in patients with IAI than those without IAI (SP-B, IAI: median 148 ng/mL, range 37.3–809 ng/mL vs. without IAI: median 7.2 ng/mL, range 0–1035 ng/mL; p = 0.005 and SP-B/total protein, IAI: median 14.1 ng/mg, range 4.3–237.5 ng/mg vs. without IAI: median 1.45 ng/mg, range 0–79.5 ng/mg; p = 0.003; see ).
Figure 2. Among patients who had received antenatal corticosteroids within 7 days prior to amniocentesis, the median amniotic fluid concentration of SP-B was significantly higher in patients with intra-amniotic infection (IAI) than those without IAI (IAI: median 148 ng/mL, range 37.3–809 ng/mL vs. without IAI: median 7.2 ng/mL, range 0–1035 ng/mL; p = 0.005). Similar result was observed after adjusting the amniotic fluid SP-B concentration according to the total protein concentration (SP-B/ total protein, IAI: median 14.1 ng/mg, range 4.3–237.5 ng/mg vs. without IAI: median 1.45 ng/mg, range 0–79.5 ng/mg; p = 0.003). *p < 0.05.
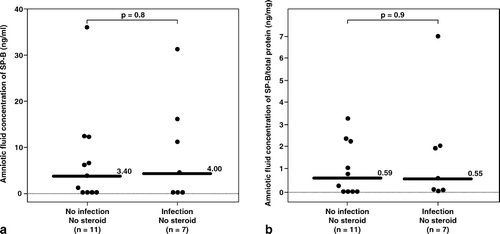
Similarly, among patients who had not received corticosteroid administration within 7 days before amniocentesis, there was no significant difference in the median gestational age at amniocentesis between patients with and without IAI (IAI: median 22.0 weeks, range 18.4–26.2 weeks vs. without IAI: median 22.0 weeks, range 18.0–27.3 weeks; p = 0.9; see ). In contrast, among patients who had not received corticosteroid within 7 days before amniocentesis, the median amniotic fluid concentrations of SP-B and of SP-B/total protein were not significantly different between patients with and without IAI (SP-B, IAI: median 4 ng/mL, range 0–31.4 ng/mL vs. without IAI: median 3.4 ng/mL, range 0–37 ng/mL; p = 0.8 and SP-B/total protein, IAI: median 0.55 ng/mg, range 0–6.96 ng/mg vs. without IAI: median 0.59 ng/mg, range 0–3.28 ng/mg; p = 0.9; see ).
Figure 3. Among patients who had not received antenatal corticosteroids within 7 days prior to amniocentesis, there was no significant difference in the median amniotic fluid concentration of SP-B between patients with and without intra-amniotic infection (IAI) (IAI: median 4 ng/mL, range 0–31.4 ng/mL vs. without IAI: median 3.4 ng/mL, range 0–37 ng/mL; p = 0.8). Similar result was observed after adjusting the amniotic fluid SP-B concentration according to the total protein concentration (SP-B/total protein, IAI: median 0.55 ng/mg, range 0–6.9 ng/mg vs. without IAI: median 0.59 ng/mg, range 0–3.3 ng/mg; p = 0.9).
The median amniotic fluid concentrations of SP-A, SP-A/total protein, SP-D, and SP-D/total protein were not significantly different between patients with and without IAI whether they had received antenatal corticosteroids or not (see ).
Table IV. Amniotic fluid concentrations of SP-A and SP-D in patients who had and who had not received antenatal corticosteroids within 7 days prior to amniocentesis.
Discussion
Principal findings of this study
(1) IAI was found to be associated with an increase in the amniotic fluid concentration of SP-B, but not SP-A and SP-D, in patients who had received corticosteroid administration within 7 days prior to amniocentesis. (2) In contrast, among patients who had not received corticosteroids, amniotic fluid concentrations of SP-A, SP-B, and SP-D were not significantly different between those with and without IAI.
Infection, microbial products, pro-inflammatory cytokines, and surfactant proteins
Intrauterine infection is associated with an increased amniotic fluid concentration of several pro-inflammatory cytokines and chemokines such as interleukin (IL)- 1, IL-8, IL-6, tumor necrosis factor (TNF)-α, monocyte chemotactic peptide-1 (MCP-1), etc. Citation[1],Citation[32],Citation[38],Citation[39]. Experimental evidence indicates that endotoxin and several cytokines regulate surfactant protein synthesis Citation[13-15]. Administration of lipopolysaccharides (LPS) or IL-1α into the amniotic fluid cavity of rabbits up-regulates SP-A and SP-B mRNA expression in lung tissue, SP-A and SP-B protein in bronchoalveolar lavage, and improves lung function Citation[19]. Recombinant IL-6 increases the production of SP-A mRNA expression and protein in H441-4, a human pulmonary adenocarcinoma cell line Citation[9]. In contrast, TNF-α has been shown to inhibit SP-A mRNA and protein synthesis but not SP-B Citation[40]. Subsequent studies have shown that the effect of infection/inflammation on the fetal lung differs depending on the gestational age and duration of exposure to intra-amniotic endotoxin/cytokines. For example, in rabbits, IL-1α has been shown to increase SP-A, SP-B, and SP-C mRNA expression in immature lung explants but decrease the expression of these SPs in mature lungs Citation[14].
Similarly, studies in sheep have suggested that intra-amniotic endotoxin in early gestation Citation[21] programs a response that results in an increased mRNA expression for SP-A, SP-B, as well as SP-C 65 days later (without a consistent effect on SP-D), and also markedly induces the processing of SP-B protein to its mature form Citation[17]. However, the response was not normal maturation, but more likely to represent maldevelopment of the surfactant system since the large increase in lung tissue surfactant lipids was not accompanied by a corresponding increase in alveolar surfactant lipids and proteins. This increase in lung tissue surfactant without effective secretion is similar to the abnormalities observed in ventilated Citation[21] preterm baboons that developed bronchopulmonary dysplasia Citation[21],Citation[41],Citation[42]. In contrast, endotoxin exposure in later gestation causes parallel increases in tissue and alveolar surfactants Citation[21].
A time-course study in late-gestation sheep has indicated that endotoxin-induced injury in the first 24 hours causes a decrease in pneumocyte type II cells as well as SP-B protein, but an increase in HSP70 expression followed by an increase in pneumocyte type II cells, SP-B mRNA, and protein in lung tissue and lavage fluid at 72 hours, which is consistent with a tissue remodeling process Citation[43]. Moreover, this group of investigators has also suggested that a single endotoxin exposure in early gestation preferentially induces surfactant without alterations in alveolarization and a subtle increase in alveolar wall thickness. In contrast, endotoxin exposure in later gestation, around the period of alveolarization, or chronic exposure prior to that period, causes a decrease in alveolar numbers and the development of a small lung Citation[21]. However, a subsequent study has shown that the fetal lung, when assessed closer to term gestation, can recover and develop relatively normally Citation[44]. Interestingly, LPS-induced changes in the lung following intra-amniotic injection required direct contact between LPS and the fetal lung, since occlusion of the airway prevented changes in the fetal lung Citation[45]. Prince et al. Citation[20] have suggested that LPS improves lung function in mice and increases the number of alveolar type II cells through stimulation of Toll-like receptor 4 and NFκB pathways.
In the present study, we did not find significant changes in the amniotic fluid concentration of any SPs in patients with IAI and without antenatal corticosteroid exposure. Since the median gestational age at aminiocentesis in this group was only 22 weeks, it is possible that there was an increased concentration of SPs in the lung tissue of patients with IAI. However, these proteins might not be secreted into the amniotic fluid cavity similar to the observation from experiment in sheep [17]. Finally, information from experimentally-induced IAI may be different from that of naturally-occurring human IAI.
Glucocorticoids and surfactant protein
Experimental evidence derived from human fetal lung explants suggests that glucocorticoids have marked dose-dependent, reversible, stimulatory effects on the mRNA expression for SP-B and SP-C Citation[28], and a modest effect on SP-D Citation[30]. In contrast, glucocorticoids have been shown to have a biphasic response in SP-A mRNA expression Citation[29]. An increased mRNA expression of SP-A has been observed in human lung explants with exposure to low concentrations of dexamethasone (≤10 nM) for less than 48 hours, but decreased expression has been observed after a longer exposure to low concentrations or incubation with high concentrations (100 nM) of dexamethasone Citation[29]. However, these results contrast with those reported in lambs. Administration of betamethasone at weekly intervals for three weeks resulted in increased mRNA expression and protein expression for both SP-A and SP-B in fetal lung tissues and bronchoalveolar lavage fluid. In contrast, animals treated for 48 hours, had overexpression of SP-B mRNA Citation[31].
Our study did not examine the effect of steroids alone on the amniotic fluid concentrations of SP in patients with and without IAI, since the median gestational age in patients who had not received antenatal steroids (22 weeks) was much lower than those who had received antenatal steroids (28 weeks), and the amniotic fluid concentrations of SP change with gestational age Citation[35],Citation[46-49].
Combined effects of cytokines and glucocorticoids on surfactant proteins
The present study shows that IAI is associated with an increased amniotic fluid concentration of SP-B, but not SP-A or SP-D, in women who have received corticosteroid administration within 7 days prior to amniocentesis. This observation is consistent with a study in rabbit lung explants Citation[50]. Vayrynen et al. Citation[50] noted the synergistic effect of glucocorticoids and cytokines on lung surfactant and concluded that dexamethasone consistently increases SP-B mRNA in explants of lung tissue from all gestational ages. This study indicated that in immature lungs, IL-1α and dexamethasone additively increase the mRNA expression of SP-A and of SP-B. In contrast, in lung explants obtained later in gestation, SP-B and SP-C expression are suppressed by IL-1α, while glucocorticoids tend to increase the expression of SP-B and SP-C and prevent the IL-1-induced suppression effects Citation[50].
Strengths and limitations of the study
This study is the first to evaluate amniotic fluid concentrations of SPs in patients with IAI. Moreover, the effects of antenatal steroid administration were examined. The majority of patients in our study had received antenatal steroids, which reflects clinical practice. After the NICHD consensus statement on the use of corticosteroids for fetal lung maturation in 1994, most patients at between 24 and 34 weeks of gestation who are at risk for preterm delivery receive antenatal steroids. However, among patients who did not receive antenatal steroids, our study did not find significant changes in the amniotic fluid concentrations of any SPs in patients with IAI. This is probably due to the fact that the median gestational age of patients in these subgroups was 22 weeks (range 18–27 weeks), which may be too early to observe a significant change in SPs because of the low concentrations of these proteins in amniotic fluid. Moreover, the ELISA assay system, which has been validated for amniotic fluid Citation[35-37], has slightly high inter- and intra-assay CV. To-date, there is no commercially available assay for surfactant proteins (except SP-D).
In conclusion, IAI was found to be associated with a variable, but significantly increased amniotic fluid concentration of SP-B in patients who received antenatal corticosteroids within 7 days prior to amniocentesis. This observation supports the view that there may be a beneficial effect of antenatal steroid administration in patients with sub-clinical intrauterine infection in late gestation (e.g. 28 weeks).
Acknowledgements
This research was supported in part by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Romero R, Mazor M, Wu Y K, Sirtori M, Oyarzun E, Mitchell M D, Hobbins J C. Infection in the pathogenesis of preterm labor. Semin Perinatol 1988; 12: 262–279
- Goncalves L F, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev 2002; 8: 3–13
- Yoon B H, Jun J K, Romero R, Park K H, Gomez R, Choi J H, Kim I O. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol 1997; 177: 19–26
- Yoon B H, Romero R, Park J S, Kim C J, Kim S H, Choi J H, Han T R. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol 2000; 182: 675–681
- Yoon B H, Park C W, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. BJOG 2003; 110(Suppl 20)124–127
- Yoon B H, Romero R, Jun J K, Park K H, Park J D, Ghezzi F, Kim B I. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1997; 177: 825–830
- Ghezzi F, Gomez R, Romero R, Yoon B H, Edwin S S, David C, Janisse J, Mazor M. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol 1998; 78: 5–10
- Yoon B H, Romero R, Kim K S, Park J S, Ki S H, Kim B I, Jun J K. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1999; 181: 773–779
- Shimoya K, Taniguchi T, Matsuzaki N, Moriyama A, Murata Y, Kitajima H, Fujimura M, Nakayama M. Chorioamnionitis decreased incidence of respiratory distress syndrome by elevating fetal interleukin-6 serum concentration. Hum Reprod 2000; 15: 2234–2240
- Whitsett J A. Surfactant proteins in innate host defense of the lung. Biol Neonate 2005; 88: 175–180
- Whitsett J A, Weaver T E. Hydrophobic surfactant proteins in lung function and disease. N Engl J Med 2002; 347: 2141–2148
- Wright J R. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 2005; 5: 58–68
- Dhar V, Hallman M, Lappalainen U, Bry K. Interleukin-1 alpha upregulates the expression of surfactant protein-A in rabbit lung explants. Biol Neonate 1997; 71: 46–52
- Glumoff V, Vayrynen O, Kangas T, Hallman M. Degree of lung maturity determines the direction of the interleukin-1- induced effect on the expression of surfactant proteins. Am J Respir Cell Mol Biol 2000; 22: 280–288
- Vayrynen O, Glumoff V, Hallman M. Regulation of surfactant proteins by LPS and proinflammatory cytokines in fetal and newborn lung. Am J Physiol Lung Cell Mol Physiol 2002; 282: L803–810
- Kramer B W, Moss T J, Willet K E, Newnham J P, Sly P D, Kallapur S G, Ikegami M, Jobe A H. Dose and time response after intraamniotic endotoxin in preterm lambs. Am J Respir Crit Care Med 2001; 164: 982–988
- Bachurski C J, Ross G F, Ikegami M, Kramer B W, Jobe A H. Intra-amniotic endotoxin increases pulmonary surfactant proteins and induces SP-B processing in fetal sheep. Am J Physiol Lung Cell Mol Physiol 2001; 280: L279–285
- Jobe A H, Newnham J P, Willet K E, Moss T J, Gore E M, Padbury J F, Sly P, Ikegami M. Endotoxin-induced lung maturation in preterm lambs is not mediated by cortisol. Am J Respir Crit Care Med 2000; 162: 1656–1661
- Bry K, Lappalainen U, Hallman M. Intraamniotic interleukin-1 accelerates surfactant protein synthesis in fetal rabbits and improves lung stability after premature birth. J Clin Invest 1997; 99: 2992–2999
- Prince L S, Okoh V O, Moninger T O, Matalon S. Lipopolysaccharide increases alveolar type II cell number in fetal mouse lungs through Toll-like receptor 4 and NF-kappaB. Am J Physiol Lung Cell Mol Physiol 2004; 287: L999–1006
- Moss T J, Newnham J P, Willett K E, Kramer B W, Jobe A H, Ikegami M. Early gestational intra-amniotic endotoxin: Lung function, surfactant, and morphometry. Am J Respir Crit Care Med 2002; 165: 805–811
- Newnham J P, Moss T J, Kramer B W, Nitsos I, Ikegami M, Jobe A H. The fetal maturational and inflammatory responses to different routes of endotoxin infusion in sheep. Am J Obstet Gynecol 2002; 186: 1062–1068
- Mulrooney N, Jobe A H, Ikegami M. Lung inflammatory responses to intratracheal interleukin-1alpha in ventilated preterm lambs. Pediatr Res 2004; 55: 682–687
- Ballard P L, Ning Y, Polk D, Ikegami M, Jobe A H. Glucocorticoid regulation of surfactant components in immature lambs. Am J Physiol 1997; 273: L1048–1057
- Newnham J P, Kallapur S G, Kramer B W, Moss T J, Nitsos I, Ikegami M, Jobe A H. Betamethasone effects on chorioamnionitis induced by intra-amniotic endotoxin in sheep. Am J Obstet Gynecol 2003; 189: 1458–1466
- Moss T J, Mulrooney N P, Nitsos I, Ikegami M, Jobe A H, Newnham J P. Intra-amniotic corticosteroids for preterm lung maturation in sheep. Am J Obstet Gynecol 2003; 189: 1389–1395
- Bolt R J, van Weissenbruch M M, Lafeber H N, Delemarre-van de Waal H A. Glucocorticoids and lung development in the fetus and preterm infant. Pediatr Pulmonol 2001; 32: 76–91
- Liley H G, White R T, Warr R G, Benson B J, Hawgood S, Ballard P L. Regulation of messenger RNAs for the hydrophobic surfactant proteins in human lung. J Clin Invest 1989; 83: 1191–1197
- Iannuzzi D M, Ertsey R, Ballard P L. Biphasic glucocorticoid regulation of pulmonary SP-A: Characterization of inhibitory process. Am J Physiol 1993; 264: L236–244
- Dulkerian S J, Gonzales L W, Ning Y, Ballard P L. Regulation of surfactant protein D in human fetal lung. Am J Respir Cell Mol Biol 1996; 15: 781–786
- Ballard P L, Ertsey R, Gonzales L W, Gonzales J. Transcriptional regulation of human pulmonary surfactant proteins SP-B and SP-C by glucocorticoids. Am J Respir Cell Mol Biol 1996; 14: 599–607
- Esplin M S, Romero R, Chaiworapongsa T, Kim Y M, Edwin S, Gomez R, Mazor M, Adashi E Y. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med 2005; 17: 365–373
- Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, Bujold E, Camacho N, Kim Y M, Hassan S, et al. Antimicrobial peptides in amniotic fluid: Defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med 2003; 13: 2–21
- Chaiworapongsa T, Romero R, Espinoza J, Kim Y M, Edwin S, Bujold E, Gomez R, Kuivaniemi H. Macrophage migration inhibitory factor in patients with preterm parturition and microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med 2005; 18: 405–416
- Pryhuber G S, Hull W M, Fink I, McMahan M J, Whitsett J A. Ontogeny of surfactant proteins A and B in human amniotic fluid as indices of fetal lung maturity. Pediatr Res 1991; 30: 597–605
- LeVine A M, Whitsett J A, Hartshorn K L, Crouch E C, Korfhagen T R. Surfactant protein D enhances clearance of influenza A virus from the lung in vivo. J Immunol 2001; 167: 5868–5873
- Kingma P S, Whitsett J A. In defense of the lung: Surfactant protein A and surfactant protein D. Curr Opin Pharmacol 2006; 6: 277–283
- Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton D B, Dinarello C A. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol 1992; 27: 117–123
- Romero R, Manogue K R, Mitchell M D, Wu Y K, Oyarzun E, Hobbins J C, Cerami A. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol 1989; 161: 336–341
- Wispe J R, Clark J C, Warner B B, Fajardo D, Hull W E, Holtzman R B, Whitsett J A. Tumor necrosis factor-alpha inhibits expression of pulmonary surfactant protein. J Clin Invest 1990; 86: 1954–1960
- Awasthi S, Coalson J J, Crouch E, Yang F, King R J. Surfactant proteins A and D in premature baboons with chronic lung injury (bronchopulmonary dysplasia). Evidence for an inhibition of secretion. Am J Respir Crit Care Med 1999; 160: 942–949
- Seidner S R, Jobe A H, Coalson J J, Ikegami M. Abnormal surfactant metabolism and function in preterm ventilated baboons. Am J Respir Crit Care Med 1998; 158: 1982–1989
- Kramer B W, Kramer S, Ikegami M, Jobe A H. Injury, inflammation, and remodeling in fetal sheep lung after intra-amniotic endotoxin. Am J Physiol Lung Cell Mol Physiol 2002; 283: L452–459
- Kallapur S G, Nitsos I, Moss T J, Kramer B W, Newnham J P, Ikegami M, Jobe A H. Chronic endotoxin exposure does not cause sustained structural abnormalities in the fetal sheep lungs. Am J Physiol Lung Cell Mol Physiol 2005; 288: L966–974
- Moss T J, Nitsos I, Kramer B W, Ikegami M, Newnham J P, Jobe A H. Intra-amniotic endotoxin induces lung maturation by direct effects on the developing respiratory tract in preterm sheep. Am J Obstet Gynecol 2002; 187: 1059–1065
- Miyamura K, Malhotra R, Hoppe H J, Reid K B, Phizackerley P J, Macpherson P, Lopez B A. Surfactant proteins A (SP-A) and D (SP-D): Levels in human amniotic fluid and localization in the fetal membranes. Biochim Biophys Acta 1994; 1210: 303–307
- King R J, Ruch J, Gikas E G, Platzker A C, Creasy R K. Appearance of apoproteins of pulmonary surfactant in human amniotic fluid. J Appl Physiol 1975; 39: 735–741
- Shelley S A, Balis J U, Paciga J E, Knuppel R A, Ruffolo E H, Bouis Jr PJ. Surfactant ‘apoproteins' in human amniotic fluid: An enzyme-linked immunosorbent assay for the prenatal assessment of lung maturity. Am J Obstet Gynecol 1982; 144: 224–228
- Dilger I, Schwedler G, Dudenhausen J W. Determination of the pulmonary surfactant-associated protein SP-B in amniotic fluid with a competition ELISA. Gynecol Obstet Invest 1994; 38: 24–27
- Vayrynen O, Glumoff V, Hallman M. Inflammatory and anti-inflammatory responsiveness of surfactant proteins in fetal and neonatal rabbit lung. Pediatr Res 2004; 55: 55–60